Abstract
Background and Purpose
The Framingham Heart Study group cardiovascular disease risk profile (FCRP) score was used to assess the relationship between baseline cardiovascular risk and subsequent changes in resting state cerebral blood flow (CBF) in cognitively normal older participants from the Baltimore Longitudinal Study of Aging (BLSA).
Methods
97 cognitively normal participants underwent annual resting-state PET scans at baseline and over a period of up to 8 years (mean interval 7.4 years). Images quantifying voxel-wise longitudinal rates of CBF change were calculated and used to examine the relationship between baseline FCRP score and changes over time in regional CBF. Individual components of the FCRP score (age, cholesterol, blood pressure, smoking status and type-II diabetes) were also correlated with changes in regional CBF to examine the independent contributions of each component to the overall pattern of change.
Results
Higher baseline FCRP scores were associated with accelerated longitudinal decline in CBF in orbitofrontal, medial frontal/anterior cingulate, insular, precuneus and brainstem regions. Of the components that make up the FCRP score, higher diastolic blood pressure and diabetes were associated independently with greater decline in the medial frontal/anterior cingulate and insular regions, respectively.
Conclusions
Baseline cardiovascular risk factors are associated with greater rates of decline in resting state regional brain function. The regions showing accelerated decline participate in higher order cognitive processes and are also vulnerable to age-related neuropathology. These results, in conjunction with other studies, encourage early treatment of cardiovascular risk factors in older individuals.
Keywords: hypertension, diabetes, cholesterol, aging, PET, fMRI
INTRODUCTION
A large body of evidence supports a relationship between cardiovascular risk factors and cognitive impairment in the elderly1. These factors include atherosclerosis, hypertension, coronary artery disease, dyslipidemia, and diabetes mellitus2, 3. Several studies also suggest that vascular factors associated with increased cardiovascular risk lead to earlier onset and faster progression of late-onset dementias4, 5.
While the precise temporal relationship between the development of cardiovascular risk factors and subsequent dementia remains unclear, previous reports indicate that elevated serum cholesterol levels and hypertension in midlife are associated with increased risk of Alzheimer's disease (AD)6, 7. These findings suggest that vascular risk factors modulate early events in AD pathogenesis that precede the onset of overt cognitive impairment by many years8.
Associations between mid-life vascular risk and later cognitive impairment are of considerable public health importance as they indicate that primary preventive strategies targeting reduction in cardiovascular risk may, in turn, also delay the onset or decrease the burden of late-onset dementia9–11. Further emphasizing the importance of vascular risk factors in aging, the American Heart Association/American Stroke Association (AHA/ASA) recently issued a statement to health care professionals suggesting that long-term vascular risk marker interventional studies may be required to prevent or postpone the onset of vascular cognitive impairment and Alzheimer's disease12. Thus, as the field of age-related neuroimaging has begun to focus on early predictors of future change, there is considerable interest in identifying early changes in brain structure and function associated with future cognitive outcome in older individuals13.
Using serial 15O-water positron emission tomography (PET) data from the neuroimaging substudy of the Baltimore Longitudinal Study of Aging (BLSA), we previously reported that hypertension was associated with significant longitudinal decreases in regional cerebral blood flow (rCBF) in a select group of older individuals with no other comorbidities14. The aim of the present study was to further investigate the relationship between risk for cardiovascular disease and longitudinal changes in rCBF, an indirect marker of neuronal activity in the human brain15.
In this study, we assess the relationship between a composite measure of cardiovascular risk (the Framingham cardiovascular disease risk profile (FCRP) score) calculated at baseline and subsequent longitudinal changes in rCBF over a 7 year period in a sample of 97 cognitively normal older adults. We also investigate the contributions of individual risk factors to these longitudinal patterns of rCBF change. Based on previously observed associations between vascular risk factors and dementia, we hypothesized that baseline cardiovascular risk would be associated with changes in rCBF over time in brain regions vulnerable to AD pathology, even in normal older individuals.
METHODS
Subjects
We used PET data from 97 cognitively normal older participants (58 males; mean age at baseline 69.5 (7.2 SD)) in the neuroimaging substudy16 of the BLSA17 (Table 1). These individuals are considered typical agers, with no history of central nervous system disease (epilepsy, stroke), psychiatric disorders, severe cardiac disease (myocardial infarction, coronary artery disease requiring angioplasty or bypass surgery), or metastatic cancer. Individuals with clinical diagnoses of mild cognitive impairment or dementia either at the beginning of the study or who developed either MCI or dementia during the study were also excluded from the analyses.
Table 1.
Participant demographics
| n | 97 |
| Baseline Age | 69.5 ± 7.2 (57–86) |
| Sex | 58M |
| Annual Scans | 8.2 ± 1.0 (5–9) |
| Scan Interval (yrs) | 7.4 ± 1.0 (4–8) |
| Systolic BP | 138.8 ± 20.8 (100–192) |
| Diastolic BP | 81.7 ± 10.4 (55–110) |
| Total Cholesterol | 213.6 ± 33.3 (149–343) |
| LDL | 115.2 ± 32.2 (46–249) |
| HDL | 50.4 ± 15.2 (22–95) |
| Smokers | 15 |
| Diabetes | 19 |
| Education (yrs) | 16.2 ± 2.8 (8–20) |
| MMSE baseline | 28.8 ± 1.3 (23–30) |
| MMSE last visit | 28.9 ± 1.2 (24–30) |
Legend: Mean values are listed for the all participants. The range of scores is shown in parentheses. Mini Mental State Examination (MMSE) scores are also shown.
This study was approved by the local Institutional Review Board. All subjects provided written informed consent prior to each assessment.
Measures of Cardiovascular Risk
At regular BLSA visits, medical examinations were performed to determine health status. Medical histories were recorded which included assessment of age, presence of Type II diabetes and smoking status. Measures of cholesterol (total and HDL), and systolic and diastolic blood pressure were also collected. These measures were used to calculate the FCRP score at the baseline imaging visit.
Neuropsychological Testing
During each neuroimaging visit, participants completed the Mini-Mental State Examination (MMSE) and a battery of 12 neuropsychological tests evaluating six cognitive domains. Memory was assessed using the California Verbal Learning Test (CVLT) and Benton Visual Retention Test (BVRT). Word knowledge and verbal ability were measured using Primary Mental Abilities Vocabulary (PMA). Verbal fluency was assessed by Letter (i.e. FAS) and Category fluency tests. Attention and working memory were measured by the Digit Span Test of the Wechsler Adult Intelligence Scale-Revised, and the Trail Making Test. Digits Backward, Trails B, and Verbal Fluency (categories and letters) assessed executive function. The Card Rotations Test assessed visuospatial function.
Data from evaluations at baseline through the last annual PET visit were used to examine the relationship between baseline FCRP score and cognitive performance. Linear mixed effects regression models were used to investigate the effects of baseline FCRP scores on baseline cognitive performance and on longitudinal rates of change in cognitive performance. We used cognitive performance measures as dependent variables and FCRP risk score, baseline age, sex, time interval (follow up time from baseline), and the interactions between these variables as predictors.
PET Scanning
Participants underwent PET scans at baseline (Year 1) and up to eight annual follow-ups. Each session included a resting PET scan in which participants were instructed to keep their eyes open and focused on a computer screen covered by a black cloth.
PET measures of regional cerebral blood flow (rCBF) were obtained using [15O]water. For each scan, 75 mCi of [15O] water were injected as a bolus. Scans were performed on a GE 4096+ scanner, which provides 15 slices of 6.5 mm thickness. Images were acquired for 60 seconds from the time the total radioactivity counts in the brain reached threshold level. Attenuation correction was performed using a transmission scan acquired prior to the emission scans.
PET Data Analysis
Data from PET scans obtained annually from baseline to the last available follow-up time points were used in the analyses. The mean interval between baseline and last follow-up PET scan was 7.4 (±1.0 SD) years. The PET scans were realigned and spatially normalized into standard stereotactic space and smoothed to full width at half maximum of 12×12×12 mm in the x, y, and z planes. Next, to control for variability in global flow, rCBF values at each voxel were ratio adjusted to the mean global flow and scaled to 50 ml/100g/min for each scan, then thresholded to exclude peripheral signal scatter in the images. For each participant, rates of change in CBF were calculated across all preprocessed scans using voxelwise linear modeling and extraction of the estimated fit parameter. An image of the voxelwise longitudinal rates of change (i.e. slope or linear temporal trends image) was then created for each subject (Statistical Parametric Mapping software, SPM2, Wellcome Trust Centre for Neuroimaging, UCL, London).
The slope images from all subjects were used in a voxel-based multiple regression analysis (SPM5) with baseline FCRP score as an independent predictor of longitudinal change in rCBF. The associations were adjusted for baseline age, sex and the interval between baseline and last scan. In secondary analyses, we examined the contributions of individual components of the FCRP score to the predicted pattern of change from the overall analysis. In a restricted search of regions from the first analysis, age, cholesterol, systolic and diastolic blood pressure, diabetes and smoking status were regressed with CBF changes individually while controlling for the other factors to examine the independent contributions of each component to the overall pattern of change. Significant associations were based on a statistical threshold of p < 0.005 and restricted to regions with a spatial extent of at least 50 voxels.
To determine the annual rates of change in CBF for each region, rCBF values were extracted from a 4mm spherical region centered on the local maxima of significant areas using the Marsbar SPM toolbox18. To determine if change in tissue volume in each specific region had an effect on the decline in CBF of that region, we repeated all analyses while controlling (covarying) for the annual rate of change in MRI volume of each cluster observed in the PET analysis.
MRI Scanning
Scanning was performed on a GE Signa 1.5 Tesla scanner (Milwaukee, WI). A 3-D T1-weighted spoiled gradient refocused (SPGR) MRI scan (35ms TR, 5ms TE, 24cm FOV, 45° flip angle, 256×256 matrix, 0.94×0.94mm voxel size, 1.5 mm slice thickness, 124 slices) was obtained annually at each imaging visit.
MRI Volume Calculation
The MRI scans were segmented into gray matter, white matter and cerebrospinal fluid and spatially normalized into stereotactic space using a high-dimensional elastic warping method19 and a volume-preserving transformation (Shen and Davatzikos, 2002). Binary maps of the clusters showing an association between cardiovascular risk and declines in CBF were generated from the PET analysis, and total volumes of gray + white matter were calculated within each cluster for each participant. The annual rate of volume change within each region was estimated using linear mixed models. The rate of change for each cluster was then included as a covariate in the follow up PET analyses.
RESULTS
Cardiovascular Risk Scores
FCRP scores were calculated at year 1 baseline yielding a mean FCRP baseline score of 13.2 (7.5 SD) with a range of 3–27 using the formula described in Wilson, et. al.20.
FCRP and Neuropsychological Performance
Analysis of the relationship between FCRP score and cognition showed that those with higher risk scores performed at lower levels on the California Verbal Learning Test at baseline (sum of 5 CVLT List A trials; estimated effect = −0.27 (0.13 SEM), p<0.039). No other significant associations were observed between FCRP score and cognition at baseline or in rates of change in cognitive performance over time.
FCRP and longitudinal change in rCBF
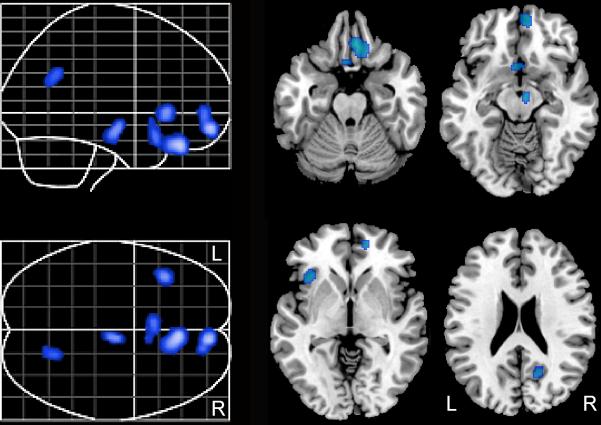
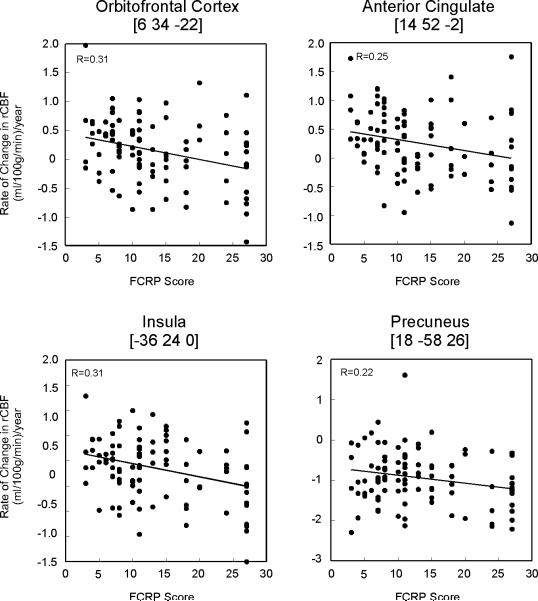
Higher baseline FCRP was associated with greater longitudinal decline in rCBF, suggesting that longitudinal rCBF declines are associated with higher cardiovascular risk. Regions that exhibit this relationship include bilateral orbitofrontal cortex (Brodmann Area (BA) 11/25) and right medial frontal/anterior cingulate cortex (BA 32), left insular cortex, right precuneus (BA 31) and right brainstem (Table 2). These results did not change when controlling for longitudinal tissue volume decline of these regions. The regional distribution of these areas is shown in Figure 1. The annual rates of rCBF change are illustrated in Figure 2. No significant associations were seen between higher FCRP scores and longitudinal increases in rCBF.
Table 2.
Regions of longitudinal change in rCBF associated with baseline FCRP score
| Coordinate | |||||||
|---|---|---|---|---|---|---|---|
| Region | Side | x | y | z | t value | p value | Voxels |
| Orbitofrontal Cortex (11) | R | 6 | 34 | −22 | 3.59 | <0.001 | 248 |
| Orbitofrontal Cortex (25) | L | −4 | 16 | −18 | 3.12 | 0.001 | 109* |
| Med Frontal/Ant Cingulate (32) | R | 14 | 52 | −2 | 3.13 | 0.001 | 157 |
| Insula | L | −36 | 24 | 0 | 3.32 | 0.001 | 84 |
| Precuneus (31) | R | 18 | −58 | 26 | 3.03 | 0.002 | 70 |
| Thalamus | R | 6 | 12 | −6 | 2.79 | 0.003 | 109* |
| Brainstem | R | 6 | −12 | −12 | 3.30 | 0.001 | 76 |
Legend: Local maxima within regions where declining rCBF over time was associated with higher baseline FCRP scores. Stereotaxic coordinates are listed, Brodmann areas are indicated in parentheses.
indicates regions contained within the same cluster.
Figure 1. Baseline cardiovascular risk and subsequent changes in CBF.
Higher baseline FCRP scores were associated with significant longitudinal CBF declines in the regions shown in blue. These declines in rCBF occurred over a 7.4 year period.
Figure 2. Baseline FCRP Score and Regional CBF change.
The relationship between baseline cardiovascular risk score and annual rates of CBF change are shown.
FCRP variables and rCBF change
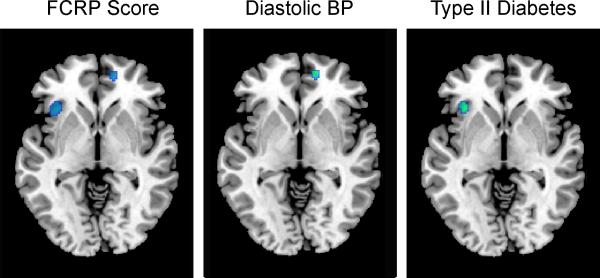
We performed secondary analysis of the individual components of the FCRP score (age, smoking status, cholesterol, blood pressure and type-II diabetes) to identify the specific factors accounting for the regional associations between FCRP and change in CBF. Within the regions showing significant changes in the primary analysis, several components of the FCRP showed significant independent associations with rCBF change. Higher baseline diastolic blood pressure was associated with greater decline in rCBF within the medial frontal/anterior cingulate region, and diabetes was associated with greater decrease in insular CBF (Figure 3). These results did not change when controlling for longitudinal tissue volume decline within these regions.
Figure 3. Contributions of risk score components to rCBF change.
Greater rCBF decline was associated with higher baseline diastolic blood pressure and presence of diabetes in anterior cingulate/medial frontal and insular regions, respectively (green). Regions exhibiting declining rCBF in relation to the total FCRP score from the primary analysis are also shown (blue).
DISCUSSION
Using the Framingham cardiovascular disease risk profile, which includes measures of age, cholesterol levels, blood pressure, diabetes, and smoking status, we found that higher baseline FCRP scores were associated with greater longitudinal CBF decline in orbitofrontal, medial frontal/anterior cingulate, insular, precuneus and brainstem regions. Although we see little effect of FPRC score on cognition in this sample of cognitively normal individuals, these regions are known to participate in higher order cognitive processes and are also regions of pathologic change in Alzheimer's disease. Whereas CBF decline in these regions was associated with the overall cardiovascular risk score, decreased CBF in medial frontal/anterior cingulate and insular regions was additionally related to independent components of the risk score.
Although previous findings suggest that the FCRP is not associated with measures of global CBF21, our observation of association between higher cardiovascular risk and decline in frontal cerebral blood flow is consistent with a study examining the relationship between FCRP and brain glucose metabolism. In the latter investigation, increased risk scores were associated with decreased glucose metabolism in medial frontal, orbitofrontal, and inferior frontal/insular areas22. Together, these findings suggest that frontal lobe regions are susceptible to functional decline over time in older individuals with a higher risk of developing cardiovascular disease. The brain regions showing associations between FCRP and rCBF include areas involved in attention, error detection and performance monitoring processes23–25 and suggest aspects of cognition that may show future vulnerability to cardiovascular risk. While there was no significant effect of risk score on rates of decline in cognitive performance over the study interval, changes in brain function may occur before changes in cognitive function become apparent.
Declines in rCBF were also observed in the precuneus. Decreased glucose metabolism in the posterior cingulate/precuneus is one of the earliest correlates of abnormal neuronal function in AD26, 27. This region also exhibits functional MRI signal changes in individuals with MCI prior to the onset of AD28, as well as neurofibrillary tangle and diffuse amyloid accumulation in AD demonstrated both pathologically29, 30 and with in vivo fibrillar amyloid tracers31.
Interestingly, we have previously shown that amyloid burden significantly increases over time in non-demented older individuals not only in the precuneus region, but also in the orbitofrontal cortex and anterior cingulate/medial frontal areas32 that show significant decrements in rCBF in relation to cardiovascular risk in the present study. This is particularly important as recent evidence points to a link between vascular dysfunction and the development of AD pathology33, and in particular amyloid accumulation6. As the associations between decreased CBF and increased risk in frontal and precuneus regions were observed in currently cognitively normal individuals, these findings may be an early marker of neurodegenerative changes that occur not only as a result of AD, but also in conjunction with vascular dysfunction that ultimately result in cognitive decline34. Taken together, these findings indicate that cardiovascular risk factors may influence neuronal function within regions susceptible to pathologic change in the aging brain.
Of the components that make up the FCRP score, higher blood pressure was associated with greater CBF decline in medial frontal/anterior cingulate cortex. This was true for diastolic pressure, and is in agreement with previous results from our laboratory which showed greater CBF decline over a 6 year period in this region in individuals with hypertension relative to those with normal blood pressure14. It is also consistent with a study by Dai, et. al.35 where older individuals with hypertension exhibited decreased CBF in the anterior cingulate. Type-II diabetes was independently associated with greater insular CBF decrease over time. Few neuroimaging studies have examined the effects of diabetes on brain function, but some studies have shown a relationship between diabetes and white matter changes in the brain36, 37 that could contribute to the functional decline observed in the insula.
Several studies have also noted structural changes related to vascular risk scores. Increased risk scores have been associated with decreased whole brain volumes and regional volume of the frontal lobes38. Hypertension alone has also been associated with decreased medial frontal and anterior cingulate volumes39, 40, whereas diabetes has been linked with decreased frontal lobe volumes including inferior regions that make up the medial wall of the insular cortex41, 42. Although the current findings suggest that volumetric changes in localized clusters of functional change do not play a significant role in the accelerated CBF decline in those regions, decreased volume in frontal lobe structures has been associated with increased vascular risk in previous studies.
Together, these results show that baseline cardiovascular risk factors are associated with future declines in regional brain function over time. In this sample, however, baseline FCRP scores were not related to rates of decline in cognitive performance over time. This may reflect the fact that these participants represent a relatively healthy subset who maintained cognitive health over time, as participants with even mild cognitive impairment were excluded from these analyses. In addition, participants with severe risk factors for cardiovascular disease were excluded from the neuroimaging study at its inception, further restricting the range of FCRP and cognitive performance. It is also important to note that our study collected only one resting scan per year. Trajectories of CBF change, however, were based on as many as 9 longitudinal scans, increasing the stability of our longitudinal estimates. Nevertheless, variance associated with acquisition of a single scan per year could reduce our power to detect additional associations.
Future investigations over longer follow-up intervals that include individuals who develop cognitive impairment will help determine whether these CBF changes will ultimately lead to cognitive decline in those at higher risk of developing cardiovascular disease. Such studies are important because emerging findings suggest that the treatment of factors such as hypertension and diabetes may reduce the risk of subsequent cognitive and functional decline43, 44 in older individuals.
Acknowledgements
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging and by Research and Development Contract N01-AG-3-2124. We are grateful to the BLSA participants and staff for their dedication to these studies and the staff of the Johns Hopkins PET facility for their assistance.
Funding This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging and by Research and Development Contract N01-AG-3-2124.
Footnotes
Disclosure Statement Drs. Sojkova and Kraut receive salary support from National Institute on Aging Research and Development Contract N01-AG-3-2124.
All authors confirm that there are no conflicts of interest with regard to this work.
This work has not been previously published except in abstract form nor is it under consideration elsewhere.
This study was approved by the local Institutional Review Board. All subjects provided written informed consent prior to each assessment.
All authors have reviewed the contents of the manuscript, approve of its contents and validate the accuracy of the data.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Waldstein SR, Wendell CR. Neurocognitive function and cardiovascular disease. J Alzheimers Dis. 2010;20:833–842. doi: 10.3233/JAD-2010-091591. [DOI] [PubMed] [Google Scholar]
- 2.de Toledo Ferraz Alves TC, Ferreira LK, Wajngarten M, Busatto GF. Cardiac disorders as risk factors for alzheimer's disease. J Alzheimers Dis. 2010;20:749–763. doi: 10.3233/JAD-2010-091561. [DOI] [PubMed] [Google Scholar]
- 3.Duron E, Hanon O. Vascular risk factors, cognitive decline, and dementia. Vasc Health Risk Manag. 2008;4:363–381. doi: 10.2147/vhrm.s1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Musicco M, Palmer K, Salamone G, Lupo F, Perri R, Mosti S, et al. Predictors of progression of cognitive decline in alzheimer's disease: The role of vascular and sociodemographic factors. J Neurol. 2009;256:1288–1295. doi: 10.1007/s00415-009-5116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sakurai H, Hanyu H, Sato T, Kanetaka H, Shimizu S, Hirao K, et al. Vascular risk factors and progression in alzheimer's disease. Geriatr Gerontol Int. 2011;11:211–214. doi: 10.1111/j.1447-0594.2010.00669.x. [DOI] [PubMed] [Google Scholar]
- 6.Craft S. The role of metabolic disorders in alzheimer disease and vascular dementia: Two roads converged. Arch Neurol. 2009;66:300–305. doi: 10.1001/archneurol.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skoog I, Lernfelt B, Landahl S, Palmertz B, Andreasson LA, Nilsson L, et al. 15-year longitudinal study of blood pressure and dementia. Lancet. 1996;347:1141–1145. doi: 10.1016/s0140-6736(96)90608-x. [DOI] [PubMed] [Google Scholar]
- 8.Kalaria RN. Vascular basis for brain degeneration: Faltering controls and risk factors for dementia. Nutr Rev. 2010;68(Suppl 2):S74–87. doi: 10.1111/j.1753-4887.2010.00352.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burgener SC, Buettner L, Coen Buckwalter K, Beattie E, Bossen AL, Fick DM, et al. Evidence supporting nutritional interventions for persons in early stage alzheimer's disease (ad) J Nutr Health Aging. 2008;12:18–21. doi: 10.1007/BF02982159. [DOI] [PubMed] [Google Scholar]
- 10.Middleton LE, Yaffe K. Promising strategies for the prevention of dementia. Arch Neurol. 2009;66:1210–1215. doi: 10.1001/archneurol.2009.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barnes DE, Yaffe K. The projected effect of risk factor reduction on alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorelick P, Scuteri A, Black S, Decarli C, Greenberg S, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the american heart association/american stroke association. American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and AnesthesiaStroke. 2011 epub ahead of print. [Google Scholar]
- 13.Karow DS, McEvoy LK, Fennema-Notestine C, Hagler DJ, Jr, Jennings RG, Brewer JB, et al. Relative capability of mr imaging and fdg pet to depict changes associated with prodromal and early alzheimer disease. Radiology. 2010;256:932–942. doi: 10.1148/radiol.10091402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beason-Held L, Moghekar A, Zonderman A, Kraut M, Resnick SM. Longitudinal changes in cerebral blood flow in the older hypertensive brain. Stroke. 2007;38:1766–1773. doi: 10.1161/STROKEAHA.106.477109. [DOI] [PubMed] [Google Scholar]
- 15.Jueptner M, Weiller C. Does measurement of regional cerebral blood flow reflect synaptic activity? Implications for pet and fmri. NeuroImage. 1995;2:148–156. doi: 10.1006/nimg.1995.1017. [DOI] [PubMed] [Google Scholar]
- 16.Resnick SM, Goldszal AF, Davatzikos C, Golski S, Kraut MA, Metter EJ, et al. One-year age changes in mri brain volumes in older adults. Cereb Cortex. 2000;10:464–472. doi: 10.1093/cercor/10.5.464. [DOI] [PubMed] [Google Scholar]
- 17.Shock NW, Greulich RC, Andres R, Arenberg D, Costa PT, Jr, Lakatta E, et al. Normal human aging: The baltimore longitudinal study of aging. U.S. Government Printing Office; Washington, D.C.: 1984. [Google Scholar]
- 18.Brett M, Anton J, Valabregue R, Poline J. Region of interest analysis using an spm toolbox. Neuroimage. 2002;16 [Google Scholar]
- 19.Davatzikos C, Genc A, Xu D, Resnick S. Voxel-based morphometry using the ravens maps: Methods and validation using simulated longitudinal atrophy. Neuroimage. 2001;14:1361–1369. doi: 10.1006/nimg.2001.0937. [DOI] [PubMed] [Google Scholar]
- 20.Wilson PW, D'Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97:1837–1847. doi: 10.1161/01.cir.97.18.1837. [DOI] [PubMed] [Google Scholar]
- 21.Glodzik L, Rusinek H, Brys M, Tsui WH, Switalski R, Mosconi L, et al. Framingham cardiovascular risk profile correlates with impaired hippocampal and cortical vasoreactivity to hypercapnia. J Cereb Blood Flow Metab. 2011;31:671–679. doi: 10.1038/jcbfm.2010.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuczynski B, Jagust W, Chui HC, Reed B. An inverse association of cardiovascular risk and frontal lobe glucose metabolism. Neurology. 2009;72:738–743. doi: 10.1212/01.wnl.0000343005.35498.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carter C, Botvinick M, Cohen J. The contribution of the anterior cingulate cortex to executive processes in cognition. Rev Neurosci. 1999;10:49–57. doi: 10.1515/revneuro.1999.10.1.49. [DOI] [PubMed] [Google Scholar]
- 24.Petrides M. The orbitofrontal cortex: Novelty, deviation from expectation, and memory. Ann N Y Acad Sci. 2007;1121:33–53. doi: 10.1196/annals.1401.035. [DOI] [PubMed] [Google Scholar]
- 25.Ullsperger M, von Cramon DY. Subprocesses of performance monitoring: A dissociation of error processing and response competition revealed by event-related fmri and erps. Neuroimage. 2001;14:1387–1401. doi: 10.1006/nimg.2001.0935. [DOI] [PubMed] [Google Scholar]
- 26.Friedland RP, Budinger TF, Ganz E, Yano Y, Mathis CA, Koss B, et al. Regional cerebral metabolic alterations in dementia of the alzheimer type: Positron emission tomography with [18f]fluorodeoxyglucose. J Comput Assist Tomogr. 1983;7:590–598. doi: 10.1097/00004728-198308000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Reiman EM, Caselli RJ, Yun LS, Chen K, Bandy D, Minoshima S, et al. Preclinical evidence of alzheimer's disease in persons homozygous for the epsilon 4 allele for apolipoprotein e. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 28.Sorg C, Riedl V, Muhlau M, Calhoun VD, Eichele T, Laer L, et al. Selective changes of resting-state networks in individuals at risk for alzheimer's disease. Proc Natl Acad Sci U S A. 2007;104:18760–18765. doi: 10.1073/pnas.0708803104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arnold SE, Hyman BT, Flory J, Damasio AR, Van Hoesen GW. The topographical and neuroanatomical disrtibution of neurofibrillary tangles and neuritic plaques in the cerebral cortex of patients with alzheimer's disease. Cerebral Cortex. 1991;1:103–116. doi: 10.1093/cercor/1.1.103. [DOI] [PubMed] [Google Scholar]
- 30.Braak H, Braak E. Frequency of stages of alzheimer-related lesions in different age categories. Neurobiol Aging. 1997;18:351–357. doi: 10.1016/s0197-4580(97)00056-0. [DOI] [PubMed] [Google Scholar]
- 31.Wolk DA, Klunk W. Update on amyloid imaging: From healthy aging to alzheimer's disease. Curr Neurol Neurosci Rep. 2009;9:345–352. doi: 10.1007/s11910-009-0051-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sojkova J, Zhou Y, Kraut M, Brasic J, Ferrucci L, Wong D, et al. Spatial pattern of longitudinal changes in amyloid deposition in nondemented older adults. J Cereb Blood Flow Metab. 2009;29:S44. [Google Scholar]
- 33.Altman R, Rutledge JC. The vascular contribution to alzheimer's disease. Clin Sci (Lond) 2010;119:407–421. doi: 10.1042/CS20100094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breteler MM. Vascular involvement in cognitive decline and dementia. Epidemiologic evidence from the rotterdam study and the rotterdam scan study. Ann N Y Acad Sci. 2000;903:457–465. doi: 10.1111/j.1749-6632.2000.tb06399.x. [DOI] [PubMed] [Google Scholar]
- 35.Dai W, Lopez OL, Carmichael OT, Becker JT, Kuller LH, Gach HM. Abnormal regional cerebral blood flow in cognitively normal elderly subjects with hypertension. Stroke. 2008;39:349–354. doi: 10.1161/STROKEAHA.107.495457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tiehuis AM, van der Graaf Y, Visseren FL, Vincken KL, Biessels GJ, Appelman AP, et al. Diabetes increases atrophy and vascular lesions on brain mri in patients with symptomatic arterial disease. Stroke. 2008;39:1600–1603. doi: 10.1161/STROKEAHA.107.506089. [DOI] [PubMed] [Google Scholar]
- 37.van Harten B, de Leeuw FE, Weinstein HC, Scheltens P, Biessels GJ. Brain imaging in patients with diabetes: A systematic review. Diabetes Care. 2006;29:2539–2548. doi: 10.2337/dc06-1637. [DOI] [PubMed] [Google Scholar]
- 38.Zade D, Beiser A, McGlinchey R, Au R, Seshadri S, Palumbo C, et al. Interactive effects of apolipoprotein e type 4 genotype and cerebrovascular risk on neuropsychological performance and structural brain changes. J Stroke Cerebrovasc Dis. 2010;19:261–268. doi: 10.1016/j.jstrokecerebrovasdis.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen X, Wen W, Anstey KJ, Sachdev PS. Effects of cerebrovascular risk factors on gray matter volume in adults aged 60–64 years: A voxel-based morphometric study. Psychiatry Res. 2006;147:105–114. doi: 10.1016/j.pscychresns.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 40.Gianaros PJ, Greer PJ, Ryan CM, Jennings JR. Higher blood pressure predicts lower regional grey matter volume: Consequences on short-term information processing. Neuroimage. 2006;31:754–765. doi: 10.1016/j.neuroimage.2006.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar A, Haroon E, Darwin C, Pham D, Ajilore O, Rodriguez G, et al. Gray matter prefrontal changes in type 2 diabetes detected using mri. J Magn Reson Imaging. 2008;27:14–19. doi: 10.1002/jmri.21224. [DOI] [PubMed] [Google Scholar]
- 42.Last D, Alsop DC, Abduljalil AM, Marquis RP, de Bazelaire C, Hu K, et al. Global and regional effects of type 2 diabetes on brain tissue volumes and cerebral vasoreactivity. Diabetes Care. 2007;30:1193–1199. doi: 10.2337/dc06-2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deschaintre Y, Richard F, Leys D, Pasquier F. Treatment of vascular risk factors is associated with slower decline in alzheimer disease. Neurology. 2009;73:674–680. doi: 10.1212/WNL.0b013e3181b59bf3. [DOI] [PubMed] [Google Scholar]
- 44.Li J, Wang YJ, Zhang M, Xu ZQ, Gao CY, Fang CQ, et al. Vascular risk factors promote conversion from mild cognitive impairment to alzheimer disease. Neurology. 2011;76:1485–1491. doi: 10.1212/WNL.0b013e318217e7a4. [DOI] [PubMed] [Google Scholar]