Abstract
Cortical oscillations in the theta (4-10 Hz) and gamma (30-100 Hz) frequency range have been hypothesized to play important roles in numerous cognitive processes and may be involved in psychiatric conditions including anxiety, schizophrenia, and autism. Here we will review background information about these oscillations and their possible roles in psychiatric illness. Then we will describe findings from recent studies that used optogenetic tools to demonstrate that (1) a particular class of inhibitory interneurons expressing the calcium binding protein parvalbumin plays a central role in gamma oscillations, (2) gamma oscillations can entrain rhythmic firing in pyramidal neurons, and (3) rhythmic firing at theta and gamma frequencies can enhance communication between neurons. Finally we will discuss how these findings may relate to the pathophysiology of psychiatric conditions, as well as questions for future studies.
Keywords: Optogenetics, interneuron, oscillations, synchrony, theta, gamma
Overview of cortical oscillations
Cortical oscillations are rhythmic patterns of neural activity that are synchronized across many neurons within or across brain regions. These oscillations can be observed at many levels including surface or intracranial EEG, local field potentials (LFPs), multiunit recordings, single unit recordings, and subthreshold fluctuations in the membrane potentials of individual neurons. Cortical oscillations are classified based on frequency, into delta (0.5-4 Hz), theta (4-10 Hz), alpha (8-12 Hz), beta (10-30 Hz), and gamma (30-100 Hz) oscillations (1). Oscillations at specific frequencies and in specific regions often occur during sleep, cognitive tasks, or sensory stimulation. Oscillations also occur during specific phases of cognitive tasks, and in many cases their amplitude or synchrony correlates with the difficulty of the task, or performance on that task (2, 3). As described below, oscillations also correlate with anxiety-related behaviors in rodents. This has led to the hypothesis that these oscillations play important roles in cognitive functions or behavior states such as anxiety.
Theta oscillations and anxiety
Theta oscillations that are synchronized between the amygdala, hippocampus, and prefrontal cortex (PFC) are thought to play a role in anxiety. Fear conditioning experiments in rats have found rhythmic theta-frequency activity in the amygdala, that is elicited by the conditioned stimulus and associated with freezing, and is synchronized with rhythmic activity in the hippocampus (4). Anxiolytic agents decrease the frequency of hippocampal theta oscillations (5), whereas mice which lack the 5-HT1A receptor, exhibit increased levels of anxiety-related behavior and increases in hippocampal and PFC theta oscillations, specifically in anxiogenic environments (6, 7). More recent work has shown that in mice, theta frequency synchronization between the medial PFC and ventral hippocampus predicts avoidance of anxiogenic regions of an open field or elevated plus maze (7). These studies are all consistent with the idea that periods of anxiety are associated with theta oscillations, synchronized across the amygdala, hippocampus, and the PFC. Prefrontal theta oscillations also play a major role in working memory (2, 3, 8), and may contribute to working memory deficits in schizophrenia (9).
Gamma oscillations in normal cognition and schizophrenia
A large literature supports the idea that gamma oscillations play important roles in various cognitive functions including attention (10-12), perception (13), and working memory (3, 14). An early hypothesis was that gamma oscillations serve to “bind together” information encoded by dispersed sets of neurons, by for example, synchronizing spikes in neurons responding to various aspects of the same stimulus (15, 16). Although experimental evidence for such “perceptual grouping” is limited, an alternative is that by synchronizing activity in different brain regions, gamma oscillations may facilitate interactions between these regions. Support for this idea has come from the recent findings that the BOLD signal measured by fMRI correlates strongly with the power of local gamma oscillations (17). By contrast, the BOLD signal is less strongly correlated with spiking activity (18), and negatively correlated with power in lower frequency bands (19). Thus, reports that networks of brain regions can be defined by covariation in the BOLD signal may indicate that gamma oscillations co-occur in these networks. Although the timescale of the BOLD signal is too slow to demonstrate that gamma oscillations in these different regions are precisely synchronized, these studies are consistent with the idea that gamma oscillations co-occur in these regions, possibly to coordinate their interactions. Long range gamma synchronization has also been observed during specific behaviors in humans (12, 13) and monkeys (20, 21). The exact mechanism by which synchronized gamma oscillations in different brain regions might facilitate interactions involving those regions are unknown, but we discuss some possibilities below. Also note that other studies have found local, but not long-range, gamma synchrony (22).
As described above, gamma oscillations are involved in cognitive processes that are disrupted in schizophrenia, and are thought to facilitate interactions between brain regions, which may be deficient in schizophrenia. This has given rise to the hypothesis that abnormal gamma oscillations may contribute to cognitive dysfunction and other symptoms of schizophrenia (23). Indeed, many studies have found that patients with schizophrenia exhibit decreases in the power or synchrony of gamma oscillations during responses to sensory stimulation or cognitive tasks (24-30). In some cases, these abnormalities correlate with the severity of cognitive dysfunction or other symptoms (25, 28). Although patients with schizophrenia as a whole typically exhibit decreased power or synchrony of gamma oscillations (especially those evoked by sensory stimuli or cognitive tasks), within this clinical population, auditory hallucinations seem to be associated with increased power or synchrony of beta and gamma oscillations (31-33). This suggests that in some cases, increased beta or gamma oscillations may contribute to positive symptoms.
The relationship between cortical gamma oscillations and parvalbumin interneurons
Altered gamma oscillations in schizophrenia are thought to result from dysfunction of a specific class of inhibitory interneurons that express the calcium-binding protein parvalbumin (PV). PV interneurons represent approximately 40% of cortical inhibitory neurons. They are characterized by a unique “fast-spiking” electrophysiological phenotype, i.e. they can fire at very high rates (>200 Hz) with minimal spike-frequency adaptation. Several groups have found alterations in PV interneurons, particularly in the PFC, in post-mortem brain tissue from patients with schizophrenia (23, 34-38). Specifically, in tissue from patients with schizophrenia, these interneurons express virtually undetectable levels of GAD67, which synthesizes the inhibitory neurotransmitter GABA, suggesting that these neurons are hypofunctional. This tissue also exhibits increases in post-synaptic GABA receptors, and decreases in GABA transporters, which may represent compensation for deficient GABA release. PV interneuron dysfunction may relate to other factors implicated in schizophrenia such as NMDA receptor hypofunction (39-41) or oxidative stress (42).
Previous studies on the role of parvalbumin interneurons in generating gamma oscillations
Several observations have led to the hypothesis that PV interneurons play a critical role in gamma oscillations. First, although pyramidal neurons and many types of interneurons fire during gamma oscillations, only PV interneurons fire rhythmically on nearly every cycle of gamma oscillations (43-45). Second, isolated networks of PV interneurons (as well as other types of interneurons) can generate gamma oscillations, whereas blocking inhibitory synapses disrupts gamma oscillations and rhythmic pyramidal neuron firing in brain slices (46). Third, PV interneurons are interconnected via chemical and electrical synapses (47, 48), such that gamma frequency spiking in one PV interneuron will entrain rhythmic firing in a second, connected PV interneuron (49). Fourth, PV interneurons target the perisomatic region of pyramidal neurons, and field potential recordings and voltage sensitive dye imaging suggests that this region is the origin for gamma frequency voltage fluctuations (50). Fifth, genetically disrupting excitatory synapses on PV interneurons suppresses gamma oscillations in brain slices (51).
Previous studies of mechanisms underlying gamma oscillations
For more information about mechanisms of gamma oscillations, or about computational models of oscillations, the reader is referred to several recent reviews that extensively discuss these topics (52-55). Besides the evidence for PV interneuron involvement in gamma oscillations described above, we will mention a few other notable aspects of this work. First, inhibitory connections between PV interneurons elicit synaptic currents with relatively large conductances and fast kinetics, which reverse at potentials between the resting membrane potential and spike threshold. All of these features play an important role in synchronizing gamma oscillations (53). Second, there are at least two possible mechanisms through which interactions between excitatory and inhibitory neurons might generate gamma oscillations in vivo (53-55). Specifically, some forms of gamma oscillations in hippocampal brain slices persist even after blocking fast, AMPA receptor-mediated excitatory synapses (46, 56), suggesting that gamma oscillations may be generated by networks of inhibitory interneurons which fire and inhibit each other, until inhibition decays and they fire again, initiating the next cycle of the oscillation. In this way, interneuron networks generate rhythmic inhibitory output that entrains excitatory neurons (interneuron gamma, “ING”). By contrast, other forms of gamma oscillations in brain slices require fast, AMPA receptor-mediated excitation (50, 57). In the latter cases, oscillations result from interactions between excitatory and inhibitory neurons, in which excitatory neurons fire, triggering interneuron firing, which, after a delay, suppresses excitatory neuron firing. The next cycle of the oscillation begins when inhibitory synaptic currents in excitatory neurons decay, allowing excitatory neurons fire again (“pyramidal-interneuron gamma”, PING). Recordings in vivo (44) and in vitro (45, 50) have shown how pyramidal neuron excitation of PV interneurons may be relevant to gamma oscillations in intact networks, although questions about the relative contributions of ING and PING to gamma oscillations in vivo (55). Third, gap junctions between PV interneurons, or between pyramidal neuron axons (58, 59), can enhance the synchrony of gamma oscillations, and indeed, gamma oscillations are impaired in hippocampal slices from connexin-36 knockout mice, which lack gap junctions between PV interneurons (60, 61).
PV interneuron dysfunction may produce abnormal gamma oscillations in psychiatric disease
Thus, a combination of neuropathological findings and studies of mechanisms that generate gamma oscillations have led to the hypothesis that PV interneuron hypofunction produces abnormal gamma oscillations which contribute to cognitive dysfunction in schizophrenia. A similar mechanism may also play a role in autism. Numerous rodent models of autism exhibit deficits in PV interneurons (62), and patients with autism spectrum disorders exhibit decreases in gamma oscillations evoked by sensory stimuli (63, 64). Other studies have found increases in baseline gamma power in patients with autism (65), which are reminiscent of the association between relatively increased gamma power and positive symptoms in schizophrenia.
Probing the role of PV interneurons in gamma oscillations using optogenetic tools
Although the studies described above provide critical evidence for a major role of PV interneurons in gamma oscillations, they nevertheless leave key questions unanswered. First, although PV interneurons are clearly involved in gamma oscillations, are they necessary or sufficient for these oscillations? More specifically, does inhibitory output from PV interneurons suffice to generate gamma oscillations, or is rhythmic activity in other interneuron subtypes also required? Second, although gamma oscillations are prominent during cognitive tasks, how exactly might they affect neural activity and information processing in a way that contributes to these tasks? Prior to the advent of optogenetic technologies, it was difficult to answer these questions directly, because it was not possible to selectively modulate either PV interneurons or gamma oscillations. Here we will describe recent studies that overcome these barriers and answer these two questions using optogenetic tools. Again, we will focus on the role of PV interneurons in gamma oscillations, and refer the reader to recent reviews for other details about mechanisms of gamma rhythms (52-55). Also, although theta oscillations clearly play an important role in psychiatric illness, studies using optogenetic tools to probe mechanisms of oscillations have thus far focused on gamma oscillations. Thus, most of the experiments described below relate to gamma oscillations, although some experiments about possible functions of cortical oscillations also considered theta oscillations.
Are PV interneurons necessary or sufficient for neocortical gamma oscillations?
Two experiments have addressed this question using optogenetic tools to control activity in PV interneurons in vivo. In first, Cardin et al. selectively expressed channelrhodopsin-2 (ChR2) in either PV interneurons or pyramidal neurons in somatosensory cortex (66). Selective expression was achieved using PV::Cre or CaMKII::Cre transgenic mice along with a virus to drive Cre-dependent expression of ChR2. This study then activated ChR2 with rhythmic trains of light flashes (8-200 Hz) while recording LFPs. Rhythmic stimulation of PV interneurons increased LFP power at the corresponding frequency, but only for stimulation frequencies in the gamma range (30-60 Hz). This rhythmic stimulation also entrained firing in pyramidal neurons. Varying the stimulation intensity did not alter the range of frequencies that exhibited increased LFP power, confirming that this effect did not simply reflect an effect of 30-60 Hz stimulation to drive PV interneuron firing particularly well (as compared to higher stimulation frequencies). By contrast, gamma frequency stimulation of pyramidal neurons failed to increase LFP power at these frequencies (this may relate to the observation that gamma-frequency stimulation of pyramidal neurons did not elicit robust rhythmic firing in those neurons). These elegant findings show that rhythmic stimulation of PV interneurons can generate LFP oscillations in vivo at gamma frequencies, but not at other frequencies, and thus demonstrate a powerful role for these interneurons in gamma oscillations.
In a complementary study (67), we used enhanced halorhodopsin (eNpHR or NpHR2.0) (68) to inhibit PV interneurons in vivo while recording LFPs in PFC. This study used the same approach described earlier to achieve selective expression in PV interneurons. This study also used the CaMKII promoter to simultaneously express ChR2 in pyramidal neurons in the same mice, and found that single flashes of blue light to activate pyramidal neurons evoked transient gamma oscillations. Delivering yellow light, to activate eNpHR and thereby inhibit PV interneurons, suppressed the power of evoked gamma oscillations by ~20%. Power at other frequencies was not affected. The incomplete suppression of power in the gamma frequency range may be explained by noting that eNpHR was only expressed in ~40% of PV interneurons, and that in these neurons, yellow light incompletely suppressed spiking. Another possibility is that other types of interneurons were able to generate attenuated gamma oscillations after PV interneuron activity was suppressed. Together, these results from Cardin et al. and our work (67), suggest that PV interneurons can powerfully drive gamma oscillations, and that this effect is a major contributor to gamma oscillations in vivo. One potential limitation of these studies is that gamma oscillations elicited by optogenetic stimulation may differ from those observed during behavior.
The latter experiment, in which transient gamma oscillations were triggered by brief stimulation of pyramidal neurons, suggests that excitatory synaptic activity may also play an important role in gamma oscillations in vivo. Indeed, Cardin et al. found that glutamate receptor antagonists suppressed gamma frequency LFP oscillations evoked by rhythmic stimulation of PV interneurons (66). More recently, a study found that continuous stimulation of cortical pyramidal neurons using ChR2 also elicits gamma-frequency LFP oscillations (69), which are eliminated by blocking either glutamate or GABA receptors. Thus, as suggested by the observations that some forms of pharmacologically induced gamma oscillations in slices require excitatory synaptic activity (50, 57), rhythmic firing in PV interneurons, which drives gamma oscillations, may arise via interactions with excitatory neurons. To elucidate how this might come about, we studied a minimal circuit consisting of one pyramidal neuron and nearby PV interneurons, by recording from one pyramidal neuron, in a brain slice that selectively expressed ChR2 in PV interneurons (67). The pyramidal neuron received noisy, non-rhythmic input under two conditions. In one condition, each pyramidal neuron spike triggered a light flash that activated nearby PV interneurons. This delivered feedback inhibition from PV interneurons to the pyramidal neuron. Comparing responses of the pyramidal neuron under this condition (spikes trigger light flashes and feedback inhibition) to responses under control conditions (when light flashes were absent), reveals how PV interneuron-mediated inhibition affects firing of the pyramidal neuron. Delivering light flashes and recruiting feedback inhibition from PV interneurons selectively enhanced gamma frequency firing. This confirms that a minimal circuit consisting of pyramidal neurons and PV interneurons is sufficient to transform noisy non-rhythmic input, into gamma-frequency rhythmic firing. Although these experiments suggest that excitatory synaptic input can drive PV interneurons in a way that leads to gamma oscillations, and that such excitatory input is necessary for optogenetically evoked gamma oscillations, they leave open some detailed questions about the role of this excitatory input, i.e. does the precise pattern of excitatory input to PV neurons influence the resulting oscillation, or does only the overall level PV interneurons excitation matter (55).
Some of the preceding results about the role of excitatory neurons in gamma oscillations may seem inconsistent. Specifically, Cardin et al. (66) found that gamma frequency stimulation of pyramidal neurons fails to induce gamma oscillations. By contrast, we (67) found that single light flashes to stimulate pyramidal neurons elicit transient gamma oscillations, and Adesnik and Scanziani (69) found that weak, continuous stimulation of pyramidal neurons induces gamma oscillations. A simple interpretation of these results is that weak or transient pyramidal neuron excitation can recruit PV interneurons and initiate gamma oscillations. By contrast, strong rhythmic excitation of pyramidal neurons may fail to elicit network oscillations because such strong stimulation may engage additional network mechanisms that suppress gamma oscillations, e.g. by recruiting activity in non-PV interneurons.
Using optogenetic tools to study putative functions of cortical oscillations
Gamma oscillations have been hypothesized to facilitate interactions between brain regions in a variety of ways. One possibility is that gamma oscillations in local populations of neurons represent a clock, and that the timing of spikes relative to this clock carries some meaning. In particular, cells that receive strong input might fire earlier in the gamma cycle, whereas cells that receive weaker input fire later or not at all (70). A second possibility is that spikes which occur in different sets of neurons but at corresponding phases of a common gamma oscillation might form a distributed representation (16). Specifically, gamma-frequency synchronization among these neurons might facilitate communication between them, by ensuring that inputs from one neuron arrive at another neuron in the same representation during a favorable portions of the gamma cycle (characterized by low levels of inhibitory current) (71, 72). A third possibility is that by synchronizing inputs to a neuron, gamma-frequency oscillations enhance the gain of that neuron, i.e. synchronized inputs will be more likely to elicit a spike than the same number of desynchronized inputs (73, 74). This might explain why long-range gamma-frequency synchrony can increase for neural activity associated with attended stimuli (10) (11, 12, 21) (but (12, 75) suggest that in primary visual cortex, attention may suppress gamma synchrony). Attention-induced increases in gamma-frequency synchrony might increase the impact of neural activity corresponding to attended stimuli on downstream targets.
To test these ideas, the studies described above also measured how gamma oscillations synchronize firing, and how synchronized rhythmic firing influences neural information processing. First, Cardin et al. found that as hypothesized, optogenetically evoked gamma oscillations synchronize neural responses in somatosensory cortex (66). Specifically, during whisker stimulation, 40 Hz optogenetic stimulation of PV interneurons, which elicits gamma oscillations, also suppresses pyramidal neuron firing at specific phases of the induced oscillation. This tends to concentrate spikes at those specific phases of the oscillation which have low levels of inhibition. Furthermore, 40 Hz stimulation of PV interneurons increases the spike timing precision of responses to whisker stimulation.
Second, we showed that as hypothesized, this kind of rhythmic synchronization of activity at theta (~ 8 Hz) or gamma (~ 40 Hz) frequencies could increase the amount of information that neurons transmit about those inputs (67). These experiments were carried out in brain slices from PFC, using random trains of light flashes to activate ChR2 in pyramidal neurons. During each train, the rate of light flashes varied, producing varying rates of pyramidal neuron firing. Some trains were non-rhythmic, whereas others were modulated at 8 or 40 Hz, and produced rhythmic modulation of pyramidal neuron firing at the corresponding frequencies. By recording responses of pyramidal neurons and downstream neurons to the same trains of light flashes, we quantified how well firing in downstream neurons encoded their inputs (which were spikes in pyramidal neurons). Rhythmic modulation of pyramidal neuron firing at theta (~ 8 Hz) or gamma (~ 40 Hz) frequencies increased the information that downstream neurons transmitted about the rate of spikes in pyramidal neurons. Thus, neurons may transmit more information about rhythmic inputs than about non-rhythmic inputs, consistent with the hypothesis attention-induced increases in rhythmic synchrony (mediated in some cases by attention) might make certain neural signals more salient to downstream targets.
Implications for psychiatric illness
As described above, theta-frequency synchronization between the amygdala, hippocampus, and PFC may relate to anxiety. The results described above suggest that theta-frequency synchronization between these structures may enhance the influence that anxiety-related signals originating in the amygdala have on decision-making processes in the PFC.
In schizophrenia and autism, PV interneuron dysfunction is thought to contribute to deficient gamma oscillations and cognitive deficits, whereas increases in beta or gamma oscillations may contribute to positive symptoms. The experiments described above confirm that PV interneurons play a critical role in gamma oscillations, and thus support the hypothesized link between PV interneuron dysfunction and disrupted gamma oscillations in schizophrenia. Furthermore, these studies demonstrate that PV interneuron-driven gamma oscillations can entrain rhythmic pyramidal neuron firing, and that such rhythmic modulation can enhance the impact of pyramidal neuron firing on downstream neurons (Fig. 1). The latter effects may explain why decreases in gamma oscillations are associated with cognitive deficits in schizophrenia. Schizophrenia (and autism) is often conceptualized as a disorder of communication between brain regions. As described above, neural oscillations represent a mechanism that can enhance communication between neurons in a dynamic and flexible way (Fig. 2). For example, during a visual task, gamma oscillations appear in higher order visual regions and the PFC (12). Moreover, these gamma oscillations in different regions are synchronized, which may facilitate communication between these regions (12). If the requirements of the task suddenly shift, and involve auditory rather than visual information, gamma oscillations would be expected to decrease in visual areas but increase in auditory areas. Correspondingly, the PFC should become less synchronized with visual areas, but more synchronized with auditory regions, allowing the PFC to attend to auditory, rather than visual information. Thus, deficits in gamma frequency synchronization could contribute to failures of communication between brain regions that are hypothesized to contribute to cognitive deficits and other symptoms of schizophrenia. In particular, corollary discharges, signals between brain regions that indicate self-generated stimuli, are thought to be deficient in schizophrenia causing deficits of self-monitoring that may manifest as symptoms including auditory hallucinations (76). Corollary discharge may be mediated by oscillations (77), such that deficient synchronization may contribute to auditory hallucinations in schizophrenia (28).
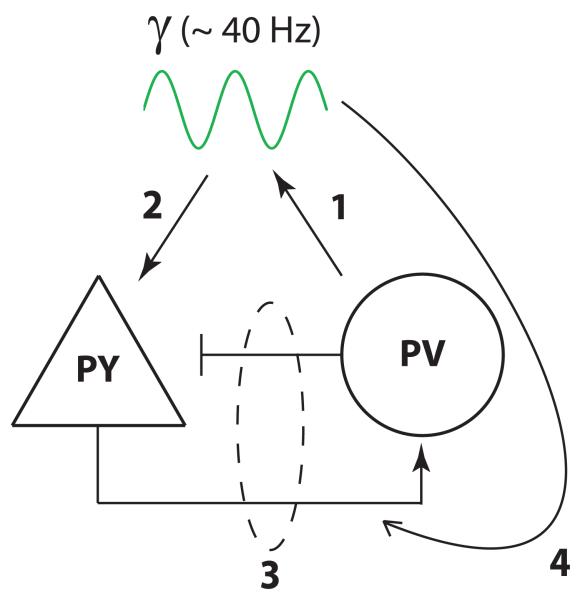
Figure 1. Optogenetic studies confirm many ideas about gamma oscillations illustrated in this diagram.
1. Parvalbumin (PV) interneurons play a critical role in generating gamma oscillations. 2. Gamma oscillations, specifically rhythmic inhibition generated by PV interneurons, can entrain and synchronize firing in pyramidal (PY) neurons. 3. Interactions between excitatory and inhibitory neurons produce emergent gamma oscillations. 4. Rhythmic, gamma-modulated firing in PY neurons can enhance the flow of information from these neurons to their post-synaptic targets.
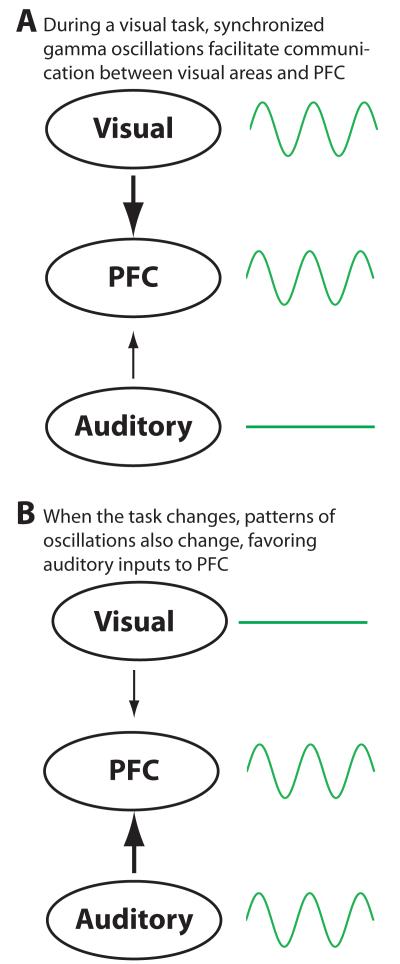
Figure 2. Diagram illustrating one mechanism by which long-range gamma synchrony might facilitate communication between brain regions in a dynamic fashion.
In this example, during a visual task, gamma oscillations occur in visual regions and PFC, and are synchronized across these regions. If requirements of the task shift, such that visual information is no longer required, but auditory information is, then changes in the patterns of oscillations can facilitate a rapid shift that favors the flow of auditory information into PFC.
Just as deficits in gamma oscillations might impair communication between brain regions, conversely, abnormally increased gamma oscillations might transmit information with heightened salience. Thus, in the context of abnormally increased gamma oscillations, auditory signals which are normally ignored may instead be misinterpreted as hallucinations. Increased gamma oscillations in schizophrenia might also cause a ceiling effect that prevents gamma recruitment during cognition. An open question is whether mechanisms that disrupt gamma oscillations in schizophrenia, e.g. PV interneuron hypofunction, might sometimes contribute to increased spontaneous beta or gamma oscillations, e.g. in the context of positive symptoms. Specifically, PV interneuron hypofunction might cause high frequency gamma oscillations that are important for cognition, to shift to lower frequencies, producing pathological beta or low gamma frequency oscillations. Alternatively, increases in spontaneous gamma oscillations might represent compensation for PV interneuron hypofunction and overall decreased gamma oscillations. Or PV interneuron hypofunction might itself represent compensation for some other process which increases gamma oscillations.
Future directions for optogenetic studies of cortical oscillations
As noted earlier, it will be important for future work to address mechanisms that generate theta oscillations, and to target non-PV interneurons in order to determine their role in gamma oscillations. Future experiments might also target distinct subpopulations of PV interneurons (e.g. basket vs. axo-axonic cells), which likely play different roles in gamma oscillations. Optogenetic tools might also be useful for exploring how oscillations are synchronized, rather than just how they are generated. For example, one might use ChR2 to entrain oscillations in one brain region, then measure the effects on a connected region in vivo.
Optogenetic tools are also increasingly being used in behavioral studies (78-83), and it will be interesting to see how future studies use optogenetic tools to control gamma oscillations in order to reproduce or amerliorate behaviors related to symptoms of schizophrenia and other conditions. In particular, a recent study used a modified version of ChR2 to increase gamma power in the medial prefrontal cortex, and found disruptions in social behavior (84). Another study stimulated PV interneurons using ChR2, and found impaired gamma oscillations in mouse with deficient NMDA-R signaling on PV interneurons (66). Future studies could use similar approaches to study gamma oscillations in mouse models of schizophrenia and autism in order to understand how abnormal PV interneurons might generate increased or decreased gamma oscillations under various conditions.
ACKNOWLEDGEMENTS
VSS is supported by the Staglin family and International Mental Health Research Organization (IMHRO), R00 MH085946-02 from NIMH, a Pilot Award from the Simons Foundation for Autism Research, and a Steve and Connie Lieber Young Investigator Award from NARSAD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
FINANCIAL DISCLOSURES: The author reports no biomedical financial interests or potential conflicts of interest.
REFERENCES
- 1.Buzsaki G. Rhythms of the Brain. Oxford University Press; Oxford: 2005. [Google Scholar]
- 2.Jensen O, Tesche CD. Frontal theta activity in humans increases with memory load in a working memory task. Eur J Neurosci. 2002;15:1395–1399. doi: 10.1046/j.1460-9568.2002.01975.x. [DOI] [PubMed] [Google Scholar]
- 3.Howard MW, Rizzuto DS, Caplan JB, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, et al. Gamma oscillations correlate with working memory load in humans. Cereb Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- 4.Seidenbecher T, Laxmi TR, Stork O, Pape HC. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science. 2003;301:846–850. doi: 10.1126/science.1085818. [DOI] [PubMed] [Google Scholar]
- 5.Zhu XO, McNaughton N. The interaction of serotonin depletion with anxiolytics and antidepressants on reticular-elicited hippocampal RSA. Neuropharmacology. 1994;33:1597–1605. doi: 10.1016/0028-3908(94)90135-x. [DOI] [PubMed] [Google Scholar]
- 6.Gordon JA, Lacefield CO, Kentros CG, Hen R. State-dependent alterations in hippocampal oscillations in serotonin 1A receptor-deficient mice. J Neurosci. 2005;25:6509–6519. doi: 10.1523/JNEUROSCI.1211-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron. 2010;65:257–269. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colgin LL. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol. 2011;21:467–474. doi: 10.1016/j.conb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sigurdsson T, Stark KL, Karayiorgou M, Gogos JA, Gordon JA. Impaired hippocampal-prefrontal synchrony in a genetic mouse model of schizophrenia. Nature. 2010;464:763–767. doi: 10.1038/nature08855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fries P, Reynolds JH, Rorie AE, Desimone R. Modulation of oscillatory neuronal synchronization by selective visual attention. Science. 2001;291:1560–1563. doi: 10.1126/science.1055465. [DOI] [PubMed] [Google Scholar]
- 11.Fries P, Womelsdorf T, Oostenveld R, Desimone R. The effects of visual stimulation and selective visual attention on rhythmic neuronal synchronization in macaque area V4. J Neurosci. 2008;28:4823–4835. doi: 10.1523/JNEUROSCI.4499-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Siegel M, Donner TH, Oostenveld R, Fries P, Engel AK. Neuronal synchronization along the dorsal visual pathway reflects the focus of spatial attention. Neuron. 2008;60:709–719. doi: 10.1016/j.neuron.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez E, George N, Lachaux JP, Martinerie J, Renault B, Varela FJ. Perception’s shadow: long-distance synchronization of human brain activity. Nature. 1999;397:430–433. doi: 10.1038/17120. [DOI] [PubMed] [Google Scholar]
- 14.Tallon-Baudry C, Mandon S, Freiwald WA, Kreiter AK. Oscillatory synchrony in the monkey temporal lobe correlates with performance in a visual short-term memory task. Cereb Cortex. 2004;14:713–720. doi: 10.1093/cercor/bhh031. [DOI] [PubMed] [Google Scholar]
- 15.Singer W. Neuronal synchrony: a versatile code for the definition of relations? Neuron. 1999;24:49–65. 111–125. doi: 10.1016/s0896-6273(00)80821-1. [DOI] [PubMed] [Google Scholar]
- 16.Engel AK, Fries P, Singer W. Dynamic predictions: oscillations and synchrony in top-down processing. Nat Rev Neurosci. 2001;2:704–716. doi: 10.1038/35094565. [DOI] [PubMed] [Google Scholar]
- 17.Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RA. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005;309:948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- 18.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412:150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 19.Scheeringa R, Fries P, Petersson KM, Oostenveld R, Grothe I, Norris DG, et al. Neuronal dynamics underlying high- and low-frequency EEG oscillations contribute independently to the human BOLD signal. Neuron. 2011;69:572–583. doi: 10.1016/j.neuron.2010.11.044. [DOI] [PubMed] [Google Scholar]
- 20.Gregoriou GG, Gotts SJ, Zhou H, Desimone R. Long-range neural coupling through synchronization with attention. Prog Brain Res. 2009;176:35–45. doi: 10.1016/S0079-6123(09)17603-3. [DOI] [PubMed] [Google Scholar]
- 21.Buschman TJ, Miller EK. Top-down versus bottom-up control of attention in the prefrontal and posterior parietal cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 22.Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsaki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron. 2008;60:683–697. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6:312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 24.Spencer KM, Nestor PG, Perlmutter R, Niznikiewicz MA, Klump MC, Frumin M, et al. Neural synchrony indexes disordered perception and cognition in schizophrenia. Proc Natl Acad Sci U S A. 2004;101:17288–17293. doi: 10.1073/pnas.0406074101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gallinat J, Winterer G, Herrmann CS, Senkowski D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin Neurophysiol. 2004;115:1863–1874. doi: 10.1016/j.clinph.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 26.Symond MP, Harris AW, Gordon E, Williams LM. “Gamma synchrony” in first-episode schizophrenia: a disorder of temporal connectivity? Am J Psychiatry. 2005;162:459–465. doi: 10.1176/appi.ajp.162.3.459. [DOI] [PubMed] [Google Scholar]
- 27.Wynn JK, Light GA, Breitmeyer B, Nuechterlein KH, Green MF. Event-related gamma activity in schizophrenia patients during a visual backward-masking task. Am J Psychiatry. 2005;162:2330–2336. doi: 10.1176/appi.ajp.162.12.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford JM, Roach BJ, Faustman WO, Mathalon DH. Synch before you speak: auditory hallucinations in schizophrenia. Am J Psychiatry. 2007;164:458–466. doi: 10.1176/ajp.2007.164.3.458. [DOI] [PubMed] [Google Scholar]
- 29.Ford JM, Roach BJ, Faustman WO, Mathalon DH. Out-of-synch and out-of-sorts: dysfunction of motor-sensory communication in schizophrenia. Biol Psychiatry. 2008;63:736–743. doi: 10.1016/j.biopsych.2007.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho RY, Konecky RO, Carter CS. Impairments in frontal cortical gamma synchrony and cognitive control in schizophrenia. Proc Natl Acad Sci U S A. 2006;103:19878–19883. doi: 10.1073/pnas.0609440103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee S-H, Wynn JK, Green MF, Kim H, Lee K-J, Nam M, et al. Quantitative EEG and low resolution electromagnetic tomography (LORETA) imaging of patients with persistent auditory hallucinations. Schizophrenia research. 2006;83:111–119. doi: 10.1016/j.schres.2005.11.025. [DOI] [PubMed] [Google Scholar]
- 32.Spencer KM, Niznikiewicz MA, Nestor PG, Shenton ME, McCarley RW. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC neuroscience. 2009;10:85–85. doi: 10.1186/1471-2202-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mulert C, Kirsch V, Pascual-Marqui R, McCarley RW, Spencer KM. Long-range synchrony of gamma oscillations and auditory hallucination symptoms in schizophrenia. International journal of psychophysiology: official journal of the International Organization of Psychophysiology. 2010 doi: 10.1016/j.ijpsycho.2010.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hashimoto T, Volk DW, Eggan SM, Mirnics K, Pierri JN, Sun Z, et al. Gene expression deficits in a subclass of GABA neurons in the prefrontal cortex of subjects with schizophrenia. J Neurosci. 2003;23:6315–6326. doi: 10.1523/JNEUROSCI.23-15-06315.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Woo TU, Whitehead RE, Melchitzky DS, Lewis DA. A subclass of prefrontal gamma-aminobutyric acid axon terminals are selectively altered in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:5341–5346. doi: 10.1073/pnas.95.9.5341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pierri JN, Chaudry AS, Woo TU, Lewis DA. Alterations in chandelier neuron axon terminals in the prefrontal cortex of schizophrenic subjects. Am J Psychiatry. 1999;156:1709–1719. doi: 10.1176/ajp.156.11.1709. [DOI] [PubMed] [Google Scholar]
- 37.Volk D, Austin M, Pierri J, Sampson A, Lewis D. GABA transporter-1 mRNA in the prefrontal cortex in schizophrenia: decreased expression in a subset of neurons. Am J Psychiatry. 2001;158:256–265. doi: 10.1176/appi.ajp.158.2.256. [DOI] [PubMed] [Google Scholar]
- 38.Volk DW, Pierri JN, Fritschy JM, Auh S, Sampson AR, Lewis DA. Reciprocal alterations in pre- and postsynaptic inhibitory markers at chandelier cell inputs to pyramidal neurons in schizophrenia. Cereb Cortex. 2002;12:1063–1070. doi: 10.1093/cercor/12.10.1063. [DOI] [PubMed] [Google Scholar]
- 39.Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch Neurol. 2006;63:1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- 40.Behrens MM, Ali SS, Dao DN, Lucero J, Shekhtman G, Quick KL, et al. Ketamine-induced loss of phenotype of fast-spiking interneurons is mediated by NADPH-oxidase. Science. 2007;318:1645–1647. doi: 10.1126/science.1148045. [DOI] [PubMed] [Google Scholar]
- 41.Belforte JE, Zsiros V, Sklar ER, Jiang Z, Yu G, Li Y, et al. Postnatal NMDA receptor ablation in corticolimbic interneurons confers schizophrenia-like phenotypes. Nature neuroscience. 2010;13:76–83. doi: 10.1038/nn.2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Do KQ, Cabungcal JH, Frank A, Steullet P, Cuenod M. Redox dysregulation, neurodevelopment, and schizophrenia. Curr Opin Neurobiol. 2009;19:220–230. doi: 10.1016/j.conb.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 43.Ylinen A, Soltesz I, Bragin A, Penttonen M, Sik A, Buzsaki G. Intracellular correlates of hippocampal theta rhythm in identified pyramidal cells, granule cells, and basket cells. Hippocampus. 1995;5:78–90. doi: 10.1002/hipo.450050110. [DOI] [PubMed] [Google Scholar]
- 44.Csicsvari J, Jamieson B, Wise KD, Buzsaki G. Mechanisms of gamma oscillations in the hippocampus of the behaving rat. Neuron. 2003;37:311–322. doi: 10.1016/s0896-6273(02)01169-8. [DOI] [PubMed] [Google Scholar]
- 45.Hajos N, Palhalmi J, Mann EO, Nemeth B, Paulsen O, Freund TF. Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci. 2004;24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Whittington MA, Traub RD, Jefferys JG. Synchronized oscillations in interneuron networks driven by metabotropic glutamate receptor activation. Nature. 1995;373:612–615. doi: 10.1038/373612a0. [DOI] [PubMed] [Google Scholar]
- 47.Galarreta M, Hestrin S. A network of fast-spiking cells in the neocortex connected by electrical synapses. Nature. 1999;402:72–75. doi: 10.1038/47029. [DOI] [PubMed] [Google Scholar]
- 48.Gibson JR, Beierlein M, Connors BW. Two networks of electrically coupled inhibitory neurons in neocortex. Nature. 1999;402:75–79. doi: 10.1038/47035. [DOI] [PubMed] [Google Scholar]
- 49.Tamas G, Buhl EH, Lorincz A, Somogyi P. Proximally targeted GABAergic synapses and gap junctions synchronize cortical interneurons. Nat Neurosci. 2000;3:366–371. doi: 10.1038/73936. [DOI] [PubMed] [Google Scholar]
- 50.Mann EO, Suckling JM, Hajos N, Greenfield SA, Paulsen O. Perisomatic feedback inhibition underlies cholinergically induced fast network oscillations in the rat hippocampus in vitro. Neuron. 2005;45:105–117. doi: 10.1016/j.neuron.2004.12.016. [DOI] [PubMed] [Google Scholar]
- 51.Fuchs EC, Zivkovic AR, Cunningham MO, Middleton S, Lebeau FE, Bannerman DM, et al. Recruitment of parvalbumin-positive interneurons determines hippocampal function and associated behavior. Neuron. 2007;53:591–604. doi: 10.1016/j.neuron.2007.01.031. [DOI] [PubMed] [Google Scholar]
- 52.Traub RD, Bibbig A, LeBeau FE, Buhl EH, Whittington MA. Cellular mechanisms of neuronal population oscillations in the hippocampus in vitro. Annu Rev Neurosci. 2004;27:247–278. doi: 10.1146/annurev.neuro.27.070203.144303. [DOI] [PubMed] [Google Scholar]
- 53.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 54.Hajos N, Paulsen O. Network mechanisms of gamma oscillations in the CA3 region of the hippocampus. Neural Netw. 2009;22:1113–1119. doi: 10.1016/j.neunet.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 55.Tiesinga P, Sejnowski TJ. Cortical enlightenment: are attentional gamma oscillations driven by ING or PING? Neuron. 2009;63:727–732. doi: 10.1016/j.neuron.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fisahn A, Contractor A, Traub RD, Buhl EH, Heinemann SF, McBain CJ. Distinct roles for the kainate receptor subunits GluR5 and GluR6 in kainate-induced hippocampal gamma oscillations. J Neurosci. 2004;24:9658–9668. doi: 10.1523/JNEUROSCI.2973-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fisahn A, Pike FG, Buhl EH, Paulsen O. Cholinergic induction of network oscillations at 40 Hz in the hippocampus in vitro. Nature. 1998;394:186–189. doi: 10.1038/28179. [DOI] [PubMed] [Google Scholar]
- 58.Schmitz D, Schuchmann S, Fisahn A, Draguhn A, Buhl EH, Petrasch-Parwez E, et al. Axo-axonal coupling. a novel mechanism for ultrafast neuronal communication. Neuron. 2001;31:831–840. doi: 10.1016/s0896-6273(01)00410-x. [DOI] [PubMed] [Google Scholar]
- 59.Traub RD, Buhl EH, Gloveli T, Whittington MA. Fast rhythmic bursting can be induced in layer 2/3 cortical neurons by enhancing persistent Na+ conductance or by blocking BK channels. J Neurophysiol. 2003;89:909–921. doi: 10.1152/jn.00573.2002. [DOI] [PubMed] [Google Scholar]
- 60.Hormuzdi SG, Pais I, LeBeau FE, Towers SK, Rozov A, Buhl EH, et al. Impaired electrical signaling disrupts gamma frequency oscillations in connexin 36-deficient mice. Neuron. 2001;31:487–495. doi: 10.1016/s0896-6273(01)00387-7. [DOI] [PubMed] [Google Scholar]
- 61.Traub RD, Pais I, Bibbig A, LeBeau FE, Buhl EH, Hormuzdi SG, et al. Contrasting roles of axonal (pyramidal cell) and dendritic (interneuron) electrical coupling in the generation of neuronal network oscillations. Proc Natl Acad Sci U S A. 2003;100:1370–1374. doi: 10.1073/pnas.0337529100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gogolla N, Leblanc JJ, Quast KB, Sudhof T, Fagiolini M, Hensch TK. Common circuit defect of excitatory-inhibitory balance in mouse models of autism. J Neurodev Disord. 2009;1:172–181. doi: 10.1007/s11689-009-9023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wilson TW, Rojas DC, Reite ML, Teale PD, Rogers SJ. Children and adolescents with autism exhibit reduced MEG steady-state gamma responses. Biol Psychiatry. 2007;62:192–197. doi: 10.1016/j.biopsych.2006.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gandal MJ, Edgar JC, Ehrlichman RS, Mehta M, Roberts TP, Siegel SJ. Validating gamma oscillations and delayed auditory responses as translational biomarkers of autism. Biol Psychiatry. 2010;68:1100–1106. doi: 10.1016/j.biopsych.2010.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orekhova EV, Stroganova TA, Nygren G, Tsetlin MM, Posikera IN, Gillberg C, et al. Excess of high frequency electroencephalogram oscillations in boys with autism. Biol Psychiatry. 2007;62:1022–1029. doi: 10.1016/j.biopsych.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 66.Cardin JA, Carlen M, Meletis K, Knoblich U, Zhang F, Deisseroth K, et al. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature. 2009;459:663–667. doi: 10.1038/nature08002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sohal VS, Zhang F, Yizhar O, Deisseroth K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2008;36:129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adesnik H, Scanziani M. Lateral competition for cortical space by layer-specific horizontal circuits. Nature. 2010;464:1155–1160. doi: 10.1038/nature08935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fries P, Nikolic D, Singer W. The gamma cycle. Trends Neurosci. 2007;30:309–316. doi: 10.1016/j.tins.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 71.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 72.Womelsdorf T, Schoffelen JM, Oostenveld R, Singer W, Desimone R, Engel AK, et al. Modulation of neuronal interactions through neuronal synchronization. Science. 2007;316:1609–1612. doi: 10.1126/science.1139597. [DOI] [PubMed] [Google Scholar]
- 73.Konig P, Engel AK, Singer W. Integrator or coincidence detector? The role of the cortical neuron revisited. Trends Neurosci. 1996;19:130–137. doi: 10.1016/s0166-2236(96)80019-1. [DOI] [PubMed] [Google Scholar]
- 74.Salinas E, Sejnowski TJ. Correlated neuronal activity and the flow of neural information. Nat Rev Neurosci. 2001;2:539–550. doi: 10.1038/35086012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chalk M, Herrero JL, Gieselmann MA, Delicato LS, Gotthardt S, Thiele A. Attention reduces stimulus-driven gamma frequency oscillations and spike field coherence in V1. Neuron. 2010;66:114–125. doi: 10.1016/j.neuron.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Frith C. Neuropsychology of schizophrenia, what are the implications of intellectual and experiential abnormalities for the neurobiology of schizophrenia? British medical bulletin. 1996;52:618–626. doi: 10.1093/oxfordjournals.bmb.a011571. [DOI] [PubMed] [Google Scholar]
- 77.Chen CM, Mathalon DH, Roach BJ, Cavus I, Spencer DD, Ford JM. The Corollary Discharge in Humans Is Related to Synchronous Neural Oscillations. J Cogn Neurosci. 2010 doi: 10.1162/jocn.2010.21589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Airan RD, Thompson KR, Fenno LE, Bernstein H, Deisseroth K. Temporally precise in vivo control of intracellular signalling. Nature. 2009;458:1025–1029. doi: 10.1038/nature07926. [DOI] [PubMed] [Google Scholar]
- 79.Tsai H-C, Zhang F, Adamantidis A, Stuber GD, Bonci A, de Lecea L, et al. Phasic firing in dopaminergic neurons is sufficient for behavioral conditioning. Science (New York, NY) 2009;324:1080–1084. doi: 10.1126/science.1168878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tye KM, Prakash R, Kim SY, Fenno LE, Grosenick L, Zarabi H, et al. Amygdala circuitry mediating reversible and bidirectional control of anxiety. Nature. 2011;471:358–362. doi: 10.1038/nature09820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Covington HE, 3rd, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, et al. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Witten IB, Lin SC, Brodsky M, Prakash R, Diester I, Anikeeva P, et al. Cholinergic interneurons control local circuit activity and cocaine conditioning. Science. 2010;330:1677–1681. doi: 10.1126/science.1193771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lobo MK, Covington HE, 3rd, Chaudhury D, Friedman AK, Sun H, Damez-Werno D, et al. Cell type-specific loss of BDNF signaling mimics optogenetic control of cocaine reward. Science. 2010;330:385–390. doi: 10.1126/science.1188472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477:171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]