Abstract
Imposed social subordination, such as that which accompanies physical defeat or alienation, has been associated with impaired cognitive function in both human and non-human animals. Here we examined whether domain-specific and/or domain-general learning abilities (c.f. general intelligence) are differentially influenced by the imposition of social subordination. Furthermore, we assessed whether the impact of subordination on cognitive abilities was the result of imposed subordination per se, or if it reflected deficits intrinsically expressed in subjects that are predisposed to subordination. Subordinate and dominant behaviors were assessed in two groups of CD-1 male mice. In one group (Imposed Stratification), social stratification was imposed (through persistent physical defeat in a colonized setting) prior to the determination of cognitive abilities, while in the second group (Innate Stratification), an assessment of social stratification was made after cognitive abilities had been quantified. Domain-specific learning abilities were measured as performance on individual learning tasks (odor discrimination, fear conditioning, spatial maze learning, passive avoidance, and egocentric navigation) while domain-general learning abilities were determined by subjects’ aggregate performance across the battery of learning tasks. We observed that the imposition of subordination prior to cognitive testing decreased exploratory tendencies, moderately impaired performance on individual learning tasks, and severely impaired general cognitive performance. However, similar impairments were not observed in subjects with a predisposition toward a subordinate phenotype (but which had not experienced physical defeat at the time of cognitive testing). Mere colonization, regardless of outcome (i.e., stratification), was associated with an increase in stress-induced serum corticosterone (CORT) levels, and thus CORT elevations were not themselves adequate to explain the effects of imposed stratification on cognitive abilities. These findings indicate that absent the imposition of subordination, individuals with subordinate tendencies do not express learning impairments. This observation could have important ramifications for individuals in environments where social stratification is prevalent (e.g., schools or workplace settings).
Keywords: subordination, aggression, stress, cognition, social status, general intelligence, stress hormones
1. Introduction
Stress has been shown to be a potent modulator of the ability to learn and to express memories. However, the direction, degree, and duration of stress effects on cognitive abilities depends greatly on the characteristics of the stressor, the type of learning being assessed, and the social structure of an organism’s environment [for reviews, see: 1–7]. The variability in reported stress effects on learning highlights the need to focus on stressors that are both ethologically relevant and conserved across both human and non-human animal species. Numerous mammalian species, including humans, live in complex social groups and are subject to intense and often unpredictable stress as a result of social interactions. As such, a relatively new area of study has emerged with the goal of investigating the learning effects induced by stressors that are primarily social in nature. One line of inquiry has focused on biobehavioral and learning challenges that arise consequent to social subordination.
In humans, subordination resulting from alienation or social defeat (e.g., bullying) has been shown to exert a negative influence on cognitive performance [8–15]. While these results are intriguing, human studies of social stress effects are limited. In the laboratory, researchers employ stressors that may be mild in comparison to the stress of actual life events. As such, the results from these studies may not fully demonstrate the impact of subordination on learning performance. Studies that examine natural instances of subordination stress in humans are similarly difficult to integrate within the larger phenomenon. For example, these studies often rely on self-reports or personal perceptions of subordination, which may not accurately reflect actual events. Further, prior history with aggression, environmental factors, and importantly, individual predispositions, may interact with instances of social subordination and may complicate the interpretation of effects on cognitive abilities and emotionality. Lastly, due to ethical constraints, it is difficult to probe the mechanisms that underlie changes in cognition in human participants. Studies of laboratory animals provide researchers with a greater degree of controllability over stressors and thus allow for a more detailed examination of the physiological substrates that may underlie alterations in cognition.
Animal studies examining the relationship between subordination and cognitive function have produced mixed results [16–21,23–28,30]. Commonly, two types of social subordination have been examined, i.e., imposed subordination or the innate predisposition toward subordination [16–21,23–28,30]. Imposed subordination refers to subordination (e.g., antagonistic encounters with conspecifics) that is inflicted upon the animals prior to assessment of cognitive function. In contrast, innate subordination (i.e. a natural predisposition toward subordination) is assessed after the assessment of cognitive abilities.
Imposed subordination has typically been observed to negatively impact learning abilities across several different measures [e.g., Spatial learning in a water maze or radial arm maze: 16, 21–25; reinforced alternation: 17; reference and working memory: 18–20]. Despite these observations, in many instances, imposed subordination has had no apparent effect on learning abilities [21, 23–28].
In contrast to studies of imposed subordination, studies of innate subordination have been far fewer in number. Yet, like those examining imposed subordination, these studies have also provided mixed results [25, 30]. One potential reason for these discrepant results is the variability in methodologies. However, it is also possible that certain forms of learning and memory are sensitive to social subordination while others are spared. Regardless of the source of the discrepancies, the mixed findings from these animal studies underscore the need for a more thorough examination of whether differences in learning abilities due to social subordination represent an innate predisposition toward subordination (of animals of lower cognitive abilities) or whether subordination-induced learning deficits arise in response to imposed subordination.
Studies of innate and imposed subordination to date have focused exclusively on domain-specific learning abilities (e.g., spatial learning). Yet, it has been established that both domain-specific (e.g., spatial abilities) as well as domain-general (general intelligence) factors influence cognition [31]. In humans, general intelligence or “g” has been called the “single most dominant cognitive trait ever discovered” [32], and the single factor that underlies g is purported to influence all domain-specific learning abilities. Like humans, CD-1 outbred mice express individual differences in their “general” cognitive abilities such that performance across tasks in a battery of diverse learning tasks is positively correlated. Through the application of principal components analysis a general learning factor can be identified that accounts for 25–48% of the variance in the performance of individual mice. This general learning factor in mice has been argued to be structurally and psychometrically analogous to general intelligence in humans [33–37].
To date, no animal studies have attempted to examine the relationship between social subordination and general learning abilities. Thus, one of the goals of the current experiment was to determine whether an individual subjects’ domain-specific and/or domain-general learning abilities are altered by the imposition of social subordination (in a colony setting) in a manner similar to that seen in previous studies. Additionally, if cognitive differences do exist in animals that undergo subordination, we would determine whether they reflect the imposition of subordination or if they represent a disposition toward poor learning in animals that are innately subordinate.
Domain-specific learning abilities were assessed on individual learning tasks while domain-general learning abilities were measured as the aggregate performance across a battery of learning tasks. Stress-induced levels of the adrenal hormone, corticosterone (CORT) were also measured since prior work has shown a differential activation of the HPA axis in subordinate and dominant subjects in response to stress [rats: 38–39; mice: 40–44; non-human primates 44–49; humans: 50–51]. Specifically, it is has been suggested that upregulation of HPA activity, such as that seen in highly stressed animals, may lead to HPA dysregulation and a dysfunctional response to subsequent stress exposure. Further, CORT has been implicated as a possible modulator of cognitive function [for review see: 1–7] thereby making any observation of differences in its expression of particular interest. Behavioral measures of stress/anxiety also vary in subordinate animals versus dominant subjects [52–63]. Consequently, we assessed performance in the elevated plus maze [EPM], Open Field [OF] and light/dark discrimination tasks. Lastly, subjects that were stratified prior to cognitive assessments underwent testing in a battery of motor tests to ensure that any deficits in learning performance that are detected are not the result of motor impairment.
2. Materials and Methods
2.1. Animals and Housing
Forty-eight outbred, male, non-sibling CD-1 mice (Harlan Sprague Dawley Inc., Indianapolis, IN) weighed 25–30 grams and were 40–45 days of age upon arrival in our laboratory. Since animals were obtained pre-pubescence, it is generally accepted that they would not had not yet stratified into social hierarchies. Subjects were non-littermates, since previous work has revealed that aggressive behaviors are more readily expressed among rodents that are unrelated [64]. Upon arrival and prior to the start of the testing, all subjects were housed individually and maintained on ad libitum food and water (unless noted otherwise) in a temperature-controlled vivarium on a 12-hour light/dark cycle. They were allowed to acclimate to the vivarium and were handled (removed from the home cage and held by the experimenter for 60s/day) for three weeks prior to behavioral testing (which began at approximately 68 days of age).
2.2. Colonization Procedure
Subjects were randomly assigned to one of two colonization conditions (imposed [IMP], n = 24, or innate [INN], n = 24]. Subjects in the imposed (IMP) group were housed in groups of three (i.e. triads) from 68–82 days of age. This imposed group colonization took place prior to testing in the learning battery so that the effects of social stratification on learning performance could be assessed. Subjects in the innate (INN) group were colonized in triads after completion of testing in the learning battery (at 133 days of age) so that the relationship between innate tendencies toward subordination/dominance and learning performance could be examined and compared with any relationships between these factors that exists as a result of the imposition of subordinance or dominance (i.e. imposed group performance) prior to tests of learning. In both conditions, animals in each triad were matched for body weight (to within ± 1.2 grams).
At the start of the colonization procedure, subjects were transported in their home cages to an isolated testing room (300 lux). To examine social interactions, three subjects were placed simultaneously in a neutral area, i.e., a novel standard shoebox cage lined with wood shavings. Behavior was observed in three evenly-spaced 10-min sessions during the light cycle (07:00–19:00) and three 10-min sessions during the dark cycle (19:00–7:00). Between observations that occurred during the light cycle and those during the dark cycle, subjects were returned to their home cages. Subjects remained housed in triads until the termination of the colonization period (14 days). Rather than the two weeks of colonization incurred by the IMP subjects (where it was the intention to induce subordination prior to testing in the cognitive battery), subjects in the innate (INN) group (where it was the intention to assess subordination after cognitive abilities had been determined) were colonized for only16 hours (after the completion of cognitive testing). This was done as it was determined from observations of group IMP that stratification of the colonized animals was complete after only 16 hours of interaction. Thus after 16 hours, social stratification could be accurately estimated, and exposing animals in Group INN to additional unnecessary aggression was deemed unwarranted. Timelines of the experimental procedures for the imposed (IMP) and the innate (INN) groups are provided in Figure 1. All behavioral interactions were recorded for offline measurement as detailed below.
Figure 1.
The timeline of the handling, colonization, exploratory, sensorimotor, learning and blood collection procedures across the experimental period for both Groups IMP (colonized before the learning battery tests) and INN (colonized after the learning battery tests). Days indicate time since the animal’s day of birth.
2.3. Assessment of Social Dominance
Three types of behavior were assessed in the colonized mice: 1) dominance-related behavior 2) submission-related behavior and 3) affiliative behavior.
Dominance-related behaviors
Number of bites made: A bite by one conspecific directed toward any area of the body (i.e. head/face or back/flanks) of another conspecific in the triad. The average of the total bites made across all six observation periods was the measure used for subsequent statistical analyses.
Number of bites received: A bite received by an individual conspecific in the triad to any area of the body (head/face or back/flanks). The average of the total bites received across all six observation periods was the measure used for statistical analyses.
Latency to first attack: The time (in sec) that elapsed from when all three individuals were placed together on Day 1 until an individual subject attacked/bit another subject in the triad.
Total number of tail rattles: A tail rattle was defined as a “rapid lateral quivering or thrashing of the tail” [65]. Tail rattles have been reported to be correlated with aggression and social dominance [66–68]. The total number of tail rattles summed across all six observation periods was the measure used for statistical analyses.
Wounding: After the last observation session on Day 1, the severity of bodily wounds was ranked for each individual subject using a scale from 1–5 (1 being the least severe wounding and 5 being the most severe wounding). This was not intended to be an absolute scale, but rather, was indicative of the relative severity of wounds in the animals that contributed to this experiment. Typically, more serious wounding is seen in subordinate subjects while dominants are often spared from any wounding.
Submission-related behavior
The number of upright/sideways defensive rears: The defensive rear is considered a sign of retreat that occurs when a subject rises up on its hindlimbs with its “forearms limp, its head angled upward, and its ears retracted” [66, 69–76]. We summed the total number of defensive rears across all six observation periods for statistical analyses.
Wounding: As described above, depending on the degree of wounding, this measure could be indicative of a dominant or submissive status. Typically, more serious wounding is seen in subordinate subjects while dominants are often spared extensive physical trauma.
Affiliative behavior
Total time spent sniffing: Sniffing is an “introductory/investigatory act” that was defined here as the time that a subjects’ nose spent in contact (or within 2 mm) with any area of the body of another conspecific in the triad [66] while engaged in sniffing. The sum of the total time spent sniffing was used for statistical analyses.
Total time spent huddling: Huddling was defined as bodily contact between two or more mice in the triad during a period of rest that exceeded 60 sec in duration. The sum of the total time spent huddling was the measure used for statistical analyses.
Since the measures of behavioral dominance assessed here were found to be highly related (see Results, Section 3.1), the average number of bites made was used to categorize animals as “dominant” “mid submissive” or “low submissive”. Only the behavioral data from the “dominant” and “low submissive” animals from each triad was used for subsequent statistical analyses. In two cases, a “low submissive” animal died, and data from the “mid submissive” from the same triad was substituted for statistical analysis.
2.4. Learning Tasks
The 48 CD-1 mice used in this experiment were assessed (in two independent replications) on the five learning tests (i.e. Lashley III maze, passive avoidance, spatial water maze, associative fear conditioning and odor guided discrimination) which make up core tasks previously used to evaluate general learning abilities. These tasks were chosen so that they place unique sensory, motor, motivational and information processing demands on the animals. The order of testing was designed so as to provide a temporal separation between any two tasks that are motivated by either food or water deprivation (to prevent excessive physical strain and to minimize any potential cross-task influences due to motivational factors). In addition, the testing order was designed to separate tasks based on similar processes or motor requirements (e.g. mazes of a similar nature, activity vs. passivity), again so as to minimize any potential transfer between tasks. All animals were assessed on tasks in the following order: Lashley maze, passive avoidance, odor discrimination, fear conditioning and spatial water maze. A different experimenter tested the animals on each of the learning tasks, and these experimenters were unaware of the animals’ history or social status.
In all learning tasks, the animals’ performance was assessed during the acquisition phase of learning (i.e., prior to reaching their stable, asymptotic level of performance). Thus the dependent measure for each task was analogous to the animals’ rate of learning on that task, and these measures of each individual’s performance could be ranked (through the application of principal component analysis and the resultant factor scores; see below) relative to other animals in the sample. To quantify an animal’s performance in tasks in which there were multiple training/test trials, performance during trials that fell within the acquisition phase were averaged. In tasks in which there was only one test trial (i.e. fear conditioning and passive avoidance), training parameters were used that were previously determined to result in sub-asymptotic responding by most animals. To characterize the general cognitive performance of individual animals, the performance on the learning tasks was subjected to a principal component analysis, which groups variables into “factors” which best account for the overall pattern of correlations between them. Based only on data in the learning tasks, a single factor was extracted (which accounted for 29% of the variance across all tasks), and this factor yielded a factor score (analogous to an average of z scores for the individual animals’ performance on each task) for each animal that served to represent its general cognitive ability. The rationale for these analysis and statistical procedures are provided in detail elsewhere [31].
2.4.1 Spatial Water Maze
This task requires animals to locate a submerged platform in a round pool of opaque water. Absent distinct intra-maze cues, animals’ performance in this task is highly dependent on the interaction of extra-maze spatial cues. The animals are motivated by their aversion to the water. The latency and path length to locate the platform decreases over successive trials, despite entering the pool from different locations.
A round black pool (140 cm diameter, 56 cm deep) was filled to within 24 cm of the top with water made opaque by the addition of a nontoxic, water soluble black paint. A hidden 11 cm diameter perforated black platform was in a fixed location 1.5 cm below the surface of the water midway between the center and perimeter of the pool. The pool was enclosed in a ceiling-high black curtain on which five different shapes (landmark cues) were variously positioned at heights (relative to water surface) ranging from 24–150 cm. Four of these shapes were constructed of strings of white LEDs (spaced at 2.5 cm intervals) and include an “X” (66 cm arms crossing at angles 40o from the pool surface), a vertical “spiral” (80 cm long, 7 cm diameter, 11 cm revolutions), a vertical line (31 cm) and a horizontal line (31 cm). The fifth cue was constructed of two adjacent 7-watt light bulbs (each 4 cm diameter). A video camera was mounted 180 cm above the center of the water surface. These cues provided the only illumination of the maze, totaling 172 lux at the water surface.
On the day prior to training, each animal was confined to the escape platform for 5 min. Training was conducted on the two subsequent days. On Day 1 of training, animals were started from one of three unique locations on each of five trials. The pool was conceptually divided into four quadrants, and one starting point was located in each of the three quadrants that did not contain the escape platform. The starting point on each trial alternated between the three available quadrants. An animal was judged to have escaped from the water (i.e., located the platform) at the moment at which all four paws were situated on the platform, provided that the animal remained on the platform for at least 5 sec. Each animal was left on the platform for a total of 20 sec, after which the trial was terminated. Trials were spaced at 10 min intervals, during which time the animals were held in their home cages. On each trial, a 90 sec limit on swimming was imposed, at which time any animal that had not located the escape platform was placed onto the platform by the experimenter, where it remained for 20 sec. The time it took for the animal to escape (latency) as well as the distance traveled (path length) to reach the platform were recorded.
Animals were observed from a remote (outside of the pool’s enclosure) video monitor, and animals’ performance was recorded on videotape for subsequent analysis. Day 2 of training proceeded, as did Day 1, albeit with four trials only. After the last training trial, a 90 min retention period began, after which animals were tested with a “probe” trial. On the probe test, the escape platform was removed from the pool, and all animals were started from the first position for that day. A 60 sec test was conducted and the animals’ time searching in the target quadrant (that in which the escape platform was previously located) and non-target quadrants was recorded.
2.4.2 Lashley III Maze
The Lashley III maze consisted of a start box, four interconnected alleys and a goal box containing a food reward. Previous studies have shown that over successive trials, the latency of rats to locate the goal box decreased, as does their number of errors (i.e., wrong turns or retracing). A Lashley III maze scaled for mice was constructed of black Plexiglas and a goal box marked by white electrical tape was located in the rear portion of the maze where 45 milligram BioServe (rodent grain) pellet served as a reinforcer. Illumination was 80 lux at the floor of the maze. The maze was isolated behind a shield of white Plexiglas to prevent the use of extra-maze landmark cues.
Food-deprived animals were acclimated and trained on two successive days. On the day prior to acclimation, all animals were provided with three food pellets in their home cages to familiarize them with the novel reinforcer. On the acclimation day, each mouse was placed in the four alleys of the maze, but the openings between the alleys were blocked so that the animals could not navigate the maze. Each animal was confined to the start and subsequent two alleys for 4 min, and for 6 min in the last (goal) alley, where three food pellets were present in the goal box. This acclimation period promotes stable and high levels of activity on the subsequent training day. On the training day, each animal was placed in the start box and allowed to traverse the maze until it reached the goal box and consumed the single food pellet present in the cup. Upon consuming the food, the animal was returned to its home cage for a 20 min interval (ITI) during which the apparatus was cleaned. After the ITI, the mouse was returned to the start box to begin the next trial, and the sequence was repeated for five trials. Both the latency and errors (i.e., a turn in an incorrect direction, including those which result in path retracing) to enter the goal box were recorded on each trial.
Associative Fear Conditioning
In this task animals received a tone (CS) paired with a mild foot shock (US). Two distinct experimental chambers (i.e., contexts;) were used, each of which was contained in a sound- and light attenuating enclosure. These boxes were designated as training and novel contexts, and differed as follows: The training chamber (16.5 × 26.5 × 20 cm) was brightly illuminated (100 lx), had clear Plexiglas walls, and parallel stainless-steel rods (5mm, 10mm spacing) forming the floor. The novel chamber (23 × 21.5 × 19 cm) was dimly illuminated (6 lx) and all of the walls and floor were constructed of clear plexiglass. In both boxes, the auditory stimulus (60dB, 2.9 kHz) was delivered by a piezoelectric buzzer.
On Day 1 subjects were acclimated in both novel and training contexts for a 20 min period in each box. On Day 2 subjects received an 18 min training session in the training chamber. All training sessions were videotaped for subsequent offline scoring. Subjects received three tone/shock presentations at 4, 10 and 16 min into the session. The CS presentation consisted of a pulsed (.7 sec on .3 sec off) 20 sec tone. Immediately following the tone offset, the shock US (0.6-mA, constant-current footshock) was presented for 500 msec.
Freezing was measured during the 20 sec before (BASELINE FREEZING), during (TONE FREEZING) and after (POST SHOCK FREEZING) the 20 sec tone presentation. A measure for freezing during the training period (TRAINING FREEZING) was calculated by subtracting the time spent freezing in baseline from the time spent freezing during the tone.
On Day 3, freezing was measured during a 5 min session in the novel chamber during which time, tone, but no shock was presented.
Odor Discrimination and Choice
Rodents rapidly learn to use odors to guide appetitively-reinforced behaviors. In a procedure based on one designed for rats [77], mice learned to navigate a square field in which unique odor-marked (e.g., almond, lemon, mint) food cups were located in three corners. Although food was present in each cup, it was accessible to the animals in only one cup, the one marked by mint odor. An animal was placed in the empty corner of the field, after which it explored the field and eventually retrieved the single piece of available food. On subsequent trials, the location of the food cups was changed, but the accessible food was consistently marked by the same odor (mint). On successive trials, animals required less time to retrieve the food and made fewer approaches (i.e., “errors”) to those food cups in which food was not available. Using this procedure, errorless performance was typically observed within three to four training trials.
A black Plexiglas 60 cm square field with 30 cm high walls was located in a dimly lit (10 fc) testing room with a high ventilation rate (3 min volume exchange). Three 4 × 4 × 2.0 cm (l, w, h) aluminum food cups were placed in three corners of the field. A food reinforcer (30 mg portions of chocolate flavored puffed rice) was placed in a 1.6 cm deep, 1 cm diameter depression in the center of each cup. The food in two of the cups was covered (1.0 cm below the surface of the cup) with a wire mesh so that it was not accessible to the animal, while in the third cup (the “target” cup), the food could be retrieved and consumed. A cotton-tipped laboratory swab, located between the center and rear corner of each cup, extended vertically 3 cm from the cups’ surface.
Immediately prior to each trial, fresh swabs were loaded with 25 μl of either lemon, almond, or mint odorants (McCormick flavor extracts). The mint odor was always associated with the target food cup. It should be noted that in pilot studies, the odor associated with food was counterbalanced across animals and no discernible differences in performance could be detected in response to the different odors.
On the day prior to test animals were given 60 min of free feeding time at the same time of day they would receive have been acclimated. On test day, animals received four training trials in the field with three food cups present. On each trial, an animal is placed in the empty corner of the field. On Trial 1, the reinforcing food was available to the animal in the cup marked by mint odor. An additional portion of food was placed on the top surface of the same cup for the first trial only. The trial continued until the animal retrieved and consumed the food from the target cup, after which the animal was left in the chamber for an additional 20 sec and then returned to its home cage to begin a 6 min ITI. On Trials 2–4, the location of the food cups was rearranged, but the baited cup remained consistently marked by the mint odor. Both the corner location of the mint odor and its position relative to the remaining odors was changed on each trial. On each trial, the latency to retrieve the food and errors was recorded. An error was recorded any time an animal made contact with an incorrect cup, or its nose crossed a plane parallel to the perimeter of an incorrect cup. Similarly, an error was recorded when an animal sampled (as above) the target cup but did not retrieve the available food.
2.4.3 One-Trial Passive Avoidance
A chamber illuminated by dim (< 20 lux) red light was used for training and testing. Animals were confined to circular (“safe”) chamber (10 cm diameter, 8 cm high). The walls and floor of this chamber were white, and the ceiling was translucent orange. The floor was comprised of plastic rods (2 mm diameter) arranged to form a pattern of 1 cm square grids. A clear exit door (3 cm square) was flush with the floor of the safe compartment, and the door was able to slide horizontally to open or close the compartment. The bottom of the exit door was located 4 cm above the floor of a second circular chamber (20 cm diameter, 12 cm high). This “unsafe” chamber had a clear ceiling and a floor comprised of 4 mm wide aluminum planks that formed a pattern of 1.5 cm square grids oriented at a 45° angle relative to the grids in the safe compartment. When an animal stepped from the safe compartment through the exit door onto the floor of the unsafe compartment, a compound aversive stimulus comprised of a bright (550 Lux) white light and “siren” (60 dB above the 50 dB background) was initiated.
Animals learn to suppress movement to avoid contact with aversive stimuli. This “passive avoidance” response is exemplified in step-down avoidance procedures, where commonly, an animal is placed on a platform, whereupon stepping off of the platform it encounters a footshock. Following just a single encounter with shock, animals are subsequently reluctant to step off of the safe platform. The animals’ reluctance to leave the platform is believed to not reflect fear, because typical fear responses are not expressed in animals engaged in the avoidance response [78–79]. Upon stepping off the platform, animals here were exposed to a compound of bright light and loud oscillating noise rather than shock, so as not to duplicate stimuli between tasks (see fear conditioning, above). Like more common procedures, our variant of this task supports learning after only a single trial (i.e., subsequent step-down latencies will be markedly increased).
Animals were placed on the platform behind the exit blocked by the Plexiglas door. After 4 min of confinement, the door was retracted and the latency of the animal to leave the platform and make contact with the grid floor was recorded. Prior to training, baseline step-down latencies typically range from 8–20 sec. Upon contact with the floor, the door to the platform was closed and the aversive stimulus (light, noise, and vibration) was presented for 4 sec, at which time the platform door was opened to allow animals to return to the platform, where they were again confined for 5 min. At the end of this interval, the door was opened and the latency of the animal to exit the platform and step onto the grid floor (with no aversive stimulation) was recorded. The ratio of post-training to pre-training step-down latencies was calculated for each animal and this served to index learning. It has been determined that asymptotic performance is apparent in group averages following 2–3 training trials; thus performance after a single trial reflects, in most instances, sub-asymptotic learning.
2.5 Exploratory and Sensory/Motor testing
All animals underwent seven assessments of physical characteristics and behavioral tendencies [31, 80–81]. These included activity in the unwalled areas of an open field (a common measure of exploration), exploration in a light/dark box and in an elevated plus maze (potential measures of anxiety), latency to lick a paw on a hotplate test (pain sensitivity), screen hanging (a measure of paw strength), latency to fall from a small elevated platform, and separately, the latency to fall from and ability to maneuver across a balance beam (measures of coordination).
2.5.1 Exploratory Tasks
2.5.1.1 Open Field
A square field (46 × 46 cm) with 13 cm high walls was constructed of white Plexiglas and was located in a brightly lit room (400 lux) with a background noise of 65 dBc. The field was conceptually comprised of a 6 × 6 grid (7.65 cm quadrants), where 20 of the quadrants abutted the outer walls of the field (i.e., “wall” quadrants), and 16 quadrants were displaced from the walls and comprised the interior (i.e., “open” quadrants) of the field.
Animals were placed in the center of the field. After 20 s had elapsed (during which the animals self-selected a “starting” location), the animals’ behavior was monitored for 4 min. Throughout this time the animal’s entries into walled and open quadrants were recorded. An entry was recorded whenever both front paws crossed the border of a quadrant. Both total activity levels (i.e., quadrant entries regardless of category) as was the percentage of entries into unwalled (open) quadrants of the field were recorded. It should be noted that a 4-min test was explicitly chosen because changes in exploratory behavior (not necessarily simple motor activity) were not detectable over time.
2.5.1.2 Elevated Plus Maze
The maze was constructed of grey Plexiglas with four arms in the form of a “plus.” Each of these arms was 6 cm wide, and the entire maze was suspended 30 cm above a black surface. Two opposing arms of the maze were enclosed in 8 cm high, grey Plexiglas walls, while the two remaining arms were left open. The maze was located in a brightly lit room (300 lux).
Animals were placed in the center of the maze facing a closed arm, and their behavior in the maze was recorded in 1-min blocks for 4 min. Of particular interest was their total number of arm entries, their percent of total arm entries that were into open arms, closed and open arm entries as well as reentries into a previously occupied arm.
2.5.1.3 Light/Dark Discrimination Test
The rectangular box used in this task (56 × 15 × 10, l × w × h) was constructed from black Plexiglas and was located in a dimly lit room (<50 lux). The box was divided by a gray Plexiglas wall to create two equal size compartments (28 cm l). The animals could travel between compartments by way of a small opening in the dividing wall (3 cm × 5 cm). The walls of one compartment were painted white while the other side remained black, resulting in a light and dark side. The lid on the apparatus was clear above the light side of the box and was opaque on the dark side. The lighting was arranged so that a 60-watt lamp was shined directly on the light side of the box, resulting in a differential illumination between the light side and the dark side (300 lux versus <50 lux).
The animals were placed in the dark side of the box and allowed to freely explore the apparatus for 5 min. During this time the latency to enter the light side (hind front and hind legs) fully pass through the door between compartments was recorded. In addition, a number of other exploratory measures were recorded including the percent of total time spent in the light side and the number of crossings between the light side and the dark side.
2.6. Sensory/Motor tasks
2.6.1 Balance Beam
Animals were placed on a 40 × 0.7 × 2 cm (l × w × h) beam suspended 30 cm above the ground. The beam was explicitly designed so that animals do not typically fall from it. Instead, movement along the beam was the variable of interest, as movement is presumed to interact with balance. In a 4-min test, mice exhibit wide variability in the amount of movement along its length.
2.6.2 Hot Plate Test of Pain Sensitivity
The animals were placed on an aluminum plate which was maintained at a surface temperature of 52.6 °C. The animals’ latency to raise a hind paw and to either lick or shake the paw served as the index of pain sensitivity.
2.6.3 Screen Hanging Test of Grip Strength
The animals were placed on the underside of a wire mesh screen (7 mm grids) tilted 40° from vertical and suspended 24 cm from ground. Both the latency to drop from the screen and the distance moved prior to dropping from the screen (cm/s; 180 maximum test duration) were recorded.
2.6.4 Balance Pole
Animals are placed on a platform atop a 4 mm rod coated with black rubber (shrink tubing). The rod was suspended 30 cm above ground. Latency to drop from the rod (an index of balance) was recorded.
2.7. Stress Procedure and Assessment of Plasma CORT
After colonization, subjects in the imposed (IMP) and innate (INN) groups underwent a mild stress procedure. For the imposed (IMP) group this procedure occurred 114 days after colonization and for the innate (INN) group this procedure took place 32 days after colonization. During the interim between the end of colonization and the initiation of the stress procedure, subjects in the imposed (IMP) and innate (INN) groups were singly housed.
To inflict the stress, animals were confined on a 10-cm diameter platform elevated 120 cm above the floor in a brightly lit, unfamiliar room for a 6 min period. Ten minutes after the procedure subjects were decapitated to collect trunk blood. Plasma CORT levels were quantified using the mouse CORT Enzyme Immunoassay (EIA) kit available from Cayman Chemicals (Ann Arbor, MI).
2.8 Statistical Analyses
This experiment was a four-group between subjects design that compared behavioral and hormonal measures in imposed dominant (IMP DOM), imposed subordinate (IMP SUB), innate dominant (INN DOM) and innate subordinate (INN SUB) mice. Statistical comparisons of groups were conducted using either analysis of variance (ANOVA) or independent samples t-tests to examine between group differences in general learning ability and performance on individual learning tasks. Correlations between factor scores (an estimate of an animal’s performance on a factor isolated with a principal component analysis), measures of behavior (exploratory, social, and sensory/motor function) and stress reactivity (stress induced CORT) were also assessed.
3. Results
3.1 Social Behavior data
To examine the inter-relationships between the measures of social behavior quantified during Day 1 observations, a Pearson’s product-moment correlation matrix was created. A negative correlation was observed between the total average number of bites made and the number of wounds received [r=−.30, n=46, p< .05], i.e., animals that made more bites were themselves less wounded. There was also a positive correlation between the total average bites made and total tail rattles [r = .30, n = 46, p < .05] and a negative correlation between total tail rattles and wounding [r= −.44, n=46, p< .05]. Wounding and time spent huddling were positively correlated [r= .46, n=46, p< .05] while time spent huddling and total tail rattles were inversely correlated [r = −.29, n = 46, p < .05]. (It is notable the animals characterized as “dominant” rarely huddled with the other two animals of the triad, i.e., only the submissive and mid-submissive animals engaged in huddling.) Lastly, there was a positive correlation between the latency to attack and bites received [r = .31, n = 46, p< .05]. These results indicate that both dominant and subordinate subjects consistently express behaviors that are in accord with previously established behavioral phenotypes for submissive and dominant rodents [50; 56; 66–70; 72; 82–84]. Thus the dominant and submissive phenotypes are easily recognized. This difference can be best summarized qualitatively: Animals made submissive through colonization were continuously attacked by their dominant cage-mates.
3.2 Exploratory Data
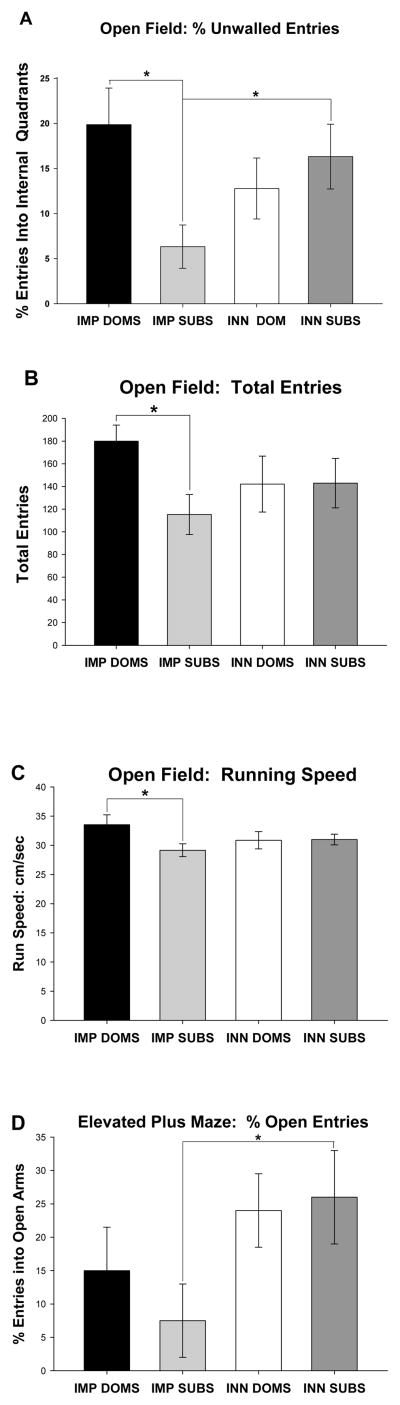
Subjects were tested prior to the assessment of learning abilities to determine whether there were pre-existing differences in exploratory tendencies. In the open field test, there was a trend toward significance for between-groups differences for the percent of internal entries, i.e., entries into unwalled quadrants, [F(3,26) = 2.67, p= .07]. Post-hoc comparisons revealed that IMP DOMS (that had been so designated based on previous stratification in a colony) had a higher percentage of entries in the internal/unwalled areas of the open field than IMP SUBS [also designated based on previous stratification in a colony; p< .01]. Similarly, INN SUBS (designated based on stratification in a colony assessed after the completion of all behavioral testing) had a higher percentage of internal entries in the open field than IMP SUBS [p< .05] (Figure 2A). This pattern of results indicates that imposed submission, but not innate submissive tendencies, tend to suppress exploratory behaviors.
Figure 2.
A. Illustrated is the percent of internal (unwalled) entries in the Open Field for the IMP DOMS, IMP SUBS, INN DOM, and INN SUBS groups. IMP DOMS (which were colonized prior to the open field test) had a higher percentage of entries in the internal areas of the open field than IMP SUBS (p=.01). Similarly, INN SUBS (that were colonized after the open field test) had a higher percentage of internal entries in the open field than IMP SUBS (p=.05). B. Open Field total entries (entries in the internal and external quadrants) for the IMP DOMS, IMP SUBS, INN DOM, and INN SUBS groups. IMP DOMS were more active (e.g. had more total entries) than IMP SUBS (p=.04). C. Open Field run speed (cm/s) for the IMP DOMS, IMP SUBS, INN DOM, and INN SUBS groups. IMP SUBS had a slower run speed (cm/sec) than IMP DOMS (p=.03). D. Elevated plus maze percent open entries for the IMP DOMS, IMP SUBS, INN DOM, and INN SUBS groups. Colonization prior to the elevated plus maze test promoted fewer entries into open arms in animals that exhibited submission, and a tendency toward fewer entries in animals that exhibited dominance. Asterisks (*) indicate significant comparisons.
A separate ANOVA for the total number of entries (combined walled and unwalled, indicative of total activity) made in the open field also showed a trend toward significance [F(3,26) = 1.57, p= .22]. Post-hoc comparison revealed that IMP DOMS were more active (e.g. had more total entries) than IMP SUBS [p< .05; Figure 2B]. Running speed in the open field also showed a trend toward significance [(F(3,24)= 1.74, p= .19] with post-hoc comparisons indicating that IMP SUBS had a slower running speed (cm/sec) than IMP DOMS [p< .05; Figure 2C], although nominally, this effect was small.
Data obtained in the elevated plus maze (after completion of the learning battery) followed a pattern similar to that in the open field. In the elevated plus maze task, there was a trend toward significance for the percent of open entries [F(3,23) =1.95, p= .15]. A post-hoc test showed that IMP SUBS had a significantly lower percentage of open entries than did INN SUBS [p< .05; Fig 2D]. There were no other significant findings for measures in the elevated plus maze (closed entries: [F(3,23) = .12, n.s.], open entries: [F(3,23) = 1.41, n.s.], reentries: [F(3,23) = 1.32, n.s.], total entries: [F(3,23) = .65, n.s.]). Again, this pattern suggests that the impairments identified here are due to imposition of subordination and are not innately expressed in subjects with a tendency toward subordination.
3.3 Sensory/Motor data
There were no significant differences between groups for any of the tests of sensory or motor abilities (Balance beam, latency to fall [F(3,26) = .33, n.s., or distance traveled: [F(3,26) = .94, n.s.]; Screen Hang, latency to fall: [F(3,26) = 1.08, n.s.] or number of grid crossings: [F(3,26) = .52, n.s.]; Balance pole, latency to fall [F(3,26) = .80, n.s.]; Hotplate, latency to lick a hind paw, [F(3,26) = 1.36, n.s.]). The lack of significant differences between groups on tests of sensory/motor function suggest that none of the animals, including those that were stratified (through colonization) prior to assessment, suffered from gross motor impairments as a result of wounding.
3.4 Learning Battery Data
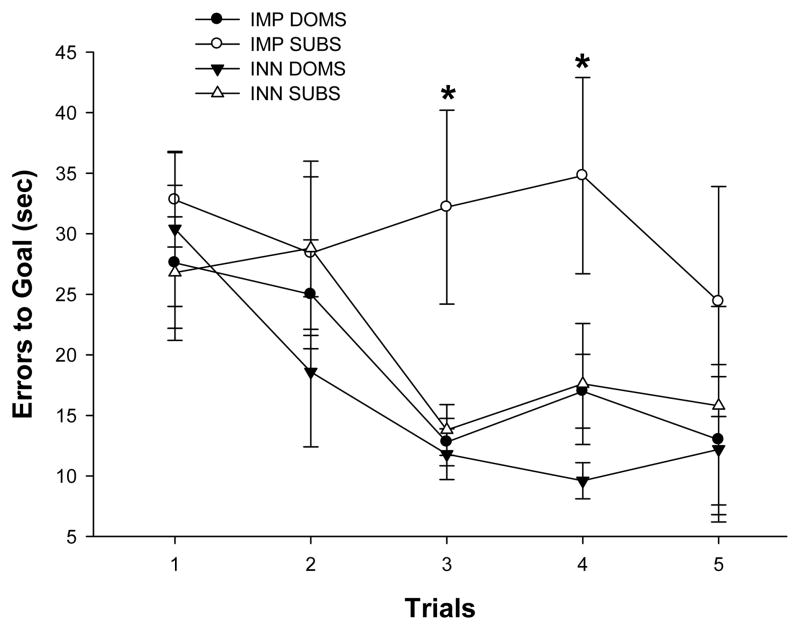
In the Lashley III maze, mice learned the task as evidenced by a significant reduction of errors across trials [F(4,104) = 11.97, p< .0001]. However, there was no difference between groups [F(3,26) = 1.13, n.s.] nor was there an interaction between group and trial [F(12,104) = .96, n.s.]. Errors to reach the goal box in the Lashley III maze also decreased across trials [F(4,104) = 4.43, p<.05], and group differences in errors were seen [F(3,26) = 3.36, p< .05] with IMP SUBS committing more errors in reaching the goal box than IMP DOMS (p< .05.; Figure 3). There were no significant group by trial interaction effects [F(12,104) = .62, n.s.].
Figure 3.
Errors across trials to find food in the Lashley III maze for the IMP DOMS, IMP SUBS, INN DOM, and INN SUBS groups. Mice learned the task as evidenced by a significant reduction of errors across trials ( p< .001), although Group IMP SUBS (which were colonized and exhibited submission prior to the test) exhibited no apparent learning and took longer to arrive at the goal box than IMP DOMS (p=.02). Asterisks (*) indicate significant comparisons.
In the associative fear conditioning task, training levels of freezing (post-pre freezing in sec) increased across trials [F(2,52) = 18.77, p< .0001], indicating that the animals learned to freeze to the tone as result of its pairing with foot shock. However there were no significant group [F(3,26) = .31, n.s.] or interaction [F(6,52) = .33, n.s.] effects. Similarly, separate repeated measures ANOVA’s carried out for freezing assessed in the absence (baseline) and presence (tone) of the tone were significant for trial only (baseline: [F(3,26) = .64, p< .05]; tone: [F(3,26) = .55, p< .05]).
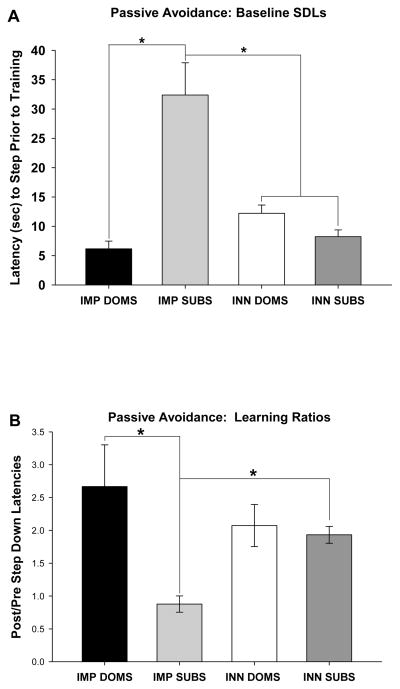
For the passive avoidance task, ANOVA revealed a significant main effect for group for baseline step-down latencies, i.e., the latency to step into a novel environment prior to pairing that step with aversive light and noise [F(3,26) = 17.52, p=< .0001]. Planned comparisons demonstrated that IMP SUBS had significantly longer baseline step-down latencies compared to IMP DOMS [p< .0001] and INN SUBS [p<. 0001] (Figure 4A). A prolonged baseline step-down latency has previously been interpreted as indicative of a reduction in exploratory behavior [86], and thus the long baseline latency exhibited by Group IMP SUB is consistent with the above observations of reduced exploration in this group. Given this difference in baseline performance, learning in the passive avoidance task was assessed by computing a ratio of the baseline step-down latency and the post-training step-down latency, where higher latencies would reflect better learning. A comparison of the four groups’ ratio of baseline step-down latencies to post-training step-down latencies was significant [F(3,26) = 4.17, p< .05]. Planned comparisons confirmed that IMP SUBS were impaired compared to IMP DOMS [p< .01] and INN SUBS [p< .05] (Figure 4B).
Figure 4.
A. Latency to step from platform prior to pairing the step with aversive stimulation in the passive avoidance task for the IMP DOMS, IMP SUBS, INN DOM, and INN SUBS groups. IMP SUBS had significantly longer baseline step-down latencies compared to IMP DOMS (p< .0001) and INN SUBS (p<. 0001). B. Ratio of the latency to step from platform before and after pairing the step with aversive stimulation in the passive avoidance task for the IMP DOMS, IMP SUBS, INN DOM, and INN SUBS groups. Subjects in the IMP SUB group exhibited impaired learning relative to the IMP DOM group (p=.03). Asterisks (*) indicate significant comparisons.
In the spatial water maze task, latencies to reach the platform decreased significantly across trials [F(9,234) = 6.50, p<.0001]. However, there were no significant group [F(3,26) = 1.16, ns] or interaction [F(27,234) = .47, n.s.] effects. Path length to the platform also decreased across the ten trials [F(9,180) = 5.80, p<.0001] and there were significant group differences for path length to the platform [F(3,20) = 5.54, p< .05], although planned comparisons revealed a significant difference between IMP SUBS and INN SUBS only on Trial 5. There was no effect of the interaction of group x trial [F(27,180) = .58, n.s.].
In the odor discrimination task, a repeated-measures ANOVA for errors was significant for trial [F(3,78) = 18.15, p<.0001] but was non-significant for group [F(3,26) = .42, n.s.] or interaction [F(9,78) = .72, n.s.]. Repeated-measures ANOVA for latency was significant for trial [F(3,78) = 25.21, p<.0001], however there were no significant group [F(3,26) = .67, n.s.] or interaction effects [F(9,78) = .83, n.s.].
3.5 Aggregate Cognitive Performance
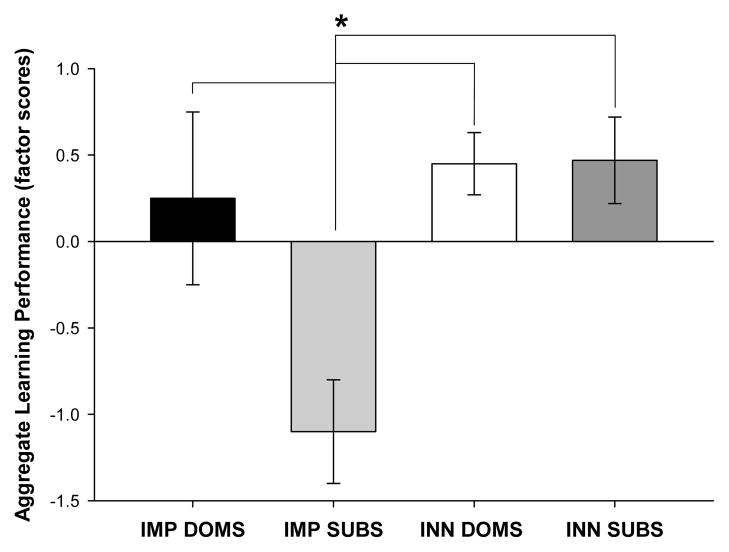
Significant impairments in cognitive performance in Group IMP SUB relative to Group INN SUB were only observed in three of the five cognitive tasks, and in the water maze, that difference was only significant for a single trial. However, it is notable that a tendency toward an impairment was exhibited by Group IMP SUB in all tasks. Such a pattern (i.e., consistent directionality of effects) is best described by factor-analytic techniques. An unrotated principal components factor analysis of the performance data for the five tasks that comprise the learning battery isolated two factors that accounted for a total of 56% of the variance in performance. Performance from all of the learning tasks loaded consistently on the primary factor, which accounted for 29% of the total variance (eigenvalue = 1.46, n = 48; Table 1). Given that performance from all learning tasks loaded in a single direction on this factor, this factor was used to extract factor scores to represent animals’ aggregate performance across all learning tasks (i.e., their general learning ability). (A factor score is analogous to an average of an animal’s z scores on each task that comprised the factor, where the z scores are weighted according to their degree of loading on that factor.) ANOVA of factor scores revealed a main of effect of group, [F(3,26) =5.21, p < .01]. Post-hoc comparisons revealed that imposed subordinates (IMP SUBS) had decrements in general learning performance compared to imposed dominants (IMP DOMS; p< .01), innate dominants (INN DOMS; p< .01), and innate subordinates (INN SUBS; p< .01; FIG 5). In total, this analysis suggests that the imposition of submission, but not the innate tendency toward submission, is associated with an impairment of general cognitive performance.
Table 1. Principal component factor analysis of learning battery performance.
An unrotated principal component factor analysis of the primary learning battery revealed primary and secondary factors (general learning abilities) explaining 29% and 27% of the total variance, respectively.
| Factor 1 | Factor 2 | |
|---|---|---|
| Passive Avoidance | 0.75 | −0.01 |
| Lashley Maze | 0.76 | 0.36 |
| Morris Water Maze | 0.09 | 0.83 |
| Odor Discrimination | 0.15 | −0.66 |
| Fear Conditioning | 0.54 | 0.32 |
| eiganvalue | 1.46 | 1.37 |
| % variance explained | 29% | 27% |
Figure 5.
Factor scores representative of animals’ aggregate le for the IMP DOMS, IMP SUBS, INN DOM, and INN SUBS groups. IMP SUBS (who had been colonized and exhibited submission prior to testing in the learning battery) exhibited significantly worse general learning performance (indicated by higher factor scores) than IMP DOMS (p=.005), INN DOMS (p=.002) and INN SUBS (p=.003). Asterisks (*) indicate significant comparisons.
The second factor extracted from our principal component analysis accounted for 27% of the variance (eigenvalue = 1.37, n=48; Table 1). Unlike the primary factory, this secondary factor was not readily interpretable (i.e., no obvious pattern of factor loadings emerged) and will not be further considered here.
We conducted a second principal component analysis on animals in the IMP groups to determine the relationship between general learning and measures of exploration and sensory/motor function. Previous work has indicated that general learning abilities are directly related to exploratory tendencies but not sensory/motor traits [80]. This unrotated principal component factor analysis produced a factor that accounted for a total of 25% of the variance in performance (eigenvalue = 4.42, n = 25; Table 2). Similar to previous reports [86–87], exploratory measures in the open field (% internal entries) and elevated plus maze (closed, open, % open entries) loaded strongly on the same factor with performance on the individual learning tasks. The low magnitude and inconsistent loading of other measures of sensory/motor performance suggest that they had little value in explaining performance on this factor. This later result indicates that sensory/motor impairments that may have been induced by the colonization procedure (from which the imposed dominant and submissive phenotypes emerged) had little value in explaining the learning deficits that were associated with this treatment.
Table 2. Principal component factor analysis of general learning scores and exploratory and sensory/motor measures.
An unrotated principal component factor analysis of exploratory, sensorimotor, and general learning abilities revealed only a primary factor explaining 25% of the total variance.
| Factor 1 | |
|---|---|
| Open field: total entries | 0.68 |
| Open field: % internal entries | 0.46 |
| Open field: run speed | −0.62 |
| Light/Dark discrimination: time in dark | −0.24 |
| Light/Dark discrimination: latency to enter light | −0.46 |
| Balance beam: latency to fall | −0.13 |
| Balance beam: distance travelled | 0.16 |
| Screen Hang: latency to fall | −0.06 |
| Screen Hang: # of crossings | −0.11 |
| Balance Pole: latency to fall | 0.26 |
| Hotplate | 0.10 |
| EPM: closed entries | 0.79 |
| EPM: open entries | 0.84 |
| EPM: reentries | 0.10 |
| EPM: total entries | 0.89 |
| EPM: % open entries | 0.82 |
| G Factor 1 | −0.29 |
| G Factor 2 | −0.27 |
| eiganvalue | 4.42 |
| % Variance Explained | 25% |
Lastly, since we observed group differences in factor scores (indicative of general learning ability), we examined how individual social behaviors were related to general learning scores. A final unrotated principal component factor analysis of general learning abilities and measures from the dominance/social behaviors produced a factor which accounted for a total of 26% of the variance in performance (eigenvalue = 2.11, n = 25; Table 3). Behavioral measures linked with submission (e.g. defensive rears and huddling) loaded in the same direction as general learning scores, which validates the conclusion that imposed subordination (but not the predisposition toward subordination) is associated with impaired learning abilities.
Table 3. Principal component factor analysis of general learning scores and social behavior measures.
An unrotated principal component factor analysis of social behaviors and general learning abilities revealed a primary and secondary factor explaining 26% and 18% of the total variance, respectively.
| Factor 1 | Factor 2 | |
|---|---|---|
| G Factor 1 | 0.40 | 0.15 |
| G Factor 2 | 0.52 | 0.27 |
| Huddling | 0.57 | −0.10 |
| Defensive Rears | 0.12 | 0.85 |
| Sniffing | −0.42 | 0.001 |
| Bites made | −0.80 | −0.23 |
| Bites received | −0.67 | 0.37 |
| Latency to attack | −0.29 | 0.64 |
| eiganvalue | 2.11 | 1.42 |
| % Variance explained | 26% | 18% |
3.6 Stress-Induced CORT Responses
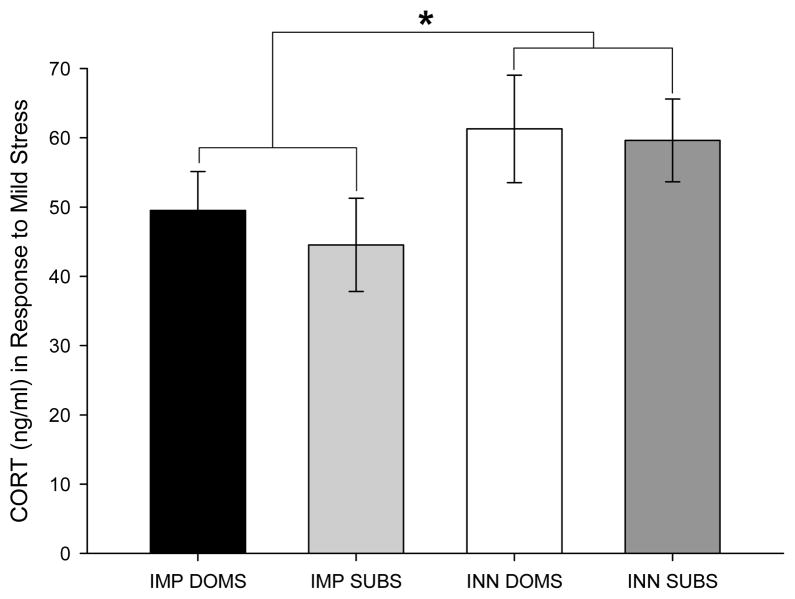
A between-groups ANOVA revealed a trend toward group differences in the stress-induced CORT elevation of the four groups of animals [F(3,26) = 1.45, p= .25], and a planned comparison post-hoc test confirmed that imposed subordinates (IMP SUBS) had significantly lower CORT levels than innate subordinates [INN SUBS; p< .05] (Figure 6). A t-test for independent samples between all subjects in the IMP groups and subjects in the INN groups was significant [t(1,28) = 2.08, p< .05], i.e., the INN groups (those who were made subordinate after learning was assessed) had higher levels of plasma CORT than subjects in the IMP groups. A t-test for independent samples between all subordinate and all dominant subjects (regardless of status) was non-significant [t(1,28) = .46, n.s.]. Thus, the experience of triadic colonization leads to an increase in stress reactivity (e.g. stress-induced CORT elevation in response to a mild stressor) that persists for at least one month after removal from the social environment (Group INN), but appears to dissipate within three months (Group IMP). In neither case was the stress-induced CORT elevation sufficient to account for the learning impairments associated with the submissive phenotype (see below). In this regard it is notable that although colonization (regardless of social stature) was associated with increased stress reactivity (and resultant CORT elevation), only animals that were deemed submissive in the colonized environment exhibited learning impairments.
Figure 6.
CORT levels (ng/ml) for the IMP DOMS, IMP SUBS, INN DOM, and INN SUBS groups. Animals were confined to an elevated platform in a bright room for five minutes (which prior work indicated induced a moderate stress response) either 124 days (Groups IMP DOM and IMP SUB) or 32 days after colonization. Regardless of phenotype, colonization within approximately one month of the CORT assay promoted an increased CORT response to mild stress, which had dissipated within four months. Asterisks (*) indicate significant comparisons.
A principal components analysis, which included stress-induced CORT and measures of dominance/submission produced a factor that accounted for 52% of the variance (eigenvalue = 4.64, n= 29; TABLE 4). Consistent with the discussion above, analysis of the structure of this factor suggests that dominance measures positive for agonstic involvement (e.g. sniffing, bites made, bites received, defensive rears, tail rattling, wounding) were highly related to stress-induced CORT levels. One could conclude that the experience of taking part in an agonstic encounter, regardless of whether an animal was subordinate or dominant, is related to stress-induced CORT reactivity.
Table 4. Principal component factor analysis of stress-induced CORT and social behavior measures.
An unrotated principal component factor analysis of social behaviors and CORT levels revealed a primary and secondary factor explaining 52% and 15% of the total variance, respectively.
| Dominance Factor 1 | Dominance Factor 2 | |
|---|---|---|
| Huddling | −0.31 | 0.06 |
| Sniffing | 0.42 | −0.33 |
| Bites Made | 0.93 | −0.02 |
| Latency | −0.03 | −0.79 |
| Bites Received | 0.10 | −0.11 |
| Defensive Rears | 0.82 | −0.33 |
| Tail rattling | 0.87 | 0.29 |
| Wounding | 0.95 | −0.05 |
| CORT | 0.51 | 0.62 |
| eiganvalue | 4.64 | 1.33 |
| % Variance Explained | 52% | 15% |
A final principal components analysis that included stress-induced CORT and sensorimotor measures produced a factor that accounted for 27% of the variance (eigenvalue = 4.53, n= 25; TABLE 5). Open field (total and % internal entries) and elevated plus maze (closed, open, % open entries) measures loaded in the same direction of stress-induced CORT levels, a pattern of results that we have previously observed [86].
Table 5. Principal component factor analysis of stress-induced CORT and exploratory and sensory/motor measures.
An unrotated principal component factor analysis of exploratory and sensorimotor behaviors and CORT levels reveals a primary and secondary factor explaining 27% and 15% of the total variance, respectively.
| Factor 1 | Factor 2 | |
|---|---|---|
| Open field: total entries | 0.65 | −0.16 |
| Open field: % internal entries | 0.42 | −0.14 |
| Open field: run speed | −0.58 | −0.09 |
| Light/Dark discrimination: time in dark | −0.18 | 0.36 |
| Light/Dark discrimination: latency to enter light | −0.45 | 0.01 |
| Balance beam: latency to fall | −0.21 | 0.64 |
| Balance beam: distance travelled | 0.05 | 0.82 |
| Screen Hang: latency to fall | −0.07 | 0.78 |
| Screen Hang: # of crossings | −0.12 | 0.76 |
| Balance Pole: latency to fall | 0.33 | 0.03 |
| Hotplate | 0.12 | −0.03 |
| EPM: closed entries | 0.80 | 0.06 |
| EPM: open entries | 0.86 | 0.20 |
| EPM: reentries | 0.11 | 0.09 |
| EPM: total entries | 0.91 | 0.14 |
| EPM: % open entries | 0.82 | 0.20 |
| CORT | 0.54 | −0.18 |
| eiganvalue | 4.53 | 2.60 |
| % Variance Explained | 27% | 15% |
4. Discussion
Our goal here was to assess and expand upon previous reports that describe the effects of social subordination on cognition, and to determine if those effects reflected the impact of subordination per se, or whether they were co-expressed with the predisposition to adopt a subordinate status. To that end, we established a triadic colony model (in which animals adopted dominant or submissive statures) and examined the impact of this type of social stress on both domain-specific and domain-general learning processes. We found that imposed, but not innate tendencies toward subordination were associated with significant impairments on three of five tests of learning, although other tests of learning were statistically, if not nominally, spared. However, general learning abilities (which are more sensitive to overall patterns of learning performance) were severely impaired in subjects exposed to social subordination prior to the learning experience. Importantly, it was also observed that the tendency to adopt a subordinate stature, absent the actual imposition of subordination, was not in itself a predisposing factor toward learning impairments. Rather, innately subordinate animals upon which subordination is actually imposed appear to develop impairments in general learning abilities. This later conclusion should be qualified in that the tendency to adopt a submissive stature may have been dependent on the composition of a particular triad of animals, and thus may not be an absolute (as opposed to relative) reflection of an innate phenotype. That is, an animal that adopted a submissive stature in one triad may have been dominant in another. However, the nature of random sampling makes it reasonable to assume that groups of animals selected from those that adopted a submissive stature within a triad are, in general, representative of a submissive phenotype. Nevertheless, in this specific case, stature of any given animal can be judged relative to only two other individuals.
In addition to its effect on cognitive performance, we found that the imposition of subordination results in a decrease in exploratory tendencies (in the open field, elevated plus maze, and the step-down avoidance task). It is often difficult to distinguish impairments in learning from impairments that reflect differences in the performance of learned responses, and it is possible that that the imposition of subordination induces deficits in both learning and performance. Thus we must fully consider whether submission-induced impairments in our tests of learning reflect modifications of underlying learning processes or whether they are the consequence of variations in exploratory tendencies (which might indirectly impact learning or its expression). In considering performance in the step-down avoidance task, learning was assessed with a ratio of pre-training to post-training step-down latencies, thus normalizing for pre-existing differences in the tendency to step off the safe platform. Despite this normalization for differences in baseline step-down latencies, the imposition of subordination was associated with impaired performance indicative of learning. The inability to properly form associations between one’s actions and negative consequences could prove detrimental and result in further adverse consequences (including further reductions in exploration). Thus at least based on this analysis, it does not appear that a reduction in exploration was itself sufficient to account for the learning deficit. Consistent with this conclusion, we have previously reported that directly increasing exploration, either through repeated exposure to novel environments [88] or through the pharmacological manipulation of stress reactivity [87], had no beneficial impact on general learning abilities. In total, these results suggest that the impairment of general learning abilities by the imposition of subordination is not attributable to the effects of this treatment on exploratory behaviors, although it is entirely possible (if not likely) that these phenotypic tendencies interact in ways that we have not detected. Similarly, we found no evidence that physical injuries that accompanied the imposition of subordination contributed to the observed learning deficits, i.e., sensory/motor performance accounted for little of the variability in learning. This is not surprising, since the tests of learning were administered more than 30 days after the termination of the colonization procedure, allowing sufficient time for wound healing and recovery from other physical injuries. Nevertheless, it is acknowledged that animals that adopted a submissive stature during colonization were exposed to rather extreme treatment by their dominant cohorts. At this point it is unknown whether a less severe treatment would result in a similar impairment of general cognitive performance.
In the current study, we also found that that stress-induced CORT levels (i.e. stress reactivity) had little predictive value in explaining variations in general learning abilities, suggesting that differences in individual stress reactivity do not underlie (under the present conditions) variations in general learning performance. Similarly, in an earlier study in our laboratory, we were able to pharmacologically disassociate stress reactivity from general learning abilities [87]. Together, this data suggests that the relationship between general learning and exploration is not necessarily mediated by stress reactivity. To more fully assess the relationship between CORT and learning abilities in socially stressed animals, we would need to quantify circulating CORT levels after colonization but prior to performance in learning tasks and determine if these measures were correlated, and if so, whether the modulation of glucocorticoids contributes to the decrements in general learning abilities induced by the imposition of subordination.
While it is unlikely that stress reactivity (i.e. stress-induced CORT elevations) can broadly explain variations in general learning abilities, it is still possible that that increases in glucocorticoid expression in response to subordination contribute to the changes in general learning abilities seen in this study. Indeed structural and functional changes in the hippocampus have been reported subsequent to social stress [22, 27, 90, 91]. However, since both imposed domination and imposed submission were associated with elevated CORT levels (one, but not four months after colonization), but only imposed subordination was associated with impaired general learning abilities, it appears that stress-induced CORT elevations (at the low levels reported here) cannot in themselves account for the cognitive deficits that arise as a consequence of imposed subordination.
It is possible that subordination may impact upon learning processes via neurolophysiological mechanisms that are independent of corticosterone. For example, it has been observed that social stress restricts dendritic arborization [92–93] and neurogenesis [27, 94–95]. Interestingly, stress-related effects on dendrtric atrophy and neurogenesis can be prevented by pre-treatment with the drug tianeptine, a selective serotonin reuptake-enhancer (SSRE) [93]. If the stress effects on hippocampal morphology and spatial learning are prevented by treatment with SSREs, it is plausible that other stress-related cognitive deficits, including its adverse effects on general learning abilities and exploration described here, could be alleviated by similar pharmacological treatments. However, in the social environments of humans (e.g., schools), it may be impractical (if not ill-advised) to attempt to moderate the effect of imposed submission (e.g., bullying) with medication. Thus, alternative treatments must be explored. Given that the tendency toward submission is not itself sufficient to promote learning deficits, an effective strategy might be to implement more active behavioral intervention to eliminate or limit exposure to subordination stress. The introduction of “anti-bullying” programs may serve to reduce the occurrences of social subordination and thereby decrease social stress and ameliorate its negative consequences on cognitive abilities. Cognitive therapies may also be useful in counteracting the negative effects of submission. In humans, working memory training has been shown to improve cognition. We have recently developed a working memory training regimen in mice that has been shown to successfully increase both selective attention and general learning performance [35,96]. Thus it is conceivable that working memory training administered either before or after social stress might prevent or alleviate the negative effects on learning which result from subordination.
5. Conclusions
The present results underscore the detrimental consequences of social stress on cognition, and furthermore, indicate that a propensity toward submission does not in itself (absent the imposition of physical defeat), detrimentally impact cognitive abilities. The social stress model that was established here is well suited to probe the biological basis of the social stress-related learning deficits that are conserved across mammalian species. Further, this model can be utilized to examine how potential pharmacological and behavioral interventions may be instituted in order to improve the quality of life for individuals who suffer due to the stress of social subordination.
Highlights.
The receipt of aggression can adversely impact cognitive performance.
Imposed submission impaired exploratory behaviors and general cognitive abilities.
Animals predisposed toward submission exhibited normal cognitive abilities.
Submissive stature, absent imposed aggression, was not associated with cognitive impairments.
Acknowledgments
This work was support by grants from the National Institute of Aging (R01 AG029289) and the Busch Foundation to LDM.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Shors TJ. Stressful Experience and Learning Across the Lifespan. Annual Rev of Psych. 2006;57:55–85. doi: 10.1146/annurev.psych.57.102904.190205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roozendaal B. Stress and Memory: Opposing Effects of Glucocorticoids on Memory Consolidation and Memory Retrieval. Neurobio of Learn and Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- 3.Roozendaal B. Systems mediating acute glucocorticoid effects on memory consolidation and retrieval. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27(8):1213–1223. doi: 10.1016/j.pnpbp.2003.09.015. [DOI] [PubMed] [Google Scholar]
- 4.Joëls M, Pu Z, Wiegert O, Oitzl MS, Krugers HJ. Learning under stress: how does it work? Trends Cogn Sci. 2006;10(4):152–8. doi: 10.1016/j.tics.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 5.de Kloet ER, Oitzl MS, Joëls M. Stress and cognition: are corticosteroids good or bad guys? Trends Neurosci. 1999;22(10):422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 6.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10(6):434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 7.McEwen BS, Sapolsky RM. Stress and cognitive function. Curr Opin Neurobiol. 1995;5(2):5–16. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- 8.Baumeister RF, Twenge JM, Nuss CK. Effects of social exclusion on cognitive processes: anticipated aloneness reduces intelligent thought. J Pers Soc Psychol. 2002;83(4):817–827. doi: 10.1037//0022-3514.83.4.817. [DOI] [PubMed] [Google Scholar]
- 9.Johnson GM. Student Alienation, Academic Achievement and WebCT Use. Educational Technology & Society. 2005;8(2):179–189. [Google Scholar]
- 10.Schmader T, Johns M, Forbes C. An integrated process model of stereotype threat effects on performance. Psychological Review. 2010;115(2):336–356. doi: 10.1037/0033-295X.115.2.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoover H, Oliver R, Hazler RJ. Bullying: Perceptions of adolescent victims in the Midwestern USA. School Psychology International. 1992;13(1):5–16. [Google Scholar]
- 12.Olweus D. Disruptive behaviours in schools. New York: Wiley; 1984. Aggressors and their victims: Bullying at school. [Google Scholar]
- 13.Knox E, Conti-Ramsden G. Bullying risks of 11-year-old children with specific language impairment (SLI): does school placement matter? Int J Lang Commun Disord. 2003;38(1):1–12. doi: 10.1080/13682820304817. [DOI] [PubMed] [Google Scholar]
- 14.Perry DG, Kusel SJ, Perry LC. Victims of peer aggression. Developmental Psychology. 1988;24(6):807–814. [Google Scholar]
- 15.Horwood J, Salvi G, Thomas K, Duffy L, Gunnell D, Hollis C, Lewis G, Menezes P, Thompson A, Wolke D, Zammit S, Harrison G. IQ and non-clinical psychotic symptoms in 12-year-olds: results from the ALSPAC birth Cohort. The British Journal of Psychiatry. 2008;193:185–191. doi: 10.1192/bjp.bp.108.051904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alzoubi KH, Abdul-Razzak KK, Khabour OF, Al-Tuweiq GM, Alzubi MA, Alkadhi KA. Adverse effect of combination of chronic psychosocial stress and high fat diet on hippocampus-dependent memory in rats. Behavioural Brain Research. 2009;204(1):117–123. doi: 10.1016/j.bbr.2009.05.025. [DOI] [PubMed] [Google Scholar]
- 17.Fitchett AE, Collins SA, Barnard CJ, Cassaday HJ. Subordinate male mice show long-lasting differences in spatial learning that persist when housed alone. Neurobiol Learn Mem. 2005;84(3):247–51. doi: 10.1016/j.nlm.2005.08.004. [DOI] [PubMed] [Google Scholar]
- 18.Krugers HJ, Douma BR, Andringa G, Bohus B, Korf J, Luiten PG. Exposure to chronic psychosocial stress and corticosterone in the rat: effects on spatial discrimination learning and hippocampal protein kinase Cgamma immunoreactivity. Hippocampus. 1997;7(4):427–36. doi: 10.1002/(SICI)1098-1063(1997)7:4<427::AID-HIPO8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 19.Ohl F, Fuchs E. Memory performance in tree shrews: effects of stressful experiences. Neurosci Biobehav Rev. 1998;23(2):319–323. doi: 10.1016/s0149-7634(98)00033-5. [DOI] [PubMed] [Google Scholar]
- 20.Ohl F, Fuchs E. Differential effects of chronic stress on memory processes in the tree shrew. Cognitive Brain Res. 1999;7:1379–1387. doi: 10.1016/s0926-6410(98)00042-1. [DOI] [PubMed] [Google Scholar]
- 21.Kvist B. Learning in mice selectively bred for high and low aggressiveness. Psychol Rep. 1989;64(1):127–130. doi: 10.2466/pr0.1989.64.1.127. [DOI] [PubMed] [Google Scholar]
- 22.Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticosterone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995;15(1):61–69. doi: 10.1523/JNEUROSCI.15-01-00061.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Touyarot K, Venero C, Sandi C. Spatial learning impairment induced by chronic stress is related to individual differences in novelty reactivity: search for neurobiological correlates. Psychoneuroendocrinology. 2004;29(2):290–305. doi: 10.1016/s0306-4530(03)00031-3. [DOI] [PubMed] [Google Scholar]
- 24.Francia N, Cirulli F, Chiarotti F, Antonelli A, Aloe L, Alleva E. Spatial memory deficits in middle-aged mice correlate with lower exploratory activity and a subordinate status: role of hippocampal neurotrophins. Eur J Neurosci. 2006;23(3):711–28. doi: 10.1111/j.1460-9568.2006.04585.x. [DOI] [PubMed] [Google Scholar]
- 25.Barnard CJ, Luo N. Acquisition of dominance status affects maze learning in mice. Behav Processes. 2002;60(1):53–59. doi: 10.1016/s0376-6357(02)00121-3. [DOI] [PubMed] [Google Scholar]
- 26.Fitchett AE, Barnard CJ, Cassaday HJ. Corticosterone differences rather than social housing predict performance of T-maze alternation in male CD-1 mice. Animal Welfare. 2009;18(1):21–31. [Google Scholar]
- 27.Buwalda B, Kole MH, Veenema AH, Huininga M, de Boer SF, Korte SM, Koolhaas JM. Long-term effects of social stress on brain and behavior: a focus on hippocampal functioning. Neuroscience & Biobehavioral Rev. 2005;29(1):83–97. doi: 10.1016/j.neubiorev.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Bartolomucci A, de Biurrun G, Czéh B, van Kampen M, Fuchs E. Selective enhancement of spatial learning under chronic psychosocial stress. Eur J Neurosci. 2002;15(11):1863–1866. doi: 10.1046/j.1460-9568.2002.02043.x. [DOI] [PubMed] [Google Scholar]
- 29.Moragrega I, Carrasco MC, Vicens P, Redolat R. Spatial learning in male mice with different levels of aggressiveness: effects of housing conditions and nicotine administration. Behav Brain Res. 2003;147(1–2):1–8. doi: 10.1016/s0166-4328(03)00112-8. [DOI] [PubMed] [Google Scholar]
- 30.Spritzer MD, Meikle DB, Solomon NG. The relationship between dominance rank and spatial ability among male meadow voles (Microtus pennsylvanicus) J Comp Psychol. 2004;118(3):332–339. doi: 10.1037/0735-7036.118.3.332. [DOI] [PubMed] [Google Scholar]
- 31.Kolata S, Light K, Matzel LD. Domain specific and domain general learning factors are expressed in genetically heterogeneous CD-1 mice. Intelligence. 2008;36(6):619–629. doi: 10.1016/j.intell.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Plomin R. Genetics and general cognitive ability. Nature. 1999;402:C25–29. doi: 10.1038/35011520. [DOI] [PubMed] [Google Scholar]
- 33.Kolata S, Light K, Grossman HC, Hale G, Matzel LD. Selective attention is a primary determinant of the relationship between working memory and general learning ability in outbred mice. Learn Mem. 2007;14(1):22–8. doi: 10.1101/lm.408507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kolata S, Light K, Townsend DA, Hale G, Grossman HC, Matzel LD. Neurobiol Learn Mem. 2005;84(3):241–246. doi: 10.1016/j.nlm.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 35.Light KR, Kolata S, Wass C, Denman-Brice A, Zagalsky R, Matzel LD. Working Memory Training Promotes General Cognitive Abilities in Genetically Heterogeneous Mice. Curr Biol. 2010;20(8):777–782. doi: 10.1016/j.cub.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kolata S, Light K, Wass CD, Colas-Zelin D, Roy D, Matzel LD. A dopaminergic gene cluster in the prefrontal cortex predicts performance indicative of general intelligence in genetically heterogeneous mice. PLoS One. 2010 Nov 17;5(11):e14036. doi: 10.1371/journal.pone.0014036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matzel LD, Kolata S. Selective attention, working memory, and animal intelligence. Neurosci Biobehav Rev. 2010 Jan;34(1):23–30. doi: 10.1016/j.neubiorev.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hardy MP, Sottas CM, Ge R, McKittrick CR, Tamashiro KL, McEwen BS, Haider SG, Markham CM, Blanchard RJ, Blanchard DC, Sakai RR. Trends of reproductive hormones in male rats during psychosocial stress: role of glucocorticoid metabolism in behavioral dominance. Biol Reprod. 2002;67(6):1750–1755. doi: 10.1095/biolreprod.102.006312. [DOI] [PubMed] [Google Scholar]
- 39.Monder C, Sakai RR, Miroff Y, Blanchard DC, Blanchard RJ. Reciprocal changes in plasma corticosterone and testosterone in stressed male rats maintained in a visible burrow system: evidence for a mediating role of testicular 11 beta-hydroxysteroid dehydrogenase. Endocrinology. 1994;134(3):1193–1198. doi: 10.1210/endo.134.3.8119159. [DOI] [PubMed] [Google Scholar]
- 40.Dong Q, Salva A, Sottas CM, Niu E, Holmes M, Hardy MP. Rapid glucocorticoid mediation of suppressed testosterone biosynthesis in male mice subjected to immobilization stress. J Androl. 2004;25(6):973–81. doi: 10.1002/j.1939-4640.2004.tb03170.x. [DOI] [PubMed] [Google Scholar]
- 41.Blanchard DC, Sakai RR, McEwen B, Weiss SM, Blanchard RJ. Subordination stress: behavioral, brain, and neuroendocrine correlates. Behav Brain Res. 1993;58(1–2):113–21. doi: 10.1016/0166-4328(93)90096-9. [DOI] [PubMed] [Google Scholar]
- 42.Blanchard DC, Spencer RL, Weiss SM, Blanchard RJ, McEwen B, Sakai RR. Visible burrow system as a model of chronic social stress: behavioral and neuroendocrine correlates. Psychoneuroendocrinology. 1995;20(2):117–34. doi: 10.1016/0306-4530(94)e0045-b. [DOI] [PubMed] [Google Scholar]
- 43.Veenema AH, Meijer OC, de Kloet ER, Koolhaas JM, Bohus BG. Differences in basal and stress-induced HPA regulation of wild house mice selected for high and low aggression. Horm Behav. 2003;43(1):197–204. doi: 10.1016/s0018-506x(02)00013-2. [DOI] [PubMed] [Google Scholar]
- 44.von Holst D. Social stress in tree shrews: problems, results and goals. Journal of Comparative Physiology. 1977;120:71–86. [Google Scholar]
- 45.Manogue KR, Leshner AI, Candland DK. Dominance status and adrenocortical reactivity to stress in squirrel monkeys (Saimiri sciureus) 1975 [Google Scholar]
- 46.Coe C, Mendoza S, Levine S. Social stastus constrains the stress response in the squirrel monkey. Physiol Behav. 1979;23(4):633–638. doi: 10.1016/0031-9384(79)90151-3. [DOI] [PubMed] [Google Scholar]
- 47.Sapolsky RM. Individual differences in cortisol secretory patterns in the wild baboon: role of negative feedback sensitivity. Endocrinology. 1983;113(6):2263–2267. doi: 10.1210/endo-113-6-2263. [DOI] [PubMed] [Google Scholar]
- 48.Bronson FH. Establishment of social rank among grouped male mice: relative effects on circulating FSH, LH, and corticosterone. Physiol Beh. 1973;10(5):947–951. doi: 10.1016/0031-9384(73)90065-6. [DOI] [PubMed] [Google Scholar]
- 49.Ely DL, Henry JP. Neuroendocrine response patterns in dominant and subordinate mice. Horm Behav. 1978;10(2):156–69. doi: 10.1016/0018-506x(78)90005-3. [DOI] [PubMed] [Google Scholar]
- 50.Wirth MM, Welsh KM, Schultheiss OC. Salivary cortisol changes in humans after winning or losing a dominance contest depend on implicit power motivation. Horm Behav. 2006;49(3):346–52. doi: 10.1016/j.yhbeh.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 51.Blanchard DC, McKittrick CR, Hardy MP, Blanchard RJ. Effects of Social Stress on Hormones, Brain and Behavior. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Vol. 1. San Diego: Academic Press; 2002. pp. 735–772. [Google Scholar]
- 52.Ruis MAW, te Brake JHA, Buwalda B, de Boer SF, Meerlo P, Korte SM, Blokhuis HJ, Koolhaas JH. Housing familiar male wildtype rats together reduces the long-term adverse behavioural and physiological effects of social defeat. Psychoneuroendocrinology. 1999;24:285–300. doi: 10.1016/s0306-4530(98)00050-x. [DOI] [PubMed] [Google Scholar]
- 53.Menzaghi F, Heinrichs SC, Vargas-Cortes M, Goldstein G, Koob GF. IRI-514, a synthetic peptide analogue of thymopentin, reduces the behavioral response to social stress in rats. Physiology and Behavior. 1996;60(2):397–401. 5. [PubMed] [Google Scholar]
- 54.Heinrichs SC, Menzaghi F, Pich EM, Baldwin HA, Rassnick S, Britton KT, Koob GF. Anti-stress action of a corticotropin-releasing factor antagonist on behavioral reactivity to stressors of varying type and intensity. Neuropsychopharmacology. 1994;111(3):179–186. doi: 10.1038/sj.npp.1380104. [DOI] [PubMed] [Google Scholar]
- 55.Heinrichs SC, Pich EM, Miczek KA, Britton KT, Koob GF. Corticotropin-releasing factor antagonist reduces emotionality in socially defeated rats via direct neurotropic action. Brain Res. 1992;29, 581(2):190–197. doi: 10.1016/0006-8993(92)90708-h. [DOI] [PubMed] [Google Scholar]
- 56.Berton O, Aguerre S, Sarrieau A, Mormede P, Chaouloff F. Differential effects of social stress on central serotonergic activity and emotional reactivity in Lewis and spontaneously hypertensive rats. Neuroscience. 1998;82(1):147–159. doi: 10.1016/s0306-4522(97)00282-0. [DOI] [PubMed] [Google Scholar]
- 57.Berton O, Durand M, Aguerre S, Mormède P, Chaouloff F. Behavioral, neuroendocrine and serotonergic consequences of single social defeat and repeated fluoxetine pretreatment in the Lewis rat strain. Neuroscience. 1999;92(1):327–341. doi: 10.1016/s0306-4522(98)00742-8. [DOI] [PubMed] [Google Scholar]
- 58.Avgustinovich DF, Gorbach OV, Kudryavtseva NN. Comparative analysis of anxiety-like behavior in partition and plus-maze tests after agonistic interactions in mice. Physiol Behav. 1997;61(1):37–43. doi: 10.1016/s0031-9384(96)00303-4. [DOI] [PubMed] [Google Scholar]
- 59.Rodgers RJ, Cole JC. Anxiety enhancement in the murine elevated plus maze by immediate prior exposure to social stressors. Physiol Behav. 1993;53(2):383–388. doi: 10.1016/0031-9384(93)90222-2. [DOI] [PubMed] [Google Scholar]
- 60.Haller J, Halász J. Mild social stress abolishes the effects of isolation on anxiety and chlordiazepoxide reactivity. Psychopharmacology. 1999;144(4):311–315. doi: 10.1007/s002130051012. [DOI] [PubMed] [Google Scholar]
- 61.Peres RC, Leite JR. The Influence of Competitive Status (Winner/Loser) on the Behavior of Male Rats in Three Models of Anxiety. Aggressive Behavior. 2002;28:164–171. [Google Scholar]
- 62.Kudryavtseva NN, Bakshtanovskaya IV, Koryakina LA. Social model of depression in mice of C57BL/6J strain. Pharmacol Biochem Behav. 1991a;38(2):315–320. doi: 10.1016/0091-3057(91)90284-9. [DOI] [PubMed] [Google Scholar]
- 63.Kudryavtseva NN, Madorskaya IA, Bakshtanovskaya IV. Social success and voluntary ethanol consumption in mice of C57BL/6J and CBA/Lac strains. Physiol Behav. 1991b;50(1):143–146. doi: 10.1016/0031-9384(91)90511-l. [DOI] [PubMed] [Google Scholar]
- 64.Zook JM, Adams DB. Competitive fighting in the rat. Journal of Comparative and Physiological Psychology. 1975;88(1):418–423. doi: 10.1037/h0076225. [DOI] [PubMed] [Google Scholar]
- 65.Schneider R, Hoffmann HJ, Schicknick H, Moutier R. Genetic analysis of isolation-induced aggression. I. Comparison between closely related inbred mouse strains. Behavioral and Neural Biology. 1992;57:198–204. doi: 10.1016/0163-1047(92)90150-3. [DOI] [PubMed] [Google Scholar]
- 66.Grant EC, Mackintosh JH. A comparison of the social postures of some common laboratory rodents. Behaviour. 1963;21(3–4):246–259. [Google Scholar]
- 67.Clark LH, Schein MW. Activities associated with conflict behavior in mice. Animal Behaviour. 1966;14(1):44–49. doi: 10.1016/s0003-3472(66)80009-x. [DOI] [PubMed] [Google Scholar]
- 68.Burg RD, Slotnick BM. Response of colony mice to intruders with different fighting experience. Aggressive Behavior. 1983;9(1):49–58. [Google Scholar]
- 69.Miczek KA, Maxson SC, Fish EW, Faccidomo S. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125(1–2):167–81. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- 70.Ginsburg B, Allee WC. Some Effects of Conditioning on Social Dominance and Subordination in Inbred Strains of Mice. Physiological Zoology. 1942;15(4):485–50. [Google Scholar]
- 71.Miczek KA, O’Donnell JM. Intruder-evoked aggression in isolated and nonisolated mice: effects of psychomotor stimulants and L-dopa. Psychopharmacology. 1978;57(1):47–55. doi: 10.1007/BF00426957. [DOI] [PubMed] [Google Scholar]
- 72.Miczek KA, Thompson ML, Shuster L. Opioid-like analgesia in defeated mice. Science. 1982;215(4539):1520–1522. doi: 10.1126/science.7199758. [DOI] [PubMed] [Google Scholar]
- 73.Miczek KA. Aggressive and social stress responses in genetically modified mice: from horizontal to vertical strategy. Psychopharmacology. 1999;147(1):17–19. doi: 10.1007/s002130051132. [DOI] [PubMed] [Google Scholar]
- 74.Blanchard RJ, Takahashi LK, Fukunaga KK, Blanchard DC. Functions of the vibrissae in the defensive and aggressive behavior of the rat. Aggress Behav. 1977;3:231–240. [Google Scholar]
- 75.Blanchard DC, Blanchard RJ, Lee EM, Nakamura S. Defensive behaviors in rats following septal and septal--amygdala lesions. J Comp Physiol Psychol. 1979 Apr;93(2):378–90. doi: 10.1037/h0077562. [DOI] [PubMed] [Google Scholar]
- 76.Pellis SM, Pellis VC. Animal play: Evolutionary, comparative, and ecological perspectives. In: Bekoff Marc, Byers John Alexander., editors. Structure-function interface in the analysis of play fighting. Animal play: Evolutionary, comparative, and ecological perspectives. New York, NY, US: Cambridge University Press; 1998. pp. 115–140. [Google Scholar]
- 77.Sara SJ, Roullet P, Przybyslawski J. Consolidation of memory for odor-reward association: β-adrenergic receptor involvement in late phase. Learning and Memory. 1999;6:88–96. [PMC free article] [PubMed] [Google Scholar]
- 78.Morris RGM. Pavlovian conditioned inhibition of fear during shuttlebox avoidance behavior. Learn Motiv. 1974;5:424–447. [Google Scholar]
- 79.Bolles RC. Avoidance and escape learning: simultaneous acquisition of different responses. J Comp Physiol Psychol. 1969;68(3):355–358. doi: 10.1037/h0027536. [DOI] [PubMed] [Google Scholar]
- 80.Matzel LD, Townsend DA, Grossman H, Han YR, Hale G, Zappulla M, Light K, Kolata S. Exploration in outbred mice covaries with general learning abilities irrespective of stress reactivity, emotionality, and physical attributes. Neurobiol Learn Mem. 2006;86(2):228–40. doi: 10.1016/j.nlm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 81.Kolata S, Wu J, Light K, Schachner M, Matzel LD. Impaired working memory duration but normal learning abilities found in mice that are conditionally deficient in the close homolog of L1. J Neurosci. 2008;28(50):13505–13510. doi: 10.1523/JNEUROSCI.2127-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blanchard RJ, Wall PM, Blanchard DC. Problems in the study of rodent aggression. Horm Behav. 2003;44(3):161–170. doi: 10.1016/s0018-506x(03)00127-2. [DOI] [PubMed] [Google Scholar]
- 83.Benton D. Is the concept of dominance useful in understanding rodent behavior? Aggressive Behavior. 1982;8(2):104– 107. [Google Scholar]
- 84.Uhrich J. The social hierarchy in albino mice. Journal of Comparative Psychology. 1938;25(2):373–413. [Google Scholar]
- 85.Brain PF, Hui SE. Variability in patterns of intra-specific biting attack in commonly used genetic lines of laboratory mice. Scand J Lab Anim Sci. 2003;3(30):113–127. [Google Scholar]
- 86.Matzel LD, Townsend DA, Grossman H, Han YR, Hale G, Zappulla M, Light K, Kolata S. Exploration in outbred mice covaries with general learning abilities irrespective of stress reactivity, emotionality, and physical attributes. Neurobiol Learn Mem. 2006;86(2):228–40. doi: 10.1016/j.nlm.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 87.Grossman HC, Hale G, Light K, Kolata S, Townsend DA, Goldfarb Y, Kusnecov A, Matzel LD. Pharmacological modulation of stress reactivity dissociates general learning ability from the propensity for exploration. Behav Neurosci. 2007;121(5):949–64. doi: 10.1037/0735-7044.121.5.949. [DOI] [PubMed] [Google Scholar]
- 88.Light KR, Kolata S, Hale G, Grossman H, Matzel LD. Up-regulation of exploratory tendencies does not enhance general learning abilities in juvenile or young-adult outbred mice. Neurobiol Learn Mem. 2008;90(2):317–29. doi: 10.1016/j.nlm.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 89.Montgomery KC. The relation between fear induced by novel stimulation and exploratory drive. Journal of Comparative and Physiological Psychology. 1955;48(4):192–194. doi: 10.1037/h0043788. [DOI] [PubMed] [Google Scholar]
- 90.Whimbey AE, Denenberg VH. Two independent behavioral dimensions in open field performance. Journal of Comparative and Physiological Psychology. 1967;63(3):500–504. doi: 10.1037/h0024620. [DOI] [PubMed] [Google Scholar]
- 91.Von Frijtag JC, Kamal A, Reijmers LG, Schrama LH, van den Bos R, Spruijt BM. Chronic imipramine treatment partially reverses the long-term changes of hippocampal synaptic plasticity in socially stressed rats. Neurosci Lett. 2001;309(3):153–156. doi: 10.1016/s0304-3940(01)02062-6. [DOI] [PubMed] [Google Scholar]
- 92.Magariños AM, McEwen BS, Flügge G, Fuchs E. Chronic psychosocial stress causes apical dendritic atrophy of hippocampal CA3 pyramidal neurons in subordinate tree shrews. J Neurosci. 1996;16(10):3534–40. doi: 10.1523/JNEUROSCI.16-10-03534.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.McKittrick CR, Magariños AM, Blanchard DC, Blanchard RJ, McEwen BS, Sakai RR. Chronic social stress reduces dendritic arbors in CA3 of hippocampus and decreases binding to serotonin transporter sites. Synapse. 2000;36(2):85–94. doi: 10.1002/(SICI)1098-2396(200005)36:2<85::AID-SYN1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 94.Czéh B, Michaelis T, Watanabe T, Frahms J, De Biurrun G, Van Kampen M, Bartolomucci A, Fuchs E. Stress-induced changes in cerebral metabolites, hippocampal volume, and cell proliferation are prevented by antidepressant treatment with tianeptine. Proc Natl Acad Sci U S A. 2001;98(22):12796–12801. doi: 10.1073/pnas.211427898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gould E, Tanapat P, McEwen BS, Flügge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA. 1998;17;95(6):3168–71. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Matzel LD, Light KR, Wass C, Colas-Zelin D, Denman-Brice A, Waddel AC, Kolata S. Longitudinal attentional engagement rescues mice from age-related cognitive declines and cognitive inflexibility. Learn Mem. 2011 Apr 26;18(5):345–56. doi: 10.1101/lm.2034711. [DOI] [PMC free article] [PubMed] [Google Scholar]