Abstract
Objectives
Alterations in miRNA expression in post-mortem brain tissue or peripheral blood have been linked to schizophrenia. Cerebrospinal fluid might provide an in vivo biomarker more directly reflecting functional changes in the brain. The goals of this study were to determine the feasibility of detecting miRNAs in cerebrospinal fluid and to compare miRNA levels in cerebrospinal fluid versus blood.
Methods
Four healthy volunteers and four patients with psychotic disorders underwent lumbar puncture and a blood draw. Expression of 378 validated microRNAs was assessed from each biofluid type for each subject using microarray technology. Five miRNAs were chosen for validation with qPCR.
Results
A substantial number of microRNAs (n=95) were exclusively or predominately detected in CSF. Levels of 35 miRNAs detected in both CSF and blood samples in all subjects were poorly correlated.
Conclusions
The investigation of microRNAs in CSF can help advance the understanding of psychiatric diseases and particularly schizophrenia.
Keywords: Schizophrenia, miRNA, cerebrospinal fluid, gene expression, psychosis, biomarker
Introduction
MicroRNAs (miRNAs) are small non-coding RNA fragments that are involved in post-transcriptional regulation of messenger RNAs. Due to their ability to simultaneously regulate the transcription of multiple mRNAs, miRNAs are appealing as potential biomarkers in the study of psychiatric diseases, where multiple mechanisms and pathways are likely implicated (Hunsberger, 2009).
Research on miRNAs has already been fruitful in the fields of oncology (Lu, 2005) and cardiology (Latronico, 2009), but has limited traction in psychiatric investigations to date. Given the inherent limitations in accessing brain tissue in vivo, most human studies have utilized port-mortem samples (Perkins, 2007; Beveridge, 2008; Beveridge 2010; Mellios, 2009; Kim 2010; Santarelli, 2011), or peripheral blood (Gardiner 2011; Rong, 2011) to study miRNA expression. However, these approaches have methodological limitations: the study of postmortem brain tissue may be confounded by long postmortem interval and prior medication exposure (McCullumsmith, 2011); alternately, peripheral blood may not closely reflect gene expression levels in brain, especially since a subset of miRNAs may be brain-specific (Lagos-Quintana 2002; Kim, 2004).
Given the intimate relationship between brain and cerebrospinal fluid (CSF), the use of CSF-derived indices represents an intriguing new approach to identify Central Nervous System (CNS) biomarkers. To date, very few studies have examined miRNA levels in CSF, in patients with Alzheimer's disease (Cogswell, 2008; Ghidoni, 2011) B-cell lymphoma of the CNS (Baraniskin, 2011) and Glioma (Baraniskin, 2012). To our knowledge, this approach has not yet been utilized in patients with psychiatric disorders. Importantly, no study has compared miRNA expression profiles in CSF and peripheral blood drawn from the same subjects, and recently-developed miRNA expression microarrays have not been tested in CSF studies.
The ultimate goal of our study is to examine miRNA levels in CSF and peripheral blood in patients with schizophrenia; however for this preliminary methodological report, we focus on the comparison in miRNA detection between CSF and peripheral blood using microarray technology. Given that gene expression patterns have shown to vary across different tissues, including brain and peripheral blood (Sullivan et al., 2006), we hypothesized that a subset of CNS-relevant miRNAs will be detected exclusively or predominately in CSF compared to peripheral blood, and that miRNA levels detected in CSF will be largely uncorrelated with levels derived from blood samples drawn from the same subjects. Data from this study can then be utilized to inform larger scale studies of the relationship between CSF miRNA and susceptibility for psychiatric illness.
Methods
Subjects
Patients with a diagnosis of schizophrenia, schizoaffective disorder, psychosis Not Otherwise Specified (NOS) and long history of antipsychotic treatment were recruited from the outpatient department at The Zucker Hillside Hospital. Healthy controls were recruited from the general population via word of mouth, newspaper and internet advertisements and poster flyers. Patients and healthy volunteers were excluded from participating in the study if they were prescribed an anticoagulant, had a history of an organic brain disorder, had a positive urine toxicology test or had clinically significant thrombocytopenia or coagulopathy based on results of CBC, PT, and APTT screening. Additionally, healthy volunteers were excluded if they had an Axis I diagnosis, or if they had a first-degree relative with a known or suspected axis I disorder, based on family history questionnaire. All subjects provided written informed consent to a protocol approved by the Institutional Review Board (IRB) of the North Shore – Long Island Jewish Health System.
Procedure
In a single session, subjects underwent a blood draw followed by a lumbar puncture, performed using a standard technique with a 25 gauge, Whitacre-point spinal needle. Two Paxgene whole blood tubes and 15–25 cc of CSF were obtained from each subject.
RNA Isolations from Human CSF and PaxGene Whole Blood
Total RNA was isolated from 7.5mls of human cerebrospinal fluid (CSF) by Asuragen, Inc. according to a proprietary large-scale biofluid RNA isolation procedure. Because RNA concentration was not measurable, single target TaqMan® miRNA qRT-PCR assays (Applied Biosystems) were tested using 150 CSF equivalents (CE) of unamplified RNA as a surrogate assessment of miRNA abundance.
Total RNA was isolated from PAXgene®-collected blood samples. The purity and quantity of total RNA samples were determined by absorbance readings at 260 and 280 nm using a NanoDrop ND-1000 UV spectrophotometer and fell within Asuragen's acceptable limits (A260/A280 greater than 1.6). The integrity of total RNA was qualified by Agilent Bioanalyzer 2100 capillary electrophoresis and samples fell within Asuragen's acceptable limits (RIN greater than 6). In addition, single target TaqMan® miRNA qRT-PCR assays (Applied Biosystems) were run using 1ng input of unamplified RNA as a surrogate assessment of miRNA abundance.
TaqMan Megaplex profiling (Human Array A)
Expression of 378 validated microRNAs (and three control transcripts) was assessed from each biofluid type for each of the subjects using microarray technology. For CSF, equal volume inputs of 112.5 CSF equivalents of the eluted RNA were used for TaqMan® Megaplex reverse transcription (RT) and Preamplification with Human Pools A. For RNA isolated from PAXgene®-collected blood samples, a mass input of 100ng was used as input.
The diluted preamplification product was loaded with an equal volume of 2× Universal Master Mix into each port of an ABI Human microRNA Array. All qPCR steps were carried out according to the manufacturer's protocol. Detection was performed on a validated ABI 7900HT Real-time PCR instrument and Sequence Detection Software (SDS) 2.3 (Applied Biosystems).
qPCR analysis
Five miRNAs were then chosen for validation using single target qPCR, based on the combination of prior reports in the literature and our Taqman Megaplex microarray results.
Single target microRNA qRT-PCR
Samples for single target microRNA qRT-PCR analysis were processed by Asuragen, Inc. For each assay, target-specific reverse transcription and qPCR were performed according to the manufacturer's instructions using Applied Biosystems' TaqMan® MicroRNA Assays. Equal mass inputs of 1ng total RNA input was used from PAXgene blood samples. Equal volume inputs of 4 microliters total RNA was used from CSF samples corresponding to 160 CSF equivalents of the original sample volume. Reverse transcription was carried out in triplicate reaction wells in incubation steps of 16°C for 30 m, 42°C for 30 m, and 85°C for 5 m. For PCR, following incubation at 95°C for 10 m, samples were amplified in 45 cycles of 95°C for 15 s, then 60°C for 30 s.
Megaplex™ microRNA qRT-PCR
Samples for Megaplex microRNA qRT-PCR analysis were processed by Asuragen, Inc. Equal volume inputs of 3 microliters of the eluted CSF total RNA, corresponding to 120 CSF equivalents of the original sample volume, were used for TaqMan® Megaplex reverse transcription (RT) and Preamplification with Human Pools A according to the manufacturer's (Applied Biosystems) protocol. For total RNA isolated from PaxGene blood samples, a mass input of 100ng was used.
Single target TaqMan® miRNA qPCR assays (Applied Biosystems) were run on a twenty-fold dilution of the Megaplex RT/Preamplification products according to Asuragen's standard operating procedure (SOP).
Detection was performed on a validated ABI 7900HT Real-time PCR instrument and Sequence Detection Software (SDS) 2.3 (Applied Biosystems). Raw data, mean Ct values, standard deviations, and threshold settings were exported from ABI's SDS software.
Statistical analysis
Mean Cycle threshold (Ct) values were normalized for each sample using mammalian U6 snRNA levels, due to its abundant detection in all CSF and peripheral blood samples in all 8 subjects. The initial comparison of CSF and peripheral blood was conducted by counting the number of samples of each fluid type in which each miRNA was detected at a given Ct cutoff. MiRNAs detected exclusively in a single fluid type at even the most liberal Ct threshold were designated as such. CSF-predominant miRNAs were those miRNAs that were detected in more CSF samples than whole blood samples, in addition to having lower overall normalized Ct values (ΔCt) in the CSF samples. Conversely, blood-predominant miRNAs were those that were detected in more whole blood samples than CSF samples, in addition to having a lower normalized Ct count (ΔCt) in the whole blood samples. For miRNAs that were detected in both fluids in all 8 subjects, we quantitatively compared expression levels across fluid type using Spearman's correlation.
Results
Overall distribution of miRNAs in CSF and whole blood
Eight subjects participated in the study. Four subjects had no Axis I psychiatric disorder, two had a diagnosis of psychosis NOS, one had schizoaffective disorder, and one had schizophrenia. All subjects were male; mean age was 40.7 (SD: 6.1).
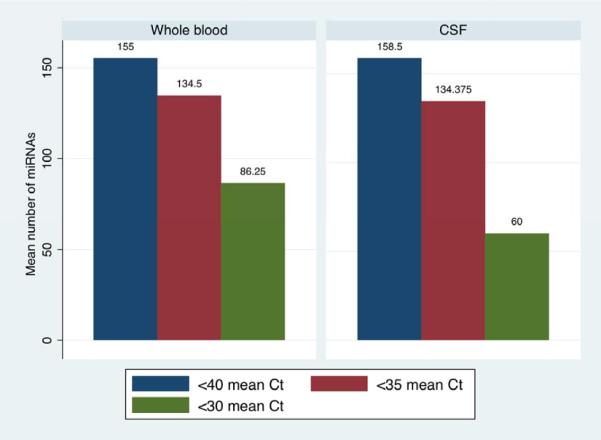
A large number of miRNAs were detected in CSF and whole blood, even when using stringent thresholds for expression (Figure 1). Using a Ct cutoff of 40 or 35, mean number of miRNAs detected were virtually identical in both fluid types. At Ct < 30, a mean of 86 miRNAs were detected in whole blood, while 60 were detected on average in CSF (paired t=1.19, df=7, p=0.27). Considering all samples, 248 distinct miRNAs were detected in CSF and 271 in whole blood samples.
Figure 1.
Number of miRNAs detected in cerebrospinal fluid and whole blood
*Ct: Cycle threshold or number of amplification cycles required to reach a given threshold. Based on microarray analysis.
Relationship between miRNA expression in CSF and whole blood
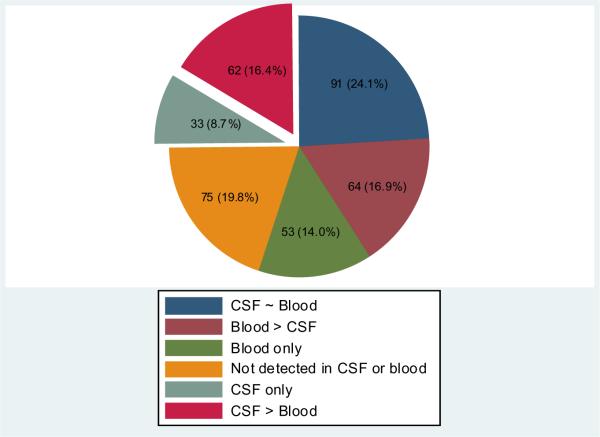
Based on microarray results, a substantial number (n=95) of miRNAs were detected more robustly in CSF compared to blood (Figure 2). Of those, 62 (65.3%) miRNAs were classified as CSF-predominant (Suppl. Table 1) and 33 (34.7%) were detected exclusively in CSF samples (Suppl. Table 2).
Figure 2.
Distribution of 378 miRNAs in cerebrospinal fluid and whole blood
Based on microarray analysis.
Given our focus on differential expression across fluid types, we used qPCR to validate results for three miRNAs that showed clear CSF-blood expression differences in the microarray analysis: let 7b, mir 9 and mir 328. As shown in Supplementary Table 3, qPCR results demonstrated statistically significant differences between blood and CSF expression levels in the same direction as the microarray results for each of these three miRNAs. We additionally used qPCR to examine two miRNAs of interest to neuroscience research, which were only weakly detected in a few CSF samples by microarray (mir-137 and mir-219-5p). While mir-137 was not detected by qPCR in either biofluid, we did obtain detected levels of mir 219-5p (mean Ct's between 34–37) for all 8 CSF samples, consistent with its prior characterization as a microRNA predominantly expressed in CNS.
Correlation between miRNA expression profiles in CSF and peripheral blood
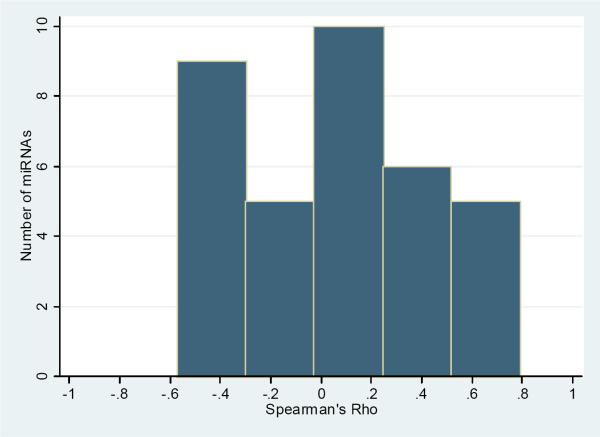
A total of 35 miRNAs were detected in both fluid types for all eight subjects. As observed in Figure 3, a histogram of the Spearman correlation coefficients comparing normalized CSF and whole blood values for each such miRNA shows that coefficients clustered around 0, indicating no systematic relationship between levels detected in the two fluids (Suppl. Table 4).
Figure 3.
Distribution of correlation coefficients comparing cerebrospinal fluid (CSF) and whole blood ΔCt values for 35 miRNAs detected in all whole blood and CSF samples.
Discussion
The results of this study provide evidence that miRNAs can be successfully detected in CSF in healthy controls as well as in patients with schizophrenia spectrum disorders. Our data suggests that the miRNA detection in CSF provides independent information that is not captured by assays derived from peripheral blood. Specifically, we found that 1) some miRNAs were expressed in CSF but not in whole blood; 2) some miRNAs were predominantly (though not solely) expressed in CSF and; 3) miRNAs levels in CSF and blood samples were poorly correlated. These preliminary data suggest that the detection of miRNAs in CSF may be complementary to existing approaches for biomarker identification in the study of psychiatric disorders.
Our results are broadly consistent with a recent study by Sullivan et al. (Sullivan, 2006), who found that gene expression in whole blood is often not strongly correlated to gene expression in different postmortem brain tissues. However, that study did not specifically measure miRNA expression and did not examine CSF samples. A study comparing brain, CSF and peripheral blood miRNA profiles in the same subjects would be ideal, but technical limitations render this study difficult to undertake, given the low feasibility of obtaining brain tissue from living subjects. To add more complexity, brain miRNA profiles may differ depending on the brain area being studied (Olsen, 2009).
A growing literature has been begun to link abnormal miRNA levels with schizophrenia in cross-sectional studies that used either post-mortem brain tissue or peripheral blood (Perkins 2007; Beveridge 2008; Beveridge, 2010; Zhu, 2009; Moreau, 2011; Santarelli, 2011). Most recently, mir 137 has attracted interest, since DNA sequence variation at this locus was found to be significantly associated in the largest genome-wide association study in schizophrenia (Ripke, 2011). Our microarray data showed detection of low levels of mir 137 in three of the CSF samples, however, that finding was not confirmed by subsequent qPCR data.
Despite the fact that a lumbar puncture is an invasive procedure, it is routinely used in clinical practice to aid in the diagnosis of illnesses such as meningitis and multiple sclerosis. However, to date, this procedure is not part of the standard diagnostic work up in patients with schizophrenia, due to the current lack of clinically useful information that can be obtained from it. On the other hand, it's likely that new technologies will facilitate the discovery of new biological markers in CSF, which could potentially impact clinical care. In this regard, miRNAs are emerging as potential useful biomarkers in various biofluids including CSF. The feasibility of performing a lumbar puncture in acutely psychotic patients was recently demonstrated by Kranaster et. al (Kranaster, 2011), who performed LPs in 155 first-episode schizophrenia patients and found that the procedure was well tolerated and had minimal adverse effects.
The exact origin of miRNAs found in CSF samples is unclear. However, Baraniskin (2011, 2012) demonstrated the presence of extracellular miRNAs in centrifuged CSF samples in patients with primary CNS lymphoma, while Cogswell (2008) used a similar approach to us by examining miRNAs in noncentrifuged CSF samples. The latter approach would probably detect a combination of extracellular miRNAs (as free circulating miRNAs) and intracellular miRNAs released to the extracellular compartment after the lysis of the few red or white blood cells present in cerebrospinal fluid. Additionally, the presence of miRNAs inside exosomes has been recently demonstrated (Skog, 2008; Ramachandran, 2011). To conclude, the fact that miRNAs are present in CSF in healthy volunteers rules out the hypothesis that miRNAs are present only in cases of pathological conditions of the CNS. In that regard, it's unlikely that miRNAs are just mere residues of cell death and it's likely that miRNAs play an active role in post-transcriptional regulation across different areas of the brain.
The small sample size in our study prevented us from looking at differences between patients and healthy volunteers. Additionally, the degree of specificity of miRNA abnormalities in CSF in psychotic disorders is unclear. It's likely that miRNA abnormalities in CSF will not respect traditional DSM-IV diagnostic categories and will probably overlap across various CNS disorders, especially those that have been associated with neurodevelopmental abnormalities. Future studies should include the recruitment of antipsychotic treatment naïve patients as part of a randomized controlled trial, which could serve as the perfect platform to study illness-specific miRNA signatures, as well as possible changes in miRNA profiles that occur with antipsychotic exposure.
In summary, miRNAs are readily detected in CSF and whole blood in patients with psychotic disorders, and certain miRNA are predominantly or exclusively expressed in CSF. Future research studies are needed to determine the value of this approach in the study of psychiatric disorders.
Supplementary Material
Acknowledgements
Funding of this study was provided by National Institute of Health grants: R01MH079800 (Malhotra), P50 MH080173-04 (Malhotra), M01RR018535 (Feinstein Institute for Medical Research- General Clinical Research Center), and UL1 RR025750 (Albert Einstein College of Medicine- CTSA). These funding agencies did not play a role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
We wish to thank Jason Gentile, Brian J. Cantley RN, Christopher Morell, M.A, Nisha Chitkara PhD and Steve M. Ventura B.S for their help with study implementation.
Footnotes
This work was presented as a poster at the International Congress on Schizophrenia Research, April 2011.
Financial Disclosures Drs. Gallego has nothing to disclose. Dr. Gordon has received research support (without direct compensation) from Eli Lilly and Forest Research Institute, and has received honoraria for speaking from Novartis and fees for consulting from Accera, Eli Lilly and GE Healthcare. Ms. Klaycomb and Bhatt are employees of Asuragen, Inc. Dr. Lencz has received consulting fees from Eli Lilly on an unrelated project. Dr. Malhotra has received grant/research support from Eli Lilly, has received honoraria for speaking from Merck, Sunovion pharmaceuticals, Inc and Shire, has served as a consultant for Eli Lilly and PGx Health, and has been on a scientific advisory board for Genomind.
Reference List
- 1.Baraniskin A, Kuhnhenn J, Schlegel U, et al. Identification of microRNAs in the cerebrospinal fluid as marker for primary diffuse large B-cell lymphoma of the central nervous system. Blood. 2011;117:3140–3146. doi: 10.1182/blood-2010-09-308684. [DOI] [PubMed] [Google Scholar]
- 2.Baraniskin A, Kuhnhenn J, Schlegel U, et al. Identification of microRNAs in the cerebrospinal fluid as biomarker for the diagnosis of glioma. Neuro Oncol. 2012 Jan;14(1):29–33. doi: 10.1093/neuonc/nor169. Epub 2011 Sep 21. PubMed PMID: 21937590; PubMed Central PMCID: PMC3245991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beveridge NJ, Tooney PA, Carroll AP, et al. Dysregulation of miRNA 181b in the temporal cortex in schizophrenia. Hum Mol Genet. 2008;17:1156–1168. doi: 10.1093/hmg/ddn005. [DOI] [PubMed] [Google Scholar]
- 4.Beveridge NJ, Gardiner E, Carroll AP, Tooney PA, Cairns MJ. Schizophrenia is associated with an increase in cortical microRNA biogenesis. Mol Psychiatry. 2010;15:1176–1189. doi: 10.1038/mp.2009.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cogswell JP, Ward J, Taylor IA, et al. Identification of miRNA changes in Alzheimer's disease brain and CSF yields putative biomarkers and insights into disease pathways. Journal of Alzheimer's disease : JAD. 2008;14:27–41. doi: 10.3233/jad-2008-14103. [DOI] [PubMed] [Google Scholar]
- 6.Gardiner E, Beveridge NJ, Wu JQ, et al. Imprinted DLK1-DIO3 region of 14q32 defines a schizophrenia-associated miRNA signature in peripheral blood mononuclear cells. Mol Psychiatry. 2011 Jul 5; doi: 10.1038/mp.2011.78. doi:10.1038/mp.2011.78. [Epub ahead of print] PubMed PMID: 21727898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghidoni R, Benussi L, Paterlini A, et al. Cerebrospinal fluid biomarkers for Alzheimer's disease: the present and the future. Neurodegener Dis. 2011;8(6):413–20. doi: 10.1159/000327756. Epub 2011 Jun 25. Review. PubMed PMID: 21709402. [DOI] [PubMed] [Google Scholar]
- 8.Hunsberger JG, Austin DR, Chen G, Manji HK. MicroRNAs in mental health: from biological underpinnings to potential therapies. Neuromolecular Med. 2009;11:173–182. doi: 10.1007/s12017-009-8070-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J, Krichevsky A, Grad Y, et al. Identification of many microRNAs that copurify with polyribosomes in mammalian neurons. Proc Natl Acad Sci U S A. 2004;101:360–365. doi: 10.1073/pnas.2333854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim AH, Reimers M, Maher B, et al. MicroRNA expression profiling in the prefrontal cortex of individuals affected with schizophrenia and bipolar disorders. Schizophr Res. 2010;124:183–191. doi: 10.1016/j.schres.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kranaster L, Koethe D, Hoyer C, Meyer-Lindenberg A, Leweke FM. Cerebrospinal fluid diagnostics in first-episode schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2011 Oct;261(7):529–30. doi: 10.1007/s00406-011-0193-7. Epub 2011 Feb 6. [DOI] [PubMed] [Google Scholar]
- 12.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific microRNAs from mouse. Current biology : CB. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 13.Latronico MV, Condorelli G. MicroRNAs and cardiac pathology. Nature reviews Cardiology. 2009;6:419–429. doi: 10.1038/nrcardio.2009.56. [DOI] [PubMed] [Google Scholar]
- 14.Lu J, Getz G, Miska EA, et al. MicroRNA expression profiles classify human cancers. Nature. 2005;435:834–838. doi: 10.1038/nature03702. [DOI] [PubMed] [Google Scholar]
- 15.McCullumsmith RE, Meador-Woodruff JH. Novel approaches to the study of postmortem brain in psychiatric illness: old limitations and new challenges. Biol Psychiatry. 2011;69:127–133. doi: 10.1016/j.biopsych.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 16.Mellios N, Huang HS, Baker SP, Galdzicka M, Ginns E, Akbarian S. Molecular determinants of dysregulated GABAergic gene expression in the prefrontal cortex of subjects with schizophrenia. Biol Psychiatry. 2009;65:1006–1014. doi: 10.1016/j.biopsych.2008.11.019. [DOI] [PubMed] [Google Scholar]
- 17.Moreau MP, Bruse SE, David-Rus R, Buyske S, Brzustowicz LM. Altered microRNA expression profiles in postmortem brain samples from individuals with schizophrenia and bipolar disorder. Biol Psychiatry. 2011;69:188–193. doi: 10.1016/j.biopsych.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olsen L, Klausen M, Helboe L, Nielsen FC, Werge T. MicroRNAs show mutually exclusive expression patterns in the brain of adult male rats. PLoS One. 2009;4:e7225. doi: 10.1371/journal.pone.0007225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perkins DO, Jeffries CD, Jarskog LF, et al. microRNA expression in the prefrontal cortex of individuals with schizophrenia and schizoaffective disorder. Genome Biol. 2007;8:R27. doi: 10.1186/gb-2007-8-2-r27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ramachandran S, Palanisamy V. Horizontal transfer of RNAs: exosomes as mediators of intercellular communication. Wiley Interdiscip Rev RNA. 2011 Oct 19; doi: 10.1002/wrna.115. doi: 10.1002/wrna.115. [Epub ahead of print] PubMed PMID: 22012863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ripke S, Sanders AR, Kendler KS, et al. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011 Sep 18;43(10):969–76. doi: 10.1038/ng.940. doi: 10.1038/ng.940. PubMed PMID: 21926974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rong H, Liu TB, Yang KJ, et al. MicroRNA-134 plasma levels before and after treatment for bipolar mania. J Psychiatr Res. 2011;45:92–95. doi: 10.1016/j.jpsychires.2010.04.028. [DOI] [PubMed] [Google Scholar]
- 23.Santarelli DM, Beveridge NJ, Tooney PA, Cairns MJ. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol Psychiatry. 2011;69:180–187. doi: 10.1016/j.biopsych.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 24.Skog J, Würdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, Curry WT, Jr, Carter BS, Krichevsky AM, Breakefield XO. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008 Dec;10(12):1470–6. doi: 10.1038/ncb1800. Epub 2008 Nov 16. PubMed PMID: 19011622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sullivan PF, Fan C, Perou CM. Evaluating the comparability of gene expression in blood and brain. American journal of medical genetics Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2006;141B:261–268. doi: 10.1002/ajmg.b.30272. [DOI] [PubMed] [Google Scholar]
- 26.Zhu Y, Kalbfleisch T, Brennan MD, Li Y. A MicroRNA gene is hosted in an intron of a schizophrenia-susceptibility gene. Schizophr Res. 2009;109:86–89. doi: 10.1016/j.schres.2009.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.