Abstract
pH-sensitive PEGylated (with PEG-PE) long-circulating liposomes (HSPC:cholesterol and Doxil®), modified with cell-penetrating TAT peptide (TATp) moieties and cancer-specific mAb 2C5 were prepared. A degradable pH-sensitive hydrazone bond between a long shielding PEG chains and PE (PEG2k-Hz-PE) was introduced. TATp was conjugated with a short PEG1k-PE spacer and mAb 2C5 was attached to a long PEG chain (2C5-PEG3.4k-PE). The “shielding” effect of TATp by long PEG chains was investigated using three liposomal models. At normal pH, surface TATp moieties are “hidden” by the long PEG chains. Upon the exposure to lowered pH, this multifunctional carrier exposes TATp moieties after the degradation of the hydrazone bond and removal of the long PEG chains. Enhanced cellular uptake of the TATp-containing immunoliposomes was observed in vitro after pre-treatment at lowered pH (using flow cytometry and fluorescence microscopy techniques). The presence of mAb 2C5 on the liposome surface further enhanced the interaction between the carrier and tumor cells but not normal cells. Furthermore, multifunctional immuno-Doxil® preparation showed increased cellular cytotoxicity of B16-F10, HeLa and MCF-7 cells when pre-incubated at lower pH, indicating TATp exposure and activity. In conclusion, a multifunctional immunoliposomal nanocarrier containing a pH-sensitive PEG-PE component, TATp, and the cancer cell-specific mAb 2C5 promotes enhanced cytotoxicity and carrier internalization by cancer cells and demonstrates the potential for intracellular drug delivery after exposure to lowered pH environment, typical of solid tumors.
Keywords: long-circulating liposomes, TAT peptide, mAb 2C5, pH-sensitive PEG-PE conjugate, Doxil®, multifunctional carriers
1. Introduction
The engineering of a multifunctional pharmaceutical nanocarrier is based on a number of properties that can act either simultaneously or sequentially to significantly enhance the efficacy of a variety of therapeutic and diagnostic protocols [1]. Among the drug delivery systems (DDS) developed, liposomes have demonstrated substantial promise as carriers for the delivery of soluble/non-soluble drugs and other therapeutic/diagnostic agents [2]. It is known that PEGylated liposomes are not readily taken up by the macrophages of the reticuloendothelial system (RES) and hence stay in the circulation for a relatively long period of time [3, 4]. This long circulating effect enables these small-sized carriers to “passively” accumulate in tumor tissue, due to the enhanced permeation and retention (EPR) effect [5]. This effect is based on the spontaneous penetration of circulating macromolecules, particulate drug carriers, and molecular aggregates into the interstitium through the leaky vasculature at certain pathological sites. This effect is typical of solid tumors, infarcts and inflammation zones [5-7].
A designed multifunctional carrier with an “active” targeted drug delivery approach, based on one or more functional groups on the carrier's surface, can further enhance its efficient accumulation in the target site and also enable delivery of the therapeutic/diagnostic agent to a specific cell organelle.Since many anticancer drugs, DNA and other therapeutic agents have their effects only in a specific cellular organelle (e.g. pro-apoptotic drugs in the mitochondrial membrane, gene therapy in the nuclear or mitochondrial genomes), enhancement of intracellular delivery of drug carriers can sharply increase the efficiency of a variety of treatment protocols. However, the receptor-mediated endocytosis of drugs, drug carriers, and DNA leads to their lysosomal delivery and subsequent considerably high degradation. These approaches for direct intra-cytoplasmic delivery that circumvent the endocytic pathway should be helpful.
Cell penetrating peptides (CPPs) (e.g. TAT-peptide, penetratin, poly-arginine, Antp and VP22) have demonstrated a capability for delivery of large variety of biologically active cargoes such as proteins, DNA, antibodies, contrast (imaging) agents, toxins and nanoparticular drug carriers, including liposomes, to the cell interior by traversing the cell's plasma membrane independent of a membrane receptor [8]. These peptides show no cell-type specificity and basically rely on the positively charged sequences of amino acids (mostly arginine and lysine) and the electrostatic interaction (prior to internalization) with negatively charged cell-surface glycoproteins. Although The mechanism of this phenomenon is still not fully understood, it has been shown that in addition to their electrostatic interactions and hydrogen bonding, responsible for the direct transduction of small molecules [9], an energy-dependent macropinocytosis is responsible for the CPP-mediated intracellular delivery of large molecules and nanoparticulates followed by consequent enhanced release from endosomes into the cell cytoplasm [10].
Furthermore, recent studies have suggested that TATp moieties (containing an arginine-arginine amino acids sequence) on the surface of liposomes and micelles are susceptible to enzymatic cleavage by enzymes present in human plasma and require steric protection by long PEG chains to maintain their CPP functionality [11].
To enable its intracellular delivery capabilities to a targeted site, the “ultimate” CPP-containing carrier should possess prolonged circulation in the blood (by PEG coating to enable an EPR effect); specific cell-surface recognition moieties (monoclonal antibodies, Fab fragments, phage display peptides); stimulus-response capabilities to take advantage of the pathological site characteristic (the ability to either release an entrapped drug or expose “hidden” active moieties by surface-attached pH- or temperature-sensitive coatings) and finally, cell penetrating capabilities that pass the lysosomal degradation and enhance intracellular drug targeting.
Here, we report the design of a multifunctional immuno-liposomal preparation containing TAT-peptide moieties, sterically shielded with a degradable pH-sensitive hydrazone bond between a long shielding PEG chains and PE (PEG2k-Hz-PE conjugate). The nucleosome-specific antibody (mAb 2C5), capable of recognition of various tumor cells via tumor cell's surface-bound nucleosomes [12], was attached to a longer PEG chain (2C5-PEG3.4k-PE) (Figure 1). We hypothesized that PEGylated liposomes accumulate in targets via the EPR effect (“passive” targeting) and the mAb 2C5 (“active” targeting). Within the “acidified” milieu (typical to solid tumors or ischemic tissues) these carriers will lose their PEG coating by hydrolysis of a hydrazone pH-sensitive bond, and penetrate inside cells via the effect of exposed TATp moieties. We have shown the liposomal PEGylation molar ratios needed for effective TATp shielding by long PEG chains using 3 liposomal preparations, including the FDA-approved and clinically effective anticancer drug Doxil® which is doxorubicin, loaded in sterically stabilized PEGylated liposomes. Upon the exposure of these multifunctional carriers to lowered pH, an enhanced cellular uptake of the TATp-containing immunoliposomes was observed in vitro (using flow cytometry and fluorescence microscopy techniques). Furthermore, increased cytotoxicity of multifunctional immuno-Doxil® formulation pre-exposed to lower pH was also found, indicating TATp exposure and effective intracellular delivery of the encapsulated doxorubicin.
Figure 1.
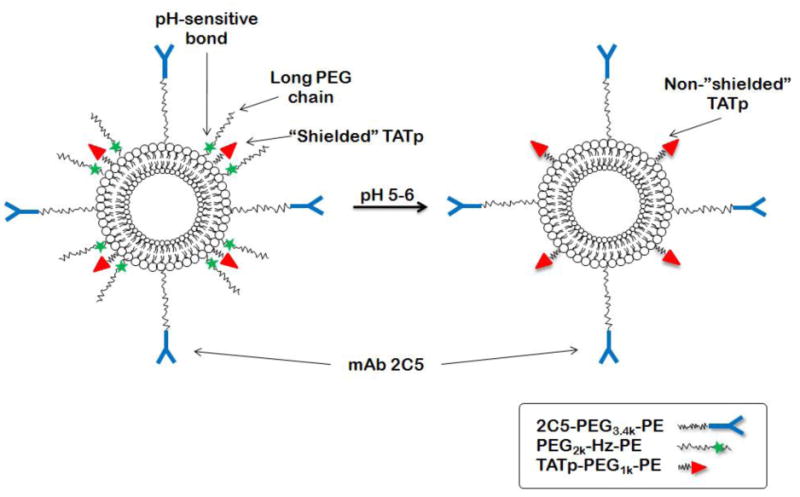
Schematic of the low pH effect on TATp-modified pH-sensitive immunoliposomes composed of a pH-degradable PEG2k-Hz-PE with long PE blocks, TATp-PEG1k-PE with short PEG blocks, and mAb2C5-PEG3.4k-PE.
In conclusion, an optimized multifunctional immuno-liposomal nanocarrier comprised of a pH-sensitive PEG-PE component, TATp, and the cancer cell-specific mAb 2C5 can promote enhanced cytotoxicity and carrier internalization by cancer cells and demonstrates the potential for in vivo intracellular drug delivery after exposure to a lowered pH environment typical of solid tumors.
2. Materials and Methods
2.1. Materials
TAT-cysteine peptide (TATp-Cys 12-mer: CysTyrGlyArgLysLysArgArgGlnArgArgArg; molecular mass 1663 Da with one reactive thiol group) was synthesized by the Tufts University Core Facility (Boston, MA). The mAb 2C5 was produced in ascites via I.P. injection of 1.5×106 hybridoma cells/ml into a primed 4 week old male Balb/C mice. The production and the purification of the mAb 2C5 were carried out by Harlan Bioproducts (Indiannapolis, IL) using the cell line from our laboratory. Control bovine antibody IgG was obtained from MP Biomedicals LLC (Ohio, USA). Doxil®, a commercially available preparation of doxorubicin in PEGylated liposomes (ALZA Corp.), was purchased from Pharmaceutics Inc. (West Roxbury, MA). Cholesterol 98% (Chol), fully hydrogenated soy phosphatidylcholine (HSPC), egg L-α-phosphatidylcholine, 1,2-Dioleoyl-sn-glycero-3-phosphoethanolamine (DOPE), 1,2-dipalmitoyl-sn-glycero-3-phosphothioethanolamine (Sodium Salt) (DPPE-SH), NHS-PEG1000-maleimide, diacyllipid polyethylene glycols (PEG1000-PE, PEG2000-PE), rhodamine-phosphatidylethanolamine (Rh-PE) and phosphatidylthioethanol (DPPE-SH) were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Poly-oxyethylene 3400-bis(p-nitrophenyl carbonate) [PEG(pNP)2] was purchased from Laysan Bio. Inc. (Arab, Alabama). Triethylamine (TEA) and fluorenylmethyloxycarbonyl chloride (FMOC-Cl) were purchased from Fluka (AG, Switzerland). N-(4-Acetylphenyl maleimide was from Acros Organics (Fairlawn, NJ, USA), methoxy poly(ethylene) glycol butyraldehyde (MW 2000), mPEG-SH - from Laysan Bio, Inc. (Huntsville, AL, USA), and 4-(4-N-maleimidophenyl) butyric acid hydrazide hydrochloride (MPBH) from Pierce Biotechnology, Inc. (Rockford, IL, USA). Fluoromount-G was from Southern Biotechnology Associates Inc. (Birmingham, AL), and CellTiter Blue cell viability assay was from Promega, Madison, WI. TATp-PEG1k-PE (TATp-conjugate), PEG2k-Hz-PE and pNP-PEG3.4k-PE were synthesized in-house (see below).
Cell lines (Human fibroblasts, 4T1, MCF-7, B16-F10, and HeLa) were purchased from the American Type Culture Collection (Manassas, VA). All cell culture media, DMEM, heat-inactivated fetal bovine serum (FBS), and concentrated solutions of penicillin/streptomycin stock solutions were from Cellgro® (Herndon, VA). All other chemicals and solvents were of analytical grade, purchased from Thermo Fisher Scientific and used without further purification.
2.2. Methods
2.2.1. Synthesis of TATp-PEG1k-PE (TATp conjugate)
The TATp-PEG1000-PE conjugate was synthesized as described previously [13] with some modifications. Briefly, an approximately 1.5-fold molar excess of NHS-PEG1k-maleimide was reacted with DOPE by stirring for 2hr in chloroform at room temperature with a 3-fold molar excess of triethylamine. A 2-fold molar excess of TATp-Cys was then added, and the reaction was continued with stirring overnight. The solvent was evaporated, and the product was freeze-dried overnight. The excess of TATp-Cys was separated from the product by gel filtration chromatography. Fractions were collected and monitored by TLC using silica plates (mobile phase of chloroform/methanol 80:20% v/v), and TATp-PEG-PE was visualized with phosphomolybdic acid and Dragendorff spray reagents. In order to confirm TATp conjugation and presence, we also analyzed for TATp conjugation using HPLC, as previously described [14].
2.2.2. Synthesis of PEG2k-Hydrazone-PE
An aldehyde-derived hydrazone-based PEG2k-Hz-PE conjugate (pH-sensitive conjugate) was synthesized by a two-steps method as previously described [15] with modifications. For step I (synthesis of acyl hydrazide-PEG derivative) 40 μmol of mPEG-SH in chloroform were mixed with a two molar excess of the acyl hydrazide cross-linker MPBH in presence of 5 molar excess of triethylamine over lipid. Following 2hr of stirring at room temperature, product was dialyzed (Spectra/Por 6 dialysis membrane, MWCO 1K, Spectrum Laboratories, Rancho Dominguez, CA) against deionized water, analyzed by TLC, freeze-dried and stored as a chloroform solution at -80°C. For step II of the synthesis (activation of phospholipid with 4-acetyl phenyl maleimide) 40 mmol of 4-acetyl phenyl maleimide were reacted with 27 mmol of 1,2-dipalmitoyl-sn-glycero-3-phosphothioethanolamine (DPPE-SH) in the presence of triethylamine overnight with continuous stirring. The activated phospholipid was separated on a silica gel column using chloroform:methanol mobile phase (9:1 v/v). The fractions containing product were identified by TLC analysis, pooled, concentrated, freeze-dried and stored as a chloroform solution at −80°C.
For the synthesis of the PEG-HZ-PE conjugate, a hydrazide activated PEG derivative was reacted over-night with a 1.5M excess of the activated phospholipid with constant stirring at room temperature. The conjugate was separated and purified by size-exclusion gel chromatography using Sepharose-CL4B media.
2.2.3. Synthesis of pNP-PEG3.4k-PE and mAb 2C5 modification
In order to attach mAb 2C5 to the liposomal preparations, we first conjugated the mAb to the distal ends of PEG blocks via p-nitrophenylcarbonyl (pNP) groups (using a pNP-PEG3.4k-PE conjugate) to form immunomicelles. Using the post-insertion method with micelles [16, 17] we formed ligand-coupled liposomes (discussed below). First, we synthesized and purified pNP-PEG3.4k -PE according to an established method as previously described [18]. Briefly, the synthesis includes the interaction of PE with a10-fold molar excess of PEG-(pNP)2 in chloroform in the presence of triethylamine. Organic solvents were removed, pNP-PEG3.4k-PE micelles were formed and separated from free PEG and pNP on a CL-4B column. The pNP-PEG3.4k-PE product was freeze-dried, extracted with chloroform and stored at −80°C.
For antibody conjugates with PEG3.4k-PE (mAb 2C5 or non-specific IgG), a 40 molar excess of pNP-PEG3.4k-PE dispersed in a 10 mg/mL micellar solution in 5 mM Na-citrate, 150 mM NaCl, pH 5.0, was added to an equal volume of a 1 mg/mL solution of protein in 100mM Tris-buffered saline (TBS), pH 8.5. The mixtures were incubated at pH 8.5 for 24 hours at 4 °C.
2.2.4. Preparation of liposomes
Two PEGylated liposomal formulations were prepared. For all, we first formed the liposomes and then attached a variety of surface-decorating polymers in different molar ratios to their surfaces (using post-insertion technique) (see below).
For the first liposomal preparation, a lipid film was obtained from a mixture of egg-phosphatidylcholine, cholesterol (Egg-PC:cholesterol, 7:3 molar ratio) in chloroform. Chloroform was removed by ventilation using N2 gas followed by freeze-drying. The film was hydrated with an appropriate buffer and vortexed at room temperature for 5 min. The second liposomal formulation was of Doxil®-mimicking composition but contained no doxorubicin. For this preparation, we used the same lipid components and the same concentrations as found in Doxil®. A lipid film was obtained from N-(carbonyl-methoxypolyethylene glycol 2000)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine sodium salt (mPEG-DSPE, 3.19 mg/mL), fully hydrogenated soy phosphatidylcholine (HSPC, 9.58 mg/mL), and cholesterol (3.19 mg/mL). Following chloroform removal with N2 gas and freeze drying, the lipid film was hydrated in HEPES-buffered saline (HBS), pH 7.4. Both liposomal formulations were sonicated with a probe-type sonicator at 11 W power for 30 min until approximately 100 nm liposomes with narrow size distribution formed (see below).
2.2.5. modification of liposomes
With the post-insertion method [16, 17] we efficiently decorated liposomal surfaces with a variety of polymeric conjugates. In order to “add” a function to the three pre-formed liposomal preparations (Egg-PC:cholesterol, HSPC:cholesterol and Doxil®), liposomes were incubated overnight with different micellar combinations from the following: TATp-PEG1k-PE (2.5 mol%), PEG2k-PE (2.5-15 mol%), PEG5k-PE (2.5-15 mol%), PEG2k-Hz-PE (15 mol%), 2C5-PEG3.4k-PE (2.5 mol%), IgG-PEG3.4k-PE (2.5 mol%) and rhodamine-PE (1 mol%). Subsequently, preparations were dialyzed against water for 24 hours. The optimal multifunctional liposomal formulation used for characterization and/or cell culture experiments included TATp-PEG1k-PE (2.5 mol%), 2C5-PEG3.4k-PE (2.5 mol%) and PEG2k-Hz-PE (15 mol%). Multifunctional immunoliposomes were pre-incubated for 30 minutes at pH 5.0 and 7.4 prior to analyses and in vitro studies.
2.2.6. Characterization of liposomes
2.2.6.1 Size and zeta-potential measurements
Liposome size measurements and size distribution analysis were performed by dynamic light scattering (DLS) using a Coulter® N4-Plus Submicron Particle Sizer (Coulter Corporation, Miami, FL). In all cases, size distribution was unimodel. Size distribution of liposomes was also confirmed by using a transmission electron microscopy (TEM) (Jeol, JEM-1010, Tokyo, Japan). Liposome surface charge analysis was performed using a Zeta Phase Analysis Light Scattering (PALS) UltraSensitive Zeta Potential Analyzer instrument (Brookhaven Instruments, Holtsville, NY).
2.2.6.2 Specific activity of mAb 2C5 on liposomal preparations
To confirm the presence of mAb 2C5 on the liposome surface, their immunological activity was estimated by a standard enzyme-linked immunosorbent assay (ELISA) as previously described [12]. We used the water-soluble fraction of calf thymus nucleohistone (Worthington Biochemical, Lakewood, USA) as an antigen and horseradish peroxidase/anti-mouse IgG conjugate (ICN Biomedical, Aurora, USA) as a secondary antibody to verify the presence of mAb 2C5 on the liposomal surface. The activity of mAb 2C5 conjugated to Doxil®, multifunctional immuno-Doxil® and HSPC:cholesterol immunoliposomes surfaces were analyzed.
2.2.7. Cell cultures
B16-F10, HeLa, MCF-7, 4T1 cells, provided from the ATCC, were grown in DMEM with 2 mM L-glutamine, supplemented with 10% (v/v) heat-inactivated fetal bovine serum, 100 units/ml penicillin G and 100 μg/ml streptomycin. Cultures were maintained at 37 °C in a humidified 5% CO2 incubator. In all studies, the cells were sub-cultured every 2-3 days and were used for experiments at passages 5-20.
2.2.8. Interaction of liposomes with cells, fluorescence microscopy
The cellular uptake and interaction of the various carriers were studied visually using multifunctional immunoliposomes labeled with 1 mol% rhodamine-PE. Adherent B16-F10 and HeLa and human fibroblast cells were grown on glass cover slips placed in six-well tissue culture plates. When the cells reached a confluency of 60-70%, they were washed twice with PBS, medium was replaced, and a further incubation was conducted for 1 hr at 37°C, 5% CO2 with pre-treated (at pH 5.0 or 7.4 for 30 minutes) multifunctional immunoliposomes. Hoechst nuclear stain (2.5μg/ml) was added for the last 30 minutes of incubation. Cells were washed three times with PBS and mounted individually cell-side down on glass slides using fluorescence-free glycerol based mounting medium (Fluoromount-G). All mounted slides were studied with a Nikon Eclipse E400 microscope under fluorescence using a Rh/TRITC filter for Rh-labeled liposomes (red) and a UV-2B filter for Hoechst-labeled nuclei (blue).
2.2.9. FACS analysis
To assess the cell binding/interaction of the multifunctional immunoliposomes, flow cytometry (FACS) analysis of the different cell types incubated with different formulations pre-treated at different pH values was performed. For HSPC liposomes, 1 mol% of rhodamine-PE was added to the formulation. 4T1, MCF-7, HeLa and B16-F10 cells (5×105 cells/ml) were plated in a six-well plate (35 mm diameter) in 1.5 ml medium and incubated for 24 h before further experiments. After the replacement of the medium with fresh serum-free medium, the pre-treated (at different pH) multifunctional rhodamine-labeled immuno-liposomes were added to the cells. The final lipid concentration was 0.1mg/ml. After 1-2 hr of incubation, the medium was discarded and the cells were washed three times with cold PBS. Cells were detached following 5 minutes of incubation with a trypsin solution (0.25%) at 37°C, washed with cold PBS, centrifuged at 1000rpm and re-suspended three times. The cellular uptake/attachment efficiency was determined by FACScan™ (Beckton Dickinson biosciences, San Jose, CA) by acquisition of 10,000 events per histogram.
2.2.10. Cytotoxicity assay
The cytotoxicity of multifunctional immuno-Doxil® liposomes against B16-F10, MCF-7 and HeLa cells was studied. Cells were seeded into 96 well microplates at a density of 3×104 cells/well. Cells were incubated for 24 hr until growth to ∼50% confluency. Multifunctional immuno-Doxil® formulations were pre-treated with pH 5 or 7.4 for 30 minutes prior their addition to cells. After 12 hr of incubation at 37°C, 5% CO2, with the various formulations, plates were washed three times with fresh media, and placed in the incubator with fresh media for an additional 48 hours. Finally, cytotoxicity of the various formulations was evaluated by the addition of 20μl/well (total vol. was 120μL/well) CellTiter Blue solution to assess viability. After 1 hr incubation at 37°C, 5% CO2, the cell survival was estimated by measuring the fluorescence intensity using a microplate reader (Synergy HT multimode microplate reader, BioTek Instrument, Winooski, VT) with 525/590 nm excitation/emission wavelengths.
2.2.11. Statistical analysis
Statistical analysis was performed using GraphPad Prism (GraphPad Software, San Diego, CA). Unless mentioned, the results are presented as the mean ± SD. Unpaired Student's t tests were performed as specified in figure legends (differences were considered significant when P ≤ 0.05).
3. Results and Discussion
The approach using a drug-encapsulated nanocarrier with cell environment-responsive functions such as elevated temperature or lower pH, cell-penetrating peptides and specific targeting surface antibodies for cellular or intracellular targeting is a significant challenge. For this study, we designed and prepared a surface-modified multifunctional liposomal carrier with a 2C5 monoclonal antibody, a cell penetrating peptide TAT and with a hydrazone-based pH-sensitive PEG polymer to serve as an effective TATp steric shield at normal pH. mAb 2C5 was attached to a long PEG chain (2C5-PEG3.4k-PE). TATp was conjugated with a short PEG1k-PE spacer and the “shielding” effect of TATp with a long PEG2k chain was investigated using three liposomal nanocarriers model. This nanocarrier was designed in such a way that during the first phase of its delivery, the mAb 2C5 moieties are exposed and available for targeting the carrier specifically to cancer cells. At normal pH, surface TATp moieties are “shielded” by the long PEG blocks. Following exposure to lowered pH, degradation of the hydrazone bond and removal of the long PEG chains occur. In turn, the exposed TATp moieties enhance TATp-modified carrier penetration through the cell membrane. A schematic of the designed carrier is presented in Figure 1.
3.1. Characterization of liposomes
We prepared, modified and characterized three liposomal preparations: Egg-PC:cholesterol, HSPC:cholesterol and the commercially available liposomal preparation of Doxil® (See Materials and Methods). Nano-vesicle modification with PEG chains prevents aggregation and fusion and prolongs blood circulation time [3, 19]. In this study we modified only the outer surface of liposomes by incorporating PEG-phosphatidylethanolamine with additional polymeric conjugates into pre-formed liposomes using a post-insertion technique [16, 17, 20]. Theoretically, the amount of PEG-lipid able to incorporate into vesicles is directly correlated with the membrane elasticity, e.g., the cholesterol content in the bilayers and the PEG chain length [21]. Leroux and co-workers showed that increasing the mol% of PEG (up to 15 mol%) not only did not increase carrier's size, but also did not influence their removal from the bloodstream [22]. The size range of all three liposomal formulations prepared in this study following the increase in the molar ratios of PEG-PE block polymer (up to 15 mol%) was within the interval of 80-100nm as confirmed by a dynamic light scattering assay. The modification of liposomes with 2.5 mol% TATp-PEG1k-PE, 2.5 mol% 2C5-PEG3.4k-PE and 15 mol% of long-chain pH-sensitive PEG blocks (PEG2k-Hz-PH) (or combinations among them), increased particle size slightly, but did not exceed 120nm (data not shown). These results were confirmed using transmission electron microscopy (Figure 2) for Doxil liposomes and for multifunctional immuno-Doxil.
Figure 2.
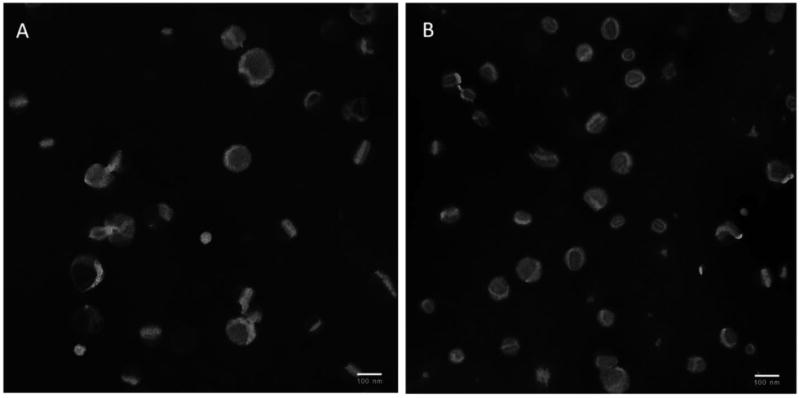
TEM images of Doxil® (A) and multifunctional immuno-Doxil® (B) liposomes.
Since TATp is a charged peptide, we were able to characterize its shielding efficiency when incorporated on the multifunctional liposome's surface, from its zeta potential. Table 1 presents zeta potentials of Doxil and HSPC-cholesterol (Doxil®-like) multifunctional liposomes. TATp moieties on the carrier's surface contributed to the positive charge of the carriers, compared to plain liposomes. While original Doxil® and plain Doxil®-like carriers had zeta potential values of −56.94±3.20 and −46.42±0.91 mV respectively, TATp-modified liposomes had values of 4.79±1.71 and −8.54±0.72 mV respectively. These values are due to the relative abundance of positively charged amino acids in TATp, such as arginine and lysine. We have previously shown that the TATp amino group couple, arginine-arginine, is susceptible to enzymatic cleavage when the peptide is presented on the surface of micelles and liposomes [11]. The concept of steric shielding was recently described as a potential way to prevent TATp cleavage, as well as its non-specific activity and to control the peptide/conjugate penetration through the cell membrane only when exposed. In this study we used a shielding but cleavable pH-sensitive PEG block that detached following a decrease in external cellular pH. Table 1 shows that both multifunctional immuno-Doxil® and immuno-HSPC liposomes possess relatively negative zeta potentials of −41.00±0.95 and −38.62±3.05 mV respectively for preparations incubated at pH 7.4. When pre-exposed to pH 5.0, multifunctional immuno-Doxil®/HSPC nanocarriers had a more positive zeta value of −4.35±2.10 and −23.72±3.55 mV respectively, in support of our proposed mechanism for a TATp shielding and de-shielding effects.
Table 1.
Zeta potentials (mV) of multifunctional HSPC:Cholesterol and Doxil® liposomal formulations (n=5, mean ± SEM).
| Liposomal preparation | HSPC:Cholesterol | Doxil® |
|---|---|---|
| Plain lip. | −46.42 ± 0.91 | −56.94±3.20 |
| TATp lip. | −8.54 ± 0.72 | 4.79±1.71 |
| 2C5 lip. | −38.61 ± 0.40 | −29.41±1.66 |
| TATp+2C5 lip. | −3.55 ± 4.09 | −19.90 ±2.40 |
| TATp+Hz+2C5 lip. pH 7.4 | −41.00 ± 0.95 | −38.62±3.05 |
| TATp+Hz+2C5 lip. pH 5 | −23.72 ± 3.55 | −4.35+2.10 |
3.2. TATp shielding by long PEG chains
When designing a “smart” multifunctional drug delivery platform, based on the ability of TAT peptide to promote cell penetration, the drug carrier should be designed so that during the first phase of delivery, the cell-penetrating function (CPP moiety) is sterically shielded (e.g. by a polymer). This is essential due to TATp's non-specificity and its susceptibility to cleavage by various proteolytic enzymes present in the circulation [11]. Upon accumulation in the target tumor area, the stimulus-sensitive steric shielding used, ideally becomes detached at the somewhat unique local conditions, exposes the TATp and allows delivery of the carrier and its cargo within cells [23]. Since TATp needs to be efficiently shielded, we hypothesized that the PEGylation degree and length might influence the liposomes' characters as well as the degree of TATp shielding.
The conformation of PEG chains on the liposomal surface relies not only on the PEG chain length, but also on the density of PEG chains covering the nanoparticle surface (recently reviewed by [24] and [25]). PEG chains of the same length will acquire the so-called “mushroom” conformation if the density of the PEG chains conjugated to the nanoparticlate is low (up to 4 mol% of grafted PEG). The same PEG molecules will obtain a “brush” conformation if the density of PEG chains on the nanoparticle is higher. In the current study we PEGylated the liposomes, using a post insertion technique, with a variety of PEG conjugates to form a “brush” PEG conformation to provide the most effective opsonin repulsion [26] and more PEG chains overlap [27]. While some investigators find destabilization of lipid lamellar structures only after the incorporation of more than 30-40 mol% of mPEG-lipid, others have reported much lower amounts [28-33]. Allen and co-workers [34] described and characterized liposomal formulations, with up to 20 mol% of mPEG. Garbuzenko et al [33] used mathematical models [35, 36] and described the effect of up to 25 mol% of PEG2k-DSPE in the presence or absence of cholesterol and the effect of phosphatidylcholine saturation on the size and the lipid bilayer packing of large unilamelar vesicles. They concluded that the combination of phosphatidylcholine, cholesterol and grafted PEG keeps the vesicles as lipid bilayers even for 20 mol% of PEG and does not cause its transition from liposomes to micelle. An explanation for reports of wide PEG mol% ranges is probably related to the different carriers/techniques used for PEG surface incorporation.
To characterize the efficiency of TATp shielding by longer PEG blocks with different molar ratios, we followed zeta potentials of the TATp-modified liposomal preparations (Figure 3). The zeta potential reflects the “exposed” TATp moieties on the carrier's surface and allowed us to optimize the effective PEG shielding content. TATp was conjugated to a short PEG1k-PE block. PEG2000-PE and PEG5000-PE were used as the shielding polymers containing longer PEG chains. We used Egg-PC:cholesterol, HSPC:cholesterol and Doxil® liposomal formulations with increasing molar ratios of PEGs from 2.5 to 15 mol%. As shown in Figure 3, a higher PEG shielding resulted in more negative zeta potential values for all three liposomal preparations suggesting effective shielding of TATp in a dose dependent manner. No significant change in zeta potentials was observed for PEGylated plain liposomes at increasing molar ratios (up to 15 mol%) (data not shown). Furthermore, the longer the PEG chain used for shielding (PEG5k-PE vs. PEG2k-PE) the more negative were the zeta potentials. The zeta potentials of Doxil and TATp-modified Doxil were -56.94±3.20 and 4.79±1.71 mV respectively. The zeta potential for the shielded TATp-modified Doxil® with 15 mol% PEG2k-PE and PEG5k-PE were −38.76±1.15 and −48.38±2.21 mV respectively.
Figure 3.
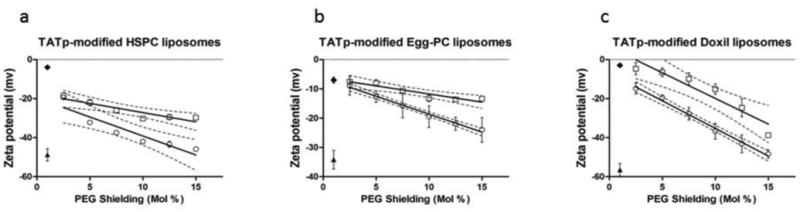
Zeta potentials of TATp-modified HSPC:cholesterol (a), Egg-PC:cholesterol (b) and Doxil® (c) liposomal preparations, versus mol% PEGylation. Plain liposomes (▴ ), TATp liposomes (◆), PEG2k-PE shielding ( □ ) and PEG5k-PE shielding ( ○ ) are shown. Note that longer PEG chains also influence TATp shielding. n=5.
Our results suggest that in addition to the role of PEGylation in promotion of long-circulation of liposomes, stimuli-sensitive PEGs can be used for the efficient shielding of TATp and potentially can also facilitate endosomal escape and prevents loss of liposomes activity [37] (referred to as the “PEG dilemma” [38]). For our in vitro experiments, we used a pH cleavable PEG-Hz-PE conjugate with 2kD PEG at 15 mol% (See section 2.2.2).
3.3. mAb 2C5-modified liposomes
One of the active targeting strategies with pharmaceutical nanocarriers is the use of monoclonal antibody as a homing ligand when attached to the carrier's surface either covalently or non-covalently. The monoclonal antibody 2C5 belongs to a subset of anti-nuclear auto-antibodies of the IgG2a isotype [39]. In addition to its ability to inhibit tumor growth in a variety of tumor models, it was found to specifically recognize an assortment of tumors with very low affinity for normal cells through the surface-bound nucleosomes. This property provides the ability to act as active targeting moiety to deliver drug carriers such as liposomes and micelles to different murine and human tumors [40, 41]. The advantage of using mAb 2C5 as the targeting ligand is that its target antigen is not tumor-type specific, but somewhat of a “universal” target that is typical of almost all tumor types [42]. Thus, it can be expected that multifunctional liposomal carrier decorated with mAb 2C5 will recognize a broad variety of murine and human cancer cell types and will actively target the carrier to the tumor. Previously [12], it was shown that PEG-PE-modified 2C5 antibody could be quantitatively incorporated into the liposomal membrane of doxorubicin-loaded liposomes (Doxil®).
2C5-targeted Doxil liposomes acquired the ability to recognize and exclusively bind to various tumor cells. Doxorubicin-loaded long-circulating liposomes modified with the mAb 2C5 were also shown to kill various tumor cells in vitro [43] and to enhance accumulation of these immunoliposomes in various tumors in mice [40]. The presence of mAb 2C5 on the multifunctional immuno-liposomal surfaces was confirmed by ELISA using nucleosomes as binding substrate [12]. To prevent the mAb from being hidden in the PEG corona, a long PEG spacer (3.4kDa) was conjugated to the antibody. Both Doxil®- and HSPC:cholesterol-2C5 modified multifunctional liposomes demonstrated immunoreactivity toward the antigen, compared to the IgG-modified carriers (figure 4). The ELISA experiments clearly demonstrated that mAb 2C5 was attached to the carrier's surface to a major extent and retained the specific activity required for the successful targeting of liposomes to cancer cells (See below). The production of multifunctional immuno-liposomes did not noticeable change the liposome size but showed slight increase in net charge (See Table 1).
Figure 4.
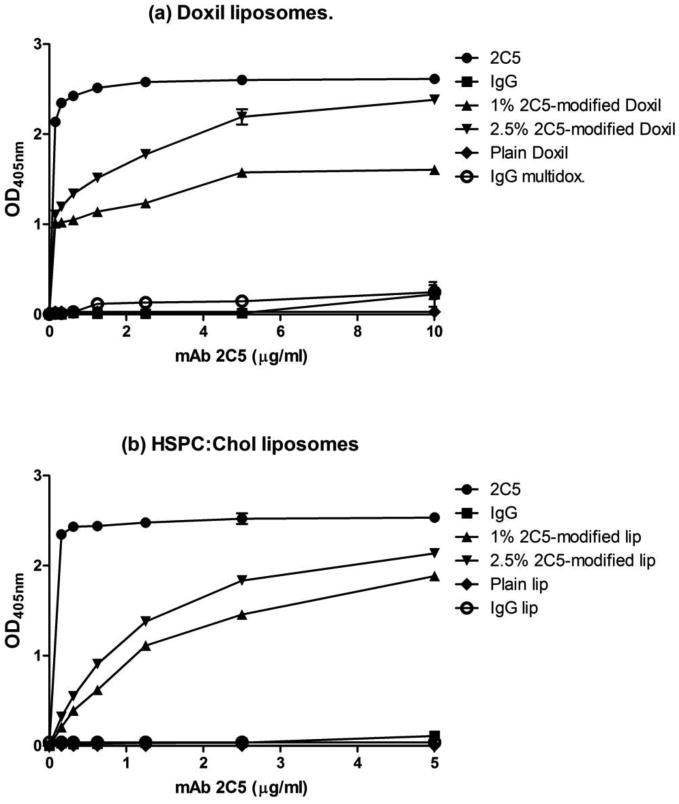
ELISA results for increasing amounts of mAb 2C5, attached to Doxil® (a) and HSPC:Cholesterol (b) multifunctional immuno-liposomal preparations. Preparations are comprised of TATp-PEG1k-PE, PEG2k-Hz-PE and 2C5mAb-PEG3.4k-PE. Data represent the mean ± SD, n=3.
3.4. Specific binding and uptake of multifunctional immunoliposomes by cancer cells in vitro
The combination of three targeting components on a single carrier should be considered a challenge. TAT peptide on the surface of liposomes was shown to enhance their efficient intracellular delivery [13] . Furthermore, the use of a combination of TATp and a PEG “shielding” by pH-sensitive PEG blocks demonstrated the ability to control TATp exposure and prevented its enzymatic degradation when attached to liposomes or micelles [11, 15]. The addition of mAb 2C5, conjugated to a long PEG block, on the surface of such a designed carrier should enhance liposome targeting and interaction with cancer cells [42]. In order to mimic the acidic environment typical in the solid tumor milieu [44, 45], multifunctional immunoliposomes were pre-incubated at pH 5.0 or 7.4 before incubation with cells. Liposomal interaction with cells was measured using fluorescence microscopy and flow cytometry.
Fluorescence microscopy showed that while TATp-modified liposomes were significantly internalized by normal fibroblasts and by MCF-7 and B16-F10 cells, 2C5-modified liposomes were internalized only by the cancer cells. Figure 5 clearly shows that pre-exposed multifunctional immunoliposomes to acidic conditions for 30 minutes enhanced internalization of the liposomal formulations to the cells, compared with liposomes incubated at pH 7.4. These results suggest that although TATp cell penetrating properties were shielded at pH 7.4, short exposure to lower pH cleaved the hydrazone pH-sensitive bond, exposed TATp moieties and promoted their activity.
Figure 5.
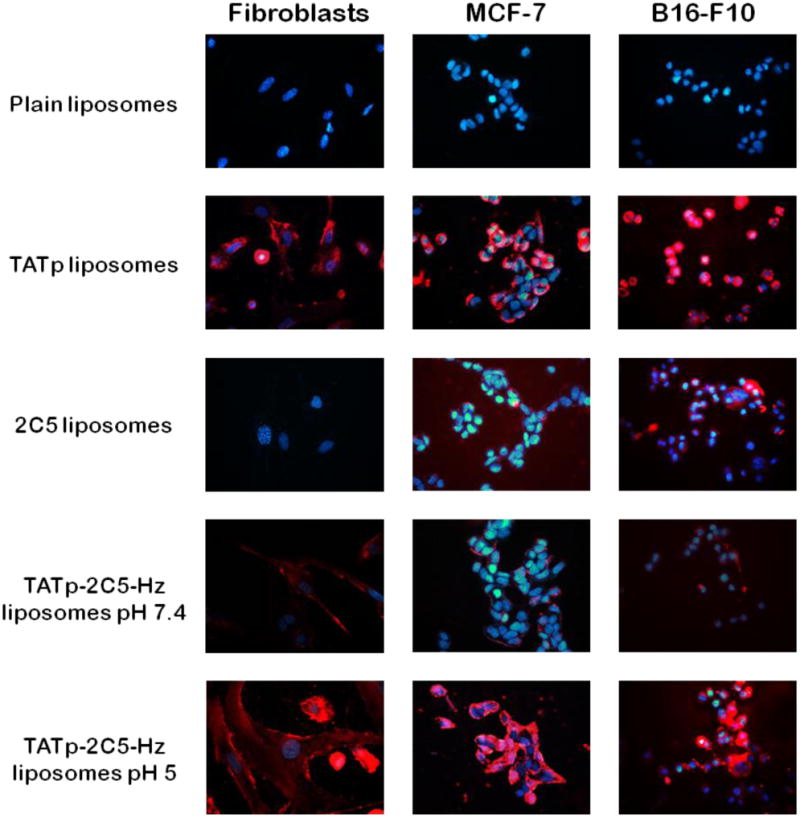
Fluorescence microscopy showing the internalization of 1 mol% Rhodamine-PE-labeled Doxil®-like immuno-liposomes by fibroblasts, MCF-7 and by B16-F10 cells, pre-incubated at pH 5 or 7.4 (Red-rhodamine; Blue-Hoechst nuclei staining).
Our in vitro results suggest that PEG-shielding of TATp moieties on the surface of liposomes will prevent or reduce the carrier's non-specific interaction with cells and allow targeting to cancer cells by the mAb 2C5.
To further investigate multifunctional immuno-liposome internalization, we performed a series of experiments using flow cytometry (Figure 6). As expected, the analysis of the geometric mean fluorescence revealed a 2-fold stronger binding of 2C5-modified immunoliposomes and 6-fold stronger interaction of TATp-modified liposomes with both B16-F10 and MCF-7 cells, when compared with plain liposomes. Binding experiments with multifunctional immunoliposomes pre-treated at pH 5.0 or 7.4 for 30 minutes showed a significant increase in fluorescence for the pH 5.0 pre-incubated group (6.68 and 7.44-fold stronger binding for B16-F10 and MCF-7 respectively) when compared to pH 7.4 conditions (2.39 and 2.21-fold stronger binding for B16-F10 and MCF-7 respectively versus plain liposomes) suggesting that the TATp was exposed and allowed liposomes to interact more efficiently with these cell lines. The increase in the multifunctional immuno-liposomes binding, incubated at pH 7.4, can be explained as the mAb 2C5 contribution to the nanocarrier-cell interaction.
Figure 6. Flow cytometry.
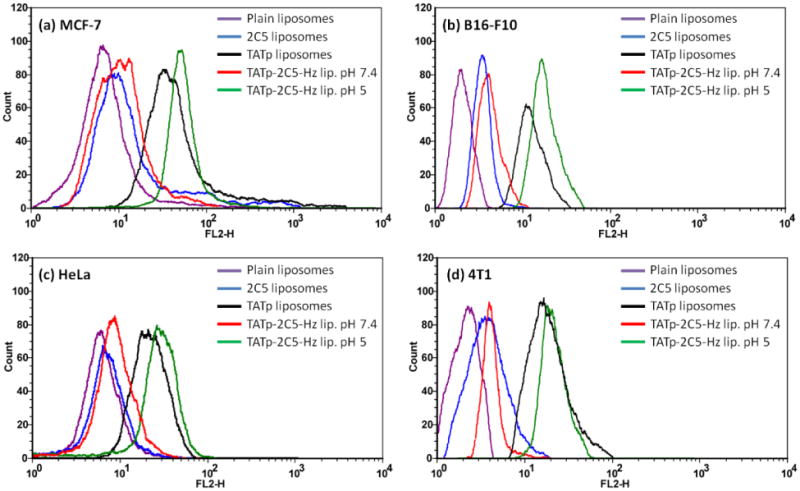
Representative histogram plots of MCF-7 cells (a) incubated for 1 hour with rhodamine-labeled Doxil®-like immunoliposomes (HSPC:cholesterol) and B16-F10, Hela and 4T1 cells (b-d respectively), incubated for 2 hours with multifunctional immuno-Doxil®. Multifunctional carriers were incubated with cells following their pre-exposure to normal or acidic conditions for 30 minutes.
3.5. In vitro cytotoxicity of multifunctional immuno-Doxil® liposomes
The liposomal formulation of doxorubicin (Doxil®) is nano-sized, with long-circulating properties, resulting from PEGylation. Its targeting depends mostly on the EPR effect [5, 46] to passively deliver doxorubicin into tumor site. Due to the variety of side-effects of doxorubicin, many attempts have been made to decrease the dose administrated while maintaining its therapeutic effect. Active targeting of this efficient drug carrier is expected to improve its safety parameters and its clinical parameters. It has been shown that 2C5 mAb-modified Doxil® increased the cytotoxicity drug effect with a reduction in the LC50 [43]. Furthermore, the attachment of the cell penetrating peptide, TATp to a Doxil preparation enhanced the cytotoxicity profile in vitro [11]. The pH-sensitive TAT-modified PEGylated liposomes also enhanced transfection of tumor cells in vivo [47]. It was also observed that TATp activity was diminished due to proteolytic cleavage. The authors concluded that TATp should be sterically shielded and described the shielding of this peptide by long PEG blocks [14].
With this in mind, we tested the cytotoxicity of our pH-sensitive multifunctional immuno-Doxil® liposomal preparation using an MCF-7, B16-F10 and HeLa cells. Multifunctional preparations were incubated for 30 minutes at pH 5.0 or 7.4 prior to their addition to the cells. Cells were incubated with the liposomal formulations for 12 hours, followed by a change of medium and an additional 48 hours of incubation (See Materials and Methods).
The liposomal formulations with equivalent concentrations of doxorubicin at 1.5 to 100μg/ml were evaluated (Figure 7). After 12+48 hours incubation, multifunctional immuno-Doxil pre-incubated at acidic conditions demonstrated higher toxicity when comprised to non-modified Doxil liposomes, 2C5-modified and multifunctional immuno-Doxil® liposomes, pre-incubated at pH 7.4 prior to their addition to the cells. TATp-modified non-shielded Doxil® was the only formulation that showed superior cytotoxicity and a lower LC50 value. Table 2 clearly shows that a TATp shielding effect at pH 7.4 is expressed at the higher LC50 values when compared to pre-incubated formulations at pH 5.0. While multi-Doxil® showed LC50 values of 8.8, 3 and 70μM/ml for MCF-7, B16-F10 and HeLa cells respectively, pre-incubated multi-Doxil® at acidic incubation were significantly lower (2, 1.2 and 9μg/ml, respectively).
Figure 7. In vitro.
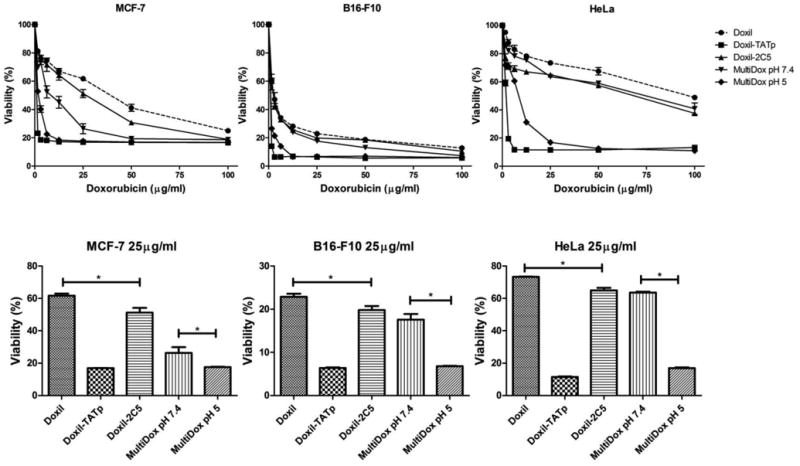
cytotoxicity of various multifunctional immuno-Doxil® preparations. Commercial Doxil® was grafted with TATp-PEG1k-PE (2.5 mol%), 2C5-PEG3.4k-PE (2.5 mol%) and PEG2k-Hz-PE (15 mol%) conjugates solely or in combination. The cytotoxicity of each preparation was expressed as % survival with untreated cells considered 100%. Upper panels show the cytotoxicity of the different preparations over a range of concentrations of doxorubicin. Lower panels compare survival of cells at 25μg/ml of doxorubicin. n=5, *p>0.05.
Table 2. LC50.
values of unmodified and surface modified Doxil® multifunctional immunoliposomes following 12 hr. of incubation. Medium was changed and additional 48hrs incubation was followed in normal media (See Materials and Methods). Results are presented as μg/ml.
| MCF-7 | B16-F10 | HeLa | |
|---|---|---|---|
| Doxil | 38.0 | 3.5 | 100.0 |
| Doxil-TATp | 1.0 | 1.0 | 1.8 |
| Doxil-2C5 | 27.0 | 3.0 | 65.0 |
| MultiDox pH 7.4 | 8.8 | 3.0 | 70.0 |
| MultiDox pH 5 | 2.0 | 1.2 | 9.0 |
Cytotoxicity of doxorubicin at 25μg/ml (Figure 7) significantly lowered survival percentage with multifunctional immuno-Doxil® (Pre-incubated at pH 5.0) compared to the other formulations but not to non-shielded TATp-Doxil formulation for all three cell lines. The high toxicity of TATp-Doxil® preparation should have been equal or less, compared to the multifunctional immuno-Doxil® with exposed TATp and mAb 2C5. Nevertheless, since we have pre-exposed the formulations to acidic conditions for only 30 minutes prior to the addition to the cells, at this point all TATp should have been fully exposed (due to the hydrazone bond cleavage and the detachment of the “shielding” PEG block). However, since it is a time-dependent kinetic reaction, the hydrazone bonds may not have been completely cleaved after 30 minutes of incubation.
4. Conclusion
A multifunctional liposomal nanocarrier, decorated with monoclonal anti-nucleosome antibody 2C5, the cell-penetrating peptide, TATp and a pH-sensitive PEG-shield was designed and characterized. This carrier can act as a stimulus-sensitive carrier targeted with antitumor antibody, with systemic long circulation characteristics and with the ability to show site-specific exposure of TAT peptide after a brief exposure to lower pH, and thereby to promote intracellular delivery of a carrier containing doxorubicin. Such an approach using a drug-encapsulated multifunctional nanocarrier can potentially minimize the interaction of the loaded nanocarrier with non-target cells, effectively accumulate at a tumor site and deliver various cargo drugs intracellularly with the potential for much improved anti-cancer therapy.
Acknowledgments
This work was supported by the NIH grants RO1 CA121838 and RO1 CA128486 to Vladimir P. Torchilin.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. Eur J Pharm Biopharm. 2009;71:431–444. doi: 10.1016/j.ejpb.2008.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 3.Klibanov AL, Maruyama K, Torchilin VP, Huang L. Amphipathic polyethyleneglycols effectively prolong the circulation time of liposomes. FEBS Lett. 1990;268:235–237. doi: 10.1016/0014-5793(90)81016-h. [DOI] [PubMed] [Google Scholar]
- 4.Barenholz Y. Liposome application: problems and prospects. Current opinion in colloid & interface science. 2001;6:66–77. [Google Scholar]
- 5.Matsumura Y, Maeda H. A new concept for macromolecular therapeutics in cancer chemotherapy: mechanism of tumoritropic accumulation of proteins and the antitumor agent smancs. Cancer Res. 1986;46:6387–6392. [PubMed] [Google Scholar]
- 6.Palmer TN, Caride VJ, Caldecourt MA, Twickler J, Abdullah V. The mechanism of liposome accumulation in infarction. Biochim Biophys Acta. 1984;797:363–368. doi: 10.1016/0304-4165(84)90258-7. [DOI] [PubMed] [Google Scholar]
- 7.Gabizon AA. Liposome circulation time and tumor targeting: implications for cancer chemotherapy. Advanced drug delivery reviews. 1995;16:285–294. [Google Scholar]
- 8.Frankel AD, Pabo CO. Cellular uptake of the tat protein from human immunodeficiency virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 9.Mai JC, Shen H, Watkins SC, Cheng T, Robbins PD. Efficiency of protein transduction is cell type-dependent and is enhanced by dextran sulfate. J Biol Chem. 2002;277:30208–30218. doi: 10.1074/jbc.M204202200. [DOI] [PubMed] [Google Scholar]
- 10.Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- 11.Koren E, Apte A, Sawant RR, Grunwald J, Torchilin VP. Cell-penetrating TAT peptide in drug delivery systems: Proteolytic stability requirements. Drug Deliv. 2011;18:377–384. doi: 10.3109/10717544.2011.567310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lukyanov AN, Elbayoumi TA, Chakilam AR, Torchilin VP. Tumor-targeted liposomes: doxorubicin-loaded long-circulating liposomes modified with anti-cancer antibody. J Control Release. 2004;100:135–144. doi: 10.1016/j.jconrel.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Torchilin VP, Rammohan R, Weissig V, Levchenko TS. TAT peptide on the surface of liposomes affords their efficient intracellular delivery even at low temperature and in the presence of metabolic inhibitors. Proc Natl Acad Sci U S A. 2001;98:8786–8791. doi: 10.1073/pnas.151247498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Grunwald J, Rejtar T, Sawant R, Wang Z, Torchilin VP. TAT peptide and its conjugates: proteolytic stability. Bioconjug Chem. 2009;20:1531–1537. doi: 10.1021/bc900081e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kale AA, Torchilin VP. Design, synthesis, and characterization of pH-sensitive PEG-PE conjugates for stimuli-sensitive pharmaceutical nanocarriers: the effect of substitutes at the hydrazone linkage on the ph stability of PEG-PE conjugates. Bioconjug Chem. 2007;18:363–370. doi: 10.1021/bc060228x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen TM, Sapra P, Moase E. Use of the post-insertion method for the formation of ligand-coupled liposomes. Cell Mol Biol Lett. 2002;7:889–894. [PubMed] [Google Scholar]
- 17.Ishida T, Iden DL, Allen TM. A combinatorial approach to producing sterically stabilized (Stealth) immunoliposomal drugs. FEBS Lett. 1999;460:129–133. doi: 10.1016/s0014-5793(99)01320-4. [DOI] [PubMed] [Google Scholar]
- 18.Torchilin VP, Levchenko TS, Lukyanov AN, Khaw BA, Klibanov AL, Rammohan R, Samokhin GP, Whiteman KR. p-Nitrophenylcarbonyl-PEG-PE-liposomes: fast and simple attachment of specific ligands, including monoclonal antibodies, to distal ends of PEG chains via p-nitrophenylcarbonyl groups. Biochim Biophys Acta. 2001;1511:397–411. doi: 10.1016/s0005-2728(01)00165-7. [DOI] [PubMed] [Google Scholar]
- 19.Woodle MC, Lasic DD. Sterically stabilized liposomes. Biochim Biophys Acta. 1992;1113:171–199. doi: 10.1016/0304-4157(92)90038-c. [DOI] [PubMed] [Google Scholar]
- 20.Uster PS, Allen TM, Daniel BE, Mendez CJ, Newman MS, Zhu GZ. Insertion of poly(ethylene glycol) derivatized phospholipid into pre-formed liposomes results in prolonged in vivo circulation time. FEBS Lett. 1996;386:243–246. doi: 10.1016/0014-5793(96)00452-8. [DOI] [PubMed] [Google Scholar]
- 21.Hristova K, Needham D. Phase behavior of a lipid/polymer-lipid mixture in aqueous medium. Macromolecules. 1995;28:991–1002. [Google Scholar]
- 22.Khalid MN, Simard P, Hoarau D, Dragomir A, Leroux JC. Long circulating poly(ethylene glycol)-decorated lipid nanocapsules deliver docetaxel to solid tumors. Pharm Res. 2006;23:752–758. doi: 10.1007/s11095-006-9662-5. [DOI] [PubMed] [Google Scholar]
- 23.Kale AA, Torchilin VP. Environment-responsive multifunctional liposomes. Methods Mol Biol. 2010;605:213–242. doi: 10.1007/978-1-60327-360-2_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schubert US, Knop K, Hoogenboom R, Fischer D. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew Chem Int Edit. 2010;49:6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 25.Howard MD, Jay M, Dziubla TD, Lu X. PEGylation of nanocarrier drug delivery systems: State of the art. J Biomed Nanotechnol. 2008;4:133–148. [Google Scholar]
- 26.Photos PJ, Bacakova L, Discher B, Bates FS, Discher DE. Polymer vesicles in vivo: correlations with PEG molecular weight. J Control Release. 2003;90:323–334. doi: 10.1016/s0168-3659(03)00201-3. [DOI] [PubMed] [Google Scholar]
- 27.Malmsten M, Emoto K, Van Alstine JM. Effect of Chain Density on Inhibition of Protein Adsorption by Poly (ethylene glycol) Based Coatings* 1. Journal of colloid and interface science. 1998;202:507–517. [Google Scholar]
- 28.Kenworthy AK, Simon SA, McIntosh TJ. Structure and phase behavior of lipid suspensions containing phospholipids with covalently attached poly(ethylene glycol) Biophys J. 1995;68:1903–1920. doi: 10.1016/S0006-3495(95)80368-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lasic D, Woodle M, Martin F, Valentincic T. Phase behavior of stealth-lipid”-lecithin mixtures. Period Biol. 1991;93:287–290. [Google Scholar]
- 30.Shimada K, Matsuo S, Sadzuka Y, Miyagishima A, Nozawa Y, Hirota S, Sonobe T. Determination of incorporated amounts of poly(ethylene glycol)-derivatized lipids in liposomes for the physicochemical characterization of stealth liposomes. Int J Pharm. 2000;203:255–263. doi: 10.1016/s0378-5173(00)00466-x. [DOI] [PubMed] [Google Scholar]
- 31.Bedu-Addo FK, Tang P, Xu Y, Huang L. Effects of polyethyleneglycol chain length and phospholipid acyl chain composition on the interaction of polyethyleneglycol-phospholipid conjugates with phospholipid: implications in liposomal drug delivery. Pharm Res. 1996;13:710–717. doi: 10.1023/a:1016091314940. [DOI] [PubMed] [Google Scholar]
- 32.Sawant RM, Hurley JP, Salmaso S, Kale A, Tolcheva E, Levchenko TS, Torchilin VP. “SMART” drug delivery systems: double-targeted pH-responsive pharmaceutical nanocarriers. Bioconjug Chem. 2006;17:943–949. doi: 10.1021/bc060080h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garbuzenko O, Barenholz Y, Priev A. Effect of grafted PEG on liposome size and on compressibility and packing of lipid bilayer. Chem Phys Lipids. 2005;135:117–129. doi: 10.1016/j.chemphyslip.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Zhang JX, Zalipsky S, Mullah N, Pechar M, Allen TM. Pharmaco attributes of dioleoylphosphatidylethanolamine/cholesterylhemisuccinate liposomes containing different types of cleavable lipopolymers. Pharmacol Res. 2004;49:185–198. doi: 10.1016/j.phrs.2003.09.003. [DOI] [PubMed] [Google Scholar]
- 35.Hristova K, Needham D. The Influence of Polymer-Grafted Lipids on the Physical-Properties of Lipid Bilayers - a Theoretical-Study. Journal of colloid and interface science. 1994;168:302–314. [Google Scholar]
- 36.Degennes PG. Polymers at an Interface - a Simplified View. Adv Colloid Interfac. 1987;27:189–209. [Google Scholar]
- 37.Remaut K, Lucas B, Braeckmans K, Demeester J, De Smedt SC. Pegylation of liposomes favours the endosomal degradation of the delivered phosphodiester oligonucleotides. J Control Release. 2007;117:256–266. doi: 10.1016/j.jconrel.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 38.Hatakeyama H, Akita H, Harashima H. A multifunctional envelope type nano device (MEND) for gene delivery to tumours based on the EPR effect: a strategy for overcoming the PEG dilemma. Adv Drug Deliv Rev. 2011;63:152–160. doi: 10.1016/j.addr.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 39.Iakoubov LZ, Torchilin VP. A novel class of antitumor antibodies: nucleosome-restricted antinuclear autoantibodies (ANA) from healthy aged nonautoimmune mice. Oncol Res. 1997;9:439–446. [PubMed] [Google Scholar]
- 40.Elbayoumi TA, Torchilin VP. Enhanced accumulation of long-circulating liposomes modified with the nucleosome-specific monoclonal antibody 2C5 in various tumours in mice: gamma-imaging studies. Eur J Nucl Med Mol Imaging. 2006;33:1196–1205. doi: 10.1007/s00259-006-0139-x. [DOI] [PubMed] [Google Scholar]
- 41.Torchilin VP, Lukyanov AN, Gao Z, Papahadjopoulos-Sternberg B. Immunomicelles: targeted pharmaceutical carriers for poorly soluble drugs. Proc Natl Acad Sci U S A. 2003;100:6039–6044. doi: 10.1073/pnas.0931428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iakoubov LZ, Torchilin VP. Nucleosome-releasing treatment makes surviving tumor cells better targets for nucleosome-specific anticancer antibodies. Cancer Detect Prev. 1998;22:470–475. doi: 10.1046/j.1525-1500.1998.00055.x. [DOI] [PubMed] [Google Scholar]
- 43.Elbayoumi TA, Torchilin VP. Enhanced cytotoxicity of monoclonal anticancer antibody 2C5-modified doxorubicin-loaded PEGylated liposomes against various tumor cell lines. Eur J Pharm Sci. 2007;32:159–168. doi: 10.1016/j.ejps.2007.05.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wike-Hooley JL, Haveman J, Reinhold HS. The relevance of tumour pH to the treatment of malignant disease. Radiother Oncol. 1984;2:343–366. doi: 10.1016/s0167-8140(84)80077-8. [DOI] [PubMed] [Google Scholar]
- 45.Gerweck LE, Seetharaman K. Cellular pH gradient in tumor versus normal tissue: potential exploitation for the treatment of cancer. Cancer Res. 1996;56:1194–1198. [PubMed] [Google Scholar]
- 46.Gabizon A, Catane R, Uziely B, Kaufman B, Safra T, Cohen R, Martin F, Huang A, Barenholz Y. Prolonged circulation time and enhanced accumulation in malignant exudates of doxorubicin encapsulated in polyethylene-glycol coated liposomes. Cancer Res. 1994;54:987–992. [PubMed] [Google Scholar]
- 47.Kale AA, Torchilin VP. Enhanced transfection of tumor cells in vivo using “Smart” pH-sensitive TAT-modified pegylated liposomes. J Drug Target. 2007;15:538–545. doi: 10.1080/10611860701498203. [DOI] [PMC free article] [PubMed] [Google Scholar]


