Abstract
Prior studies have found abnormalities of functional brain asymmetry in patients having a major depressive disorder (MDD). This study aimed to replicate findings of reduced right hemisphere advantage for perceiving dichotic complex tones in depressed patients, and to determine whether patients having “pure” dysthymia show the same abnormality of perceptual asymmetry as MDD. It also examined gender differences in lateralization, and the extent to which abnormalities of perceptual asymmetry in depressed patients are dependent on gender. Unmedicated patients having either a MDD (n=96) or “pure” dysthymic disorder (n=42) and healthy controls (n=114) were tested on dichotic fused-words and complex-tone tests. Patient and control groups differed in right hemisphere advantage for complex tones, but not left hemisphere advantage for words. Reduced right hemisphere advantage for tones was equally present in MDD and dysthymia, but was more evident among depressed men than depressed women. Also, healthy men had greater hemispheric asymmetry than healthy women for both words and tones, whereas this gender difference was not seen for depressed patients. Dysthymia and MDD share a common abnormality of hemispheric asymmetry for dichotic listening.
Keywords: Dysthymia, major depression, hemispheric laterality, dichotic listening, gender
1. Introduction
Studies using neurocognitive, electrophysiologic, and neuroimaging measures have found evidence of abnormalities of functional brain asymmetry in depressed patients, most of whom had major depressive or bipolar disorders (Henriques & Davidson, 1991; George et al., 1994; Heller et al.,1995; Reid et al., 1998; Deldin et al., 2000; Kayser et al., 2000; Bruder, 2003; Rabe et al., 2005; Moratti et al., 2008; Stewart et al., 2011). Our studies of cerebral laterality using dichotic listening tests demonstrated that major depression is characterized by abnormal laterality, which is related to the patient's diagnostic subtype and response to antidepressants (Bruder, 2003). In dichotic tests, a different stimulus (e.g., word or tone) is simultaneously presented to the left and right ear, and the accuracy for perceiving stimuli in the right and left ear provides a measure of perceptual asymmetry (PA). Dichotic listening tests have been shown to provide reliable and valid measures of left hemisphere dominance for language processing (Wexler & Halwes, 1983; Zatorre, 1989; Hugdahl, et al., 2003) and right hemisphere dominance for complex pitch perception (Sidtis, 1981; Zatorre, 2003). The most consistent finding for patients having depressive disorders has been a reduction or absence of the normal right hemisphere advantage for nonverbal dichotic listening (Johnson & Crockett 1982; Bruder et al., 1989; Overby et al., 1989; Bruder et al., 1995). This agrees with evidence of right hemisphere dysfunction in depression in neurocognitive tests (Flor-Henry, 1976; Heller et al., 1995; Miller et al., 1995) and decreased right parietotemporal activity in neurophysiologic tests (Post et al., 1987; Henriques & Davidson, 1991; Bruder et al., 1995; Reid et al., 1998; Deldin et al., 2000; Kayser et al., 2000; Moratti et al., 2008; Stewart et al., 2011). Findings for verbal dichotic listening tests have been more variable and some studies have found a normal left hemisphere advantage in depressed patients (Hugdahl et al., 2003). The present study further examined cerebral laterality for relatively both verbal and nonverbal dichotic listening in large samples of depressed patients and healthy controls, which enabled us to also address diagnostic and gender issues not adequately studied in this area.
An important question addressed in this report is whether patients having a “pure” dysthymic disorder display the same abnormality of dichotic listening as patients having a MDD. If so, this would provide new evidence that they share a common abnormality of functional brain asymmetry. The diagnosis of dysthymic disorder was added to DSM-III in 1980 to categorize mild chronic depressions, which were less severe than major depression and were previously termed “neurotic depression” in DSM-II (Keller & Russell, 1996). It is a common disorder, affecting 3–6% of people during a lifetime and appears strongly associated with major depression, which supervenes in about 77% of the cases (Klein et al., 2000). Systematic review of controlled trial evidence has shown that antidepressant medications of various classes are effective in treating dysthymia (De Lima & Hotopf, 2003). Biological studies of “pure” dysthymia, that is dysthymia uncomplicated by major depression, have been few and, apart from its response to antidepressant medication, it is unclear whether dysthymia shares biologic characteristics with major depression (Howland & Thase, 1991).
A further purpose was to evaluate gender differences in dichotic listening asymmetry among patients having depressive disorders. Gender differences in the prevalence of depressive disorders (Weissman et al., 1984) and in hemispheric lateralization (McGlone, 1980; Kimura, 1999) point to the potential importance of gender. Epidemiological studies have consistently found greater lifetime prevalence of major depression and dysthymia in women than men, although the gender difference in dysthymia was smaller in some studies (Hasin et al., 2011). Studies have found gender differences in dichotic listening, with men generally having greater lateralization than women (Bryden, 1988; Hiscock et al., 1995). The extent to which differences in dichotic listening among patients having a depressive disorder and healthy controls are dependent on gender is therefore evaluated.
The following hypotheses were tested in this study: (1) depressed patients will show decreased right hemisphere advantage for perceiving dichotic tones, but will not differ from healthy controls in PA for dichotic words; (2) patients with a dysthymic disorder will show the same abnormalities of PA as patients having MDD; (3) men will show greater PA than women; and (4) differences in PA between depressed patients and controls will depend on gender.
2. Method
2.1 Subjects
Patients were right-handed outpatients between the ages of 18 and 65 who were attending a university-affiliated research clinic at New York State Psychiatric Institute. Patients were excluded for any of the following: serious suicide risk, substance abuse disorders (including alcohol abuse) within the last 6 months, psychotic disorders, antisocial personality disorder, seizure disorder, organic mental disorder, unstable medical disorder, taking psychoactive medication, history of head trauma, or other neurological disorder. Diagnostic assessment was by Structured Interview for Clinical Diagnosis, patient version (SCID-P; First et al., 1994) conducted by research psychiatrists before dichotic listening tests. Patients met DSM-IV criteria for either current MDD without a lifetime history of dysthymia (n= 96) or current “pure” dysthymic disorder, without a lifetime history of MDD (n=42). All cases were reviewed after structured interview to compare the clinical history with the SCID-P diagnosis, and dysthymic patients with a history of major depression were removed from the sample.
Right-handed controls (n=114) were recruited through notices to hospital staff and college students and through advertisements in local newspapers. Potential participants were screened with a semistructured interview to exclude those with current or past psychopathology. They were also excluded if they had current substance abuse or a history of head trauma or other neurological disorder. Control subjects and patients were excluded if they had a hearing loss greater than 30 dB in either ear at 500, 1000 or 2000 Hz or if they had an ear difference greater than 10 dB. The study was approved by the Institution Review Board at New York State Psychiatric Institute and Columbia University Department of Psychiatry. All participants gave written informed consent before participating in the study. Both patients and controls received $15 per hour for their participation.
2.2 Procedure
Patients were unmedicated a minimum of 7 days before testing, although most patients were drug free for a considerably longer period or were not previously treated with an antidepressant. No patient was tested within 6 weeks of receiving fluoxetine. Handedness was determined with the Edinburgh Inventory (Oldfield, 1971) and severity of depression was assessed using the Beck Depression Inventory (BDI; Beck et al., 1961). All patients and controls were tested on the dichotic fused words and complex tones tests described below, with the order of the tests counterbalanced across subjects.
The Fused Rhymed Words Test (Wexler & Halwes, 1983) consists of 15 different single-syllable word pairs, in which each member of every pair differs from the other only in the initial consonant (e.g., coat, goat). All words begin with one of six stop consonants (b, d, p, t, g, k) and are natural speech spoken by a male voice. When dichotically presented, the members of each pair fuse into a single percept. Participants indicate what word they heard by marking a line through it on a prepared answer sheet that has four possible responses, both members of the dichotic pair and two other words differing from the dichotic stimuli only in the initial consonant. Following practice trials, each participant received four 30-item blocks for a total of 120 trials. Orientation of headphones was reversed after the first and third quarters to control for channel differences and ear of presentation. The words were presented via a matched pair of TDH-49 headphones at a comfortable level of 75 dB sound pressure level (SPL).
The Complex Tone Test (Sidtis, 1981) requires participants to compare the pitch of a binaural complex tone with the pitches of a dichotic pair of complex tones presented 1 second earlier. Subjects point to a response card labeled Yes when the probe tone is the same as either member of the previous dichotic pair or to a card labeled No when it differs from both. The complex tones are square waves with fundamental frequencies corresponding to eight notes in the octave between C4 and C5. After 16 binaural and 16 dichotic practice trails, participants were tested on four blocks of 28 trials in which half of the probe tones matched a member of the dichotic pair and half did not. Orientation of headphones was reversed after the first and third blocks. The tones were presented at 74 dB SPL.
2.3 Statistical Analyses
Correct responses in the fused words and complex tones tests were computed for right- and left-ear presentations. These scores were used to compute an index of perceptual asymmetry, PA= 100 (Right Correct − Left Correct)/(Right Correct + Left Correct). An initial 2 by 2 by 2 ANOVA of PA scores included the between-subject variables of Group (MDD, controls) and Gender (women, men) and one repeated-measure factor of Test (words, tones). Given significant interactions involving Group, Gender and Test, separate ANOVA were performed to evaluate the significance of group differences on each test in women and men. Analyses were also performed on the absolute accuracy scores for the right and left ear for the complex tone test, with the variables being Group (MDD, controls), Gender (women, men) and one repeated-measure factor of Ear (right, left). This analysis was not performed on the data for the fused-words test because overall accuracy was close to 100% for the single response required on each trial. Correlational analyses were also used to examine the relationship of PA scores to Beck Depression Inventory scores, age and education.
An ANOVA was also used compare the PA scores for patients having a MDD and those having a dysthymic disorder with the between subject variables of Group (MDD, Dysthmia), Gender (women, men) and Test (words, tones). An ANOVA also compared accuracy scores for MDD and dysthmic patients using Group, Gender and Ear as the variables. Parallel analyses were also performed to compare the PA scores for patients having a MDD and controls or those having a dysthymic disorder and controls.
3. Results
3.1 Depressed Patients and Healthy Controls
Table 1 summarizes the characteristics of depressed patients and healthy controls. Although the groups differed significantly in gender, the impact of gender on group differences in PA is examined below. There were relatively small differences in age and education between groups, but PA scores on the dichotic word and tone tests were not significantly correlated with either age or education of patients and controls (r≤ .05, P>.50). Also, entering age and education as covariates did not alter the significance of group differences in PA, and these variables are therefore not dealt with further. All subjects were right-handed and there was no difference among groups in the Edinburgh Inventory handedness scores. As would be expected, BDI scores were significantly higher in depressed patients compared to healthy controls.
Table 1.
Characteristics of Depressed Patients and Healthy Controls
| Variable | Patients (n = 138) | Controls (n = 114) |
|---|---|---|
| Gendera | ||
| Women | 63 | 67 |
| Men | 75 | 47 |
| Age (years)b | ||
| M | 36.4 | 30.6 |
| SD | 11.3 | 8.3 |
| Education (years)c | 15.2 | 15.8 |
| 2.3 | 1.9 | |
| Handedness Laterality Quotient | 83.0 | 80.9 |
| 19.4 | 19.5 | |
| Beck Depression Inventoryd | 22.2 | 1.9 |
| 8.5 | 2.6 |
Significant difference in gender between groups (x2 = 14.6, df = 1, p < .001).
Significant difference in age between groups (t = 4.59, df = 250, p < .001).
n = 135 for patients; n = 112 for controls; significant difference in education between groups (t = 2.40, df = 245, p < .05).
n = 134 for patients; n = 110 for controls; significant difference in depression between groups (t = 24.08, df = 242, p < .001).
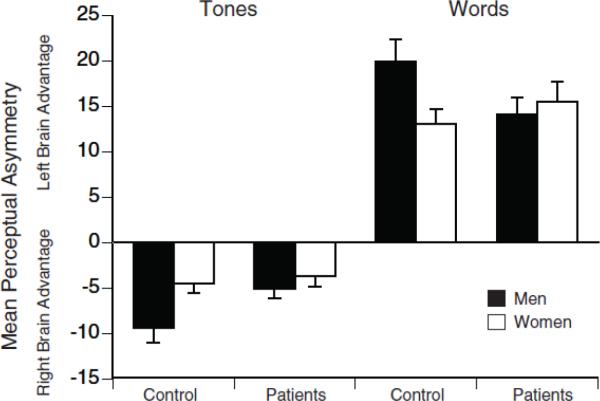
Figure 1 shows the mean PA scores for depressed patients and healthy controls on the word and tone tests. An ANOVA yielded the expected main effect of test (F=335.38, df= 1, 248, P<.001), with the words test yielding a left hemisphere advantage (i.e., positive PA scores indicating better accuracy in the right than left ear) and the complex tones test yielding a right hemisphere advantage (i.e., negative PA scores indicating better accuracy in the left than right ear). Differences among groups in PA depended on test and gender (Group by Gender by Test interaction: F=6.41, df= 1,248, P=.012). Separate analyses for each test revealed a significant difference between the depressed patients and controls in right hemisphere advantage for tones (F= 4.35, df= 1,248, P=.038), but not left hemisphere advantage for words (F= 0.69, df= 1,248, P=.41). The Group by Test interaction was, however, significant for men (F= 8.15, df=1,120, P=.005), but not women (F= 0.30, df= 1,128, P=.59), which would account for the 3-way interaction among group, test and gender. Men having a depressive disorder had significantly smaller right hemisphere advantage for tones when compared to male controls (t=2.32, df=120, P= .022), but only a nonsignificant trend for a smaller left hemisphere advantage for words (t=1.93, df= 120, P=.056). Among women, there was no significant difference in PA between those having a depressive disorder and controls for either dichotic word or tone tests (t≤0.89, df=128, P≥.38).
Figure 1.
Mean perceptual asymmetry scores and standard errors on the fused-words and complex tones tests for men and women having a depressive disorder and healthy controls. Perceptual asymmetry score= 100 (right−left)/(right +left), based on the number of correct responses for right and left ear presentations. Scores greater than zero indicate a left hemisphere (right ear) advantage; scores less than zero indicate a right hemisphere (left ear) advantage.
Among healthy controls, men showed overall greater PA than women on both the word and tone tests (see Figure 1). When compared to women, healthy men had significantly greater left hemisphere advantage for words (t=2.46, df=112, P=.015) and greater right hemisphere advantage for tones (t=2.56, df=112, P=.012). In contrast, these gender effects in healthy controls were not present in patients for either words or tones (t≤0.88, df=136, P≥ .38). The lack of normal gender effects in patients was clearly due to the reduced PA scores for depressed men (see Figure 1).
The complex tones test also permitted analyses of absolute accuracy scores for each ear. An ANOVA of percent correct scores for patients and controls confirmed the overall greater accuracy for tones presented to left ear (M=88.0, SD=11.2) than the right ear (M=79.9, SD= 15.4; F= 92.54, df= 1, 248, P<.001). There was a difference in left ear but not right ear accuracy between patients and controls (Group by Ear interaction, F=3.97, df= 1,248, P=.047). Accuracy for tones presented to the left ear was significantly poorer in patients (M=86.7, SD= 11.9) when compared to controls (M=89.7, SD= 10.1; t= 2.16, df=250, P=.032), but accuracy for right ear tones did not differ in patients (M=79.9, SD= 14.7) and controls (M=80.0, SD= 16.3; t=0.08, df=250, P=.94). The ANOVA also yielded a significant Gender by Ear interaction (F=5.08, df= 1,248, P=.025), which reflects an overall gender difference in accuracy for right ear but not left ear tones among both patients and controls. There was better right ear accuracy in women (M= 82.3, SD= 13.7) than in men (M= 77.4, SD=16.8; t= 2.52, df= 250, P=.01), whereas there was no difference in left ear accuracy between women (M= 88.8, SD= 10.9) and men (M=87.3, SD=11.6; t= 1.07, df=250, P=.28). There was, however, no significant Group by Gender by Ear interaction (F=1.13, df= 1,248, P=.29).
3.2 Major Depression and Dysthymia
Table 2 gives the characteristics of the patients having a MDD or dysthymia. There was a significant gender difference between groups, which stems from the smaller number of women in the dysthymia group. There was relatively little difference in age, education and handedness laterality quotient between these groups. As would be expected, patients having a MDD had higher Beck depression scores than dysthymic patients.
Table 2.
Characteristics of Patients having a Major Depressive or Dysthymic Disorder
| Variable | Major Depressive Disorder (n = 96) | Dysthymia (n = 42) |
|---|---|---|
| Gendera | ||
| Women | 52 | 11 |
| Men | 44 | 31 |
| Age (years)b | ||
| M | 35.0 | 39.7 |
| SD | 11.5 | 10.2 |
| Education (years)c | 15.0 | 15.6 |
| 2.4 | 2.1 | |
| Handedness Laterality Quotient | 82.0 | 85.4 |
| 20.4 | 16.8 | |
| Beck Depression Inventoryd | 24.9 | 15.5 |
| 7.9 | 6.0 |
Significant difference in gender between groups (x2 = 9.21, df = 1, p < .01).
Significant difference in age between groups (t = 2.25, df = 136, p < .05).
n = 94 for MDD group; n = 41 for dysthymia group.
n = 96 for MDD group; n = 38 for dysthymia group; significant difference in depression between groups (t = 6.63, df = 132, p < .001).
Figure 2 shows the mean PA scores for patients having a MDD or dysthymia. An ANOVA of these PA scores showed no significant difference in asymmetry between MDD and dysthymia groups for either tones or syllables and no significant interactions involving the variables of group, gender or test (F≤ 1.03, df=1,134, P≥ .31). Nor was there a significant gender effect in asymmetry scores for these groups. Separate analyses comparing the PA scores for each of these groups to controls yield essentially the same findings as seen above for patient vs. control comparisons. An ANOVA of the PA scores for MDD and controls yielded a significant Group by Gender by Test interaction (F= 5.44, df= 1,206, P= .021). Follow-up analyses showed a significant Group by Test interaction of men (F=5,28, df=1,89, P= .024), but not for women (F=0.68, df=1,117, P= .41). Men having a MDD had a smaller right hemisphere advantage for tones when compared to controls (t= 2.07, df= 89, P= .041), but did not differ significantly from controls in left hemisphere advantage for words (t= 1.44, df= 89, P= .153). An ANOVA comparing the PA scores for patients having a dysthymic disorder and controls yielded a Group by Test interaction (F=4.13, df= 1,152, P=.044). Separate analyses for each test revealed a significant difference between the dysthymic patients and controls in right hemisphere advantage for tones (F= 4.14, df= 1,152, P<.044), but not left hemisphere advantage for words (F= 1.10, df= 1,152, P=.30). Thus, the results for both patients having a MDD and those having a dysthymic disorder showed evidence of reduced right hemisphere advantage for tones, which was dependent on gender in MDD but not in dysthymia.
Figure 2.
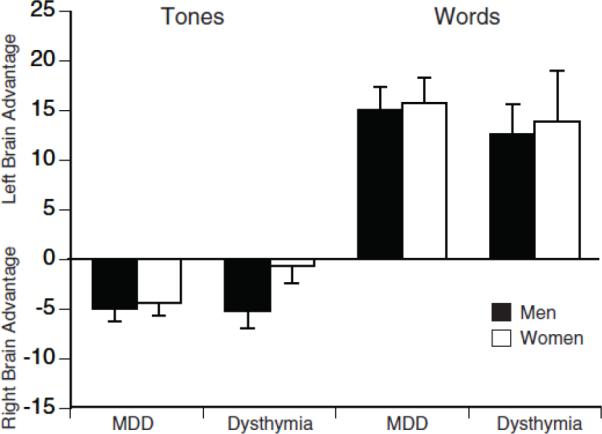
Mean perceptual asymmetry scores and standard errors on the fused-words and complex tones tests for men and women having a major depressive disorder (MDD) or dysthymic disorder. Perceptual asymmetry score= 100 (right−left)/(right +left), based on the number of correct responses for right and left ear presentations. Scores greater than zero indicate a left hemisphere (right ear) advantage; scores less than zero indicate a right hemisphere (left ear) advantage.
An ANOVA of accuracy scores on the complex tone test for patients having a MDD or dysthymic disorder showed the expected left ear (right hemisphere) advantage (F= 19.08, df= 1, 134, P<.001), but no significant Group by Ear interaction (F= 0.54, df= 1,134, P= .46). This agrees with the lack of a difference in PA scores for between patients having a MDD and dysthymic disorder. There was also no significant difference in overall accuracy of complex tone perception between the MDD and dysthymic groups (F= 1.24, df= 1, 134, P=.27).
3.3 Correlational Analyses
The severity of depression among patients, as indexed by their BDI scores, was not significantly related to PA for either words (r= .10, ns) or tones (r= −.003, ns). Nor was severity related to accuracy for perceiving complex tones presented to the right ear (r= .05, ns) or left ear (r= .06, ns).
4. Discussion
The findings replicate prior reports of reduced right hemisphere advantage in depressed patients for perceiving nonverbal dichotic stimuli (Johnson & Crockett 1982; Bruder et al., 1989; Overby et al., 1989; Bruder et al., 1995). Given the predominant projections between each ear and the contralateral hemisphere, the poorer left ear accuracy for perceiving dichotic complex tones in patients is supportive of other evidence of right hemisphere dysfunction in depression (Flor-Henry, 1976; Heller et al., 1995; Miller et al., 1995; Bruder, 2003). A new finding is that this abnormality of hemispheric asymmetry was present in both MDD and “pure” dysthymia, and was not related to severity of depression. Dysthymia has many clinical characteristics in common with major depression: most subjects with dysthymia subsequently develop major depression, subjects with dysthymia have a higher risk of major depression in their families than those with episodic major depression (Klein et al., 2000), and dysthymia responds to antidepressant medications that are effective for major depression, though possibly at a lower rate (De Lima & Hotopf, 2003). Biologic studies have been equivocal, with some studies finding similar neurophysiologic abnormalities in dysthymia and major depression and other studies finding differences in neuroendocrine function (Howland & Thase, 1991). This is one of the first studies to show that “pure” dysthymia shares an abnormality of functional brain asymmetry with major depression. This finding supports the inclusion of dysthymia among the mood disorders in Axis I and provides no support for a distinction between dysthymia and MDD. It suggests that biologic studies of major depression need not exclude those with dysthymia and that dysthymia should be studied with the modern neurophysiologic and genetic methods.
Reduced right hemisphere advantage for complex tones is particularly evident among depressed patients who respond to a selective serotonin reuptake inhibitor (SSRI) and does not change following successful antidepressant treatment, which suggests that it represents a stable, trait characteristic (Bruder et al., 1996). This is consistent with the suggestion that trait-dependent abnormalities in depressed patients on biological measures, such as shortened rapid eye movement (REM) latency and reduced resting skin conductance, are found equally in dysthymia and MDD, whereas state-dependent differences, such as in dexamethasone nonsupression, are more evident in MDD than in dysthymia (Howland & Thase 1991; Oshima et al., 2000). The finding of reduced skin conductance, indicative of lower physiologic arousal in both dysthymia and MDD, is of interest because autonomic and behavioral components of emotional arousal have been linked to right parietotemporal activity (Heller et al., 1995; Moratti et al., 2008). Reduced right hemisphere advantage for tones in MDD and dysthymia is consistent with evidence from PET (Post et al., 1987), MEG (Moratti et al., 2008), and electrophysiologic studies (Henriques and Davidson, 1991;Bruder et al., 1995; Heller et al., 1995; Reid et al., 1998; Deldin et al., 2000; Kayser et al., 2000; Stewart et al., 2011) indicative of right parietotemporal hypoactivity in depression.
The reduction of right hemisphere advantage for complex tones in depressed patients was more evident in depressed men than women. Reduced right hemisphere advantage for tones in patients who respond to treatment with a SSRI antidepressant, when compared to nonresponders, was also found to be evident in men but not in women (Bruder et al., 2004). Gur et al. (1984) reported that abnormalities of regional cerebral blood flow in depressed patients during cognitive tasks were different in men and women, with only men showing a pattern of activation on verbal and spatial tasks consistent with right hemisphere dysfunction.
Although there have been conflicting reports concerning gender differences in hemispheric lateralization among adults, our findings are consistent with evidence of greater lateralization in healthy men than women (Bryden 1988; Kimura 1999). In healthy controls, men showed greater left hemisphere advantage for words and right hemisphere advantage for complex tones when compared to women. In reviewing gender differences in neurocognitive function and depression, Heller (1993) suggested that the greater prevalence of depression among women could, in part, be related to differences in hemispheric organization between women and men. There are also gender differences in serotonergic function among depressed patients (e.g., Staley et al., 2006), and it has been suggested that these may be related to gender-related differences in hemispheric lateralization and the development of depression (Arato et al., 1991; Steiner et al., 1997). Importantly, depressed patients in our study did not show the gender differences in hemispheric asymmetry for dichotic words or tones seen in healthy adults. However, depressed men did show reduced right hemisphere advantage for perceiving complex tones, which was less evident in depressed women. Studies using more direct electrophysiologic or neuroimaging measures during cognitive tasks are needed to further define gender-related differences in regional hemispheric activation in depression and their relation to clinical responsiveness to SSRI antidepressants.
Acknowledgements
Supported in part by NIMH grants MH36295 (GEB) and R10 MH 56058 (PJM) and by the State of New York The authors thank Dr. Deborah Deliyannides and other members of the Depression Evaluation Service for assistance with patient evaluations, and Carlye Griggs, Jennifer Schaller, Paul Leite and Barbara Stuart for assistance with data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arato M, Frecska E, Tekes K, MacCrimmon DJ. Serotonergic interhemispheric asymmetry: Gender difference in the orbital cortex. Acta Psychiatrica Scandinavica. 1991;84:110–111. doi: 10.1111/j.1600-0447.1991.tb01431.x. [DOI] [PubMed] [Google Scholar]
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Archives of General Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Bruder GE. Frontal and parietotemporal asymmetries in depressive disorders: Behavioral, electrophysiologic, and neuroimaging findings. In: Hugdahl K, Davidson RH, editors. The Asymmetrical Brain. MIT Press; Cambridge, MA: 2003. pp. 719–742. [Google Scholar]
- Bruder GE, Otto MW, Stewart JW, McGrath P, Fava M, Rosenbaum JF, Quitkin FM. Dichotic listening before and after fluoxetine treatment for major depression: Relations of laterality to therapeutic response. Neuropsychopharmacology. 1996;15:171–179. doi: 10.1016/0893-133X(95)00180-L. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Quitkin FM, Stewart JW, Martin C, Voglmaier M, Harrison WM. Cerebral laterality and depression: Differences in perceptual asymmetry among diagnostic subtypes. Journal of Abnormal Psychology. 1989;98:177–186. doi: 10.1037//0021-843x.98.2.177. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Stewart JW, McGrath PJ, Deliyannides D, Quitkin FM. Dichotic listening tests of functional brain asymmetry predict response to fluoxetine in depressed women and men. Neuropsychopharmacology. 2004;29:1752–1761. doi: 10.1038/sj.npp.1300519. [DOI] [PubMed] [Google Scholar]
- Bruder GE, Tenke CE, Stewart JW, Towey JP, Leite P, Voglmaier M, Quitkin FM. Brain event-related potentials to complex tones in depressed patients: Relations to perceptual asymmetry and clinical features. Psychophysiology. 1995;32:373–381. doi: 10.1111/j.1469-8986.1995.tb01220.x. [DOI] [PubMed] [Google Scholar]
- Bryden MP. An overview of the dichotic listening procedure and its relation to cerebral organization. In: Hugdahl K, editor. Handbook of Dichotic Listening: Theory, Methods and Research. John Wiley & Sons; Chichester, England: 1988. pp. 1–43. [Google Scholar]
- De Lima M, Hotopf M. Benefits and risks of pharmacotherapy for dysthymia: a systematic appraisal of the evidence. Drug Safety. 2003;26:55–64. doi: 10.2165/00002018-200326010-00006. [DOI] [PubMed] [Google Scholar]
- Deldin PJ, Keller J, Gergen JA, Miller GA. Right-posterior face processing anomaly in depression. Journal of Abnormal Psychology. 2000;109:116–121. doi: 10.1037//0021-843x.109.1.116. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J. The structural clinical interview for DSM-IV Axis I Disorders. Patient Edition (SCID-I/P, Verson 2.0) Biometric Research Department, New York State Psychiatric Institute; New York: 1994. [Google Scholar]
- Flor-Henry P. Lateralized temporal limbic dysfunction and pychopathology. Annuals of the New York Academy of Sciences. 1976;280:770–797. doi: 10.1111/j.1749-6632.1976.tb25541.x. [DOI] [PubMed] [Google Scholar]
- George MS, Ketter TA, Post RM. Prefrontal cortex dysfunction in clinical depression. Depression. 1994;2:59–72. [Google Scholar]
- Gur RE, Skolnick BE, Gur RC, Caroff S, Rieger W, Obrist WD, Younkin D, Reivich M. Brain function in psychiatric disorders. Archives of General Psychiatry. 1984;41:695–699. doi: 10.1001/archpsyc.1984.01790180065008. [DOI] [PubMed] [Google Scholar]
- Hasin DS, Fenton M, Weissman MM. Epidemiology of depressive disorders. In: Tsuang M, Tohen M, Jones P, editors. Textbook in Psychiatric Epidemiology. John Wiley & Sons, Ltd.; Chichester, England: 2011. pp. 289–309. [Google Scholar]
- Heller W. Gender differences in depression: Perspectives from neuropsychology. Journal of Affective Disorders. 1993;29:129–143. doi: 10.1016/0165-0327(93)90028-i. [DOI] [PubMed] [Google Scholar]
- Heller W, Etienne MA, Miller GA. Patterns of perceptual asymmetry in depression and anxiety: Implications for neuropsychological models of emotion and psychopathology. Journal of Abnormal Psychology. 1995;104:327–333. doi: 10.1037//0021-843x.104.2.327. [DOI] [PubMed] [Google Scholar]
- Henriques JB, Davidson RJ. Left frontal hypoactivation in depression. Journal of Abnormal Psychology. 1991;100:535–545. doi: 10.1037//0021-843x.100.4.535. [DOI] [PubMed] [Google Scholar]
- Hiscock M, Israelian M, Inch R, Jacek C, Hiscock-Kalil C. Is there a sex difference in human laterality? II. An exhaustive survey of visual laterality studies from six neuropsychology journals. Journal of Clinical and Experimental Neuropsychology. 1995;17:590–610. doi: 10.1080/01688639508405148. [DOI] [PubMed] [Google Scholar]
- Hugdahl K, Rund BR, Lund A, Asbjørnsen A, Egeland J, Landrø NI, Roness A, Stordal KI, Sundet K. Attentional and executive dysfunctions in schizophrenia and depression: Evidence from dichotic listening performance. Biological Psychiatry. 2003;53:609–616. doi: 10.1016/s0006-3223(02)01598-6. [DOI] [PubMed] [Google Scholar]
- Howland RH, Thase ME. Biological studies of dysthymia. Biological Psychiatry. 1991;30:283–304. doi: 10.1016/0006-3223(91)90112-y. [DOI] [PubMed] [Google Scholar]
- Johnson O, Crockett D. Changes in perceptual asymmetries with clinical improvement of depression and schizophrenia. Journal of Abnormal Psychology. 1982;91:45–54. doi: 10.1037//0021-843x.91.1.45. [DOI] [PubMed] [Google Scholar]
- Kayser J, Bruder GE, Tenke CE, Stewart JW, Quitkin FM. Event-related potentials (ERPs) to hemified presentations of emotional stimuli: Differences between depressed patients and healthy adults in P3 amplitude and asymmetry. International Journal of Psychophysiology. 2000;36:211–236. doi: 10.1016/s0167-8760(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Keller M, Russell C. Dysthymia. In: Widiger T, Frances A, Pincus H, Ross R, First M, Davis W, editors. DSM-IV Sourcebook. vol 2. American Psychiatric Press; Washington, DC: 1996. pp. 21–35. [Google Scholar]
- Kimura D. Sex and Cognition. MIT Press; London: 1999. [Google Scholar]
- Klein D, Schwartz J, Rose S, Leader J. Five-year course and outcome of dysthymic disorder: A prospective, naturalistic follow-up study. American Journal of Psychiatry. 2000;157:931–933. doi: 10.1176/appi.ajp.157.6.931. [DOI] [PubMed] [Google Scholar]
- McGlone J. Sex differences in human brain asymmetry: A critical survey. Behavioral and Brain Sciences. 1980;3:215–263. [Google Scholar]
- Miller EN, Fujioka TAT, Chapman LJ, Chapman JP. Hemispheric asymmetries of function in patients with major affective disorders. Journal of Psychiatric Research. 1995;29:173–183. doi: 10.1016/0022-3956(95)00011-s. [DOI] [PubMed] [Google Scholar]
- Moratti S, Rubio G, Campo P, Keil A, Ortiz T. Hypofunction of right temporoparietal cortex during emotional arousal in depression. Archives of General Psychiatry. 2008;65:532–541. doi: 10.1001/archpsyc.65.5.532. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Oshima A, Yamashita S, Owashi T, Murata T, Tadokoro C, Miyaoka H, Kamijima K, Higuchi T. The differential ACTH responses to combined dexamethasone/CRH administration in major depressive and dysthymic disorders. Journal of Psychiatric Research. 2000;34:325–328. doi: 10.1016/s0022-3956(00)00021-2. [DOI] [PubMed] [Google Scholar]
- Overby LA, III, Harris AE, Leek MR. Perceptual asymmetry in schizophrenia and affective disorder: Implications from a right hemisphere task. Neuropsychologia. 1989;27:861–870. doi: 10.1016/0028-3932(89)90009-2. [DOI] [PubMed] [Google Scholar]
- Post RM, DeLisi LE, Holcomb HH, Uhde TW, Cohen R, Buchsbaum MS. Glucose utilization in the temporal cortex of affectively ill patients: Positron emission tomography. Biological Psychiatry. 1987;22:545–553. doi: 10.1016/0006-3223(87)90182-x. [DOI] [PubMed] [Google Scholar]
- Rabe S, Debener S, Brocke B, Beauducel A. Depression and its relation to posterior cortical activity during performance of neuropsychological verbal and spatial tasks. Personality and Individual Differences. 2005;39:601–611. [Google Scholar]
- Reid SA, Duke LM, Allen JJB. Resting frontal electroencephalographic asymmetry in depression: Inconsistencies suggest the need to identify mediating factors. Psychophysiology. 1998;35:389–404. [PubMed] [Google Scholar]
- Sidtis JJ. The complex tone test: implications for the assessment of auditory laterality effects. Neuropsychologia. 1981;19:103–112. doi: 10.1016/0028-3932(81)90050-6. [DOI] [PubMed] [Google Scholar]
- Staley JK, Sanacora G, Tamagnan G, Maciejewski PK, Malison RT, Berman RM, Vythilingam M, Kugaya A, Baldwin RM, Seibyl JP, Charney D, Innis RB. Sex differences in diencephalons serotonin transporter availability in major depression. Biological Psychiatry. 2006;59:40–47. doi: 10.1016/j.biopsych.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Steiner M, Lepage P, Dunn EJ. Serotonin and gender-specific psychiatric disorders. International Journal of Psychiatry in Clinical Practice. 1997;1:3–13. doi: 10.3109/13651509709069200. [DOI] [PubMed] [Google Scholar]
- Stewart JL, Towers DN, Coan JA, Allen JJB. The oft-neglected role of parietal EEG asymmetry and risk for major depressive disorder. Psychophysiology. 2011;48:82–95. doi: 10.1111/j.1469-8986.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Leaf PJ, Holzer CE, III, Myers JK, Tischler GL. The epidemiology of depression: An update on sex differences in rates. Journal of Affective Disorders. 1984;7:179–188. doi: 10.1016/0165-0327(84)90039-9. [DOI] [PubMed] [Google Scholar]
- Wexler BE, Halwes T. Increasing the power of dichotic methods: The fused rhymed words test. Neuropsychologia. 1983;21:59–66. doi: 10.1016/0028-3932(83)90100-8. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ. Perceptual asymmetry on the dichotic fused words test and cerebral speech lateralization determined by the carotid sodium amytal test. Neuropsychologia. 1989;27:1207–1219. doi: 10.1016/0028-3932(89)90033-x. [DOI] [PubMed] [Google Scholar]
- Zatorre RJ. Hemispheric asymmetries in the processing of tonal stimuli. In: Hugdahl K, Davidson RJ, editors. The Asymmetrical Brain. MIT Press; Cambridge, MA: 2003. pp. 411–440. [Google Scholar]