Abstract
Introduction
Brain tumors are a unique class of cancers since they are anatomically shielded from normal immunosurveillance by the blood brain barrier, lack a normal lymphatic drainage system and reside in a potently immunosuppressive environment. Of the primary brain cancers, glioblastoma multiforme (GBM) is the most common and aggressive in adults. Although treatment options include surgery, radiation and chemotherapy, the average lifespan of GBM patients remains at only 14.6 months post-diagnosis.
Areas covered
A review of key cellular and molecular immune system mediators in the context of brain tumors including TGF-β, cytotoxic T cells, Tregs, CTLA-4, PD-1, and IDO, is discussed. In addition, prognostic factors, currently utilized immunotherapeutic strategies, on-going clinical trials, and a discussion of new or potential immunotherapies for brain tumor patients are considered.
Expert opinion
Current drugs that improve the quality of life and overall survival in patients with brain tumors, especially for GBM, are poorly effective. This disease requires a re-analysis of currently accepted treatment strategies, as well as newly designed approaches. Here, we review the fundamental aspects of immunosuppression in brain tumors, new and promising immunotherapeutic drugs, as well as combinatorial strategies that focus on the simultaneous inhibition of immunosuppressive hubs, both in immune- and brain tumor-cells, which is critical to consider for achieving future success for the treatment of this devastating disease.
Keywords: Glioblastoma, glioma, Treg, Rindopepimut, CTLA-4, PD-1, IDO, TGF-β
1. Background
Brain cancer is a malignancy with diverse origins. These may include cells that normally reside within the central nervous system (CNS) such as astrocytes, oligodendrocytes, neurons, ependymal cells, and cells of the meninges, which upon transformation give rise to astrocytoma, oligodendroglioma, neuroblastoma, ependymoma and meningioma, respectively.
Brain cancer is categorically unique from cancer that resides outside of the CNS, since it is segregated from normal immunosurveillance by the blood-brain-barrier (BBB) [1]. Brain tumors are further distinguished from non-CNS tumors by residing in an anatomical compartment that lacks a normal lymphatic drainage system [2]. Moreover, brain cancer includes those tumors that localize within the CNS, but have an initial origin from somewhere outside of the brain (i.e. metastases). However, this review will focus on primary brain tumors; arising from CNS-resident glial cells.
Of the primary brain cancers, astrocytoma grade IV, otherwise known as glioblastoma multiforme (GBM), is the most common, aggressive and most difficult to treat in adults [3]. GBM accounts for 52% of all primary brain tumors. Although treatment options include surgery, radiation and chemotherapy, the average lifespan of GBM patients is only 14.6 months post-diagnosis [4]. This may be due to the lack of normal immune surveillance, presence of the BBB, radio-resistance of both GBM and stromal (glial) cells, low levels of MHC II expression on CNS-resident microglia and the potently immunosuppressive CNS parenchyma. Furthermore, in spite of the presence of immune cells in GBM, the overall tumor environment is highly immunosuppressive [5–9]. Experimentally, brain tumor models that recapitulate GBM-like pathology also show a high degree of immunosuppressive leukocyte infiltration, as well as cytokine expression [10–12]. Importantly, data from clinical trials suggest that future immunotherapeutic strategies should target the removal or depletion of immunosuppressive cells and molecules in GBM [11, 13] which will promote normal immune cell-mediated tumor rejection.
1.1 Immune competence of brain tumors
The presence of T cells in brain tumors was first described in an immunofluorescent study performed in 1977, which identified T cell infiltrates in both human gliomas, as well as rat brain tumors induced by N-methyl-N-nitrosourea [14]. Since then, a more defined mechanism describing the effects of brain tumor-derived factors, such as the immunosuppressive cytokines, interleukin 10 (IL-10) and transforming growth factor-beta (TGF-β), as well as the various anti- and pro-inflammatory T cell subsets that accumulate, has emerged. Figure 1 briefly summarizes the major interactions between T cell subtypes and the immunosuppressive signals that promote the persistence and progression of brain tumors.
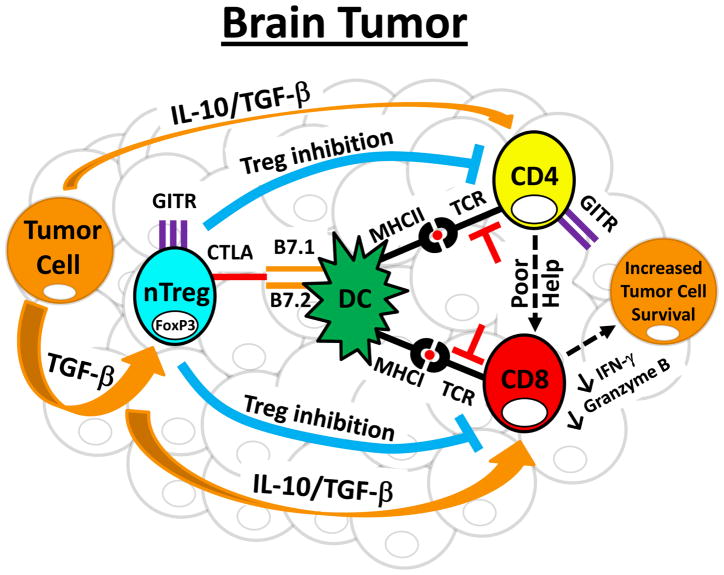
Figure 1. Model of immune dysfunction in brain tumors.
CD4+ and CD8+ T cells actively infiltrate brain tumors. CD4+ T cells are enriched for immunosuppressive regulatory T cells (Treg; CD4+FoxP3+), which express high levels of CTLA-4 and GITR. Importantly, the Tregs that accumulate in brain tumors are primarily thymus-derived, or natural Tregs (nTregs), rather than converted from CD4+FoxP3− conventional T cells. Within the brain tumor microenvironment, nTregs directly and indirectly suppress CD4+ and CD8+ T cells. This occurs through 3 main mechanisms. First, nTregs directly interact with dendritic cells (DCs) and induce an immunosuppressive phenotype that acts to suppress full CD4+ and CD8+ T cell activation, thus promoting tumor cell survival. In addition, nTregs produce IL-10 and TGF-β that directly acts on effector T cells to downregulate cytotoxic programs that would otherwise promote brain tumor clearance. Finally, nTregs interact via cell surface expression with effector T cells to induce T cell anergy. In addition to these processes, brain tumor cells produce large amounts of TGF-β that aides in increasing immunosuppressive effector T cell programs, leading to decreased pro-inflammatory cytokine help and increased GITR by CD4+ effectors, as well as decreased IFN-γ and granzyme B levels by CD8+ T cell effectors. Collectively, these immunosuppressive processes allow for tumor survival and outgrowth. Note: TCR inhibition (denoted in red) symbolizes low TCR activation.
TGF-β has been a focus throughout tumor biology for nearly 4 decades. It was initially found to be expressed by human glial tumors in 1988 [15] and has since become a major focus due to its effects on the maintenance of tumor progression. At the same time, CD4+ T cells were isolated from human malignant astrocytomas [16]. The majority of CD4+ T cells expressing IL-2 receptor alpha (CD25) were not appreciated at the time of the discovery. However, it is now understood that those initial observations were actually describing highly immunosuppressive cellular mediators that we now refer to as regulatory T cells (Tregs; CD4+FoxP3+CD25+) [17–19]. Functionally, our laboratory has demonstrated that those brain-tumor resident Tregs contribute to the pathogenesis of tumor progression, since depleting them results in significantly lower brain tumor-resident Treg levels, as well as a significantly increased survival in experimental mouse brain tumor models [17]. Since that work, a concerted effort has focused on decreasing Treg levels in brain tumors to improve the length of survival [11, 12, 20–23].
Aside from immunosuppressive cytokines and T cells, dendritic cells (DC), macrophages, microglia, B cells, natural killer T (NKT) cells, and NK cells have also been described to be deregulated in brain tumors [12, 24, 25]. Table 1 includes a comprehensive list of agents that have previously been shown to promote immunosuppression in the tumor microenvironment. Accordingly, there is now unanimous agreement that host immune cells succumb to the immunosuppressive forces induced by the brain tumor cells and in-so-doing, become converted into active participants that promote tumor progression. Based on this central tenet, it is now understood that to be maximally effective in treating brain tumors, immunotherapeutic strategies must both reverse the immunosuppression in leukocytes, as well as target the central hubs within tumor cells that regulate those immunosuppression-inducing pathways.
Table 1.
Agents involved in creating an immunosuppressive environment.
| Agent | Acronym | Known Receptor |
|---|---|---|
| Interleukin-10 | IL-10 | IL-10R |
| Transforming growth factor beta | TGF-β | TGF-βR(s) 1 and 2 |
| Cytotoxic T-Lymphocyte Antigen 4 | CTLA-4 | CD80, CD86 |
| B-and T-lymphocyte attenuator | BTLA-4 | TNFRSF14 |
| V-set domain containing T cell activation inhibitor 1 | B7-H4 | n/a |
| Programmed cell death 1 ligand 1 | PD-L1 | PD-1 |
| Glucocorticoid-induced TNFR-related protein | GITR | - |
| Indoleamine 2,3 dioxygenase | IDO | - |
| Tryptophan 2,3 dioxygenase | TDO | - |
| Kynurenine | Kyn. | Ahr |
| Regulatory T cell | Treg | - |
| Myeloid suppressor cell | mSC | - |
| Signal transducer and activator of transcription 3 | STAT 3 | - |
| Signal transducer and activator of transcription 5 | STAT 5 | - |
-: No receptor interaction required
1.2 Blood brain barrier
Normally, the central nervous system has a very low level of T cell infiltration due, in part, to an anatomical barrier composed of endothelial cells, pericytes, astrocytes and microglia; although astrocytic interaction with neurons also indirectly contributes to architecture and stability [26–28]. The capillaries of the BBB consist of a monolayer of endothelial cells coupled by tight junctions. Pericytes, which belong to the vascular smooth muscle lineage [29], share a common basement membrane with the capillaries. Pericytes not only provide mechanical stability to the endothelial-based capillaries, but also have the intrinsic ability to directly regulate endothelial cell differentiation and quiescence [30]. However, it is thought that astrocytes actually provide the specific signals that program endothelial cells to produce and maintain tight junctions [31]. The maintenance of tight junctions between endothelial cells of the brain means that for immunotherapies to effectively penetrate the BBB, they must be non-polar small molecules with a molecular weight of less than 500Da, or be able to use active transport mechanisms, since the BBB is functional in the peripheral and active areas of GBM. Moreover, since immunosuppressive leukocytes actively infiltrate brain tumors, the mechanism of migration can be selectively targeted in the future, immunotherapeutically. Figure 2 schematically demonstrates a generalized mode of leukocyte migration to- and through-the BBB. It is important to keep in mind that different molecules recruit different subsets of leukocyte subsets. Accordingly, the chemokine, CCL22, preferentially recruits immunosuppressive Tregs that promote tumorigenesis [32, 33]. In contrast, CXCL10 (IP-10) recruits pro-inflammatory IFN-γ-expressing CD4+ T cells that promote tumor rejection [34]. This selectivity in chemokine-recruiting potential at the level of the BBB has highlighted the anatomical and molecular regulation of the BBB as yet another aspect to consider for future therapeutic design.
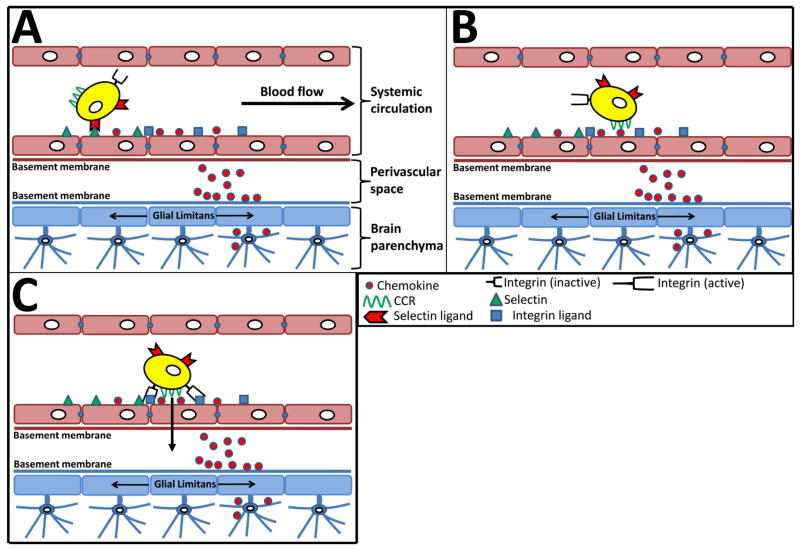
Figure 2. The multi-step paradigm of T cell recruitment and trafficking across the blood brain barrier.
T cells normally traffic through the systemic circulation continuously. In the context of the brain, during periods of stress or inflammation, selectins are upregulated on the vasculature. (A) During the rolling phase, T cells that express selectin receptors interact with the selectins on endothelial cells of the brain. This interaction results in a conformational change in the integrin receptors on T cells, converting from low affinity- to high affinity-receptors. (B) The interaction of chemokine receptors on T cells with chemokines on the basement membrane and cell surface of endothelial cells activates the T cells. (C) Once activation has taken place, T cells are capable of strongly tethering to the cell surface of endothelial cells via high affinity interaction of integrin-integrin receptor interactions, prior to transmigration into the perivascular space. However, the form of transit from the perivascular space into the brain parenchyma is a hotly debated subject and is not yet firmly established.
1.3 Future directions
Great strides have been made into understanding how the immune system is regulated to promote tumor progression versus rejection. Using this knowledge, Table 2 summarizes a variety of clinical trials that are now focused on arming the effector phase of the immune response through multiple strategies including, 1) CD8+ T cell stimulation with IL-2, 2) toll-like receptor (TLR) stimulation in combination with GBM-specific epitope targeting, 3) depleting Tregs, 4) stimulating DC with brain tumor-specific lysates, and 5) combining more than 3 different immuno-/chemo-therapies with TMZ (i.e. the kitchen sink approach). Under the best case scenario, these approaches combine to decrease the level of CD8+ T cell anergy/tolerization, allowing the CTL response to carry out antigen-specific tumor rejection.
Table 2.
On-going clinical trials for patients with brain tumors.
| Protocol ID | Immunotherapeutic Strategy | Phase | Treatment Strategy(s) |
|---|---|---|---|
| NCT00002572 | CTL’s and IL-2 | I | Repeated allogeneic CTL/IL-2 brain infusions for recurrent brain tumors. |
| NCT00014573 | Chemotherapy/Stem Cell Transplantation/Interleukin-2 | II | Combining chemotherapy, stem cell transplantation and IL-2 for refractory or recurrent brain tumors. |
| NCT00047879 | Phase II Trial of Peginterferon Alpha-2b and Thalidomide in Adults With Recurrent Gliomas | II | Peginterferon alpha-2b (PEG-Intron) alone and together with thalidomide in patients with gliomas. |
| NCT00504660 | 6-TG, Capecitabine and Celecoxib with TMZ or CCNU | II | The combination of capecitabine, celecoxib with temozolomide or CCNU in the treatment of recurrent or progressive anaplastic glioma or GBM in patients who have failed previous treatments. |
| NCT01454596 | White Blood Cells With Anti-EGFR-III | I/II | Using anti-EGFR-expressing leukocytes for advanced gliomas. |
| NCT00331526 | Cellular Adoptive Immunotherapy | II | IL-2 stimulated adoptive transfer of leukocytes in patients with GBM. |
| NCT01235845 | Combined CK/DC Treatment | I/II | Dendritic cell-activated cytokine killer (CK) treatment combined with DCs following tumor resection and radiotherapy for malignant glioma. |
| NCT01081223 | TVAX | I/II | TVAX generates large numbers of ‘killer’ white blood cells ex vivo for adoptive transfer into recurrent glioma (stage IV) for tumor destruction. |
| NCT01144247 | Allo CTL Therapy | I | Using allogeneic (donor) CTLs to destroy brain tumors. |
| NCT01082926 | Modified CTL Adoptive Transfer | I | Using an allogeneic CD8+ T cell line modified to express the IL 13-Zetakine and HyTK and to be resistant to glucocorticoids, in combination with IL-2 for recurrent/ refractory malignant glioma. |
| NCT00626483 | Treg depletion | I/II | Using daclizumab (humanized anti-CD25α) in treating patients with newly diagnosed GBM and during TMZ-induced lymphopenia. |
| NCT00795457 | Peptide/Adjuvant Delivery | 0 | Utilizing HLA-A2-restricted glioma antigen-peptides in combination with poly-ICLC for adult grade II astrocytomas and oligo-astrocytomas. |
| NCT00002965 | Interferon alpha (IFN-α) | II | Using IFN-α in patients with recurrent unresectable meningiomas and malignant meningiomas. |
| NCT01171469 | DC Vaccination | I | Autologous dendritic cells (DCs) loaded with allogeneic brain tumor stem cells administered as a vaccination in children and adults with recurrent brain tumors. |
CTL: cytotoxic T lymphocyte (CD8+ T cell); IL-2: interleukin-2; 6-TG: 6-thioguanine; TMZ: temozolomide; CCNU: Lomustine; EGFR: epidermal growth factor; GBM: glioblastoma multiforme; CK: cytokine killer; DC: dendritic cell; TVAX: immunotherapy made by TVAX biomedical; poly-ICLC: carboxymethylcellulose, polyinosinic-polycytidylic acid, and poly-L-lysine double-stranded RNA; IFN: interferon.
2. Medical Need
2.1 Clinical severity
The severity of symptoms in GBM patients varies widely depending on the location of the tumor. Neurological deficits, focal or diffuse, are common and progressive. Up to one third of patients have seizures before or after surgery and therefore benefit from peri-operative anti-epileptic medication [35]. During disease progression, all patients develop symptoms of increased intracranial pressure, headaches, nausea/vomiting, blurred vision and drowsiness. This high intracranial pressure results from both the tumor mass, as well as the associated altered properties of the BBB, culminating with marked peri-tumoral vasogenic edema and brain herniation. In the absence of treatment, symptoms progress rapidly. Moreover, due to the severity and lack of therapies that cross the BBB, the overall prognosis has not increased dramatically for GBM patients over the last three decades [36], highlighting the critical need for newly designed approaches.
2.2 Poor outcome
Surgical resection of GBM, alone, results in a median survival of approximately 6 months. Combining surgical resection with radiotherapy extends the median survival to 12.1 months and further combination with the chemotherapeutic agent, TMZ, leads to an additional 2.5 months of average lifespan [4]. TMZ was most effective in the group of GBM patients that possessed a methylated MGMT (O6-methylguanine DNA methyltransferase) gene [37]. This is due to the fact that TMZ introduces epigenetic modifications to rapidly dividing cells by akylating/methylating guanine residues at N-7 or O-6 locations. The MGMT complex repairs this type of TMZ-induced DNA damage. Thus, MGMT methylation is one of the few positive prognostic factors for GBM patients. Other prognostic criteria that suggest a better success rate for GBM patients include: young age (< 40 years), Karnofsky performance status (> 70; able to care for themselves) and gross total resection [38]. However, these prognostic criteria are not used to guide therapy. Moreover, data from The Cancer Genome Atlas (TCGA) project has suggested that GBM should be classified into four distinct genetic subtypes [39]. The analysis of each subtype found differences in both prognosis, as well as potential benefit from intense chemotherapeutic regimens or growth factor blocking agents [40].
The first classical subtype of GBM is characterized by highly proliferative cells that express elevated levels of the neural precursor marker, Nestin, as well as activated Notch and Sonic-hedgehog pathways. This subtype bears chromosomal alterations that lead to EGFR upregulation and loss of the PTEN or CDKN2A gene loci. In contrast, cell cycle check point regulator proteins, TP53 and NF1, are not altered and function properly. This may explain the better response rates to current chemo-radiotherapies in this group of patients.
The second subtype of GBM is known as the mesenchymal form and is defined by a genetic profile that resembles mesenchyme with highly active angiogenesis. Mutations frequently inactivate NF1, TP53 and PTEN resulting in the over-expression or unregulated activation of the NFκB, Ras and PI3K signaling pathways. These tumors respond better to a more aggressive chemo-irradiation regimen, when combinatorially administered angiogenesis inhibitors [40].
The third subtype of GBM is known as the pro-neural form and is associated with a gene activation profile resembling neuronal development with high expression of oligodendrocytic and neural development genes, such as Olig2, PDGFRA and Sox. This subtype tends to be associated with the best prognosis of any of the classical GBM subtypes. Paradoxically, the pro-neural subtype does not seem to benefit from a more aggressive chemo-irradiation schedule.
The fourth and final subtype of GBM is known as the neural form and is associated with the shortest survival. Interestingly, this subtype benefits from the highest levels of chemotherapy and irradiation. Moreover, it is characterized by a gene expression signature that is the most similar to normal brain tissue when compared to the other GBM subtypes. Whether these subtypes possess different genotypic and epigenetic signatures originating from different cellular origins remains to be determined. In summary, based on recent advances from the TCGA project, this genetic classification scheme may be become useful for defining molecular targets and antigenic variants for the specific design of future immunotherapeutic development.
2.3 Recurrent disease
Using current treatment paradigms, most GBM patients experience tumor relapse and outgrowth within 7 months after initial chemo-irradiation. The recurrence of GBM resembles a primary tumor and is thought to derive from a population of cells that are resistant to current therapies [41]. This population of brain cancer initiating and/or perpetuating cells is also collectively known as glioma stem cells (GSC) based on the genetic signature that resembles neural stem cells. The GSCs have been found to be more resistant to irradiation due to more active DNA repair mechanisms [42]. Also, they express a high level of multi-drug resistance genes, including the ABCG2 transporter, which actively excretes chemotherapeutic agents extracellularly [43]. In addition, GSCs are more immunosuppressive than differentiated cells [44]. Thus, to effectively improve patient survival, future immunotherapies may be more successful if GSC-targeting is employed. Moreover, GBM cells in different areas of the same tumor have a range of sensitivity to current chemotherapies [45]. Limited drug distribution in poorly perfused areas of brain tumors is a major determinant of resistance to therapy, since these areas are also resistant to radiation therapy [46].
2.4 Research questions
Many of the research questions that need to be answered for achieving better therapeutic design for treating brain tumors is listed in Table 3. Among the many questions, one of the most relevant is how immunotherapy modifies the tumor environment to promote the rejection of GSCs; destroying a lifeline for solid tumor survival. In addition, it is still unknown if the immunotherapeutic efficacy will differ among the subtypes of GBM, which is highly relevant since different gene mutations that lead to tumor survival may be the result of the various deregulated immunosuppressive pathways. Moreover, we still have a long way to go in understanding the combinatorial nature of central hubs that are critical to the regulation of immunosuppression in the tumor microenvironment. It is becoming increasingly clear that the most important hubs controlling immunological status are fundamentally based on deregulated metabolic pathways [47]. However, these observations have only been observed in cancers outside of the CNS and have yet to be thoroughly studied in the context of brain tumors.
Table 3.
Research questions regarding treatment of brain tumor patients.
| Research Questions |
|---|
| Which signaling pathways are required to be targeted to inhibit immunosuppression? |
| Can immunostimulants and immunosuppresants be used simultaneously? |
| How do Tregs get selectively depleted, while avoiding the depletion of cytotoxic T cells? |
| What role do GSCs play in promoting immunosuppression? |
| Are there genes expressed by brain tumors that centrally regulate immune dysfunction? |
| Can combinatorial immunotherapy lead to greater success than single targeting modalities? |
3. Existing Treatment
3.1 Temozolomide
In 2005, Stupp et al. [4] reported that the addition of TMZ to radiotherapy resulted in a statistically significant survival benefit to patients with GBM. Based on those clinical benefits, TMZ is now considered a standard-of-care therapy given to most GBM patients (Table 4). TMZ acts by introducing epigenetic modifications to rapidly dividing cells by akylating/methylating guanine residues at N-7 or O-6 locations. While much of the work on TMZ initially focused on its ability to induce apoptosis in rapidly dividing tumor cells, more recent findings have demonstrated that TMZ also affects the immune system. It is now recognized that TMZ induces lymphopenia [48], a condition whereby circulating lymphocytes persist at abnormally low levels. Moreover, low-dose metronomic administration of TMZ has been demonstrated to decrease circulating Tregs in a pre-clinical rat model [21]. In contrast, concomitant radiotherapy (RT) with TMZ increased the proportion of functional Tregs while decreasing the absolute number of CD3−CD56+ (NK cells) cells in the circulation of GBM patients, suggesting an increased immunosuppressive environment [49]. Thus, the combination of RT-TMZ is likely to produce an alternative outcome with regard to Treg levels when compared to administration of TMZ, alone. To dampen the homeostatic proliferation of Tregs during combined RT-TMZ, Mitchell et al. [11] showed that further combination of humanized anti-CD25 (Treg-depleting antibody) with RT-TMZ synergized to decrease circulating Treg levels in GBM patients. Most importantly, the Treg reduction was associated with a significant expansion of the vaccine-stimulated anti-tumor effector T cell levels. Thus, TMZ appears to be an important step forward in the treatment of GBM and may have increased efficacy in the right context of immunomodulatory therapies.
Table 4.
Treatments being tested or discontinued for brain tumor patients.
| Compound | Company | Evidence Level | Therapeutic Description |
|---|---|---|---|
| TP-38 | Teva | II | Recurrent and refractory GBM |
| Bevacizumab | Hoffmann-La Roche | III | Recurrent GBM |
| CRM-107 | Celtic Pharma | III | Inoperable and recurrent glioma |
| Everolimus | Novartis | II | Recurrent GBM |
| Imatinib | Novartis | Discontinued | Recurrent GBM |
| Sirolimus | Pfizer | II | Anticancer, immunological |
| Resveratrol | GlaxoSmithKline | Discontinued | Anticancer |
| Temozolomide | Cancer Research Technology | III | GBM |
| Temsirolimus | Pfizer | I/II | Relapsed/refractory neuroblastoma, rhabdomyosarcoma and high-grade glioma |
| IL-13 toxin | Insys Therapeutics | III | GBM and anaplastic astrocytoma |
| IL-4 PE | Neurocrine Biosciences | IIa | GBM |
3.2 Bevacizumab
Glioblastoma is one of the most vascularized tumors known and can thus be used as a model of angiogenesis [50]. Vascular endothelial growth factor (VEGF) is a critical regulator of angiogenesis [51] and is highly expressed within tumors of the brain and CNS. The expression of VEGF is not only correlated to the aggressiveness of the brain tumor, but also with clinical outcomes including recurrence and survival [52, 53]. Strategies that target angiogenic pathways may be potentially effective in tumor treatments, since angiogenesis is generally absent in normal healthy individuals. To this end, bevacizumab, otherwise known as Avastin, is a humanized monoclonal antibody that inhibits vascular endothelial growth factor A (VEGF-A) and has been established as a treatment option in clinical trials for patients with recurrent GBM that have a failed response to temozolomide treatment. The improved outcome after treatment with bevacizumab in humans was found in two phase II clinical trials for GBM and has been approved by the US Food and Drug Administration. The first trial found that a 32 patient cohort co-administered irinotecan and bevacizumab led to a median progression-free survival of 20- and 30-weeks in astrocytoma grade IV and III patients, respectively [54]. A separate study found that a cohort of 167 patients administered bevacizumab alone, vs. co-administration of irinotecan with bevacizumab, led to a 6-month progression free survival rate of 42.6% and 50.3%, respectively [55]. Paradoxically, it was recently reported that single-agent usage of bevacizumab in anaplastic astrocytoma patients provides radiographic response and clinical benefits, while not providing any advantage to progression-free survival [56].
While data for patients with recurrent and anaplastic disease have been encouraging, overall, studies of non-small cell lung cancer patients with CNS metastases treated with bevacizumab have demonstrated lower rates of CNS hemorrhage, providing similar lines of evidence for the safety of this treatment [57]. Although the patient sample size was small, Chira et al. [58] have also reported low neurological toxicity in breast cancer patients with brain metastases.
Various mechanisms have been attributed to the anti-tumor effects of bevacizumab, including reduced angiogenesis, reduced growth of tumor cells expressing the VEGF receptor, as well as the disruption of the cancer stem cell microvasculature niche [50, 59–61]. The cancer stem cell niche is of particular interest since resident glioma stem cells (described as CD133+, nestin+) are both radio- and chemo-resistant [42, 60].
4. Market review
In the year 2011, brain cancer in adults was estimated to lead to an estimated 10,080 and 12,260 newly diagnosed cases in women and men, respectively [62]. Also, it has been estimated that 5,670 women- and 7,440 men-died from brain cancer in that year [62]. Although brain cancer is relatively rare, when compared to other forms of cancer, a 2004 study found that patients with brain cancer reported that their average costs per month of treatment was equal to $7,081 [63]. Later in 2007, it was reported that 47% of individuals paid for medical and non-medically-related expenses by incurring credit card debt, 42% borrowed money from family/friends, 15% took out a second and/or third home mortgage, 8.4% cashed in on life- and/or retirement- plans, while 7.2% were forced to declare bankruptcy [64].
There were a total of 139,000 brain cancer cases in the U.S. during the year, 2010, and that number is expected to increase to 176,000 by the year, 2020 [65]. Moreover, the average cost of treatment within the initial year of diagnosis was $129,802 and $138,300 for women and men less than 65 years of age, respectively. Interestingly, of all of the different types of cancer, brain cancer has the highest cost of treatment during the final year of life, averaging $251,230 and $269,280 for women and men less than 65 years of age, respectively [65]. This is an important consideration given that the average monthly cost for TMZ treatment, alone, is over $10,000/month. Thus, the financial aspect of brain tumor treatment adds a significant layer of complexity to an already difficult-to-treat disease. Moreover, some of the reasons for such a high cost of treatment are the result of a high cost of training those who treat the disease, combined with an expensive drug development program for a relatively small patient population.
5. Current research goals
Finding immunotherapies that impact brain tumor progression is an important goal. However, to understand which immunotherapeutic strategies are most effective will depend on an investment into understanding the interactions between brain tumor cells and the immune system. In the context of brain tumors, our laboratory is among many that have chosen to pursue this line of research [12, 17, 19, 66–69]. One consideration for determining the potential success of immunotherapy in patients is finding good prognostic indicators that identify patient populations that will respond to treatment.
Characterization of cells that are relevant to the immune system for prognostic importance is still in the phase of infancy. It is well established that Tregs play a pathogenic role in brain tumor progression [17–19]. Thus, utilizing Tregs as a prognostic indicator for brain tumor patients was thought to have a high potential for success. Unfortunately, several studies have demonstrated that Treg levels act as a non-informative prognostic indicator for GBM patients [70, 71].
While Tregs may not serve as a good indicator of prognosis, cytokines and receptors that regulate Treg activity may a higher potential for success. Accordingly, high TGF-β expression and/or SMAD-related activity [72, 73] have been shown to act as good prognostic indicators in large-scale analysis of GBM patients.
Smad proteins are intracellular mediators of TGF-β signaling. Upon activation of the TGF-β receptor complex, Smad2/3 becomes phosphorylated and associates with Smad4. This complex translocates to the nucleus where it binds to DNA, thereby regulating transcription. It was recently demonstrated that high TGF-β-Smad activity is present in aggressive and highly proliferative gliomas; ultimately correlated with a poor prognosis in glioma patients. The TGF-β-Smad pathway promotes proliferation via the induction of methylation by the PDGF-B gene [72]. Smad proteins activated by TGF-β form a complex with FoxO proteins and turn on the growth inhibitory gene, p21Cip1, which is negatively regulated by the PI3K pathway. The activity of this network confers resistance to TGF-β-mediated cytostasis in GBM cells [74].
An additional immunosuppressive cytokine, IL-10, may also have prognostic importance [75], although this has yet to be shown in a large scale gene expression analysis. Likewise, TLR9, a toll-like receptor that recognizes unmethylated CpG dinucleotides, is a prognostic factor for GBM patients [76]. It is therefore interesting that TLR9 has been associated with decreasing IL-10-secreting Treg function [77], as well as synergizing with vaccine strategies to induce cytotoxic T cell-mediated rejection in a pancreatic tumor model [78].
In the future, factors such as TGF-β, IL-10 and TLR9 may be individually useful for prognostically identifying progression free survival. However, it may be even more effective to analyze this group of genes, collectively. Moreover, with large scale gene expression analysis now becoming more affordable, it may be time to analyze brain tumor biopsies for a range of immunosuppressive biomarkers that while, individually may not be a useful prognostic indicator, collectively they represent a powerful tool to assess those patients that have the highest likelihood of responding to different immunotherapeutic regimens.
6. Scientific rationale
Glioblastoma is the most invasive and malignant of the primary brain tumors. It is a progressive tumor that accumulates genetic mutations as it increases in aggressiveness. Due to the high degree of invasiveness, it is often impossible to completely remove the tumor mass, which can later contribute to recurrence. Despite recent advances in treatment, the mean survival post-treatment remains at 14.6 months, while only 10% of patients survive up to 5 years post-diagnosis [79]. Thus, it is critical to develop novel treatment therapies that improve the survival of patients with GBM.
7. Competitive environment
Although current immunomodulatory drugs and therapies for treating brain tumors have been discussed, new and potentially groundbreaking strategies are on the horizon. These alternative immunotherapies include those found in Table 5. Here we will review several current and futuristic approaches based on results from pre-clinical models and clinical trials. This section will focus on immunotherapies that have shown some form of success in clinical trials, as well as those targets that have shown promise in pre-clinical models of cancer.
Table 5.
Future immunological targets for the treatment of brain tumors.
| Target/Strategy | Rationale | Evidence |
|---|---|---|
| EGFRvIII | EGFRvIII is overexpressed at a high frequency in transformed but not normal brain tissue. | [105, 150–152] |
| IL-13Rα2 | IL-13Rα2 is overexpressed at a high frequency in transformed but not normal brain tissue. | [153, 154] |
| IL-4R | IL-4R is overexpressed at a high frequency in brain tumors. | [84, 90, 155, 156] |
| DC Vaccine | DC-lysate strategies have shown pre-clinical and clinical efficacy in high grade glioma. | [109, 157–162] |
| IL-2 | Exogenous IL-2 administration induces LAK cells. | [163–168] |
| TGF-β2 | TGF-β2 antisense therapy has shown clinical response in GBM patients. | [169–172] |
| TGF-βR | TGF-βR blockade has shown anti-tumor activity in pre-clinical GBM models. | [173–175] |
| IFN-α | Combinatorial treatment utlizing IFN-α has shown clinical success in patients. | [176–179] |
| CTLA-4 | CTLA-4 blockade has shown anti-tumor activity in pre-clinical GBM models. | [110, 180] |
| Tregs | Treg depletion leads to more effective anti-tumor responses in pre-clinical GBM models. | [17, 21, 180, 181] |
| STAT-3 | The STAT-3 plays a tumor-promoting role in pre-clinical GBM models. | [69, 182, 183] |
7.1 Therapies from clinical trials
7.1.1 Rindopepimut
In 24 – 67% of GBM specimens, EGFRvIII has been shown to be over-expressed [80]. Since EGFRvIII is not normally expressed by surrounding stromal brain cells, this mutant form of EGFR is ideal for targeting through immunotherapeutic approaches. Rindopepimut is a 14-mer injectable vaccine designed to stimulate immunity against a specific antigen of EGFRvIII [81] and its mechanism of action is reviewed in Figure 3. Both Phase I and II clinical trials have indicated a high efficacy in stimulating anti-tumor immunity in GBM patients with consequently longer survival times. During the Phase II clinical trial for rindopepimut, it was demonstrated that 31% of newly diagnosed GBM patients overexpressed EGFRvIII. Phase III clinical trials were launched at the end of 2011, entitled, “ACT IV Study” by the drug company, Celldex. Currently, the company is also testing the efficacy of this therapeutic agent in the setting of recurrent disease in a double-blinded phase II study called “ReACT”. In this study, rindopepimut is being used together with bevacizumab. This study aims to elucidate the role of rindopepimut in progression free survival of patients with EGFRvIII+, bevacizumab naïve- or resistant recurrent-glioma. Although rindopepimut has shown both pre-clinical and clinical success in treating GBM patients that express EGFRvIII, future work is required for the approximately 69% of patients that do not overexpress the target antigen in GBM cells.
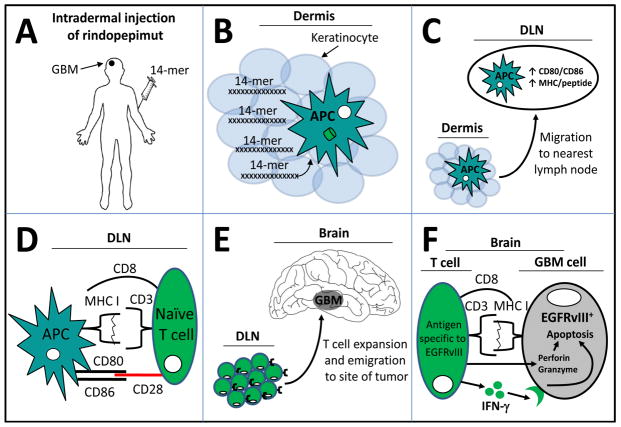
Figure 3. Intradermal injection of rindopepimut results in a systemic immune response to EGFRvIII+ glioblastoma multiforme cells.
(A) Rindopepimut is synthesized as a 14-mer peptide vaccine and is being tested in the context of co-incident administration of GM-CSF or keyhole limpet hemocyanin adjuvants. The 14-mer is intradermally injected into GBM patients with the expectation that (B) dermis-resident Langerhans (dendritic) cells, or other types of antigen presenting cells (APCs) will phagocytize the peptides. In the context of adjuvant with rindopepimut, the DCs emigrate from the skin to a draining lymph node (DLN). (C) During transit and/or once in the lymph node, the DCs finish their maturation program of processing the 14-mer into MHC molecules for future presentation to T cells. This maturation is co-incident with co-stimulatory molecule upregulation of CD80 and CD86. (D) Once in the DLN, the newly immigrated APCs loaded with the 14-mer present peptide-MHC I in the context of CD8 (although peptide-MHC II in the context of CD4 also occurs; not shown). (E) Activated T cells undergo a rapid and clonal expansion, before emigrating from the DLN to sites of EGFRvIII-expressing GBM cells. (F) Inside the tumor, CD8+ T cells interact directly with EGFRvIII+ cells and target those cells for apoptosis by secreting a combination of perforin, granzyme, and interferon-gamma (IFN-γ). The end result is decreased tumor size and an increased lifespan of GBM-bearing patients.
7.1.2 Immunotoxins
Immunotoxins are a class of recombinant molecules that bind selectively to cell surface receptors over-expressed by tumors and induce internalization for toxin-delivery of apoptosis-triggered pathways. These consist of a tumor-specific monoclonal antibody conjugated to a toxin or a recombinantly generated immunotoxic molecule. The toxin is the most consistent part of the molecule, typically originating from Pseudomonas aeruginosa (PE) or Diphteria (DT) exotoxin. Immunotoxins developed for GBM target molecules that are over-expressed, including receptors for IL-13 [82, 83], IL-4 [84], epidermal growth factor (EGF) [80] and urokinase-type plasminogen activator (uPAR) [85, 86]. The antibody-toxin fusion is selectively internalized by glioma cells and inhibits protein synthesis, which induces apoptosis without affecting normal brain cells. Immunotoxins have been shown to be very effective against tumor cells that are radio-and chemo-resistant. They have also been shown to be relatively safe in early clinical trials. Here, we briefly describe some of the immunotoxins that are currently being tested as possible treatments for GBM.
IL-13 receptors are high affinity tumor-specific targets. The immunotoxin, IL-13-PE. has been shown to be cytotoxic to glioma cell lines in vitro and has been tested in Phase I and II clinical trials using convection enhanced delivery (CED) for patients with recurrent or progressive WHO grade III/IV malignant gliomas [87]. Overall, median survival for GBM patients was 42.7 weeks or 55.6 weeks for patients with optimally positioned catheters [88]. The recombinant fusion protein IL-4-PE is cytotoxic to glioma cell lines in vitro, while less cytotoxic to hematopoietic and normal brain cells. An extended Phase I/II clinical trial of IL-4-PE in histologically verified grade III and IV astrocytomas determined that ~70% of patients showed discernible glioma necrosis as evidenced by decreased tumor size on MRI [89], without systemic cytotoxicity [90]. The overall median survival was 8.2 months with a median survival of 5.8 months for the GBM patients.
Despite promising results from early clinical trials, the PRECISE study, a randomized Phase III clinical trial, did not show a significant survival benefit of cintredekin besudotox (CB; IL-13PE) when compared with Gliadel wafers (GW) in adult patients with GBM at first recurrence [91]. One drawback from the design of this study was the inclusion of any GBM patient, without prior verification of GBM-expressed, IL-13R. Since IL-13R expression is highly variable among GBM specimens, this factor may have contributed to the overall lack of efficacy. Alternatively, variations in catheter placement may have resulted in poor perfusion of CB into the GBM. However, the impact of catheter placement on long-term clinical outcome has been scrutinized by Mueller et al, [92] finding no improvement in local perfusion with better catheter positioning.
The extracellular domain of EGFR binds to either EGF or TGF-α, resulting in receptor dimerization. TP-38 is an immunotoxin that targets EGFR. This recombinant protein is a fusion of the toxin, PE-38, with TGF-α. A phase I clinical trial was conducted with recurrent primary or metastatic malignant brain tumor patients where the dose-escalation of TP-38 demonstrated a median survival of 28 weeks post-TP-38 treatment and a median survival of 20 weeks or 33 weeks for those with residual disease or no evidence of residual disease, respectively [93]. However, the potential efficacy of TP-38 may be severely influenced by the ineffective infusion into the brain tumor mass, as was evidenced by imaging the co-infused I123-albumin.
The immunotoxin DTAT targets uPAR expressed on both GBM cells and on tumor neovasculature [94–96]. This recombinant protein was highly selective in vitro for human GBM and when used in vivo, caused the regression of subcutaneous uPAR-expressing tumors with a low level of toxicity to critical organs [95]. An additional immunotoxin targeting uPAR, DTAT13, is a bispecific immunotoxin synthesized to target GBM cells expressing both uPAR and IL-13R [97]. This recombinant protein is highly selective and synergistic for human GBM. It caused the regression of small tumors, as well as GBM, with less cytotoxicity than DTAT [98, 99].
7.1.3 DC-based therapies
Most tumors develop measures to suppress or circumvent the development of an effective immune response. To combat this challenge, therapies are being developed to target antigen presenting cells (APC), such as DC that, would allow for the development of an anti-tumor specific immune response. Included in these approaches are vaccinations utilizing autologous DC pulsed with tumor lysates, in vitro, prior to adoptive transfer of those cells into the host systemic circulation [100–103] or vaccination that targets the tumor-specific epitope of EGFRvIII, which is not expressed in normal adult human brain [104, 105]. Dendritic cells are the most potent APC, due to their ability to express MHC at relatively high levels, effectively inducing tumor specific CD8+- and CD4+- T cell-mediated anti-tumor responses [106]. This property of DC is constantly being explored, as evidenced by the many Phase I and II on-going DC-focused clinical trials. Table 6 lists all of the on-going trials, both domestically and internationally. In addition, the FMS-like tyrosine kinase 3 ligand (Flt3L) leads to the differentiation of precursor cells into DC through a STAT3-dependent mechanism. Recent work has shown that the expression of human Flt3L via adenoviral transduction of pre-clinical brain tumors leads to both the recruitment of bone marrow-derived DC to the brain tumor microenvironment, as well as the induction of in situ priming against brain tumor antigens [107–109].
Table 6.
On-going clinical trials using dendritic cell-based immunotherapy.
| TITLE | PHASE | LOCATION | AGENT | FORMAT |
|---|---|---|---|---|
| Surgical resection with gliadel wafer followed by DC vaccination of malignant glioma patients | Phase 1 | Cedars-Sinai Medical Center | DC | Surgery + Gliadel wafer + 3 DC injections at 2 week intervals |
| DC vaccine for patients with brain tumors | Phase II (new and recurrent) | UCLA | Autologous DC co-incubated with tumor lysate | Cohort 1: Surgery + Tumor lysate-pulsed DC; Cohort 2: Surgery + Tumor lysate-pulsed DC + (TLR7 agonist); Cohort 3: Surgery + Tumor lysate-pulsed DC + (TLR3 agonist) |
| DC-activated CIKCs combined with DC treatment for glioma | Phase I and II (new and recurrent) | Qingdao University (China) | DC-activated CIKCs | Surgery + Irradiation + 4 DC injections (pulsed with tumor lysate) at weekly intervals + intrathecally administered DC-activated CIKCs |
| DC cancer vaccine for high-grade glioma (GBM-Vax) | Phase II (multi-center) | Trimed Biotech GmbH (Austria) | Trivax: IL-12-secreting DC co-incubated with tumor lysate | Therapy cohort: Surgery + TMZ + Irradiation + Trivax; Control cohort: Surgery +TMZ + Irradiation |
| Efficacy and safety of autologous DC vaccination in GBM after complete surgical resection | Phase II (new) | Instituto Cientifico y Tecnologico de Navarra, Universidad de Navarra | Autologous DC; leukapheresis-extracted monocytes and co-incubated with tumor lysate | Therapy cohort: Surgery + Chemotherapy + Irradiation + Subcutaneous vaccination with autologous DC; Control cohort: Surgery + Chemotherapy + Irradiation |
| Vaccine therapy with or without sirolimus in treating patients with NY-ESO-1-expressing solid tumors | Phase I (all solid tumors) | Roswell Park Cancer Institute | DEC-205-NY-ESO-1 fusion protein vaccine | Cohort 1a:DEC-205-NY-ESO-1 at days 1, 29, 57, and 113; Cohort 1b: DEC-205- NY-ESO-1 + sirolimus at days 1–14, 29–42, and 57–70; Cohort 1c: DEC-205-NY-ESO-1 + sirolimus at days 15–28, 43–56, and 71–84; Cohort 1d: DEC-205-NY- ESO-1 vaccine + sirolimus at days 1–84 |
| Study of the drug [DCVax®-L] for treating newly diagnosed GBM | Phase II (new) | Northwest Biotherapeutics | DCVax®-L, Autologous DC pulsed with tumor lysate antigen | Therapy cohort: Surgery + TMZ + Irradiation + 2 intradermal injections of DCVax®-L; Control cohort: Surgery + TMZ + Irradiation + Autologous PBMCs |
| Vaccine therapy for treating patients undergoing surgery for recurrent GBM | Phase I (recurrent) | Duke University | Autologous CD133+ BTSC mRNA-pulsed autologous DC | Surgical resection + Autologous BTSC mRNA-loaded DC at weekly intervals (×3), then at monthly intervals in the absence of disease progression or high toxicity |
| A study of ICT-107 immunotherapy in GBM | Phase IIb (randomized, double blind) | ImmunoCellular Therapeutics, Ltd. | Autologous DC pulsed with immunogenic tumor peptides | Therapy cohort: Surgery + TMZ + Irradiation + ICT-107; Control cohort: Surgery + TMZ + Irradiation + Autologous DCs not pulsed with Ags |
COND: condition; DC: dendritic cells; CIKC: cytokine-induce killer cell; BTSC: brain tumor stem cell; IL-12: interleukin 12; Ags: antigens.
7.1.4 Daclizumab
Several groups, including ours, have shown the survival benefits of depleting Tregs from pre-clinical mouse models through targeting IL-2Rα (CD25), a receptor constitutively expressed by Tregs [12, 17, 19]. Based on the success of CD25 antibodies in targeting Tregs in pre-clinical mouse cancer models, humanized anti-CD25 has recently been brought to the market and is referred to by its trade name, daclizumab. Recent work has demonstrated that this drug has potent effects in controlling immunosuppressive Treg levels when combined with other forms of immunotherapy in patients with GBM [11]. Notably, glioma-resident Tregs have been shown to be decreased after systemic administration of anti-CD25, post-intracranial injection of brain tumor cells, in a pre-clinical mouse brain tumor model [12]. These data suggest that, the Treg-depleting antibody possesses some level of access to the Tregs within the brain tumor compartment.
7.1.5 Ipilimumab
CTLA-4 is constitutively expressed by Tregs, transiently upregulated on activated T effectors and has been shown to inhibit effector T cell responses via interaction with CD80/CD86. Pre-clinical brain tumor models have confirmed this effect, demonstrating that systemically-administered neutralizing anti-CTLA-4 antibodies lead to tumor rejection [110]. Ipilimumab is a humanized anti-CTLA-4 antibody that has recently been FDA approved for the use in melanoma patients. Although the effects of ipilimumab have yet to be tested in GBM patients, melanoma patients have witnessed a durable and potentially curable tumor regression after treatment [111].
7.1.6 Other therapies
In order to target tumor-specific antigens, other strategies are currently also being evaluated. These include radio-immunotherapy, consisting of radiolabeled monoclonal antibodies specific to antigens overexpressed in GBM, such as EGFR [112] or integrin alphavbeta3 [113]. Labeling tumor-specific monoclonal antibodies with 125I allows for targeted GBM cell destruction through radiation-mediated DNA damage. These treatments currently show promising results in pre-clinical models and early clinical-studies.
Oncolytic viral vectors, such as the Herpes simplex virus-thymidine kinase (HSV1-TK) are also being evaluated as anti-tumor therapies [114]. The advantage of using oncolytic viruses is the selectivity for replication within tumor cells, but not in untransformed tissues. To further enhance the efficacy of HSV1-TK, this viral vector is being combined with gancyclovir and immunostimulatory co-treatments, such as TNF-α [115], Flt3L [116–118] and IL-4 [119], in an attempt to increase tumor destruction. The dual gene therapy with TNF-α and HSV-TK resulted in direct cytotoxicity through binding of cognate receptors. The combined therapy of Flt3L with HSV-TK and gancyclovir resulted in the combined effects of DC recruitment, killing of tumor cells and long-term survival. Thus, the use of immunostimulatory molecules, in combination with oncolytic viruses, enables increased tumor killing and rejection.
Recent work has demonstrated thatCpG-based immunotherapies are a treatment option for various tumors, including GBM. CpG is a synthetic oligodeoxynucleotide (ODN) with unmethylated CG dinucleotides. CpG mimics microbial DNA and activates the immune system by activating TLR9. DC and B cells express TLR9 in the endoplasmic reticulum. TLR9 is also expressed in breast[120], gastric[121], lung- [122] and prostate cancer, [123] as well as glioma.
Previous work has shown that the expression of TLR9 increases according to the histopathological grade of glioma [76]. Furthermore, TLR9 expression correlates with shorter PFS and OS in patients with GBM [124]. Stimulation of TLR9-expressing breast cancer cells, astrocytoma and GBM cells with CpG oligonucleotides increased in vitro invasion- and MMP-13 levels. However, neutralizing the MMP-13 reduced invasive properties [125]..
In vivo, treatment of immunocompetent experimental mouse brain tumor models with CpG-ODN inhibited glioma growth and significantly increased survival of tumor-bearing mice [126, 127]. The combination of local CpG-ODN and radiotherapy has also been shown to induce complete tumor remission in treated animals, significantly higher than treatment with CpG-ODN, alone [128]. CpG-ODN treatment induced TLR9 down-regulation, apoptosis of GL261 cells and enhancement of antigen presenting capability by microglia, leading to a decreased level of tumor-resident Tregs[127].
7.2 Pre-clinical testing
7.2.1 Denileukin diftitox
IL-2Rα is the high affinity aspect of the IL-2R and is expressed by Tregs. Although some therapies, such as daclizumab, directly target IL-2Rα via a monoclonal antibody, this response may lead to secondary inflammation due to an Fc-triggered immune response. Under normal conditions, this may not be considered a problem. However, in the context of GBM, whereby the disease is perpetuated by inflammation, this issue may be necessary to avoid. In contrast to the actions of daclizumab, denileukin diftitox is a compound whereby IL-2 directly conjugated to the Diphtheria toxin. This combination may be able to induce apoptosis directly in Tregs and have a similar effect as daclizumab. However, investigation into the benefits of denileukin diftitox in brain tumors has yet to be explored.
7.2.2 LY2109761
TGF-β is a prevalent cytokine in the brain tumor microenvironment and its negative role in tumor progression has been recognized in many ways. Different approaches have been utilized to decrease TGF-β levels including siRNA-mediated neutralization and monoclonal antibody-mediated depletion. However, recent work has shown that instead of decreasing TGF-β, directly, targeting the TGF-β receptor (TGF-βR) may be a more effective way to limit tumor growth. The TGF-βR I kinase inhibitor, LY2109761, has been shown sensitize GBM cells, as well as GBM cancer stem cells to radiation, resulting in increased apoptosis during co-incident inhibition of DNA damage repair, mesenchymal transition and angiogenesis [129] in a mouse model that utilized human GBM. Furthermore, combining LY2109761 with the standard clinical treatment of radiation and TMZ, significantly reduced tumor size in a nude mouse model implanted with human GBM. Although these data are very promising in nude mouse models, it is important to establish if these inhibitors also convey a similar clinically-relevant benefit in an immunocompetent mouse model, since TGF-βR I is expressed by many infiltrating T cells.
7.2.3 MDX-1106
The expression of the inhibitory ligand, B7-H1, on tumor cells, and the corresponding T cell co-inhibitory receptor, PD-1, has been demonstrated in several mouse models of cancer [130]. In addition, a recent Phase I clinical trial of the anti-PD-1 immunotherapy, MDX-1106, showed tolerability and anti-tumor activity in patients with non-GBM solid tumors [131]. The effects of anti-PD1 immunotherapy appears to be critical in reversing CD8+ cytotoxic T cell anergy in melanoma [132]. Moreover, the data strongly suggest that although solo anti-PD-1 immunotherapy has a clear benefit by inducing tumor immunity, combining PD-1 inhibition with Lag3 inhibition synergizes to functionally reverse immunosuppression in T cells [133]. Furthermore, PD-L1 has been demonstrated to induce Tregs from conventional T cells, as well as maintain their suppressive phenotype [134], potentially contributing to the expansion of GBM-resident Tregs. Recent work support this observation by demonstrating that human Th1 cells can be converted into Tregs via a PD-1-dependent pathway [135]. Regardless, a synergy between induction/conversion of CD4+ T cells into immunosuppressive Tregs, in combination with suppression of cytotoxicity by CD8+ T cells appears to lead to the potent immunosuppression mediated by PD-1 in the tumor microenvironment.
7.2.4 1-MT
Indoleamine 2,3 dioxygenase (IDO) is an inducible enzyme that catabolizes the essential amino acid, tryptophan, to kynurenine. IDO is expressed by cultured glioma cells and increases in expression in the presence of IFN-γ [136, 137] via the Jak/STAT pathway. Recent work has shown that IFN-γ increases the expression of IDO in cultured glioma cells. Interestingly, IDO expression by monocytes or DCs directly and potently induces Tregs [138, 139]. In addition, recent work has found that the Treg-inducing cytokine, TGF-β [140], can also regulate IDO expression via interaction with NF-κB pathway. Functionally, stromal-cell deficiency of IDO results in slower tumor progression and increased papilloma-free survival in a mouse model of skin carcinogenesis. This effect may be associated with the ability of IDO-expressing antigen presenting cells to suppress T cell responses [141]. Moreover, one of the powerful aspects of targeting IDO is that it is an induced enzyme and therefore, not normally expressed in tissues, as well as its inhibition not being associated with significant autoimmune side effects [142]. Moreover, we have reviewed the IDO inhibitor in addition to the other immunotherapeutic inhibitors available in Figure 4. However, while the IDO inhibitor, 1-MT, is currently in clinical trials for solid tumors, the effects of this drug in brain tumor patients has yet to be investigated.
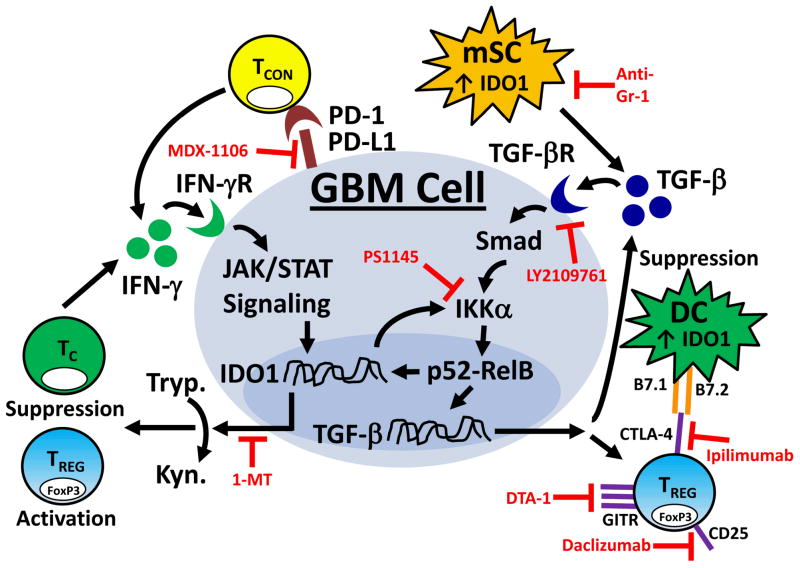
Figure 4. Representative model of the potential targets for effective immunotherapy in brain tumors.
Here we present a schematic of the immunosuppressive pathways throughout the brain tumor and molecular inhibitors that reverse the potent immunosuppression. It has previously been demonstrated that glioblastoma multiforme (GBM) cells, as well as tumor-resident dendritic cells (DC) and myeloid-derived suppressor cells (mSC) express indoleamine 2,3 dioxygenase 1 (IDO1). IDO1 expression is regulated by the Jak/STAT and NF-κB pathways, which can be induced by IFN-γ- and TGF-β-receptor activation, respectively. IDO1 is a cytoplasmic enzyme that metabolizes tryptophan (Tryp.) to kynurenine (Kyn.), the latter of which has a potently suppressive effect on T effector responses and an inducing effect on Treg function. In contrast, IDO1 can also directly activate NF-κB signaling which is able to maintain and/or upregulate TGF-β expression. The increased TGF-β levels reinforce Treg-expressed CTLA-4 and GITR. CTLA-4 directly interacts with B7.1 (CD80) and B7.2 (CD86) on DCs, resulting in downregulation of further T cell stimulation. The GBM and mSC both express TGF-β, which can synergize with PD-L1 to suppress the T cell effector response. By targeting one of more of these immunoregulatory pathways regulated by GBM, it may be possible to reverse the immunosuppression. Candidate targets for immunoregulatory suppression are shown in red. Note: Although IDO1 expression and signaling are only shown in GBM cells, a similar mechanistic pattern is presumed to also occur in DC and mSC. TCON: conventional CD4+FoxP3− T cell; TREG: regulatory CD4+FoxP3+ T cell; TC: cytotoxic CD8+ T cell; 1-MT (1-methyl tryptophan): inhibitor of IDO1; PS1145: inhibitor of the NF-κB pathway; DTA-1: monoclonal agonistic antibody for GITR; Ipilimumab: humanized monoclonal antibody for CTLA-4; LY2109761: TGF-β receptor kinase inhibitor; MDX-1106: monoclonal antibody to PD-1; Anti-Gr1: mSC-depleting antibody; Daclizumab: Humanized anti-CD25 (IL-2Rα). [110, 131, 137, 140, 145–149]
8. Potential development issues
The main focus of research for the novel treatment of GBM is increasing the survival rate and prognosis of the disease. Despite the various combinatorial therapies currently in existence and on-going in clinical trials, most GBM patients eventually die from the disease. This is complicated by the presence of the BBB which prevents normal immune surveillance, a high level of angiogenesis and additive immunosuppressive signals in the context of an already highly immunosuppressive environment. Moreover, many therapies that go to clinical trials do not proceed past Phase II due to unforeseen side effects. Moreover, while pre-clinical animal models are highly advantageous to the investigation of new immunotherapies, these research tools still possess significant limitations. To think of new ways to deal with these impediments, we discuss critical aspects of these challenges, here.
8.1 Clinical trials
Although much of the testing for future immunotherapies can be performed in pre-clinical GBM rodent models to establish a proof-of-concept, clinical trials involving patients with GBM are the gold standard. However, there are key challenges to keep in mind during the planning stages of clinical development in the academic setting. Testing simple compounds for clinical efficacy can cost as little as $20,000/patient/year [143]. Thus, to have an effective sample size for statistical purposes, the cost of performing a trial reaches a very expensive level quickly. In addition, adding patients to trials can be burdensome due to regulatory requirements. Moreover, the reporting standards differ between institutions, which can raise challenges when comparing data between trials. Finally, as we have tried to convey here, it is possible that there are many different combinations of immunotherapies that synergize to form an effective therapy for GBM patients. However, testing the many different combinations is expensive, time consuming and difficult to perform in a disease with such a low patient case load.
8.2 Side effects
All pharmacotherapies are associated with side effects that range from very mild to severe symptoms. Some of the well-established drugs to treat the GBM-related symptoms of edema and seizures, including decadron or dilantin, have minor side effects including euphoria, insomnia and increased appetite or rash and clumsiness, respectively. In contrast, emerging immunotherapies that have been shown to- or have the potential to-treat GBM, have been associated with more severe symptoms. The side effects of daclizumab include an increased risk of infection that may manifest as fever, chills, sore throat, coughing, pain or swelling around a skin wound, as well as a burning sensation during urination. Similarly, ipilumumab is associated with diarrhea, colon inflammation, skin rash, hepatitis, hypophysitis, uveitis, and nephritis. Important to note, those side effects are observed often, in as many as 84% of patients treated with ipilimumab. In addition, denileukin difitox treatment has been shown to result in blurred vision, generalized swelling, easy bruising, tachycardia, fainting, back pain and difficulty with swallowing. The various side effects associated with treatment of daclizumab, ipilimumab and denileukin difitox highlight the problematic symptoms that may occur when the immunotherapy has systemic implications and lack of specificity. In contrast, the highly specific action of rindopepimut is associated with very few side effects of which only pertain to soreness of the site of injection. Thus, in order for future immunotherapies to be well-tolerated, specificity of the effector response should be taken into account.
8.3 Pre-clinical brain tumor models
Two primary forms of pre-clinical brain tumor models exist with competing advantages and limitations when considering the rational design of immunotherapy: immunocompetent and immunodeficient. These models compose a spectrum of animal paradigms that possess a normal immune system to those that lack specific immune-related molecules to those models that are completely deficient for cells required for an adaptive immune response (i.e. T and B cell-deficient). To properly utilize immunocompetent models in an orthotopic context, implantation of genetically compatible tumor cells is required to prevent graft vs. host immunity. This would be analogous to implanting tumor cells derived from a donor C57BL/6 mouse into a host C57BL/6 mouse. This method is useful for studying how immune cells infiltrate-, respond to- and mediate- anti-tumor immunity. A different way an immunotherapy can be studied is by utilizing an immunodeficient model. This type of model can be useful in several ways. First, it can act as a litmus test to determine whether a particular therapy requires a specific immune molecule or cell type to mediate an anti-tumor effect. For example, anti-CTLA-4 immunotherapy promotes brain tumor rejection in immunocompetent, but not in T cell-deficient (immunodeficient) mice intracranially-injected orthotopic brain tumors [110]. This knowledge is useful since it allows researchers to understand that 1) investigating the mechanism of action for anti-CTLA-4 in a brain tumor model that has an immunodeficiency for T cells would lead to an unproductive investigation and 2) that T cell functionality is required for translating this therapy into patients with brain tumors.
Additional ways immunodeficient models can be useful is through allowing for the study of human patient-derived GBM, in vivo, without inducing graft vs. host immunity. For example, nude mice are athymic and therefore, T cell-deficient, which allows for the implantation of human GBM and the study of different therapies that are specifically designed to reject ‘human’ brain tumors. This is particularly helpful for understanding therapies that do not require immune-cell intermediates. For example, the monoclonal antibody therapy, bevacizumab, independently inhibits vascular growth by targeting human VEGF and can be further investigated in a nude mouse implanted with human GBM.
There are limitations to any system and studying brain tumors in animal models is an attempt to mirror the effects of GBM that normally occurs in humans, in a context whereby investigators can tease out complex mechanisms under carefully-monitored studies. Utilizing these methods, understanding the role of the immune system in immunocompetent and immunodeficient brain tumor models allows for a better understanding of a drug, which is critically important for understanding how to most effectively treat brain tumor patients in the future.
9. Conclusion
Combining surgical resection, irradiation and temozolomide treatments has become standard practice for patients with GBM. However, average GBM patient survival remains at only 14.6 months post-diagnosis, suggesting that other therapeutic avenues need to be pursued. Immunotherapeutic approaches have established themselves as first-line agents in a variety of advanced solid cancers and are currently being tested in several clinical trials for GBM. Here we reviewed different immunotherapies and highlight recent developments and pitfalls.
On-going clinical trials focused on immunotherapy are investigating various ways to manipulate the microenvironment in brain tumors, whether by stimulating cytotoxic T cell responses that can directly interact with GBM cells to induce apoptosis (i.e. Rindopepimut), depleting immunosuppressive cells (i.e. Tregs) or by altering immunosuppressive environmental mechanisms (i.e. PD-1, IDO) that exist in GBM. These different immunotherapeutic approaches allow for potential combinatorial strategies by targeting different aspects of immunosuppressive processes active in brain tumors.
10. Expert Opinion
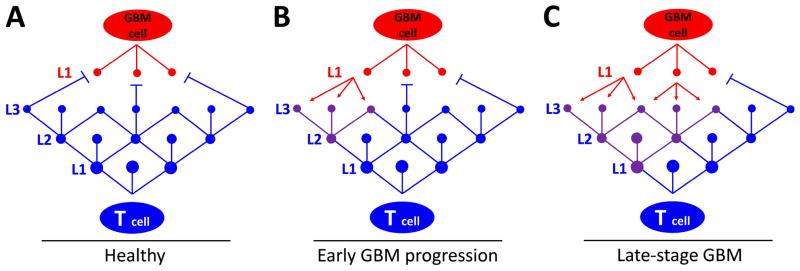
Recent work from our laboratory and others has highlighted the contextual immunosuppressive nature of the immune system in brain tumors [12, 19, 67, 144]. In this environment ‘immunosuppression begets immunosuppression’. Importantly, this highlights a concept that must be appreciated when considering the most effective design for immunotherapies of the future: brain tumor cells re-program the local microenvironment by promoting immune cell dysfunction that inhibits tumor rejection. To illustrate this point further, Figure 5 schematically represents a ball-and-stick model that highlights the competing hubs in brain tumor- and T-cells that vary in the degree of importance to survival of each cell, as well as the hubs that are critical for regulating tumor tolerance vs. rejection.
Figure 5. A model for understanding how central hubs regulate T cell-mediated rejection vs. progression in GBM.
The red and blue dots are hubs within GBM and T cells, respectively. The lines connecting the hubs represent pathways within each cellular network that cross-talk. This model focuses on T cell hubs that are both critical to overall health, as well as co-ordination of activities impacted by extracellular and intrinsic stimuli. Level 1 (L1) in T cells contains the central hubs that control fundamental T cell programming responsible for regulating T cell viability and tolerance. Level 2 (L2) hubs are classified as intermediary hubs that are important, but not required for viability. L2 hubs are associated with serving as the co-ordination centers for the majority of transcriptional activity required for signaling from the cell surface. This level links incoming (extracellular) signals with intracellular transcription networks, such as Jak/STAT signaling. Level 3 (L3) is associated with the most basic functions of T cells involving mechanisms that govern cytokine regulation, perforin/granzyme production, as well as regulation of receptor expression for molecules such as PD-1, GITR and/or TGF-βR I/II. L3 is classified as a peripheral hub. L1 of GBM cells involve pathways that overcome T cell tolerance resistance mechanisms including enzymes that regulate metabolic processes, such as IDO, upregulation of cell-surface molecules, such as B7-H1, or expression of immunosuppressive cytokines, such as IL-10 and TGF-β. (A) Under normal conditions (i.e. healthy state), T cells have a ramified network of hubs that function to both counteract pathways that induce immunosuppression by GBM cells, as well as to destroy GBM cells via cytotoxic mechanisms. (B) During early GBM progression, normal T cell defenses have been weakened and/or maladaptive responses occur. At this point, several peripheral hubs, as well as an intermediary hub in T cells are compromised (purple dots) and counteracting intracellular signals to co-ordinate tumor rejection (purple lines). (C) During late-stage tumor progression, at least one central hub in T cells has been compromised as a result of the maladaptive peripheral and intermediary hub signaling responses arising from factors derived from the GBM cell(s). At this time, T cells are primarily regulated by the GBM to promote tumor progression. This model points out that there are several pathways, both in T cells and GBM cells, with various levels of importance that can be combinatorially targeted for immunotherapeutic purposes.
In the future, we suggest that the following are important concepts to keep in mind when investigating future drugs for the treatment of brain tumors to achieve maximal efficacy:
Immunotherapies inherently mediate or initiate immune responses that can have toxic and unforeseen side effects. One of the primary side effects is typically associated with non-specific autoimmune attack of non-tumor-associated organs/tissues. However, investigation of the non-specific immune activation in pre-clinical brain tumor models may help to predict the level of peripheral immuno-toxicity to brain tumor patients during clinical trials of new immunotherapies.
Testing models of tumors in the periphery (outside of the CNS) does not recapitulate the key immunological aspects that are specific to brain tumors due to lack of appropriate context; i.e. the BBB, lack of lymphatics and highly radio-resistant stroma.
Combinatorial treatment of brain tumors with drugs designed to inhibit multiple hubs, both in T cells, as well as brain tumor cells, will likely lead to better efficacy in reversing immunosuppression due to the multiple pathways that can be compromised that combine to form the barrier of resistance to treatment.
Given these considerations, we are very optimistic that in the near future, more effective immunotherapeutic approaches will be available for our patients. For nearly 30 years, there have been very few treatments to convey optimism to patients with brain tumors. However, by re-focusing and increasing the awareness about the role of the immune system in brain cancer, the future may hold very promising success.
Footnotes
Declaration of interest:
This work was supported by the NIH grants R01 CA138587, NIH R01 CA122930, NIH U01 NS069997 and ACS RSG-07-276-01 to MS Lesniak. It was also supported by the NIH grant F32 NS073366 to DA Wainwright. The authors declare no other conflicts of interest.
Contributor Information
Dr Derek Wainwright, Email: dwainwr@surgery.bsduchicago.edu, University of Chicago, Chicago, United States.
Dr Pragati Nigam, Email: pnigam@surgery.bsd.uchicago.edu, University of Chicago, Chicago, United States.
Dr Bart Thaci, Email: bthaci@surgery.bsd.uchicago.edu, University of Chicago, Chicago, United States.
Dr Mahua Dey, Email: Mahua.dey@uchospitals.edu, University of Chicago, Chicago, United States.
Dr Maciej S Lesniak, Email: mlesniak@surgery.bsd.uchicago.edu, The University of Chicago, Division of Neurological Surgery Pritzker School of Medicine, 5841 S. Maryland Avenue, MC3026 Chicago, Chicago, IL 60637 United States.
References
- 1.de Micco C. Immunology of tumors of the central nervous system. Bull Cancer. 1989;76(1):17–31. [PubMed] [Google Scholar]
- 2.Sawamura Y, de Tribolet N. Immunobiology of brain tumors. Adv Tech Stand Neurosurg. 1990;17:3–64. doi: 10.1007/978-3-7091-6925-4_1. [DOI] [PubMed] [Google Scholar]
- 3.Reardon DA, Wen PY. Therapeutic advances in the treatment of glioblastoma: rationale and potential role of targeted agents. Oncologist. 2006;11(2):152–64. doi: 10.1634/theoncologist.11-2-152. [DOI] [PubMed] [Google Scholar]
- 4.Stupp R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 5.Bhondeley MK, et al. Imbalances in T cell subpopulations in human gliomas. J Neurosurg. 1988;68(4):589–93. doi: 10.3171/jns.1988.68.4.0589. [DOI] [PubMed] [Google Scholar]
- 6.Black KL, et al. Inflammatory leukocytes associated with increased immunosuppression by glioblastoma. J Neurosurg. 1992;77(1):120–6. doi: 10.3171/jns.1992.77.1.0120. [DOI] [PubMed] [Google Scholar]
- 7.Huettner C, et al. Interleukin 10 is expressed in human gliomas in vivo and increases glioma cell proliferation and motility in vitro. Anticancer Res. 1997;17(5A):3217–24. [PubMed] [Google Scholar]
- 8.Huettner C, Paulus W, Roggendorf W. Increased amounts of IL-10 mRNA in anaplastic astrocytomas and glioblastoma multiforme. Verh Dtsch Ges Pathol. 1994;78:418–22. [PubMed] [Google Scholar]
- 9.Huettner C, Paulus W, Roggendorf W. Messenger RNA expression of the immunosuppressive cytokine IL-10 in human gliomas. Am J Pathol. 1995;146(2):317–22. [PMC free article] [PubMed] [Google Scholar]
- 10.Tran TT, et al. Inhibiting TGF-beta signaling restores immune surveillance in the SMA-560 glioma model. Neuro Oncol. 2007;9(3):259–70. doi: 10.1215/15228517-2007-010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell DA, et al. Monoclonal antibody blockade of IL-2 receptor alpha during lymphopenia selectively depletes regulatory T cells in mice and humans. Blood. 2011;118(11):3003–12. doi: 10.1182/blood-2011-02-334565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Curtin JF, et al. Treg depletion inhibits efficacy of cancer immunotherapy: implications for clinical trials. PLoS One. 2008;3(4):e1983. doi: 10.1371/journal.pone.0001983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rolle CE, Sengupta S, Lesniak MS. Challenges in clinical design of immunotherapy trials for malignant glioma. Neurosurg Clin N Am. 2010;21(1):201–14. doi: 10.1016/j.nec.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stavrou D, et al. Immunofluorescence study of lymphocytic infiltration in gliomas. Identification of T-lymphocytes. J Neurol Sci. 1977;33(1–2):275–82. doi: 10.1016/0022-510x(77)90200-3. [DOI] [PubMed] [Google Scholar]
- 15.Clark WC, Bressler J. Transforming growth factor-beta-like activity in tumors of the central nervous system. J Neurosurg. 1988;68(6):920–4. doi: 10.3171/jns.1988.68.6.0920. [DOI] [PubMed] [Google Scholar]
- 16.Sawamura Y, et al. Isolation and in vitro growth of glioma-infiltrating lymphocytes, and an analysis of their surface phenotypes. J Neurosurg. 1988;69(5):745–50. doi: 10.3171/jns.1988.69.5.0745. [DOI] [PubMed] [Google Scholar]
- 17.El Andaloussi A, Han Y, Lesniak MS. Prolongation of survival following depletion of CD4+CD25+ regulatory T cells in mice with experimental brain tumors. J Neurosurg. 2006;105(3):430–7. doi: 10.3171/jns.2006.105.3.430. [DOI] [PubMed] [Google Scholar]
- 18.El Andaloussi A, Lesniak MS. An increase in CD4+CD25+FOXP3+ regulatory T cells in tumor-infiltrating lymphocytes of human glioblastoma multiforme. Neuro Oncol. 2006;8(3):234–43. doi: 10.1215/15228517-2006-006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fecci PE, et al. Increased regulatory T-cell fraction amidst a diminished CD4 compartment explains cellular immune defects in patients with malignant glioma. Cancer Res. 2006;66(6):3294–302. doi: 10.1158/0008-5472.CAN-05-3773. [DOI] [PubMed] [Google Scholar]
- 20.Kong LY, et al. A novel phosphorylated STAT3 inhibitor enhances T cell cytotoxicity against melanoma through inhibition of regulatory T cells. Cancer Immunol Immunother. 2009;58(7):1023–32. doi: 10.1007/s00262-008-0618-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banissi C, et al. Treg depletion with a low-dose metronomic temozolomide regimen in a rat glioma model. Cancer Immunol Immunother. 2009;58(10):1627–34. doi: 10.1007/s00262-009-0671-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maes W, et al. DC vaccination with anti-CD25 treatment leads to long-term immunity against experimental glioma. Neuro Oncol. 2009;11(5):529–42. doi: 10.1215/15228517-2009-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sengupta S, et al. Short hairpin RNA-mediated fibronectin knockdown delays tumor growth in a mouse glioma model. Neoplasia. 2010;12(10):837–47. doi: 10.1593/neo.10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morimura T, et al. Monocyte subpopulations in human gliomas: expression of Fc and complement receptors and correlation with tumor proliferation. Acta Neuropathol. 1990;80(3):287–94. doi: 10.1007/BF00294647. [DOI] [PubMed] [Google Scholar]
- 25.Kiefer R, et al. In situ detection of transforming growth factor-beta mRNA in experimental rat glioma and reactive glial cells. Neurosci Lett. 1994;166(2):161–4. doi: 10.1016/0304-3940(94)90475-8. [DOI] [PubMed] [Google Scholar]
- 26.Rossler K, et al. Expression of leucocyte adhesion molecules at the human blood-brain barrier (BBB) J Neurosci Res. 1992;31(2):365–74. doi: 10.1002/jnr.490310219. [DOI] [PubMed] [Google Scholar]
- 27.Stewart PA, et al. A quantitative analysis of blood-brain barrier ultrastructure in the aging human. Microvasc Res. 1987;33(2):270–82. doi: 10.1016/0026-2862(87)90022-7. [DOI] [PubMed] [Google Scholar]
- 28.Lowe D, et al. The effect of “ouabain” on the ultrastructure of cerebral arterioles and surrounding tissue, studied by a cannulation of a cerebral artery. Res Exp Med (Berl) 1975;166(2):97–114. doi: 10.1007/BF01851178. [DOI] [PubMed] [Google Scholar]
- 29.Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells Tissues Organs. 2001;169(1):1–11. doi: 10.1159/000047855. [DOI] [PubMed] [Google Scholar]
- 30.Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circ Res. 2005;97(6):512–23. doi: 10.1161/01.RES.0000182903.16652.d7. [DOI] [PubMed] [Google Scholar]
- 31.Janzer RC, Raff MC. Astrocytes induce blood-brain barrier properties in endothelial cells. Nature. 1987;325(6101):253–7. doi: 10.1038/325253a0. [DOI] [PubMed] [Google Scholar]
- 32.Curiel TJ, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 33.Jordan JT, et al. Preferential migration of regulatory T cells mediated by glioma-secreted chemokines can be blocked with chemotherapy. Cancer Immunol Immunother. 2008;57(1):123–31. doi: 10.1007/s00262-007-0336-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang H, Liu Y, Xiang J. Synergistic effect of adoptive T-cell therapy and intratumoral interferon gamma-inducible protein-10 transgene expression in treatment of established tumors. Cell Immunol. 2002;217(1–2):12–22. doi: 10.1016/s0008-8749(02)00508-7. [DOI] [PubMed] [Google Scholar]
- 35.Riva M, et al. Tumour-associated epilepsy: clinical impact and the role of referring centres in a cohort of glioblastoma patients. A multicentre study from the Lombardia Neurooncology Group. Neurol Sci. 2006;27(5):345–51. doi: 10.1007/s10072-006-0708-6. [DOI] [PubMed] [Google Scholar]
- 36.Van Meir EG, et al. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60(3):166–93. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hegi ME, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 38.Gorlia T, et al. Nomograms for predicting survival of patients with newly diagnosed glioblastoma: prognostic factor analysis of EORTC and NCIC trial 26981–22981/CE.3. Lancet Oncol. 2008;9(1):29–38. doi: 10.1016/S1470-2045(07)70384-4. [DOI] [PubMed] [Google Scholar]
- 39.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455(7216):1061–8. doi: 10.1038/nature07385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Verhaak RG, et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell. 2010;17(1):98–110. doi: 10.1016/j.ccr.2009.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murat A, et al. Stem cell-related “self-renewal” signature and high epidermal growth factor receptor expression associated with resistance to concomitant chemoradiotherapy in glioblastoma. J Clin Oncol. 2008;26(18):3015–24. doi: 10.1200/JCO.2007.15.7164. [DOI] [PubMed] [Google Scholar]
- 42.Bao S, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444(7120):756–60. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 43.Bleau AM, et al. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 2009;4(3):226–35. doi: 10.1016/j.stem.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei J, et al. Glioma-associated cancer-initiating cells induce immunosuppression. Clin Cancer Res. 2010;16(2):461–73. doi: 10.1158/1078-0432.CCR-09-1983. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Pistollato F, et al. Intratumoral hypoxic gradient drives stem cells distribution and MGMT expression in glioblastoma. Stem Cells. 2010;28(5):851–62. doi: 10.1002/stem.415. [DOI] [PubMed] [Google Scholar]
- 46.Spence AM, et al. Regional hypoxia in glioblastoma multiforme quantified with [18F]fluoromisonidazole positron emission tomography before radiotherapy: correlation with time to progression and survival. Clin Cancer Res. 2008;14(9):2623–30. doi: 10.1158/1078-0432.CCR-07-4995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wynn ML, Merajver SD, Schnell S. Unraveling the complex regulatory relationships between metabolism and signal transduction in cancer. Adv Exp Med Biol. 2012;736:179–89. doi: 10.1007/978-1-4419-7210-1_9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Khan RB, et al. A phase II study of extended low-dose temozolomide in recurrent malignant gliomas. Neuro Oncol. 2002;4(1):39–43. doi: 10.1215/15228517-4-1-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fadul CE, et al. Immune modulation effects of concomitant temozolomide and radiation therapy on peripheral blood mononuclear cells in patients with glioblastoma multiforme. Neuro Oncol. 2011;13(4):393–400. doi: 10.1093/neuonc/noq204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jain RK, et al. Angiogenesis in brain tumours. Nat Rev Neurosci. 2007;8(8):610–22. doi: 10.1038/nrn2175. [DOI] [PubMed] [Google Scholar]
- 51.Salmaggi A, et al. Intracavitary VEGF, bFGF, IL-8, IL-12 levels in primary and recurrent malignant glioma. J Neurooncol. 2003;62(3):297–303. doi: 10.1023/a:1023367223575. [DOI] [PubMed] [Google Scholar]
- 52.Schmidt NO, et al. Levels of vascular endothelial growth factor, hepatocyte growth factor/scatter factor and basic fibroblast growth factor in human gliomas and their relation to angiogenesis. Int J Cancer. 1999;84(1):10–8. doi: 10.1002/(sici)1097-0215(19990219)84:1<10::aid-ijc3>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 53.Zhou YH, et al. The expression of PAX6, PTEN, vascular endothelial growth factor, and epidermal growth factor receptor in gliomas: relationship to tumor grade and survival. Clin Cancer Res. 2003;9(9):3369–75. [PubMed] [Google Scholar]
- 54.Vredenburgh JJ, et al. Phase II trial of bevacizumab and irinotecan in recurrent malignant glioma. Clin Cancer Res. 2007;13(4):1253–9. doi: 10.1158/1078-0432.CCR-06-2309. [DOI] [PubMed] [Google Scholar]
- 55.Friedman HS, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27(28):4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 56.Kreisl TN, et al. A phase II trial of single-agent bevacizumab in patients with recurrent anaplastic glioma. Neuro Oncol. 2011;13(10):1143–50. doi: 10.1093/neuonc/nor091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Braganca KC, et al. Efficacy and safety of bevacizumab in active brain metastases from non-small cell lung cancer. J Neurooncol. 2010;100(3):443–7. doi: 10.1007/s11060-010-0200-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chira C, et al. Preliminary experience of whole-brain radiation therapy (WBRT) in breast cancer patients with brain metastases previously treated with bevacizumab-based chemotherapy. J Neurooncol. 2011;105(2):401–8. doi: 10.1007/s11060-011-0607-4. [DOI] [PubMed] [Google Scholar]
- 59.Ferrara N. VEGF as a therapeutic target in cancer. Oncology. 2005;69(Suppl 3):11–6. doi: 10.1159/000088479. [DOI] [PubMed] [Google Scholar]
- 60.Calabrese C, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11(1):69–82. doi: 10.1016/j.ccr.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 61.Reardon DA, et al. Glioblastoma multiforme: an emerging paradigm of anti-VEGF therapy. Expert Opin Biol Ther. 2008;8(4):541–53. doi: 10.1517/14712598.8.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Society AC. Cancer facts and figures. 2011;2011 [Google Scholar]
- 63.Kutikova L, et al. How costly is brain cancer? Healthcare services use and costs from across the US. Journal of Clinical Oncology. 2004;22(14S):1559. [Google Scholar]
- 64.Patterson H. The financial impact of brain tumors on patients and families: a summary of findings. National Brain Tumor Foundation; 2007. [Google Scholar]
- 65.Mariotto AB, et al. Projections of the cost of cancer care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–28. doi: 10.1093/jnci/djq495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmed AU, et al. Bone marrow mesenchymal stem cells loaded with an oncolytic adenovirus suppress the anti-adenoviral immune response in the cotton rat model. Mol Ther. 2010;18(10):1846–56. doi: 10.1038/mt.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wainwright DA, et al. Thymus-derived rather than tumor-induced regulatory T cells predominate in brain tumors. Neuro Oncol. 2011;13(12):1308–23. doi: 10.1093/neuonc/nor134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tran Thang NN, et al. Immune infiltration of spontaneous mouse astrocytomas is dominated by immunosuppressive cells from early stages of tumor development. Cancer Res. 2010;70(12):4829–39. doi: 10.1158/0008-5472.CAN-09-3074. [DOI] [PubMed] [Google Scholar]
- 69.Heimberger AB, et al. The role of tregs in human glioma patients and their inhibition with a novel STAT-3 inhibitor. Clin Neurosurg. 2009;56:98–106. [PubMed] [Google Scholar]
- 70.Heimberger AB, et al. Incidence and prognostic impact of FoxP3+ regulatory T cells in human gliomas. Clin Cancer Res. 2008;14(16):5166–72. doi: 10.1158/1078-0432.CCR-08-0320. [DOI] [PubMed] [Google Scholar]
- 71.Jacobs JF, et al. Prognostic significance and mechanism of Treg infiltration in human brain tumors. J Neuroimmunol. 2010;225(1–2):195–9. doi: 10.1016/j.jneuroim.2010.05.020. [DOI] [PubMed] [Google Scholar]
- 72.Bruna A, et al. High TGFbeta-Smad activity confers poor prognosis in glioma patients and promotes cell proliferation depending on the methylation of the PDGF-B gene. Cancer Cell. 2007;11(2):147–60. doi: 10.1016/j.ccr.2006.11.023. [DOI] [PubMed] [Google Scholar]
- 73.Jung Y, et al. Identification of prognostic biomarkers for glioblastomas using protein. Int J Oncol. 2012;40(4):1122–32. doi: 10.3892/ijo.2011.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seoane J, et al. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117(2):211–23. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 75.Nitta T, et al. Selective expression of interleukin-10 gene within glioblastoma multiforme. Brain Res. 1994;649(1–2):122–8. doi: 10.1016/0006-8993(94)91055-3. [DOI] [PubMed] [Google Scholar]
- 76.Wang C, et al. TLR9 expression in glioma tissues correlated to glioma progression and the prognosis of GBM patients. BMC Cancer. 2010;10:415. doi: 10.1186/1471-2407-10-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Urry Z, et al. Ligation of TLR9 induced on human IL-10-secreting Tregs by 1alpha,25-dihydroxyvitamin D3 abrogates regulatory function. J Clin Invest. 2009;119(2):387–98. doi: 10.1172/JCI32354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacobs C, et al. An ISCOM vaccine combined with a TLR9 agonist breaks immune evasion mediated by regulatory T cells in an orthotopic model of pancreatic carcinoma. Int J Cancer. 2011;128(4):897–907. doi: 10.1002/ijc.25399. [DOI] [PubMed] [Google Scholar]
- 79.Wen PY, Kesari S. Malignant gliomas in adults. N Engl J Med. 2008;359(5):492–507. doi: 10.1056/NEJMra0708126. [DOI] [PubMed] [Google Scholar]
- 80.Heimberger AB, et al. Prognostic effect of epidermal growth factor receptor and EGFRvIII in glioblastoma multiforme patients. Clin Cancer Res. 2005;11(4):1462–6. doi: 10.1158/1078-0432.CCR-04-1737. [DOI] [PubMed] [Google Scholar]
- 81.Del Vecchio CA, Wong AJ. Rindopepimut, a 14-mer injectable peptide vaccine against EGFRvIII for the potential treatment of glioblastoma multiforme. Curr Opin Mol Ther. 2010;12(6):741–54. [PubMed] [Google Scholar]
- 82.Debinski W, et al. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Clin Cancer Res. 1995;1(11):1253–8. [PubMed] [Google Scholar]
- 83.Debinski W, et al. Receptor for interleukin 13 is abundantly and specifically over-expressed in patients with glioblastoma multiforme. Int J Oncol. 1999;15(3):481–6. doi: 10.3892/ijo.15.3.481. [DOI] [PubMed] [Google Scholar]
- 84.Joshi BH, et al. In situ expression of interleukin-4 (IL-4) receptors in human brain tumors and cytotoxicity of a recombinant IL-4 cytotoxin in primary glioblastoma cell cultures. Cancer Res. 2001;61(22):8058–61. [PubMed] [Google Scholar]
- 85.Mohanam S, et al. Proteolysis and invasiveness of brain tumors: role of urokinase-type plasminogen activator receptor. J Neurooncol. 1994;22(2):153–60. doi: 10.1007/BF01052890. [DOI] [PubMed] [Google Scholar]
- 86.Yamamoto M, et al. Expression and localization of urokinase-type plasminogen activator in human astrocytomas in vivo. Cancer Res. 1994;54(14):3656–61. [PubMed] [Google Scholar]
- 87.Li YM, Hall WA. Targeted toxins in brain tumor therapy. Toxins (Basel) 2010;2(11):2645–62. doi: 10.3390/toxins2112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kunwar S, et al. Direct intracerebral delivery of cintredekin besudotox (IL13-PE38QQR) in recurrent malignant glioma: a report by the Cintredekin Besudotox Intraparenchymal Study Group. J Clin Oncol. 2007;25(7):837–44. doi: 10.1200/JCO.2006.08.1117. [DOI] [PubMed] [Google Scholar]
- 89.Rand RW, et al. Intratumoral administration of recombinant circularly permuted interleukin-4-Pseudomonas exotoxin in patients with high-grade glioma. Clin Cancer Res. 2000;6(6):2157–65. [PubMed] [Google Scholar]
- 90.Puri RK. Development of a recombinant interleukin-4-Pseudomonas exotoxin for therapy of glioblastoma. Toxicol Pathol. 1999;27(1):53–7. doi: 10.1177/019262339902700111. [DOI] [PubMed] [Google Scholar]
- 91.Kunwar S, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. Neuro Oncol. 2010;12(8):871–81. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mueller S, et al. Effect of imaging and catheter characteristics on clinical outcome for patients in the PRECISE study. J Neurooncol. 2011;101(2):267–77. doi: 10.1007/s11060-010-0255-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sampson JH, et al. Progress report of a Phase I study of the intracerebral microinfusion of a recombinant chimeric protein composed of transforming growth factor (TGF)-alpha and a mutated form of the Pseudomonas exotoxin termed PE-38 (TP-38) for the treatment of malignant brain tumors. J Neurooncol. 2003;65(1):27–35. doi: 10.1023/a:1026290315809. [DOI] [PubMed] [Google Scholar]
- 94.Rustamzadeh E, et al. Targeting the over-expressed urokinase-type plasminogen activator receptor on glioblastoma multiforme. J Neurooncol. 2003;65(1):63–75. doi: 10.1023/a:1026238331739. [DOI] [PubMed] [Google Scholar]
- 95.Rustamzadeh E, et al. Intracranial therapy of glioblastoma with the fusion protein DTAT in immunodeficient mice. Int J Cancer. 2007;120(2):411–9. doi: 10.1002/ijc.22278. [DOI] [PubMed] [Google Scholar]
- 96.Vallera DA, et al. Targeting urokinase-type plasminogen activator receptor on human glioblastoma tumors with diphtheria toxin fusion protein DTAT. J Natl Cancer Inst. 2002;94(8):597–606. doi: 10.1093/jnci/94.8.597. [DOI] [PubMed] [Google Scholar]
- 97.Todhunter DA, et al. A bispecific immunotoxin (DTAT13) targeting human IL-13 receptor (IL-13R) and urokinase-type plasminogen activator receptor (uPAR) in a mouse xenograft model. Protein Eng Des Sel. 2004;17(2):157–64. doi: 10.1093/protein/gzh023. [DOI] [PubMed] [Google Scholar]
- 98.Rustamzadeh E, et al. Immunotoxin pharmacokinetics: a comparison of the anti-glioblastoma bi-specific fusion protein (DTAT13) to DTAT and DTIL13. J Neurooncol. 2006;77(3):257–66. doi: 10.1007/s11060-005-9051-7. [DOI] [PubMed] [Google Scholar]
- 99.Rustamzadeh E, et al. Intracranial therapy of glioblastoma with the fusion protein DTIL13 in immunodeficient mice. Int J Cancer. 2006;118(10):2594–601. doi: 10.1002/ijc.21647. [DOI] [PubMed] [Google Scholar]
- 100.Heimberger AB, Sampson JH. The PEPvIII-KLH (CDX-110) vaccine in glioblastoma multiforme patients. Expert Opin Biol Ther. 2009;9(8):1087–98. doi: 10.1517/14712590903124346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Heimberger AB, et al. Immunological responses in a patient with glioblastoma multiforme treated with sequential courses of temozolomide and immunotherapy: case study. Neuro Oncol. 2008;10(1):98–103. doi: 10.1215/15228517-2007-046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wheeler CJ, et al. Clinical responsiveness of glioblastoma multiforme to chemotherapy after vaccination. Clin Cancer Res. 2004;10(16):5316–26. doi: 10.1158/1078-0432.CCR-04-0497. [DOI] [PubMed] [Google Scholar]
- 103.Liau LM, et al. Dendritic cell vaccination in glioblastoma patients induces systemic and intracranial T-cell responses modulated by the local central nervous system tumor microenvironment. Clin Cancer Res. 2005;11(15):5515–25. doi: 10.1158/1078-0432.CCR-05-0464. [DOI] [PubMed] [Google Scholar]
- 104.Sampson JH, et al. Immunologic escape after prolonged progression-free survival with epidermal growth factor receptor variant III peptide vaccination in patients with newly diagnosed glioblastoma. J Clin Oncol. 2010;28(31):4722–9. doi: 10.1200/JCO.2010.28.6963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sampson JH, et al. An epidermal growth factor receptor variant III-targeted vaccine is safe and immunogenic in patients with glioblastoma multiforme. Mol Cancer Ther. 2009;8(10):2773–9. doi: 10.1158/1535-7163.MCT-09-0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Schuler G, Schuler-Thurner B, Steinman RM. The use of dendritic cells in cancer immunotherapy. Curr Opin Immunol. 2003;15(2):138–47. doi: 10.1016/s0952-7915(03)00015-3. [DOI] [PubMed] [Google Scholar]
- 107.Curtin JF, et al. Fms-like tyrosine kinase 3 ligand recruits plasmacytoid dendritic cells to the brain. J Immunol. 2006;176(6):3566–77. doi: 10.4049/jimmunol.176.6.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ali S, et al. Inflammatory and anti-glioma effects of an adenovirus expressing human soluble Fms-like tyrosine kinase 3 ligand (hsFlt3L): treatment with hsFlt3L inhibits intracranial glioma progression. Mol Ther. 2004;10(6):1071–84. doi: 10.1016/j.ymthe.2004.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mineharu Y, et al. Engineering the brain tumor microenvironment enhances the efficacy of dendritic cell vaccination: implications for clinical trial design. Clin Cancer Res. 2011;17(14):4705–18. doi: 10.1158/1078-0432.CCR-11-0915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fecci PE, et al. Systemic CTLA-4 blockade ameliorates glioma-induced changes to the CD4+ T cell compartment without affecting regulatory T-cell function. Clin Cancer Res. 2007;13(7):2158–67. doi: 10.1158/1078-0432.CCR-06-2070. [DOI] [PubMed] [Google Scholar]
- 111.Prieto PA, et al. CTLA-4 Blockade with Ipilimumab: Long-Term Follow-up of 177 Patients with Metastatic Melanoma. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Li L, et al. A Phase II study of anti-epidermal growth factor receptor radioimmunotherapy in the treatment of glioblastoma multiforme. J Neurosurg. 2010;113(2):192–8. doi: 10.3171/2010.2.JNS091211. [DOI] [PubMed] [Google Scholar]
- 113.Veeravagu A, et al. Integrin alphavbeta3-targeted radioimmunotherapy of glioblastoma multiforme. Clin Cancer Res. 2008;14(22):7330–9. doi: 10.1158/1078-0432.CCR-08-0797. [DOI] [PubMed] [Google Scholar]
- 114.Aghi M, Martuza RL. Oncolytic viral therapies - the clinical experience. Oncogene. 2005;24(52):7802–16. doi: 10.1038/sj.onc.1209037. [DOI] [PubMed] [Google Scholar]
- 115.Niranjan A, et al. Effective treatment of experimental glioblastoma by HSV vector-mediated TNF alpha and HSV-tk gene transfer in combination with radiosurgery and ganciclovir administration. Mol Ther. 2000;2(2):114–20. doi: 10.1006/mthe.2000.0101. [DOI] [PubMed] [Google Scholar]
- 116.King GD, et al. Flt3L in combination with HSV1-TK-mediated gene therapy reverses brain tumor-induced behavioral deficits. Mol Ther. 2008;16(4):682–90. doi: 10.1038/mt.2008.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.King GD, et al. Flt3L and TK gene therapy eradicate multifocal glioma in a syngeneic glioblastoma model. Neuro Oncol. 2008;10(1):19–31. doi: 10.1215/15228517-2007-045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ghulam Muhammad AK, et al. Antiglioma immunological memory in response to conditional cytotoxic/immune-stimulatory gene therapy: humoral and cellular immunity lead to tumor regression. Clin Cancer Res. 2009;15(19):6113–27. doi: 10.1158/1078-0432.CCR-09-1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Okada H, et al. Gene therapy of malignant gliomas: a phase I study of IL-4-HSV-TK gene-modified autologous tumor to elicit an immune response. Hum Gene Ther. 2000;11(4):637–53. doi: 10.1089/10430340050015824. [DOI] [PubMed] [Google Scholar]
- 120.Ilvesaro JM, et al. Toll-like receptor 9 mediates CpG oligonucleotide-induced cellular invasion. Mol Cancer Res. 2008;6(10):1534–43. doi: 10.1158/1541-7786.MCR-07-2005. [DOI] [PubMed] [Google Scholar]
- 121.Schmausser B, et al. Toll-like receptors TLR4, TLR5 and TLR9 on gastric carcinoma cells: an implication for interaction with Helicobacter pylori. Int J Med Microbiol. 2005;295(3):179–85. doi: 10.1016/j.ijmm.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 122.Droemann D, et al. Human lung cancer cells express functionally active Toll-like receptor 9. Respir Res. 2005;6:1. doi: 10.1186/1465-9921-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ilvesaro JM, et al. Toll like receptor-9 agonists stimulate prostate cancer invasion in vitro. Prostate. 2007;67(7):774–81. doi: 10.1002/pros.20562. [DOI] [PubMed] [Google Scholar]
- 124.Leng L, Jiang T, Zhang Y. TLR9 expression is associated with prognosis in patients with glioblastoma multiforme. J Clin Neurosci. 2012;19(1):75–80. doi: 10.1016/j.jocn.2011.03.037. [DOI] [PubMed] [Google Scholar]
- 125.Merrell MA, et al. Toll-like receptor 9 agonists promote cellular invasion by increasing matrix metalloproteinase activity. Mol Cancer Res. 2006;4(7):437–47. doi: 10.1158/1541-7786.MCR-06-0007. [DOI] [PubMed] [Google Scholar]
- 126.Grauer OM, et al. TLR ligands in the local treatment of established intracerebral murine gliomas. J Immunol. 2008;181(10):6720–9. doi: 10.4049/jimmunol.181.10.6720. [DOI] [PubMed] [Google Scholar]
- 127.El Andaloussi A, et al. Stimulation of TLR9 with CpG ODN enhances apoptosis of glioma and prolongs the survival of mice with experimental brain tumors. Glia. 2006;54(6):526–35. doi: 10.1002/glia.20401. [DOI] [PubMed] [Google Scholar]
- 128.Meng Y, et al. Successful combination of local CpG-ODN and radiotherapy in malignant glioma. Int J Cancer. 2005;116(6):992–7. doi: 10.1002/ijc.21131. [DOI] [PubMed] [Google Scholar]
- 129.Zhang M, et al. Blockade of TGF-beta signaling by the TGFbetaR-I kinase inhibitor LY2109761 enhances radiation response and prolongs survival in glioblastoma. Cancer Res. 2011;71(23):7155–67. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 130.Topalian SL, Drake CG, Pardoll DM. Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol. 2012 doi: 10.1016/j.coi.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brahmer JR, et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J Clin Oncol. 2010;28(19):3167–75. doi: 10.1200/JCO.2009.26.7609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fourcade J, et al. CD8+ T Cells Specific for Tumor Antigens Can Be Rendered Dysfunctional by the Tumor Microenvironment through Upregulation of the Inhibitory Receptors BTLA and PD-1. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Woo SR, et al. Immune Inhibitory Molecules LAG-3 and PD-1 Synergistically Regulate T-cell Function to Promote Tumoral Immune Escape. Cancer Res. 2012 doi: 10.1158/0008-5472.CAN-11-1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Francisco LM, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Amarnath S, et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci Transl Med. 2011;3(111):111ra120. doi: 10.1126/scitranslmed.3003130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Miyazaki T, et al. Indoleamine 2,3-dioxygenase as a new target for malignant glioma therapy. Laboratory investigation. J Neurosurg. 2009;111(2):230–7. doi: 10.3171/2008.10.JNS081141. [DOI] [PubMed] [Google Scholar]
- 137.Avril T, et al. Distinct effects of human glioblastoma immunoregulatory molecules programmed cell death ligand-1 (PDL-1) and indoleamine 2,3-dioxygenase (IDO) on tumour-specific T cell functions. J Neuroimmunol. 2010;225(1–2):22–33. doi: 10.1016/j.jneuroim.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 138.Chung DJ, et al. Indoleamine 2,3-dioxygenase-expressing mature human monocyte-derived dendritic cells expand potent autologous regulatory T cells. Blood. 2009;114(3):555–63. doi: 10.1182/blood-2008-11-191197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sharma MD, et al. Plasmacytoid dendritic cells from mouse tumor-draining lymph nodes directly activate mature Tregs via indoleamine 2,3-dioxygenase. J Clin Invest. 2007;117(9):2570–82. doi: 10.1172/JCI31911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Pallotta MT, et al. Indoleamine 2,3-dioxygenase is a signaling protein in long-term tolerance by dendritic cells. Nat Immunol. 2011;12(9):870–8. doi: 10.1038/ni.2077. [DOI] [PubMed] [Google Scholar]
- 141.Mellor AL, et al. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J Immunol. 2002;168(8):3771–6. doi: 10.4049/jimmunol.168.8.3771. [DOI] [PubMed] [Google Scholar]
- 142.Huang L, et al. Dendritic cells, indoleamine 2,3 dioxygenase and acquired immune privilege. Int Rev Immunol. 2010;29(2):133–55. doi: 10.3109/08830180903349669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Heimberger AB, Sampson JH. Immunotherapy coming of age: what will it take to make it standard of care for glioblastoma? Neuro Oncol. 2011;13(1):3–13. doi: 10.1093/neuonc/noq169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wainwright DA, et al. The presence of IL-17A and T helper 17 cells in experimental mouse brain tumors and human glioma. PLoS One. 2010;5(10):e15390. doi: 10.1371/journal.pone.0015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nogueira L, et al. The NFkappaB pathway: a therapeutic target in glioblastoma. Oncotarget. 2011;2(8):646–53. doi: 10.18632/oncotarget.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Curti A, et al. Modulation of tryptophan catabolism by human leukemic cells results in the conversion of CD25- into CD25+ T regulatory cells. Blood. 2007;109(7):2871–7. doi: 10.1182/blood-2006-07-036863. [DOI] [PubMed] [Google Scholar]
- 147.Cohen AD, et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS One. 2010;5(5):e10436. doi: 10.1371/journal.pone.0010436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Raychaudhuri B, et al. Myeloid-derived suppressor cell accumulation and function in patients with newly diagnosed glioblastoma. Neuro Oncol. 2011;13(6):591–9. doi: 10.1093/neuonc/nor042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wing K, Yamaguchi T, Sakaguchi S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. 2011;32(9):428–33. doi: 10.1016/j.it.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 150.Nishikawa R, et al. A mutant epidermal growth factor receptor common in human glioma confers enhanced tumorigenicity. Proc Natl Acad Sci U S A. 1994;91(16):7727–31. doi: 10.1073/pnas.91.16.7727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nagane M, et al. A common mutant epidermal growth factor receptor confers enhanced tumorigenicity on human glioblastoma cells by increasing proliferation and reducing apoptosis. Cancer Res. 1996;56(21):5079–86. [PubMed] [Google Scholar]
- 152.Shinojima N, et al. Prognostic value of epidermal growth factor receptor in patients with glioblastoma multiforme. Cancer Res. 2003;63(20):6962–70. [PubMed] [Google Scholar]
- 153.Rahaman SO, et al. IL-13R(alpha)2, a decoy receptor for IL-13 acts as an inhibitor of IL-4-dependent signal transduction in glioblastoma cells. Cancer Res. 2002;62(4):1103–9. [PubMed] [Google Scholar]
- 154.Wu AH, Low WC. Molecular cloning and identification of the human interleukin 13 alpha 2 receptor (IL-13Ra2) promoter. Neuro Oncol. 2003;5(3):179–87. doi: 10.1215/S1152-8517-02-00051-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Joshi BH, et al. IL-4 receptors on human medulloblastoma tumours serve as a sensitive target for a circular permuted IL-4-Pseudomonas exotoxin fusion protein. Br J Cancer. 2002;86(2):285–91. doi: 10.1038/sj.bjc.6600034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Puri RK. Cytotoxins directed at interleukin-4 receptors as therapy for human brain tumors. Methods Mol Biol. 2001;166:155–76. doi: 10.1385/1-59259-114-0:155. [DOI] [PubMed] [Google Scholar]
- 157.Heimberger AB, et al. Dendritic cells pulsed with a tumor-specific peptide induce long-lasting immunity and are effective against murine intracerebral melanoma. Neurosurgery. 2002;50(1):158–64. doi: 10.1097/00006123-200201000-00024. discussion 164–6. [DOI] [PubMed] [Google Scholar]
- 158.Heimberger AB, et al. Bone marrow-derived dendritic cells pulsed with tumor homogenate induce immunity against syngeneic intracerebral glioma. J Neuroimmunol. 2000;103(1):16–25. doi: 10.1016/s0165-5728(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 159.Cho DY, et al. Adjuvant Immunotherapy with Whole-Cell Lysate Dendritic Cells Vaccine for Glioblastoma Multiforme: A Phase II Clinical Trial. World Neurosurg. 2011 doi: 10.1016/j.wneu.2011.08.020. [DOI] [PubMed] [Google Scholar]
- 160.Chang CN, et al. A phase I/II clinical trial investigating the adverse and therapeutic effects of a postoperative autologous dendritic cell tumor vaccine in patients with malignant glioma. J Clin Neurosci. 2011;18(8):1048–54. doi: 10.1016/j.jocn.2010.11.034. [DOI] [PubMed] [Google Scholar]
- 161.Fadul CE, et al. Immune response in patients with newly diagnosed glioblastoma multiforme treated with intranodal autologous tumor lysate-dendritic cell vaccination after radiation chemotherapy. J Immunother. 2011;34(4):382–9. doi: 10.1097/CJI.0b013e318215e300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Okada H, et al. Induction of CD8+ T-cell responses against novel glioma-associated antigen peptides and clinical activity by vaccinations with {alpha}-type 1 polarized dendritic cells and polyinosinic-polycytidylic acid stabilized by lysine and carboxymethylcellulose in patients with recurrent malignant glioma. J Clin Oncol. 2011;29(3):330–6. doi: 10.1200/JCO.2010.30.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Miyatake S, et al. Induction of human glioma-specific cytotoxic T-lymphocyte lines by autologous tumor stimulation and interleukin 2. J Neurooncol. 1986;4(1):55–64. doi: 10.1007/BF02158003. [DOI] [PubMed] [Google Scholar]
- 164.Jacobs SK, et al. Interleukin-2 and autologous lymphokine-activated killer cells in the treatment of malignant glioma. Preliminary report. J Neurosurg. 1986;64(5):743–9. doi: 10.3171/jns.1986.64.5.0743. [DOI] [PubMed] [Google Scholar]
- 165.Jacobs SK, et al. Interleukin-2 or autologous lymphokine-activated killer cell treatment of malignant glioma: phase I trial. Cancer Res. 1986;46(4 Pt 2):2101–4. [PubMed] [Google Scholar]
- 166.Jacobs SK, et al. Interleukin-2 and lymphokine activated killer (LAK) cells in the treatment of malignant glioma: clinical and experimental studies. Neurol Res. 1986;8(2):81–7. doi: 10.1080/01616412.1986.11739735. [DOI] [PubMed] [Google Scholar]
- 167.Kitahara T, et al. Establishment of interleukin 2 dependent cytotoxic T lymphocyte cell line specific for autologous brain tumor and its intracranial administration for therapy of the tumor. J Neurooncol. 1987;4(4):329–36. doi: 10.1007/BF00195603. [DOI] [PubMed] [Google Scholar]
- 168.Yoshida S, et al. Local administration of autologous lymphokine-activated killer cells and recombinant interleukin 2 to patients with malignant brain tumors. Cancer Res. 1988;48(17):5011–6. [PubMed] [Google Scholar]
- 169.Hau P, Jachimczak P, Bogdahn U. Treatment of malignant gliomas with TGF-beta2 antisense oligonucleotides. Expert Rev Anticancer Ther. 2009;9(11):1663–74. doi: 10.1586/era.09.138. [DOI] [PubMed] [Google Scholar]
- 170.Hau P, et al. TGF-beta2 Signaling in High-Grade Gliomas. Curr Pharm Biotechnol. 2011 doi: 10.2174/138920111798808347. [DOI] [PubMed] [Google Scholar]
- 171.Hau P, et al. Inhibition of TGF-beta2 with AP 12009 in recurrent malignant gliomas: from preclinical to phase I/II studies. Oligonucleotides. 2007;17(2):201–12. doi: 10.1089/oli.2006.0053. [DOI] [PubMed] [Google Scholar]
- 172.Schlingensiepen KH, et al. Antisense therapeutics for tumor treatment: the TGF-beta2 inhibitor AP 12009 in clinical development against malignant tumors. Recent Results Cancer Res. 2008;177:137–50. doi: 10.1007/978-3-540-71279-4_16. [DOI] [PubMed] [Google Scholar]
- 173.Zhang M, et al. Blockade of TGF-beta Signaling by the TGFbetaR-I Kinase Inhibitor LY2109761 Enhances Radiation Response and Prolongs Survival in Glioblastoma. Cancer Res. 2011;71(23):7155–7167. doi: 10.1158/0008-5472.CAN-11-1212. [DOI] [PubMed] [Google Scholar]
- 174.Zhang M, et al. Trimodal glioblastoma treatment consisting of concurrent radiotherapy, temozolomide, and the novel TGF-beta receptor I kinase inhibitor LY2109761. Neoplasia. 2011;13(6):537–49. doi: 10.1593/neo.11258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lohr J, et al. Effector T-cell infiltration positively impacts survival of glioblastoma patients and is impaired by tumor-derived TGF-beta. Clin Cancer Res. 2011;17(13):4296–308. doi: 10.1158/1078-0432.CCR-10-2557. [DOI] [PubMed] [Google Scholar]
- 176.Olson JJ, et al. Phase I analysis of BCNU-impregnated biodegradable polymer wafers followed by systemic interferon alfa-2b in adults with recurrent glioblastoma multiforme. J Neurooncol. 2008;90(3):293–9. doi: 10.1007/s11060-008-9660-z. [DOI] [PubMed] [Google Scholar]
- 177.Buckner JC, et al. Phase II evaluation of recombinant interferon alpha and BCNU in recurrent glioma. J Neurosurg. 1995;82(3):430–5. doi: 10.3171/jns.1995.82.3.0430. [DOI] [PubMed] [Google Scholar]
- 178.Groves MD, et al. Two phase II trials of temozolomide with interferon-alpha2b (pegylated and non-pegylated) in patients with recurrent glioblastoma multiforme. Br J Cancer. 2009;101(4):615–20. doi: 10.1038/sj.bjc.6605189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dillman RO, et al. Interferon-alpha2a and 13-cis-retinoic acid with radiation treatment for high-grade glioma. Neuro Oncol. 2001;3(1):35–41. doi: 10.1093/neuonc/3.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Grauer OM, et al. CD4+FoxP3+ regulatory T cells gradually accumulate in gliomas during tumor growth and efficiently suppress antiglioma immune responses in vivo. Int J Cancer. 2007;121(1):95–105. doi: 10.1002/ijc.22607. [DOI] [PubMed] [Google Scholar]
- 181.Grauer OM, et al. Elimination of regulatory T cells is essential for an effective vaccination with tumor lysate-pulsed dendritic cells in a murine glioma model. Int J Cancer. 2008;122(8):1794–802. doi: 10.1002/ijc.23284. [DOI] [PubMed] [Google Scholar]
- 182.Hussain SF, et al. A novel small molecule inhibitor of signal transducers and activators of transcription 3 reverses immune tolerance in malignant glioma patients. Cancer Res. 2007;67(20):9630–6. doi: 10.1158/0008-5472.CAN-07-1243. [DOI] [PubMed] [Google Scholar]
- 183.Brantley EC, Benveniste EN. Signal transducer and activator of transcription-3: a molecular hub for signaling pathways in gliomas. Mol Cancer Res. 2008;6(5):675–84. doi: 10.1158/1541-7786.MCR-07-2180. [DOI] [PMC free article] [PubMed] [Google Scholar]