Abstract
Background
Patients with high blood pressure (BP) visit a physician an average of 4 times or more per year in the U.S., yet BP is controlled in fewer than half. Practical, robust and sustainable models are needed to improve BP in patients with uncontrolled hypertension.
Objectives
The Home Blood Pressure Telemonitoring and Case Management to Control Hypertension study (HyperLink) is a clinic-randomized trial designed to determine whether an intervention that combines home BP telemonitoring with pharmacist case management improves BP control compared to usual care at 6 and 12 months in patients with uncontrolled hypertension. Secondary outcomes are maintenance of BP control at 18 months, patient satisfaction with their health care, and costs of care.
Methods
HyperLink enrolled 450 hypertensive patients with uncontrolled BP from 16 primary care clinics. Eight clinics were randomized to provide usual care (UC) to their patients (n = 222) and 8 were randomized to provide the telemonitoring intervention (TI) (n = 228). TI patients received home BP telemonitors that internally store and electronically transmit BP data to a secure database. Pharmacist case managers adjust antihypertensive therapy based on the home BP data under a collaborative practice agreement with the clinics’ primary care teams. The length of the intervention is 12 months, with follow-up to 18 months to determine the durability of the intervention.
Conclusions
We will test in a real primary care setting whether combining BP telemonitoring and pharmacist case management can achieve and maintain high rates of BP control compared to usual care.
Keywords: hypertension, telemedicine, pharmacist, randomized trial
Introduction
High blood pressure (BP) is the most common chronic condition for which patients see primary care physicians, affecting about 25% of U.S. adults [1]. Decades of research have shown that treatment of hypertension prevents cardiovascular events, and a large number of well-tolerated, effective, and relatively inexpensive drugs are readily available [2-5]. Although BP control has improved over the past two decades, BP is controlled to recommended levels in less than half of American adults with hypertension [6-8].
Patients with hypertension visit a physician an average of 4 or more times annually, theoretically providing ample opportunity for therapeutic intervention [9]. Patient, physician, and social factors have been invoked to explain why action to address poor BP control is often delayed, insufficient, or does not occur at all [10-11]. Many interventions have been tested to improve the quality of hypertension care, the most effective which have been shown to involve a re-organization of office practice and empowerment of non-physician practitioners to make changes to antihypertensive therapy [12]. Other promising interventions include patient self-management education and alternative methods for collecting and relaying BP data [12]. Still, hypertension control in most primary care practice remains elusive, and few physicians have altered the physician-centered, office visit-based model of hypertension care.
Telemedicine, the electronic exchange of medical information, has emerged as a promising tool for managing chronic conditions. Using telemonitoring to convey home BP data is a logical application of this technology, especially given substantial evidence that home BP measurement yields better prediction of cardiovascular risk compared to office BP measurement [13-14]. Telemonitoring has the advantage of eliminating underreporting of high home BP readings, a well-recognized problem with self-reported BP data [15-16]. Several recent studies have suggested that a combined intervention of telemedicine with nurse - or pharmacist-led care may be effective for improving hypertension management [17-21].
We designed a study combining home BP telemonitoring with pharmacist case management to address several gaps in knowledge that could hamper a wider adoption of this approach. First, we sought to enroll a population of patients with uncontrolled hypertension that broadly reflected the wide range of hypertension severity and co-morbidity in typical primary care practices. Second, our 18-month study includes an intensive intervention during the first 6 months of follow-up, a short-term maintenance phase with continued telemonitoring and a lower level of pharmacist contact during months 7-12, and a longer-term maintenance phase without intervention during months 13-18. Third, we plan to conduct a formal cost analysis, an element that has been absent from previous studies of telemonitoring.
Trial design and methods
Overview
HyperLink is a two-arm clinic-randomized controlled trial comparing usual care of hypertension by primary care providers to an intervention that combines home BP telemonitoring with pharmacist case management. The primary aim of the study is to improve BP control at 6 and 12 months. The trial enrolled 450 hypertensive patients with uncontrolled BP from 16 primary care clinics. Half of the clinics were randomized to continue to provide usual care (UC)1 to their patients and half were randomized to the telemonitoring intervention (TI).
TI patients received home monitors provided free of charge by the study (A&D Medical 767PC automatic oscillometric BP monitor, San Jose, CA) that store and transmit BP data through a simple touch-tone or cellular phone modem to a secure website. This monitor received an A/A grade by the British Hypertension Society protocol and a satisfactory rating (the best possible) using the Association for the Advancement of Medical Instrumentation protocol [22]. Pharmacist case managers then review data from the website and determine need for adjustments to patients’ antihypertensive therapy. The pharmacist case managers are located within the TI clinics and work directly with patients under a collaborative practice agreement with the clinics’ primary care teams. Pharmacists meet with patients in the clinic setting for one intake visit, and subsequently discuss all changes to treatment during telephone visits. During the first 6 months, phone visits occur every two weeks until BP control is sustained for 6 weeks, and then frequency is reduced to monthly. During months 7-12, phone visits take place every two months. The pharmacists communicate with the patients’ primary care teams through the shared electronic medical record (EMR) and securely message the results of each phone visit to the physician. After 12 months, pharmacists have no further contact with patients.
Patients in both the TI and UC arms visit a separate research clinic for screening prior to enrollment in the study, and at 6, 12, and 18 months of follow-up. Clinical data (including the BP outcome measurements) and survey data are collected at each research clinic visit. While the intervention lasts for 12 months, the 18-month follow-up will determine the durability of the intervention effects.
The specific aims of the study are to assess the effect of the intervention relative to usual care on (a) the proportion of patients with controlled BP at both the 6 and 12 month research clinic visits (defined as <140 mmHg systolic and <90 mmHg diastolic; <130 mmHg systolic and <80 mmHg diastolic in patients with diabetes or kidney disease); (b) maintenance of BP control defined as the proportion of patients at the 18 month research clinic visit with controlled BP; (c) patient satisfaction with their health care; (d) cost of care.
Study setting
This study is being conducted at HealthPartners Medical Group (HPMG), a multi-specialty practice in the Minneapolis-St. Paul metropolitan area. Approximately 200,000 adult HPMG patients are cared for by 138 primary care providers at 21 clinics. Most patients with hypertension receive care from family physicians or general internists, with fewer than 5% of hypertension patients referred to hypertension specialists per year.
Eligibility and exclusion criteria
Study eligibility and exclusion criteria were intended to capture a population broadly representative of patients with uncontrolled hypertension, while excluding patients with hypertension complicated by major co-morbidities who would not be appropriate for management by a pharmacist. To increase the generalizability of our results, patients were not excluded due to an upper level of BP or number of drugs, since patients with the most severe hypertension who are on multiple classes of drugs may derive the most benefit from the intervention.
All participants meet each of four eligibility criteria: (1) age 21 years or older, (2) a patient of a primary care physician at one of the 16 HPMG study clinics, (3) had at least two primary care outpatient encounters in 12 months prior to screening with BP >140/90 mmHg at the two most recent visits, and (4) the average of three BP measurements at the research clinic screening visit was above the goal recommended by the 7th Joint National Commission for the Detection, Evaluation and Treatment of High Blood Pressure (JNC7) [3].
Blood pressure is considered above the JNC7 goal if either the systolic or diastolic goal is exceeded. The JNC7 goal is <140 mmHg for systolic BP and <90 mmHg for diastolic BP (<130 mmHg systolic and <80 mmHg diastolic if the patient has diabetes or kidney disease). Diabetes is considered to be present if diagnosed by a physician in the EMR, if patient has been treated with insulin or another hypoglycemic agent, or if diagnostic levels of hyperglycemia have been documented on two occasions in the EMR (>126 mg/dl fasting plasma glucose or >200 mg/dl random plasma glucose). Kidney disease is considered to be present if the estimated glomerular filtration rate (eGFR) is <60 ml/min/1.73 m2 using the CKD-Epi equation or if the albumin-creatinine ratio is >30 mg/g creatinine on at least two spot urine specimens [23]. For patients aged ≥70 years, we used a more conservative eGFR of <45 ml/min/1.73 m2.
Medical exclusion criteria include the following: stage 4 or 5 kidney disease (eGFR <30 ml/min/1.73 m2 on the most recent determination of eGFR) or if the albumin-creatinine ratio is ≥700 mg/g creatinine in absence of urinary symptoms); acute coronary syndrome, coronary revascularization or stroke, within the past three months; known secondary causes of hypertension like coarctation of the aorta, pheochromocytoma, adrenal cortical hypertension or renal vascular hypertension; class III (marked limitation of physical activity) or IV (symptoms at rest) New York Heart Association heart failure, or known left ventricular ejection fraction <30%. Additional exclusion criteria relate to the likelihood of being able to carry out the intervention or use the telemonitoring equipment: unwillingness to be followed for a period of 18 months; pregnancy or intention to become pregnant in next two years for females; unwillingness to use birth control for women of child-bearing potential; participation in another clinical trial; difficulty with communication in English without an interpreter over the telephone; dementia, mental illness or any condition that would limit ability to give informed consent; or arm circumference >18 inches (indicating BP cuff on telemonitoring device may not be accurate without modification).
Recruitment
Electronic medical records were used to identify patients who met the first three eligibility criteria in the previous 12 months. The EMR query was refreshed every 2-3 months for the 26-month recruitment period to identify patients who newly fit the criteria. Patients meeting the criteria received up to two mailings 3-4 months apart, followed by telephone calls to non-responders. Primary care physicians were invited to refer appropriate patients to the study, although few did so. The study was also advertised in patient and employee newsletters and other HPMG corporate communications.
Patients who expressed interest in participation by responding to a mailing or referral were screened over the phone, and those who remained eligible after the phone screen were screened in the research clinic. During the research clinic screening, the patient must have met the fourth eligibility criterion (an average of three BP measurements meeting JNC7 goal), and if eligible signed a consent form and completed study questionnaires, a medication inventory, and laboratory measurements.
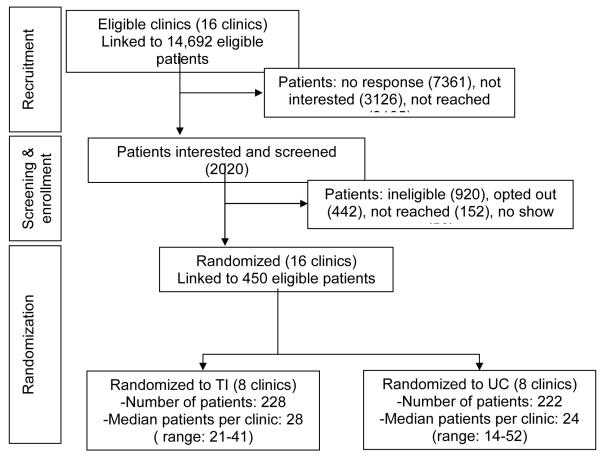
During the 26-month recruitment period from March 2009 to April 2011, 14 692 letters were mailed to patients identified as potentially eligible by EMR data, and 2 020 (14%) responded as interested either from a mailing or recruitment phone call. Of these, 450 (22%) were enrolled (Figure 1). Of the 920 patients who were ineligible at screening, the majority (652) were ineligible due to normal level of blood pressure at the clinic screening visit (despite the EMR suggesting some history of elevated BP). The other main reasons for ineligibility were: no land-line phone (we did not have Bluetooth capability until near the end of recruitment), medical exclusions (e.g., stage 4 or 5 chronic kidney disease, cardiovascular disease event within last 3 months, significant mental illness), and plans to change primary care clinics.
Figure 1.
Consort-style diagram for Hyperlink
Group randomization
The trial was randomized at the level of the primary care clinic in order to avoid exposing physicians in the same practice to the two different treatment arms. Of the 21 HPMG primary care clinics, 16 met the study requirements for clinic inclusion of an on-site pharmacist who sees patients at least one day per week and a clinical practice agreement in place between the pharmacist and primary care physicians this allows the pharmacists to prescribe and change antihypertensive therapy within specified parameters.
The 16 primary care clinics were randomized to either the UC (n = 8) or TI (n = 8) study arm. Clinics were blocked by size and clinic-level baseline BP control in 2008 in order to balance those factors across study arms. Patients were linked to their primary care clinic by self-report and were assigned to the intervention based on which clinic they attended, resulting in n = 228 patients assigned to TI and n = 222 patients assigned to UC. All consenting patients and primary care providers were blinded to the study design and intervention assignment of the clinics, although each patient and their primary care provider were informed of their treatment assignment after randomization. Research clinic coordinators were not blinded to clinics’ treatment assignments, but were trained to treat patients in both study arms identically.
Study Arms
Patients in both arms attend an identical schedule of research clinic visits at screening/baseline, 6 months, 12 months, and 18 months follow-up, where all outcome measurements are taken.
Patients who are eligible at the screening visit, have baseline data collected and are enrolled in the study. At this visit, study staff discuss clinic BP goal based on their age and co-morbidities. Patients are then given their randomization assignment, and then review and take home an NIH publication on blood pressure self-management [24]. Both UC and TI patients return for identical follow-up visits. After each research clinic visit, clinic staff file a visit summary in the patients’ EMR, copied to the primary care physician, which includes the recommended BP goal for that patient, whether the goal was met, and an alert if the research clinic BP is ≥160/100 mmHg. During the 18 months of follow-up, UC patients have no other contact with study staff while TI patients follow the intervention protocol, below.
Telemonitoring Intervention
The underlying conceptual model for the telemonitoring intervention is a closed feedback loop in which patient self-monitoring and education is coupled with therapeutic management by a clinical pharmacist. The pharmacists hold doctoral pharmacy degrees and participate in the primary care clinics as caregivers under the Medication Therapy Management (MTM) program [25]. As implemented at HPMG, the MTM program provides patients free, confidential appointments with the pharmacist to review and advise on use, effects, and interactions of over-the-counter and prescription medications. Additionally, the pharmacists monitor conditions affected by medications, like hypertension, cholesterol, and diabetes. For this intervention, the pharmacists have an expanded role to deliver individualized patient education and monitor patients’ BP and medication adherence. They are authorized to initiate therapy, adjust dosages, change medication or order refills, and order lab tests to assess treatment and monitor for adverse effects of the drug therapy. Diuretic treatment is the cornerstone of hypertension therapy, combined with synergistic drugs from other classes as necessary to achieve both systolic and diastolic BP control. Four MTM pharmacists work with the intervention patients from the 8 TI clinics.
TI patients meet with their assigned pharmacist for an intake visit in the patient’s primary care clinic within two weeks following enrollment. At this visit, the pharmacist measures the patient’s BP using the home monitor and reviews their medical history, medications, and adherence to pharmacotherapy and lifestyle approaches to lowering BP. At this time, the pharmacist may make adjustments in antihypertensive therapy if changes are indicated based on BP level, drug interactions, or side effects. Then the pharmacist reviews general teaching points about hypertension with the patient and discusses the BP goal. A home BP goal is set 5 mmHg below the primary outcome goal measured in the clinic in accordance with expert recommendations (i.e., <135/85 mmHg or <125/75 mmHg for patients with diabetes or kidney disease) [26-27]. Finally, the pharmacist instructs the patient on setting up the monitor, properly taking BP readings, and installing and using the modem for data transmission. For accurate home readings, the patient is instructed to sit quietly for five minutes with both feet flat on the floor, with back and arms supported, before measuring their BP. The patient is shown how to correctly place the cuff on their upper arm. The patient is instructed to transmit at least six BP measurements weekly (three in the morning and three in the evening).
Telemonitoring equipment is shipped to patients’ homes from the monitoring company, and patients are provided with a toll-free number for customer support. Patients connect their telemonitors to their modems (using a cord for a land-line modem or Bluetooth technology for a cellular modem) and press a ‘transmit’ button at least once weekly, which automatically transmits readings since the last transmission and uploads the data to a secure website. After transmission, a computer system automatically calls the patient and asks them to respond to two questions: 1) ’Have you missed more than one dose of your blood pressure medication in the past week?’ and 2) ’Are you having any problems that you would like your study pharmacist to call you about before your next regularly scheduled call?’. If the patient responds ‘yes’ to either of these questions, an e-mail alert is automatically sent from the website to the pharmacist case manager. TI patients can request the pharmacist to contact them each time they transmit BP data. Pharmacists also receive automatic e-mail alerts if the patient has a dangerously high (>180/110 mmHg) or low (<80/50 mmHg) reading.
During the first 6 months, patients have phone visits with pharmacists every two weeks until BP control is achieved at three consecutive visits, after which call frequency may decrease to monthly. Thus, the number of calls in the first 6 months can range from 7 to 11. In months 7-12, patients continue to use the home BP monitor and have three maintenance phone visits with the pharmacist, occurring every two months. There is no requirement for greater call frequency if BP is uncontrolled, but additional calls can take place at the pharmacist’s discretion.
During all phone visits, pharmacists emphasize therapeutic lifestyle changes and adherence to therapy. Pharmacists assess the need for changes in antihypertensive drug therapy based on an algorithm using the percentage of the patient’s home BP readings that meet goal, and JNC7-derived treatment guidelines for appropriate changes in drug treatment (Tables 1 and 2). This algorithm is also the basis for the HPMG collaborative practice agreement between primary care physicians and MTM pharmacists. At each visit, if at least 75% of readings since the last visit meet BP goal, then no medication changes are suggested. However, if fewer than 75% of readings meet goal, then the algorithm calls for treatment intensification. “Milepost visits” are designated at months 1.5, 2.5, and 3.5. If intensification is indicated at a milepost visit, the pharmacist is required to add a new medication. If intensification is indicated at a non-milepost visit, the pharmacist may either add a medication or increase the dosage of an existing medication at that time. Regardless of BP control, if patients are experiencing adverse effects the dose of a drug may be lowered or the drug may be stopped and another substituted. Each pharmacist was observed conducting a telephone visit on two occasions by one of the study investigators or coordinators to verify fidelity to the intervention.
Table 1. Antihypertensive Drug Therapy Options.
| When Drug Added |
Drug class | Examples of generic agents and dose range |
Daily Dosing |
|---|---|---|---|
| 1st | Thiazide diuretic | chlorthalidone, 12.5 - 25 mg | Once |
| Potassium sparing diuretic combination |
hydrochlorothiazide 25-50/ triamterene 50 mg |
Once | |
| 2nd | ß-blocker | metoprolol succinate, 25-200 mg | Once |
| Angiotensin converting enzyme inhibitor |
lisinopril, 10-40 mg | Once | |
| Dihydropyridine calcium channel blocker |
amlodipine, 5-10 mg | Once | |
| Non-dihydropyridine calcium channel blocker |
extended release diltiazem, 120-360 mg |
Once | |
| Angiotensin II receptor blocker | losartan 25-100 mg | Once | |
| 3rd | α-blocker | doxazosin, 2-8 mg | Once |
| Central α-adrenergic agonist | clonidine, 0.1-0.3 mg | Twice | |
| Direct-acting vasodilator | hydralazine, 50 -100 mg | Twice | |
| Peripheral adrenergic neuron antagonist |
reserpine, 0.05 - 0.10 mg | Once | |
| Aldosterone antagonist | spironolactone 25-100 mg | Once | |
| Other | Potassium chloride (KCl) | KCl tablets or capsules, 10-20 mEq | Varies |
Table 2. Algorithm for Drug Initiation and Titration.
| Patient Circumstances at Visit | Pharmacist Action |
|---|---|
| Not on drug treatment SBP 1-19 mmHg and DBP 1-9 mmHg above goal |
Diuretic, reinforce lifestyle modification |
| Not on drug treatment SBP ≥20 mmHg or DBP ≥10 mmHg above goal |
Begin with combination of diuretic and second- line/add-on drug (see Table 1), consider compelling reasons for choice of one or more drugs, reinforce lifestyle modification |
| Either SBP or DBP uncontrolled according to JNC7 criteria Not adhering to already prescribed medication regimen |
Address reasons for non-adherence, adjust regimen, monitor adherence. |
| SBP 1-19 mmHg and DBP 1-9 mmHg above goal Adhering to 1-2 BP control medications |
Add thiazide-like diuretic if not part of regimen, otherwise add synergistic second-line drug, reinforce lifestyle modification |
| SBP ≥20mmHg or DBP ≥10mmHg above goal Adhering to 1-2 BP control medications |
Add synergistic combination of two more drugs, reinforce lifestyle modification |
| SBP ≥180mmHg or DBP ≥110mmHg, or BP uncontrolled despite reported adherence to 4 BP control medications |
Probe more for non-adherence, consult primary care physician and study investigators regarding reasons for resistant hypertension, refer for work-up for secondary causes of hypertension if needed |
| BP and DBP below goal, adhering to therapeutic regimen consistent with JNC7 therapeutic recommendations, no barriers to ongoing adherence |
Continue present treatment, reinforce lifestyle modification |
Patients return the telemonitors after 12 months, return to the care of their primary providers, and receive no telemonitoring or pharmacist support during months 13-18. Thus, the total length of the intervention is 12 months, with follow up through 18 months to determine the durability of the intervention effects.
Usual Care
Following enrollment in the study, UC patients are managed by their primary care providers as usual. This may include referral to hypertension specialists, referral to MTM pharmacists for regular consultation, and conventional home BP measurement. Under usual care circumstances, MTM pharmacist referrals result in one or two clinic visits and do not include telephone follow-up or prolonged monitoring. Other components of usual care at HPMG clinics include promotion of regional and national hypertension management guidelines [3, 28], individual and group classes for dietary and weight management advice, and monthly feedback from the medical group to physicians regarding BP levels in patients with diabetes.
Study variables and outcomes
Demographics, non-pharmacologic therapy, and healthcare
Patient age, sex, race, clinic, and primary care provider data are obtained from administrative and EMR sources and confirmed by self-report at the baseline research clinic visit. At baseline, 6, 12, and 18 month research clinic visits, self-reported survey items measure: education, income, marital status, employment status, physical activity, smoking, alcohol and salt intake, alternative and lifestyle therapies used for hypertension, use of home BP monitoring, time and money spent on weight management and exercise, and travel time to primary care clinic. Finally, patient quality of life and general health are measured by the Medical Outcomes Study Short Form 12 questionnaire (version 2), and self-efficacy for managing BP is measured using a 13-item subset of questions that assess patient self-efficacy for pharmacist communication, health care team communication, home BP monitoring, BP medication adherence, and controlling BP [18, 29].
Blood pressure
The primary study outcome is BP control at the 6 and 12 month research clinic visits. The average of three measurements taken at these visits must fall beneath goal for both systolic and diastolic measures for BP to be classified as controlled. Blood pressure is measured at these visits using an automated monitor identical to the home BP telemonitor but not connected to a modem. Three measurements are taken with at least 30 seconds between cuff inflations, and the three measurements are averaged. Measurements are performed in the right upper arm with an appropriate sized cuff after the patient has been seated quietly for five minutes with the back supported, arm supported at the level of the heart, and feet placed flat on the floor. Secondary outcomes are BP control at 18 months and the mean change from baseline in systolic and diastolic BP at each time period, also measured in the research clinic.
Antihypertensive drug treatment
At baseline, 6, 12, and 18 month research clinic visits, the number and type of BP medications and medication classes are assessed with a medication inventory. Adherence to those medications is assessed by self-report, using Morisky’s 4-item scale (modified to focus on BP medications) [30]. In addition, the number and types of BP medications and additional adherence data among the TI group are reported by pharmacist case managers after visits. The electronic pharmacy claims can confirm the treatment data for the subset of patients with HealthPartners insurance pharmacy benefits.
Satisfaction with care
Six items concerning satisfaction with health care and providers were selected from the Consumer Assessment of Healthcare Providers and Systems (CAHPS) adult survey v4.0, and are collected at each research clinic visit (baseline, 6, 12, and 18 months) [31]. The items concern how successfully providers listen carefully, explain things clearly, show respect, and spend enough time with the patient, as well as the patient’s ability to obtain care when needed and an overall rating of health care in the past 12 months. In addition, the Patient Assessment of Chronic Care (PACIC) [32] is completed at each research clinic visit. The 20-item PACIC assesses patient perceptions of care from the perspective of the Chronic Care Model in the areas of patient activation, delivery system design, decision support, goal setting, problem-solving, and follow-up. The PACIC was modified slightly to be specific to care for hypertension [33].
Cost measures
Cost of TI care data include the pharmacist time spent on study-related visits and calls, including preparation and documentation time as measured by self-report; the cost of telemonitoring equipment as measured by study records of the cost of equipment procurement, distribution, and instruction; and the amounts paid by the insurer or out-of-pocket by the patient for health care utilization (including pharmaceuticals), as reported in HealthPartners insurance claims data.
Data Monitoring and Analysis
Adverse events reporting
Adverse event data are collected primarily through self-report at research clinic visits at 6, 12, and 18 months. Since these clinic visits are identical in frequency and content for both study arms, they produce the least biased source of data. At these visits, subjects report any hospitalization longer than 12 hours, emergency room visits, or same-day clinic visits in the past 6 months as well as the reason for those events.
Study staff obtain and review medical records related to all potential adverse events, including: deaths, hospitalizations, major cardiac events, incident kidney failure and urgent evaluations for hypertension, hypotension, loss of consciousness or fainting, allergic reactions, hives, and angioedema. These are evaluated for severity, expectedness, and potential relatedness to the intervention and study participation. While the clinical pharmacists do not systematically screen for adverse events, they inform study staff if they become aware of a potentially reportable event during routine patient contact.
Sample size and power
This study was powered at 80% to detect a difference in the proportion of patients with controlled BP at 6 and 12 months of 40% in UC and 60% in TI. The calculations were downwardly adjusted to obtain effective sample sizes that reflected the clustering of patients within clinics (using an expected intra-class correlation ICC = 0.03) and assuming a Type I error of 0.05 (two-sided test). The sample size is based on the recruitment goal of n = 450 from 16 clinics, for an average of 28 participants per clinic. We expect that n = 405 (90%) of these participants will complete the 6 month clinic visit, and n = 360 (80%) will complete both the 6 and 12 month clinic visits. The expected effects for the primary outcome is that 40% of UC patients will have controlled BP at 6 and 12 months, 70% of TI patients will have controlled BP at 6 months, and 60% of TI patients will have controlled BP at both 6 and 12 months. Power was conservatively estimated using the smaller sample size and effect size estimates expected at 12 months. Thus, power will be the same or higher at 6 months providing assumptions about sample size and effect size hold. Assumptions regarding size of clinic-level ICCs for BP were based on data from patients receiving care at HPMG clinics in 2007. We based our expectation of BP control in the UC group on control groups of previous clinical trials, and the intervention groups of clinical trials that included only less-effective interventions like patient-physician education, feedback, or medication reminders [34-38]. Assumptions regarding BP control in the TI group based on data from trials such as ALLHAT, INVEST, CONVINCE, and ACCOMPLISH, which achieved BP control rates of approximately 70% [39-42].
Statistical analyses
Our primary hypotheses are:
BP control will be achieved at 6 months in more than 70% of TI patients, compared to less than 40% of UC patients,
BP control will be maintained at both 6 and 12 months in more than 60% of TI patients, compared to less than 40% of UC patients.
We will use generalized linear mixed models with a logit link to test the effect of the intervention on a binary outcome of BP control. Because patients are the primary unit of observation and are nested within primary care providers and clinics, these models will include patient-level covariates (e.g., age, gender, race) that are not balanced across study arms as well as random terms to account for the nested data structure. The binary BP control outcome is based on the mean of the three BP measurements made at research clinic visits 6 and 12 months. Patients with systolic and diastolic BP control at 6 months (and both at 6 and 12 months) will be considered to have successfully achieved and maintained BP control. BP control at 18 months will be used to assess the durability of the intervention when TI patients return to the care of their primary care physicians.
Additional planned analyses include a mediational analysis to assess possible mechanisms through which the intervention increases BP control. Factors hypothesized to mediate the intervention effect include medication intensification and medication adherence. A process analysis utilizing data from TI patients will help establish which components of the intervention are more strongly related to BP control (e.g., adherence to pharmacist visits, adherence to home BP monitoring).
We will compare patient satisfaction with care in TI and UC groups as measured by a composite of six items selected from the CAHPS 4.0 survey at each research clinic visit [31]. General linear mixed models will test the intervention’s effect on patient satisfaction at each time point. As above, these models will include patient covariates if balance across arms is not achieved as well as random terms to account for nesting of patients.
We will also compare cost of care in the TI and UC groups. The study sample for cost analysis will be participants with continuous HealthPartners insurance coverage, including pharmacy benefits (approximately 60% of the study sample). We will calculate the incremental cost of TI compared to UC, and compute cost-effectiveness as the incremental cost per additional patient who achieves JNC7 BP goals at both 6 and 12 months. While the study is not powered to detect statistically significant difference in costs, we will compute boot-strapped 95% confidence intervals of the incremental cost per participant and incremental cost per success of TI compared to UC as measures of the multivariate sensitivity of the results. To assist decision makers who wish to consider alterations to the intervention, we will report incremental costs by detailed categories including telemonitoring costs, pharmacist case management costs, the net impact on visits, labs, and prescriptions, as well as the utilization of behavioral health, emergency room, and inpatient hospital resources. All costs will be calculated from both the health care system and societal perspectives, where the latter includes patient time for travel and care.
Discussion
The traditional physician-centered office visit model of care hails from an era when most patients sought care for acute conditions. Increasingly, this model is proving ill-suited in many ways for chronic illness care. Within the office visit model, physicians and other care providers are faced with very limited time for gathering and integrating data and making complex treatment decisions. The Chronic Care Model (CCM) is a widely accepted framework for thinking about organizational changes necessary for improving chronic illness care in clinical practice [38, 43-44]. The CCM posits that there are six domains important for effective care of chronic illness: health care organization (the structure and support processes of the organization), delivery system redesign, clinical information systems, decision support, self-management support, and community linkages. Interventions that have incorporated one or more elements of the CCM have shown beneficial effects on clinical outcomes and processes of care for patients with a variety of chronic illness diagnoses, with the largest effect sizes for delivery system redesign and self-management support [45]. The HyperLink study incorporates these CCM elements. The patient-centered medical home is another recently developed concept that is promising for preventive services and chronic disease care [46]. A central feature of the medical home is the presence of a various practice systems designed to ensure consistency in delivery of evidence-based care. HyperLink is also in keeping with this model [47].
Over the past three decades, quality improvement research trials for hypertension care have shown that organizational interventions that included the delegation of care to a health professional other than the physician achieved the largest reductions of blood pressure [12, 45, 48-66]. In most cases, these types of interventions included a nurse or pharmacist, and were variously called “team change,” “case management,” “disease management,” or “nurse- or pharmacist-led care.” In these studies, average systolic BP dropped by 10 mmHg, diastolic BP dropped by 4 mmHg, and the absolute proportion of patients who achieved BP control improved by 20% [12]. The most successful interventions incorporated a protocol that did not depend upon the physician responding to recommendations. The strategies associated with the second largest improvements in BP include the closely related concepts of patient self-management and self-monitoring (access to resources or devices like home blood pressure monitors that enhance patients’ ability to manage their condition) [12, 48, 50, 56, 67-77]. Home monitoring has also been associated with increased adherence to pharmacological therapy [78]. A recent study by Green and colleagues [18] found that home BP monitoring combined with internet-based pharmacist-led care resulted in improved systolic and diastolic BP and BP control compared to usual care over a period of 12 months (systolic: 14 mmHg vs. 5 mmHg, p<0.001; diastolic: 7 mmHg vs. 4 mmHg, p<0.001; BP control 56% vs. 31%, p<0.001). However, unlike HyperLink, this study excluded patients with diabetes, renal disease or cardiovascular disease.
Several recent studies have examined home BP telemonitoring, in which home blood pressure data are transmitted directly to health care providers, in some cases combined with nurse- or pharmacist-led care. Artinian and colleagues [17] studied 387 urban African Americans with uncontrolled BP randomly assigned to community nurse-managed telemonitoring vs. usual care. At 12 months, the intervention group had a greater reduction in systolic BP (13 mmHg vs. 8 mmHg, p = 0.04), but diastolic BP did not differ (6 mmHg vs. 4 mmHg, p = 0.12), nor did BP control to <140/90 mmHg. There were also no differences between the two groups in self-reported treatment intensity, so the greater degree of systolic BP lowering in the intervention group was likely achieved through other mechanisms, such as better adherence to drug therapy or lifestyle changes. A British study conducted in 24 general practices randomized patients with uncontrolled BP on up to two antihypertensive drugs to usual care or an intervention that included telemonitoring of home BP data and self-titration of medications based on two changes or increases in dose agreed upon in advance with their family doctor [20]. Systolic BP decreased more in the intervention group after 12 months (18 mmHg vs. 12 mmHg, p=0.0004). Most patients in the intervention group made at least one medication change, and intervention group patients were less likely to be treated with monotherapy at 12 months. Another recent study among U.S. veterans compared a telemonitoring intervention combining nurse-administered behavioral management, nurse- and physician-administered medication management, or a combination of behavioral and medication management to usual care [21]. In that study, the largest effect on systolic BP was observed for the combined intervention, which was 4 mmHg lower than in the control group at 12 months, but was not significantly different at 18 months. A larger difference was observed for the post-hoc subgroup with inadequate BP control at baseline (15 mmHg at 12 months and 8 mmHg at 18 months, both significantly different than the control group).
HyperLink brings together several features that distinguish it from previous similar interventions and are likely to improve its effectiveness and generalizability: 1) the broad eligibility criteria ensured that we enrolled a population with a range of co-morbidities representative of patients with uncontrolled hypertension, 2) incorporation of the intervention into the primary care setting, and 3) use of pharmacists who were able to work directly with the patients to alter treatment plans.
BP control in the U.S. and in clinical practice is inadequate. For the most part, the reasons for this inadequate control are understood, and the methods to achieve the highest level of BP control are known from previous research. Although it is a randomized trial, and thus will likely enroll highly motivated volunteers, the goal of HyperLink is to test an intervention combining BP telemonitoring and pharmacist case management to achieve and maintain BP control among hypertension patients in a real primary care setting. The exclusion criteria are consistent with those that would be used to identify patients for whom telemonitoring and telephone-based support is appropriate in practice. Our study includes patients with undiagnosed and untreated hypertension, as well as patients whose BP is treated but uncontrolled. The intervention design is well-grounded in previous research, incorporates principles from a theoretical model for improving chronic illness care, and takes advantage of newer technology and team care [79]. Most importantly, the intervention builds upon and extends existing health care roles, collaborations, and systems in primary care clinics of a large managed care organization. The planned formal cost analysis will be key to wider adoption of BP telemonitoring and pharmacist case management, should it prove effective. This project is a rigorous test of a new model of hypertension treatment that frees patients from the constraints of office-based care. Its results will have important implications for efforts to improve care for millions of Americans with uncontrolled hypertension.
Acknowledgements
This study is supported by a grant from the National Heart, Lung, and Blood Institute (R01HL090965).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Abbreviations used: UC, Usual Care; TI, telemonitoring intervention; EMR, electronic medical record; HPMG, HealthPartners Medical Group; JNC7, 7th Joint National Commission for the Detection, Evaluation and Treatment of High Blood Pressure; MTM, Medication Therapy Management; CAHPS, Consumer Assessment of Healthcare Providers and Systems; PACIC,Patient Assessment of Chronic Care; CCM, Chronic Care Model
References
- 1.Hsiao C, Cherry D, Beatty P, Rechtsteiner E. National Ambulatory Medical Care Survey: 2007 summary. National Center for Health Statistics2010; Hyattsville, MD: Report No.: No. 27 Contract No.: 27. [PubMed] [Google Scholar]
- 2.Collins R, Peto R, MacMahon S, Hebert P, Fiebach N, Eberlein K, Godwin J, Qizilbash N, Taylor J, Hennekens C. Blood pressure, stroke, and coronary heart disease. Part 2, short-term reductions in blood pressure: Overview of randomised drug trials in their epidemiological context. The Lancet. 1990;335:827–38. doi: 10.1016/0140-6736(90)90944-z. [DOI] [PubMed] [Google Scholar]
- 3.Chobanian A, Bakris G, Black H, Cushman W, Green L, Izzo J, Jones D, Materson B, Oparil S, Wright J, Roccella E. The Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: The JNC 7 Report. JAMA. 2003;289:2560–71. doi: 10.1001/jama.289.19.2560. [DOI] [PubMed] [Google Scholar]
- 4.Blood Pressure Lowering Treatment Trialists’ Collaboration Effect of different blood-pressure-lowering regimens on major cardiovascular events: Results of prospectively-designed overviews of randomised trials. The Lancet. 2003;362:1527–35. doi: 10.1016/s0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 5.Blood Pressure Lowering Treatment Trialists’ Collaboration Effects of ACE inhibitors, calcium antagonists, and other bloodpressure-lowering drugs: Results of prospectively designed overviews of randomised trials. The Lancet. 2000;355:1955–64. doi: 10.1016/s0140-6736(00)03307-9. [DOI] [PubMed] [Google Scholar]
- 6.Egan BM, Zhao Y, Axon RN. US trends in prevalence, awareness, treatment, and control of hypertension, 1988-2008. JAMA. 2010 May 26;303(20):2043–50. doi: 10.1001/jama.2010.650. [DOI] [PubMed] [Google Scholar]
- 7.Yoon SS, Ostchega Y, Louis T. Recent trends in the prevalence of high blood pressure and its treatment and control, 1999-2008. NCHS Data Brief. 2010 Oct;(48):1–8. [PubMed] [Google Scholar]
- 8.Gillespie C, Kuklina E, Briss P, Blair N, Hong Y. Vital Signs: Prevalence, treatment, and control of hypertension--United States, 1999-2002 and 2005-2008. MMWR. 2011 Feb 4;60(4):101–8. [Google Scholar]
- 9.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988-2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 10.Hyman D, Pavlik V. Characteristics of patients with uncontrolled hypertension in the United States. N Engl J Med. 2001;345:479–86. doi: 10.1056/NEJMoa010273. [DOI] [PubMed] [Google Scholar]
- 11.Ashton C, Haidet P, Paterniti D, Collins T, Gordon H, O’Malley K, Peterson L, Sharf B, Suarez-Almazor M, Wray N, Street R. Racial and ethnic disparities in the use of health services: Bias, preferences, or poor communication? J Gen Intern Med. 2003;18:146–52. doi: 10.1046/j.1525-1497.2003.20532.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Walsh JM, McDonald KM, Shojania KG, Sundaram V, Nayak S, Lewis R, Owens DK, Goldstein MK. Quality improvement strategies for hypertension management: a systematic review. Med Care. 2006 Jul;44(7):646–57. doi: 10.1097/01.mlr.0000220260.30768.32. [DOI] [PubMed] [Google Scholar]
- 13.Mancia G, Facchetti R, Bombelli M, Grassi G, Sega R. Long-Term Risk of Mortality Associated With Selective and Combined Elevation in Office, Home, and Ambulatory Blood Pressure. Hypertension. 2006 May 1;47(5):846–53. doi: 10.1161/01.HYP.0000215363.69793.bb. [DOI] [PubMed] [Google Scholar]
- 14.Stergiou GS, Baibas NM, Kalogeropoulos PG. Cardiovascular risk prediction based on home blood pressure measurement: the Didima study. J Hypertens. 2007 Aug;25(8):1590–6. doi: 10.1097/HJH.0b013e3281ab6c69. [DOI] [PubMed] [Google Scholar]
- 15.Myers MG. Self-measurement of blood pressure at home: the potential for reporting bias. Blood Press Monit. 1998;3(Suppl 1):S19–S22. [Google Scholar]
- 16.Mengden T, Hernandez Medina RM, Beltran B, Alvarez E, Kraft K, Vetter H. Reliability of reporting self-measured blood pressure values by hypertensive patients. Am J Hypertens. 1998;11:1413–7. doi: 10.1016/s0895-7061(98)00241-6. [DOI] [PubMed] [Google Scholar]
- 17.Artinian NT, Flack JM, Nordstrom CK, Hockman EM, Washington OGM, Jen KC, Fathy M. Effects of nurse-managed telemonitoring on blood pressure at 12-month follow-up among urban African Americans. Nurs Res. 2007;56(5):312–6. doi: 10.1097/01.NNR.0000289501.45284.6e. [DOI] [PubMed] [Google Scholar]
- 18.Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, Carrell D, Tyll L, Larson EB, Thompson RS. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008 Jun 25;299(24):2857–67. doi: 10.1001/jama.299.24.2857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bosworth HB, Olsen MK, Grubber JM, Neary AM, Orr MM, Powers BJ, Adams MB, Svetkey LP, Reed SD, Li Y, Dolor RJ, Oddone EZ. Two self-management interventions to improve hypertension control: a randomized trial. Ann Intern Med. 2009 Nov 17;151(10):687–95. doi: 10.1059/0003-4819-151-10-200911170-00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McManus RJ, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, Kaambwa B, Banting M, Bryan S, Little P, Williams B, Hobbs FD. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. The Lancet. 2010 Jul 17;376(9736):163–72. doi: 10.1016/S0140-6736(10)60964-6. [DOI] [PubMed] [Google Scholar]
- 21.Bosworth HB, Powers BJ, Olsen MK, McCant F, Grubber J, Smith V, Gentry PW, Rose C, Van Houtven C, Wang V, Goldstein MK, Oddone EZ. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med. 2011 Jul 11;171(13):1173–80. doi: 10.1001/archinternmed.2011.276. [DOI] [PubMed] [Google Scholar]
- 22.Rogoza A, Pavlova T, Sergeeva M. Validation of A&D UA-767 device for the self-measurement of blood pressure. Blood Press Monit. 2000;5(4):227–31. doi: 10.1097/00126097-200008000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.National Institutes of Health, National Heart, Lung and Blood Institute . Your guide to lowering blood pressure. Bethesda, MD: 2003. Report No.: Publication No. 03-5232. [Google Scholar]
- 25.2010 Medicare Part D Medication Therapy Management (MTM) Programs. Centers for Medicare & Medicaid Services [Google Scholar]
- 26.Pickering T. Recommendations for the use of home (self) and ambulatory blood pressure monitoring. Am J Hypertens. 1996;9:1–11. doi: 10.1016/0895-7061(95)00341-x. [DOI] [PubMed] [Google Scholar]
- 27.Staessen JA, Den Hond E, Celis H, Fagard R, Keary L, Vandenhoven G, O’Brien ET. Treatment of Hypertension Based on Home or Office Blood Pressure Trial I. Antihypertensive treatment based on blood pressure measurement at home or in the physician’s office: a randomized controlled trial.[see comment] JAMA. 2004;291(8):955–64. doi: 10.1001/jama.291.8.955. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz G, Kerandi H, Luehr D, O’Connor P, Margolis K, Reddy G, Woolley A, Canzanello V, Pereira C, Schlichte A. Hypertension diagnosis and treatment (Healthcare guideline) Minneapolis, MN: 2010. [Google Scholar]
- 29.Ware JE, Kosinski M, Turner-Bower D, Gandek B. How to score version 2 of the SF-12 health survey (with a supplement documenting version 1) QualityMetric Incorporated; Lincoln, RI: 2002. [Google Scholar]
- 30.Morisky D, Green L, Levine D. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care. 1986;24(1):67–74. doi: 10.1097/00005650-198601000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Szlosarek P, Charles KA, Balkwill FR. Tumor necrosis factor-α as a tumour promoter. Eur J Cancer. 2006;42:745–50. doi: 10.1016/j.ejca.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 32.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ, Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC) Med Care. 2005 May;43(5):436–44. doi: 10.1097/01.mlr.0000160375.47920.8c. [DOI] [PubMed] [Google Scholar]
- 33.Green BB, Ralston JD, Fishman PA, Catz SL, Cook A, Carlson J, Tyll L, Carrell D, Thompson RS. Electronic communications and home blood pressure monitoring (e-BP) study: design, delivery, and evaluation framework. Contemp Clin Trials. 2008 May;29(3):376–95. doi: 10.1016/j.cct.2007.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oliveria S, Lapuerta P, McCarthy B, L’Italien G, Berlowitz DR, Asch S. Physician-related barriers to the effective management of uncontrolled hypertension. Arch Intern Med. 2002;162:413–20. doi: 10.1001/archinte.162.4.413. [DOI] [PubMed] [Google Scholar]
- 35.Berlowitz DR, Ash AS, Hickey EC, Friedman RH, Glickman M, Kader B, Moskowitz MA. Inadequate management of blood pressure in a hypertensive population. N Engl J Med. 1998;339:1957–63. doi: 10.1056/NEJM199812313392701. [DOI] [PubMed] [Google Scholar]
- 36.Hyman D, Pavlik V. Self-reported hypertension treatment practices among primary care physicians: Blood pressure thresholds, drug choices, and the role of guidelines and evidence-based medicine. Arch Intern Med. 2000;160:2281–6. doi: 10.1001/archinte.160.15.2281. [DOI] [PubMed] [Google Scholar]
- 37.Ardery G, Carter BL, Milchak JL, Bergus GR, Dawson JD, James PA, Franciscus C, Kim Y. Explicit and implicit evaluation of physician adherence to hypertension guidelines. J Clin Hypertens. 2007;9(2):113–9. doi: 10.1111/j.1524-6175.2007.06112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bodenheimer T, Wagner EH, Grumbach K. Improving primary care for patients with chronic illness. JAMA. 2002;288:1775–9. doi: 10.1001/jama.288.14.1775. [DOI] [PubMed] [Google Scholar]
- 39.Cushman W, Ford C, Cutler J, Margolis K, Davis B, Grimm R, Black H, Hamilton B, Holland J, Nwachuku C, Papdemetriou V, Probstfield J, Wright J, Alderman M, Weiss R, Piller L, Bettencourt J, Walsh S. Success and predictors of blood pressure control in diverse North American settings: the Antihypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT) J Clin Hypertens. 2002;4:393–404. doi: 10.1111/j.1524-6175.2002.02045.x. [DOI] [PubMed] [Google Scholar]
- 40.Black H, Elliot W, Grandits G, Grambsch P, Lucente T, Neaton J, Grimm R, Hansson L, Lacourciere Y, Muller J, Sleight P, Weber M, Williams G, Wittes J, Zanchetti A, Anders R. Principal results of the Controlled Onset Verapamil Investigations of Cardiovasular End Points Trial (CONVINCE) JAMA. 2003;289:2073–82. doi: 10.1001/jama.289.16.2073. [DOI] [PubMed] [Google Scholar]
- 41.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, Mancia G, Cangiano JL, Garcia-Barreto D, Keltai M, Erdine S, Bristol HA, Kolb HR, Bakris GL, Cohen JD, Parmley WW. A Calcium Antagonist vs a Non-Calcium Antagonist Hypertension Treatment Strategy for Patients With Coronary Artery Disease: The International Verapamil-Trandolapril Study (INVEST): A Randomized Controlled Trial. JAMA. 2003;290(21):2805–16. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 42.Jamerson K, Bakris GL, Dahlof B, Pitt B, Velazquez E, Gupta J, Lefkowitz M, Hester A, Shi V, Kjeldsen SE, Cushman WC, Papademetriou V, Weber MA. Exceptional early blood pressure control rates: The ACCOMPLISH (Avoiding Cardiovascular Events Through Combination Therapy in Patients Living with Systolic Hypertension) Trial. Arch Intern Med. 2007 in press. [Google Scholar]
- 43.Wagner EH. Quality improvement in chronic illness care: a collaborative approach. Jt Comm J Qual Improv. 2001;27:63–80. doi: 10.1016/s1070-3241(01)27007-2. [DOI] [PubMed] [Google Scholar]
- 44.Wagner EH. Meeting the needs of chronically ill people. BMJ. 2001;323:945–6. doi: 10.1136/bmj.323.7319.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tsai AC, Morton SC, Mangione CM, Keeler EB. A meta-analysis of interventions to improve care for chronic conditions. Am J Manag Care. 2005;11:478–88. [PMC free article] [PubMed] [Google Scholar]
- 46.AHIP Board of Directors Statement on the Core Principles Integral to the Development of the Patient-Centered Medical Homes. 2008 Jun 18; [November 2010]; Available from: http://www.ahip.org/content/default.aspx?bc=31|44|23691.
- 47.Rosenthal T. The medical home: growing evidence to support a new approach to primary care. J Am Board Fam Med. 2008;21(5):427–40. doi: 10.3122/jabfm.2008.05.070287. [DOI] [PubMed] [Google Scholar]
- 48.Fahey T, Schroeder K, Ebrahim S. Educational and organizational interventions used to improve the management of hypertension in primary care: a systematic review. Br J Gen Pract. 2005;55:875–82. [PMC free article] [PubMed] [Google Scholar]
- 49.Schroeder K, Fahey T, Ebrahim S. How can we improve adherence to blood pressure-lowering medication in ambulatory care? Systematic review of randomized controlled trials. Arch Intern Med. 2004 Apr 12;164(7):722–32. doi: 10.1001/archinte.164.7.722. [DOI] [PubMed] [Google Scholar]
- 50.Chodosh J, Morton SC, Mojica W, Maglione M, Suttorp MJ, Hilton L, Rhodes S, Shekelle P. Meta-analysis: chronic disease self-management programs for older adults. Ann Intern Med. 2005;143:427–38. doi: 10.7326/0003-4819-143-6-200509200-00007. [DOI] [PubMed] [Google Scholar]
- 51.Weingarten SR, Henning JM, Badamgarav E, Knight K, Hasselblad V, Gano A, Ofman J. Interventions used in disease management programmmes for patients with chronic illness - which ones work? Meta-analysis of published reports. BMJ. 2002;325:925–32. doi: 10.1136/bmj.325.7370.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cappuccio FP, Kerry SM, Forbes L, Donald A. Blood pressure control by home monitoring: meta-analysis of randomised trials. BMJ. 2004 Jul 17;329(7458):145. doi: 10.1136/bmj.38121.684410.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hill MN, Han H-R, Dennison CR, Kim MT, Roary MC, Blumenthal RS, Bone LR, Levine DM, Post WS. Hypertension care and control in underserved urban African American men: behavioral and physiologic outcomes at 36 months. Am J Hypertens. 2003;16(11):906. doi: 10.1016/s0895-7061(03)01034-3. [DOI] [PubMed] [Google Scholar]
- 54.Hla KM, Feussner JR, Blessing-Feussner CL, Neelon FA, Linfors EW, Starmer CF, McKee PA. BP control. Improvement in a university medical clinic by use of a physician’s associate. Arch Intern Med. 1983 May 1;143(5):920–3. doi: 10.1001/archinte.143.5.920. [DOI] [PubMed] [Google Scholar]
- 55.Vivian EM. Improving blood pressure control in a pharmacist-managed hypertension clinic. Pharmacotherapy. 2002;22:1533–40. doi: 10.1592/phco.22.17.1533.34127. [DOI] [PubMed] [Google Scholar]
- 56.Artinian N, Washington O, Templin T. Effects of home telemonitoring and community-based monitoring on blood pressure control in urban African Americans: A pilot study. Heart Lung. 2001;30:191–9. doi: 10.1067/mhl.2001.112684. [DOI] [PubMed] [Google Scholar]
- 57.Schroeder K, Fahey T, Hollinghurst S, Peters TJ. Nurse-led adherence support in hypertension: a randomized controlled trial. Fam Pract. 2005 Apr;22(2):144–51. doi: 10.1093/fampra/cmh717. [DOI] [PubMed] [Google Scholar]
- 58.Woollard J, Burke V, Beilin LJ. Effects of general practice-based nurse-counselling on ambulatory blood pressure and antihypertensive drug prescription in patients at increased risk of cardiovascular disease. J Hum Hypertens. 2003 Oct;17(10):689–95. doi: 10.1038/sj.jhh.1001593. [DOI] [PubMed] [Google Scholar]
- 59.Rudd P, Miller NH, Kaufman J, Kraemer HC, Bandura A, Greenwald G, Debusk RF. Nurse management for hypertension. A systems approach. Am J Hypertens. 2004 Oct;17(10):921–7. doi: 10.1016/j.amjhyper.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 60.Zillich AJ, Sutherland JM, Kumbera PA, Carter BL. Hypertension outcomes through blood pressure nomintoring and evalutation by pharmacists (HOME Study) J Gen Intern Med. 2005;20:1091–6. doi: 10.1111/j.1525-1497.2005.0226.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bogden PE, Abbott RD, Williamson P, Onopa JK, Koontz LM. Comparing standard care with a physician and pharmacist team approach for uncontrolled hypertension. J Gen Intern Med. 1998 Nov;13(11):740–5. doi: 10.1046/j.1525-1497.1998.00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garcia-Pena C, Thorogood M, Armstrong B, Reyes-Frausto S, Munoz O. Pragmatic randomized trial of home visits by a nurse to elderly people with hypertension in Mexico. Int J Epidemiol. 2001 Dec;30(6):1485–91. doi: 10.1093/ije/30.6.1485. [DOI] [PubMed] [Google Scholar]
- 63.Glynn LG, Murphy AW, Smith SM, Schroeder K, Fahey T. Interventions used to improve control of blood pressure in patients with hypertension. Cochrane Database Syst Rev. 2010;(3):CD005182. doi: 10.1002/14651858.CD005182.pub4. [DOI] [PubMed] [Google Scholar]
- 64.Clark CE, Smith LF, Taylor RS, Campbell JL. Nurse led interventions to improve control of blood pressure in people with hypertension: systematic review and meta-analysis. BMJ. 2010;341:c3995. doi: 10.1136/bmj.c3995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carter BL, Ardery G, Dawson JD, James PA, Bergus GR, Doucette WR, Chrischilles EA, Franciscus CL, Xu Y. Physician and pharmacist collaboration to improve blood pressure control. Arch Intern Med. 2009 Nov 23;169(21):1996–2002. doi: 10.1001/archinternmed.2009.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Carter BL, Rogers M, Daly J, Zheng S, James PA. The potency of team-based care interventions for hypertension: a meta-analysis. Arch Intern Med. 2009 Oct 26;169(19):1748–55. doi: 10.1001/archinternmed.2009.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Friedman RH, Kazis LE, Jette A, Smith MB, Stollerman J, Torgerson J, Carey K. A Telecommunications System for Monitoring and Counseling Patients With Hypertension: Impact on Medication Adherence and Blood Pressure Control. Am J Hypertens. 1996;9(4):285. doi: 10.1016/0895-7061(95)00353-3. [DOI] [PubMed] [Google Scholar]
- 68.Cappuccio FP. Self-monitoring of blood pressure in primary care: is it useful? Am J Hypertens. 2005 Nov;18(11):1421. doi: 10.1016/j.amjhyper.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 69.Halme L, Vesalainen R, Kaaja M, Kantola I. Self-monitoring of blood pressure promotes achievement of blood pressure target in primary health care. Am J Hypertens. 2005 Nov;18(11):1415–20. doi: 10.1016/j.amjhyper.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 70.Figar S, Galarza C, Petrlik E, Hornstein L, Rodriguez Loria G, Waisman G, Rada M, Soriano ER, de Quiros FG. Effect of education on blood pressure control in elderly persons: a randomized controlled trial. Am J Hypertens. 2006;19(7):737–43. doi: 10.1016/j.amjhyper.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 71.Carnahan JE, Nugent CA. The effects of self-monitoring by patients on the control of hypertension. Am J Med Sci. 1975 Jan-Feb;269(1):69–73. doi: 10.1097/00000441-197501000-00008. [DOI] [PubMed] [Google Scholar]
- 72.Mehos BM, Saseen JJ, MacLaughlin EJ. Effect of pharmacist intervention and initiation of home blood pressure monitoring in patients with uncontrolled hypertension. Pharmacotherapy. 2000 Nov;20(11):1384–9. doi: 10.1592/phco.20.17.1384.34891. [DOI] [PubMed] [Google Scholar]
- 73.Midanik LT, Resnick B, Hurley LB, Smith EJ, McCarthy M. Home blood pressure monitoring for mild hypertensives. Public Health Rep. 1991 Jan-Feb;106(1):85–9. [PMC free article] [PubMed] [Google Scholar]
- 74.Rogers MA, Small D, Buchan DA, Butch CA, Stewart CM, Krenzer BE, Husovsky HL. Home monitoring service improves mean arterial pressure in patients with essential hypertension. A randomized, controlled trial. Ann Intern Med. 2001 Jun 5;134(11):1024–32. doi: 10.7326/0003-4819-134-11-200106050-00008. [DOI] [PubMed] [Google Scholar]
- 75.Soghikian K, Casper S, Fireman B, Hunkeler E, Hurley L, Tekawa I, Vogt T. Home blood pressure monitoring: Effect on use of medical services and medical care costs. Med Care. 1992;30(9):855–65. [PubMed] [Google Scholar]
- 76.Staessen JA, Den Hond E, Celis H, Fagard R, Keary L, Vandenhoven G, O’Brien ET. Antihypertensive treatment based on blood pressure measurement at home or in the physician’s office: a randomized controlled trial. JAMA. 2004 Feb 25;291(8):955–64. doi: 10.1001/jama.291.8.955. [DOI] [PubMed] [Google Scholar]
- 77.Zarnke KB, Feagan BG, Mahon JL, Feldman RD. A randomized study comparing a patient-directed hypertension management strategy with usual office-based care. Am J Hypertens. 1997 Jan;10(1):58–67. doi: 10.1016/s0895-7061(96)00305-6. [DOI] [PubMed] [Google Scholar]
- 78.White W. Out-of-office blood pressure monitoring. In: Izzo J, Black H, editors. Hypertension Primer. 2nd ed American Heart Association; Dallas, TX: 1999. [Google Scholar]
- 79.Scherger JE. Primary care needs a new model of office practice: fewer in-person visits, more patient contacts. BMJ. 2005;330:358–9. doi: 10.1136/bmj.330.7504.E358. [DOI] [PubMed] [Google Scholar]