Abstract
We evaluated weight loss response to 16 months of supervised exercise (45 minutes/d, 5 d/wk, 75% heart-rate-reserve) in sedentary, overweight/obese participants without energy restriction in the Midwest Exercise Trial (MET1). Results indicated men lost weight, women did not. The gender differences were associated with differences in the energy expenditure of exercise (EEEx) (men = 667 ± 116; women = 439 ± 88 kcal/session) when exercise was prescribed by frequency, intensity and duration. MET2 is a randomized control trial designed and powered to examine differences in weight loss and gender in response to EEEx for men and women of 400 or 600 kcal/session, 5d/wk, for 10 months without energy restriction. One hundred forty one participants will be randomized to 1 of 2 exercise groups or a non-exercise control. EEEx will be verified by indirect calorimetry monthly during the intervention. This study will evaluate: (1) the weight change response to two levels of EEEx versus non-exercise control; (2) gender differences in weight response to two levels of EEEx; (3) potential compensatory changes in energy intake and/or daily physical activity that may explain the observed weight changes. Results from this study may impact how exercise is prescribed for weight loss and prevention of weight regain and may clarify if men and women differ in response to exercise.
Keywords: Supervised Exercise, Energy Expenditure, Gender, Weight loss, Energy Balance
1. Introduction
The prevalence of overweight (BMI > 25.0) and obesity (BMI > 30.0) among US adults is ~68% and 34%, respectively [1]. Overweight and obesity contribute to heart disease, hypertension, diabetes, and some cancers as well as psychosocial and economic difficulties [2–5]. The cost of treatment for weight reduction is now estimated to exceed 147 billion annually [6].
Exercise is recommended by virtually every public health organization for weight loss and prevention of weight regain [7–13]. However, the role of exercise is generally considered secondary to energy restriction [14–16]. Indeed, an argument can be made that exercise is ineffective for weight loss. Wing [17] reviewed the literature on the role of exercise on weight loss and concluded that exercise alone results in a minimal weight loss of 2 kg compared to control conditions. The American College of Sports Medicine Position Stand on “Appropriate Physical Activity Intervention Strategies for Weight loss and Prevention of Weight Regain for Adults,” suggested that 150–250 minutes/week of moderate intensity exercise does not result in clinically significant weight loss [12].
The literature on the effects of exercise for weight loss is influenced by the absence of studies that prescribe exercise with equivalent levels of exercise energy expenditure (EEEx) across individuals and genders, as well as the lack of verification that the exercise was competed at the prescribed level of EEEx. Verification of exercise completion is critical as self-reported exercise is frequently over-estimated [18]. Previously, Donnelly et al. [19] reported results from the Midwest Exercise Trial (MET1) where exercise was prescribed to previously sedentary overweight/obese men and women by frequency, intensity and duration for 16 months without energy restriction. EEEx was measured at three month intervals and was higher in men (667 ± 116 kcal/session) than women (439 ± 88 kcal/session), which is expected due to gender differences in body weight. The gender differences in EEEx (228 kcal/session) resulted in a mean weight loss for men of 5.2 ± 4.7 kg and a small weight gain for women of 0.6 ± 3.8 kg. The gender differences in EEEx diminished our ability to conclude that differences in the weight response to exercise were due to gender.
The Midwest Exercise Trial II (MET2) study was designed to evaluate gender differences in weight response to exercise prescribed at the same level of EEEx without diet restriction. Overweight and obese men and women will be randomly assigned to 10 months of supervised exercise 5 days/week with a verified EEEx of 400 kcal/session or 600 kcal/session (2,000 or 3,000 kcal/week) or a non-exercise control group. This study will evaluate: (1) the weight change response to two levels of EEEx versus non-exercise control; (2) gender differences in weight response to two levels of EEEx; (3) potential compensatory changes in energy intake and/or daily physical activity that may explain the observed weight changes.
2. Materials and methods
2.1. Eligibility/recruitment/randomization
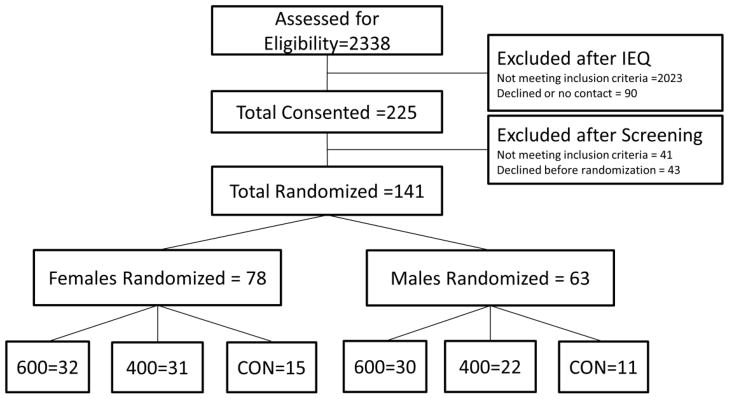
Participant recruitment and randomization for MET2 has been completed. Participants were recruited from Lawrence, KS and surrounding communities and will be compensated for their participation. Approval for this study was obtained from the Human Subjects Committee at the University of Kansas-Lawrence. The following inclusion/exclusion criteria were used: age = 18–30 yrs., BMI = 25.0 to 39.9 kg/m2, sedentary defined as < 500 kcal/week in planned exercise as assessed by questionnaire [20]. Participants with a history of chronic disease (i.e., diabetes, heart disease, etc.), elevated blood pressure (≥ 140/90), lipids (cholesterol > 6.72 mmol/L; triglycerides > 5.65 mmol/L), or fasting glucose (> 7.8 mmol/L) were excluded. Additionally, smokers, those taking medications that affect physical performance (i.e., beta blockers) or metabolism (i.e., thyroid, steroids), or those lacking the ability to perform laboratory tests or participate in moderate-to-vigorous intensity exercise were excluded. Participants were randomized at a 2:2:1 ratio (~65% to the exercise groups and ~35% to the control group), stratified by gender under the supervision of the project statistician. The blinding of participants to group assignment is not possible with an exercise intervention. Investigators will be blinded at the level of outcome assessments, data entry and data analysis. A consort diagram describing recruitment and randomization is presented in Figure 1.
Figure 1.
Recruitment and randomization diagram or participants randomized to 400 or 600 kcal/session and control groups.
One hundred forty one participants were recruited, consented, and randomized into the 400 or 600 kcal/session exercise groups or non-exercise control. This slightly exceeded our targeted enrollment of 136 participants needed to meet power requirements. The sample is comprised of 55.3% women and 14.8% minorities. Sample baseline demographics by gender and group are presented in Table 1.
Table 1.
Baseline characteristics for participants randomized to 400 or 600 kcal/session and control groups
| Female
|
Male
|
|||||
|---|---|---|---|---|---|---|
| Control (n=15) | 400 kcal (n=31) | 600 kcal (n=32) | Control (n=11) | 400 kcal (n=22) | 600 kcal (n=30) | |
| Age (yrs) | 21.7±2.8 | 23.3±3.1 | 22.1±3.1 | 23.2±3.1 | 23.1±3.4 | 22.8±3.1 |
| Weight (kg) | 78.7±13.3 | 84.5±19.1 | 82.9±12.8 | 98.8±12.5 | 97.9±17.8 | 104.7±14.9 |
| BMI (kg/m2) | 29.5±3.8 | 29.1±5.5 | 29.5±3.8 | 31.1±4.0 | 31.6±5.2 | 32.9±4.1 |
| Fat (%) | 44.6±5.1 | 44.4±5.5 | 44.5±5.2 | 37.0±4.1 | 34.5±6.5 | 37.0±6.1 |
| VO2 (ml/kg·min−1) | 29.9±3.7 | 29.9±3.8 | 30.0±4.1 | 34.1±5.2 | 37.8±6.1 | 35.7±6.2 |
Note. Values are means ± standard deviation
2.2. Exercise groups
Exercise will consist primarily of walking/jogging on motor-driven treadmills; however, alternate activities such as stationary biking, walking/jogging outside, or walking on stationary elliptical trainers will be allowed for 20% of the total exercise sessions (1 session/week). Exercise prescriptions will progress from 150 kcal/session at intervention onset to reach the target EEEx (400 or 600 kcal/session) at the end of month 4 and remain at target over the final six months of the study (Table 2). These levels for EEEx were selected based on recommendations from The American College of Sports Medicine Position Stand “Appropriate Physical Activity Intervention Strategies for Weight Loss and Prevention of Weight Regain for Adults,” [21]. These levels of EEEx are also consistent with the recommendations for weight loss provided by Health and Human Services “2008 Physical Activity Guidelines for Americans” [22] and were associated with no weight change (~400 kcal/session) or clinically significant weight loss (>600 kcal/session) in MET1.
Table 2.
Ten month exercise progression by kcal/session and intensity.
| Month | 400 kcal/session | 600 kcal/session | Heart rate (% max) days/week* |
|---|---|---|---|
| 1 | 150–175 | 150–250 | 70% (5) |
| 3 | 200–225 | 275–375 | 70% (4) / 80% (1) |
| 3 | 250–325 | 400–500 | 70% (3) / 80% (2) |
| 4 | 350–400 | 525–600 | 70% (0–2) / 80% (3–5) |
| 5–10 | 400 | 600 | 70% (0–1) 80% (4–5) |
Days per week exercise sessions were completed at either 70 or 80% max HR. For example, in week three, 4 exercise sessions were completed at 70% and 1 session at 80% max HR
2.3. Energy expenditure of exercise
Changes in both body weight and aerobic fitness influence EEEx when performed at the same intensity. Therefore, EEEx will be assessed at baseline and monthly during the intervention to determine the duration of treadmill exercise required to achieve the EEEx goals (Table 2). EEEx will be assessed by indirect calorimetry (ParvoMedics TrueOne 2400 System, ParvoMedics Inc, Sandy, UT) at one minute intervals. The Weir equation [23] will be used to calculate EEEx from measured oxygen consumption and carbon dioxide production. Prior to each EEEx assessment participants will perform a brief warm-up (~ 2 minute, 3–4 mph, 0% grade). At the baseline assessment, treadmill speed/grade will begin at 3 mph/0% grade and will be adjusted by increments of 0.5 mph/1% grade until the participant reaches 70% HR max ± 4 beats/minute. At the end of months 1 to 3, EEEx will be calculated at both 70% and 80% of heart rate (HR) maximum to accommodate personal preferences for walking or running. EEEx will be assessed at both 70% (15 minutes) and 80% HR max (15 minutes) with a 2 minute interval between assessments to allow participants to remove the mouthpiece and obtain water if desired. Either speed or grade will be adjusted depending on participant preference. The average EEEx over a 15 minute interval (kcal/minute) will be used determine the duration of exercise sessions performed during the first month. For example: EEEx = 9.2 kcal/minute, prescribed exercise = 400 kcal/session, exercise duration = 400/9.2 = 44 minutes/session. All subsequent EEEx assessments will begin at the participant’s current treadmill speed and grade with adjustments made as described for the baseline assessment. At the end of months 4–10 EEEx will also be assessed in 15 minute blocks; however, the number of blocks will be selected (maximum 3 blocks) to approximate the current duration of exercise training sessions (nearest 15 minutes). This procedure was designed to adjust EEEx during exercise training sessions over the course of the 10 month trial.
2.4. Exercise compliance
MET2 is an efficacy study designed to evaluate the effects of a verified dose of exercise (400 and 600 kcal/session) on weight change; therefore, compliance with the exercise protocol is critical. We will define compliance as successfully completing > 90% of scheduled exercise sessions. Successful completion of an exercise session will be defined as maintaining target HR ± 4 beats/minute for the prescribed exercise duration. Participants falling below this requirement during any 3 month interval (months 0–3, 3–6, 6–9) or during the final month of the study (month 10) will be non-compliant with the exercise protocol and will be dismissed from the study. The compliance requirement during month 10 will assure that participants complete a minimum of 90% of scheduled exercise sessions during the assessment of energy balance (energy intake and energy expenditure).
2.5. Exercise supervision
Research assistants will be responsible for documenting compliance to the prescribed exercise protocol. The duration and intensity of each exercise session will be verified by a downloadable heart rate monitor (RS 400; Polar Electro Inc., Woodbury, NY) set to collect HR in 1-minute epochs. After completing a short warm-up on the treadmill the speed and grade will be increased to achieve the desired target HR. Additionally, the level of perceived exertion, treadmill speed and grade and HR will be recorded by the research assistant at 10 minute intervals during each exercise session. This procedure assures interaction between study staff and participants which may help to maintain compliance, as well as providing a detailed description of each exercise session.
2.6. Control group
Participants assigned to the non-exercise control group will be instructed to continue their typical patterns for physical activity and dietary intake over the duration of the 10 month study. With the exception of assessment of EEEx, the same outcome assessments will be completed with the control and exercise groups.
2.7. Outcome Assessments
All outcome assessments will be completed in the Energy Balance Laboratory at The University of Kansas. The schedule for outcome assessments is presented in Table 3
Table 3.
Assessment Timeline.
| Month
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
| Anthropometric | |||||||||||
| Body weight | x | x | x | x | x | x | x | x | x | x | x |
| Body composition (DXA) | x | x | x | ||||||||
| Waist Circumference | x | x | x | ||||||||
| Energy Intake | |||||||||||
| Picture-plate waste | x | x | x | x | |||||||
| 3-day diet record | x | x | x | x | |||||||
| Energy Expenditure | |||||||||||
| Resting metabolic rate | x | x | |||||||||
| Exercise session | x | x | x | x | x | x | x | x | x | x | x |
| Doubly labeled water | x | x | |||||||||
| Fitness/Physical Activity | |||||||||||
| Maximal Aerobic capacity | x | x | x | ||||||||
| Accelerometry | x | x | x | x | |||||||
| Blood Chemistry/Pressure | |||||||||||
| Blood sample | x | x | x | ||||||||
| Blood pressure | x | x | |||||||||
Note. DXA = dual-energy X-ray absorptiometry
2.7.1. Anthropometrics (Weight/height/body composition/ waist circumference)
Body weight will be measured between the hours of 7 a.m. and 9 a.m. using a digital scale accurate to ± 0.1 kg. Participants will be weighed prior to breakfast and after attempting to void and will wear a standardized hospital gown at the time of weighing. Dual energy x-ray absorptiometry (DXA) will be used to determine fat-free mass, fat mass, and percent body fat (Lunar DPX-IQ). Women will undergo pregnancy testing prior to each DXA test. Waist circumference will be assessed using the procedures described by Lohman et al. [24].
2.7.2. Resting metabolic rate (RMR)
RMR will be determined by indirect calorimetry (ParvoMedics TrueOne 2400 System, ParvoMedics Inc., Sandy, UT) between 6 a.m. and 10 a.m. after a 12 hour fast and 48 hour abstention from exercise [25]. Following 15 minutes of supine rest in an isolated temperature controlled (21–24°C) room; participants will be placed under a ventilated hood for a minimum of 35 minutes. Criteria for a valid RMR assessment will be a minimum of 30 minutes of steady state defined as less than 10% fluctuation in minute ventilation, oxygen consumption and respiratory quotient. RMR (kcal/day) will be calculated using the Weir equations [23].
2.7.3. Aerobic capacity
Maximal aerobic capacity will be assessed on a motor-driven treadmill using a modified Balke protocol [26]. Expired gases will be collected in 20-second epochs (Parvo Medics TrueOne 2400) and HR will be monitored continually using a three lead electrocardiogram (Marquette Electronics, Milwaukee, WI, USA). Maximal aerobic capacity tests will be valid if participants meet three of the following four criteria: (1) heart rate ± 10 beats min-1 of the age-predicted maximal HR (220 – age), (2) rating of perceived exertion greater than 17, (3) respiratory exchange ratio greater than 1.10, and (4) oxygen consumption plateau (i.e., no increase in oxygen consumption with increased workload.
2.7.4. Energy intake/macronutrient composition
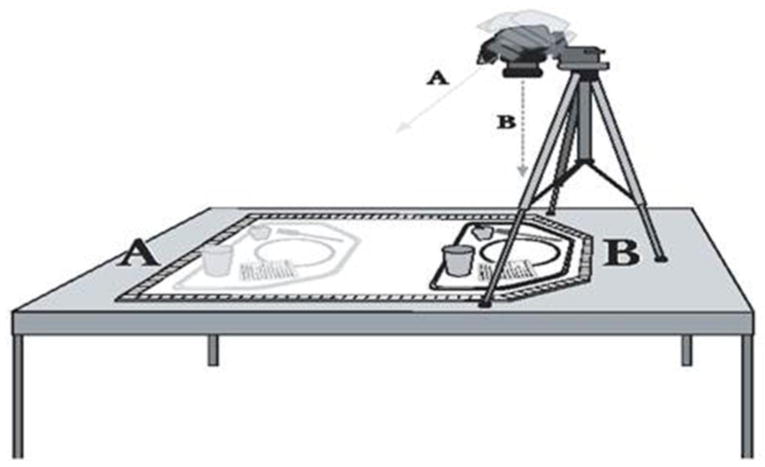
Energy intake and macronutrient composition will be assessed over a 1 week period 4 times over the course of the study during ad libitum eating in a University of Kansas cafeteria. Digital photographs will be obtained before and after consumption (Figure 2). The type and amount of foods and beverages consumed will be quantified by research staff that has been trained in this technique. Food consumed outside the cafeteria (i.e., snacks) will be assessed using multiple pass 24-hour recall procedures using food models and standardized, neutral probing questions [27]. We have previously demonstrated our ability to measure energy intake using picture-plate-waste combined with 24-hour recalls within 3% of that measured by traditional weigh and measure [28] and have demonstrated that 7 days of dietary data adequately characterize usual energy and macronutrient intake [29]. Results from the digital photographs will be entered into the current version of Nutrition Data System for Research (University of Minnesota, Minneapolis, MN) for determination of total energy intake, and energy intake from fat, protein and carbohydrate.
Figure 2.
Picture-plate waste illustration. Pictures are taken before and after meal consumption from two angles (A, B) to aide in portion measurement by providing an overhead view and a view at ~45 degrees for depth perception
In addition to outcome measures of diet and macronutrient content in the cafeteria, we will use 3-day diet records (2 weekdays, 1 weekend day) at 2, 5, 8, and 9 mos. These records will be used during the 10 month intervention to provide diet counseling should participants change from their normal diet to a diet consistent with volitional weight loss (i.e. 500 to 1,000 kcal below estimated weight maintenance values). Participants will be asked to complete food records and bring them to a designated exercise session where they will be reviewed by a registered dietitian and clarification will be obtained if necessary. To facilitate recording of diet intake participants will receive verbal instructions from a registered dietitian as well as a pamphlet titled “How to record your food record.” Food record data will be entered into the current version of Nutrient Data System for Research (University of Minnesota, Minneapolis, MN) for energy and macronutrient analysis.
2.7.5. Energy expenditure by doubly labeled water (DLW)
DLW will be used to assess average total daily energy expenditure (TDEE) over a 14 day period at baseline and 10 months using the procedures described by Coward et al [30]. Participants will report to the Energy Balance Laboratory between 8 a.m. and 9 a.m. after an over-night fast of at least 12 hours. A baseline urine sample will be collected prior to oral dosing with a mixed solution of approximately 0.10g/kg body weight of 2H2O and 0.15g/kg of H218O followed by a rinse solution of 100ml of tap water. A weighed 1:400 dilution of each dose and a sample of the tap water will be stored at −70°C for later analysis. Additional urine samples, collected at least 3 hours apart, will be collected on days 1 and 14. All urine samples will be stored in sealed containers at −70°C for later analysis. Samples will be analyzed in duplicate for 2H2O and H218O by isotope ratio mass spectrometry as previously described by Herd et al. [31]. TDEE will be estimated using the equation of Elia [32]:
Physical activity energy expenditure (PAEE) will be estimated from TDEE and RMR [33]:
This approach assumes that the thermic effect of food represents 10% of TDEE [34]. Energy expenditure from physical activity outside the exercise program will be calculated as (PAEE- EEEx).
2.7.6. Physical activity (PA)
An ActiGraph Model GT1M (Actigraph, LLC, Pensacola, FL) portable accelerometer will be used to determine day-to-day and within day variation in PA (i.e., compare exercise to non-exercise days, exercise time vs. non-exercise time), as well as to estimate the time spent in a range of PA intensity levels (moderate, vigorous). Participants will wear the ActiGraph on a belt over the non-dominant hip for 7 consecutive days in conjunction picture-plate waste assessments at baseline, 3, 6, and 10 months. The data collection interval will be set at one min with a minimum of 12 hours constituting a valid monitored day. We will apply the intensity cut-points used in the National Health and Nutrition Examination Survey as described by Troiano et al [35]; moderate (> 3 METS: >2020 to 5999 counts/minute), vigorous (> 6 METS: >5999 counts/minute). ActiGraph analysis will be completed with a custom SAS program.
2.7.7 Blood Pressure and Blood Chemistry
Blood pressure will be obtained with a Dinamap automated sphygmomanometer (Pro Care 100, GE HealthCare, Madison, WI) between 8 a.m. and 10 a.m., subsequent to the measurement of height, weight, and waist circumference. The participant will be seated with the arm bared, supported, and positioned at the heart level. Measurement will begin after 5 minutes of quiet rest. The appropriate size cuff will be used such that the rubber bladder encircles at least 2/3 of the arm. Two measures will be averaged and additional measures will be obtained if those measures differ by more than 5 mmHG [36, 37].
Fasting blood samples (12 hour overnight) will be obtained by a certified phlebotomist subsequent to the measurement of blood pressure, for the assessment of blood lipids, glucose, and insulin. Following collection, blood samples will be separated by centrifugation for 15 min at 2000g. The separated plasma will be transferred to cryogenic vials and stored at −70° C for later analysis. Total serum cholesterol and triglyceride concentrations will be measured using an automated analyzer (Du Pont Co), using standard enzymatic techniques. HDL will be measured after removal of VLDL and LDL by precipitation with phosphotungstate [38]. Glucose will be measured using an autoanalyzer (Beckman) and insulin will be measured using a double-label antibody technique [39].
2.8. Sample size and analysis plan
2.8.1. Sample size
This study will evaluate: (1) the weight change response to two levels of EEEx versus non-exercise control; (2) gender differences in weight response to two levels of EEEx; (3) potential compensatory changes in energy intake and/or daily physical activity that may explain the observed weight changes. Sample size was determined to provide adequate statistical power for the analysis of aims 1 and 2. Aim 1 will compare weight change (10 months- baseline) across the three groups. From our previous work (DK49181) we expect participants randomized to the control arm to gain weight (~ 5%), the 400 kcal/session group to remain weight stable, and participants in the 600 kcal/session group to lose weight (~5%). In our previous studies the 5% gain and 5% loss were equivalent to an average change of approximately 0.5 standard deviations. Given these assumptions, and a conservative rate of attrition of 33%, 136 participants will be randomized to the 400 kcal/session and 600 kcal/session groups and a control group in a 2:2:1 ratio to insure a total sample of 90. A sample of 90 completers will provide 88% power to detect the hypothesized difference across the groups using a one-way analysis of variance with a type I error rate of 5%. Aim 2 will determine if the change in weight (10 months- baseline) is equivalent between males and females for both the 400 and 600 kcal/session groups, respectively. Equivalence will be defined as a ratio of the average weight change in males versus females between 0.85 and 1.15. Our previous data indicates that the coefficient of variation (standard deviation/mean) is 0.10 for change in weight at 10 months. Given these assumptions, 18 males and 18 females will allow us to determine if the ratio of the means is equivalent with 95% power assuming a type I error rate of 0.025. Each statistical test will be conducted at two levels of exercise, therefore we will use a type I error rate of 0.025 for each test.
2.8.2. Analysis plan-Aim 1: Compare weight change across the 3 randomized groups
Weight change (10 months-baseline) for each group will be described using means and standard deviations and compared using a one-way analysis of variance. Body weight assessments will be completed monthly. Therefore, we will perform an exploratory analysis using mixed linear models to longitudinally compare weights among the three groups both unadjusted and adjusted for baseline measures and demographic characteristics. Similar analyses will be performed on other body composition measures including fat-free mass, fat mass, percent body fat, and BMI as well as maximal aerobic capacity.
2.8.3 Analysis plan-Aim 2: Evaluate gender differences in the weight response to 2 levels of EEEx
The equivalence for weight change (10 months-baseline) between males and females for both the 400 kcal/session and 600 kcal/session groups will be tested using the method described in Hauschke et al [40]. The hypotheses to be tested will be:
Weight change between males and females is considered equivalent if H0 is rejected. Similar analysis will be performed on fat mass, fat-free mass, percent body fat, and BMI. If equivalence is not demonstrated, we will examine the effect of gender on change in weight in conjunction with treatment using a two-factor ANOVA with interaction.
2.8.4. Analysis plan-Aim 3: Potential compensatory changes in energy intake and total daily energy expenditure
We will examine the effects of changes in energy intake, physical activity, and TDEE as well as treatment on weight over time for participants randomized to the 400 kcal/session and 600 kcal/session groups. Mixed linear models will be utilized to examine if the energy intake, physical activity, and TDEE explain variance in weight change above that explained by exercise group. Both weight loss and weight gain might be a function of changes in energy intake, physical activity and/or TDEE or a combination of the three.
We are conducting an efficacy study, thus our primary analyses will be conducted only on participants who comply with the study protocol and complete all outcome assessments for the analysis of our primary and secondary outcomes (Aims 1 and 2).
2.9. Data management
Data will be categorized and entered in separate tables in a relational Access data base. For example, body weight/composition will be in one table, energy intake in another table, with all tables linked by participant ID number. At logical time points, data will be checked for outliers and normalcy. Questionable data (i.e., > 3 standard deviations from the mean) will be checked and re-entered if necessary. All data will be archived daily on a secure server.
3.0. Discussion
MET2 is designed and adequately powered to evaluate the level of EEEx associated with weight loss and potential gender differences in the weight change response to exercise in previously sedentary overweight men and women. Unique aspects of this study include: long duration exercise training program; supervised exercise with strict compliance requirements (90% of scheduled sessions); precise control of the level of EEEx (400 and 600 kcal/session) achieved by the assessment of EEEx by indirect calorimetry at regular intervals over the course of the intervention; assessments of energy intake and energy expenditure using state-of-the-art techniques to evaluate the potential for compensatory changes in these variables which may impact both the weight loss response to exercise in general, or differentially by gender. The results of MET2 will provide a more complete understanding of the weight change response to exercise in both men and women and will provide preliminary information regarding compensatory changes in energy intake and daily energy expenditure that may affect this response. This information will be important for the design of more effective and perhaps targeted strategies for the use of exercise for weight management in men and women.
Acknowledgments
Funding: The National Institutes of Health (DK49181).
Abbreviations
- BMI
Body Mass Index
- DLW
Doubly-labeled water
- DXA
dual-energy X-ray absorptiometry
- EEEx
Energy Expenditure of Exercise
- EI
Energy Intake
- HR
Heart Rate
- kcal
kilocalories
- Kg
kilograms
- MET1
Midwest Exercise Trial 1
- MET2
Midwest Exercise Trial 2
- MJ
Megajoules
- PAEE
Physical Activity Energy Expenditure
- TDEE
Total Daily Energy Expenditure
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Flegal KM, Carroll MD, Ogden CL, Curtin LR. Prevalence and trends in obesity among US adults, 1999–2008. JAMA. 2010;303:235–41. doi: 10.1001/jama.2009.2014. [DOI] [PubMed] [Google Scholar]
- 2.U.S. Department of Health and Human Services P. DHHS Publication No. (PHS) 91–50212. Washington, D.C: U.S. Government Printing Office, Public Health Service; 1990. Healthy People 2000: National health promotion and disease prevention objectives. [Google Scholar]
- 3.Gortmaker SL, Must A, Perrin JM, Sobol AM, Dietz WH. Social an economic consequences of overweight in adolescence and young adulthood. N Engl J Med. 1993;329(14):1008–12. doi: 10.1056/NEJM199309303291406. [DOI] [PubMed] [Google Scholar]
- 4.Mokdad AH, Ford ES, Bowman BA, Dietz WH, Vinicor F, Bales VS, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 5.Must A, Spadano J, Coakley EH, Field AE, Colditz G, Dietz WH. The disease burden associated with overweight and obesity. JAMA. 1999;282:1523–9. doi: 10.1001/jama.282.16.1523. [DOI] [PubMed] [Google Scholar]
- 6.Finkelstein EA, Trogdon JG, Cohen JW, Dietz W. Annual Medical Spending Attributable To Obesity: Payer-And Service-Specific Estimates. Health Affairs. 2009;28:w822–w31. doi: 10.1377/hlthaff.28.5.w822. [DOI] [PubMed] [Google Scholar]
- 7.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health. A recommendation from the Centers for Disease Control and Prevention and the American College of Sports Medicine. JAMA. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 8.Shephard RJ, Lankenau B, Pratt M, Neiman A, Puska P, Benaziza H, et al. Physical Activity Policy Development: a synopsis of the WHO/CDC Consultation, September 29 through October 2, 2002, Atlanta, Georgia. Public Health Rep. 2004;119:346–51. doi: 10.1016/j.phr.2004.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Centers for Disease Control. Healthy people 2000 review 1997. Healthy People 2000. 1997:1–223. [Google Scholar]
- 10.U.S. Department of health and human services. Physical activity and health: a report of the surgeon general. USDepartment of Health and Human Services; 1996. [Google Scholar]
- 11.U.S. Department of Health and Human Services Public Health Service. Healthy People 2010. Washington D.C: 2000. Conference ed. [Google Scholar]
- 12.Donnelly JE, Blair SN, Jakicic JM, Manore MM, Rankin JW, Smith BK. American College of Sports Medicine Position Stand. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Med Sci Sports Exerc. 2009;41:459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 13.Seidler RD, Stelmach GE. Reduction in sensorimotor control with age. Quest. 1995;47:386–94. [Google Scholar]
- 14.Katzel LI, Bleecker ER, Colman EG, Rogus EM, Sorkin JD, Goldberg AP. Effects of weight loss vs aerobic exercise training on risk factors for coronary disease in healthy, obese, middle-aged and older men. A randomized controlled trial. JAMA. 1995;274:1915–21. doi: 10.1001/jama.1995.03530240025035. [DOI] [PubMed] [Google Scholar]
- 15.Jakicic JM. Exercise in the treatment of obesity. Endocrinol Metab Clin North Am. 2003;32:967–80. doi: 10.1016/s0889-8529(03)00075-6. [DOI] [PubMed] [Google Scholar]
- 16.Janssen I, Katzmarzyk PT, Ross R. Body mass index, waist circumference, and health risk: evidence in support of current National Institutes of Health guidelines. Arch Intern Med. 2002;162:2074–9. doi: 10.1001/archinte.162.18.2074. [DOI] [PubMed] [Google Scholar]
- 17.Wing RR. Physical activity in the treatment of the adulthood overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. 1999;31:S547–52. doi: 10.1097/00005768-199911001-00010. [DOI] [PubMed] [Google Scholar]
- 18.Jakicic JM, Polley BA, Wing RR. Accuracy of self-reported exercise and the relationship with weight loss in overweight women. Med Sci Sports Exerc. 1998;30(4):634–8. doi: 10.1097/00005768-199804000-00024. [DOI] [PubMed] [Google Scholar]
- 19.Donnelly JE, Kirk EP, Jacobsen DJ, Hill JO, Sullivan DK, Johnson SL. Effects of 16-months of verified, supervised aerobic exercise on macronutrient intake in overweight men and women: The midwest exercise trial (MET) American Journal of Clinical Nutrition. 2003;78(5):950–6. doi: 10.1093/ajcn/78.5.950. [DOI] [PubMed] [Google Scholar]
- 20.Taylor H, Jacobs D, Schucker B, Knudsen J, Leon A, Debacker G. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis. 1978;31:741–55. doi: 10.1016/0021-9681(78)90058-9. [DOI] [PubMed] [Google Scholar]
- 21.Donnelly JE. Appropriate physical activity intervention strategies for weight loss and prevention of weight regain for adults. Medicine & Science in Sports & Exercise. 2009;41(2):459–71. doi: 10.1249/MSS.0b013e3181949333. [DOI] [PubMed] [Google Scholar]
- 22.Office of Disease Prevention & Health Promotion. 2008 Physical Activity Guidelines for Americans. US Department of Health and Human Services; 2008. http://wwwhealthgov/paguidelines. [Google Scholar]
- 23.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, Ill: Human Kinetics Books; 1988. [Google Scholar]
- 25.Melanson EL, Sharp TA, Seagle HM, Donahoo WT, Grunwald GK, Peters JC, et al. Resistance and aerobic exercise have similar effects on 24-h nutrient oxidation. Medicine & Science in Sports & Exercise. 2002;34:1793–800. doi: 10.1097/00005768-200211000-00016. [DOI] [PubMed] [Google Scholar]
- 26.American College of Sports Medicine Guidelines for exercise testing and prescription. 6. Philadelphia: Lippincott Williams & Wilkins; 2000. [Google Scholar]
- 27.Gibson RS. Principles of nutrition assessment. Oxford: Oxford University Press; 1990. [Google Scholar]
- 28.Hise ME, Sullivan DK, Jacobsen DJ, Johnson SL, Donnelly JE. Validation of energy intake measurements determined from observer-recorded food records and recall methods compared with the doubly labeled water method in overweight and obese individuals. Am J Clin Nutr. 2002;75:263–7. doi: 10.1093/ajcn/75.2.263. [DOI] [PubMed] [Google Scholar]
- 29.Grunwald GK, Sullivan DK, Hise M, Donnelly JE, Jacobsen DJ, Johnson SL, et al. Number of days, number of subjects, and sources of variation in longitudinal intervention or crossover feeding trials with multiple days of measurement. Br J Nutr. 2003;90:1087–95. doi: 10.1079/bjn2003989. [DOI] [PubMed] [Google Scholar]
- 30.Coward WA. Stable isotopic methods for measuring energy expenditure. The doubly-labelled-water (2H2(18)O) method: principles and practice. Proc Nutr Soc. 1988;47:209–18. doi: 10.1079/pns19880037. [DOI] [PubMed] [Google Scholar]
- 31.Herd S, Vaughn W, Goran M. Comparison of zinc reduction with platinum reduction for analysis of deuterium-enriched water samples for the doubly-labeled water technique. Obes Res. 1999;8:302–8. doi: 10.1038/oby.2000.36. [DOI] [PubMed] [Google Scholar]
- 32.Elia M. Converting carbon dioxide production to energy expenditure. In: Prentice AM, editor. The doubly-labeled water method for measuring energy expenditure Technical recommendations for use in humans A consensus report by the IDECG working group. Vienna: NAHRES-4; 1990. pp. 193–211. [Google Scholar]
- 33.Hunter GR, Wetzstein CJ, Fields DA, Brown A, Bamman MM. Resistance training increases total energy expenditure and free-living physical activity in older adults. J Appl Physiol. 2000;89:977–84. doi: 10.1152/jappl.2000.89.3.977. [DOI] [PubMed] [Google Scholar]
- 34.Weststrate JA, van het Hof KH, van den Berg H, Velthuis-te-Wierik EJ, de Graaf C, Zimmermanns NJ, et al. A comparison of the effect of free access to reduced fat products or their full fat equivalents on food intake, body weight, blood lipids and fat-soluble antioxidants levels and haemostasis variables. Eur J Clin Nutr. 1998;52:389–95. doi: 10.1038/sj.ejcn.1600570. [DOI] [PubMed] [Google Scholar]
- 35.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–8. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 36.U.S. Department of Health and Human Services P. NIH Publication No 88–1088. Washington, D.C: U.S. Government; 1988. The 1988 report of the Joint National Committee on detection, evaluation and treatment of high blood pressure. [Google Scholar]
- 37.Luepker RV, Jacobs DR, Prineas RJ, Sinaiko AR. Secular trends of blood pressure and body size in a multi-ethnic adolescent population: 1986 to 1996. J Pediatrics. 1999;134:668–74. doi: 10.1016/s0022-3476(99)70279-9. [DOI] [PubMed] [Google Scholar]
- 38.Burnstein M, Scholnick HR, Morfin R. Rapid method for the isolation of lipoproteins from human serum by precipitation with polyanions. J Lipid Res. 1970;11:583–95. [PubMed] [Google Scholar]
- 39.Morgan CR, Larner J. Immunoassay of insulin: two antibody system: plasma insulin levels of normal, subdiabetic rats. Diabetes. 1963;12:115–26. [Google Scholar]
- 40.Hauschke D, Kieser M, Diletti E, Burke M. Sample size determination for proving equivalence based on the ratio of two means for normally distributed data. Stat Med. 1999;18:93–105. doi: 10.1002/(sici)1097-0258(19990115)18:1<93::aid-sim992>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]