Cell death is an event important for the survival of multicellular organisms. Cell death can occur with the active cooperation of the dying cell, a process known variously as programmed cell death, target cell apoptosis, or cell suicide. Cell death, however, also occurs without cooperation of the dying cells by cell-mediated cytotoxicity or target cell lysis. Target cell lysis is mediated by dedicated, professional killer cells, primarily cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells, which constitute immune defense against intracellular parasites, such as viruses, and against tumor cells. CTLs and NK cells mediate both membrane damage and apoptotic events in target cells. Are the apoptotic events mediated by killer cells classifiable as programmed cell death or programmed cell suicide of the target cell? The answer is—not always.
There are principally two ways by which killer cells induce apoptosis in target cells. The pathways of bona fide apoptosis are triggered by death ligands on the killer cell and death receptors on the target cell, the best known of which are Fas-ligand and Fas-receptor, respectively (1). The other pathway used by killer cells employs perforin (2) and granzymes (3, 4) and causes “involuntary suicide” or “involuntary apoptosis” in target cells. Perforin and granzymes do not require the presence of death receptors and their signaling machinery in the target cell. DNA degradation (apoptosis; ref. 5) is the end result of both types of cell death. What, then, is meant by involuntary apoptosis, an expression that sounds like an oxymoron but conveys the correct sense? Is there such a thing as involuntary suicide?
An interesting piece of information in this drama is provided by Pham and Ley (6), who show that an important step in generating death signals requires dipeptidyl peptidase I (DPPI) in the killer cell. Ablation of the DPPI gene in mice and the generation of DPPI-deficient killer cells results in CTLs that are unable to induce DNA degradation, i.e., involuntary apoptosis in target cells. In other words, DPPI-deficient CTLs or lymphokine-activated killer cells cannot induce death receptor (e.g., Fas) independent apoptosis. DPPI is required in the killer cell for the intracellular processing of granzyme A and granzyme B and for processing of other lysosomal proteases (7, 8). Granzymes A and B assume an enzymatically active state only after cleavage of the N-terminal dipeptide by DPPI. They are stored in the enzymatically active form in the lysosomal granules (granulosomes; ref. 9) of killer cells along with perforin. There is evidence (10) that perforin also may need to be processed by an as yet unidentified protease to expose its Ca-dependent phospholipid-binding site, the C2 domain homologous to other phospholipid-binding proteins.
Active granzyme A and granzyme B can trigger apoptosis on internalization into target cells (Fig. 1). Granzyme B has aspase activity similar to that of caspases and can directly activate several upstream and downstream caspases (11). Granzyme B, in triggering involuntary apoptosis, skips death receptors and the signaling cascades and does not require cellular cooperation. The mechanism of granzyme A action is less well understood even though it was the first granzyme to be identified (as benzyloxyl carbonyl-l-lysine thiobenzylester esterase; ref. 3) and subsequently shown to reside in the same granules as perforin (12). Granzyme A is a tryptase cleaving next to basic amino acid residues. Granzyme A has been shown to form complexes with several intracellular proteins (13, 14), some of which are cleaved, and may cause apoptosis in a caspase-independent pathway. Interestingly, granzyme A knockout mice are especially susceptible to ectromelia virus, a pox virus able to block caspases via crmA (15). The array of granzymes found in granules may have evolved to counter viral strategies to block apoptosis of infected cells to facilitate their own replication.
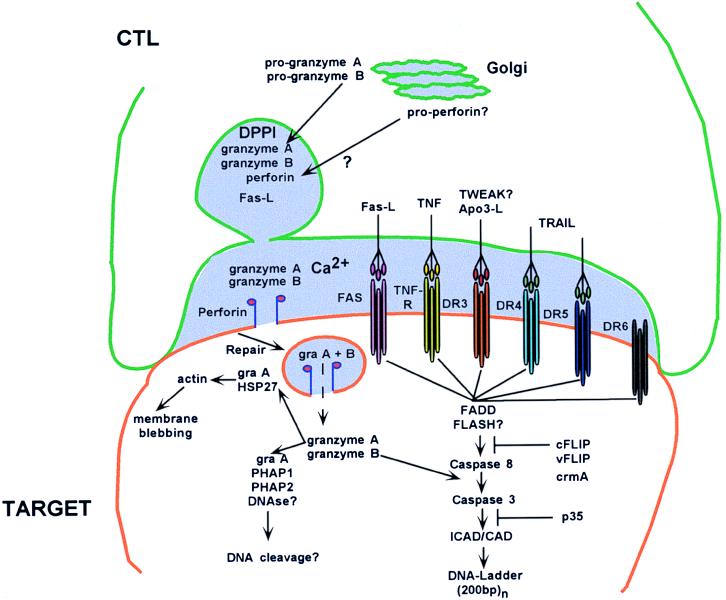
Figure 1.
CTL cytotoxic mechanisms. The killer cell has formed a conjugate “synapse” with the target cell and is in the process of secreting granules (light purple) in the synaptic space. Granule material including perforin is derived from the Golgi complex; granzymes are processed by DPPI and stored in the granule. After secretion into the synaptic space and pore formation, granzymes enter into the target cell through repair endocytosis. Granzyme B activates downstream apoptotic pathways, whereas granzyme A may activate caspase-independent apoptosis. TNF, tumor necrosis factor; TWEAK?, ligand in the TNF family that weakly induces apoptosis; TRAIL, TNF-related apoptosis-inducing ligand; DRn, death receptor n; gra, granzyme; PHAPn, putative HLA-associated protein n; FADD, FAS-associated death domain factor; FLASH?, FLICE-associated huge protein; ICAD/CAD, inhibitor caspase-activated DNase/caspase-activated DNase; cFLIP, cellular FLICE inhibitory protein; vFLIP, viral FLICE inhibitory protein; crmA, cytokine response modifier A.
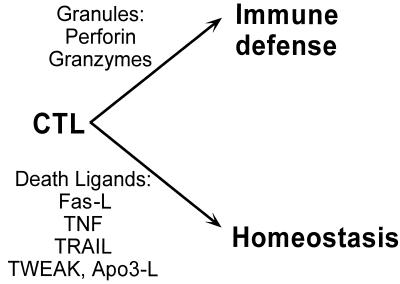
What are the biological functions of voluntary versus involuntary apoptosis? Or to put the question in molecular terms—what are the biological functions of Fas-ligand (death-ligand) versus granzyme-induced apoptosis? Both functions are mediated by CTLs and NK cells, a fact which brings us to their biological function of destroying parasitized cells (Fig. 2). Virally or oncogenically transformed cells usually are not compliant in mediating apoptosis, because the transformation down-regulates death receptors, blocks apoptosis-signaling pathways, and up-regulates antiapoptotic mechanisms. Therefore, transformed cells must be killed by mechanisms that do not depend on compliance by the target.
Figure 2.
Biological functions of CTLs and effector molecules.
Granzymes are delivered into target cells by perforin after synapse formation of the killer with the target cell and secretion of granules into the synaptic space (Fig. 1). Perforin, by Ca-dependent binding to phospholipid head groups and polymerization, forms transmembrane channels of variable size up to 16 nm. The cell is forced to repair these lesions, or it would die from loss of membrane potential and ionic equilibration across the membrane. Repair endocytosis removes the lesion from the membrane but inevitably causes pinocytosis of extracellular fluids containing granzymes (Fig. 1; ref. 16). Granzymes released into the cytoplasm of target cells activate downstream apoptotic pathways, even in cells with down-regulated death receptors. Thus, granzymes trigger involuntary apoptosis in cells that are attempting only to repair membrane damage. However, if cells did not repair the damage, they would die of perforin membrane lesions; however, if they do, they die of involuntary apoptosis.
How granzymes escape from endosomes in the target cell and trigger apoptosis is still unclear (17). However, it is known that, instead of perforin, other endosomolytic agents can deliver granzymes. It is likely that the extreme proteolytic resistance of perforin is critical in the delivery of granzymes from the endosome to the cytoplasm and nucleus of the target cell (18).
The DPPI knockout in some measure resembles perforin knockout in CTLs: both types of deficiency fail to cause target cell apoptosis. DPPI knockout CTLs are deficient in generating granzyme activity even though granzymes are present in granules and presumably are delivered to target cells via perforin. However, without proteolytic activity, granzymes are unable to trigger DNA degradation. Perforin-deficient CTLs are unable to deliver granzymes into target cells, because transmembrane pores cannot be formed. Perforin deficiency therefore also has the phenotype functional granzyme deficiency. Perforin-deficient mice are unable to clear lymphocytic choriomeningitis virus (19), and they have a reduced capacity to reject tumors and diminished immunosurveillance (20). DPPI-deficient CTLs produce inactive granzymes. In regard to diminished DNA degradation, DPPI- and perforin-deficient CTLs are similar. However, DPPI-deficient mice are expected to express functional perforin, may be able to mediate membrane damage similar to granzyme A × B knockout CTLs (21), and may express some antiviral activity. An in vivo comparison of DPPI- and perforin-deficient mice with regard to virus and oncogene control could allow the dissection of the contribution of perforin to the immune defense with and without the help of granzymes.
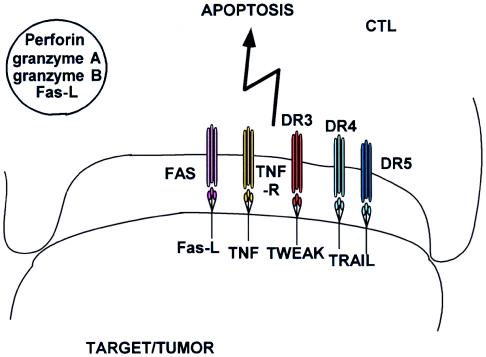
The function of apoptosis by death ligands and death receptors is largely confined to homeostatic regulation of lymphocytes and other hemopoietic elements. Fas-ligand has been reported to be present in perforin- and granzyme-containing granules in CTLs (Fig. 1) and therefore may function in immune defense as well (22). Deficiency of either Fas or Fas-L leads to lymphoproliferation and autoimmunity (23). CTLs are able (E.R.P., unpublished observation) to express TNF, Fas-L, TRAIL (24, 25), and TWEAK (26), but they also can express the corresponding death receptors (27). CTLs, therefore, are able to commit fratricide, potentially killing each other after an immune response. However, death-ligand expression is not restricted to CTLs; it is found in many tissues including tumors (28) and may be used to provide immune privilege by restricting lymphocyte activation (Fig. 3). Apoptosis mediated by death ligands and receptors seems to proceed bidirectionally: CTLs can induce apoptosis of targets, but organ tissues and tumors, too, can induce T cell down-regulation including apoptosis, a phenomenon known as immune privilege.
Figure 3.
Reverse apoptosis of CTL. Death ligands expressed by tissues and tumors may cause apoptosis or down-regulation of death receptor-expressing killer cells.
In contrast, immune defense by perforin and granzymes seems to be unidirectional: there is no equivocation and talking back by the target cell, thanks to the exclusive expression of granzymes and perforin in activated T cells and NK cells. Are there exceptions to this rule? Possibly. Keratinocytes have been reported to express both perforin and granzymes (in addition to Fas and Fas-ligand; ref. 29). Thus, maybe there is talking back to perforin and granzymes after all.
Acknowledgments
Work by the author is supported by National Cancer Institute Grants CA39201, CA57904, and CA80228.
Footnotes
A commentary on this article begins on page 8627.
References
- 1.Rouvier E, Luciani M F, Golstein P. J Exp Med. 1993;177:195–200. doi: 10.1084/jem.177.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dennert G, Podack E R. J Exp Med. 1983;157:1483–1495. doi: 10.1084/jem.157.5.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pasternack M S, Verret C R, Liu M A, Eisen H N. Nature (London) 1986;322:740–743. doi: 10.1038/322740a0. [DOI] [PubMed] [Google Scholar]
- 4.Nakajima H, Henkart P A. J Immunol. 1994;152:1057–1063. [PubMed] [Google Scholar]
- 5.Russell J H, Dobos C B. J Immunol. 1980;125:1256–1261. [PubMed] [Google Scholar]
- 6.Pham C T N, Ley T J. Proc Natl Acad Sci USA. 1999;96:8627–8632. doi: 10.1073/pnas.96.15.8627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brown G R, McGuire M J, Thiele D L. J Immunol. 1993;150:4733–4742. [PubMed] [Google Scholar]
- 8.Pham C T, Thomas D A, Mercer J D, Ley T J. J Biol Chem. 1998;273:1629–1633. doi: 10.1074/jbc.273.3.1629. [DOI] [PubMed] [Google Scholar]
- 9.Podack E R, Hengartner H, Lichtenheld M G. Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- 10.Uellner R, Zvelebil M J, Hopkins J, Jones J, MacDougall L K, Morgan B P, Podack E, Waterfield M D, Griffiths G M. EMBO J. 1997;16:7287–7296. doi: 10.1093/emboj/16.24.7287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darmon A J, Nicholson D W, Bleackley R C. Nature (London) 1995;377:446–448. doi: 10.1038/377446a0. [DOI] [PubMed] [Google Scholar]
- 12.Masson D, Tschopp J. Cell. 1987;49:679–685. doi: 10.1016/0092-8674(87)90544-7. [DOI] [PubMed] [Google Scholar]
- 13.Beresford P J, Kam C M, Powers J C, Lieberman J. Proc Natl Acad Sci USA. 1997;94:9285–9290. doi: 10.1073/pnas.94.17.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beresford P J, Jaju M, Friedman R S, Yoon M J, Lieberman J. J Immunol. 1998;161:161–167. [PubMed] [Google Scholar]
- 15.Mullbacher A, Ebnet K, Blanden R V, Hla R T, Stehle T, Museteanu C, Simon M M. Proc Natl Acad Sci USA. 1996;93:5783–5787. doi: 10.1073/pnas.93.12.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Podack E R, Lowrey D M, Lichtenheld M, Hameed A. Ann NY Acad Sci. 1988;532:292–302. doi: 10.1111/j.1749-6632.1988.tb36347.x. [DOI] [PubMed] [Google Scholar]
- 17.Shi L, Mai S, Israels S, Browne K, Trapani J A, Greenberg A H. J Exp Med. 1997;185:855–866. doi: 10.1084/jem.185.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Trapani J A, Jans P, Smyth M J, Froelich C J, Williams E A, Sutton V R, Jans D A. Cell Death Differ. 1998;5:488–496. doi: 10.1038/sj.cdd.4400373. [DOI] [PubMed] [Google Scholar]
- 19.Kagi D, Ledermann B, Burki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Nature (London) 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 20.van den Broek M E, Kagi D, Ossendorp F, Toes R, Vamvakas S, Lutz W K, Melief C J, Zinkernagel R M, Hengartner H. J Exp Med. 1996;184:1781–1790. doi: 10.1084/jem.184.5.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon M M, Hausmann M, Tran T, Ebnet K, Tschopp J, ThaHla R, Mullbacher A. J Exp Med. 1997;186:1781–1786. doi: 10.1084/jem.186.10.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bossi G, Griffiths G M. Nat Med. 1999;5:90–96. doi: 10.1038/4779. [DOI] [PubMed] [Google Scholar]
- 23.Nagata S. J Hum Genet. 1998;43:2–8. doi: 10.1007/s100380050029. [DOI] [PubMed] [Google Scholar]
- 24.Wiley S R, Schooley K, Smolak P J, Din W S, Huang C P, Nicholl J K, Sutherland G R, Smith T D, Rauch C, Smith C A, et al. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 25.Walczak H, Degli-Esposti M A, Johnson R S, Smolak P J, Waugh J Y, Boiani N, Timour M S, Gerhart M J, Schooley K A, Smith C A, et al. EMBO J. 1997;16:5386–5397. doi: 10.1093/emboj/16.17.5386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chicheportiche Y, Bourdon P R, Xu H, Hsu Y M, Scott H, Hession C, Garcia I, Browning J L. J Biol Chem. 1997;272:32401–32410. doi: 10.1074/jbc.272.51.32401. [DOI] [PubMed] [Google Scholar]
- 27.Ashkenazi A, Dixit V M. Curr Opin Cell Biol. 1999;11:255–260. doi: 10.1016/s0955-0674(99)80034-9. [DOI] [PubMed] [Google Scholar]
- 28.Hahne M, Rimoldi D, Schroter M, Romero P, Schreier M, French L E, Schneider P, Bornand T, Fontana A, Lienard D, et al. Science. 1996;274:1363–1366. doi: 10.1126/science.274.5291.1363. [DOI] [PubMed] [Google Scholar]
- 29.Berthou C, Michel L, Soulie A, Jean-Louis F, Flageul B, Dubertret L, Sigaux F, Zhang Y, Sasportes M. J Immunol. 1997;159:5293–5300. [PubMed] [Google Scholar]