Abstract
The synthesis and evaluation of several benzothiazole based compounds are described in an attempt to identify novel dual-acting 5HT1A receptor and SERT inhibitors as new antidepressants. Binding affinities at the 5HT1A receptor and the serotonin transporter do not appear to be congruent and other areas of the binding sites would need to be explored in order to improve binding simultaneously at both sites. Compounds 20 and 23 show moderate binding affinity at the 5HT1A receptor and the SERT site and thus, have the potential to be further explored as dual-acting agents. In addition, compound 20 binds with low affinity to the dopamine transporter (DAT), the norepinephrine transporter (NET) and 5HT2C receptor, which are desirable properties as selectivity for SERT (and not DAT or NET) is associated with an absence of cardiovascular side-effects.
Keywords: Benzothiazoles, SERT inhibition, 5HT1A binding, dual-acting antidepressants, 5HT1A agents, potential SERT inhibitors
1. Introduction
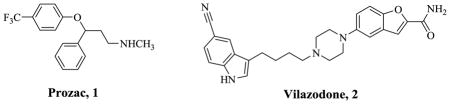
According to the National Institute of Mental Health (NIMH), depressive disorders affect approximately 19 million American adults or 9.5% of the U.S. population age 18 and older in a given year. This includes major depressive disorder, dysthymic disorder, and bipolar disorder [1]. The most widespread treatment option for depression is the use of antidepressants. Selective serotonin reuptake inhibitors (SSRIs) such as Prozac (1), have changed the landscape of antidepressant therapy for some time now and have several advantages over their predecessors, the tricyclic antidepressants (TCAs). The superior clinical profile of SSRIs is said to be related to their overall selectivity resulting in the absence of cardiovascular disease (<0.0003%) and a high therapeutic index [2]. However, antidepressants in general, including SSRIs suffer from a variety of drawbacks including the fact that up to a third of patients do not respond to treatment. There is also a delay of about 4 – 6 weeks in the onset of action of SSRIs. One hypothesis suggests that the delay in the onset of action is due to a negative feedback control exerted by 5HT1A autoreceptors on nerve terminal 5HT release [3]. According to this hypothesis, onset of action is initiated only when this impulse flow is restored following desensitization of 5HT1A autoreceptors and coincident increases in postsynaptic 5HT levels are achieved. Clinical proof of this hypothesis has been suggested in studies that found a significant augmentation of the effect of SSRIs when the β-adrenergic/5HT1A receptor antagonist pindolol was co-administered with SSRI treatment [4]. Indeed, the FDA has recently approved the first drug developed on the basis of this hypothesis, vilazodone (Viibryd®), for the treatment of depression [5]. Vilazodone has been demonstrated to act as an inhibitor of SERT and a partial agonist at the 5HT1A receptor, binding with very high affinities at these sites [6] (Table 1). Its binding affinities at other receptors such as DAD2 and 5HT2A receptors, DAT and NET are said to be low and this selectivity appears to support the basis for its superior therapeutic profile [7–9].
Table 1.
Binding affinity constants of benzothiazoles at selected CNS receptors.
| Compd # | Binding data; Ki ± SEM (nM)c |
||||||
|---|---|---|---|---|---|---|---|
| 5HT1A | SERT | 5HT2A | 5HT2C | 5HT7 | DAD2 | DAD4 | |
| Prozac, 1a | ND | 1.1 ± 0.01 | ND | 72 ± 1 | ND | ND | ND |
| Vilazodone, 2b | 0.3±0.06 | 0.5±0.4 | ND | ND | ND | 666±75 | ND |
| 3 | 216±30 | 34.0±3.9 | 696±51 | MP | 2060±353 | MP | 431±41 |
| 4 | 263 | 65.0 | 159 | 1420 | 413±61 | 259±48 | 28.6±3.4 |
| 5 | MP | 5.3 | 1038 | MP | 356 | MP | 126 |
| 6 | 495±91 | 65.0±6.0 | 1003±96 | 5920±769 | 2810±658 | 1983±163 | 1215±67 |
As part of our on-going drug discovery effort to identify new leads for the treatment of mental illnesses, we evaluated several benzothiazoles at DA and 5HT receptors [10] (Table 1). Further screening at other CNS receptors has led to the identification of compounds 3 and 6 as SERT inhibitors as well as 5HT1A receptor ligands (Chart 1). Based on the pharmacological properties of various CNS receptors, we have hypothesized that an agent which antagonizes the 5HT1A receptor, inhibits SERT and does not interact avidly with DAT, NET, DA D2-like subtypes, 5HT2C and H1 receptors will have a potentially superior therapeutic profile as novel antidepressants [11–17]. Thus, the aim of this research was to study the structure-activity relationships of newly designed benzothiazoles in order to understand the contributions of the component parts towards selectivity for the 5HT1A receptor and inhibition of SERT.
Chart 1.
2. Chemistry
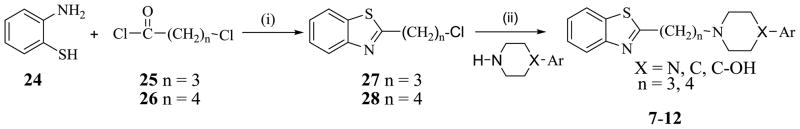
The syntheses of compounds 3 – 6 were previously reported [10]. To obtain key alkylating agents 27, 28, 34 and 35, the method of Chikashita et al [18] as reported in Peprah, et al [10] was followed, taking advantage of the reactivity of 2-lithiobenzothiazole to various electrophiles. Alkylating agent 2-(3-chloropropyl)benzo[d]thiazole 27 was prepared by reacting 2-aminothiophenol 24 and 4-chlorobutanoyl chloride 25 in toluene, followed by purification on silica gel. Alkylating agent 2-(4-chlorobutyl)benzo[d]thiazole 28 was obtained in a similar manner using 5-chloropentanoyl chloride as described in Scheme 1. Target compounds 7 – 12, were obtained by coupling each alkylating agent 27 and 28 with different amines in the presence of K2CO3 and KI (Scheme 2).
Scheme 1.
Synthesis of Target Compounds 7–12. Reagents and conditions: (i) Toluene, rt; (ii) KI, K2CO3, CH3CN, reflux.
Scheme 2.
Synthesis of Target Compounds. Reagents and conditions: (i) KI, K2CO3, CH3CN, reflux.
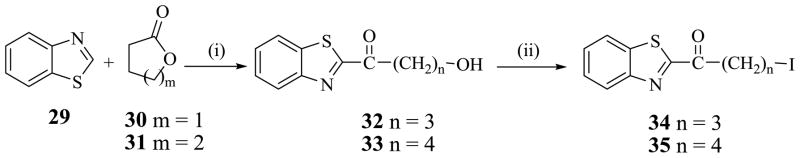
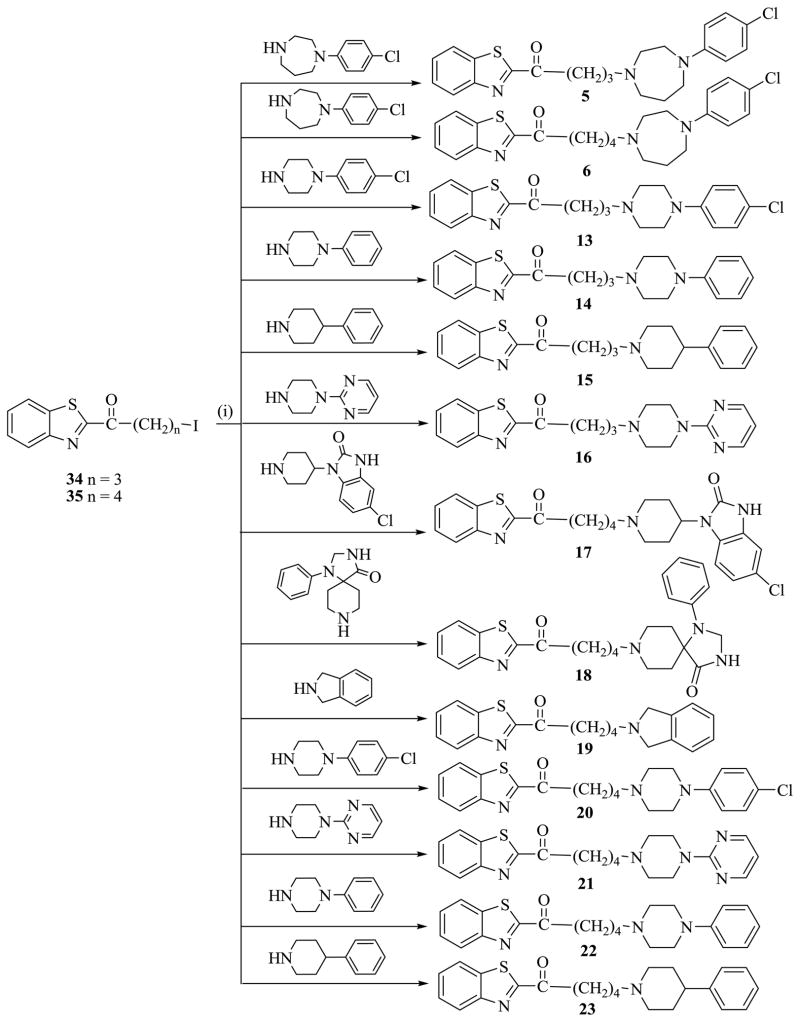
Alkylating agent, 1-(benzo[d]thiazol-2-yl)-4-iodobutan-1-one 34 was prepared by a two-step transformation starting from benzothiazole 29. Deprotonation of 29 with n-BuLi in THF at −78 °C was followed by treatment with lactone 30 to obtain 1-(benzo[d]thiazol-2-yl)-4-hydroxybutan-1-one 32. Treatment of alcohol 32 with I2 in the presence of imidazole and Ph3P in CH2Cl2 afforded 1-(benzo[d]thiazol-2-yl)-4-iodobutan-1-one 34. Using the same procedure, alkylating agent, 1-(benzo[d]thiazol-2-yl)-5-iodopentan-1-one 35 was synthesized, as described in Scheme 3. Compounds 13–23 were synthesized by reacting each alkylating agent, 34 and 35 with different amines in the presence of K2CO3 and KI in CH3CN as solvent as shown in Scheme 4.
Scheme 3.
Synthesis of alkylating agents, 34 and 35. Reagents and conditions: (i) n-BuLi, −78 °C, THF; (ii) TPP, imidazole, I2, DCM, 0 °C - rt.
Scheme 4.
Synthesis of Target Compounds. Reagents and conditions: (i) KI, K2CO3, CH3CN, reflux.
3. Results and Discussion
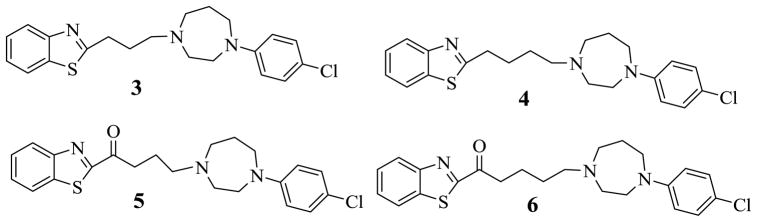
During a search for new atypical antipsychotic agents [10], we synthesized and screened several compounds, among which were compounds 3 – 6, for their binding affinities at dopamine D2, D3 and D4 receptor subtypes as well as serotonin 5HT1A, 5HT2A and 5HT2C receptors. Screening of these compounds at additional 5HT receptors and at SERT showed that compounds 3, 4 and 6 have affinity to SERT as well as the 5HT1A receptor (Table 1). This observation provided the impetus for the current study to design new agents and conduct a structure-activity relationship study of benzothiazole-based compounds as new potential dual-acting antidepressants.
Compound 3 is a benzothiazole linked by a propyl chain to a 4-chlorophenyl homopiperazine moiety. Compound 4 differs from 3 by a methylene group which extends the propyl to a butyl linkage. This modification has resulted in minimal change in binding at both the 5HT1A receptor and SERT. Insertion of a carbonyl group in compound 3 to form 5 or in 4 to form 6, resulted in loss of binding affinity to the 5HT1A receptor. However, at SERT, compound 5 binds with over 6-fold increase compared to 3 and compound 6 binds with the same affinity compared to 4 suggesting that the presence of the carbonyl group has a mixed effect on binding to the 5HT1A receptor and the SERT site.
The next design strategy was to replace the 4-chlorophenyl homopiperazine moiety with arylcycloalkyl amine pharmacophores with affinity to CNS receptors including the 5HT1A receptor. The results are recorded in Table 2. Compounds 7 and 8 are obtained by replacement of the homopiperazine ring in compounds 3 and 4 respectively with a piperazine ring. Compound 7 binds with less affinity to both the 5HT1A receptor and SERT as compared to compound 3, suggesting that the homopiperazine ring is better tolerated at these receptors when the chain length is three. Compound 8 on the other hand, showed about a 3-fold increase in binding to the 5HT1A receptor but a 2.6-fold decrease in binding at the SERT site. Comparing the binding affinities of 7 and 8 suggests that increasing chain length from 3 to 4 with the piperazine ring in place, is better tolerated at both receptors. Interestingly, the carbon chain length in vilazodone is 4 as well. Considering compounds 9 – 11, the contributions of 4-chlorophenyl piperidinol, 2-(piperazin-1-yl)pyrimidine and tetrahydroisoquinoline moieties respectively, were probed by substituting them in place of the 4-chlorophenyl piperazine moiety in 8. Evaluation of compound 9, with the 4-chlorophenyl piperidinol moiety resulted in significant decreases in binding at both the 5HT1A receptor and SERT.
Table 2.
Binding Affinity constants of benzothiazoles at selected CNS receptors
| Binding data; Ki ± SEM (nM)a | |||||||
|---|---|---|---|---|---|---|---|
| Compd # | 5HT1A | SERT | 5HT2A | 5HT2C | 5HT7 | DAD2 | DAD4 |
| 7 | 312±31 | 1184±244 | 36.0±4.0 | 4647±632 | 790±120 | 2321±314 | 31.0±4.0 |
| 8 | 90.5±31.0 | 166±16 | 132±11 | 6210±1452 | 329±52 | 219±12 | 4.0±0.0 |
| 9 | 771 | 922 | 4255 | MP | 983 | 201 | 140 |
| 10 | 6.6 | MP | 224 | >10000 | 228.9±33.7 | 26.5±4.5 | 0.8±0.09 |
| 11 | 5.1 | 1325 | 3812 | 6265±1397 | 35.6±5.9 | 30.8±4.2 | 65.5±9.7 |
| 12 | 111.0 | 186.0 | >10000 | MP | 211.0 | 990.0 | 141.0 |
Where no SEM is given, SEM is within 20% of the mean value.
MP = Missed primary assay threshold of 50% inhibition.
In previous publications, [17, 19] we observed that 2-(piperazin-1-yl)pyrimidine moiety enhanced binding affinity to the 5HT1A receptor. Replacing the 4-chlorophenyl moiety in 8 with the pyrimidinyl moiety to form 10 led to an increase of over 13-fold binding affinity over compound 8. In a similar manner, replacement of the 4-chlorophenyl piperazine with a tetrahydroisoquinoline ring produced compound 11 and about 18-fold increase in the binding affinity to the 5HT1A receptor. Unfortunately, both compounds have significantly less binding at the SERT. Compound 12 in which the 4-chlorophenyl moiety is replaced by an isoindole ring, had only moderate binding at both receptors.
Compound 13 was obtained by insertion of a carbonyl group into compound 7 and compound 14 is the unsubstituted analog of 13. Evaluation of 13 and 14 showed that the presence of the carbonyl group produced a decrease on binding affinity at both the 5HT1A receptor and SERT as shown in Table 3. Interestingly, the absence of N-1 from the piperazine ring in compound 14 to form 15 improved the binding affinity at both 5HT1A and SERT. Replacement of the phenyl group in compound 14 with a 2-pyrimidinyl ring to form 16, also led to an improvement in binding affinity to the 5HT1A receptor as expected. However, compound 16 has little or no affinity to SERT.
Table 3.
Binding Affinity constants of benzothiazoles at selected CNS receptors
| Binding data; Ki ± SEM (nM)a | |||||||
|---|---|---|---|---|---|---|---|
| Compd # | 5HT1A | SERT | 5HT2A | 5HT2C | 5HT7 | DAD2 | DAD4 |
| 13 | >10000 | 491±65 | 1996±263 | MP | MP | 8355 | 342±34 |
| 14 | 350 | >10000 | >10000 | MP | MP | >10000 | MP |
| 15 | 85.0 | 706 | 287 | MP | 425.8±66.8 | 530±146 | 44.4±5.0 |
| 16 | 63.0 | MP | 2206 | MP | 2467± 400 | 1522±489 | 51.5±5.8 |
| 17 | 252 | 565 | 1564 | MP | 127.0 | 217 | 1710 |
| 18 | 103 | 284 | 3720 | MP | 518 | 25.0 | 162 |
| 19 | 214.0 | 237 | >10000 | MP | 242 | 2931 | 512 |
Where no SEM is given, SEM is within 20% of the mean value.
MP = Missed primary assay threshold of 50% inhibition.
Replacement of the 2-(piperazin-1-yl)pyrimidine moiety in compound 16 with 5-chloro-1-(piperidin-4-yl)-1H-benzo[d]imidazol-2(3H)-one to form compound 17 resulted in diminished binding to the 5HT1A receptor and only a low binding affinity to SERT. Chain extension by one methylene group and exploration of two arylcycloalkyl amine groups; 1-phenyl-1,3,8-triazaspiro[4.5]decan-4-one (Compound 18) and isoindoline (Compound 19) by replacing the 4-chlorophenyl homopiperazine moiety did not result in significant improvements in binding affinity at 5HT1A and SERT.
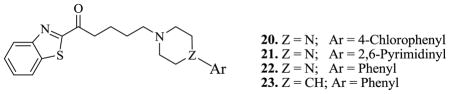
Compound 20 was obtained by inserting a carbonyl group between the benzothiazole ring and the first methylene group in compound 8. Evaluation of its binding affinities showed that binding to the 5HT1A receptor was enhanced by over 3-fold and to SERT by over 2-fold. In compound 21, the 4-chlorophenyl ring is replaced by 2,6-pyrimidinyl ring. As expected, the binding to the 5HT1A receptor again increased about 8-fold over compound 20 and more than 25-fold over compound 8. Surprisingly, compound 21 demonstrated little or no binding to SERT. The contribution of the 4-chloro group to binding affinity was investigated by evaluating compound 22. The presence of the chloro group imparted a 7.9 fold increase in the binding affinity at the 5HT1A receptor but binding to the SERT site showed over 3-fold decrease. This observation suggests the possibility that substituents on the phenyl ring may affect binding to these receptors. Finally, when the piperazine ring in compound 22 was replaced by a piperidine ring to form compound 23, binding affinity decreased by 2-fold at the 5HT1A receptor but increased to 64 nM at the SERT site.
In addition to the evaluations at the 5HT1A and SERT, it was also of interest to investigate the binding of the compounds at receptors which might influence antipsychotic and antidepressant pharmacology and/or side effects, including 5HT2A, 5HT7, 5HT2C, D2 and D4 receptors, H1, DAT and NET. Only two compounds, 7 and 23 (Ki = 36 and 3.4 nM respectively) bind with moderately high affinity to the 5HT2A receptor. Similarly, only compounds 11 and 22 bind with moderately high affinity at the 5HT7 receptor (Ki = 35.6 and 62.6 nM respectively). At the 5HT2C receptor, none of the compounds has affinity better than 1000 nM which is desirable as high affinity to this receptor may be associated with weight gain [19–21]. We have previously presented a set of criteria for compounds to be considered for further screening as new antipsychotic agents [19]. These include binding to dopamine D2 receptor within 10 < Ki < 150 nM range, high affinity for D4 receptor (Ki ≤ 10 nM), high affinity for 5HT1A and 5HT2A receptors and a low affinity for 5HT2C and H1 receptors. Only compounds 10, 11 and 18 meet the dopamine D2 binding requirement and will be further screened at relevant receptors. At the D4 receptor, only compounds 8 and 10 (Ki = 4.0 and 0.8 nM respectively) have binding affinity better than 10 nM. Interestingly, compound 10 turned out to be the most potent and D4 selective agent (with selectivity index, D2/D4 = 33.1) among the compounds evaluated.
4. Conclusion
Overall, the binding affinities at the 5HT1A receptor and the SERT site do not appear to be congruent and other areas of the binding sites would need to be explored in order to improve binding simultaneously at both sites. Only compounds 20 and 23 demonstrate simultaneously relatively moderate affinity binding at both 5HT1A receptor and the SERT site and thus have the potential to be further explored as dual-acting agents. Compound 20 shows low affinity for DAT, NET and 5HT2C receptor, which are desirable properties as selectivity for SERT (and not DAT or NET) is associated with an absence of cardiovascular problems. The low affinity for 5HT2C is also desirable because of its association with weight gain and type II diabetes [20]. The moderate affinity for the H1 receptor is undesirable for the same reasons indicated for the 5HT2C receptor [21]. For compound 23, there is a need to decrease the binding affinity to NET and the H1 receptor for the same reasons stated. Efforts in this direction are ongoing. Plans are also ongoing to conduct functional assays to determine whether compounds with high affinity to the 5HT1A receptor are agonists or antagonists.
5. Experimental
5.1 Reagents and general procedures
Melting points were determined on a Gallenkamp (UK) apparatus and are uncorrected. 1H NMR spectra were obtained on a Varian 300 MHz Mercury Spectrometer. Elemental analyses were carried out by Atlantic Microlab, Inc., Norcross, GA, and are within 0.4% of theory unless otherwise noted. Flash chromatography was performed on Combi-Flash (Teledyne Isco) using RediSep columns. N,N Dimethylformamide was distilled from CaSO4 and stored over 4Å molecular sieves. Starting materials were obtained from Sigma-Aldrich and were used without further purification.
5.2. General procedure for synthesis of alkylating agents (27, 28)
To a solution of 2-aminothiophenol (5 g, 39.9 mmol) in toluene (100 mL), 5-chlorobutanoyl chloride (25) or 5-chloropentanoyl chloride (26) (43.9 mmol) was added drop wise over a 15 min period and during the addition, an off-white precipitate was formed. The reaction mixture was stirred at room temperature (rt) overnight, then water (100 mL) was added, the two layers were separated and the aqueous layer was extracted with EtOAc (2 × 100 mL). The combined organic extract was washed with water (100 mL) and saturated NaCl solution, dried over Na2SO4 and concentrated in vacuo. The crude product was purified on Combiflash using EtOAc/Hexanes, to afford 2-(3-chloropropyl)benzo-[d]thiazole 27 or 2-(4-chlorobutyl)benzo[d]thiazole 28 as an oily liquid.
5.2.1 2-(3-Chloropropyl)benzo[d]thiazole (27)
Oily liquid (72% yield). 1H NMR (CD3OD): δ 8.14 (d, 1H, J = 4.1 Hz), 8.02 (d, 1H, J = 4.1 Hz), 7.72-7.59 (m, 2H), 3.64-3.57 (m, 2H), 3.38-3.28 (m, 2H), 1.95-1.86 (m, 2H).
5.2.2 2-(4-Chlorobutyl)benzo[d]thiazole (28)
Oily liquid (56% yield). 1H NMR (CDCl3) : δ 7.98-7.95 (m, 1H), 7.85-7.82 (m, 1H), 7.48-7.42 (m, 1H), 7.37-7.32 (m, 1H), 3.60 (t, 2H, J = 7.5 Hz), 3.15 (t, 2H, J = 7.5 Hz), 2.09-1.97 (m, 2H), 1.95-1.90 (m, 2H).
5.3. General procedure for synthesis of alkylating agents (34, 35)
A stirred solution of benzo[d]thiazole 29 (10 g, 74 mmol) in dry THF (37 mL) under N2 was cooled to −78 °C (dry ice /acetone bath) and 10% excess of n-BuLi (37 mL 1M solution in THF) was added in a drop-wise manner. Just before the addition was completed, the solution gave rise to a clear orange colored solution. Thereafter, a solution of lactone, 30 (7.0 g, 81 mmol) or 31 (8.14 g, 81 mmol) in dry THF (37 mL) was added to the reaction mixture at −78 °C, and the mixture was stirred at −78 °C for 1h. After removal of the cold bath, the reaction mixture was continuously stirred for 30 minutes and then quenched with a large excess of 0.1 M HCl (300 mL). The aqueous mixture was extracted with EtOAc (3 × 150 mL) and the combined organic extracts was washed with H2O (2 × 100 mL) and saturated NaCl (100 mL) and dried over Na2SO4. The solution was concentrated in vacuo, the crude product was dissolved in EtOAc (50 mL), hexane (200 mL) was added to precipitate an orange solid. The precipitate was filtered, washed with 10% EtOAc/Hexane (200 mL) and dried in vacuo to obtain the pure products, 1-(benzo[d]thiazol-2-yl)-4-hydroxybutan-1-one 32 and 1-(benzo[d]thiazol-2-yl)-5-hydroxypentan-1-one 33, as solids.
5.3.1. 1-(Benzo[d]thiazol-2-yl)-4-hydroxybutan-1-one (32)
Solid (49 % yield), mp: 93–94 °C. 1H NMR (CDCl3) : δ 8.20-8.17 (m, 1H), 8.00-7.97 (m, 1H), 7.61-7.51 (m, 2H), 3.79-3.75 (m, 2H), 3.41 (t, 2H, J = 6.9 Hz), 2.14-2.05 (m, 2H).
5.3.2. 1-(Benzo[d]thiazol-2-yl)-5-hydroxypentan-1-one (33)
Solid (37% yield), mp: 81–83 °C; 1H NMR (CDCl3) : δ 8.18 (dd, 1H, J = 1.8, 6.9 Hz), 7.97 (dd, 1H, J = 1.5, 7.2 Hz), 7.62-7.52 (m, 2H), 3.71 (t, 2H, J = 6.3 Hz), 3.40 (t, 2H, J = 7.2 Hz), 2.00-1.88 (m, 2H), 1.76-1.67 (m, 2H).
To a solution of TPP (3.12 g, 11.9 mmol), imidazole (810 mg) in CH2Cl2 (30 mL) was added iodine (3.02 g, 11.9 mmol) at 0–5 °C. The reaction mixture was stirred at 0–5 °C for 30 minutes and a solution of 1-(benzo[d]thiazol-2-yl)-4-hydroxybutan-1-one (32) (1.9 g, 8.6 mmol) or 1-(benzo[d]thiazol-2-yl)-5-hydroxypentan-1-one (33) (2.0 g, 8.5 mmol) in CH2Cl2 (15 mL) was added drop wise for 5 min. The reaction mixture was stirred at 0–5 °C for another 30 min, and then the ice bath was removed and stirring continued at rt for 12 h. When TLC showed the reaction was complete, the reaction mixture was treated with water (100 mL), the two layers were separated, and the aqueous layer was extracted with CH2Cl2 (2 × 50 mL). The combined organic extracts was washed with water (2 × 100 mL) and 10% sodium thiosulfate solution (50 mL), water (100 mL) then saturated aqueous NaCl solution (75 mL), dried over Na2SO4 and concentrated in vacuo. The crude product was further purified on Combiflash using EtOAc/Hexane (1 : 9) to obtain the pure product as 1-(benzo[d]thiazol-2-yl)-4-iodobutan-1-one (34) or 1-(benzo[d]thiazol-2-yl)-5-iodopentan-1-one (35) as a solid.
5.3.3 1-(Benzo[d]thiazol-2-yl)-4-iodobutan-1-one (34)
Solid (43 % yield), mp: 91–92 °C; 1H NMR (CDCl3): δ 8.21-8.18 (m, 1H), 8.00-7.97 (m, 1H), 7.62-7.52 (m, 2H), 3.45 (t, 2H, J = 6.9 Hz), 3.34 (t, 2H, J = 6.6 Hz), 2.35 (q, 2H, J = 6.90 Hz).
5.3.4 1-(Benzo[d]thiazol-2-yl)-5-iodopentan-1-one (35)
Solid (61 % yield), mp: 88–89 °C; 1H NMR (CDCl3) : δ 8.20 (dd, 1H, J = 1.5, 7.8 Hz), 8.98 (dd, 1H, J = 1.2, 7.8 Hz), 7.61-7.51 (m, 2H), 3.34-3.23 (m, 4H), 2.02-1.90 (m, 4H).
5.4. General alkylation procedure for compounds (7–12)
A mixture of 2-(3-chloropropyl)benzo[d]thiazole (27) (1.33 mmol), or 2-(4-chlorobutyl)benzo[d]thiazole (28), the appropriate amine, (1.33 mmol), KI (100 mg), K2CO3 (13.3 mmol), and CH3CN (15 mL) was heated to reflux for 12-24 h. The mixture was cooled to room temperature and then loaded onto a cartridge and purified by flash chromatography using EtOAc and hexane (9:1) to give the desire products.
5.4.1 2-(3-(4-(4-Chlorophenyl)piperazin-1-yl)propyl)benzo[d]thiazole hydrochloride (7)
Yield (32 %), mp: 158–160 °C; 1H NMR (CD3OD): δ 7.94–8.01 (m, 2H), 7.52–7.57 (m, 1H), 7.43–7.49 (m, 1H), 7.26 (dd, 2H, J = 6.6, 2.4 Hz), 7.01 (dd, 2H, J = 6.8, 2.4 Hz), 3.62–3.94 (m, 4H), 3.34–3.42 (m, 4H), 3.29–3.32 (m, 4H), 2.39–2.46 (m, 2H). Anal.Calcd for C20H24Cl3N3S.0.6 H2O: C, 52.72; H, 5.31; N, 9.22. Found: C, 52.60; H, 5.57; N, 9.15.
5.4.2. 2-(4-(4-(4-Chlorophenyl)piperazin-1-yl)butyl)benzo[d]thiazole (8)
Yield (20%), mp: 104–106 °C; 1H NMR (CDCl3) : δ 7.98-7.95 (m, 1H), 7.86-7.83 (m, 1H), 7.48-7.43 (m, 1H), 7.38-7.35 (m, 1H), 7.20 (dd, 2H, J = 2.4, 9.3 Hz), 6.84 (dd, 2H, J = 2.4, 9.0 Hz), 3.19-3.14 (m, 6H), 2.58 (t, 2H, J = 5.1 Hz), 2.45 (t, 2H, J = 7.5 Hz), 1.97-1.92 (m, 2H), 1.70-1.65 (m, 2H). Anal. Calcd for C21H24ClN3S: C, 65.35; H, 6.27; N, 10.89. Found: C, 65.11; H, 6.05; N, 10.71.
5.4.3. 2-(4-(4-(4-Chlorophenyl)piperazin-1-yl)butyl)benzo[d]thiazole (8)hydrochloride
Yield (48 %), mp: 214–216 °C. 1H NMR (CD3OD): δ 8.22 (d, 1H, J = 8.1 Hz), 8.07 (d, 1H, J = 8.1 Hz), 7.79-7.74 (m, 1H), 7.70-7.65 (m, 1H), 7.27 (d, 2H, J = 8.4 Hz), 7.02 (d, 2H, J = 9 Hz), 3.91-3.63 (m, 4H), 3.52-3.46 (m, 2H), 3.40-3.20 (m, 6H), 2.18-1.98 (m, 4H). Anal. Calcd for C21H26Cl3N3S.H2O: C, 51.72; H, 5.37; N, 8.62. Found: C, 51.61; H, 5.68; N, 8.35.
5.4.4. 1-(4-(Benzo[d]thiazol-2-yl)butyl)-4-(4-chlorophenyl)piperidin-4-ol (9)
Yield (18%), mp: 143–145 °C; 1H NMR (CDCl3) : δ 7.97-7.94 (m, 1H), 7.86-7.83 (m, 1H), 7.48-7.42 (m, 3H), 7.37-7.26 (m, 3H), 3.16 (t, 2H, J = 7.8 Hz), 2.98-2.88 (m, 2H), 2.60-2.52 (m, 4H), 2.30-2.18 (m, 2H), 1.97-1.90 (m, 2H), 1.80-1.70 (m, 4H). Anal. Calcd for C22H25ClN2OS: C, 65.90; H, 6.28; N, 6.99. Found: C, 65.16; H, 6.17; N, 6.91.
5.4.5 2-[4-(4-Pyrimidin-2-yl-piperazin-1-yl)-butyl]-benzo[d]thiazole hydrochloride (10)
The product was converted into HCl salt, followed by crystallization from MeOH-Et2O to give the pure compound. Yield (19%), mp: 236–237 °C; 1H NMR (DMSO-d6): δ 11.44 (brs, 1H), 8.44 (d, 2H, J = 5.1 Hz), 8.04 (d, 1H, J = 8.1 Hz), 7.91 (d, 1H, J = 8.1 Hz), 7.50-7.44 (m, 1H), 7.41-7.36 (m, 1H), 6.77 (t, 1H, J = 5.1 Hz), 4.66 (d, 2H, J = 14.4 Hz), 3.53-3.42 (m, 4H), 3.19-3.10 (m, 4H), 3.04-2.97 (2H, m), 1.88-1.82 (m, 4H). Anal. Calcd for C19H24ClN5S: C, 58.52; H, 6.20; N, 17.96. Found: C, 58.40; H, 6.17; N, 17.86.
5.4.6 2-(4-Benzo[d]thiazol-2-yl-butyl)-1,2,3,4-tetrahydro-isoquinoline p-toluenesulfonate (11)
The product was converted into the tosylate salt, followed by crystallization from MeOH-Et2O to afford the pure compound. Yield (18%), mp: 154–155 °C; 1H NMR (DMSO-d6): δ 9.65 (brs, 1H), 8.05 (d, 1H, J = 7.8 Hz), 7.92 (d, 1H, J = 8.1 Hz), 7.49 (d, 1H, J = 7.5 Hz), 7.46-7.42 (m, 4H), 7.38 (d, 1H, J = 8.1 Hz), 7.29 - 7.19 (m, 3H), 7.14 (d, 4H, J = 7.5 Hz), 7.04-7.01 (m, 1H), 4.54 (d, 2H, J = 13.5 Hz), 4.28 (dd, 1H, J = 7.8, 15.6 Hz), 3.72-3.69 (m, 1H), 3.34-3.26 (m, 3H), 3.19-3.15 (m, 2H), 3.11-3.03 (m, 2H), 2.26 (s, 3H), 1.89-1.85 (m, 4H). Anal. Calcd for C34H38N2O6S3: C,61.24; H, 5.74; N, 4.20. Found: C, 61.23; H, 5.92; N, 4.12.
5.4.7 2-(4-(Isoindolin-2-yl)butyl)benzo[d]thiazole hydrochloride (12)
The product was converted into the HCl salt, followed by crystallization from MeOH-Et2O to give the pure compound. Yield (7%), mp: 188–190 °C; 1H NMR (DMSO-d6): δ 11.08 (s, 1H), 8.05 (dd, 1H, J = 0.9, 7.2 Hz), 7.91 (dd, 1H, J = 0.6, 7.8 Hz), 7.50-7.44 (m, 1H), 7.41-7.32 (m, 4H), 7.22 (s, 1H), 4.75 (dd, 2H, J = 5.7, 13.8 Hz), 4.45 (dd, 2H, J = 6.9, 13.8 Hz), 3.41 (q, 2H, J = 5.7 Hz), 3.17 (t, 2H, J = 6.6 Hz), 1.90-1.87 (m, 4H). Anal. Calcd for C21H24ClN3S 2HCl 0.3H2O: C, 59.00; H, 5.73; N, 7.24. Found: C, 59.00; H, 5.73; N, 7.24.
5.6 General alkylation procedure for compounds (13–23)
A mixture of 1-(4-chlorophenyl)piperazine (0.65 mmol), 1-(benzo[d]thiazol-2-yl)-5-iodopentan-1-one (35) (0.84 mmol), K2CO3 (5.18 mmol), and CH3CN (15 mL) was heated to reflux for 12-24 h. The mixture was cooled to room temperature and then loaded onto a cartridge and purified by flash chromatography using EtOAc and hexane (9.5:0.5) to give the desired products.
5.6.1. 1-(Benzo[d]thiazol-2-yl)-4-(4-(4-chlorophenyl)piperazin-1-yl)butan-1-one hydrochloride (13)
The product was converted into the HCl salt, followed by crystallization from MeOH-Et2O to give the pure compound. Yield (19%), mp: 251–253 °C; 1H NMR (CD3OD): δ 8.19-8.16 (m, 1H), 8.13-8.09 (m, 1H), 7.66-7.57 (m, 2H), 7.26 (d, 2H, J = 6.6 Hz), 7.03 (d, 2H, J = 7.2 Hz), 3.88-3.73 (m, 4H), 3.49 (t, 2H, J = 7.2 Hz), 3.39-3.31 (m, 4H), 3.19-3.06 (m, 2H), 2.30-2.25 (m, 2H). Anal. Calcd for C21H23Cl2N3OS 0.15MeOH: C, 56.96; H, 5.33; N, 9.42. Found: C, 57.13; H, 5.60; N 9.04.
5.6.2 1-(Benzo[d]thiazol-2-yl)-4-(4-phenyl-piperazin-1-yl)-butan-1-one (14)
Yield (48%), mp: 160–161 °C; 1H NMR (CDCl3): δ 8.17 (d, 1H, J = 7.8 Hz), 7.94 (d, 1H, J = 8.1 Hz), 7.59-7.54 (m, 1H), 7.53-7.48 (m, 1H), 7.26-7.20 (m, 2H), 6.84 - 6.80 (m, 3H), 3.27 (t, 2H, J = 6.6 Hz), 2.97-2.94 (m, 4H), 2.55-2.47 (m, 6H), 2.13-2.08 (m, 2H). Anal.Calcd for C21H23N3OS: C, 69.10; H, 6.34; N, 11.50. Found: C, 68.89; H, 6.31; N, 11.37.
5.6.3. 1-(Benzo[d]thiazol-2-yl)-4-(4-phenyl-piperidin-1-yl)-butan-1-one hydrochloride (15)
The product was converted into the HCl salt, followed by crystallization from MeOH-Et2O to afford the pure compound. Yield (48%), mp: 269–270 °C; 1H NMR (DMSO-d6): δ 10.37 (brs, 1H), 8.27-8.22 (m, 2H), 7.69 -7.67 (m, 2H), 7.34-7.30 (m, 2H), 7.24-7.19 (m, 3H), 3.58 (d, 2H, J = 14.7 Hz), 3.44-3.37 (m, 2H), 3.20-3.14 (m, 2H), 3.09-2.99 (m, 2H), 2.86-2.78 (m, 1H), 2.20-2.08 (m, 2H), 2.08-2.04 (m, 2H), 1.98-1.93 (m, 2H). Anal. Calcd for C22H25ClN2OS: C, 65.90; H, 6.28; N, 6.99. Found: C, 65.70; H 6.22; N, 6.86.
5.6.4. 1-(Benzo[d]thiazol-2-yl)-4-(4-pyrimidin-2-yl-piperazin-1-yl)-butan-1-one hydrochloride (16)
The product was converted into the HCl salt, followed by crystallization from MeOH-Et2O to afford the pure HCl salt. Yield (42%), mp: 242–243 °C; 1H NMR (DMSO-d6): δ 10.76 (brs, 1H), 8.42 (d, 2H, J = 4.8 Hz), 8.26-8.21 (m, 2H), 7.69-7.60 (m, 2H), 6.75 (t, 1H, J = 4.8 Hz), 4.68 (d, 2H, J = 14.1 Hz), 3.64-3.58 (m, 2H), 3.47-3.34 (m, 4H), 3.24-3.17 (m, 2H), 3.06-3.01 (m, 2H), 2.162.11 (m, 2H). Calcd for C19H23Cl2N5OS: C 51.82, H 5.26, N 15.90; Found: C 51.86, H 5.35, N 15.77.
5.6.5 1-(1-(5-(Benzo[d]thiazol-2-yl)-5-oxopentyl)piperidin-4-yl)-5-chloro-1H-benzo[d]imidazol-2(3H)-one (17)
Yield (49%), mp: 221–222 °C; 1H NMR (DMSO-d6) : δ 10.99 (s, 1H), 8.24-8.21 (m, 2H), 7.64-7.60 (m, 2H), 7.12 (d, 1H, J = 8.7 Hz), 6.96-6.90 (m, 2H), 4.04-4.16 (m, 1H), 3.28 (t, 4H, J = 8.4 Hz), 3.00-2.97 (m, 2H), 2.44-2.30 (m, 2H), 2.27-2.19 (m, 2H), 2.10-2.00 (m, 2H), 1.76-1.68 (m, 2H), 1.63-1.52 (m, 4H). Anal. Calcd for C24H25ClN4O2S: C, 61.46; H, 5.37; N, 11.95. Found: C, 61.16; H, 5.45; N, 11.66.
5.6.6. 8-(5-(Benzo[d]thiazol-2-yl)-5-oxopentyl)-1-phenyl-1,3,8-triazaspiro[4.5]decan-4-one (18)
Yield (60%), mp: 206–207 °C; 1H NMR (DMSO-d6) : δ 8.58 (s, 1H), 8.24-8.19 (m, 2H), 7.63-7.60 (m, 2H), 7.17 (t, 2H, J = 8.4 Hz), 6.81 (d, 2H, J = 7.8 Hz), 6.69 (t, 1H, J = 7.2 Hz), 4.54 (s, 2H), 3.29 (t, 4H, J = 6.6 Hz), 2.80-2.54 (m, 4H), 2.36 (t, 2H, J = 7.2 Hz), 1.80-1.70 (m, 2H), 1.58-1.52 (m, 4H). Anal. Calcd for C25H28N4O2S 0.075 EtOAc: C, 65.97; H, 6.20; N, 12.31. Found: C, 65.99; H, 6.37; N, 12.03
5.6.7. 1-(Benzo[d]thiazol-2-yl)-5-(isoindolin-2-yl)pentan-1-one hydrochloride (19)
The product was converted into the HCl salt, followed by crystallization from MeOH-Et2O to give the pure compound. Yield (29%), mp: 248–250 °C; 1H NMR (DMSO-d6): δ 11.36 (s, 1H), 8.22-8.19 (m, 2H), 7.64-7.60 (m, 2H), 7.32-7.24 (m, 4H), 4.37 (s, 4H), 3.33 (t, 2H, J = 6.6 Hz), 3.20 (brs, 2H), 1.75 (t, 4H, J = 2.7 Hz). Calculated for C20H21ClN2OS: C, 64.42; H, 5.68; N, 7.51; Found: C, 64.51; H, 5.92; N, 7.31.
5.6.8. 1-(Benzo[d]thiazol-2-yl)-5-(4-(4-chlorophenyl)piperazin-1-yl)pentan-1-one hydrochloride (20)
The product was converted into the HCl salt, followed by crystallization from MeOH-Et2O to give the pure compound. Yield (58%), mp; 227–229 °C; 1H NMR (CD3OD) : δ 8.18-8.15 (m, 1H), 8.12-8.09 (m, 1H), 7.65-7.56 (m, 2H), 7.26 (d, 2H, J = 6.6 Hz), 7.00 (d, 2H, J = 6.6 Hz), 3.85 (d, 2H, J = 14.1 Hz), 3.70 (d, 2H, J = 12.6 Hz), 3.39 (t, 2H, J = 6.9 Hz), 3.32-3.22 (m, 6H), 3.19-3.04 (m, 2H), 1.93-1.90 (m, 4H). Anal. Calcd for C22H26Cl3N3OS: C, 54.27; H, 5.38; N 8.63. Found: C, 54.35; H, 5.12; N 8.90.
5.6.9 1-(Benzo[d]thiazol-2-yl)-5-(4-pyrimidin-2-yl-piperazin-1-yl)-pentan-1-one hydrochloride (21)
The product was converted into the HCl salt, followed by crystallization from MeOH-Et2O to give the pure compound. Yield (35%), mp: 202–203 °C; 1H NMR (DMSO-d6): δ 11.06 (brs, 1H), 8.41 (d, 2H, J = 4.8 Hz), 8.25-8.21 (m, 2H), 7.68-7.61 (m, 2H), 6.74 (t, 1H, J = 4.8 Hz), 4.65 (d, 2H, J = 7.8 Hz), 3.54-3.50 (m, 2H), 3.47-3.38 (m, 2H), 3.34-3.30 (m, 2H), 3.17-3.11 (m, 2H), 3.05-2.94 (m, 2H), 1.87-1.82 (m, 2H), 1.79-1.71 (m, 2H). Anal. C20H25Cl2N5OS: C, 52.86; H, 5.55; N, 15.41. Found: C, 52.66; H, 5.66; N, 15.36.
5.6.10. 1-(Benzo[d]thiazol-2-yl)-5-(4-phenyl-piperazin-1-yl)-pentan-1-one hydrochloride (22)
The product was converted into the HCl salt, followed by crystallization from MeOH-Et2O to give the pure compound. Yield (37%). mp: 216–217 °C; 1H NMR (DMSO-d6): δ 10.85 (brs, 1H), 8.26-8.22 (m, 2H), 7.68-7.59 (m, 2H), 7.24 (dd, 2H, J = 7.2, 8.7 Hz), 6.97 (d, 2H, J = 7.8 Hz), 6.84 (t, 1H, J = 7.2 Hz), 3.80-3.77 (m, 2H), 3.55-3.52 (m, 2H), 3.35-3.31 (t, 2H, J = 6.6 Hz), 3.17-3.07 (m, 6H), 1.85-1.81 (m, 2H), 1.78-1.71 (m, 2H). Anal. Calcd for C22H26ClN3OS 0.7H2O: C, 61.65; H, 6.11; N, 9.80. Found: C, 61.82; H, 6.45; N 9.62.
5.6.11. 1-(Benzo[d]thiazol-2-yl)-5-(4-phenyl-piperidin-1-yl)-pentan-1-one hydrochloride (23)
The product was converted into the HCl salt, followed by crystallization from MeOH-Et2O to give the pure compound. Yield (39%), mp: 204–205 °C; 1H NMR (DMSO-d6): δ 10.71 (brs, 1H), 8.26-8.22 (m, 2H), 7.68-7.59 (m, 2H), 7.34-7.29 (m, 2H), 7.23-7.18 (m, 3H), 3.54-3.51 (m, 2H), 3.30-3.24 (m, 2H), 3.12-3.05 (m, 2H), 2.93-3.01 (m, 2H), 2.83-2.75 (m, 1H), 2.02 – 2.15 (m, 2H), 1.90-1.94 (m, 2H), 1.88-1.82 (m, 2H), 1.77-1.70 (m, 2H). Anal. Calcd for C23H28Cl2N2OS: C, 66.57; H, 6.56; N, 6.75. Found: C, 66.37; H, 6.56; N, 6.77.
5.7. Receptor binding studies
Binding affinities reported in Tables 1–4 were conducted by the National Institute of Mental Health Psychoactive Drug Screening Program (NIMH-PDSP). Details of the methods and radioligands used for the binding assays were previously reported [22].
Table 4.
Binding affinity constants of benzothiazoles at selected CNS receptors
| Binding data; Ki ± SEM (nM)a | |||||||
|---|---|---|---|---|---|---|---|
| Compd # | 5HT1A | SERT | 5HT2A | 5HT2C | 5HT7 | DAD2 | DAD4 |
| 20 | 28.3±9.2 | 81.0±7.0 | 523±68 | MP | 2917±464 | 4793±356 | 100±10 |
| 21 | 3.6 | MP | 6982 | MP | 337±35 | 505±77 | 346±35 |
| 22 | 3.6 | 298 | 204.0 | 2140±464 | 62.6±6.8 | 198±27 | 105±10 |
| 23 | 7.3 | 64.0 | 3.4 | 3738±807 | 107±12 | 642±153 | 180±17 |
Where no SEM is given, SEM is within 20% of the mean value.
MP = Missed primary assay threshold of 50% inhibition.
Table 5.
Binding affinity constants of compounds 20 and 23 at selected CNS receptors
| Compd # | Binding data; Ki ± SEM (nM)a | |||||
|---|---|---|---|---|---|---|
| 5HT1A | SERT | DAT | NET | H1 | 5HT2C | |
| 20 | 28.3±9.2 | 81.0±7.0 | 4742±938.01 | >10,000 | 46.0±5.0 | MP |
| 23 | 7.3 | 64.0 | ND | 49.0 | 52.0 | 3738±808 |
Where no SEM is given, SEM is within 20% of the mean value.
MP = Missed primary assay threshold of 50% inhibition. ND = Not determined.
Research Highlights.
Four old and 17 new benzothiazoles have been synthesized and screened for binding to CNS receptors related to neuropsychiatric illnesses.
Several benzothiazoles have been identified as potential leads in the development of dual-acting agents at 5HT1A and SERT sites.
One of the identified agents has little or no binding to receptors implicated in the off-target pharmacology of known SSRIs and thus will undergo an elaborate SAR studies.
Acknowledgments
We acknowledge the financial support of the National Institute of General Medical Studies (NIGMS) MBRS Grant # 1SC1GM088451-01, NIMH Psychoactive Drug Screening Program, and a Title III Grant to Florida A&M University. This work is supported in part by the Pharmaceutical Research Center NIH/NCRR 1 C06-RR12512-01 Grant. These funding sources had no involvement in the study design, data collection and interpretation, or article preparation and submission of this manuscript. The authors would like to acknowledge Mrs. Barbara Bricker for her editorial assistance during the writing of this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and Notes
- 1.http://www.nimh.nih.gov/health/publications/the-numbers-count-mental-disorders-inamerica.shtml.
- 2.(a) Pacher P, Kecskemeti V. Cardiovascular side effects of new antidepressants and antipsychotics: new drugs, old concerns? Curr Pharm Des. 2004;10:2463–2475. doi: 10.2174/1381612043383872. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Howell C, Wilson AD, Waring WS. Cardiovascular toxicity due to venlafaxine poisoning in adults: a review of 235 consecutive cases. Br J Clin Pharmacol. 2007;64:192–197. doi: 10.1111/j.1365-2125.2007.02849.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Starr KR, Price GW, Watson JM, Atkinson PJ, Arban R, Melotto S, Dawson LA, Hagan JJ, Upton N, Duxon MS. A novel 5-HT1A/B autoreceptor antagonist and serotonin reuptake inhibitor, is anxiolytic and displays fast onset activity in the rat high light social interaction test. Neuropsychopharmacol. 2007;32:2163–2172. doi: 10.1038/sj.npp.1301341. [DOI] [PubMed] [Google Scholar]; (b) Blier P, De Montigny C. Current advances and trends in the treatment of depression. Trends Pharmacol Sci. 1994;15:220–226. doi: 10.1016/0165-6147(94)90315-8. [DOI] [PubMed] [Google Scholar]
- 4.Guilloux JP, David DJ, Guiard BP, Chenu F, Repérant C, Toth M, Bourin M, Gardier AM. Blockade of 5HT1A receptors by (+/−)-pindolol potentiates cortical 5HT outflow, but not antidepressant-like activity of paroxetine: microdialysis and behavioral approaches in 5HT1A receptor knockout mice. Neuropsychopharmacol. 2006;31:2162–2172. doi: 10.1038/sj.npp.1301019. [DOI] [PubMed] [Google Scholar]
- 5.Khan A. Vilazodone: a novel dual-acting serotonergic antidepressant for managing major depression. Expert Opin Investig Drugs. 2009;18:1753–1764. doi: 10.1517/13543780903286396. [DOI] [PubMed] [Google Scholar]
- 6.Birch AM, Bradley PA, Gill JC, Kerrigan F, Needham PL. N-Substituted (2,3-dihydro-1,4-benzodioxin-2-yl)methylamine derivatives as D(2) antagonists/5HT(1A) partial agonists with potential as atypical antipsychotic agents. J Med Chem. 1999;42:3342– 3355. doi: 10.1021/jm9910122. [DOI] [PubMed] [Google Scholar]
- 7.Tatsumi M, Groshan K, Blakely RD, Richelson E. Pharmacological profile of antidepressants and related compounds at human monoamine transporters. Eur J Pharmacol. 1997;340:249–258. doi: 10.1016/s0014-2999(97)01393-9. [DOI] [PubMed] [Google Scholar]
- 8.Howland RH. Vilazodone: another novel atypical antidepressant drug. J Psychosoc Nurs Ment Health Serv. 2011;49:19–22. doi: 10.3928/02793695-20110203-98. [DOI] [PubMed] [Google Scholar]
- 9.Frampton JE. Vilazodone (Viibryd): a new antidepressant. Med Lett Drugs Ther. 2011;53:53–54. [PubMed] [Google Scholar]
- 10.Peprah K, Zhu XY, Eyunni SVK, Etukala JR, Setola V, Roth BL, Ablordeppey SY. Structure-activity relationship studies of SYA 013, a homopiperazine analog of haloperidol. Bioorg Med Chem. 2012;20:1671–1678. doi: 10.1016/j.bmc.2012.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Mnie-Filali O, Lambás-Señas L, Zimmer L, Haddjeri N. 5-HT7 Receptor Antagonists as a New Class of Antidepressants. Drug News Perspect. 2007;20:613–618. doi: 10.1358/dnp.2007.20.10.1181354. [DOI] [PubMed] [Google Scholar]; (b) Bromidge SM, Bertani B, Borriello M, Faedo S, Gordon LJ, Granci E, Hill M, Marshall HR, Stasi LP, Zucchelli V, Merlo G, Vesentini A, Watson JM, Zonzini L. 6-[2-(4-aryl-1-piperazinyl)ethyl]-2H-1,4-benzoxazin-3(4H)-ones: dual-acting 5-HT1 receptor antagonists and serotonin reuptake inhibitors. Bioorg Med Chem Lett. 2008;18:5653–5656. doi: 10.1016/j.bmcl.2008.08.084. [DOI] [PubMed] [Google Scholar]
- 12.Millan MJ. Dual- and triple-acting agents for treating core and co-morbid symptoms of major depression: novel concepts, new drugs. Neurotherapeutics. 2009;6:53–77. doi: 10.1016/j.nurt.2008.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatzenbuhler NT, Baudy R, Evrard DA, Failli A, Harrison BL, Lenicek S, Mewshaw RE, Saab A, Shah U, Sze J, Zhang M, Zhou D, Chlenov M, Kagan M, Golembieski J, Hornby G, Lai M, Smith DL, Sullivan KM, Schechter LE, Andree TH. Advances toward new antidepressants with dual serotonin transporter and 5-HT1A receptor affinity within a class of 3-aminochroman derivatives. J Med Chem. 2008;51:6980–7004. doi: 10.1021/jm8007097. [DOI] [PubMed] [Google Scholar]
- 14.Watson JM, Dawson LA. Characterization of the potent 5-HT(1A/B) receptor antagonist and serotonin reuptake inhibitor, preclinical evidence for hastened onset of antidepressant/anxiolytic efficacy. CNS Drug Rev. 2007;13:206–223. doi: 10.1111/j.1527-3458.2007.00012.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heinrich T, Böttcher H, Gericke R, Bartoszyk GD, Anzali S, Seyfried CA, Greiner HE, Amsterdam CV. Synthesis and structure--activity relationship in a class of indolebutylpiperazines as dual 5-HT(1A) receptor agonists and serotonin reuptake inhibitors. J Med Chem. 2004;47:4684–4692. doi: 10.1021/jm040793q. [DOI] [PubMed] [Google Scholar]
- 16.(a) Martínez-Esparza J, Ana MO, Silvia PS, Begon H, Lara O, Juan AP, Berta L, Joan R, Marisa M, Ana B, Juan CD, Rosa T, Joaquín DR, Antonio M. New 1-Aryl-3-(4-arylpiperazin-1-yl)propane Derivatives, with Dual Action at 5-HT1A Serotonin Receptors and Serotonin Transporter, as a New Class of Antidepressants. J Med Chem. 2001;44:418–428. doi: 10.1021/jm001059j. [DOI] [PubMed] [Google Scholar]; (b) Zhou D, Stack GP, Lo J, Failli AA, Evrard DA, Harrison BL, Hatzenbuhler NT, Tran M, Croce S, Yi S, Golembieski J, Hornby GA, Lai M, Lin Q, Schechter LE, Smith DL, Shilling AD, Huselton C, Mitchell P, Beyer CE, Andree TH. Synthesis, potency, and in vivo evaluation of 2-piperazin-1-ylquinoline analogues as dual serotonin reuptake inhibitors and serotonin 5-HT1A receptor antagonists. J Med Chem. 2009;52:4955–4959. doi: 10.1021/jm900374r. [DOI] [PubMed] [Google Scholar]
- 17.Peprah K, Zhu XY, Eyunni SVK, Setola V, Roth BL, Ablordeppey SY. Multi-receptor drug design: Haloperidol as a scaffold for the design and synthesis of atypical antipsychotic agents. Bioorg Med Chem. 2011;20:1291–1297. doi: 10.1016/j.bmc.2011.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chikashita H, Ishibaba M, Ori K, Ito K. General reactivity of 2-lithiobenzothiazole to various electrophiles and the use as a formyl anion equivalent in the synthesis of α-hydroxy carbonyl compounds. Bull Chem Soc Jpn. 1988;61:3637–3648. [Google Scholar]
- 19.Ablordeppey SY, Ramazan A, Bricker B, Zhu XY, Eyunni SK, Jackson T, Khan A, Roth BL. Identification of a butyrophenone analog as a potential atypical antipsychotic agent: 4-[4-(4-chlorophenyl)-1,4-diazepan-1-yl]-1-(4-fluorophenyl)butan-1-one. Bioorg Med Chem. 2008;16:7291–7301. doi: 10.1016/j.bmc.2008.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.(a) Reynolds GP, Hill MJ, Kirk SL. The 5-HT2C receptor and antipsychotic induced weight gain – mechanisms and genetics. J Pharmacol. 2006;20:15–18. doi: 10.1177/1359786806066040. [DOI] [PubMed] [Google Scholar]; (b) Matsui-Sakata A, Ohtani H, Sawada Y. Receptor occupancy-based analysis of the contributions of various receptors to antipsychotics-induced weight gain and diabetes mellitus. Drug Metab Pharmacokinet. 2005;20:368–378. doi: 10.2133/dmpk.20.368. [DOI] [PubMed] [Google Scholar]
- 21.Kroeze WK, Hufeisen SJ, Popadak BA, Renock SM, Steinberg S, Ernsberger P, Jayathilake K, Meltzer HY, Roth BL. H1-histamine receptor affinity predicts short-term weight gain for typical and atypical antipsychotic drugs. Neuropsychopharmacology. 2003;28:519–526. doi: 10.1038/sj.npp.1300027. [DOI] [PubMed] [Google Scholar]
- 22.Shapiro DA, Renock S, Arrington E, Chiodo LA, Liu LX, Sibley DR, Roth BL, Mailman R. Aripiprazole, a novel atypical antipsychotic drug with a unique and robust pharmacology. Neuropsychopharmacology. 2003;28:1400–1411. doi: 10.1038/sj.npp.1300203. [DOI] [PubMed] [Google Scholar]