1.1 Introduction
The gene encoding the 25-hydroxyvitamin D-1 hydroxylase (CYP27B1) is expressed in cells from a variety of tissues of endodermal, ectodermal and mesenchymal origin [1]. In spite of this fact, the physiological impact of extrarenal CYP27B1 remains controversial. Studies in vitro using a variety of non-renal cell types have shown that the CYP27B1 catalyzes localized conversion of pro-hormone 25-hydroxyvitamin D (25OHD) to active 1,25-dihydroxyvitamin D (1,25(OH)2D; reviewed in [2]). However, the relevance of this event in vivo is less clear. While global knockout models of the mouse Cyp27b1 [3,4] has shed some light on this issue, it continues to be difficult to delineate between effects of circulating 1,25(OH)2D generated by the kidney and tissue-specific effects of 1,25(OH)2D synthesized by the extrarenal CYP27B1. In studies using a promoter-reporter cDNA to knockout the mouse Cyp27b1 gene DeLuca and colleagues observed that active transcription of Cyp27b1 was only evident in two tissues, the kidney and the placenta [4]. The overall conclusion drawn from these data was that expression of the CYP27B1 in healthy mice is very limited without an additional stimulus. However, more recent work using a chondrocyte-specific knockout of Cyp27b1 in the mouse resulted in an animal possessing a wide range of bone and growth plate abnormalities [5] with the opposite phenotype being observed in mice with transgenic over-expression of the Cyp27b1 in the chondrocyte [6]. It is these kinds of tissue/cell specific gene results that lend credence to the idea that expression of CYP27B1 in other tissues/cells, such as in bone-forming osteoblasts [7,8], is associated with vitamin D function in vivo. Similar models of tissue-specific CYP27B1 expression, synthesis of 1,25(OH)2D and concomitant localized, intracrine, signaling via the vitamin D receptor (VDR) have been proposed for other tissues such as the skin [9], parathyroid glands [10], prostate gland [11] and breast [12]. Conclusive evidence for local generation of 1,25(OH)2D from 25(OH)D in these tissues in vivo has yet to be published but is currently being addressed in tissue specific knockout and transgenic mouse models.
Nonetheless, so far in humans quantitative expression of the functional gene product to a level that contributes to the circulating concentrations of 1,25(OH)2D and acts in an endocrine mode occurs only in disease-activated, tissue macrophages and the placenta. In the former situation production of the vitamin D hormone may be so prolific that the endocrine actions of 1,25(OH)2D at the level of the skeleton are sufficient to promote accelerated bone resorption with a pathological increase in the circulating calcium concentration. The placenta, on the other hand, is capable of providing far less hormone to the circulating pool [13]. Along with the maternal kidney, the placenta is capable of contributing to a rise in the serum level of 1,25(OH)2D during pregnancy; the elevated serum 1,25(OH)2D concentration of pregnancy does not lead to a pathological increase of bone resorption [13]. In view of the clear-cut pathophysiological and physiological contributions of the macrophage and placenta, respectively, to a documented increase in the circulating serum 1,25(OH)2D level the following review will feature the placenta and macrophage as bona fide cites for expression of the CYP27B1 gene outside of the kidney.
1.2 The phylogeny of 1,25-(OH)2D function
The human genome contains just a little over 25,000 structural genes. With such a limited repertoire of genes, humans have evolved to use a single structural gene product for functionally distinct purposes. The CYP27B1, the product of a single structural gene, is one such example. As depicted in Table 1, it is proposed that the CYP27B1 gene has evolved to produce 1,25(OH)2D for two distinct functions. The more recent function of 1,25(OH)2D from phylogenic point of view is that of a circulating endocrine factor, a hormone, designed to guarantee a readily accessible source of calcium and phosphate to the host for skeletal maintenance. The 1,25(OH)2D hormone is made almost exclusively by the CYP27B1 in the proximal tubular epithelial (PTE) cell at the human kidney. Like other hormones, it is designed to find its way back into the general circulation and to act at a distance from its site of synthesis. The production of 1,25(OH)2D in the proximal tubular epithelial cell of the kidney is regulated by other hormones, namely PTH and FGF23, which up- and down-regulate expression of the CYP27B1 gene, respectively [14]. Importantly, when the concentration of substrate 25-hydroxyvitamin D (25OHD) is diminished, as is seen in states of vitamin D deficiency, production of 1,25(OH)2D in the healthy kidney increases.
Table 1.
Proposed, evolutionarily distinct functions of 1,25-dihydroxyvitamin D.
| Hormone | Cytokine |
|---|---|
| Advanced function | Primitive function |
| Skeletal homeostasis | Host protection |
| Made by the PTE cell | Made by macrophages |
| Acts at a distance | Locally acting |
| Regulated by other hormones; PTH, FGF23 | Regulated by immune factors; IFNs, IL15 |
| ⬆ 25D leads to ⬇ 1,25D synthetic rate | ⬆ 25D leads to ⬆ 1,25D synthetic rate |
By contrast, we propose that the more phylogenetically ancient function of 1,25(OH)2D is that of an inflammatory mediator meant to regulate the innate immune response and direct the adaptive immune response to invading microbes. Orthologs of the specific intracellular receptor for 1,25(OH)2D, the vitamin D receptor (VDR), can be found in single cell organisms (e.g., yeast), and both the VDR and its specific ligand 1,25(OH)2D are found in the lamprey eel, an ancient vertebrate lacking a calcified skeleton and teeth [15]; this is evidence that interaction of the VDR with its cognate ligand 1,25(OH)2D plays a role distinct from provision of calcium and phosphate for skeletal mineralization. In its role as a functioning cytokine (see Table 1), 1,25(OH)2D is made locally by inflammatory cells, specifically the macrophage and the dendritic cell [16], to act locally on other immune cells that express the VDR upon activation (Figure 1). In view of its local synthesis and action, it’s not surprising that the inflammatory cell CYP27B1 is regulated by other inflammatory cytokines (e.g., IFN-γ and IL15; [1]). At least partially owing to the transcription of an amino-terminally truncated, enzymatically-defunct CYP24A1 (24-hydroxylase; [17]), synthesis of 1,25(OH)2D the cytokine and the activity of the CYP27B1 in the inflammatory cell is substrate dependent, such that the extracellular concentration of available, free 25OHD is the major determinant of how much 1,25(OH)2D will be produced by that cell. In other words, when the serum 25OHD level goes down the inflammatory cell 1,25(OH)2D synthetic rate will also fall [13].
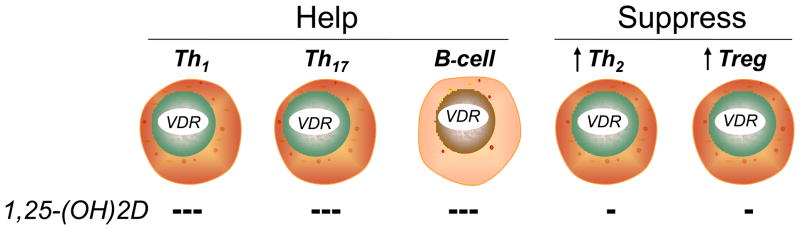
Figure 1.
Classes of lymphocytes designed to “help” (left; Th1, Th17 and immunoglobulin-producing B lymphocytes) and “suppress” (right; Th2 and Tregulatory [Treg] lymphocytes) the human immune response. When activated, all classes of lymphocytes express the vitamin D receptor (VDR). When exposed to 1,25-(OH)2D, the proliferation of each population is inhibited; the inhibition of proliferation is most profound in the “helper cell” classes, resulting in a net suppression of the adaptive immune response; the resultant relative increase in suppressor cell activity is indicated by the arrows.
Finally, as will be discussed later in this review, it must be noted that although extrarenal synthesis of 1,25(OH)2D was first described nearly thirty years ago in association with the human disease sarcoidosis [18,19]. The key advance in linking this to normal human physiology arose from the cloning of the gene for 1α-hydroxylase (CYP27B1) [20]. Interestingly, the human CYP27B1 cDNA was cloned from keratinocytes [21], not the kidney, as one might have expected. The keratinocyte as a bona fide extrarenal source of 1,25(OH)2D in normal human physiology remains debatable. Analysis of mice in which both copies of the Cyp27b1 gene has been replaced by a LacZ reporter cDNA suggests that the enzyme is poorly expressed in the skin [4]. Nevertheless, other studies using mice with gene ablation of Cyp27b1 have confirmed that synthesis of 1,25(OH)2D is required for normal differentiation and function of epidermal keratinocytes [22,23]; similar observations in humans that are null for the CYP27B1 gene have not been made. Regardless, in the absence of a tissue specific knockout of the Cyp27b1, the importance of epidermal 1,25(OH)2D production versus renal synthesis of the hormone in mediating its effects on the skin has yet to be confirmed.
1.3 The 1-hydroxylase and extrarenal synthesis of 1,25(OH)2D in normal physiology
Placenta
The human placenta is a chimeric structure consisting of elements originating from both the mother and the fetus. The placenta is connected to i) the umbilical artery coming from the fetus; ii) the umbilical vein draining a mixture of maternal and fetal blood back to the fetus, iii) maternal arteries delivering oxygenated blood and nutrients, including 25OHD, to the placenta and iv) maternal veins draining a mixture of maternal and fetal blood back to the general maternal circulation. The placenta was one of the first sites reported to exhibit extrarenal 1α-hydroxylase activity [24], with the CYP27B1 being detected in both maternal decidual, stromal cells and resident macrophages and fetal trophoblast [25,26]; all of the 1,25(OH)2D producing cells in the placenta are of mesenchymal origin as are the tubular epithelial cells of the kidney, the home to the endocrine source of 1,25(OH)2D. As such, during human pregnancy, there are five potential cellular sites for expression of the CYP27B1 gene: 1] the maternal kidney; 2] the decidual, stromal cells of the maternal side of the placenta; 3] the fetal kidney; 4] the trophoblast on the fetal side of the placenta; and 5] the macrophage on either side of the placenta. To make the issue even more complicated is the fact that the intervillous space of the placenta can serve as a site for admixture of 25OHD from the maternal circulation and potentially 1,25(OH)2D originating from cells in the fetus as well as on the fetal side of the placenta (see below). The question remains which one or combinations of these sites harboring the CYP27B1 gene and CYP27B1 enzyme contributes to the circulating concentration of 1,25(OH)2D in the mother and in the fetus.
The first point to be made in this regard is that, while 25OHD crosses the placental from mother to child [27], 1,25(OH)2D does not [28, 29, 30, 31, 32]. This suggests that the maternal kidney is not a source for 1,25(OH)2D in the fetal circulation. In fact, there is no correlation between the maternal and fetal serum levels of 1,25(OH)2D [33] with total concentrations of 1,25(OH)2D on the fetal side of the placenta, from either fetal artery of vein, always lower than that of the mother [34]. The second point to be emphasized is that the increase in maternal 1,25(OH)2D levels during pregnancy is at least partially due to expression of the CYP27B1 and 1-hydroxylating activity in the maternal kidney and not the result of decreased clearance of the hormone from the maternal circulation [35, 36]. With these two realities in mind then, what is the evidence that 1,25(OH)2D produced by the placenta, incompassing both fetal and maternal elements, is a source for 1,25(OH)2D reaching the circulation of the mother? The most convincing evidence that this situation does exist comes from pregnant, nephrectomized rodents and functionally anephric humans. Injection of 25OHD3 to a nephrectomized rat results in the appearance of 1,25(OH)2D3 in the maternal serum of pregnant but not of non-pregnant animals [37]. A human example is the case of a woman with chronic renal failure and barely detectable serum 1,25(OH)2D whose 1,25(OH)2D levels increased significantly during pregnancy [38]. In situations like those alluded to above, where maternal renal 1,25(OH)2D synthesis is dramatically impaired or absent, it is arguable that the 1,25(OH)2D in the maternal circulation may derive from the maternal placenta, the fetal side of the placenta or the fetal kidney. There are two lines of evidence to suggest that 1,25(OH)2D in the maternal circulation does not originate from the fetal side of the placenta or the fetal kidney. The first comes from experiments performed in the CYP27B1-null Hanover pig [37]. These animals show no increase in serum 1,25(OH)2D levels through pregnancy, even when the mother is carrying CYPY2B1 +/− offspring. The other line of evidence comes from the early human studies of Steichen et al [33] who demonstrated that preterm infants harbor serum 1,25(OH)2D levels that are only about 25% of those observed in the mother and do not increase significantly until 24 hours after birth. In sum and recalling that 1,25(OH)2D does not readily cross placenta, the above-described data indicates that the maternal side of the placenta contributes to the circulating level of 1,25(OH)2D in the mother. Less certain is how much the fetal side of the placenta contributes to the 1,25(OH)2D level of the fetus.
Working from the supposition that the neither fetal nor the maternal side of placenta is generating 1,25(OH)2D to be used for calcium and phosphate homeostasis by the fetus [39], it has been suggested that 1,25(OH)2D made by the placenta is not meant to function as a hormone, but as an immunomodulatory cytokine to i) prevent rejection of the fetus, ii) prevent a graft-(fetus)-versus-host (mother) reaction and iii) promote the orchestrated innate and adaptive immune response to prevent microbial invasion of the fetal-placental unit during pregnancy [40]. If this is the case then the CYP27B1 in the placenta functions more as it does in inflammatory cells and less like it does in the kidney (see Table 1). One indication that this is the situation is the fact that activity of the CYP27B1 in human placenta, especially during the in 1st and 2nd trimester, is paralleled by similar patterns of expression for VDR in the placenta [41,42]. In the kidney, a rise in the serum 1,25(OH)2D would result in an increase in expression of CYP24A1 gene that encodes the 1,25(OH)2D and 25(OH)D catabolic 24-hydroxylase enzyme. However, just as is the case with the CYP24A1 in the macrophage (see below), 24-hydroxylating activity actually decreases, not increases, in 1st and 2nd trimester placentas [41]. This results in a net increase in 1,25(OH)2D synthesis by the placental unit, an event that is particularly pronounced during the first six months of pregnancy. The decrease in CYP24A1 gene expression and CPY24A1-hydroxylating activity appears to be due to extensive methylation of the CYP24A1 in placental tissue leading to transcriptional silencing of the enzyme [43]; such a means for silencing 24-hydroxylating activity may be specific for the placenta and independent of alternative splicing of the CYP24A1 gene as occurs in macrophages (see below).
Furthermore, the presence of VDR in a variety of placental tissues is consistent with an intracrine, autocrine or paracrine mode of vitamin D action at the fetal-maternal interface, similar to that observed in the immune system [44, 45]. This stems in part from the recognized immune activity within the wide variety of cells that make up the placenta as a whole. Both maternal and fetal cells are involved in mediating innate [46, 47, 48] and adaptive [49, 50] immune responses. In particular, the placenta expresses a wide range of antibacterial [51, 52, 53] and antiviral factors [54], which may act as targets for the high levels of local 1,25(OH)2D production within the placenta. Experiments using human placental tissue and cells have shown that 25OHD and 1,25(OH)2D induce expression of the antimicrobial cathelicidin gene in both the maternal decidua and fetal trophoblast, leading to enhanced bacterial killing [55]. Similar studies have also highlighted the anti-inflammatory action of 25OHD in placental cells following local conversion via the CYP27B1 [56]. The significance of this has been further endorsed in vivo using mice with ablation of the Cyp27b1 gene; knockout of Cyp27b1 within the fetal component of the placenta alone was sufficient to greatly exacerbate inflammatory responses to the pathogen-associated membrane pattern (PAMP) and Toll-like receptor-4 (TLR-4) ligand, lipopolysaccharide (LPS) [55]; see section entitled “The extrarenal synthesis of 1,25(OH)2D in pathopysiological states” below.
In view of the above in vitro and animal data that strongly suggest that a key function of CYP27B1 in the placenta is to support antibacterial and anti-inflammatory actions, it is not surprising that maternal vitamin D-insufficiency is associated with increased rates of bacterial vaginosis in the 1st trimester of pregnancy [57]. Other reports have described association between impaired vitamin D status in pregnant women and risk of maternal to child transmission of the human immunodeficiency virus (HIV) [58]; it is hypothesized that the lower levels of HIV transmission under conditions of vitamin D-sufficiency may be due to improved innate immune response to infection in these women. Given the fundamental role of the placenta in vertical transmission of HIV from mother to the fetus [59], it is possible that 1,25(OH)2D-induced innate immune responses within the placenta plays a role in combating viral infection during pregnancy. Other groups have reported altered levels of the CYP27B1 in placentas from preeclampsia pregnancies [59, 60], and this may be linked to epidemiology indicating that vitamin D deficiency significantly increases the risk of preeclampsia [61, 62, 63]. In conclusion and in concert with current evidence suggesting that placental production of 1,25(OH)2D is not needed for development of the fetal skeleton or the maintenance of maternal skeleton [13], an immunomodulatory role for placental 1,25(OH)2D production seems highly likely [64].
1.4 The extrarenal synthesis of 1,25(OH)2D in pathophysiological states
Clinical evidence for disease-associated, 1,25(OH)2D-directed dysregulation of calcium balance (Table 2)
Table 2.
Human granuloma-forming diseases associated with the extrarenal overproduction of 1,25-dihydroxyvitamin D.
| Noninfectious
|
Infectious
|
Neoplastic
|
|---|---|---|
| sarcoidosis | tuberculosis | B-cell lymphoma |
| Crohn’s disease | leprosy | Hodgkin’s disease |
| silicone granulomata | candidiasis | lymphomatoid granulomata |
| paraffin granulomata | histoplasmosis | dysgerminoma |
| berylliosis | coccidiomycosis | seminoma |
| Wegener’s | cat scratch fever | mesothelioma |
| infantile fat necrosis | mMar. infection | |
| slack skin disease |
The association of dysregulated calcium homeostasis and the prototypical granuloma-forming disease sarcoidosis was established in 1939 by the work of Harrell and Fisher [65]; in fact, data suggest that i) mild to severe hypercalcemia is detected in 10% of patients with sarcoidosis and ii) up to 50% of patients will become hypercalciuric at some time during the course of their disease [66]. Two early clinical observations suggested dysregulated metabolism and action of vitamin D was implicated in the pathogenesis of abnormal calcium metabolism: 1] patients with sarcoidosis who had hypercalcemia or hypercalciuria (or both) absorbed high amounts of dietary calcium; and 2] normocalcemic patients were prone to hypercalcemia after receiving small amounts of vitamin D or ultraviolet light [67]. Based on the seminal observations of Fuller Albright [68] that i) a diet low in calcium seldom induces a normocalcemic state in sarcoidosis patients with moderate to severe hypercalcemia and ii) urinary calcium excretion often exceeds dietary calcium intake, Fallon [69] proposed that accelerated bone resorption was the most important contributor to the pathogenesis of hypercalciuria and hypercalcemia in these patients. More recent studies have demonstrated that generalized, accelerated trabecular bone loss occurs in patients with sarcoidosis before institution of steroid therapy [70]; these investigators showed that i) bone mass was significantly decreased in patients with active sarcoidosis, ii) bone loss was most marked in patients with hypercalcemia and/or hypercalciuria and iii) bone loss was most prominent in postmenopausal women with long-standing disease
There are four major lines of clinical evidence to suggest that the endogenous extrarenal synthesis of 1,25(OH)2D in hypercalcemic/hypercalciuric patients with granuloma-forming disease or lymphoma is not subject to normal, physiologic (endocrine) regulatory influences [71, 72, 73]. First, hypercalcemic patients possess high or inappropriately elevated serum 1,25(OH)2D concentrations, although serum immunoreactive parathyroid hormone levels (iPTH) are suppressed and serum phosphorus concentrations are relatively elevated; if 1,25(OH)2D synthesis were under the trophic control of PTH and phosphorus as it is in the kidney, then 1,25(OH)2D concentrations would be low, not high or even normal. Second, unlike in normal individuals whose serum 1,25(OH)2D levels are not influenced by small to moderate increments of circulating 25OHD concentration, serum 1,25(OH)2D levels in sarcoidosis patients with widespread, active disease may increase substantially with relatively small increments in the serum 25OHD level. Third, serum calcium and 1,25(OH)2D concentrations are positively correlated to indices of disease activity; patients with sarcoidosis who have widespread disease are more likely to hypercalcuric or hypercalcemic. Finally, the rate of endogenous 1,25(OH)2D production, which is significantly increased in patients with sarcoidosis, is unusually sensitive to inhibition by immunomodulatory drugs and factors (e.g., glucocorticoids) that do not directly influence the renal CYP27B1 enzyme responsible for the synthesis of 1,25(OH)2D the hormone. These kinds of clinical data suggested three likely explanations for discrepancies in the regulation of vitamin D metabolism and action in health and in granuloma-forming disease: 1] there was more than one gene product regulating the synthesis of 1,25(OH)2D in these two states; 2] 1,25(OH)2D was the product of a single gene product but one which was differentially regulated in different tissues (cells); and/or 3] the active vitamin D metabolite in granuloma-forming disease co-purified with native 1,25(OH)2D, being bound with high affinity by the VDR but was structurally distinct 1,25(OH)2D. As detailed below, the second of these three options appears to be operative.
Cellular Source of 1,25(OH)2D in granuloma-forming diseases
Barbour et al [18] described an anephric patient with sarcoidosis, hypercalcemia, and a high serum concentration of an active vitamin D metabolite that was detected as 1,25(OH)2D. Subsequent structural studies showed that the hypercalcemia-causing metabolite was 1,25(OH)2D [74], and that the extrarenal source of 1,25(OH)2D was the macrophage [19], the cells that make up a significant portion in the prototypical inflammatory granulomatous lesions characteristic of the disease. More recent work has revealed that macrophage synthesis of 1,25(OH)2D is the consequence of expression of a single gene, the CYP27B1 [75]. As shown in Table 2, clinical evidence for the extrarenal overexpression of the CYP27B1 gene can also be observed in a number of other granuloma-forming diseases, including be lymphomas and other malignancies; for example, immunohistochemical analysis of the human B-cell lymphomas associated with hypercalcemia and raised circulating levels of 1,25(OH)2D in the host suggests that the tumor itself is not a source of the steroid hormone. Rather macrophages adjacent to the tumor are likely to be the major site of 1,25(OH)2D synthesis [76]. Similar observations have been made for other hypercalcemia-causing tumors such as dysgerminomas [41].
1.5 Regulation of the CYP27B1-hydroxylase in the macrophage
Like its counterpart in the kidney, the extrarenal CYP27B1 functions as a mitochondrial, mixed function oxidase with cytochrome P450 activity [77]. Using reconstituted mitochondrial extracts it has been shown that electron transfer to the cytochrome P450 and subsequent insertion of an oxygen atom in the substrate 25OHD requires the following: flavoprotein; ferredoxin reductase; an electron source, and molecular oxygen [77]. Both renal and extrarenal 1α-hydroxylase requires a secosterol (vitamin D sterol molecule with an open B-ring) as substrate [78], and in the case of the kidney and macrophage the enzyme has a particular affinity for secosterols bearing a carbon-25-hydroxy group (i.e., 25OHD and 24,25-dihydroxyvitamin D [24,25(OH)2D]) [78]; the calculated Km (affinity) of the CYP27B1 in pulmonary alveolar macrophages for these two substrates is in the range of 50–100 nM, which is similar to that observed in renal proximal tubule cells. Both renal and extrarenal 1-hydroxylase activities are inhibited by napthoquinones, molecules which compete with reductase for donated electrons and by the imidazoles which compete with the enzyme for receipt of O2 [79]. While the substrate-specificity and enzyme kinetics for the CYP27B1 appears to be the same for both kidney cells and macrophages [78], as just noted the regulation of 1,25(OH)2D synthesis at these sites appears to be very different. For example, as previously noted in Table 1 the macrophage CYP27B1 is not induced by PTH, but it is very sensitive to stimulation by immunoactivators such as lipopolysaccharide [78] and Th1 cytokines, particularly interferon-γ (IFN-γ; [80]). The molecular mechanism underpinning the expression of the CYP27B1 in the human macrophage has recently been elucidated employing ex vivo models of tuberculosis, another prototypical human granuloma-forming disease (see below).
Vitamin D, tuberculosis and innate immunity
Innate immunity encompasses the ability of the host immune system to recognize and respond to an offending antigen. In 1986 Rook and colleagues [81] described studies using cultured human macrophages in which they showed that 1,25(OH)2D can inhibit the growth of Mycobacterium tuberculosis (mTB). Although this seminal report was widely cited, it is only in the last few years that more comprehensive appraisals of the antimicrobial effects of vitamin D metabolites have been published. In silico screening of the human genome revealed the presence of a vitamin D response element (VDRE) in the promoter of the human cathelicidin gene, whose product LL37 is an antimicrobial peptide capable of killing bacteria [82]. Subsequent investigations confirmed the ability of 1,25(OH)2D [83] and its precursor 25OHD [84] to induce expression of cathelicidin in cells of the monocyte/macrophage and epidermal lineage, highlighting the potential for intracrine/autocrine induction of antimicrobial responses in cells that also express the 25(OH)D-activating enzyme, CYP27B1. Although detectable in many immune cell types, functionally significant expression and activity of CYP27B1 appears to be dependent on cell-specific stimulation of a broad spectrum of immune surveillance proteins, the Toll-like receptors (TLRs). The TLRs are an extended family of noncatalytic, host cell transmembrane pattern-recognition receptors (PRRs) that interact with specific pathogen-associated molecular patterns or PAMPs shed by infectious agents and trigger the innate immune response in the host [85].
In this regard, Liu and colleagues [86] used DNA array to characterize changes in gene expression following activation of the TLR2/1 dimer in human macrophages and dendritic cells by one of the PAMPs for mTB. Macrophages, but not dendritic cells, so-treated showed increased expression of both CYP27B1 and VDR genes and gene products with demonstrated intracrine induction of the antimicrobial cathelicidin gene with subsequent mycobacterial killing in response to exposure of cells to physiologically-relevant concentrations 25OHD as well as 1,25(OH)2D. In fact, microbial killing was more efficiently achieved with the pro-hormone 25OHD than with 1,25(OH)2D at similar extracellular concentrations, indicating that the robustness of the human innate response to microbial challenge is dependent upon the serum 25OHD status of the host. This concept was confirmed in these studies by the ability of 25OHD-sufficient serum to rescue a deficient, cathelicidin-driven antimicrobial response in human macrophages conditioned in vitamin D-deficient serum [87]. A similar vitamin D-directed antimicrobial generating capacity has been recently observed in wounded skin [88], suggesting that TLR-driven expression of cathelicidin, requiring the intracellular synthesis and genomic action of 1,25(OH)2D, is a common response feature to infectious agent invasion. Reinforcing these events is the ability of locally-generated 1,25(OH)2D to escape the confines of the cell in which it is made to act on neighboring, VDR-expressing monocytes to promote their maturation to mature macrophages [89], thus acting as a feed-forward signal to further enhance the innate immune response. While clinical evidence in support of this notion is beginning to accumulate [1], the utility of vitamin D or 25OHD as adjuvant therapy to boost the human innate immune response in a therapeutic or preventive sense needs more in depth, prospective investigation in humans.
Macrophage and innate immunity
Subsequent studies have expanded the spectrum of macrophage innate immune responses regulated via intracrine activation of 25OHD to 1,25(OH)2D. These include the induction of other antibacterial proteins such as β-defensin 2 (DEFB4), which appears to require local generation of 1,25(OH)2D in conjunction with the transcription factor nuclear factor-kappa B (NF-κB) [90, 91]. A macrophage NF-κB response may be generated by a variety of means, including in response to the inflammatory cytokine interleukin-1 (IL-1; [91]) and activation of the intracellular PRR NOD2 [90]. As noted above, 1,25(OH)2D induces expression of both LL37 and DEFB4 due to direct transcriptional regulation of their parent genes by 1,25(OH)2D-liganded VDR acting via an enhancing VDRE within the proximal promoters of the cathelicidin and DEFB4 genes [82, 83].
Microbial killing in the human infectious diseases delineated in Table 1 occurs after internalization of the offending microbe by the macrophage. The internal environment of the fused lysosome-phagosome in which microbial killing takes place i) is strongly acidic in nature and ii) permits incorporation of specific antibacterial factors such as LL37 and DEFB4 [92]. Recent attention has focused on autophagy as a process that may enhance the management of phagocytosed pathogens. Autophagy is a cellular mechanism common to all eukaryotic organisms that involves membrane encapsulation of organelles or cell proteins in an autophagosome prior to fusion with lysosomes and degradation of the autolysosomal contents. In addition to its well-recognized function as a pivotal factor in the maintenance of cytosolic homeostasis [93], autophagy also plays a key role in cellular response to infection, with pathogens contained in autophagosomes being either eliminated or degraded prior to presentation to PRRs, (e.g., TLRs; [94, 95]). Recent studies have shown that TLR2-induction of CYP27B1 is associated with enhanced autophagy in the macrophage, indicating that this mechanism is also likely to be strongly influenced by bioavailability of 25OHD and localized intracellular generation of 1,25(OH)2D [96, 97].
1,25(OH)2D and the adaptive immune response
The adaptive immune response is generally defined by T- and B-lymphocytes and their ability to produce cytokines and immunoglobulins, respectively, to specifically combat the source of the antigen presented to them by cells (i.e. macrophages, dendritic cells, etc) of the innate immune response. As previously noted (Figure 1), the presence of VDR in activated, but not resting, human T- and B-lymphocytes was the first observation implicating these cells as targets for the noncalciotropic responses to 1,25(OH)2D [98]. Contrary to the role of locally-produced 1,25(OH)2D to promote the innate immune response, the hormone exerts a generalized dampening effect on lymphocyte function and the adaptive immune response. With respect to B cells, acting through the VDR 1,25(OH)2D suppresses proliferation and immunoglobulin production and retards the differentiation of B-lymphocyte precursors to mature, immunoglobulin-producing plasma cells. With regard to T cells, 1,25(OH)2D, again acting through the VDR, inhibits the proliferation of proinflammatory IFNγ-elaborating, macrophage-stimulating Th(helper)1 and chemokine-producing Th17 cells [99]. A much less robust antiproliferative effect of 1,25(OH)2D is exerted on immunosuppressive Th2 and regulatory T cells (Tregs) [100], promoting their relative (compared to helper cells) accumulation at sites of inflammation by stimulating expression of the dendritic cell-derived T cell homing molecule, CCL22 [101]. In fact, it is this generalized ability of 1,25(OH)2D to quell the adaptive immune response which has prompted the use of the hormone and it analogues in the adjuvant treatment of inflammatory and neoplastic disorders.
Concerted immunoactions of 1,25(OH)2D
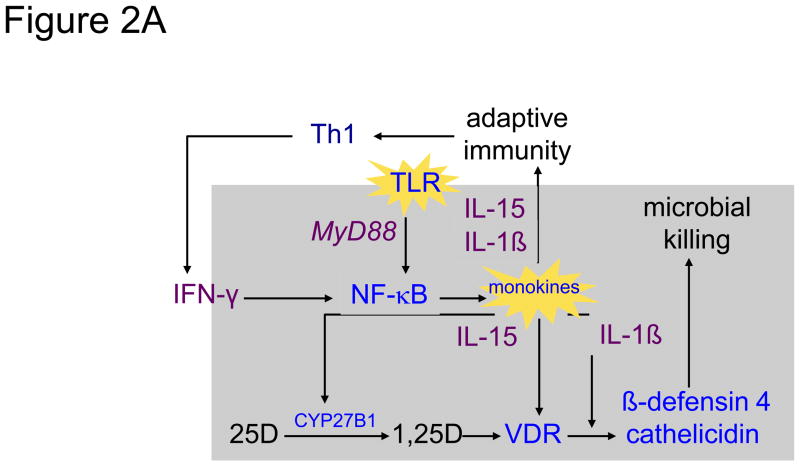
In sum, the collective action of 1,25(OH)2D is to promote the host’s innate response to an invading pathogen, while simultaneously acting to limit what might be an overzealous adaptive immune response to that pathogen, representative of the process of ‘tolerance’. Figure 2 shows a molecular schema by which 1,25(OH)2D-directed microbial killing is, on the one hand, amplified by the T-cell-driven adaptive immune response, but on the other hand kept in balance by a monokine-derived negative feedback control on T cell activity. Figure 2A depicts the example of stimulation of the TLR 2/1 by an mTB PAMP. Stimulation of TLR results in MyD88-coupled signaling through to NK-κB to elicit a monokine synthetic response [86]. Included among those TLR-responsive monokines are IL-15 and IL-1β. 1L-15 initiates expression of both the CYP27B1 and VDR genes in the host macrophage [102], while IL1-β amplifies 1,25(OH)2D-VDR-driven expression of the antimicrobial cathelicidin and DEFB4 genes [91], leading to phagolysosomal/autophagic killing of the ingested mTB [96, 103]. Release of IL-15 and IL-1β into the local microenvironment promotes the proliferation and lymphokine production by Th1 cells. Central among these Th1 lymphokines is IFN-γ, long recognized to be the most potent stimulator of the macrophage CYP27B1 [74]. Presumably through interaction with its receptor on the macrophage, IFN-γ promotes further upregulation of TLR/IL-15-driven CYP27B1 and VDR gene expression [102, 103], enhancing antimicrobial production and microbial killing [96].
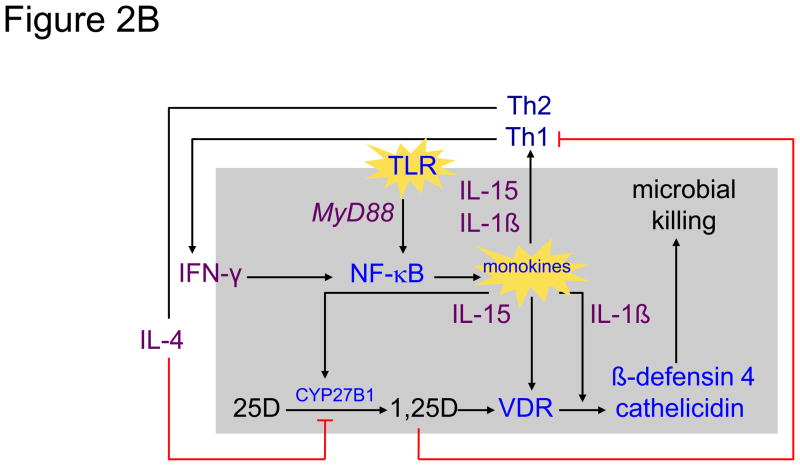
Figure 2.
Shown in the left panel 2A is a simplified schematic of the 25-hydroxyvitamin D (25D)-regulated innate immune response to Toll-like receptor (TLR) activation of the human macrophage in response to interaction with a pathogen-associated membrane pattern (PAMP) shed from Mycobacterium tuberculosis (mTB) [86]. Upon activation of the TLR 2/1 receptor by an mTB-specific PAMP, the activated receptor engages the intracellular coupling molecule MyD88 which, in turn, stimulates a kinase cascade ultimately leading to the nuclear localization of NF-κB and transcriptional activation of monokine genes, including IL-15 [102] and IL1-β [91]. In an autocrine fashion IL-15 promotes the expression of the CYP27B1 (1-hydroxlase) and vitamin D receptor (VDR) genes. In the presence of intracellularly-generated 1,25-dihydroxyvitamin D (1,25D), the ligand-bound VDR transactivates antimicrobial genes, cathelicidin and β-defensin 4, whose gene products, amplified under the autocrine actions of IL-1β, team with a 1,25D-directed autophagic response to promote microbial killing [96]. In a paracrine mode, IL-1β and IL-15 act to promote T helper-1 (Th1) cell generation of lymphokines, including interferon-gamma (IFN-γ), the most potent known stimulator of IL-15-driven CYP27B1 expression [103]. The net result is a feed-forward amplification loop to enhance microbial killing. The right panel 2B demonstrates interruption of the feed-forward loop by movement of 1,25D from the cell into the local inflammatory microenvironment when the CYP27B1-hydroxylating activity of the cell is robust. Escape of 1,25D permits it to interact with VDR-expressing helper (represented by Th1) and suppressor (represented Th2) cells [1]. Inhibition of “help” results in a decrease in IFN-γ production and downturn in macrophage 1,25D synthesis. Because the action of 1,25D to limit the proliferation and action of “suppressor” T cells is less strong, unopposed secretion of IL-4, a natural inhibitor of the CYP27B1, results in a further downturn in intracellular 1,25D synthesis and action.
If substantial amounts of 1,25(OH)2D escape the confines of the macrophage (Figure 2B), then the immunostimulation and subsequent proliferation of VDR-expressing, activated, helper Th1 and Th17 (helper) T-cell populations, immunoglobulin producing B-cells and Th2 and Treg (suppressor) T-cell in that environment will be quelled (see Figure 1). Because the antiproliferative effects on the helper cell populations are more robust than they are on suppressor cells, the net result is relative suppression of the adaptive immune response, with the major suppressive effect at the level of the CYP27B1-hydroxylating activity mediated by the Th2 product IL4 [103]. Only when the innate immune response is extreme and enough 1,25(OH)2D finds its way into the general circulation, as may occur in human granuloma-forming disorders (see Table 2), are the endocrine effects of the hormone (i.e., hypercalciuria and hypercalcemia) observed.
Summary and conclusions
Although the existence of extrarenal production of 1,25(OH)2D by the human placenta and disease-activated macrophages has been recognized for many years now, the potential for this vitamin D metabolite to function normally in the host as something other than a calcium-regulating hormone is becoming more likely. The importance of 1,25(OH)2D as a normal functioning, locally-produced, locally-active immunomodulatory molecule in humans in vivo at the fetal-maternal interface in the placenta and/or at other sites of microbial/cancer cell invasion has been stymied by our inability to measure changes in the local concentration of 1,25(OH)2D at sites of immunoactivity in vivo. While there are examples of step-up gradients in the concentration of 1,25(OH)2D between the serum and loculated inflammatory sites, like the pleural fluid of patients with active tuberculosis [104, 105], the potential for 1,25(OH)2D to balance the innate and adaptive immune response to foreign agents (e.g., fetus, mycobacteria, etc., see Figure 2) at a micro-scale in vivo remains real but unproven. Breakthroughs in the field await: 1] prospective clinical trial evidence that provision of 25OHD substrate to vitamin D-deficient individuals will i) improve maternal-fetal outcomes and/or ii) limit disease or protect against microbial infection in exposed individuals (e.g., close family contacts of patients with active tuberculosis); and 2] development of a means for detecting changes in the level and bioactivity of 1,25(OH)2D in the local intra- and extracellular inflammatory microenvironment of the host.
Highlights.
The macrophage and placenta as the extrarenal sites for the CYP27B1-hydroxylase.
The role of 1,25-dihydroxyvitamin D in the management of the immune response.
The elicited immune response may be compromised by vitamin D deficiency.
Acknowledgments
Funding source. This work is supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS; RO1 1AI073539) and National Center for Research Resources (NCRR; UL1 RR033176).
Footnotes
Policy and ethics. The work described in this article has been carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans http://www.wma.net/en/30publications/10policies/b3/index.html; EC Directive 86/609/EEC for animal experimentshttp://ec.europa.eu/environment/chemicals/lab_animals/legislation_en.htm; Uniform Requirements for manuscripts submitted to Biomedical journals http://www.icmje.org.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 2011 doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- 2.Hewison M, Burke F, Evans KN, Lammas DA, Sansom DM, Liu P, Modlin RL, Adams JS. Extra-renal 25-hydroxyvitamin D3-1alpha-hydroxylase in human health and disease. J Steroid Biochem Mol Biol. 2007;103:316–321. doi: 10.1016/j.jsbmb.2006.12.078. [DOI] [PubMed] [Google Scholar]
- 3.Panda DK, Miao D, Tremblay ML, Sirois J, Farookhi R, Hendy GN, Goltzman D. Targeted ablation of the 25-hydroxyvitamin D 1alpha-hydroxylase enzyme: evidence for skeletal, reproductive, and immune dysfunction. Proc Natl Acad Sci U S A. 2001;98:7498–7503. doi: 10.1073/pnas.131029498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vanhooke JL, Prahl JM, Kimmel-Jehan C, Mendelsohn M, Danielson EW, Healy KD, DeLuca HF. CYP27B1 null mice with LacZreporter gene display no 25-hydroxyvitamin D3-1alpha-hydroxylase promoter activity in the skin. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:75–80. doi: 10.1073/pnas.0509734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St-Arnaud R, Dardenne O, Prud’homme J, Hacking SA, Glorieux FH. Conventional and tissue-specific inactivation of the 25-hydroxyvitamin D-1alpha-hydroxylase (CYP27B1) J Cell Biochem. 2003;88:245–251. doi: 10.1002/jcb.10348. [DOI] [PubMed] [Google Scholar]
- 6.Naja RP, Dardenne O, Arabian A, St Arnaud R. Chondrocyte-specific modulation of Cyp27b1 expression supports a role for local synthesis of 1,25-dihydroxyvitamin D3 in growth plate development. Endocrinology. 2009;150:4024–4032. doi: 10.1210/en.2008-1410. [DOI] [PubMed] [Google Scholar]
- 7.Atkins GJ, Anderson PH, Findlay DM, Welldon KJ, Vincent C, Zannettino AC, O’Loughlin PD, Morris HA. Metabolism of vitamin D3 in human osteoblasts: evidence for autocrine and paracrine activities of 1 alpha,25-dihydroxyvitamin D3. Bone. 2007;40:1517–1528. doi: 10.1016/j.bone.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 8.van Driel M, Koedam M, Buurman CJ, Hewison M, Chiba H, Uitterlinden AG, Pols HA, van Leeuwen JP. Evidence for auto/paracrine actions of vitamin D in bone: 1alpha-hydroxylase expression and activity in human bone cells. FASEB J. 2006;20:2417–2419. doi: 10.1096/fj.06-6374fje. [DOI] [PubMed] [Google Scholar]
- 9.Pillai S, Bikle DD, Elias PM. Vitamin D and epidermal differentiation: evidence for a role of endogenously produced vitamin D metabolites in keratinocyte differentiation. Skin Pharmacol. 1988;1:149–160. doi: 10.1159/000210769. [DOI] [PubMed] [Google Scholar]
- 10.Kawahara M, Iwasaki Y, Sakaguchi K, Taguchi T, Nishiyama M, Nigawara T, Tsugita M, Kambayashi M, Suda T, Hashimoto K. Predominant role of 25OHD in the negative regulation of PTH expression: clinical relevance for hypovitaminosis D. Life Sci. 2008;82:677–683. doi: 10.1016/j.lfs.2007.12.027. [DOI] [PubMed] [Google Scholar]
- 11.Flanagan JN, Young MV, Persons KS, Wang L, Mathieu JS, Whitlatch LW, Holick MF, Chen TC. Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006;26:2567–2572. [PubMed] [Google Scholar]
- 12.McCarthy K, Laban C, Bustin SA, Ogunkolade W, Khalaf S, Carpenter R, Jenkins PJ. Expression of 25-hydroxyvitamin D-1-alpha-hydroxylase, and vitamin D receptor mRNA in normal and malignant breast tissue. Anticancer Res. 2009;29:155–157. [PubMed] [Google Scholar]
- 13.Liu NQ, Hewison M. Vitamin D, the placenta and pregnancy. Arch Biochem Biophys. 2011 doi: 10.1016/j.abb.2011.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Bikle D, Adams JS, Christakos S. Primer on Metabolic Bone Diseases and Disorders of Mineral Metabolism. 8. Washington, D.C: American Society for Bone and Mineral Research; 2011. Vitamin D: Production, metabolism and mechanism of action. in press. [Google Scholar]
- 15.Whitfield GK, Dang HT, Schluter SF, Bernstein RM, Bunag T, Manzon LA, Hsieh G, Dominguez CE, Youson JH, Haussler MR, Marchalonis JJ. Cloning of a functional vitamin D receptor from the lamprey (Petromyzon marinus), an ancient vertebrate lacking a calcified skeleton and teeth. Endocrinology. 2003;144:2704–2716. doi: 10.1210/en.2002-221101. [DOI] [PubMed] [Google Scholar]
- 16.Lagishetty V, Chun RF, Liu NQ, Lisse TS, Adams JS, Hewison M. 1alpha-hydroxylase and innate immune responses to 25-hydroxyvitamin D in colonic cell lines. J Steroid Biochem Mol Biol. 2010;121:228–233. doi: 10.1016/j.jsbmb.2010.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ren S, Nguyen L, Wu S, Encinas C, Adams JS, Hewison M. Alternative splicing of vitamin D-24-hydroxylase: a novel mechanism for the regulation of extrarenal 1,25-dihydroxyvitamin D synthesis. The Journal of biological chemistry. 2005;280:20604–20611. doi: 10.1074/jbc.M414522200. [DOI] [PubMed] [Google Scholar]
- 18.Barbour GL, Coburn JW, Slatopolsky E, Norman AW, Horst RL. Hypercalcemia in an anephric patient with sarcoidosis: evidence for extrarenal generation of 1,25-dihydroxyvitamin D. N Engl J Med. 1981;305:440–443. doi: 10.1056/NEJM198108203050807. [DOI] [PubMed] [Google Scholar]
- 19.Adams JS, Sharma OP, Gacad MA, Singer FR. Metabolism of 25-hydroxyvitamin D3 by cultured pulmonary alveolar macrophages in sarcoidosis. J Clin Invest. 1983;72:1856–1860. doi: 10.1172/JCI111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Takeyama K, Kitanaka S, Sato T, Kobori M, Yanagisawa J, Kato S. 25-Hydroxyvitamin D3 1alpha-hydroxylase and vitamin D synthesis. Science. 1997;277:1827–1830. doi: 10.1126/science.277.5333.1827. [DOI] [PubMed] [Google Scholar]
- 21.Fu GK, Lin D, Zhang MY, Bikle DD, Shackleton CH, Miller WL, Portale AA. Cloning of human 25-hydroxyvitamin D-1 alpha-hydroxylase and mutations causing vitamin D-dependent rickets type 1. Mol Endocrinol. 1997;11:1961–1970. doi: 10.1210/mend.11.13.0035. [DOI] [PubMed] [Google Scholar]
- 22.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM. Mice lacking 25OHD 1alpha-hydroxylase demonstrate decreased epidermal differentiation and barrier function. J Steroid Biochem Mol Biol. 2004;89–90:347–353. doi: 10.1016/j.jsbmb.2004.03.113. [DOI] [PubMed] [Google Scholar]
- 23.Bikle DD, Chang S, Crumrine D, Elalieh H, Man MQ, Choi EH, Dardenne O, Xie Z, Arnaud RS, Feingold K, Elias PM. 25 Hydroxyvitamin D 1 alpha-hydroxylase is required for optimal epidermal differentiation and permeability barrier homeostasis. J Invest Dermatol. 2004;122:984–992. doi: 10.1111/j.0022-202X.2004.22424.x. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka Y, Halloran B, Schnoes HK, DeLuca HF. In vitro production of 1,25-dihydroxyvitamin D3 by rat placental tissue. Proceedings of the National Academy of Sciences of the United States of America. 1979;76:5033–5035. doi: 10.1073/pnas.76.10.5033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gray TK, Lester GE, Lorenc RS. Evidence for extra-renal 1 alpha-hydroxylation of 25-hydroxyvitamin D3 in pregnancy. Science. 1979;204:1311–1313. doi: 10.1126/science.451538. [DOI] [PubMed] [Google Scholar]
- 26.Weisman Y, Harell A, Edelstein S, David M, Spirer Z, Golander A. 1 alpha, 25-Dihydroxyvitamin D3 and 24,25-dihydroxyvitamin D3 in vitro synthesis by human decidua and placenta. Nature. 1979;281:317–319. doi: 10.1038/281317a0. [DOI] [PubMed] [Google Scholar]
- 27.Wieland P, Fischer JA, Trechsel U, Roth HR, Vetter K, Schneider H, Huch A. Perinatal parathyroid hormone, vitamin D metabolites, and calcitonin in man. The American journal of physiology. 1980;239:E385–390. doi: 10.1152/ajpendo.1980.239.5.E385. [DOI] [PubMed] [Google Scholar]
- 28.Hillman LS, Slatopolsky E, Haddad JG. Perinatal vitamin D metabolism. IV. Maternal and cord serum 24,25-dihydroxyvitamin D concentrations. The Journal of clinical endocrinology and metabolism. 1978;47:1073–1077. doi: 10.1210/jcem-47-5-1073. [DOI] [PubMed] [Google Scholar]
- 29.Lund B, Selnes A. Plasma 1,25-dihydroxyvitamin D levels in pregnancy and lactation. Acta endocrinologica. 1979;92:330–335. doi: 10.1530/acta.0.0920330. [DOI] [PubMed] [Google Scholar]
- 30.Fleischman AR, Rosen JF, Cole J, Smith CM, Deluca HF. Maternal and fetal serum 1,25-dihydroxyvitamin D levels at term. The Journal of pediatrics. 1980;97:640–642. doi: 10.1016/s0022-3476(80)80030-8. [DOI] [PubMed] [Google Scholar]
- 31.Seki K, Furuya K, Makimura N, Mitsui C, Hirata J, Nagata I. Cord blood levels of calcium-regulating hormones and osteocalcin in premature infants. Journal of perinatal medicine. 1994;22:189–194. doi: 10.1515/jpme.1994.22.3.189. [DOI] [PubMed] [Google Scholar]
- 32.Hollis BW, Pittard WB., 3rd Evaluation of the total fetomaternal vitamin D relationships at term: evidence for racial differences. The Journal of clinical endocrinology and metabolism. 1984;59:652–657. doi: 10.1210/jcem-59-4-652. [DOI] [PubMed] [Google Scholar]
- 33.Steichen JJ, Tsang RC, Gratton TL, Hamstra A, DeLuca HF. Vitamin D homeostasis in the perinatal period: 1,25-dihydroxyvitamin D in maternal, cord, and neonatal blood. N Engl J Med. 1980;302:315–319. doi: 10.1056/NEJM198002073020603. [DOI] [PubMed] [Google Scholar]
- 34.Walker VP, Zhang X, Rastegar I, Liu PT, Hollis BW, Adams JS, Modlin RL. Cord blood vitamin D status impacts innate immune responses. The Journal of clinical endocrinology and metabolism. 2011;96:1835–1843. doi: 10.1210/jc.2010-1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paulson SK, Ford KK, Langman CB. Pregnancy does not alter the metabolic clearance of 1,25-dihydroxyvitamin D in rats. The American journal of physiology. 1990;258:E158–162. doi: 10.1152/ajpendo.1990.258.1.E158. [DOI] [PubMed] [Google Scholar]
- 36.Delvin EE, Gilbert M, Pere MC, Garel JM. In vivo metabolism of calcitriol in the pregnant rabbit doe. Journal of developmental physiology. 1988;10:451–459. [PubMed] [Google Scholar]
- 37.Lachenmaier-Currle U, Harmeyer J. Placental transport of calcium and phosphorus in pigs. Journal of perinatal medicine. 1989;17:127–136. doi: 10.1515/jpme.1989.17.2.127. [DOI] [PubMed] [Google Scholar]
- 38.Turner M, Barre PE, Benjamin A, Goltzman D, Gascon-Barre M. Does the maternal kidney contribute to the increased circulating 1,25-dihydroxyvitamin D concentrations during pregnancy? Mineral and electrolyte metabolism. 1988;14:246–252. [PubMed] [Google Scholar]
- 39.Kovacs CS, Woodland ML, Fudge NJ, Friel JK. The vitamin D receptor is not required for fetal mineral homeostasis or for the regulation of placental calcium transfer in mice. Am J Physiol Endocrinol Metab. 2005;289:E133–144. doi: 10.1152/ajpendo.00354.2004. [DOI] [PubMed] [Google Scholar]
- 40.Christakos S, DeLuca HF. Minireview: Vitamin D: is there a role in extraskeletal health? Endocrinology. 2011;152:2930–2936. doi: 10.1210/en.2011-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Evans KN, Bulmer JN, Kilby MD, Hewison M. Vitamin D and placental-decidual function. J Soc Gynecol Investig. 2004;11:263–271. doi: 10.1016/j.jsgi.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 42.Zehnder D, Evans KN, Kilby MD, Bulmer JN, Innes BA, Stewart PM, Hewison M. The ontogeny of 25-hydroxyvitamin D(3) 1alpha-hydroxylase expression in human placenta and decidua. Am J Pathol. 2002;161:105–114. doi: 10.1016/s0002-9440(10)64162-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Novakovic B, Sibson M, Ng HK, Manuelpillai U, Rakyan V, Down T, Beck S, Fournier T, Evain-Brion D, Dimitriadis E, Craig JM, Morley R, Saffery R. Placenta-specific methylation of the vitamin D 24-hydroxylase gene: implications for feedback autoregulation of active vitamin D levels at the fetomaternal interface. The Journal of biological chemistry. 2009;284:14838–14848. doi: 10.1074/jbc.M809542200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cross NA, Hillman LS, Allen SH, Krause GF, Vieira NE. Calcium homeostasis and bone metabolism during pregnancy, lactation, and postweaning: a longitudinal study. The American journal of clinical nutrition. 1995;61:514–523. doi: 10.1093/ajcn/61.3.514. [DOI] [PubMed] [Google Scholar]
- 45.Dahlman T, Sjoberg HE, Bucht E. Calcium homeostasis in normal pregnancy and puerperium. A longitudinal study. Acta obstetricia et gynecologica Scandinavica. 1994;73:393–398. doi: 10.3109/00016349409006250. [DOI] [PubMed] [Google Scholar]
- 46.Seki K, Makimura N, Mitsui C, Hirata J, Nagata I. Calcium-regulating hormones and osteocalcin levels during pregnancy: a longitudinal study. American journal of obstetrics and gynecology. 1991;164:1248–1252. doi: 10.1016/0002-9378(91)90694-m. [DOI] [PubMed] [Google Scholar]
- 47.Rasmussen N, Frolich A, Hornnes PJ, Hegedus L. Serum ionized calcium and intact parathyroid hormone levels during pregnancy and postpartum. British journal of obstetrics and gynaecology. 1990;97:857–859. doi: 10.1111/j.1471-0528.1990.tb02585.x. [DOI] [PubMed] [Google Scholar]
- 48.Gallacher SJ, Fraser WD, Owens OJ, Dryburgh FJ, Logue FC, Jenkins A, Kennedy J, Boyle IT. Changes in calciotrophic hormones and biochemical markers of bone turnover in normal human pregnancy. European journal of endocrinology/European Federation of Endocrine Societies. 1994;131:369–374. doi: 10.1530/eje.0.1310369. [DOI] [PubMed] [Google Scholar]
- 49.Baksi SN, Kenny AD. Acute effect of estradiol on the renal vitamin D hydroxylases in Japanese quail. Biochemical pharmacology. 1978;27:2765–2768. doi: 10.1016/0006-2952(78)90187-9. [DOI] [PubMed] [Google Scholar]
- 50.Spanos E, Colston KW, Evans IM, Galante LS, Macauley SJ, Macintyre I. Effect of prolactin on vitamin D metabolism. Molecular and cellular endocrinology. 1976;5:163–167. doi: 10.1016/0303-7207(76)90080-0. [DOI] [PubMed] [Google Scholar]
- 51.Spanos E, Brown DJ, Stevenson JC, MacIntyre I. Stimulation of 1,25-dihydroxycholecalciferol production by prolactin and related peptides in intact renal cell preparations in vitro. Biochimica et biophysica acta. 1981;672:7–15. doi: 10.1016/0304-4165(81)90273-7. [DOI] [PubMed] [Google Scholar]
- 52.Pitkin RM. Calcium metabolism in pregnancy and the perinatal period: a review. American journal of obstetrics and gynecology. 1985;151:99–109. doi: 10.1016/0002-9378(85)90434-x. [DOI] [PubMed] [Google Scholar]
- 53.Shinki T, Ueno Y, DeLuca HF, Suda T. Calcitonin is a major regulator for the expression of renal 25-hydroxyvitamin D3-1alpha-hydroxylase gene in normocalcemic rats. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:8253–8258. doi: 10.1073/pnas.96.14.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhong Y, Armbrecht HJ, Christakos S. Calcitonin, a regulator of the 25-hydroxyvitamin D3 1alpha-hydroxylase gene. The Journal of biological chemistry. 2009;284:11059–11069. doi: 10.1074/jbc.M806561200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu NQ, Kaplan AT, Lagishetty V, Ouyang YB, Ouyang Y, Simmons CF, Equils O, Hewison M. Vitamin D and the regulation of placental inflammation. J Immunol. 2011;186:5968–5974. doi: 10.4049/jimmunol.1003332. [DOI] [PubMed] [Google Scholar]
- 56.Evans KN, Nguyen L, Chan J, Innes BA, Bulmer JN, Kilby MD, Hewison M. Effects of 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 on cytokine production by human decidual cells. Biol Reprod. 2006;75:816–822. doi: 10.1095/biolreprod.106.054056. [DOI] [PubMed] [Google Scholar]
- 57.Bodnar LM, Krohn MA, Simhan HN. Maternal vitamin D deficiency is associated with bacterial vaginosis in the first trimester of pregnancy. J Nutr. 2009;139:1157–1161. doi: 10.3945/jn.108.103168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mehta S, Hunter DJ, Mugusi FM, Spiegelman D, Manji KP, Giovannucci EL, Hertzmark E, Msamanga GI, Fawzi WW. Perinatal outcomes, including mother-to-child transmission of HIV, and child mortality and their association with maternal vitamin D status in Tanzania. J Infect Dis. 2009;200:1022–1030. doi: 10.1086/605699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Diaz L, Arranz C, Avila E, Halhali A, Vilchis F, Larrea F. Expression and activity of 25-hydroxyvitamin D-1 alpha-hydroxylase are restricted in cultures of human syncytiotrophoblast cells from preeclamptic pregnancies. The Journal of clinical endocrinology and metabolism. 2002;87:3876–3882. doi: 10.1210/jcem.87.8.8730. [DOI] [PubMed] [Google Scholar]
- 60.Fischer D, Schroer A, Ludders D, Cordes T, Bucker B, Reichrath J, Friedrich M. Metabolism of vitamin D3 in the placental tissue of normal and preeclampsia complicated pregnancies and premature births. Clin Exp Obstet Gynecol. 2007;34:80–84. [PubMed] [Google Scholar]
- 61.Bodnar LM, Catov JM, Simhan HN, Holick MF, Powers RW, Roberts JM. Maternal vitamin D deficiency increases the risk of preeclampsia. The Journal of clinical endocrinology and metabolism. 2007;92:3517–3522. doi: 10.1210/jc.2007-0718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bodnar LM, Simhan HN, Powers RW, Frank MP, Cooperstein E, Roberts JM. High prevalence of vitamin D insufficiency in black and white pregnant women residing in the northern United States and their neonates. J Nutr. 2007;137:447–452. doi: 10.1093/jn/137.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hypponen E. Vitamin D for the prevention of preeclampsia? A hypothesis. Nutr Rev. 2005;63:225–232. doi: 10.1301/nr.2005.jul.225-232. [DOI] [PubMed] [Google Scholar]
- 64.Rebut-Bonneton C, Demignon J. Effects of 1,25-dihydroxyvitamin D3 on in vitro lymphocyte reactions: arguments for a role at the maternofetal interface. Gynecol Obstet Invest. 1991;32:134–138. doi: 10.1159/000293014. [DOI] [PubMed] [Google Scholar]
- 65.Harrell GT, Fisher S. Blood Chemical Changes in Boeck’s Sarcoid with Particular Reference to Protein, Calcium and Phosphatase Values. J Clin Invest. 1939;18:687–693. doi: 10.1172/JCI101084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Studdy PR, Bird R, Neville E, James DG. Biochemical findings in sarcoidosis. J Clin Pathol. 1980;33:528–533. doi: 10.1136/jcp.33.6.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bell NH, Gill JR, Jr, Bartter FC. On the Abnormal Calcium Absorption in Sarcoidosis. Evidence for Increased Sensitivity to Vitamin D. Am J Med. 1964;36:500–513. doi: 10.1016/0002-9343(64)90099-3. [DOI] [PubMed] [Google Scholar]
- 68.Albright F, Carroll EL, Dempsey EF, Henneman PH. The cause of hypercalcuria in sarcoid and its treatment with cortisone and sodium phytate. J Clin Invest. 1956;35:1229–1242. doi: 10.1172/JCI103378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fallon MD, Perry HM, 3rd, Teitelbaum SL. Skeletal sarcoidosis with osteopenia. Metab Bone Dis Relat Res. 1981;3:171–174. doi: 10.1016/0221-8747(81)90004-7. [DOI] [PubMed] [Google Scholar]
- 70.Rizzato G, Montemurro L, Fraioli P. Bone-Mineral Content in Sarcoidosis. Seminars in Respiratory Medicine. 1992;13:411–423. [Google Scholar]
- 71.Sandler LM, Winearls CG, Fraher LJ, Clemens TL, Smith R, O’Riordan JL. Studies of the hypercalcaemia of sarcoidosis: effect of steroids and exogenous vitamin D3 on the circulating concentrations of 1,25-dihydroxy vitamin D3. Q J Med. 1984;53:165–180. [PubMed] [Google Scholar]
- 72.Meyrier A, Valeyre D, Bouillon R, Paillard F, Battesti JP, Georges R. Resorptive versus absorptive hypercalciuria in sarcoidosis: correlations with 25-hydroxy vitamin D3 and 1,25-dihydroxy vitamin D3 and parameters of disease activity. Q J Med. 1985;54:269–281. [PubMed] [Google Scholar]
- 73.Insogna KL, Dreyer BE, Mitnick M, Ellison AF, Broadus AE. Enhanced production rate of 1,25-dihydroxyvitamin D in sarcoidosis. The Journal of clinical endocrinology and metabolism. 1988;66:72–75. doi: 10.1210/jcem-66-1-72. [DOI] [PubMed] [Google Scholar]
- 74.Adams JS, Gacad MA. Characterization of 1 alpha-hydroxylation of vitamin D3 sterols by cultured alveolar macrophages from patients with sarcoidosis. J Exp Med. 1985;161:755–765. doi: 10.1084/jem.161.4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Smith SJ, Rucka AK, Berry JL, Davies M, Mylchreest S, Paterson CR, Heath DA, Tassabehji M, Read AP, Mee AP, Mawer EB. Novel mutations in the 1alpha-hydroxylase (P450c1) gene in three families with pseudovitamin D-deficiency rickets resulting in loss of functional enzyme activity in blood-derived macrophages. J Bone Miner Res. 1999;14:730–739. doi: 10.1359/jbmr.1999.14.5.730. [DOI] [PubMed] [Google Scholar]
- 76.Hewison M, Freeman L, Hughes SV, Evans KN, Bland R, Eliopoulos AG, Kilby MD, Moss PA, Chakraverty R. Differential regulation of vitamin D receptor and its ligand in human monocyte-derived dendritic cells. J Immunol. 2003;170:5382–5390. doi: 10.4049/jimmunol.170.11.5382. [DOI] [PubMed] [Google Scholar]
- 77.Shany S, Ren SY, Arbelle JE, Clemens TL, Adams JS. Subcellular localization and partial purification of the 25-hydroxyvitamin D3 1-hydroxylation reaction in the chick myelomonocytic cell line HD-11. J Bone Miner Res. 1993;8:269–276. doi: 10.1002/jbmr.5650080304. [DOI] [PubMed] [Google Scholar]
- 78.Reichel H, Koeffler HP, Barbers R, Norman AW. Regulation of 1,25-dihydroxyvitamin D3 production by cultured alveolar macrophages from normal human donors and from patients with pulmonary sarcoidosis. The Journal of clinical endocrinology and metabolism. 1987;65:1201–1209. doi: 10.1210/jcem-65-6-1201. [DOI] [PubMed] [Google Scholar]
- 79.Adams JS, Ren SY, Arbelle JE, Horiuchi N, Gray RW, Clemens TL, Shany S. Regulated production and intracrine action of 1,25-dihydroxyvitamin D3 in the chick myelomonocytic cell line HD-11. Endocrinology. 1994;134:2567–2573. doi: 10.1210/endo.134.6.8194484. [DOI] [PubMed] [Google Scholar]
- 80.Adams JS, Singer FR, Gacad MA, Sharma OP, Hayes MJ, Vouros P, Holick MF. Isolation and structural identification of 1,25-dihydroxyvitamin D3 produced by cultured alveolar macrophages in sarcoidosis. The Journal of clinical endocrinology and metabolism. 1985;60:960–966. doi: 10.1210/jcem-60-5-960. [DOI] [PubMed] [Google Scholar]
- 81.Rook GA, Steele J, Fraher L, Barker S, Karmali R, O’Riordan J, Stanford J. Vitamin D3, gamma interferon, and control of proliferation of Mycobacterium tuberculosis by human monocytes. Immunology. 1986;57:159–163. [PMC free article] [PubMed] [Google Scholar]
- 82.Wang TT, Nestel FP, Bourdeau V, Nagai Y, Wang Q, Liao J, Tavera-Mendoza L, Lin R, Hanrahan JW, Mader S, White JH. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi: 10.4049/jimmunol.173.5.2909. [DOI] [PubMed] [Google Scholar]
- 83.Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- 84.Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjo A, Torma H, Stahle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124:1080–1082. doi: 10.1111/j.0022-202X.2005.23687.x. [DOI] [PubMed] [Google Scholar]
- 85.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449:819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 86.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zugel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 87.Adams JS, Ren S, Liu PT, Chun RF, Lagishetty V, Gombart AF, Borregaard N, Modlin RL, Hewison M. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Schauber J, Dorschner RA, Coda AB, Buchau AS, Liu PT, Kiken D, Helfrich YR, Kang S, Elalieh HZ, Steinmeyer A, Zugel U, Bikle DD, Modlin RL, Gallo RL. Injury enhances TLR2 function and antimicrobial peptide expression through a vitamin D-dependent mechanism. J Clin Invest. 2007;117:803–811. doi: 10.1172/JCI30142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kreutz M, Andreesen R, Krause SW, Szabo A, Ritz E, Reichel H. 1,25-dihydroxyvitamin D3 production and vitamin D3 receptor expression are developmentally regulated during differentiation of human monocytes into macrophages. Blood. 1993;82:1300–1307. [PubMed] [Google Scholar]
- 90.Wang TT, Dabbas B, Laperriere D, Bitton AJ, Soualhine H, Tavera-Mendoza LE, Dionne S, Servant MJ, Bitton A, Seidman EG, Mader S, Behr MA, White JH. Direct and indirect induction by 1,25-dihydroxyvitamin D3 of the NOD2/CARD15-defensin beta2 innate immune pathway defective in Crohn disease. The Journal of biological chemistry. 2009;285:2227–2231. doi: 10.1074/jbc.C109.071225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu PT, Schenk M, Walker VP, Dempsey PW, Kanchanapoomi M, Wheelwright M, Vazirnia A, Zhang X, Steinmeyer A, Zugel U, Hollis BW, Cheng G, Modlin RL. Convergence of IL-1beta and VDR activation pathways in human TLR2/1-induced antimicrobial responses. PLoS One. 2009;4:e5810. doi: 10.1371/journal.pone.0005810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sorensen OE, Follin P, Johnsen AH, Calafat J, Tjabringa GS, Hiemstra PS, Borregaard N. Human cathelicidin, hCAP-18, is processed to the antimicrobial peptide LL-37 by extracellular cleavage with proteinase 3. Blood. 2001;97:3951–3959. doi: 10.1182/blood.v97.12.3951. [DOI] [PubMed] [Google Scholar]
- 93.Klionsky DJ, Emr SD. Autophagy as a regulated pathway of cellular degradation. Science. 2000;290:1717–1721. doi: 10.1126/science.290.5497.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gutierrez MG, Master SS, Singh SB, Taylor GA, Colombo MI, Deretic V. Autophagy is a defense mechanism inhibiting BCG and Mycobacterium tuberculosis survival in infected macrophages. Cell. 2004;119:753–766. doi: 10.1016/j.cell.2004.11.038. [DOI] [PubMed] [Google Scholar]
- 95.Deretic V, Levine B. Autophagy, immunity, and microbial adaptations. Cell Host Microbe. 2009;5:527–549. doi: 10.1016/j.chom.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fabri M, Stenger S, Shin DM, Yuk JM, Liu PT, Realegeno S, Lee HM, Krutzik SR, Schenk M, Sieling PA, Teles R, Montoya D, Iyer SS, Bruns H, Lewinsohn DM, Hollis BW, Hewison M, Adams JS, Steinmeyer A, Zugel U, Cheng G, Jo EK, Bloom BR, Modlin RL. Vitamin D is required for IFN-gamma-mediated antimicrobial activity of human macrophages. Sci Transl Med. 2011;3:104ra102. doi: 10.1126/scitranslmed.3003045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shin DM, Yuk JM, Lee HM, Lee SH, Son JW, Harding CV, Kim JM, Modlin RL, Jo EK. Mycobacterial lipoprotein activates autophagy via TLR2/1/CD14 and a functional vitamin D receptor signalling. Cell Microbiol. 2011;12:1648–1665. doi: 10.1111/j.1462-5822.2010.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Provvedini DM, Tsoukas CD, Deftos LJ, Manolagas SC. 1,25-dihydroxyvitamin D3 receptors in human leukocytes. Science. 1983;221:1181–1183. doi: 10.1126/science.6310748. [DOI] [PubMed] [Google Scholar]
- 99.Bruce D, Ooi JH, Yu S, Cantorna MT. Vitamin D and host resistance to infection? Putting the cart in front of the horse. Exp Biol Med (Maywood) 2010;235:921–927. doi: 10.1258/ebm.2010.010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 101.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, Adorini L. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 102.Krutzik SR, Hewison M, Liu PT, Robles JA, Stenger S, Adams JS, Modlin RL. IL-15 links TLR2/1-induced macrophage differentiation to the vitamin D-dependent antimicrobial pathway. J Immunol. 2008;181:7115–7120. doi: 10.4049/jimmunol.181.10.7115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Edfeldt K, Liu PT, Chun R, Fabri M, Schenk M, Wheelwright M, Keegan C, Krutzik SR, Adams JS, Hewison M, Modlin RL. T-cell cytokines differentially control human monocyte antimicrobial responses by regulating vitamin D metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:22593–22598. doi: 10.1073/pnas.1011624108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Barnes PF, Modlin RL, Bikle DD, Adams JS. Transpleural gradient of 1,25-dihydroxyvitamin D in tuberculous pleuritis. J Clin Invest. 1989;83:1527–1532. doi: 10.1172/JCI114048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Adams JS, Modlin RL, Diz MM, Barnes PF. Potentiation of the macrophage 25-hydroxyvitamin D-1-hydroxylation reaction by human tuberculous pleural effusion fluid. The Journal of clinical endocrinology and metabolism. 1989;69:457–460. doi: 10.1210/jcem-69-2-457. [DOI] [PubMed] [Google Scholar]