Abstract
Physical activity (PA) is associated with decreased levels of arterial stiffness in adults, but the relationship between PA and multiple measures of arterial stiffness in adolescents and young adults is not clear. The objective of this study was to test the hypothesis that PA is an independent predictor of multiple measures of arterial stiffness in adolescents and young adults. A total of 548 participants were enrolled in a study of the cardiovascular effects of obesity and type 2 diabetes mellitus (T2DM) (lean, 201; obese, 191; T2DM, 156). Anthropometrics, blood pressure, central and peripheral measures of arterial stiffness (pulse wave velocity, brachial distensibility, and augmentation index), blood (lipids and metabolic tests), and accelerometry data were collected. General linear modeling was performed to test for the independent relationship of PA on arterial stiffness. The mean age of the participants was 17.9 years (standard deviation, 3.5 years). After adjusting for other cardiovascular disease risk factors such as age, sex, body size, mean arterial pressure, and the presence of obesity or T2DM, PA was an independent predictor of augmentation index and brachial distensibility (P < .001). A greater effect of PA on pulse wave velocity was found in participants with T2DM (P = .009) compared with participants in the lean or obese groups. Physical activity is significantly and independently associated with multiple measures of arterial stiffness in adolescents and young adults. The role of PA in the prevention of cardiovascular disease target organ damage in youth, independent of energy balance, merits further exploration.
1. Introduction
Cardiovascular diseases (CVDs) have been the leading cause of death in the United States for several decades, accounting for 1 of every 3 deaths [1]. In adults, noninvasive measures of arterial stiffness predict coronary artery disease [2,3] and CVD mortality [4]. Increased arterial stiffness has been found in young adults and is associated with other CVD risk factors, such as elevated body size, blood pressure, glucose, and low-density lipoprotein cholesterol [5]. Obese youth and youth with type 2 diabetes mellitus (T2DM) also have higher levels of arterial stiffness [6].
Physical activity (PA) is protective against CVDs, and this may be partially mediated through its effect on arterial stiffness [7]. In adults, several studies have shown a favorable effect of habitual exercise on arterial health, such as arterial stiffness, endothelial function, and intima media thickness [8]. Few studies have investigated the relationship between PA and arterial stiffness in younger populations and have been limited to one measure of arterial stiffness [9,10].
The atherosclerosis that underlies CVDs develops in a nonuniform fashion throughout the arterial tree [11], and CVD risk factors affect arterial parameters differently in the central and peripheral arterial tree [12]. Therefore, we performed a comprehensive assessment of arterial stiffness in the central arterial tree (pulse wave velocity [PWV]) and peripheral arterial tree (brachial artery distensibility [BrachD]) and included a mixed measure that incorporates features of central stiffness and wave reflection (augmentation index [AI]). The purpose of this study was to use these measures to test our hypothesis that PA is a significant and independent predictor of multiple measures of arterial stiffness in adolescents and young adults.
2. Methods
2.1. Population and design
As part of an Institutional Review Board–approved study on the effects of T2DM on the cardiovascular system, subjects with T2DM were identified from local clinics (n = 156). Each subject with diabetes was matched to one obese (n = 191, ≥95th percentile for body mass index [BMI]) subject without diabetes (normal 2-hour oral glucose tolerance test result) and one lean (n = 201, 5th-85th percentile for BMI) subject by age, race, and sex. Exclusion criteria included positive pregnancy status and T2DM requiring insulin in the basal state to prevent ketoacidosis.
2.2. Measures
After participants granted consent/assent, historical information and anthropometric data were collected (including height and weight; BMI was calculated); blood pressure and arterial stiffness were measured; and blood was collected for (fasting) laboratory tests.
Physical activity was measured using a multidirectional accelerometer (Actical, Phillips Respironics, Bend, OR); participants were instructed to wear the accelerometer on the right hip at waist level for 7 consecutive days, except when sleeping, bathing, or engaging in activities harmful to the device (eg, collision sports). Participants were assigned to 1 of 3 groups of PA (Low, Middle, and High) using tertiles of mean counts per minute (cpm) over the time the device was worn on valid days. A valid day started at the first minute with at least 60 counts followed by 4 consecutive minutes with at least 10 cpm, stopped when 7 of 10 consecutive minutes had at least 10 counts followed by 120 consecutive minutes with less than 10 cpm, and had 10 to 20 hours of wear time.
Three measures of arterial stiffness were performed: BrachD (DynaPulse Pathway instrument; Pulse Metric, San Diego, CA) [5], PWV (carotid-femoral; SphygmoCor SCOR-PVx System; Atcor Medical, Sydney, Australia), and AI (Sphygmo-Cor) [13]. A validated generalized transfer function was used to analyze the pulse, and AI values were adjusted to a standard heart rate of 75 beats per minute [14]. All 3 measures have been used previously by the authors and have proven reproducible [6].
2.3. Analysis
All statistical analyses were performed using SAS version 9.1.3 (SAS Institute, Cary, NC). Means and proportions of the demographic, anthropometric, laboratory, and arterial data were calculated and examined. Nonnormal distributions were transformed using variance-stabilizing measures. An α level of .05 was used to indicate significance, and all tests were 2-sided. Differences by PA tertile were tested by analysis of variance; differences between categorical variable groups were tested using χ2 analysis. Using correlation analyses, general linear models were constructed and then reduced using parsimony and model stability as goals.
3. Results
The mean PA levels for the Low, Middle, and High tertiles of PA were 127, 216, and 377 cpm, respectively (Table 1). The participants in the High PA tertile were significantly younger (P < .0001) and more likely to be boys (P < .0001), but the proportion of participants of color did not differ significantly between PA tertiles (P = .994). There were significantly fewer participants with obesity or T2DM in the High PA tertile (P < .0001).
Table 1.
Demographic, anthropometric, blood measurements, and hemodynamics by physical activity level
| Variable | Low tertile (n = 183) | Middle tertile (n = 182) | High tertile (n = 183) |
|---|---|---|---|
| PA (cpm) a | 127 (35) | 216 (25) | 377 (106) |
| Age (y) a | 19 (3.1) | 18 (3.2) | 17 (3.7) |
| Sex (male) b | 28% | 32% | 50% |
| Race (persons of color) | 62% | 62% | 62% |
| Group b | |||
| Lean | 26% | 30% | 54% |
| Obese | 36% | 42% | 26% |
| T2DM | 38% | 28% | 20% |
| Height (cm) | 167 (10) | 167 (11) | 166 (12) |
| Weight (kg) b | 96 (33) | 91 (29) | 76 (27) |
| BMI (kg/m2) b | 34 (11) | 32 (9.3) | 27 (8.5) |
| Blood measurements | |||
| Cholesterol, total (mg/dL) | 175 (37) | 173 (37) | 168 (36) |
| Low-density lipoprotein cholesterol (mg/dL) d | 107 (34) | 102 (30) | 98 (31) |
| High-density lipoprotein cholesterol (mg/dL) a | 47 (12) | 50 (12) | 53 (14) |
| Triglycerides (mg/dL) b | 111 (72) | 102 (73) | 87 (66) |
| Glucose, fasting (mg/dL) | 119 (69) | 109 (52) | 105 (46) |
| Insulin, fasting (μU/mL) b | 20 (17) | 18 (12) | 15 (14) |
| Hemoglobin A1c (%) c | 6.6 (2.6) | 6.1 (1.9) | 5.9 (1.7) |
| C-reactive protein (mg/L) b | 4.7 (5.4) | 4.3 (5.9) | 2.5 (4.3) |
| Hemodynamics | |||
| Blood pressure, systolic (mm Hg) b | 116 (12) | 117 (13) | 112 (12) |
| Blood pressure, diastolic (mm Hg) b | 67 (13) | 65 (12) | 61 (12) |
| Mean arterial pressure (mm Hg) b | 84 (10) | 83 (9.4) | 81 (9.0) |
| Heart rate (beats/min) | 68 (10) | 65 (8.0) | 67 (12) |
| AI (%) c | 6.2 (12) | 2.6 (11) | 0.7 (11) |
| BrachD (mm/mm Hg) b | 5.7 (1.2) | 5.9 (1.3) | 6.3 (1.4) |
| PWV (m/s) b | 6.4 (1.5) | 6.2 (1.2) | 5.6 (0.9) |
All values presented as mean (standard deviation) except sex, race, and group proportions.
P ≤ .05 for differences by PA tertile determined by analysis of variance on normalized variables and χ2 for categorical variables, and indicated by:
Low ≠ Middle ≠ High.
Low and Middle ≠ High.
Low ≠ Middle and High.
Low ≠ High.
Several hemodynamic measures were correlated with PA (Table 1). Blood pressure (systolic, diastolic, and mean arterial) was significantly lower among participants in the High PA tertile (P = .0005, P < .0001, and P = .006, respectively). Augmentation index was significantly higher in the Low PA tertile (P < .0001). Brachial artery distensibility was significantly higher in the High PA tertile (P < .0001), and PWV was significantly lower in the High PA tertile (P < .0001). In all arterial stiffness measures, there was a trend of decreased stiffness for each step up in PA tertile (Fig. 1).
Fig. 1.
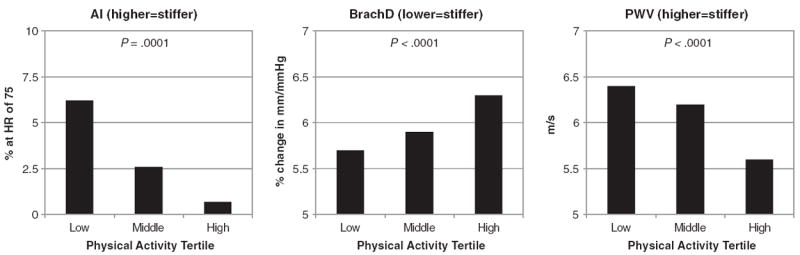
Arterial stiffness by physical activity group.
After controlling for CVD risk factors, including age, sex, body size, mean arterial pressure as a measure of distending pressure, and obesity or T2DM status, PA remained a significant independent predictor of arterial stiffness measured by AI (parameter estimate [cpm], −0.011) and BrachD (parameter estimate [cpm], 0.0001) (both P < .05). In the PWV model, significant interaction was present between PA and group (obesity or T2DM) status (P < .0001). A greater effect of PA on PWV was present in participants with T2DM (P = .009).
4. Discussion
In this study, we showed that PA in adolescents and young adults, after adjustment for demographic variables and other CVD risk factors, was an independent predictor of 2 measures of arterial stiffness in youth: BrachD, a measure of arterial stiffness in a medium-sized muscular artery, and AI, a mixed measure combining features of arterial stiffness and wave reflection. The independent association of PA with one of the arterial stiffness measures, PWV, a measure of stiffness in a central elastic artery, was significant only in participants with T2DM, with a nonsignificant trend in nondiabetic obese youth.
These findings are consistent with other studies of the effect of PA and physical fitness on arterial stiffness in adults [8,15]. Fewer studies using PWV as a measure of arterial stiffness have been performed in younger populations [10,16]. In a study of 573 children in Australia (mean age, 10.1 years), pedometer-measured PA was negatively correlated with PWV [10]. Schack-Nielsen et al [16] found that PWV was associated with PA at age 10 years. Farpour-Lambert et al [9] conducted a randomized controlled trial with 44 obese children (mean age, 8.9 years) and found that the exercise group (60 minutes, 3 times per week, 12 weeks) had lower levels of arterial stiffness at 6 months.
The relationship between PA and non-PWV measures of arterial stiffness, as used in this study, is less clear. Tanaka et al [19] studied 53 adult women and found that highly physically active participants did not demonstrate age-related increases in arterial stiffness as measured by PWV and AI. In a recent study, participants with less arterial stiffness measured by distensibility and compliance at age 36 years spent more time in vigorous PA from age 13 to 36 years [20]. Our findings are consistent with these studies in older and younger adults.
Physical activity is a complex, multifaceted behavior; objective measures of PA (eg, accelerometry) are limited in their ability to capture and quantify PA [17]. Energy expenditure predicted from accelerometer counts may be underestimated [18]; in this context, interpretations about the PA in this study may not be valid if energy expenditure is the primary measure of interest.
In adolescents and young adults, higher PA is an independent predictor of lower arterial stiffness using multiple measures, more so in obese and diabetic youth. Although some of the relationship between vascular health and PA in youth may be mediated through energy balance, the independent relationship shown here merits further mechanistic and epidemiologic investigation.
Acknowledgments
This study gratefully acknowledges the work of the CVD in T2DM study team and the generosity of the participants and their families.
Funding
This study was supported in part by: National Institutes of Health (NIH) (National Heart, Lung, and Blood Institute) R01 HL076269 (CVD in Adolescents with T2DM), US Public Health Service UL1 RR026314 (National Center for Research Resources [NCRR]), and NIH KL2RR026315 (NCRR). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NCRR or the NIH.
Footnotes
Author contributions: Edwards contributed to the analysis, data interpretation, and manuscript writing. Daniels, Claytor, Dolan, Kimball, and Urbina contributed to the design and conduct of the study, data collection and analysis, data interpretation, and manuscript writing. Khoury contributed to the data collection and analysis and manuscript writing.
Conflict of Interest
There are no potential conflicts of interest.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Budoff MJ, Flores F, Tsai J, et al. Measures of brachial artery distensibility in relation to coronary calcification. Am J Hypertens. 2003;16:350–5. doi: 10.1016/s0895-7061(03)00051-7. [DOI] [PubMed] [Google Scholar]
- 3.Fischer-Rasokat U, Brenck F, Zeiher AM, et al. Radial augmentation index unmasks premature coronary artery disease in younger males. Blood Press Monit. 2009;14:59–67. doi: 10.1097/MBP.0b013e32832941ce. [DOI] [PubMed] [Google Scholar]
- 4.Vlachopoulos C, Aznaouridis K, Stefanadis C. Prediction of cardiovascular events and all-cause mortality with arterial stiffness: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;55:1318–27. doi: 10.1016/j.jacc.2009.10.061. [DOI] [PubMed] [Google Scholar]
- 5.Urbina EM, Brinton TJ, Elkasabany A, et al. Brachial artery distensibility and relation to cardiovascular risk factors in healthy young adults (The Bogalusa Heart Study) Am J Cardiol. 2002;89:946–51. doi: 10.1016/s0002-9149(02)02244-0. [DOI] [PubMed] [Google Scholar]
- 6.Urbina EM, Kimball TR, Khoury PR, et al. Increased arterial stiffness is found in adolescents with obesity or obesity-related type 2 diabetes mellitus. J Hypertens. 2010;28:1692–8. doi: 10.1097/HJH.0b013e32833a6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tanaka H, Dinenno FA, Monahan KD, et al. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–5. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- 8.Seals DR, Desouza CA, Donato AJ, et al. Habitual exercise and arterial aging. J Appl Physiol. 2008;105:1323–32. doi: 10.1152/japplphysiol.90553.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farpour-Lambert NJ, Aggoun Y, Marchand LM, et al. Physical activity reduces systemic blood pressure and improves early markers of atherosclerosis in pre-pubertal obese children. J Am Coll Cardiol. 2009;54:2396–406. doi: 10.1016/j.jacc.2009.08.030. [DOI] [PubMed] [Google Scholar]
- 10.Sakuragi S, Abhayaratna K, Gravenmaker KJ, et al. Influence of adiposity and physical activity on arterial stiffness in healthy children: the lifestyle of our kids study. Hypertension. 2009;53:611–6. doi: 10.1161/HYPERTENSIONAHA.108.123364. [DOI] [PubMed] [Google Scholar]
- 11.Solberg LA, Eggen DA. Localization and sequence of development of atherosclerotic lesions in the carotid and vertebral arteries. Circulation. 1971;43:711–24. doi: 10.1161/01.cir.43.5.711. [DOI] [PubMed] [Google Scholar]
- 12.Urbina EM, Wadwa RP, Davis C, et al. Prevalence of increased arterial stiffness in children with type 1 diabetes mellitus differs by measurement site and sex: the SEARCH for Diabetes in Youth Study. J Pediatr. 2010;156:731–7. doi: 10.1016/j.jpeds.2009.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27:2588–605. doi: 10.1093/eurheartj/ehl254. [DOI] [PubMed] [Google Scholar]
- 14.O’Rourke MF, Gallagher DE. Pulse wave analysis. J Hypertens Suppl. 1996;14:S147–57. [PubMed] [Google Scholar]
- 15.Boreham CA, Ferreira I, Twisk JW, et al. Cardiorespiratory fitness, physical activity, and arterial stiffness: the Northern Ireland Young Hearts Project. Hypertension. 2004;44:721–6. doi: 10.1161/01.HYP.0000144293.40699.9a. [DOI] [PubMed] [Google Scholar]
- 16.Schack-Nielsen L, Molgaard C, Larsen D, et al. Arterial stiffness in 10-year-old children: current and early determinants. Br J Nutr. 2005;94:1004–11. doi: 10.1079/bjn20051518. [DOI] [PubMed] [Google Scholar]
- 17.Corder K, Brage S, Ekelund U. Accelerometers and pedometers: methodology and clinical application. Curr Opin Clin Nutr Metab Care. 2007;10:597–603. doi: 10.1097/MCO.0b013e328285d883. [DOI] [PubMed] [Google Scholar]
- 18.Rothney MP, Schaefer EV, Neumann MM, et al. Validity of physical activity intensity predictions by ActiGraph, Actical, and RT3 accelerometers. Obesity (Silver Spring) 2008;16:1946–52. doi: 10.1038/oby.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tanaka H, DeSouza CA, Seals DR. Absence of age-related increase in central arterial stiffness in physically active women. Arterioscler Thromb Vasc Biol. 1998;18:127–32. doi: 10.1161/01.atv.18.1.127. [DOI] [PubMed] [Google Scholar]
- 20.van de Laar RJ, Ferreira I, van Mechelen W, et al. Habitual physical activity and peripheral arterial compliance in young adults: the Amsterdam growth and health longitudinal study. Am J Hypertens. 2011;24:200–8. doi: 10.1038/ajh.2010.201. [DOI] [PubMed] [Google Scholar]


