Abstract
Studies over the past 5 or so years have indicated that the traditional clustering of mechanisms for translation initiation in eukaryotes into cap-dependent and cap-independent (or IRES-mediated) is far too narrow. From individual studies of a number of mRNAs encoding proteins that are regulatory in nature (i.e. likely to be needed in small amounts such as transcription factors, protein kinases, etc.), it is now evident that mRNAs exist that blur these boundaries. This review seeks to set the basic ground rules for the analysis of different initiation pathways that are associated with these new mRNAs as well as related to the more traditional mechanisms, especially the cap-dependent translational process that is the major route of initiation of mRNAs for housekeeping proteins and thus, the bulk of protein synthesis in most cells. It will become apparent that a mixture of descriptions is likely to become the norm in the near future (i.e. m7G-assisted internal initiation).
Keywords: eukaryotic protein synthesis, cap-dependent translation, IRES-mediated translation, mRNAs
1. Introduction
The purpose of this review is to pose some questions, and perhaps provide a few answers, as relates to the process of translation initiation and the regulation of this process, termed translation control. To do this requires that one have a feel for the total picture of translation so that the unique bits and pieces can be fit into particular quarters. In contrast to bacterial systems where transcriptional control is predominant, in eukaryotic systems roughly 30% of the mass of cellular protein made is subject to translational control. A part of this reflects the unique difference between eukaryotes and prokaryotes which is that in eukaryotes the mRNA emerges from the nucleus as an mRNP (Glisovic et al., 2008) that is approximately half RNA/half protein while in bacterial systems the mRNA is essentially available as a naked transcript in the same compartment as the translating ribosome where often the mRNA is in bound to ribosomes before transcription has been completed. Secondly, regulation of gene expression at the level of translation not only offers an additional point for fine tuned control, but also a more rapid response system (i.e. conversion of an inactive mRNA to an active mRNA would not require transcription, processing or transport).
2. Cap-dependent translation
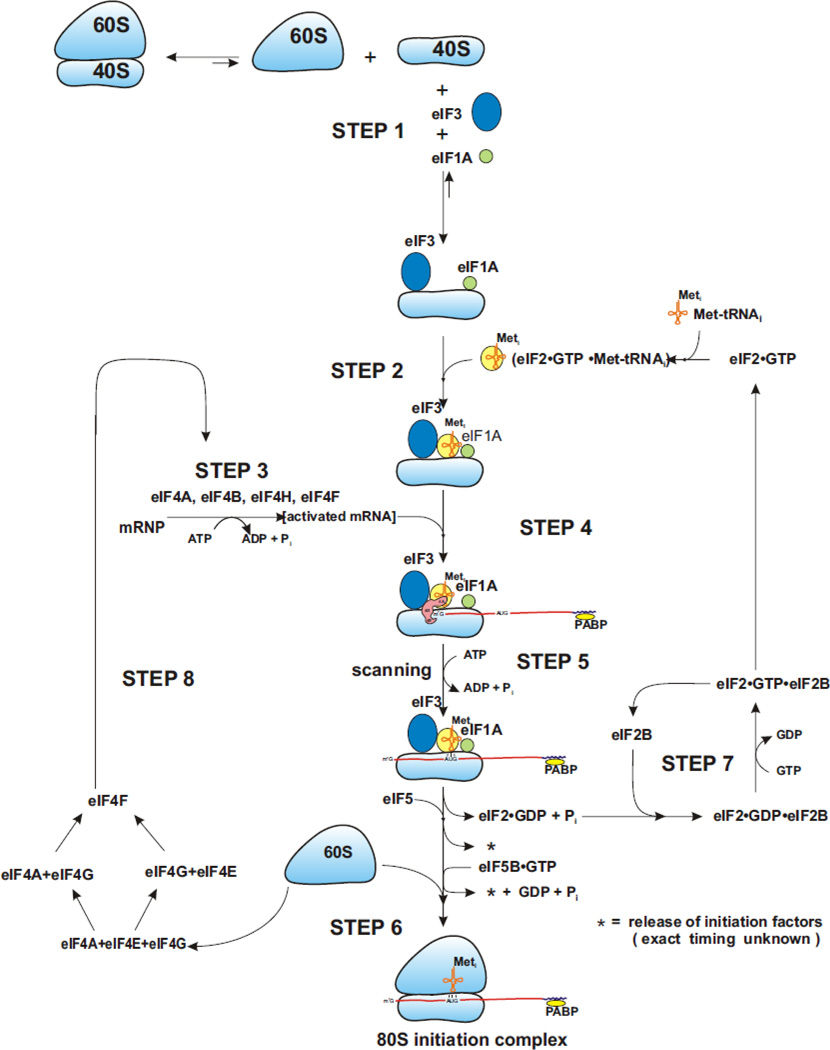
As the result of numerous studies in the 1970’s and 1980’s, a loose description of cap-dependent translation was formulated (Merrick, 1992). Although based upon a few test mRNAs, the general principles derived seemed to apply to most cellular mRNAs. In it’s simplest, the steps involved were:
binding of eIF3 to 40S subunits to block joining with the 60S subunit and thus provide a pool of small ribosomal subunits on which to build an initiation complex.
Binding of the ternary complex of eIF2•GTP•Met-tRNAi to the 40S subunit (to form a 43S complex).
Binding of eIF4F (and eIF4A and eIF4B) to mRNPs for ATP-dependent activation (generally the removal of proteins and/or secondary structure from the 5’ end of the mRNA).
Binding of the activated mRNA to 43S subunits.
Scanning of the mRNA to identify the initiating AUG codon.
Hydrolysis of the GTP in the ternary complex (and possible release of factors) and eIF5B-directed subunit joining (with GTP hydrolysis).
Recycling of the eIF2•GDP complex to eIF2•GTP
Reassembly of eIF4F from free eIF4A, eIF4E and eIF4G generated during the initiation process.
As presented, this flow scheme (see Figure 1) is over simplified in that there are no kinetic statements as to where the fast and slow steps might be and the observation that, relative to prokaryotes, there is a considerably larger number of initiation factors that participate in the process and that are critical for both efficiency and accuracy. In particular, eIF1, eIF1A, eIF2γ and eIF5 have been associated with the accurate selection of an AUG start codon as influenced by the surrounding nucleotide sequence (especially for the nucleotide at position −3 where the A in AUG is +1). This topic is reviewed extensively in Hinnebusch, 2011. And, although it is likely that for most mRNAs, eIF4A is capable of providing the motor for ATP-dependent scanning, it is becoming apparent that other RNA helicases may also participate, in particular for either long 5’ UTRs or for 5’ UTRs with extensive secondary structure (DHX29, Pisareva et al., 2008, Parsyan et al., 2009; DED1, Berthelot et al., 2004; Hilliker et al., 2011; SLH1, Daugeron et al., 2011; RNA helicase A, Ranji et al., 2011; HELZ, Hasgall et al., 2011). Thus, although the number of “refinements” seems to be growing, the basic steps of 1 through 8 seem to account for the translation of most mRNAs (especially the “traditional” housekeeping mRNAs). At the same time, the global regulation of translation initiation appears to be at either step 7, recycling of eIF2•GDP or at step 8, the reassembly of eIF4F. Both of these steps are subject to regulation through the action of protein kinases (Sonenberg and Hinnebusch, 2009).
Figure 1. The “traditional” 80S pathway.
Pictured above is an 80S pathway that has been supported by studies examining the requirements for the formation of different intermediates of the pathway and some kinetic analyses. Limitations on the accuracy of this pathway are discussed in the text. A key feature to note is that steps 7 and 8 are the primary sites of global regulation of protein synthesis. In theory, regulation at step 7 should reduce expression of all mRNAs equally while regulation at step 8 should drive mRNA competition such that less efficient mRNAs are affected much more than highly competitive mRNAs. This figure is from Merrick, 2010 and used with permission from the Journal of Biological Chemistry, American Society for Biochemistry and Molecular Biology.
3. Problematic mRNA translation
Given the 80S pathway described above, experiments from the 1980’s defined a number of characteristics of mRNAs that would decrease their efficiency in translation. These included the length of the 5’ UTR (ideal to be less than 150 nucleotides), a lack of secondary structure, a good context around the AUG start codon (A/GXXAUGG in mammalian systems), and an AUG start codon (Kozak, 1984c; Kozak, 1986; Kozak, 1989, Kozak, 1990; Pelletier, J. and Sonenberg, N., 1985). While these characteristics were contained in the mRNAs studied at the time, most of these mRNAs represented “house keeping” proteins that often were expressed at high level (0.2 to 2% of the cellular protein). Interest in the biological function of proteins then led to the study of mRNAs that encoded less abundant proteins and several problems became apparent with the “standard” 80S pathway.
The first was leaky scanning, as noted by Marilyn Kozak (Kozak, 1984a, b). Her experimental observation was that an AUG start codon might be missed if it was not in an optimal context (with the purine at minus 3 being most important; Kozak, 1984c). This property is used biologically for the glycyl-tRNA synthetase where the first AUG encodes the mitochondrial enzyme and the second, in-frame AUG encodes the cytosolic form of the enzyme (Mudge et al., 1998; Chihara et al., 2007). The protein coding region in between contains the mitochondirial import signal. Thus, two proteins are generated with the same function but destined for different cellular compartments. Although limited examples are available, it is also possible that a sequence or structure between the two AUGs might bind a protein and thus render the resulting structure stable enough to stall the movement of the scanning ribosome and thereby lead to the preferred utilization of the upstream AUG.
Other forms of leaky scanning are likely also at work for a number of mRNAs. With the advancement of the sequence databases, it became apparent that a number of cellular mRNAs contain upstream open reading frames (uORFs) or upstream start codons (uAUGs, either in-frame or out of frame), perhaps in as much as 50% of the total sequences available (Iacono et al., 2005; Calvo et al., 2009). While these different start codons might generate multiple functional proteins as noted above, these obstacles to maximal translation may also serve one of two other purposes. The first is to make the overall expression from this mRNA inefficient and thus limit the amount of protein made (Calvo et al., 2009). This “necessity” may reflect the inability to make only a single mRNA once a transcription start site is activated and if the number of mRNAs can not be well regulated to a low level, moderate level expression of the mRNA followed by poor expression from translation would yield the desired affect of limited protein production.
The second possibility is that in some manner the upstream uORF is associated with regulation of expression of the downstream protein. For many such mRNAs, the process of re-initiation appears to be applicable. In this, after the efficient recognition of the uORF, other cellular conditions influence the downstream expression of the protein. In the case of GCN4 or ATF4, this is influenced by the level of ternary complexes (eIF2•GTP•Met-tRNAi) available (Mueller and Hinnebusch, 1986; Dever et al., 1992; Lu et al., 2004). In the case of S-adenosylmethionine decarboxylase, the uORF and levels of polyamines influence the expression of the enzyme (Law et al., 2001). In another example, the peptide made by the upstream uORF can regulate the expression of the downstream protein, in this case the small subunit of the arginine-specific carbamoyl phosphate synthetase (Fang et al., 2004). In each of these instances, the important feature is the regulated expression of a limited amount of protein. In the case of GCN4, the regulated expression goes from a basal level of 1 to 3 or 8 depending on the yeast strain (Meuller and Hinnebusch, 1986; Komar et al., 2005). In contrast, removal of all the upstream AUGs in the GCN4 mRNA results in a 60-fold increase in the level of GCN4 expression. Clearly the biologically important feature is the ability to regulate GCN4 expression more than make lots of GCN4 protein (Meuller and Hinnebusch 1986).
Another consideration is elements in the mRNA that appear to enhance expression. These may be associated with either the normal 80S pathway or with IRES-mediated expression (see below). These elements come in two flavors. The first is complimentarity to the 18S rRNA, similar to the Shine-Delgano sequence found in bacterial mRNAs (Chappell et al., 2004; Yang et al., 2003). In this instance one imagines that either this sequence stabilizes the mRNA on the 40S subunit (either to increase mRNA occupancy or to facilitate AUG recognition). A second element is secondary structure just downstream of the initiating AUG which likely stalls the mRNA in the proximity of the AUG thereby facilitating the pairing of the mRNA with the anticodon of the initiator tRNA (the DLP element in SV 26S RNA; Ventoso et al, 2006). This latter feature may be present in many mRNAs as by computer prediction, most mRNAs have little secondary structure in the 5’ UTR, but more extensive secondary structure in the coding region (Dr. Harry Vorma, personal communication, 1986). As an example, globin mRNA is roughly 60% double stranded (Lockard et al., 1986), in spite of having a 5’ UTR lacking secondary structure that leads to efficient expression to reporter constructs. This example is similar to that cited above for leaky scanning interrupted by a stable secondary structure (Kozak, 1990).
If secondary structure is indeed a block to efficient translation than one would expect 5’ UTRs to be unstructured and less than 150 nucleotides. However, several exceptions to these expectations have been noted. The long (and predicted to be structured) 5’ UTRs of caulifower mosaic virus 35S RNA (Futterer et al, 1993) and late adenovirus mRNAs containing the tripartite leader (Yeuh and Schneider, 1996) appear to undergo ribosomal shunting. In this process, the m7G cap of the mRNA is recognized, but then much of the intervening nucleotides (but perhaps not all) in the 5’ UTR are bypassed to reach the initiating AUG codon. This type of mechanism has a more modern interpretation which pictures a stem-loop being bypassed during the normal process of scanning to locate an AUG start codon (Abaeva et al., 2011). A similar scanning may be occurring with the LINE-1 mRNA which has a 900 nucleotide 5’ UTR that is GC-rich. This mRNA appears to be translated in a cap-dependent manner and likely does not shunt as insertion of AUGs in the 5’ UTR alters expression (Dmitiev et al., 2007). Further experimentation is needed to determine if all the nucleotides in the LINE-1 5’ UTR are sensed or perhaps only those not within secondary structure.
Another series of mRNAs that don’t appear to adhere to the standard 80S pathway are those with short 5‘ UTRs as characterized by the TISU element (translation initiator of short 5’ UTR) or mRNAs from Giardia (Elfakess et al., 2011; Li and Wang, 2004) These mRNAs have 5‘ UTRs that vary from 1 to 15 nucleotides. Given the general site size of either eIF4A or eIF4F of 12 or more nucleotides, it is difficult to envision how these mRNAs might be recognized by eIF4F and still placed on the surface of the 40S subunit. Given the shortness of the 5’ UTR, it is likely that these mRNAs are not scanned. What is not clear is whether they require the full set of canonical initiation factors.
But that may not be the real problem .…
Most of the studies that have yielded the information cited above (and below for IRES-mediated translation) were determined in “artificial” systems which included purified protein systems, cell free extracts, or cell cultures where the cells (yeast, insect, mammalian) were in log phase growth. The cell free, purified protein systems are often not catalytic and are generally less active than the cells from which they were isolated and devoid of mRNA competition. The in vivo systems monitor protein synthesis in cells that double every 2 to 48 hours. Yet in adult, multi-cellular organisms, cell growth is much slower. In humans, the 70 kg man turns over about a 450 grams of protein a day or about 0.6% of his body weight (or correcting for 70% water, about 2% of his body dry weight). Thus, doubling in mass (if possible) would occur in about 50 days. If one were to do polysome analysis from most tissues, one would find generally few polysomes and many 40S, 60S and 80S complexes (in contrast to most logarithmically growing cells where most of the ribosomes are in polysomes with relatively few ribosomes as 40S, 60S or 80S, i.e. less than 30%; Drs. Maria Hatzoglou (Case Western Reserve University) and Scot Kimball (Pennsylvania State University at Hershey), personal communication). Thus, while there is plenty of mRNA in the cell, much of it and the translational machinery are not being used. This leaves open the possibility that a non-standard translational mechanism may have an opportunity when not faced with the very competitive process of a high level of utilization of translational components in cap-dependent translation.
One such possibility has an analogous assay and that is the poly(U)-directed synthesis of polyphenylanine, a standard elongation assay in use for over 40 years. This assay is conducted in 10 mM MgCl2 which would normally render the ribosomes to be present as 80S couples. How does the mRNA get on the ribosome? The two most likely scenarios are that the poly(U) binds to the few 40S subunits available or that the 80S ribosome breaths and this allows for poly(U) binding. Subsequent synthesis of polyphenylalanine results from either limited binding of Phe-tRNA to the P site followed by normal elongation or by the translocation of A site bound Phe-tRNA to the P site by eEF2 (Rheinberger et al., 1981). Because the phenylalanine was radiolabelled, it was possible to follow this relatively inefficient synthesis that yields less than a pmol of product (Cavallius and Merrick, 1998). This type of initiation may also account for the observed increase in the downstream reporter expression when a (UC)15 sequence was inserted into the intragenic region of a bicistronic reporter (Anthony and Merrick, 1991).
Could a similar type of initiation be occurring in whole animals? An initial test tube answer is yes as the nuclease-treated rabbit reticuloyte assay system is well known to be somewhat m7G-cap-independent and independent of a poly(A) tail, two features associated with mRNAs being well translated in vivo. A similar event may also be taking place with repeat associated non-ATG (RAN) translation. These mRNAs are derived from trinucloetide expansions leading to the insertion of 100 or more repeats in the mRNA (CAG repeats) that begin coding for poly-glutamine, poly-serine or poly-alanine depending on the reading frame (Zu et al., 2011). A similar type of event may be associated with the expression of MHC class I peptides (Starck et al., 2008, Starck and Shastri, 2011). Like the poly(U) assay, the simplest interpretation is that these proteins begin with an elongation event (binding of aminoacyl-tRNA to the A site followed by translocation) or an initiation event (either misrecognition by Met-tRNAi, non-enzymatic binding of an aminoacyl-tRNA to the P site, or use of eIF2-less intiation which appears to have a broader range of initiating tRNAs (Merrick and Anderson, 1975, Choi et al., 1998, Dmitriev et al., 2010). A potential key element of these proteins, particularly as associated with microsatellite diseases, would be their stability as it would be anticipated that the mRNA encoding the protein would not be well translated and thus protein accumulation would only occur if the protein was unusually stable.
4. IRES-mediated translation
Around 1990, several reports emerged on unique RNA sequences that would allow an mRNA to bind to 40S subunits in the vicinity of the AUG codon bypassing many upstream AUG codons (Pelletier and Sonenberg, 1988; Jang and Wimmer, 1990). This process has come to be known as internal ribosome entry and the RNA sequences associated with the process as internal ribosome entry sites or IRESs. In the first few findings, the IRES element was just 5’ of the initiating AUG. For one of these examples, polio virus, the process of infection was also associated with the inactivation of eIF4F via proteolytic cleavage of the eIF4G subunit (Grifo et al. 1984; Lee and Sonenberg, 1982). In doing this, the process of infection rendered cap-dependent translation impossible. The loss of this competitive pathway meant that now the polio virus mRNA could be translated. The early mRNAs with identified IRES elements were from viruses but raised the question as to whether there might be cellular mRNAs that were also translated via this same general mechanism. And although the answer would be yes, the strength of that conviction was tempered by the observation that the cellular IRES elements did not promote translation anywhere near as well as the viral IRES elements did (Kozak, 2005; Merrick, 2004; Shatsky et al., 2010, Gilbert, 2010). Despite the recent progress in the field, the question remains how do IRES elements recruit the 40S ribosomes to initiate translation.
The efficiency of the viral IRES elements has allowed for these elements to be studied in model systems and has provided some preliminary data on the possible mechanism of initiation. The IRES element of the polio virus mRNA binds the proteolyzed eIF4F and thus serves as an artificial cap to establish the eIF4F/eIF3 interaction necessary to dock the mRNA on the 40S subunit (Marcotrigiano et al., 2001). However, the hepatitis C virus (HCV) mRNA appears to bind to 40S subunits independent of eIF4F (Pestova et al., 1998). The cricket paralysis virus mRNA also binds independently of eIF4F but curiously begins initiation from the A site of the ribosome (in contrast to the usual binding of the initiator met-tRNAi in the P site) where the first amino acid is directed to the ribosome by an elongation factor (eEF1A) rather than an initiation factor (Wilson et al, 2000). Thus, it would appear that there are at least three different mechanisms for translating an IRES-containing viral mRNA.
Further, it appeared that at least for the viral IRES elements their 2D and 3D structures would be extremely important for their function. Much information regarding the structure of viral IRES elements came from a combination of in silico structure prediction experiments, RNA structure probing assays by RNA footprinting (using a variety of chemical and enzymatic probes) (Filbin and Kieft, 2009) and, recently, from direct Cryo-EM and/or NMR and X-ray analysis of IRES structures in their free and ribosome bound forms (Spahn, et al, 2001; Lukavsky et al., 2003; Spahn et al, 2004; Schüler et al., 2006; Pfingsten et al., 2006; Zhao et al., 2008; Berry et al., 2011).
These experiments showed that viral IRES elements possess complex secondary and tertiary structures that fold into domains with distinct functions. The prototype model for many studies was the HCV IRES, which is composed of a ~370 nt long RNA fragment and folds into four major structural domains (Lukavsky, 2009). A small (~ 15 nt; Fig. 2 from Honda et al., 1999) Domain I appeared to be dispensable for translational activity, but has been shown to be important for viral replication (Lukavsky, 2009). A 75 nt long hairpin Domain II enhances IRES activity, however it has been found to not be absolutely essential for function (Lukavsky, 2009). Domain III harbors several extremely important functional subdomains (termed IIIa – IIIf), that are essential for ribosomal recruitment and domain IV was found to contain the initiator AUG codon (Figure 2).
Figure 2. The HCV IRES element.
Shown above is the two-dimensional structure of the HCV IRES as determined by Honda et al., 1999 (used with the permission of the author and the Journal of Virology, American Society for Microbiology, publisher). As is described in the text, various parts of this structure are required for optimal protein synthesis and a number of these elements are also required for optimal replication of the virus. This can be contrasted to the smaller and less complicated cellular IRES found in the Ure2p mRNA (see Figure 4).
The overall organization of functional domains (II–IV) was found to be quite conserved among related IRES elements from the Flaviviridae family of viruses, such as the classical swine fever virus (CSFV), the bovine viral diarrhea virus (BVDV), and GB virus B (GBV-B) (see Lukavsky, 2009, for a review). However, it also appeared that structurally related viral IRESs may utilize distinct mechanisms of ribosome requitment, based on requirements for canonical and non-canonical initiation factors (Jackson et al., 2010).
At present, the actual mechanism of IRES-mediated translation is “inferred” from the translation factor requirements for the utilization of the mRNA (Piserev et al., 2007; Andreev et al., 2007). In this light, as the polio virus mRNA requires the same translation factors that are required for cap-dependent translation (although it will also use the proteolyzed eIF4F in lieu of intact eIF4F), it is possible that the sequence of steps for binding the mRNA to the 40S subunit are similar to those outlined above for cap-dependent translation. In particular, the binding of the ternary complex could precede the binding of the mRNA to the 40S subunit. However, other studies have shown that a number of such virus elements appear to be resistant to the loss of ternary complexes (eIF2•GTP•met-tRNAi) and the term “eIF2-less initiation” has been used (Pestova et al., 2008; Dmitriev et al, 2010; Thakor and Holcik, 2011; Robert et al., 2006; Ventoso et al., 2006; Kim et al., 2011). Proteins that have been suggested to serve the function of eIF2 are eIF2A, eIF2D, eIF5B or the combination of MCT-1 and DENR (Dmitriev et al., 2010; Ventoso et al., 2006; Pestova et al., 2008). Of these, eIF5B would normally seem to be a good candidate as it is the homolog of bacterial IF2 which directs the binding of the initiator tRNA to the 30S subunit in prokaryotic protein synthesis initiation.
In addition to canonical initiation factors, many IRESs also require IRES trans-acting factors (ITAFs) for their function. ITAFs have been proposed to stabilize the functional IRES conformation and/or serve as additional bridges between the IRES and the ribosome (Lewis and Holcik, 2008; Komar and Hatzoglou, 2011). For example, the encephalomyocarditis virus (EMCV) IRES activity is known to be stimulated by pyrimidine tract binding protein (PTB), and the foot-and-mouth disease virus (FMDV) IRES has been shown to require PTB and ITAF45 (Agol, 2001). Recent experiments provide clear evidence suggesting that these ITAFs promote similar conformational changes in the EMCV and FMDV IRESs resulting in compaction of these IRESs and thereby making them more competent and efficient in ribosome recruitment (Yu et al., 2011). A striking feature of many ITAFs is that they belong to the group of heterogeneous nuclear ribonucleoproteins (HnRNP A1, C1/C2, I, E1/E2, K and L) known to shuttle between the nucleus and the cytoplasm (Lewis and Holcik, 2008; Komar and Hatzoglou, 2011). Several studies have suggested that the subcellular (nuclear/cytoplasmic) distribution of ITAFs is an important determinant of IRES activity, however many aspects of ITAFs function are yet to be determined.
While the biology of using this mysterious route of internal initiation makes sense in generally finding a way to defeat or down regulate cellular (or host) cap-dependent translation to allow for the preferential translation of viral mRNAs, it was not immediately apparent how cellular IRES-containing mRNAs might also use a similar mechanism. However, the elucidation of the mTOR regulatory pathway and the activation of 4E-BP to sequester the eIF4E cap binding subunit of eIF4F provided a scheme to regulate the levels of eIF4F activity with the understanding that down regulation of cap-dependent translation was permissive for IRES-mediated translation (Raught and Gingras, 2007; Komar et al., 2003). This led to the early experimental projection that the cellular content of IRES-containing mRNAs was roughly 3% of the total mRNA pool (Johannes et al., 1999).
At the same time, it was recognized that the reduction of eIF4F activity due to stress often up regulated the synthesis of proteins that would be expected to assist the cell in recovery from that stress and that these mRNAs appeared to contain IRES elements (or at least had rather long 5’ UTRs that by folding appeared structured; reviewed in Elroy-Stein and Merrick, 2007). Thus, in a manner similar to the viral mRNAs, it was anticipated that these mRNAs might use the IRES-mediated mechanism for translation. It was later suggested that up to 10–15% of cellular mRNAs may rely on cap-independent mechanisms of translation initiation, even independently of stress (Spriggs et al, 2005). Compared to their viral counterparts, in general, cellular IRES elements appeared to be shorter in their length (~100–200 nt compared to ~300–600 nt; Komar and Hatzoglou, 2005, 2011), more diverse in their secondary structures and less stable (much reduced ΔG; Komar and Hatzoglou 2011). Cellular IRES elements appear to have no common structural motif, in contrast to viral IRES elements (Baird et al., 2007).
Many of the studies used to determine if a given mRNA might contain an IRES were based upon the use of a bi-cistronic mRNA where cap-dependent initiation would be monitored via the 5’ cistron and the suspected IRES element would be 3’ of the first cistron and 5’ to the second cistron (Pelletier and Sonenberg, 1988). With the evolution of the bi-cistronic vector containing the firefly and renella luciferase enzymes, the sensitivity for expression from either cistron became quite sensitive allowing for the detection of very low levels of protein synthesis. An RNA sequence was deemed to be an IRES if it led to more relative synthesis from the second cistron than a control RNA sequence. Because of the sensitivity of the luciferase assay or the use of radiolabelled amino acids, one might generally see a several-fold increase in expression from the second cistron without it truly being an IRES (Anthony and Merrick, 1991). Second, a number of technical issues also arose which included cryptic promoters in the IRES sequence yielding a monocistronic mRNA for the second cistron or alternative splicing. Given the small fold increase, it was often hard to tell when an IRES element had been authenticated (Kozak, 2003; Shatsky et al., 2010; Gilbert, 2010; Merrick, 2010).
In response to these concerns, a variety of alternatives were developed as tests of whether expression from the second cistron was truly IRES-mediated. The first was whether the mRNA sequence encoding the second cistron was in the bicistronic mRNA or some smaller RNA by the use of Northern blots. A second was the use of an siRNA directed against the first cistron of a bi-cistronic mRNA (Van Eden et al., 2004). In this case, an mRNA made properly would be degraded whereas an mRNA made inappropriately (i.e. cryptic promotor) would persist. A third test was to transfect RNA into the host cell. By bypassing transcription and processing, in theory the above artifacts would be avoided. In practice, most investigators used at least two of these tests as proof of the IRES element.
The story has become even more complicated when several new concepts, such as cap-assisted internal initiation and cap-independent, but 5’ -end and scanning-dependent initiation mechanisms have emerged (Gunnery, et al, 1997; Miller et al, 2007; Andreev et al, 2009; Shatsky et al, 2010; Martin et al, 2011). As all the cellular mRNAs are expected to be capped, the question is whether IRES-containing mRNAs may be translated via both mechanisms, and/or whether the cap-structure may indeed assist internal initiation becames very important. Indeed, does utilization of a cellular IRES element mean that it must be “cap-independent” (i.e. the addition of a 5’ m7G cap does not enhance translation)? In the simplest terms, no. It is possible that an mRNA might be recruited to the 40S subunit and then bypasses extensive secondary structure and bind at the IRES-AUG border. This might in fact be the process that is currently referred to as “shunting” (Yueh and Schneider, 2005) and as mentioned above, the alternate term for this process might be “m7G cap-assisted internal initiation”. Currently, many laboratories are trying to figure out what might be the reason for why a particular sequence or structure would behave as an IRES element. A reasonable guess (awaiting verification) could be that the structured/folded RNA adopts an RNA configuration similar to a tRNA and thus has some affinity for the A, P or E site of the ribosome as noted above for several viral IRESs (see above). Alternatively, the IRES element may bind with high affinity to eIF4F and serve as a “cap mimic” as was seen with poliovirus or EMCV mRNA. Or there may be proteins that specifically bind to the IRES element forming an mRNP that has properties to mimic one of the normal protein/RNA complexes that binds to the ribosome. It is clear that the level of speculation is high in the absence of more definitive data. For both cap-dependent and IRES-mediated mechanisms the main question is does a specific initiation pathway (with a strictly defined order of events) exist as seen in Figure 1? It seems that in both cases, even for one and the same mRNA, the order of events may differ (see Figure 3 for examples) and the exact pathway at a given point in time (of cellular metabolism) would depend on a number of parameters:
Relative affinities of the key players in the process to each other (mRNA, mRNA associated proteins, initiation factors, ribosomal subunits).
Presence/absence of specific modifications that may alter these affinities. This includes modifications of initiation factors (i.e. phosphorylation), modifications of ribosomal proteins as well as modifications of ribosomal RNA, (such as methylation and pseudouridylation; see below).
Availability of the key players for interaction with each other. Compartmentalization and the change in intracellular distribution of certain factors (i.e. ITAFs) are known to be critical in this regard (reviewed in Lewis and Holcik, 2008; Komar and Hatzoglou, 2011).
Figure 3. Utilization pathways of IRES-containing mRNAs.
Pictured above are five potential pathways for the initiation of protein synthesis, the pathway on the left being a simplified version of that seen in Figure 1 for cap-dependent translation. The details featured are the sequence in which the mRNA and initiating tRNA are bound to the ribosome, when subunit joining occurs and whether the binding of the initiating tRNA might be through eIF2 (the most likely) or through some other protein (less likely). The complete set of initiation factors or elongation factors necessary for any of the particular events remains to be determined in the case of cellular IRES containing mRNAs and has only been worked out for a few viral mRNAs.
With this in mind, listed below are several tests/criteria that could be used by the cell biologist to answer the question as to whether their mRNA of interest does indeed use IRES-mediated initiation.
bicistronic test (in vivo and in vitro) – use of an mRNA with an upstream ORF as cap-dependent and a down stream ORF as IRES-mediated.
monocistronic test utilizing a reporter mRNA containing hairpin structure in the 5’ UTR to prevent scanning (in the case of in vivo translation, the 5’ end should be blocked with an A-cap, preventing interaction with eIF4E or possible degradation).
polysomal abundance of the endogenous IRES-containing mRNA should be tested under normal conditions and conditions favoring IRES activity (i.e. under conditions of inhibition of cap-dependent translation).
thorough verification of RNA integrity in all the above mentioned cases should be performed.
use of under methylated (Basu et al, 2011) or under pseudouridylated (Yoon et al., 2006) ribosomes (or in yeast, the use of strains that lack ribosomal protein S25; Landry, Hertz and Thompson, 2009) as a test for the presence of the cellular IRES element.
The skepticisms (Kozak, 2005; Shatsky et al., 2010) on the authenticities of many cellular IRESs are fueled by the fact that reconstitution of the translation initiation complexes using purified components have not been reported for any of the cellular IRESs thus far in a way researchers have demonstrated this for many viral IRESs (Pestova et al., 1996; Pestova et al., 1998; Lomakin et al., 2000; Kolupaeva et al., 2003). This could be due to the many essential (yet undiscovered) ITAFs that are required to achieve successful reconstitution. It is also possible that many of these cellular IRESs may require almost all canonical initiation factors, thereby making the pathway indistinguishable from cap-dependent initiation in terms of factor requirements, however not excluding the possibility of a direct landing of the ribosome in the vicinity of the internal AUG codon. Many more experiments need to be done before one will be able to fully reconstitute and understand the cellular IRES-mediated pathway.
However, as we mentioned above, even in the case where an mRNA element could be proven to be an IRES, it would be very difficult to establish (especially in vivo) the exact order of events (pathway) leading to the formation of the elongation competent ribosomes. As illustrated in Figure 3, even in the simplest case (CrPV IRES), when the number of the key players is limited just to the IRES element, 40S and 60S ribosomal subunits and the elongation factor 1A (eEF1A) (Jan et al., 2001), the order of events might differ: the IRES might bind the 40S ribosomal subunits first (pathway B) or the IRES element may first bind the preformed 80S ribosome (pathway E, Spahn et al., 2004). With the increasing number of the key players, the number of pathways may increase dramatically and for HCV IRES one may envision already 4 (or more) pathways, which could be realized depending on the conditions and factor availability (Figure 3, pathways B, C, D and E; Ji et al., 2004; Pestova et al., 2008; Dmitriev et al., 2010) In this regard, quantitative fluorescence techniques allowing single molecule kinetic analysis of the dynamic interactions within the complexes forming along the translation initiation pathway are becoming of immense importance (Stevenson et al., 2010). However, labeling of ribosomes and initiation factors with fluorophores without compromising their function and activity still remains a challenging task. A recent breakthrough in solving the crystal structure of the yeast 80S ribosome (Ben-Shem et al., 2011) may substantially facilitate this kind of study and make the yeast system a unique one in which to address the many questions above.
5. Yeast as a model system for IRES-mediated translation
Given the various problems that have been observed both in vivo and in vitro, we have chosen to focus on yeast as the model system. This system allows one to address many questions, employing powerful genetic tools. However, the original problem was to find a yeast cellular IRES-containing mRNA as numerous unpublished reports indicated that yeast would not translate the IRES-containing mammalian mRNAs, especially the large viral IRES elements and translation of certain smaller viral IRES elements would require very specific conditions and genetic manipulations (Thompson et al., 2001). The first IRES-containing yeast mRNA for which a comprehensive in vivo and in vitro analysis was done, was that for the Ure2 protein which is one of the two best studied yeast prion-like proteins (Komar et al., 2003). Both in vivo and in vitro experiments indicated that two polypeptides were made from this mRNA, one full-length, initiating at the first AUG, and the other shortened, initiating at AUG 94 downstream. The long form of the protein was produced preferentially over the short form by a ratio of about 4 to 1. Mutation of either the first AUG or the AUG 94 resulted in only the short or the long form of the protein being made, respectively. Stress tests (e.g. heat stress) indicated that there were certain conditions that favored the long form of Ure2p and certain conditions that favored the use of the short form. Thus, nature had generated an mRNA that would satisfy all the requirements through the synthesis of the two proteins via one mRNA rather than two mRNAs.
The discovery of this IRES element required, in part, the development of an expression system with which to monitor translation. The two key elements were that the reporter mRNA was under the control of the inducible Gal promoter and that there was a stable stem-loop hairpin (ΔG >−30 kcal/mol) just 5’ of the reporter mRNA sequence to block any possible cap-dependent translation. In yeast, a secondary structure with a stability of about −20 kcal/mol (in the 5’ mRNA leader) was found to be strongly inhibitory for translation via the cap-dependent mechanism (Altmann et al, 1993). The initial reporter protein was β-Gal, but subsequent experiments have indicated that equivalent results can be obtained with luciferase as the reporter protein (and in our hands, this protein yielded better reproducibility). When tested in wild type cells, reporter expression was increased 5-fold when growth was changed from 27° to 37° C and in the yeast strain carrying the eIF4E temperature sensitive gene, the induction from a switch from the permissive to non-permissive temperature was 10-fold (Komar et al. 2003; 2005). Thus, we had established a robust assay system for IRES-mediated translation even though the level of expression was still less than that possible via a cap-dependent pathway and one where the background activity was from 1% to 4% of the induced value. However, the URE2 IRES satisfied the criteria of a “classical” IRES, i.e. its expression was eIF4E and cap-independent (Komar et al., 2003; 2005). It should be mentioned that subsequent experiments by Dr. Wendy Gilbert and colleagues discovered a number of IRES-containing yeast mRNAs associated with glucose deprivation (Gilbert et al., 2007).
A. URE2 mRNA
Having identified that an IRES element existed in the Ure2p mRNA, the minimal sequence and structure required for activity were determined (Reineke et al., 2008; Reineke and Merrick, 2009). These studies revealed that the minimal number of nucleotides necessary for IRES activity is 104 nucleotides which forms a stem loop structure with a ΔG of about −15 kcal/mol. While there is some plasticity in the structure to be functional (especially deletion of nucleotides in the loop), the stem portion with bulged nucleotides is less flexible suggesting specific sites of recognition. Curiously, changes in the stability of the stem had both positive and negative consequences. Our current model of the specific nucleotides and structure required for complete activity is pictured in Figure 4.
Figure 4. The URE2 mRNA and its IRES element.
Shown in the upper figure is a representation of the URE2 mRNA indicating the start site for cap-dependent translation, 1(AUG), and the start site for IRES-mediated initiation, 94(AUG). In the lower figure is a representation of the minimal URE2 IRES element as determined by both structure mapping and assessment of IRES strength based upon downstream protein production. N = any nucleotide; X = nucleotides for which there is no information for a particular functional role in initiation (although base pairing is required as indicated), • = base pair interactions that are partially covariable, the box indicates nucleotides that are completely covariable and the arrow points to nucleotides that are variable in single stranded regions of the minimal IRES (Reineke and Merrick, 2009). Note that the size of the loop can be reduced to 12 nucleotides although the “native” loop is 24 nucleotides.
At the same time, studies on a recently identified translation factor, eIF2A, indicated that its presence was detrimental to expression from the Ure2p IRES and that this protein appeared to locate uniquely in 80S ribosomes although some eIF2A was also found associated with 40S ribosomal subunits (Komar et al., 2005). Given the biochemical background for eIF2A (Adams et al., 1975), the simplest mechanism to explain these results was that eIF2A directed the binding of initiator tRNA to 40S subunits but then failed to be released quickly following subunit joining thus delaying the process of elongation. Although not studied as of yet, this model presumed the prior binding of the mRNA to the 40S subunit (as required by model eIF2A assays) followed by the binding of eIF2A-directed met-tRNAi. Consistent with this interpretation was the finding that HA-tagged eIF2A was found in 80S ribosomes (Zoll et al., 2002; Komar et al., 2005).
B. GIC1 and PAB1 mRNAs
As part of a diagnostic search for the genes associated with the yeast response to glucose starvation, Wendy Gilbert and colleagues identified a number of mRNAs that had long (for yeast) 5’ UTRs and were likely to contain extensive secondary structure (Gilbert et al., 2007). Among these were YMR191c, GPR1, BOI1, FLO8, NCE102, MSN1, GIC1 and PAB1. Although the minimal IRES elements were not defined for these mRNAs, the folding of the 5’ UTRs did not appear to yield any consensus structure. Second, for the YMR181c IRES element, it was confined to the −60 to +1 nucleotides (with +1 as the A in AUG of the start codon) and this sequence appeared to be unstructured and A-rich. Curiously, the poly(A)-binding protein was required for efficient IRES-mediated translation of this mRNA. This same element was found in the BOI1 and PAB1 5’ UTRs and was also necessary for efficient expression. This contrasts with a similar mRNA sequence for the PABP mRNA in which a regulatory A-rich stretch serves as a possible negative regulatory element (Patel et al., 2005; Xia et al., 2011).
A second part of the Gilbert study was how efficient these mRNAs might be. Previous studies from numerous laboratories (mostly mammalian systems) found cellular IRES-elements to direct only 1% or less the expression of a cap-dependent reporter in the same cell. As a consequence, the difference between a negative control RNA sequence and the IRES-element was generally either not impressive (several fold) or questionable. In the Gilbert et al. study, most of the mRNAs led to expression levels at least 10% of the equivalent expression from a good cap-dependent leader (Gilbert et al., 2007).
6. Proteins that may regulate IRES-mediated expression
In this review, we are considering primarily the yeast URE2, GIC1 and PAB1 mRNAs, but the evidence that the proteins to be discussed (or others) may be an integral part of IRES-mediated expression is extensive. As posed above, PABP is a positive regulatory protein for the PAB1 and BOI1 IRES elements and the A-rich stretch just upstream of the initiating AUG is the cis-acting sequence for this stimulation (Gilbert et al., 2007). In contrast, eIF2A is a negative regulatory protein that suppresses IRES-mediated expression from the URE2, GIC1 and PAB1 IRES elements (Reineke and Merrick, 2009). At present, eIF2A has suppressed all of the IRES-elements tested (all 3 that is; in our hands, the FLO8, TIF4632, YMR181c and NCE102 5’ UTRs had extensive cryptic promoter activity and thus could not be assessed accurately). Given an apparent lack of identity of cis-acting elements that might recruit eIF2A, it is possible that eIF2A is specific for the IRES-mediated pathway of translation initiation.
A curious feature of the IRESs discovered by Gilbert et al. was that they all were dependent on eIF4G for optimal translation, suggesting perhaps a mode of initiation similar to that observed for a number of mammalian IRES-containing mRNAs (Gilbert et al., 2007). As these mRNAs would normally not compete well with the cap-dependent pathway, it is clear that the association of eIF4E with eIF4G, as regulated via the mTOR pathway, can be considered as a positive or negative effecter for IRES-mediated translation (intact eIF4F as a negative regulator, eIF4G alone as a positive regulator).
Although there is limited data, it has been observed that eIF2 restriction (as the ternary complex) may not be a regulating element for some IRES elements, especially viral IRES elements. As noted earlier, a part of the answer for eIF2-less initiation may be the utilization of eIF2A, eIF2D or eIF5B as the binder of the initiator tRNA (Jan et al., 2001, Ventoso et al., 2006, Pestova et al., 2008, Dmitriev et al., 2010, Redondo et al., 2011, Verma et al, 2011, White et al., 2011). However, one also needs to consider what the pathway for IRES-mediated translation might look like. If one assume that under physiological conditions that the 40S and 60S subunits tend to bind tightly in the absence of other components, it would not be surprising to see eIF3 as an early step in the IRES-mediated pathway. However, subsequent steps seem less certain. An attractive hypothesis would be that the mRNA is bound next and before the binding of the ternary complex. This process may take advantage of the RNA structure/sequence of the IRES element and facilitate the correct placement of the mRNA on the 40S subunit (Boehringer et al., 2005; Spahn et al., 2004). This could then provide a template that might bind ternary complexes more efficiently that just the 40S•eIF3 complex and thus render this complex less sensitive to reductions in ternary complexes which often accompany most stress conditions. This would be similar to the competition between mRNAs when eIF4F becomes limiting and the more efficient mRNAs continue to be translated well while less efficient mRNAs have their expression greatly reduced (Merrick, 2003).
In contrast, if the mRNA bound to the 40S subunit after the ternary complex, then one would expect that its level of expression would directly reflect the levels of ternary complex. That is, a 50% reduction in ternary complexes should yield a 50% reduction in expression from the IRES-containing mRNA. Another possibility would be that the pathway for the binding of the IRES-containing mRNA is not linear, but rather forked such that under some conditions the ternary complex is bound first and under other conditions the mRNA is bound first. Obviously, the kinetics of expression could become much more complicated.
7. The role of the ribosome
Much of what has been discussed above implies that there is a unique property of the mRNA, or of a subset of initiation factors or ITAFs that is responsible for the placement of the IRES-containing mRNA on the 40S subunit. However, several recent reports indicate that perhaps there may be a role played by the ribosome as well. These reports indicate that pseudouridylation, methylation or the presence of ribosomal protein S25 is necessary to allow for internal initiation (Yoon et al., 2006; Landry, Hertz and Thompson, 2009; Basu et al., 2011; Jack et al., 2011). These findings would be consistent with the interpretation that there may indeed be something unique about IRES-mediated translation other than the lack of a requirement for eIF4E. At the same time, it also begs the question as to whether the ribosomes in a cell are a homogenous population of particles or whether there exists subpopulations that might have different constitutions or different post-translational/ post-transcriptional modifications that enable the ensemble to have specialized ribosomes for individual mRNAs or classes of mRNAs (Gilbert, 2011).
8. A new framework for understanding cap-dependent and cap-independent translation
Although most of the discussion above has focused upon IRES-mediated translation, control of translation at the level of initiation occurs for both cap-dependent and cap-independent translation (the latter usually being IRES-mediated). Each of these terms has an implicit understanding of mechanism. For cap-dependent translation, the mRNA is bound to 40S subunits at its 5’ end and then scanned until the ternary complex recognizes an initiating AUG codon. For cap-independent translation, an element in the mRNA recruits the 40S subunit and directs the binding such that the initiating AUG is located close to or at the P site of the ribosome. Although these descriptions often work for the processes they describe, it is becoming more and more clear that these two mechanisms do not adequately describe the many possible pathways of protein synthesis initiation although they may describe the bulk of the translation that occurs (either housekeeping mRNAs or viral mRNAs). The major exceptions that are turning up tend to be for mRNAs encoding “regulatory molecules” as either transcription factors, RNA binding proteins, stress response proteins or signaling components which are generally not needed in large amounts.
As an alternative, it may be more useful to describe the behavior of the mRNA under study without having to prove whether the mRNA is using one of the two “simple” pathways. To do this, the process may be broken down into four separate categories.
Initiation effector – does the mRNA cis-acting element enhance or reduce the level of translation initiation? Effectors of this type may be the m7G cap, an IRES element, an RNA sequence/structure that is bound by protein, the position minus 3 which influences met-tRNAi binding and accuracy, etc.
Role of the effector – how does the effector element work? The effector may bind directly to 40S subunits, eIF3 or eIF4F to assist in loading the mRNA on the 40S subunits. The effector (potentially with bound protein) may be a mimic of a tRNA (or derivative such as the ternary complex) that binds to the A, P or E site of the 40S subunit. The effector RNA sequence may be complimentary to the 40S rRNA and thereby directs binding to the 40S subunit in a manner similar to that seen in bacterial systems with the Shine-Delgarno sequence (Chappepell, et al., 2006) or may block scanning past the AUG as seen in Kozak (1990).
Binding of initiator tRNA – What protein does the mRNA in question use to direct the binding of the initiator tRNA? It is anticipated that eIF2 will perform the bulk of this function. However, under conditions of limiting or severely limiting ternary complexes, it is possible that eIF2-less initiation takes place, perhaps using eIF2A, eIF2D or eIF5B (or other proteins to be determined). Alternately, a mechanism as odd as that seen with the cricket paralysis virus may be operative where initiation is affected through the A-site of the ribosome with the complex of eEF1A•GTP•aminoacyl-tRNA (Jan et al., 2001; Zu et al., 2011).
Location of the AUG start codon – Is scanning involved? For the standard cap-dependent initiation, it is assumed that scanning will occur. However, it is possible that for short 5’ UTRs that the placement of the initiating AUG codon in the P site will occur with binding of the mRNA to the 40S subunit and thus, there might be no scanning (TISU mRNAs; Elfakess et al., 2011). And while it is generally anticipated that only limited scanning occurs with IRES-mediated translation, clearly this is a possibility as well. Thus, it is possible for either pathway that scanning might or might not occur.
As is discouraged with characterizing people, avoiding the stereotypes of either cap-dependent or cap-independent translation is undoubtedly a useful endeavor. The difficulty is that then one needs to be much more precise about the different bits and pieces that are involved in the mechanism of initiation for a given mRNA or its regulation. Clearly this is discouraging for those who wish to teach protein synthesis to graduate or undergraduate students or whose research problem brought them into this mess as a simple answer was not at hand (and this seems all the more likely for those mRNAs whose expression is acutely regulated). Additionally, this is often challenging experimentally as many of these mRNAs may not yield robust protein expression and will therefore be more difficult to study. On the other hand, for those working more in depth in the area, these subtle distinctions are what the biology is generally all about.
Highlights.
Cap-dependent vs. cap-independent initiation of protein synthesis
Role of cis-acting sequences in the 5’ UTR of mRNAs
Possible use of eIF2-less initiation of protein synthesis
Methods to test for IRES-mediated translation initiation
Acknowledgements
This work was supported in part by grants from the Human Frontiers in Science Program (RGP0024; AAK), the Ohio Scholars Program (AAK) and the National Institutes of Health (HL079164; BM).
Abbreviations
- eIF
eukaryotic initiation factor
- UTR
untranslated region
- IRES
internal ribosome entry site
- ORF
open reading frame
- EMCV
encephalomyocarditis virus
- HCV
hepatitis C virus
- HA
heme agglutinin
- PABP
poly(A)-binding protein
- ITAF
IRES trans-acting factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abaeva IS, Marintchev A, Pisareva VP, Hellen CU, Pestova TV. Bypassing of stems versus linear base-by-base inspection of mammalian mRNAs during ribosomal scanning. EMBO J. 2011;30:115–129. doi: 10.1038/emboj.2010.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams SL, Safer B, Anderson WF, Merrick WC. Eukaryotic initiation complex formation: evidence for two distinct pathways. J. Biol. Chem. 1975;250:9083–9089. [PubMed] [Google Scholar]
- Agol VI. Translational control of the picornavirus phenotype. Mol. Biol. 2001;35:691–701. [PubMed] [Google Scholar]
- Altman M, Muller PP, Wittmer B, Ruchti F, Lanker S, Trachsel H. A Saccharomyces cerevisiae homologue of mammalian translation initiation factor 4B contributes to RNA helicase activity. EMBO J. 1993;12:3997–4003. doi: 10.1002/j.1460-2075.1993.tb06077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev DE, Fernandez-Miragall O, Ramajo J, Dmitriev SE, Terenin IM, Martinez-Salas E, Shatsky IN. Differential factor requirement to assemble translation initiation complexes at the alternative start codons of foot-and-mouth disease virus RNA. RNA. 2007;13:1366–1374. doi: 10.1261/rna.469707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreev DE, Dmitriev SE, Terenin IM, Prassolov VS, Merrick WC, Shatsky IN. Differential contribution of the m7G-cap to the 5' end-dependent translation initiation of mammalian mRNAs. Nuc. Acids Res. 2009;37:6135–6147. doi: 10.1093/nar/gkp665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony DD, Merrick WC. Eukaryotic initiation factor (eIF)-4F: Implications for a role in internal initiation of translation. J. Biol. Chem. 1991;266:10218–10226. [PubMed] [Google Scholar]
- Baird SD, Lewis SM, Turcotte M, Holcik M. A search for structurally similar cellular internal ribosome entry sites. Nuc. Acids Res. 2007;35:4664–4677. doi: 10.1093/nar/gkm483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Das P, Chaudhuri S, Bevilacqua E, Andrews J, Barik S, Hatzoglou M, Komar AA, Mazumder B. Requirement of rRNA methylation for 80S ribosome assembly on a cohort of cellular Internal Ribosome Entry Sites. Mol. Cell. Biol. 2011;31:4482–4499. doi: 10.1128/MCB.05804-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shem A, Garreau de Loubresse N, Melnikov S, Jenner L, Yusupova G, Yusupov M. The structure of the eukaryotic ribosome at 3.0 Å resolution. Science. 2011;334:1524–1529. doi: 10.1126/science.1212642. [DOI] [PubMed] [Google Scholar]
- Berry KE, Waghray S, Mortimer SA, Bai Y, Doudna JA. Crystal structure of the HCV IRES central domain reveals strategy for start-codon positioning. Structure. 2011;19:1456–1466. doi: 10.1016/j.str.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthelot K, Muldoon M, Rajkowitsch L, Hughes J, McCarthy JE. Dynamics and processivity of 40S ribosome scanning on mRNA in yeast. Mol. Microbiol. 2004;51:987–1001. doi: 10.1046/j.1365-2958.2003.03898.x. [DOI] [PubMed] [Google Scholar]
- Boehringer D, Thermann R, Ostareck-Lederer A, Lewis JD, Stark H. Structure of the hepatitis C virus IRES bound to the human 80S ribosome: remodeling of the HCV IRES. Structure. 2005;13:1695–1706. doi: 10.1016/j.str.2005.08.008. [DOI] [PubMed] [Google Scholar]
- Boussadia O, Miepmann M, Creancier L, Prats A-C, Dauty F, Jacquemin-Sablon H. Unr is required in vivo for efficient initation of translation from the interal ribosome entry sites of both rhinovirus and poliovirus. J. Virol. 2003;77:3353–3359. doi: 10.1128/JVI.77.6.3353-3359.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvo SE, Pagliarini DJ, Mootha VK. Upstream open reading frames cause wide spread reduction of protein expression and are polymorphic among humans. Proc. Natl. Acad. Sci. USA. 2009;106:7507–7512. doi: 10.1073/pnas.0810916106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallius J, Merrick WC. Site-directed mutagenesis of yeast eEF1A. J. Biol. Chem. 1998;273:28752–28758. doi: 10.1074/jbc.273.44.28752. [DOI] [PubMed] [Google Scholar]
- Chappell SA, Edelman GM, Mauro VP. Biochemical and functional analysis of a 9-nt RNA sequence that affects translation efficiency in eukaryotic cells. Proc. Natl. Acad. Sci. USA. 2004;101:9590–9594. doi: 10.1073/pnas.0308759101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell SA, Dresios J, Edelman GM, Mauro VP. Ribosomal shunting mediated by a translational enhancer element that base pairs to 18S rRNA. Proc. Natl. Acad. Sci. USA. 2006;103:9488–9493. doi: 10.1073/pnas.0603597103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SK, Lee JH, Zoll WL, Merrick WC, Dever TE. Promotion of met-tRNAiMet binding to ribosomes by yIF2, a bacterial IF2 homolog in yeast. Science. 1998;280:1757–1760. doi: 10.1126/science.280.5370.1757. [DOI] [PubMed] [Google Scholar]
- Chihara T, Luginbuhl D, Luo L. Cytoplasmic and mitochondrial protein translation in axonal and dendritic terminal arboization. Nat. Neurosci. 2007;10:828–837. doi: 10.1038/nn1910. [DOI] [PubMed] [Google Scholar]
- Daugeron MC, Prouteau M, Lacroute F, Seraphin B. The highly conserved eukaryotic DRG factors are required for efficient translation in a manner redundant with the putative RNA helicase Slh1. Nuc. Acids Res. 2011;39:2221–2233. doi: 10.1093/nar/gkq898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dever TE, Feng L, Wek RC, Cigan AM, Donahue TF, Hinnebusch AG. Phosphorylation of initiation factor 2 alpha by protein kinase GCN2 mediates gene-specific translational control of GCN4 in yeast. Cell. 1992;68:585–596. doi: 10.1016/0092-8674(92)90193-g. [DOI] [PubMed] [Google Scholar]
- Dmitriev SE, Andreev DE, Terenin IM, Olovnikov IA, Prassolov VS, Merrick WC, Shatsky IN. Efficient translation initiation directed by the 900-nucleotide and GC-rich 5’ untranslated region of the human retroposon LINE-1 mRNA is strictly cap-dependent rather than internal ribosome entry site mediated. Mol. Cell. Biol. 2007;27:4685–4697. doi: 10.1128/MCB.02138-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dmitriev SE, Terenin IM, Andreev DE, Ivanov PA, Dunaevsky JE, Merrick WC, Shatsky IN. GTP-independent tRNA delivery to the ribosomal P-site by a novel eukaryotic translation factor. J. Biol. Chem. 2010;285:26779–26787. doi: 10.1074/jbc.M110.119693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elfakess R, Sinvani H, Haimov O, Svitkin Y, Sonenberg N, Dikstein R. Unique translation initiation of mRNAs-containing TISU element. Nuc. Acids Res. 2011;39:7598–7609. doi: 10.1093/nar/gkr484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elroy-Stein O, Merrick WC. Translation initiation by cellular internal ribosome entry sites. In: Sonenberg N, Hershey JWH, Matthews M, editors. Translational control in biology and medicine. Cold Spring Harbor, New York: Cold Spring Harbor Press; 2007. pp. 155–172. [Google Scholar]
- Fang P, Spevak CC, Wu C, Sachs MS. A nascent polypeptide domain that can regulate translation elongation. Proc. Natl. Acad. Sci. USA. 2004;101:4059–4064. doi: 10.1073/pnas.0400554101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbin ME, Kieft JS. Toward a structural understanding of IRES RNA function. Curr. Opin. Struct. Biol. 2009;19:267–276. doi: 10.1016/j.sbi.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futterer J, Kiss-Laszlo Z, Hohn T. Nonlinear ribosome migration on cauliflower mosaic virus 35S RNA. Cell. 1993;73:789–802. doi: 10.1016/0092-8674(93)90257-q. [DOI] [PubMed] [Google Scholar]
- Gilbert WV, Zhou K, Butler TK, Doudna JA. Cap-independent translation is required for starvation-induced differentiation in yeast. Science. 2007;317:1224–1227. doi: 10.1126/science.1144467. [DOI] [PubMed] [Google Scholar]
- Gilbert WV. Alternate ways to think about cellular internal ribosome entry. J. Biol. Chem. 2010;285:29033–29038. doi: 10.1074/jbc.R110.150532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert WV. Functional specialization of ribosomes? Trends Biochem. Sci. 2011;36:127–132. doi: 10.1016/j.tibs.2010.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008;582:1977–1986. doi: 10.1016/j.febslet.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grifo JA, Abramson RD, Satler CA, Merrick WC. RNA-stimulated ATPase activity of eukaryotic initiation factors. J. Biol. Chem. 1984;259:8648–8654. [PubMed] [Google Scholar]
- Gunnery S, Maivali U, Mathews MB. Translation of an uncapped mRNA involves scanning. J. Biol. Chem. 1997;272:21642–21646. doi: 10.1074/jbc.272.34.21642. [DOI] [PubMed] [Google Scholar]
- Hasgall PA, Hoogewijs D, Faza MB, Panse VG, Wenger RH, Camenisch G. The putative RNA helicase HELZ promotes cell proliferation, translation initiation and ribosomal protein S6 phosphorylation. PLoS One. 2011;6(7):e22107. doi: 10.1371/journal.pone.0022107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilliker A, Gao Z, Jankowsky E, Parker R. The DEAD-box protein Ded1 modulates translation by the formation and resolution of an eIF4F-mRNA complex. Mol. Cell. 2011;43:962–972. doi: 10.1016/j.molcel.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnebusch AG. Molecular mechanism of scanning and start codon selection in eukaryotes. Microbiol. Mol. Biol. Rev. 2011;75:434–467. doi: 10.1128/MMBR.00008-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Beard MR, Ping LH, Lemon SM. A phylogenetically conserved stem–loop structure at the 5' border of the internal ribosome entry site of hepatitis C virus is required for cap-independent viral translation. J. Virol. 1999;73:1165–1174. doi: 10.1128/jvi.73.2.1165-1174.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iacono M, Mignone F, Pesole G. uAUG and uORFs in human and rodent 5’ untranslated mRNAs. Gene. 2005;349:97–105. doi: 10.1016/j.gene.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Jack K, Bellodi C, Landry DM, Niederer R, Meskauskas A, Musalgaonkar S, Kopmar N, Krasnykh O, Dean AD, Thompson SR, Ruggero D, Dinman JD. rRNA Pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol. Cell. 2011;44:660–666. doi: 10.1016/j.molcel.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat. Rev. Mol. Cell Biol. 2010;11:113–127. doi: 10.1038/nrm2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan E, Thompson SR, Wilson JE, Pestova TV, Hellen CU, Sarnow P. Initiator Met-tRNA-independent translation mediated by an internal ribosome entry site element in cricket paralysis virus-like insect viruses. Cold Spring Harb. Symp. Quant. Biol. 2001;66:285–292. doi: 10.1101/sqb.2001.66.285. [DOI] [PubMed] [Google Scholar]
- Jang SK, Wimmer E. Cap-independent translation of encephalomyocarditis virus RNA: structural elements of the internal ribosomal entry site and involvement of a cellular 57-kD RNA-binding protein. Genes Dev. 1990;9:1560–1572. doi: 10.1101/gad.4.9.1560. [DOI] [PubMed] [Google Scholar]
- Ji H, Fraser CS, Yu Y, Leary J, Doudna JA. Coordinated assembly of human translation initiation complexes by the hepatitis C virus internal ribosome entry site RNA. Proc. Natl. Acad. Sci. USA. 2004;101:16990–16995. doi: 10.1073/pnas.0407402101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes G, Carter MS, Eisen MB, Brown PO, Sarnow P. Identification of eukaryotic mRNAs that are translated at reduced cap binding complex eIF4F concentrations using a cDNA microarray. Proc. Natl. Acad. Sci. USA. 1999;96:13118–13123. doi: 10.1073/pnas.96.23.13118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Park SM, Park JH, Keum SJ, Jang SK. eIF2A mediates translation of hepatitis C viral mRNA under stress conditions. EMBO J. 2011;30:2454–2464. doi: 10.1038/emboj.2011.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolupaeva VG, Lomakin IB, Pestova TV, Hellen CU. Eukaryotic initiation factors 4G and 4A mediate conformational changes downstream of the initiation codon of the encephalomyocarditis virus internal ribosomal entry site. Mol. Cell. Biol. 2003;23:687–698. doi: 10.1128/MCB.23.2.687-698.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar AA, Lesnik T, Cullin C, Merrick WC, Trachsel H, Altmann M. Internal initiation drives the synthesis of Ure2 protein lacking the prion domain and affects [URE3] propagation in yeast cells. EMBO J. 2003;22:1199–1209. doi: 10.1093/emboj/cdg103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komar AA, Gross SR, Barth-Baus D, Strachan R, Hensold JO, Kinzy TG, Merrick WC. Novel characteristics of the biological properties of the yeast Saccharomyces cerevisiae initiation factor eIF2A. J. Biol. Chem. 2005;280:15601–15611. doi: 10.1074/jbc.M413728200. [DOI] [PubMed] [Google Scholar]
- Komar AA, Hatzoglou M. Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle. 2011;10:229–240. doi: 10.4161/cc.10.2.14472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Adherence to the first-AUG rule when a second AUG codon follows closely upon the first. Proc. Natl. Acad. Sci. USA. 1984a;92:2662–2666. doi: 10.1073/pnas.92.7.2662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Selection of initiation sites by eukaryotic ribosomes: effect of inserting AUG triplets upstream from the coding sequence for preproinsulin. Nuc. Acids Res. 1984b;12:3873–3893. doi: 10.1093/nar/12.9.3873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations close to the AUG initiator codon affect the efficiency of translation of rat preproinsulin in vivo. Nature. 1984c;308:241–246. doi: 10.1038/308241a0. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translatin by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kozak M. Circumstances and mechanisms of inhibition of translation by secondary structure in eucaryotic mRNAs. Mol. Cell. Biol. 1989;11:5134–5142. doi: 10.1128/mcb.9.11.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Downstream secondary structure facilitates recognition of initiator codons by eukaryotic ribosomes. Proc. Natl. Acad. Sci. USA. 1990;87:8301–8305. doi: 10.1073/pnas.87.21.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Alternative ways to think about mRNA sequences and proteins that appear to promote internal initiation of translation. Gene. 2003;318:1–23. doi: 10.1016/s0378-1119(03)00774-1. [DOI] [PubMed] [Google Scholar]
- Kozak M. A second look at cellular mRNA sequences said to function as internal ribosome entry sites. Nuc. Acids Res. 2005;33:6593–6602. doi: 10.1093/nar/gki958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry DM, Hertz MI, Thompson RR. RPS25 is essential for translation initiation by the dicistroviridae and hepatitis C viral IRESs. Genes Dev. 2009;23:2753–2764. doi: 10.1101/gad.1832209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law GL, Raney A, Heusner C, Morris DR. Polyamine regulation of ribosome pausing at the upstream open reading frame of S-adenosylmethionine decarboxylase. J. Biol. Chem. 2001;276:38036–38043. doi: 10.1074/jbc.M105944200. [DOI] [PubMed] [Google Scholar]
- Lee KA, Sonenberg N. Inactivation of cap-binding proteins accompanies the shut-off of host protein synthesis by poliovirus. Proc. Natl. Acad. Sci. USA. 1982;79:3447–3451. doi: 10.1073/pnas.79.11.3447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SM, Holcik M. For IRES trans-acting factors, it is all about location. Oncogene. 2008;27:1033–1035. doi: 10.1038/sj.onc.1210777. [DOI] [PubMed] [Google Scholar]
- Li L, Wang CC. Capped mRNA with a single nucleotide leader is optimally translated in a primitive eukaryote, Giardia lamblia. J. Biol. Chem. 2004;279:14656–14664. doi: 10.1074/jbc.M309879200. [DOI] [PubMed] [Google Scholar]
- Lockard RE, Currey K, Browner M, Lawrence C, Maizel J. Secondary structure model for mouse beta Maj globin mRNA derived from enzymatic digestion data, comparative sequence and computer analysis. Nuc. Acids Res. 1986;14:5827–5841. doi: 10.1093/nar/14.14.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin IB, Hellen CU, Pestova TV. Physical association of eukaryotic initiation factor 4G (eIF4G) with eIF4A strongly enhances binding of eIF4G to the internal ribosomal entry site of encephalomyocarditis virus and is required for internal initiation of translation. Mol. Cell. Biol. 2000;20:6019–6029. doi: 10.1128/mcb.20.16.6019-6029.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu PD, Harding HP, Ron D. Translation reinitiation at alternative open reading frames regulates gene expression in an integrated stress response. J. Cell Biol. 2004;167:27–33. doi: 10.1083/jcb.200408003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukavsky PJ, Kim I, Otto GA, Puglisi JD. Structure of HCV IRES domain II determined by NMR. Nat. Struct. Biol. 2003;10:1033–1038. doi: 10.1038/nsb1004. [DOI] [PubMed] [Google Scholar]
- Lukavsky PJ. Structure and function of HCV IRES domains. Virus Res. 2009;139:166–171. doi: 10.1016/j.virusres.2008.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotrigiano J, Lomakin IB, Sonenberg N, Pestova TV, Hellen CU, Burley SK. A conserved HEAT domain within eIF4G directs assembly of the translation initiation machinery. Mol. Cell. 2001;1:193–203. doi: 10.1016/s1097-2765(01)00167-8. [DOI] [PubMed] [Google Scholar]
- Martin F, Barends S, Jaeger S, Schaeffer L, Prongidi-Fix L, Eriani G. Cap-assisted internal initiation of translation of histone H4. Mol. Cell. 2011;41:197–209. doi: 10.1016/j.molcel.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Merrick WC, Anderson WF. Purification and characterization of homogeneous protein synthesis initiation factor M1 from rabbit reticulocytes. J. Biol. Chem. 1975;250:1197–1206. [PubMed] [Google Scholar]
- Merrick WC. Mechanism and regulation of eukaryotic protein synthesis. Microbiol. Rev. 1992;56:291–315. doi: 10.1128/mr.56.2.291-315.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick WC. Initiation of protein biosynthesis in eukaryotes. Bioc. Mol. Biol. Ed. 2003;31:378–385. [Google Scholar]
- Merrick WC. Cap-dependent and cap-independent translation in eukaryotic systems. Gene. 2004;332:1–11. doi: 10.1016/j.gene.2004.02.051. [DOI] [PubMed] [Google Scholar]
- Merrick WC. Eukaryotic Protein Synthesis: Still a Mystery. J. Biol. Chem. 2010;285:21197–21201. doi: 10.1074/jbc.R110.111476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller WA, Wang Z, Treder K. The amazing diversity of cap-independent translation elements in the 3'-untranslated regions of plant viral RNAs. Biochem. Soc. Trans. 2007;35:1629–1633. doi: 10.1042/BST0351629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudge S, Williams J, Sutherland G, Cowan P, Power D. Complex organization of the 5’-end of the humane glycine tRNA synthetase gene. Gene. 1998;209:45–50. doi: 10.1016/s0378-1119(98)00007-9. [DOI] [PubMed] [Google Scholar]
- Meuller PP, Hinnebusch AG. Multiple upstream AUG codons mediate translational control of GCN4. Cell. 1986;45:201–207. doi: 10.1016/0092-8674(86)90384-3. [DOI] [PubMed] [Google Scholar]
- Parsyan A, Shahbazian D, Martineau Y, Petroulakis E, Alain T, Larsson O, Mathonnet G, Tettweiler G, Hellen CU, Pestova TV, Svitkin YU, Sonenberg N. The helicase protein DHX29 promotes translation initiation, cell proliferation and tumorigenesis. Proc. Natl. Acad. Sci. USA. 2009;106:22217–22222. doi: 10.1073/pnas.0909773106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel GP, Ma S, Bag J. The autoregulatory translation control element of poly(A)-binding protein mRNA forms a heteromeric ribonucleoprotein complex. Nuc. Acids. Res. 2005;33:7074–7089. doi: 10.1093/nar/gki1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J, Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985;40:515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Pelletier J, Sonenberg N. Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature. 1988;334:320–325. doi: 10.1038/334320a0. [DOI] [PubMed] [Google Scholar]
- Pestova TV, Hellen CU, Shatsky IN. Canonical eukaryotic intiation factors determine initiation of translation by internal ribosomal entry. Mol. Cell. Biol. 1996;16:6859–6869. doi: 10.1128/mcb.16.12.6859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Shatsky IN, Fletcher SP, Jackson RJ, Hellen CU. A prokaryotic-like mode of cytoplasmic eukaryotic ribosome binding to the initiation codon during internal translation initiation of hepatitis C and classical swine fever virus RNAs. Genes Dev. 1998;12:67–83. doi: 10.1101/gad.12.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, de Breyne S, Pisarev AV, Abaeva IS, Hellen CU. eIF2-dependent and eIF2-independent modes of initiation on the CSFV IRES: a common role of domain II. EMBO J. 2008;27:1060–1072. doi: 10.1038/emboj.2008.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfingsten JS, Costantino DA, Kieft JS. Structural basis for ribosome recruitment and manipulation by a viral IRES RNA. Science. 2006;314:1450–1454. doi: 10.1126/science.1133281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisarev AV, Unbehaun A, Hellen CU, Pestova TV. Assembly and analysis of eukaryotic translation initiation complexes. Meth. Enzymol. 2007;430:147–177. doi: 10.1016/S0076-6879(07)30007-4. [DOI] [PubMed] [Google Scholar]
- Pisareva VP, Pisarev AV, Komar AA, Hellen CU, Pestova TV. Translation initiation on mammalian mRNAs with structured 5’ UTRs requires DExH-box protein DhX29. Cell. 2008;135:1237–1250. doi: 10.1016/j.cell.2008.10.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranji A, Shkriabai N, Kyaratskhelia M, Musier-Forsyth K, Boris-Lawrie K. Features of double-stranded RNA-binding domains of RNA helicase A are necessary for selective recognition and translation of complex mRNAs. J. Biol. Chem. 2011;286:5328–5337. doi: 10.1074/jbc.M110.176339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raught B, Gingras A-C. Singnaling to translation initiation. In: Mathews MB, Sonenberg N, Hershey JWB, editors. Translational Control in Biology and Medicine. Cold Spring Harbor, NY: Cold Spring Harbor Press; 2007. pp. 369–400. [Google Scholar]
- Redondo N, Sanz MA, Welnowska E, Carrasco L. Translation without eIF2 promoted by poliovirus 2A protease. PLoS One. 2011;6:e25699. doi: 10.1371/journal.pone.0025699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke LC, Komar AA, Caprara MG, Merrick WC. A small stem loop element directs internal initiation of the URE2 internal ribosome entry site in Saccharomyces cerevisiae. J. Biol. Chem. 2008;283:19011–19025. doi: 10.1074/jbc.M803109200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reineke LC, Merrick WC. Characterization of the functional role of nucleotides within the URE2 IRES element and the requirements for eIF2A-mediated repression. RNA. 2009;15:2264–2277. doi: 10.1261/rna.1722809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rheinberger H-J, Sternbach H, Nierhaus KH. Three tRNA binding sites on Escherichia coli ribosomes. Proc. Natl. Acad. Sci USA. 1981;78:5310–5314. doi: 10.1073/pnas.78.9.5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert F, Kapp LD, Khan SN, Acker M, Kaufman RJ, Merrick WC, Lorsch JR, Pelletier J. Translation initiation by the HCV IRES is refractory to reduced eIF2•GTP•Met-tRNAi ternary complex availability. Mol. Biol. Cell. 2006;17:4632–4644. doi: 10.1091/mbc.E06-06-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schüler M, Connell SR, Lescoute A, Giesebrecht J, Dabrowski M, Schroeer B, Mielke T, Penczek PA, Westhof E, Spahn CM. Structure of the ribosome-bound cricket paralysis virus IRES RNA. Nat. Struct. Mol. Biol. 2006;13:1092–1096. doi: 10.1038/nsmb1177. [DOI] [PubMed] [Google Scholar]
- Shatsky IN, Dmitriev SE, Terenin IM, Andreev DE. Cap- and IRES-independent scanning mechanism of translation initiation as an alternative to the concept of cellular IRESs. Mol. Cell. 2010;30:285–293. doi: 10.1007/s10059-010-0149-1. [DOI] [PubMed] [Google Scholar]
- Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: Mechanisms and biological targets. Cell. 2009;136:731–745. doi: 10.1016/j.cell.2009.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn CM, Kieft JS, Grassucci RA, Penczek PA, Zhou K, Doudna JA, Frank J. Hepatitis C virus IRES RNA-induced changes in the conformation of the 40S ribosomal subunit. Science. 2001;291:1959–1962. doi: 10.1126/science.1058409. [DOI] [PubMed] [Google Scholar]
- Spahn CM, Jan E, Mulder A, Grassucci RA, Sarnow P, Frank J. Cryo-EM visualization of a viral internal ribosome entry site bound to human ribosomes: the IRES functions as an RNA-based translation factor. Cell. 2004;118:465–475. doi: 10.1016/j.cell.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Spriggs KA, Bushell M, Mitchell SA, Willis AE. Internal ribosome entry segment-mediated translation during apoptosis: the role of IRES-trans-acting factors. Cell Death Differ. 2005;12:585–591. doi: 10.1038/sj.cdd.4401642. [DOI] [PubMed] [Google Scholar]
- Starck SR, Ow Y, Jiang V, Tokuyama M, Rivera M, Qi X, Roberts RW, Shastri N. A distinct translation initation mechanism generates cryptic peptides for immune surveillance. PLoS ONE. 2008;3:e3460. doi: 10.1371/journal.pone.0003460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starck SR, Shastri N. Non-conventional sources of peptides presented by MHC class 1. Cell. Mol. Life Sci. 2011;68:1471–1479. doi: 10.1007/s00018-011-0655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson AL, Juanes PP, McCarthy JE. Elucidating mechanistic principles underpinning eukaryotic translation initiation using quantitative fluorescence methods. Biochem. Soc. Trans. 2010;38:1587–1592. doi: 10.1042/BST0381587. [DOI] [PubMed] [Google Scholar]
- Thakor N, Holcik M. IRES-mediated translation of cellular messenger RNA operates in eIF2{alpha}-independent manner during stress. Nuc. Acids Res. 2011;40:541–552. doi: 10.1093/nar/gkr701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SR, Gulyas KD, Sarnow P. Internal initiation in Saccharomyces cerevisiae mediated by an initiator tRNA/eIF-2 independent internal ribosome entry site element. Proc. Natl. Acad. Sci. USA. 2001;98:12972–12977. doi: 10.1073/pnas.241286698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eden ME, Byrd MP, Sherrill KW, Lloyd RE. Demonstrating internal ribosome entry sites in eukaryotic mRNAs using stringent RNA test procedures. RNA. 2004;10:720–730. doi: 10.1261/rna.5225204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventoso I, Sanz MA, Molina S, Berlanga JJ, Carrasco L, Esteban M. Translational resistance of late alphavirus mRNA to eIF2alpha phosphorylation: a strategy to overcome the antiviral effect of protein kinase PKR. Genes Dev. 2006;20:87–100. doi: 10.1101/gad.357006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma B, Ponnuswamy A, Gnanasundram SV, Das S. Cryptic AUG is important for 48S ribosomal assembly during internal initiation of translation of coxsackievirus B3 RNA. J. Gen. Virol. 2011;92:2310–2319. doi: 10.1099/vir.0.032151-0. [DOI] [PubMed] [Google Scholar]
- White JP, Reineke LC, Lloyd RE. Poliovirus switches to an eIF2-independent mode of translation during infection. J. Virol. 2011;85:8884–8893. doi: 10.1128/JVI.00792-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JE, Pestova TV, Hellen CU, Sarnow P. Initiation of protein synthesis from the A site of the ribosome. Cell. 2000;102:511–520. doi: 10.1016/s0092-8674(00)00055-6. [DOI] [PubMed] [Google Scholar]
- Xia X, Holcik M. Strong eukaryotic IRESs have weak secondary structure. PLoS One. 2009;4:e4136. doi: 10.1371/journal.pone.0004136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia X, MacKay V, Yao X, Wu J, Miura F, Ito T, Morris DR. Translation initiation: A regulatory role for poly(A) tracts in front of the AUG codon in Saccharomycves cerevisiae. Genetics. 2011;189:469–478. doi: 10.1534/genetics.111.132068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Cheung P, Sun Y, Yuan J, Zhang H, Carthy CM, Anderson DR, Bohunek L, Wilson JE, McManus BM. A Shine-Delgarno-like sequence mediates in vitro ribosomal internal entry and subsequent scanning for translation initiation of coxsackievirus B3 RNA. Virology. 2003;305:31–43. doi: 10.1006/viro.2002.1770. [DOI] [PubMed] [Google Scholar]
- Yoon A, Peng G, Brandenburger Y, Zollo O, Xu W, Rego E, Ruggero D. Impaired control of IRES-mediated translation in X-linked dyskeratosis congenita. Science. 2006;312:902–906. doi: 10.1126/science.1123835. [DOI] [PubMed] [Google Scholar]
- Yu Y, Abaeva IS, Marintchev A, Pestova TV, Hellen CU. Common conformational changes induced in type 2 picornavirus IRESs by cognate transacting factors. Nuc. Acids Res. 2011;39:4851–4865. doi: 10.1093/nar/gkr045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yueh A, Schneider RJ. Translation by ribosome shunting on adenovirus and hsp70 mRNAs facilitated by complementarity to 18S rRNA. Genes Dev. 2000;14:414–421. [PMC free article] [PubMed] [Google Scholar]
- Zhao Q, Han Q, Kissinger CR, Hermann T, Thompson PA. Structure of hepatitis C virus IRES subdomain IIa. Acta Crystallogr. D. Biol. Crystallogr. 2008;64:436–443. doi: 10.1107/S0907444908002011. [DOI] [PubMed] [Google Scholar]
- Zoll WL, Horton LE, Komar AA, Hensold JO, Merrick WC. Characterization of mammalian eIF2A and identification of the yeast homolog. J. Biol. Chem. 2002;277:37079–37087. doi: 10.1074/jbc.M207109200. [DOI] [PubMed] [Google Scholar]
- Zu T, Gibbens B, Doty NS, Gomes-Pereira M, Huguet A, Stone MD, Margolis J, Peterson M, Markowski TW, Ingram MA, Nan Z, Forster C, Low WC, Schoser B, Somia NV, Clark HB, Schmechel S, Bitterman PB, Gourdon G, Swanson MS, Moseley M, Ranum LP. Non-ATG-initiated translation directed by microsatellite expansions. Proc. Natl. Acad. Sci. USA. 2011;108:260–265. doi: 10.1073/pnas.1013343108. [DOI] [PMC free article] [PubMed] [Google Scholar]