Abstract
We reported that weight loss induces bone loss which is prevented by exercise training; however, the mechanism for this observation remains unclear. Sclerostin, an inhibitor of bone formation, has been found to increase in states of unloading and may mediate the changes in bone metabolism associated with weight loss and exercise. The objective of the study was to determine the effect of lifestyle intervention in obese older adults on sclerostin levels, and on hip geometry parameters. One-hundred-seven obese (BMI ≥30 kg/m2) older (≥65 yrs) adults were randomly assigned to control, diet, exercise and combined diet-exercise for 1 year. Sclerostin levels were measured by ELISA at baseline, 6, and 12 months, while hip geometry parameters were obtained from bone mineral density (BMD) images done by dual-energy x-ray absorptiometry using hip structure analysis at baseline and 12 months. Both the diet and diet-exercise groups had significant decreases in body weight (−9.6% and −9.4%, respectively), while weight was stable in the exercise and control groups. Sclerostin levels increased significantly and progressively in the diet group (6.6±1.7% and 10.5±1.9% at 6 and 12 months, respectively, all P<0.05), while they were unchanged in the other groups; in particular, they were stable in the diet-exercise group (0.7±1.6% and 0.4±1.7% at 6 and 12 months, respectively, all P=NS). Hip geometry parameters showed significant decreases in cross-sectional area, cortical thickness, and BMD; and increases in buckling ratio at the narrow neck, intertrochanter and femoral shaft. These negative changes on bone geometry were not observed in the diet-exercise group. Significant correlations between changes in sclerostin and changes in certain hip geometry parameters were also observed (P<0.05). In conclusion, the increase in sclerostin levels with weight loss which was prevented by exercise may partly mediate the negative effects of weight loss on bone metabolism and the osteoprotective effect of exercise training.
Keywords: sclerostin, hip geometry, weight loss, exercise, mechanical loading, obesity
Introduction
Weight loss therapy to improve overall health in obese older adults is limited by concomitant loss of bone mineral density (BMD).(1) However, we recently reported that the addition of exercise training (ET) to weight loss attenuates the decrease in hip BMD induced by weight loss.(2,3) The precise mechanisms for weight loss-induced bone loss are not known. Although alterations in hormones regulating bone metabolism that occur during weight loss have been proposed, results from prior studies showed inconsistent results.(4–6) Our recent study(2,6) found no correlation between the changes in bone-active hormones and the changes in BMD. Moreover, we found that the protective effect of ET against weight loss-induced bone loss occurred despite decline in bone-active hormones (e.g. estradiol, leptin). On the other hand, the changes in lean body mass were strongly correlated with the changes in BMD, particularly at the weight-bearing site of the hip.(2) These findings support the hypothesis that decreased mechanical stress on the weight-bearing skeleton may be the primary mechanism underlying weight loss-induced bone loss. However, the mediator or the signaling pathway involved in the changes in BMD when obese older adults undergo weight loss therapy remains undetermined.
Sclerostin is a secreted Wnt antagonist which regulates bone mass by binding to LRP5/6 and inhibiting the canonical Wnt/β-catenin signaling resulting in inhibition of osteoblastic proliferation and differentiation, thus reduced bone formation.(7,8) Animal studies indicate an increase in sclerostin levels in experimental models of skeletal unloading,(9) while bone loading and intermitent PTH injection inhibit sclerostin release.(10) Theoretically, by inhibiting bone formation, an increase in sclerostin levels in obese patients undergoing voluntary weight loss would lead to bone loss and perhaps deterioration in bone quality. Therefore, our objective in the present study is to determine the effect of lifestyle intervention on sclerostin levels and hip geometry parameters in obese older adults undergoing weight loss, exercise or combined weight loss and exercise. We hypothesized that bone loss in obese subjects undergoing weight loss therapy is mediated by an increase in sclerostin levels, which in turn is prevented by ET. Furthermore, we hypothesized that bone loss leads to deterioration in hip geometry, a measure of bone quality,(11) which is prevented by ET.
METHODS
Subjects
This project was a secondary analysis of data from a previous study on the effect of lifestyle intervention on physical function in obese older adults.(3) This study was done in accordance withthe guidelines in the Declaration of Helsinki for the ethicaltreatment of human subjects. It was conducted at Washington University School of medicine and was approved by the Institutional ReviewBoard. Participants were recruited through advertisements and written informed consent was obtained from each subject. Briefly, eligibility criteria included: 1) older age (≥65 years), 2) obese (BMI ≥ 30 kg/m2), 3) sedentary lifestyle, 4) stable body weight (±2 kg) over the past year, and 5) on stable medications for 6 months before enrollment. Subjects who were treated with bone-acting drugs (e.g. biphosphonates, glucocorticoids, sex-steroid compounds) during the previous year were excluded from participation. Additional inclusion and exclusion criteria have been previously described in detail.(3) The effects of weight loss and/or ET on measures of frailty, body composition, BMD, bone turnover, specific physical functions, and quality of life on these subjects were reported previously.(2,3) The present study reports the effects of weight loss and/or ET on serum sclerostin levels and parameters of hip geometry.
Study Design
Subjects were randomized, with stratification for sex, for a 52-week study, to 1 of 4 groups: 1) control group, 2) diet-induced weight loss (diet group), 3) exercise training (exercise group), and 4) diet-induced weight loss and exercise training (diet-exercise group). Subjects in the control group did not receive advice to change their diet or activity habits. They were prohibited from concurrently participating in any weight loss or exercise program. They were provided general information about a healthy diet during monthly visits with the staff. The diet group was prescribed a balanced diet to provide an energy deficit of 500–750 kcal/day from daily energy requirement. Subjects met weekly as a group with a dietitian for caloric intake adjustments and behavioral therapy. They were instructed to set weekly behavioral goals and to attend weekly weigh-in sessions. Food diaries were reviewed and new goals were based on diary reports. The goal was to achieve a ~10% weight loss at 6 months and weight maintenance for an additional 6 months. Subjects in the exercise group were given information regarding a diet that would maintain their current weight and counseled on maintaining a weight-stable diet and participated in a multi-component ET program supervised by a physical therapist. Each session was approximately 90 minutes in duration; 15 min of flexibility exercises, 30 min of aerobic exercise,30 min of progressive resistance training, and15 min of balance exercises. Additional details about the interventions including exercise intensity have been described previously.(3) Subjects in the diet-exercise group participated in both weight management and ET programs described above, which were conducted separately from the other groups. All subjects were provided supplements to ensure an intake of ~1500 mg of calcium/day and ~1000 IU vitamin D/day.(1) Additional details about the interventions including compliance data have been reported previously.(3)
Outcome assessments
Parameters of hip geometry
Hip geometry was measured at baseline and after 12 months of the diet and exercise interventions. Hip geometry parameters were determined using hip structure analysis (HSA) software as previously described.(12,13) This program uses mineral mass and dimensional data from conventional dual energy absorptiometry (DXA) images of the hip of bone cross-sections traversing the proximal femur. The regions of interest include the narrow neck (which corresponds to the narrowest area on the femoral neck), the intertrochanter (which traverses the bisector of the neck and shaft axes), and the femoral shaft (situated at a distance equal to 1.5 times the neck width distal to the intersection of the neck and shaft axes. Five parallel profiles are generated and averaged using the algorithm by Beck,(12) to calculate the following parameters: 1) bone mineral density (grams per square centimeter), 2) outer cortical diameters (centimeters), 3) bone cross sectional area (square centimeter), and 4) the cross-sectional moment of inertia (CSMI). The CSMI was used to calculate the section modulus (cm3), which is the CSMI divided by the maximum distance from the center of mass to the outer cortical margin. The estimated average cortical thickness of the narrow neck, intertrochanter and shaft were modeled as circular annuli with 60%, 70%, and 100% of the measured mass in the cortex, respectively. Buckling ratios were computed as the maximum distance from the center of mass to the outer diameter divided by the mean cortical thickness. The precision for HSA parameters ranges from 1–5%.(14)
Serum concentrations of sclerotin
Venous blood samples were obtained in the morning after subjects fasted for at least 12 hours at baseline, 6 months, and 12 months. An aliquot of the sample was stored at −80 C until time of assay. All samples were assayed together in a single batch by a trained blinded technician. Enzyme-linked immune absorbent assay kit was used to measure serum sclerostin level (TECO Sclerostin, TECOmedical AG, Sissach, Switzerland). The coefficient of variation for this assay in our laboratory was < 10%.
Body weight and body composition
Body weight was measured in the morning after subjects had fasted for 12 hours. Body composition was also measured using dual energy x-ray absorptiometery (Delphi 4500-W; Hologic In. Walthan, MA) as previously reported.(3)
Statistical Analyses
The primary outcome was the changes in sclerostin level from baseline at months 6 and 12. Secondary outcomes included changes in parameters of hip geometry. Intention-to-treat analyses were performed using SAS version 9.2, with inclusion of all participants who provided any data after baseline. Baseline characteristics were compared using analyses of variance or Fisher’s exact tests. Longitudinal changes between groups were tested with mixed-model repeated-measures analyses of variance, adjusting for baseline values and sex. Within the framework of the mixed model, when the P value for an interaction was significant, the specific contrasts were used to test the null hypothesis that changes between 2 specific time points in 1 group were equal to corresponding changes in another group. Analyses testing for within-group changes also were performed using mixed-model repeated-measures ANOVA. Pearson’s correlation was used to examine relationships among changes in selected variables. Data are presented as mean±SE. P≤0.05 was considered statistically significant. Our study was powered to detect a difference of 5 percentage points in the percentage change in sclerostin between study arms with 80% power and α=0.05.
RESULTS
The results of enrollment, randomization, and follow-up have been reported previously. (3) Briefly, of the 107 volunteers who were randomized, ninety-three (87%) completed the study. Fourteen participants discontinued the intervention due to personal or medical reasons but were included in the intention-to-treat analyses. Compliance based on mean attendance at exercise sessions was 88% (interquartile range, 85 to 92) among participants in the exercise group and 83% (interquartile range, 80 to 88) among those in the diet-exercise group.(3)
The 4 groups did not significantly differ in baseline characteristics including age, sex, race, weight, BMI, hip BMD and T-score, and serum concentration of sclerostin (Table 1). In addition, the 4 groups did not significantly differ in baseline parameters from hip structure analyses across the sites of the narrow neck, intertrochanter, and femoral shaft (Table 2).
TABLE 1.
Baseline characteristics of study participants
| Control (n = 27) | Diet (n = 26) | Exercise (n = 26) | Diet-exercise (n = 28) | P value | |
|---|---|---|---|---|---|
| Age (yr) | 69±0.8 | 70±0.8 | 70±0.8 | 70±0.8 | 0.85 |
| Female sex, No. (%) | 18 (67) | 17 (65) | 16 (61) | 16 (57) | 0.89 |
| White race, No. (%) | 22 (81) | 23 (88) | 21 (81) | 25 (89) | 0.78 |
| Weight (kg) | 101±3.1 | 104±2.9 | 99±3.3 | 99±3.2 | 0.66 |
| BMI (kg/m2) | 37.3±0.9 | 37.2±0.9 | 36.9±1.1 | 37.2±1.0 | 0.93 |
| BMD at total hip (g/cm2) | 0.962±0.026 | 1.021±0.027 | 0.958±0.030 | 1.014±0.028 | 0.25 |
| T-score at total hip | −0.18±0.18 | 0.34±0.19 | −0.25±0.22 | 0.18±0.21 | 0.07 |
| Serum sclerostin (ng/ml) | 1.51±0.08 | 1.50±0.07 | 1.41±0.05 | 1.57±0.07 | 0.54 |
Values are means ± SE. BMD, bone mineral density; BMI, = body mass index
TABLE 2.
Baseline Parameters from Hip Structure Analyses
| Control | Diet | Exercise | Diet-exercise | P value | |
|---|---|---|---|---|---|
| Narrow neck | |||||
| Cross-sectional area (cm2) | 3.13±0.10 | 3.34± 0.14 | 3.22±0.13 | 3.40±0.12 | 0.38 |
| Section modulus (cm2) | 1.59±0.08 | 1.79±0.11 | 1.70±0.10 | 1.88±0.10 | 0.17 |
| Cortical thickness (cm2) | 0.183±0.01 | 0.190±0.01 | 0.183±0.01 | 0.193±0.01 | 0.55 |
| Buckling ratio | 10.97±0.42 | 10.83±0.44 | 11.31±0.46 | 10.65±0.36 | 0.73 |
| BMD (g/cm2) | 0.952±0.03 | 0.987± 0.03 | 0.951±0.03 | 1.174±0.03 | 0.54 |
| Intertrochanter | |||||
| Cross-sectional area (cm2) | 5.17±.0.20 | 5.77± 0.26 | 5.31±0.20 | 5.59±0.23 | 0.23 |
| Section modulus (cm2) | 4.89±0.28 | 5.69±0.34 | 5.07±0.27 | 5.26±0.28 | 0.46 |
| Cortical thickness (cm2) | 0.398±0.02 | 0.433±0.02 | 0.395±0.02 | 0.426±0.02 | 0.20 |
| Buckling ratio | 8.25±0.31 | 7.77±0.30 | 8.68±0.42 | 7.90±0.31 | 0.23 |
| BMD (g/cm2) | 0.954±0.03 | 1.028± 0.04 | 0.966±0.03 | 1.025±0.03 | 0.27 |
| Femoral shaft | |||||
| Cross-sectional area (cm2) | 4.68±0.21 | 5.30±0.25 | 4.91±0.19 | 5.05±0.19 | 0.23 |
| Section modulus (cm2) | 2.82±0.11 | 3.18±0.20 | 3.02±0.16 | 3.00±0.15 | 0.46 |
| Cortical thickness (cm2) | 0.611±0.02 | 0.667±0.03 | 0.598±0.03 | 0.645±0.02 | 0.20 |
| Buckling ratio | 2.67±0.10 | 2.54±0.12 | 2.91±0.16 | 2.59±0.11 | 0.15 |
| BMD (g/cm2) | 1.609±0.05 | 1.725±0.06 | 1.589±0.05 | 1.674±0.05 | 0.22 |
Values are means ± SE. BMD, bone mineral density
As previously reported,.(3) body weight decreased significantly and comparably in the diet group (−9.6±1.2%) and diet-exercise group (−9.4±0.8%) but not in the exercise group (−0.6±0.7%) and control group (−0.2±0.7%) (P<0.001 for the between-group differences). Lean body mass declined less in the diet-exercise group (−3.2%±0.5%) than in the diet group (−5.3%±0.7%) while it increased in the exercise group (2.4%±0.5%).
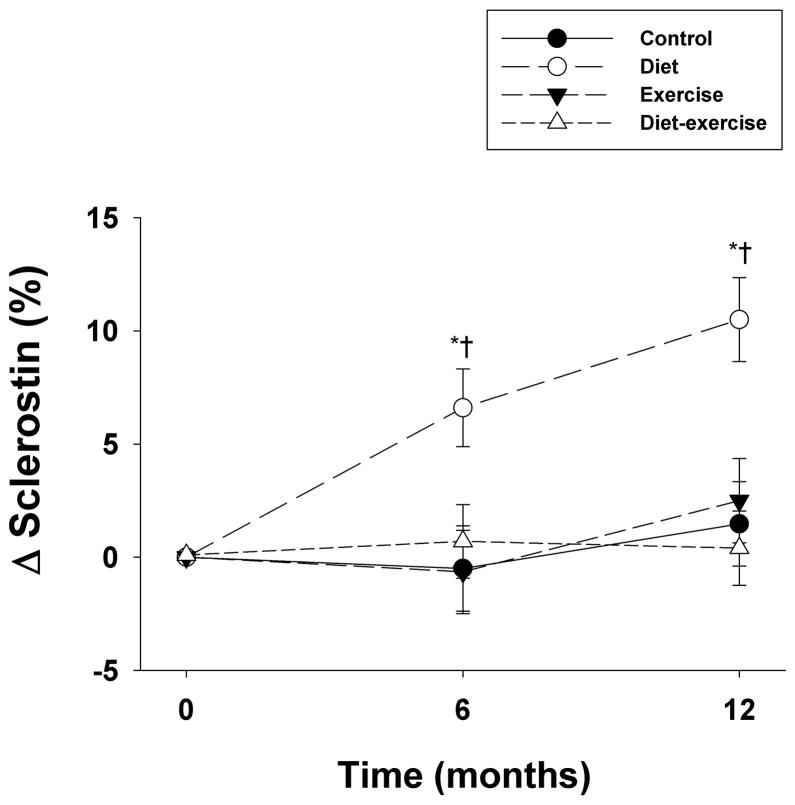
Serum sclerostin levels significantly and progressively increased at 6 months and at 12 months in the diet group compared to baseline (Figure 1). In contrast, sclerostin levels did not significantly change in the control, exercise, and diet-exercise groups compared to baseline. Group comparisons revealed a significant difference in the changes in sclerostin levels in the diet group compared to the other groups at each time point (P<.001 for the between-group differences).
Figure 1.
Changes from baseline in circulating sclerostin levels in obese older adults during the 1-year interventions. Values are mean ± SE. *P<0.05 for the comparison of the value to baseline, calculated using mixed-model repeated measures analyses of variance. †P<0.05 for the comparison of the value to control group, exercise group, and diet-exercise group, each calculated using mixed-model repeated measures analyses of variance contrasts.
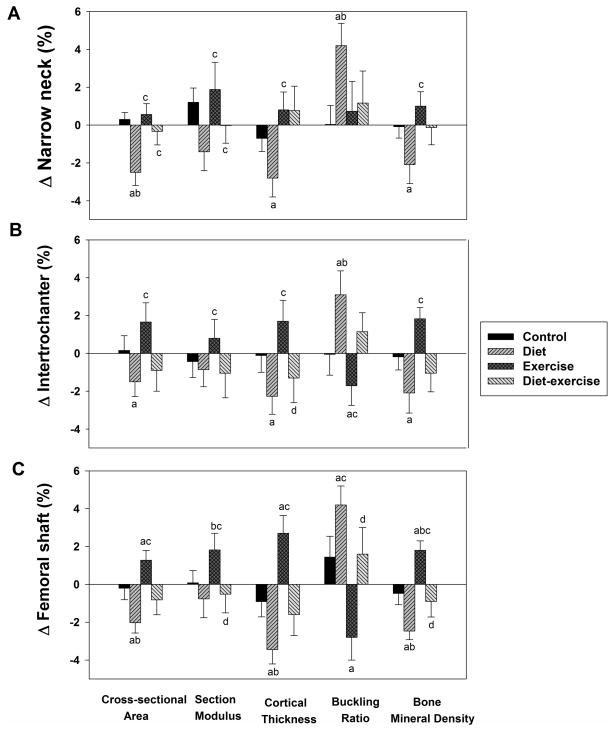
The changes in parameters of hip geometry are depicted in Figure 2. Compared to baseline, significant decreases in cross-sectional area, cortical thickness, and BMD and significant increase in buckling ratio at the a) narrow neck (Figure 2A), b) intertrochanter (Figure 2B) and c) femoral shaft (Figure 2C) occurred in the diet group. In comparison, significant increases in cross-sectional area, cortical thickness, and BMD and significant decrease in the buckling ratio at the femoral shaft (Figure 2C) were observed in the exercise group. By contrast, there were no significant changes in any of these parameters in the diet-exercise group.
Figure 2.
Changes from baseline in parameters of hip geometry in obese older adults during the 1-year interventions. Values are given as mean ± SE. aP<0.05 for the comparison of the value to baseline, as calculated using mixed-model repeated measures analyses of variance. bP<0.05 for the comparison of the value to control group, cP<0.05 for the comparison of the value to diet group, dP<0.05 for the comparison of the value to exercise group, each calculated using mixed-model repeated measures analyses of variance contrasts.
Group comparisons revealed significant differences in the hip geometry changes among the different groups (all P<.05 for the between-group differences). In the narrow neck (Figure 2A), significant between-group differences were observed in a) cross-sectional (diet vs. control, exercise, and diet-exercise groups); b) section modulus (diet vs. exercise); c) cortical thickness (diet vs. exercise and diet-exercise) d) buckling ratio (diet vs. control), and e) BMD (diet vs. exercise). In the intertrochanter (Figure 2B), significant between-group differences were observed in the: a) cross-sectional area (diet vs. exercise); b) section modulus (diet vs. exercise; c) cortical thickness (exercise vs. diet and diet-exercise); d) buckling ratio (diet vs. exercise); and BMD (diet vs. exercise). Finally for the femoral shaft (Figure 2C), the following between-group differences were observed: a) cross-sectional area (diet vs. control and exercise); b) section modulus (exercise vs. diet, control and diet-exercise); c) cortical thickness (diet vs. control and exercise; d) buckling ratio (exercise vs. diet and diet-exercise); and 5) BMD (diet vs. control, and exercise and exercise vs. control and diet-exercise). These data are also presented in Table 1 in the Supplementary Appendix.
Pearson correlation analysis for the entire cohort revealed that changes in sclerostin levels correlated negatively with changes in lean body mass (r= −0.24, P=0.03). Importantly, changes in sclerostin levels also correlated significantly with changes in cortical thickness (r= −0.23, P=0.04) and with changes in BMD (r= −0.22, P=0.05) at the narrow neck. In addition, significant correlations were also found between changes in sclerostin and changes in section modulus (r= −0.25, P=0.03) and changes in buckling ratio (r=0.24, P=0.04) at the intertrochanter. No significant correlations were found with parameters of hip geometry at the femoral shaft. In addition, there were no significant correlations (r=−0.06 to 0.13, all P>0.05) between changes in sclerostin and previously reported(3,15) changes in leptin, markers of bone turnover (CTX, PINP, and osteocalcin), and markers of exercise intensity (changes inVO2peak and total one-repetition strength).
DISCUSSION
Our results showed that weight loss among subjects in the diet group was associated with a significant increase in sclerostin levels. However, the increase in sclerostin was prevented by the addition of ET in a similar way that ET prevented bone loss and the increase in markers of bone turnover in the combined diet and exercise group as previously reported.(2) Likewise, weight loss was associated with deterioration in hip geometry parameters while the addition of ET appeared to result in attenuation of these negative effects. In addition, an inverse relationship was found between the changes in sclerostin and lean body mass highlighting the important influence of mechanical stress on circulating sclerostin levels.
Diet therapy is the cornerstone in every management program designed to reduce weight and treat obesity. However, our group showed that weight loss from diet alone in older adults is associated with loss of bone and muscle mass, which could exacerbate osteopenia and sarcopenia respectively.(3,4,16) The cause for the weight-loss associated bone loss is multifactorial and complex. Some investigators believed the change in hormone levels is the primary cause for bone loss among these subjects,(17,18) while others proposed that the reduction in skeletal stress from unloading as a result of weight loss is the main cause for bone loss.(19) We previously reported that changes in estradiol and leptin in these same subjects are not major determinants of bone loss.(2) Although there were significant reductions in estradiol and leptin among patients in the weight loss groups, changes in lean body mass and muscle strength as well as bone marker were the only major determinants of bone loss, suggesting that perhaps the relative reduction in skeletal stress from unloading could be the primary driver for bone loss. To date, the exact mediator for bone loss in this setting remains unclear.
The discovery of the canonical Wnt signaling pathway as a key regulator of bone homeostasis has led to an understanding of the mechanism of skeletal adaptation to mechanical loading and unloading.(9,20,21) Activation of the pathway leads to osteoblastic differentiation, proliferation and activity resulting in enhanced bone formation. This pathway can be antagonized by secreted inhibitors, which include secreted Frizzled-related protein, Dickoff or DKK-1, and sclerostin.(22) Sclerostin is secreted almost exclusively by the osteocytes, the bone mechanostat, and binds to LRP5/6 to inhibit the canonical Wnt signaling. Sclerostin appears to be the main inhibitor involved in states of mechanical loading and unloading.(9,21) Animal studies revealed that tail suspension is associated with an increase in sclerostin staining of unloaded hindlimbs in comparison to the exercising forelimbs.(9) Human studies likewise demonstrated an increase in sclerostin levels in patients immobilized by stroke relative to age-matched subjects.(23) Our findings of an increase in sclerostin with weight loss in the diet group which was prevented by the addition of ET in the combined diet and exercise group (despite the same amount of weight loss as the diet group) may provide additional proof as to the role of mechanical stress in modulating sclerostin levels. Realizing the effect of sclerostin on bone metabolism, it is possible that the resulting rise in sclerostin levels or lack thereof among subjects in the diet and diet-exercise group, respectively, in turn mediate the skeletal effects of lifestyle interventions in both bone mineral density(2) and hip geometry parameters in our subjects.
We did not find any correlation between changes in sclerostin and changes in leptin and markers of bone turnover. This is not totally surprising because of mixed results reported in other smaller intervention studies (mostly drug trials). In two studies using teriparatide, one showed a reduction in sclerostin while the other did not.(10,24) Furthermore, in both studies there was no correlation between changes in sclerostin and markers of bone turnover.(10,24) Both raloxifene and biphosphonates are antiresorptive agents, but raloxifene was associated with a lowering(25) while bisphosphonates either had no effect(25) or increased sclerostin levels.(24) In addition, a correlation between changes in sclerostin and markers of bone turnover was only reported in the raloxifene-treated patients. Given these conflicting results, data from larger intervention trials may shed light on correlations with changes in sclerostin associated with different types of therapies.
We anticipated a reduction in sclerostin from baseline in the exercise group but found none. A possible explanation is that there may be a floor effect of mechanical loading on the osteocyte’s response in these chronically overloaded obese subjects. On the other hand, a lack of increase in sclerostin levels in response to mechanical loading by exercise has also been reported recently(26) in younger nonobese subjects. This study considered the effect of an intensive physical training program compared to sedentary lifestyle on sclerostin level.(26)
Despite the substantial body of information on the effect of diet, exercise or both on BMD in the context of lifestyle interventions,(4,19,27,28) little is known regarding the effect of these interventions on bone quality, most especially in elderly obese. In addition, little information is available on the role of sclerostin as a potential determinant of bone quality in humans. As presented in the results section of this manuscript, there was a significant deterioration in hip geometry parameters among subjects in the diet group. On the other hand, this decline in bone quality at 12 months with weight loss appeared to be prevented by the addition of ET as hip geometry in the diet-exercise group showed no significant change from baseline. Overall, these changes are consistent with the changes in BMD by hologic DXA reported earlier,(2) indicating that the decline in BMD in the diet group was also associated with negative effects on bone quality. More importantly, however, our data suggest that the addition of ET did not only prevent further bone loss but also resulted in preservation of bone quality as assessed using hip geometry parameters.
Using hip structure analysis, prior studies reported superior bone geometrical parameters in young athletes relative to non-athletic controls.(29,30) Longitudinal data showed that exercise score was predictive of cortical thickness at both narrow neck and femoral shaft in young women ages 21 and 22 years old.(31) A 6 year study in boys and girls aged 4 to 12 at the study initiation also showed that moderate and vigorous physical activity was a positive independent predictor of femoral neck cross-sectional area and section modulus.(32) Our study demonstrates that mechanical loading does have the same positive affect on the skeleton of elderly subjects as it does on younger individuals. Moreover, the significant correlations between changes in sclerostin with changes in certain hip geometry parameters would imply that sclerostin may mediate the deterioration in bone quality from unloading in patients who are undergoing voluntary weight loss and its preservation with the addition of ET.
Our study is the first to report the independent and combined effect of weight loss and ET on sclerostin levels and hip geometry parameters in obese older adults based on a one year randomized controlled trial. As previously reported,(3) we used comprehensive diet and exercise programs with a high rate of compliance to the interventions. However, this study has some limitations. Given the intensity of the interventions and testings involved, we have relatively small number of subjects per group. In addition, we do not have data on trabecular microarchitecture which directly assesses bone structure, and should be included in future studies. We used HSA to assess hip geometry, which is inherently limited to analyses in a single plane, and, thus, may not fully reflect bone strength.(33) However, HSA has been found to compare favorably with volumetric QCT, which supports the validity of a projective technique such as DXA to derive hip geometry parameters.(34) Additional limitations of HSA include sensitivity to variations in the positioning of the patient; and blurred scan images in heavier patients may result in edge detection issues..(35) We minimized these limitations by ensuring accurate femur positioning (performed by a single expert ISCD certified technician), scanning at a low speed to minimize noise and improve the scan image, and using a state-of-the art Hologic densitometer. Although another limitation of HSA is that it is based on the assumption that average tissue mineralization does not change much through adult life, this is a reasonable assumption in our study.(35) Besides, because our study is a randomized controlled trial, we would expect any uncertainties related to the method to average out in all the randomized groups. Accordingly, despite equal weight loss (~10%) between the diet group and diet-exercise group, HSA analyses were clearly able to demonstrate that hip geometry deteriorated in the diet group but not in the diet-exercise group, indicating an osteoprotective effect of exercise. More importantly, despite its limitations, HSA-derived hip geometry parameters have been found to be useful noninvasive and inexpensive surrogate measures of bone strength and may predict hip fractures.(36–38) For instance, investigators from the Study of Osteoporotic Fractures found that cortical thickness and average buckling ratio predicted incident hip fractures equally well as areal BMD.(38)
In summary, our results suggest that sclerostin may partially mediate bone loss and deterioration in bone structure among obese elderly patients undergoing weight loss. Theoretically, the reduction in skeletal stress associated with weight loss leads to an increase in sclerostin production by the mechanostat in bone tissues, the osteocytes. By binding to the LRP5/6 receptors, sclerostin inhibits signaling through the canonical Wnt signaling resulting in an inhibition of osteoblastic differentiation, thus bone formation. Our results indicate that skeletal loading from ET increases BMD and improves bone geometry, and when added to diet, inhibits the weight loss-induced increase in sclerostin, resulting in the attenuation of bone loss and preservation of bone geometry. Increased understanding of the mechanisms for weight loss-induced bone loss could lead to more effective interventions to prevent bone loss most especially in those who already have osteoporosis. An antibody to sclerostin is currently in drug development. In addition to exercise, this agent may be used as a potential tool to counteract weight loss-induced bone loss, in particular, in obese older subjects who may already have low bone mass prior to lifestyle therapy.
Supplementary Material
Acknowledgments
This study was supported by grants RO1-AG025501, R01-AG031176, P30-DK56341 (Clinical Nutrition Research Unit), UL1-RR024992 (Clinical and Translational Science Award), and DK20579 (Diabetes Research and Training Center) from the National Institutes of Health.
We thank the participants for their cooperation and the staff of the Intensive Research Unit of the Institute of Clinical and Translational Sciences for their skilled assistance in the performance of this study.
Footnotes
This work was presented in part to the 2011 annual meeting of the American Society for Bone and Mineral Research, San Diego, CA, USA.
DISCLOSURE
All authors state that they have no conflicts of interest.
Authors’ roles: Study design: DTV and DRS. Study conduct: DTV, KS, NN, SC: Data analysis: DTV, RA, CS, NN, DRS, CQ. Data interpretation: DTV, RA, CS, NN, DRS, CQ. Revising manuscript content: DTV, RA, CS, NN, KS, SC, DRS, CQ. Approving final version of manuscript: DTV, RA, CS, NN, KS, SC, DRS, CQ. DTV takes responsibility for the integrity of the data analysis.
REFERENCE LIST
- 1.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–934. doi: 10.1093/ajcn/82.5.923. [also published in: Obes Res. 2005: 13:1849–1863] [DOI] [PubMed] [Google Scholar]
- 2.Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip BMD induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26:2852–2859. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Villareal DT, Chode S, Parimi N, et al. Weight loss, exercise, or both and physical function in obese older adults. N Engl J Med. 2011;364:1218–1229. doi: 10.1056/NEJMoa1008234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Villareal DT, Fontana L, Weiss EP, et al. Bone Mineral Density Response to Caloric Restriction-Induced Weight Loss or Exercise-Induced Weight Loss: A Randomized Controlled Trial. Arch Intern Med. 2006;166:2502–2510. doi: 10.1001/archinte.166.22.2502. [DOI] [PubMed] [Google Scholar]
- 5.Cifuentes M, Riedt CS, Brolin RE, Field MP, Sherrell RM, Shapses SA. Weight loss and calcium intake influence calcium absorption in overweight postmenopausal women. Am J Clin Nutr. 2004;80:123–130. doi: 10.1093/ajcn/80.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31(5):547–555. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 7.Semenov M, Tamai K, He X. SOST is a ligand for LRP5/LRP6 and a Wnt signaling inhibitor. J Biol Chem. 2005;280:26770–26775. doi: 10.1074/jbc.M504308200. [DOI] [PubMed] [Google Scholar]
- 8.Sutherland MK, Geoghegan JC, Yu C, et al. Sclerostin promotes the apoptosis of human osteoblastic cells: a novel regulation of bone formation. Bone. 2004;35:828–835. doi: 10.1016/j.bone.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 9.Robling AG, Niziolek PJ, Baldridge LA, et al. Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem. 2008;283:5866–5875. doi: 10.1074/jbc.M705092200. [DOI] [PubMed] [Google Scholar]
- 10.Drake MT, Srinivasan B, Modder UI, et al. Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab. 2010;95:5056–5062. doi: 10.1210/jc.2010-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seeman E, Delmas PD. Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med. 2006;354:2250–2261. doi: 10.1056/NEJMra053077. [DOI] [PubMed] [Google Scholar]
- 12.Beck TJ, Looker AC, Ruff CB, Sievanen H, Wahner HW. Structural trends in the aging femoral neck and proximal shaft: analysis of the Third National Health and Nutrition Examination Survey dual-energy X-ray absorptiometry data. J Bone Miner Res. 2000;15:2297–2304. doi: 10.1359/jbmr.2000.15.12.2297. [DOI] [PubMed] [Google Scholar]
- 13.Bonnick SL. HSA: beyond BMD with DXA. Bone. 2007;41(1 Suppl 1):S9–12. doi: 10.1016/j.bone.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Khoo BC, Beck TJ, Qiao QH, et al. In vivo short-term precision of hip structure analysis variables in comparison with bone mineral density using paired dual-energy X-ray absorptiometry scans from multi-center clinical trials. Bone. 2005;37:112–121. doi: 10.1016/j.bone.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Shah K, Armamento-Villareal R, Parimi N, et al. Exercise training in obese older adults prevents increase in bone turnover and attenuates decrease in hip bone mineral density induced by weight loss despite decline in bone-active hormones. J Bone Miner Res. 2011;26:2851–2859. doi: 10.1002/jbmr.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Weiss EP, Racette SB, Villareal DT, et al. Lower extremity muscle size and strength and aerobic capacity decrease with caloric restriction but not with exercise-induced weight loss. J Appl Physiol. 2007;102:634–640. doi: 10.1152/japplphysiol.00853.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reid IR, Cornish J, Baldock PA. Nutrition-related peptides and bone homeostasis. J Bone Miner Res. 2006;21:495–500. doi: 10.1359/jbmr.051105. [DOI] [PubMed] [Google Scholar]
- 18.Shapses SA, Riedt CS. Bone, body weight, and weight reduction: what are the concerns? J Nutr. 2006;136:1453–1456. doi: 10.1093/jn/136.6.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jensen LB, Kollerup G, Quaade F, Sorensen OH. Bone minerals changes in obese women during a moderate weight loss with and without calcium supplementation. J Bone Miner Res. 2001;16:141–147. doi: 10.1359/jbmr.2001.16.1.141. [DOI] [PubMed] [Google Scholar]
- 20.Baron R, Rawadi G. Wnt signaling and the regulation of bone mass. Curr Osteoporos Rep. 2007;5:73–80. doi: 10.1007/s11914-007-0006-0. [DOI] [PubMed] [Google Scholar]
- 21.Turner CH, Warden SJ, Bellido T, et al. Mechanobiology of the skeleton. Sci Signal. 2009;2:pt3. doi: 10.1126/scisignal.268pt3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Elston MS, Clifton-Bligh RJ. Identification of Wnt family inhibitors: A pituitary tumor directed whole genome approach. Mol Cell Endocrinol. 2010;326:48–54. doi: 10.1016/j.mce.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 23.Gaudio A, Pennisi P, Bratengeier C, et al. Increased Sclerostin Serum Levels Associated with Bone Formation and Resorption Markers in Patients with Immobilization-Induced Bone Loss. J Clin Endocrinol Metab. 2010;95:2248–2253. doi: 10.1210/jc.2010-0067. [DOI] [PubMed] [Google Scholar]
- 24.Polyzos SA, Anastasilakis AD, Bratengeier C, Woloszczuk W, Papatheodorou A, Terpos E. Serum sclerostin levels positively correlate with lumbar spinal bone mineral density in postmenopausal women-the six-month effect of risedronate and teriparatide. Osteoporos Int. 2011 doi: 10.1007/s00198-010-1525-6. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 25.Chung YE, Lee SH, Lee SY, et al. Long-term treatment with raloxifene, but not bisphosphonates, reduces circulating sclerostin levels in postmenopausal women. Osteoporos Int. 2011 doi: 10.1007/s00198-011-1675-1. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Bergstrom I, Parini P, Gustafsson SA, Andersson G, Brinck J. Physical training increases osteoprotegerin in postmenopausal women. J Bone Miner Metab. 2011 doi: 10.1007/s00774-011-0304-6. [DOI] [PubMed] [Google Scholar]
- 27.Pritchard JE, Nowson CA, Wark JD. Bone loss accompanying diet-induced or exercise-induced weight loss: a randomised controlled study. Int J Obes Relat Metab Disord. 1996;20:513–520. [PubMed] [Google Scholar]
- 28.Jensen LB, Quaade F, Sorensen OH. Bone loss accompanying voluntary weight loss in obese humans. J Bone Miner Res. 1994;9:459–463. doi: 10.1002/jbmr.5650090404. [DOI] [PubMed] [Google Scholar]
- 29.Breban S, Chappard C, Jaffre C, Khacef F, Briot K, Benhamou CL. Positive influence of long-lasting and intensive weight-bearing physical activity on hip structure of young adults. J Clin Densitom. 2011;14:129–137. doi: 10.1016/j.jocd.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 30.Hind K, Gannon L, Whatley E, Cooke C, Truscott J. Bone cross-sectional geometry in male runners, gymnasts, swimmers and non-athletic controls: a hip-structural analysis study. Eur J Appl Physiol. 2011 doi: 10.1007/s00421-011-2008-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 31.Petit MA, Beck TJ, Lin HM, Bentley C, Legro RS, Lloyd T. Femoral bone structural geometry adapts to mechanical loading and is influenced by sex steroids: the Penn State Young Women’s Health Study. Bone. 2004;35:750–759. doi: 10.1016/j.bone.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Janz KF, Gilmore JM, Levy SM, Letuchy EM, Burns TL, Beck TJ. Physical activity and femoral neck bone strength during childhood: the Iowa Bone Development Study. Bone. 2007;41:216–222. doi: 10.1016/j.bone.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouxsein ML. Technology insight: noninvasive assessment of bone strength in osteoporosis. Nat Clin Pract Rheumatol. 2008;4:310–318. doi: 10.1038/ncprheum0798. [DOI] [PubMed] [Google Scholar]
- 34.Prevrhal S, Shepherd JA, Faulkner KG, Gaither KW, Black DM, Lang TF. Comparison of DXA hip structural analysis with volumetric QCT. J Clin Densitom. 2008;11:232–236. doi: 10.1016/j.jocd.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 35.Beck TJ. Extending DXA beyond bone mineral density: understanding hip structure analysis. Curr Osteoporos Rep. 2007;5:49–55. doi: 10.1007/s11914-007-0002-4. [DOI] [PubMed] [Google Scholar]
- 36.Leslie WD, Pahlavan PS, Tsang JF, Lix LM. Prediction of hip and other osteoporotic fractures from hip geometry in a large clinical cohort. Osteoporos Int. 2009;20:1767–1774. doi: 10.1007/s00198-009-0874-5. [DOI] [PubMed] [Google Scholar]
- 37.LaCroix AZ, Beck TJ, Cauley JA, et al. Hip structural geometry and incidence of hip fracture in postmenopausal women: what does it add to conventional bone mineral density? Osteoporos Int. 2010;21:919–929. doi: 10.1007/s00198-009-1056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaptoge S, Beck TJ, Reeve J, et al. Prediction of incident hip fracture risk by femur geometry variables measured by hip structural analysis in the study of osteoporotic fractures. J Bone Miner Res. 2008;23:1892–1904. doi: 10.1359/JBMR.080802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.