Abstract
Objective
Examine temporal alterations in vascular angiotensin II (ANG II) receptors (AT1R and AT2R) and determine vascular response to ANG II in growth-restricted offspring.
Study design
Offspring of pregnant rats fed low-protein (6%) and control (20%) diet were compared.
Results
Prenatal protein restriction reprogrammed AT1aR mRNA expression in males’ mesenteric arteries to cause 1.7- and 2.3-fold increases at 3 and 6 months of age associated with arterial pressure increases of 10 and 33 mmHg, respectively; however, in females, increased AT1aR expression (2-fold) and arterial pressure (15 mmHg) occurred only at 6 months. Prenatal protein restriction did not affect AT2R expression. Losartan abolished hypertension, suggesting that AT1aR plays a primary role in arterial pressure elevation. Vasoconstriction to ANG II was exaggerated in all protein-restricted offspring, with greater potency and efficacy in males.
Conclusion
Prenatal protein restriction increased vascular AT1R expression and vasoconstriction to ANG II, possibly contributing to programmed hypertension.
Keywords: angiotensin, fetal programming, hypertension, intrauterine growth restriction, rats, vascular
INTRODUCTION
Perturbation of nutrition during critical periods of fetal growth induces long-term structural and functional defects in the developing organism, predisposing it to increased risk for development of hypertension and cardiovascular disease later in life.1 Animal studies support the concept of developmental programming of hypertension,2–6 although the mechanisms are still not completely understood.
Evidence suggests that the renin angiotensin system (RAS), a regulatory system important in the long-term control of blood pressure, is altered in the offspring of protein-restricted dams and may contribute to the etiology of programmed hypertension. The blocking of angiotensin II (ANG II) formation with angiotensin converting enzyme blocker7 or inhibition of ANG II action at ANG II type-1 receptor (AT1R) with antagonist8 prevents elevation of blood pressure in the offspring of protein-restricted dams. RAS components are highly expressed in the brain9 and kidneys,10 and numerous investigators have associated the changes in central and intrarenal RAS to increases in arterial pressure.9;11 Increased expression of AT1R is observed in cardiovascular regulating regions of the brain in adult male protein-restricted offspring. Further, intracerebroventricular injection of the ACE inhibitor enalaprilat or of the AT1R antagonist losartan is shown to normalize blood pressure in the offspring of protein-restricted dams.12 Offspring of rat dams fed a low-protein diet during gestation display significantly altered mRNA and protein levels of AT1R and AT2R in kidneys.13;14 Renal AT1R and AT2R receptor protein expression was significantly lower in prenatal protein-restricted rats from fetal day 18 to postnatal day 10.15 In 4-week-old protein-restricted offspring, the renal AT1R protein level was greater.16;17 In adult 16-week-old protein-restricted rats, AT1R protein was upregulated while AT2R protein was downregulated, 15 suggesting that the ontogeny of the intrarenal RAS is altered throughout the perinatal and early postnatal period and through adult life.14 Vehaskari and colleagues reported that plasma renin activity is increased in prenatally protein-restricted adult rats, suggesting inappropriate activation of the peripheral RAS.14;18 Circulating ANG II, in addition to acting at the central and intrarenal levels, can impact the vascular system to increase blood pressure. However, information on expression of vascular AT1R and AT2R and the functional effect of ANG II in the resistance mesenteric arteries, the major determinant of systemic blood pressure in prenatally protein-restricted adult rats, is lacking.
Studies examining the role of RAS in hypertension induced by maternal protein restriction are focused in males, as females were found to be relatively protected.19 Since cardiovascular dysfunction in women is rapidly increasing, mechanistic studies in females, as well as males, are critical to understanding the gender differences in the pathogenesis of hypertension. We use a rat model of maternal dietary protein restriction (6% protein diet compared to 20% in controls) that leads to hypertension in both sexes, with more pronounced effects in adult males than females,3;5;6 similar to effects observed in population settings. Therefore, the purpose of this study was to examine the male and female offspring of control and protein-restricted groups to determine 1) whether the expression of vascular AT1R and AT2R mRNA is altered in the resistance arteries and if they relate to onset and magnitude of hypertension and 2) whether peripheral vascular response (mesenteric arteries) to ANG II is altered in adult male and female protein-restricted offspring compared to respective control offspring.
MATERIALS AND METHODS
Animals
The animal protocol was approved by the Animal Care Committee of University of Texas Medical Branch and is in accordance with the National Institutes of Health guidelines (NIH Publication No. 85–23, revised 1996). Timed pregnant Sprague Dawley rats at gestational day 3 were purchased from Harlan Inc (Houston, TX). The rats were allocated to ad libitum isocaloric diet containing either 20% (control, n = 8) or 6% (protein-restricted group, n = 9) casein, as in our previous studies.3;5;6 After the delivery of pups, dams were returned to regular chow. Pups were weaned at 3 weeks of age to regular chow, and males and females were housed separately and examined at 1, 3, and 6 months of age for arterial pressure, mesenteric vascular reactivity and expression of ANG II receptors (AT1aR and AT2R). At each time period, blood pressure was measured and the animals were sacrificed, mesenteric arteries were collected, RNA was isolated, and expression of ANG II receptors was determined. In the 6-month-old offspring, a portion of the mesenteric arteries was separated for vascular reactivity studies, and the remaining was quickly frozen for RNA isolation. Unless specified otherwise, one animal per litter in each sex was used for the different studies.
Experimental Procedures
Mean Arterial Pressure
Mean arterial pressure in conscious free-moving male and female offspring of control and protein-restricted dams was determined at 1, 3, and 6 months of age using indwelling carotid arterial catheters as described in our previous publications.6 Briefly, rats under anesthesia (ketamine—45 mg/kg; xylazine—5 mg/kg; Burns Veterinary Supply, Westbury, NY) were surgically instrumented with flexible catheters (PE 50 tubing) in the left carotid artery. The catheters were tunneled to the nape of the neck and exteriorized. After 24-hour recovery period, when the animals are fully conscious and in free moving state, arterial catheter was connected to a pressure transducer and arterial blood pressure was obtained using a data acquisition system (DBP001 direct BP system and Workbench for Windows software; both from Kent Scientific, Litchfield, CT). Following a 30-minute stabilization period, the arterial pressure was monitored continuously for 30 minutes and averaged to determine the baseline values.
Losartan Treatment
Another set of male and female offspring at 6 months of age were gavaged with AT1R antagonist losartan (20 mg·kg−1·day−1)20 for 1 week. Following losartan treatment, changes in arterial pressure were recorded as above using an indwelling arterial catheter.
Quantitative Real-time (qRT)-PCR
Immediately following the measurements of baseline blood pressure the whole mesenteric arteries were collected from 1-, 3-, and 6-month-old animals. Mesenteric arteries were instantly frozen in liquid nitrogen and then processed for the total RNA extraction (TRIZOL, Invitrogen, Carlsbad, CA). All RNA isolates were made DNA free by treatment with DNAse and further purified with RNeasy clean up kit (QIAGEN Inc, Valencia, CA). Total RNA concentration and integrity were determined using an ND-1000 Nanodrop spectrophotometer (Thermo Fisher Scientific, Newark, DE). One microgram of total RNA was reverse transcribed using a modified Maloney murine leukemia virus-derived RT (New England Biolabs Inc, Ipswich, MA) and a blend of oligo (dT) and random hexamer primers (Invitrogen). The reaction was carried out at 28°C for 15 minutes and 42°C for 50 minutes, then stopped by heating at 94°C for 5 minutes followed by 4°C before storage at −20°C until further analysis. One microliter of the diluted cDNA corresponding to 100 ng RNA was amplified by real-time PCR using FAM (Invitrogen) as the fluorophore in a CFX96 real-time thermal cycler (Bio-Rad, Hercules, CA). PCR conditions used were 2 minutes at 50°C for 1 cycle; 10 minutes at 95°C, 15 seconds at 95°C, and 1 minute at 60°C for 40 cycles; and a final dissociation step (0.05 seconds at 65°C and 0.5 seconds at 95°C). Results were calculated using the 2−ΔΔCT method and expressed in folds increase/decrease of the gene of interest in protein-restricted vs. control rats. All reactions were performed in duplicate, and 18S was used as an internal control. The following TaqMan assays were done in 10 μl volume for real-time PCR at a final concentration of 250nM TaqMan probe and 900nM of each primer; Assays-on-Demand for AT1aR (Rn01435427_m1), AT1bR (Rn02132799_s1), and AT2R (Rn00560677_s1) were obtained from Applied Biosystems.
Ex Vivo Vascular Reactivity Studies
Freshly excised third-order mesenteric arteries from 6-month-old male and female offspring were placed in ice cold modified Krebs bicarbonate solution (KBS) of the following composition (in mM): 118 NaCl, 4.7 KCl, 25 NaHCO3, 2.5 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, and 11 dextrose. The mesenteric arteries were cleaned of adherent connective tissue and precisely cut into rings of same length (2 mm). Two to four rings from 1 rat were used for 1 experiment. The n presented with each figure represents the number of animals studied. In the presence of endothelium, ANG II does not elicit a stable contraction ex vivo, presumably due to the AT2 receptor-mediated vasodilatory effects. Hence, studies were done in endothelium-denuded vessels. Further, our study shows that AT2 receptor expression in the mesenteric arteries was unchanged, and endothelium denudation will help focus on the role of AT1 receptors. Furthermore, we have previously shown that protein-restricted offspring have endothelial dysfunction5; hence, studies were done in endothelium-denuded arterial rings to avoid confounding and to assess the AT1 receptor-mediated effects on the smooth muscle. Endothelium was denuded by gently rubbing with tungsten wires. Two 25-μm tunsten wires were threaded through the lumen, and the rings were mounted in an isometric wire myograph system (model 610M wire myography; Danish Myotechniques, Aarhaus, Denmark). The rings were bathed in 6 mL KBS, gassed with 95% oxygen and 5% carbon dioxide, maintained at a temperature of 37°C, and allowed to equilibrate for 30 minutes before normalization to an internal diameter of 0.9 of L13.3kPa by using a normalization software package (Myodata; Danish Myotechnologies). This corresponds to a transmural pressure of ~90 mmHg.
Following normalization, rings were repeatedly exposed to KCl (80mM) to test their viability and to determine a standard contractile response for each of them. The rings were contracted with phenylephrine (3μM; Sigma, St. Louis, MO), and when responses were stable, endothelium-denudation was confirmed by absence of relaxation to acetylcholine (10μM; Sigma). Rings were then allowed to recover for 60 minutes, after which cumulative concentration-response curves were generated with ANG II (10−13 to 10−8M; Sigma) in the presence and absence of losartan (10μM; AT1R antagonist; Sigma). Antagonists were added to the bath 30 minutes before cumulative concentration-response curves. Cumulative concentration-response curves were also generated with phenylephrine (10−9 to 10−5M) and serotonin (10−9 to 10−5M; Sigma).
Statistical Analysis
For the comparison of arterial pressure, analysis was performed using ANOVA, with adjustments for multiple comparisons. For comparison of genes expressed between the control and protein-restricted groups, unpaired Student t test was used. Cumulative concentration-response curves were analyzed by computer fitting to a 4-parameter sigmoid curve using the Prism 5 program (GraphPad, San Diego, CA) to evaluate the half-maximal effective concentration (EC50) and Emax, the maximum asymptote of the curve. All values are expressed as means ± SE. A P < 0.05 was considered significant.
RESULTS
Mean Arterial Pressure
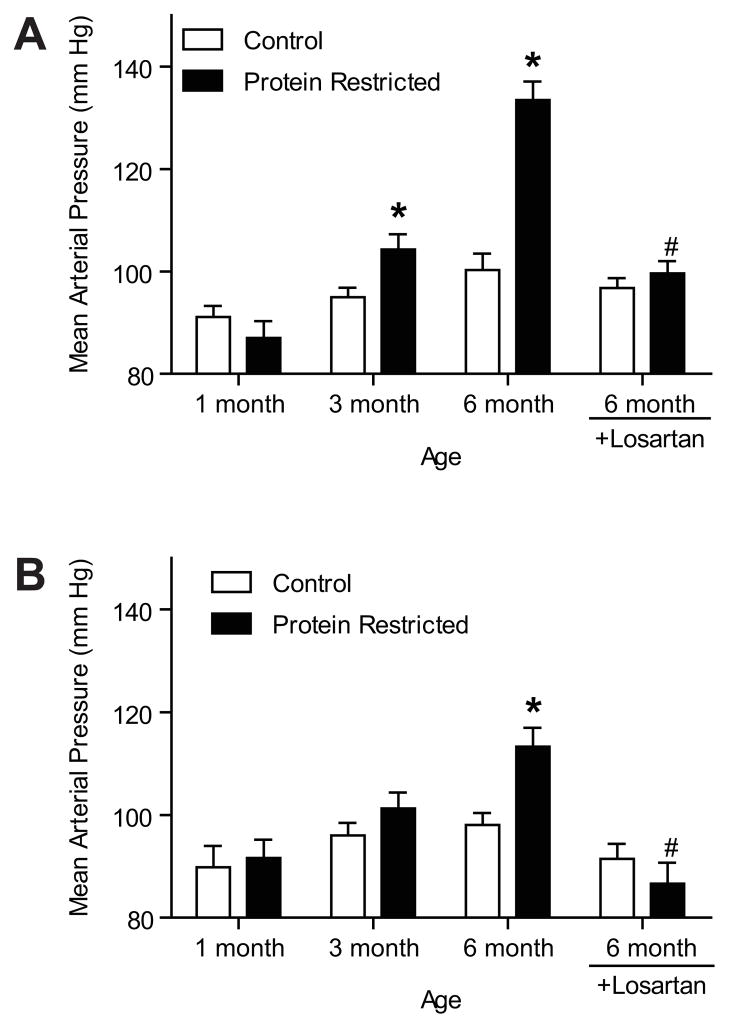
Mean arterial pressure measured in conscious free-moving rats via indwelling carotid catheter was comparable between control and protein-restricted males at 1 month of age, but protein-restricted males had higher arterial pressure than controls at 3 months (10 mmHg higher; n=8; P < 0.05) and 6 months of age (33 mmHg higher; n=8; P < 0.05; Figure 1A). In the female offspring, arterial pressure was similar between control and protein-restricted groups at 1 and 3 month of age, but protein-restricted females had significantly higher arterial pressure at 6 months of age (15 mmHg higher; n=8; P < 0.05; Figure 1B). Changes in both systolic and diastolic blood pressures were similar to that in mean arterial blood pressure; therefore, this data is not presented to reduced redundancy.
Figure 1.
Temporal changes in mean arterial pressure in male (A) and female (B) offspring of control and low-protein fed dams. Mean arterial pressure was measured in conscious free-moving rats through carotid arterial catheters at 1, 3, and 6 months of age (n=8 in each group). At 6 months of age, control and protein-restricted offspring were treated with vehicle or losartan (20 m·kg−1·day−1, n=5 in each group) by gavage for 1 week, and then mean arterial pressure was recorded. *P < 0.05 vs. respective control. #P < 0.05 vs. untreated 6-month-old offspring.
Effect of AT1R Blockade on Mean Arterial Pressure
Losartan treatment significantly decreased the blood pressure in protein-restricted male (mean decrease of 34 mmHg; n=5; P < 0.05; Figure 1A) and female (mean decrease of 27 mmHg; n=8; P < 0.05; Figure 1B) offspring. Mean arterial pressure did not significantly differ in losartan-treated control male and female offspring (n=5 each; Figure 1).
Changes in mRNA of AT1 and AT2 Receptors in the Mesenteric Artery
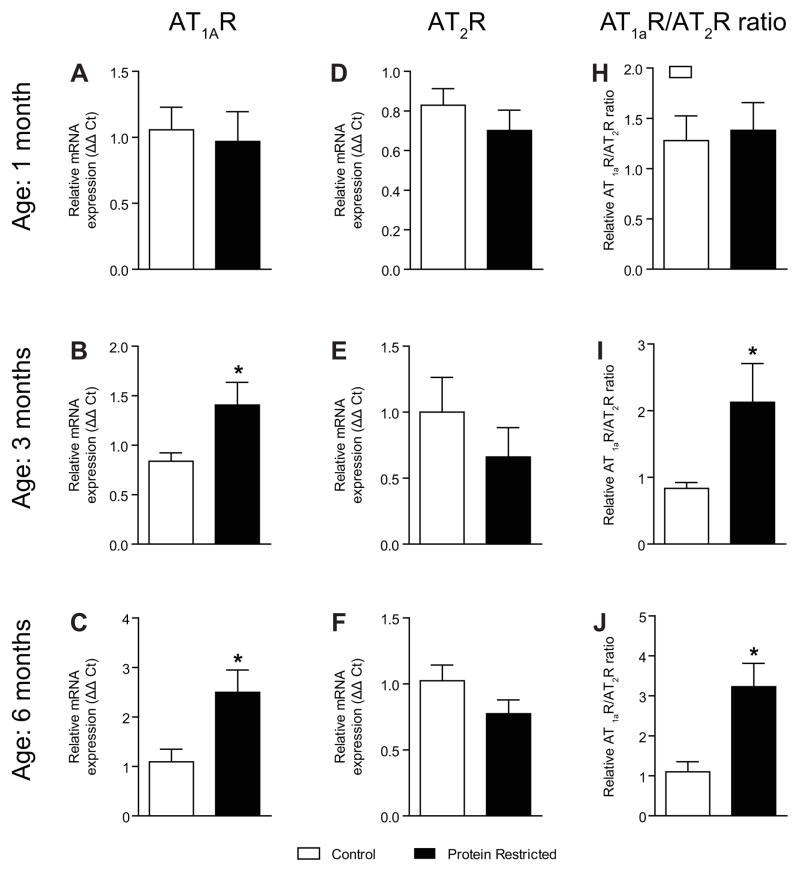
At the mRNA level, rodents possess 2 AT1 receptor isoforms, designated AT1aR and AT1bR. AT1bR was undetectable in the rat mesenteric arteries similar to previous studies.21 The mesenteric vascular expression of AT1aR mRNA was comparable between control and protein-restricted males at 1 month of age (n=6 in each group; Figure 2A). Expression of AT1aR mRNA in the mesenteric arteries was significantly increased by 1.7-fold in protein-restricted male offspring (n=7) relative to control male offspring (n=8; P < 0.05; Figure 2B) at 3 months. At 6 months of age, a 2.3-fold increase in expression of AT1aR in mesenteric arteries was observed in protein-restricted male vs. control male offspring (n=8 in each group; P < 0.05; Figure 2C). AT2R mRNA expression in the mesenteric arteries was similar between control and protein-restricted males at 1 (n=8; Figure 2D), 3 (Figure 2E), and 6 months of age (Figure 2F). In protein-restricted males, the changes in vascular AT1aR/AT2R ratio followed a trend similar to the changes in AT1aR. The AT1R/AT2R ratio in protein-restricted males was similar to controls at 1 month of age (n=7 in each group; Figure 2G) but significantly increased by 2.1-fold and 3.2-fold at 3 (n=6; P < 0.05; Fig. 2H) and 6 months of age (n=6; P < 0.05; Figure 2I), respectively.
Figure 2.
Temporal changes in vascular angiotensin receptors in male control and protein-restricted offspring. Real-time PCR was used to assess vascular AT1aR mRNA expression at 1 month of age (A), 3 months of age (B), and 6 months of age (C) and vascular AT2R mRNA expression of in 1 month of age (D), 3 month of age (E), and 6 month of age (F). Quantitation of vascular RAS components was normalized relative to 18S levels. The ratio of AT1aR/AT2R at 1 month of age (G), 3 months of age (H), and 6 months of age (I) is presented. n=6–8 in each group.*P < 0.05 vs. control.
Figure 3.
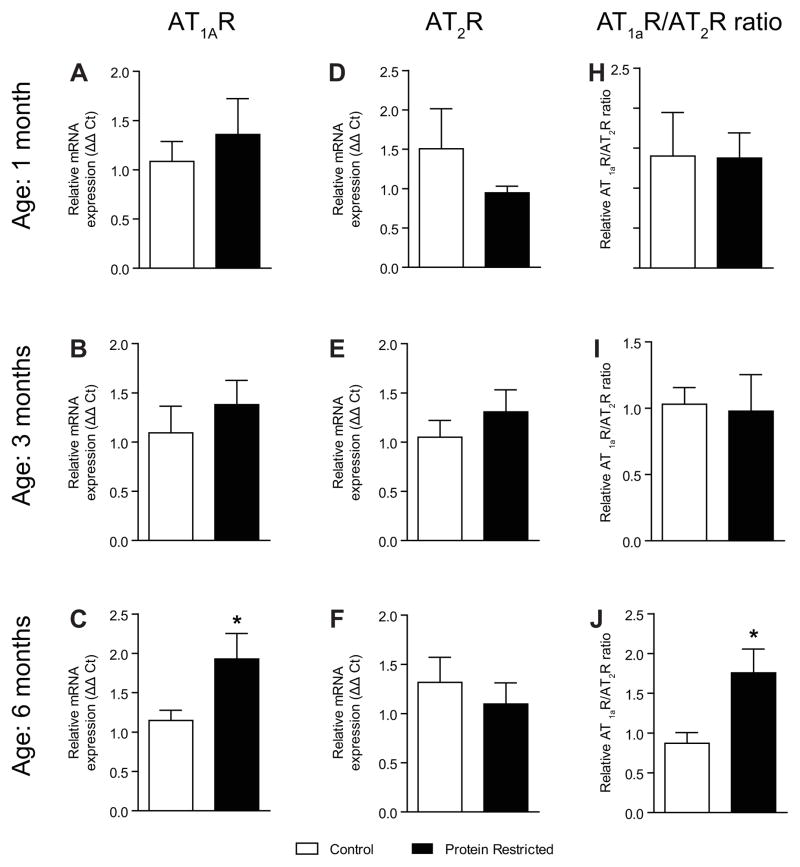
Temporal changes in vascular angiotensin receptors in female control and protein-restricted offspring. Real-time PCR was used to assess vascular AT1aR mRNA expression at 1 month of age (A), 3 months of age (B), and 6 months of age (C) and vascular AT2R mRNA expression of in 1 month of age (D), 3 months of age (E), and 6 months of age (F). Quantitation of vascular RAS components was normalized relative to 18S levels. The ratio of AT1aR/AT2R at 1 month of age (G), 3 months of age (H), and 6 months of age (I) is presented. n=5–8 in each group.*P < 0.05 vs. control.
In females, the mesenteric vascular expression of AT1aR mRNA was comparable between control and protein-restricted females at 1 and 3 months of age (n=8; Figures 3A and B), but protein-restricted females have 2-fold higher AT1aR mRNA expression at 6 months of age (n=5; P < 0.05; Figure 3C). AT2R mRNA was similar between control and protein-restricted females at 1, 3, and 6 months of age (n=6 in each group; Figures 3D, E, and F). The vascular AT1R/AT2R ratio was comparable between control and protein-restricted females at 1 and 3 months of age (Figures 3G and H) but was significantly higher by 2.0-fold at 6 months of age in protein-restricted females compared with control females (n=5; P < 0.05; Figure 3I).
Ex Vivo Vasomotor Responses
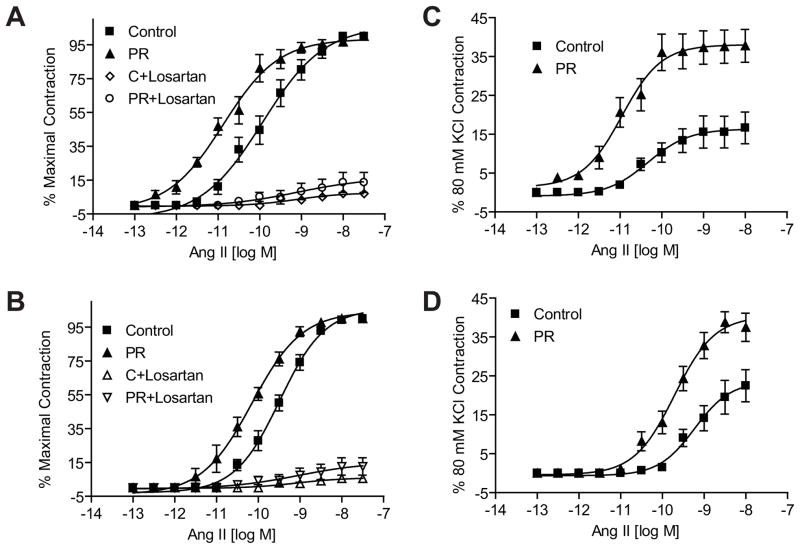
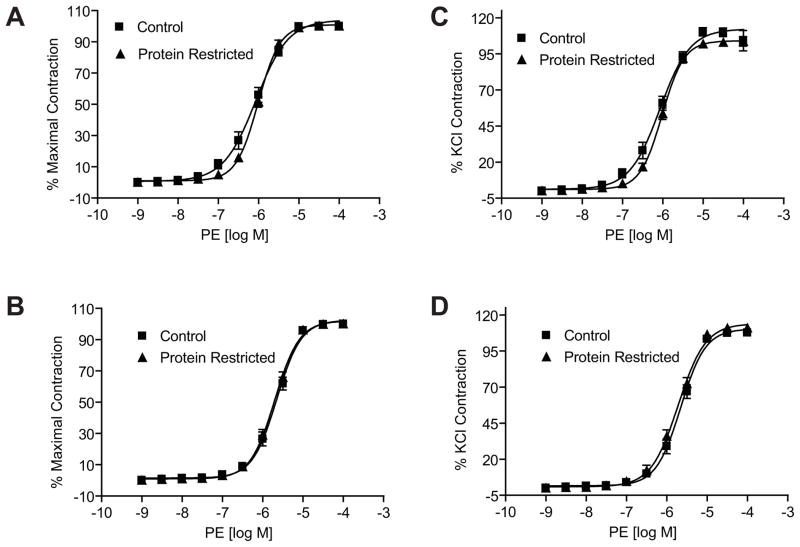
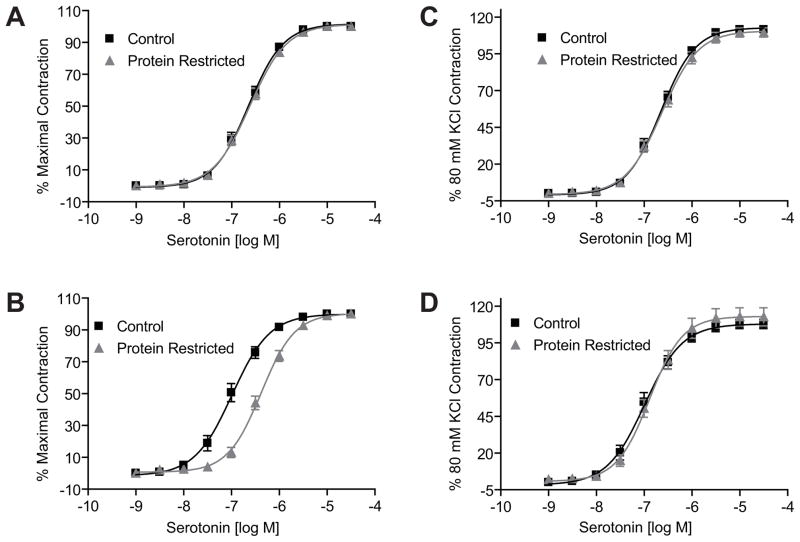
ANG II induced a dose-dependent increase in contractile responses in mesenteric arterial rings in both males and females. However, the ANG II-induced contractile responses were exaggerated with a leftward shift in the dose-response curves as well as an increase in maximal responses in the protein-restricted compared to control rats. The magnitude of leftward shift, compared to respective controls, was greater in the protein-restricted males (n=5; P < 0.05; Figure 4A and Table 1) than protein-restricted females (Figure 4B and Table 1). Similarly, the ANG II-induced maximal responses were greater in the protein-restricted males (n=5; P < 0.05; Figure 4C and Table 1) than protein-restricted females (n=5; P < 0.05; Figure 4D and Table 1). Pretreatment of the vascular rings with losartan inhibited the ANG II-induced vasoconstriction (n=5; P < 0.05; Figures 4A and B). On the other hand, contractile responses to phenylephrine (n=5 in each group; Figure 5 and Table 1) and serotonin (n=5 in each group; Figure 6 and Table 1) were not increased in protein-restricted males and females compared to respective controls. Indeed, the contractile response to serotonin was reduced in protein-restricted females compared to control females (n=5 in each group; P < 0.05; Figure 6B and Table 1).
Figure 4.
Angiotensin II-induced contraction in mesenteric arterial rings of control and protein-restricted offspring. Endothelium-denuded mesenteric arterial rings were incubated in Krebs buffer and then stimulated with increasing concentrations of angiotesin II. Angiotensin II contraction was measured and presented as percentage of maximal contraction in the male (A) and female (B) offspring. ANG II-induced contractions were also calculated as percentage of 80mM KCl contractions in males (C) and females (D). n= 10 to 12 mesenteric arterial rings from 5 to 6 rats of each group. *P<0.05 compared with control offspring. PR, protein restricted.
Table 1.
Vasomotor responses in offspring of control and protein-restricted groups at 6 months of age.
| Vasoconstrictor Agent | Gender | ED50 (expressed in M) | Emax (expressed as %) | ||
|---|---|---|---|---|---|
| Control | PR | Control | PR | ||
| Ang II | Male | 1.16 × 10−10 | 13.2 ×10−10* | 16.64 ± 4.09 | 38.16 ± 4.08* |
| Female | 3.24 ×10−10 | 70.6 ×10−10* | 22.48 ± 4.11 | 37.47 ± 3.63* | |
| Phenylephrine | Male | 8.53 ×10−7 | 9.71 ×10−7 | 104.16 ± 7.06 | 103.65 ± 3.31 |
| Female | 2.21 ×10−6 | 1.99 ×10−6 | 107.65 ± 1.27 | 110.95 ± 1.62 | |
| Serotonin | Male | 2.26 ×10−7 | 2.42 ×10−7 | 111.52 ± 1.30 | 109.51 ± 3.04 |
| Female | 1.03 ×10−7 | 4.18 ×10−7* | 107.81 ± 3.16 | 112.67 ± 6.17 | |
Abbreviation: PR, protein restricted.
P < 0.05 compared to respective control
Figure 5.
Phenyleprine-induced contraction in mesenteric arterial rings of control and protein-restricted offspring. Endothelium-denuded mesenteric arterial rings were incubated in Krebs buffer and then stimulated with increasing concentrations of phenyleprine. Phenylephrine contraction was measured and presented as percentage of maximal contraction in the male (A) and female (B) offspring. Phenylephrine-induced contractions were also calculated as percentage of 80mM KCl contraction in males (C) and females (D). n=10 to 12 mesenteric arterial rings from 5 to 6 rats of each group.
Figure 6.
Serotonin-induced contraction in mesenteric arterial rings of control and protein-restricted offspring. Endothelium-denuded mesenteric arterial rings were incubated in Krebs buffer and then stimulated with increasing concentrations of serotonin. Serotonin contraction was measured and presented as percentage of maximal contraction in the males (A) and female (B) offspring. Serotonin-induced contractions were also calculated as percentage of 80mM KCl contraction in male (C) and females (D). n=10 to 12 mesenteric arterial rings from 5 to 6 rats of each group.
DISCUSSION
For the first time, we show that prenatal protein restriction causes a temporal increase in mesenteric vascular AT1aR mRNA transcripts with little or no change in AT2R mRNA transcripts relating to the onset and magnitude of hypertension in males and females. Acute blockade of AT1R with losartan abolished hypertension in adult protein-restricted offspring of both sexes, suggesting that RAS is a cause and not a consequence of hypertension development. The increased AT1aR expression in the mesenteric arteries was associated with exaggerated vascular contractile responses in a sex- and agonist-specific manner. The mesenteric vasomotor response mediated by ANG II was greater in males than females, and responses to other vasoconstrictors, phenylephrine and serotonin, are not increased in protein-restricted compared to control offspring. Therefore we suggest that increases in vascular AT1R and ANG II stimulated responses may mediate the onset and maintenance of hypertension in the offspring that are exposed to prenatal protein restriction.
It is now well established that a variety of insults, when experienced in the prenatal period, can have long-term influences on the health of the individual. Factors, such as maternal undernutrition 22 and placental insufficiency, 23 that lead to impaired fetal growth are known to cause hypertension and other cardiovascular abnormalities in the offspring. In many animal models, the males and females are not equally affected; usually, males experience more profound effects.24;25 In the present study, the onset and magnitude of hypertension is different between male and female offspring of protein-restricted dams, with males having an earlier onset (3 months vs 6 months in females) and more pronounced effect (mean increase of 33.2 mmHg in males vs 15.2 mmHg in females at 6 months). This suggests that the prenatally protein-restricted females are less susceptible; hypertension is less severe and occurs later than in males. This is consistent with our previous report of systolic blood pressure measurements (tail-cuff) in prenatally protein-restricted animals.3 The finding that prenatal protein restriction predominantly affected male offspring is in agreement with those reported in the Wistar rat model of nutrition restriction26 and other models of programming induced by placental insufficiency.24 Recently, we have demonstrated that in female prenatally protein-restricted rats, ovariectomy caused increases in arterial pressure to the levels similar to that in males,6 suggesting that estrogens may contribute to gender differences in BP regulation.
Inappropriate activation of the peripheral RAS, noted by a marked increase in plasma renin activity, is observed in conjunction with established hypertension in the prenatally protein-restricted adult rats.14;18 ANG II, the main effector of RAS, acts through AT1R and AT2R. In the present study, we show that the expression of AT1aR in the mesenteric arteries of prenatally protein-restricted offspring increases with age and also varies depending on the gender of rats. In male rats, AT1aR upregulation is observed as early as 3 months of age, with a more pronounced increase at 6 months of age. In females, a significant increase in AT1aR is observed only at 6 months of age. Interestingly, the increase in AT1aR expression mirrored the blood pressure changes observed in these male and female protein-restricted offspring. Although AT1R exists as AT1aR and AT1bR in rodents, AT1aR is known to mediate most of the well-known effects of ANG II including vasoconstriction and hypertension.27 AT1bR is reported to be undetectable in vascular smooth muscle cells,21 similar to what was observed in this study. Actions of the AT2R are less clear, but seem to counterbalance some of the actions of the AT1R leading to vasodilation. Studies indicate that the AT1aR/AT2R ratio plays a crucial role in the development of the hypertensive phenotype,28 and the vascular AT1R/AT2R ratio relates to the magnitude of blood pressure elevation observed in spontaneously hypertensive rats. 28 Since AT2R expression in this study was unaltered in protein-restricted male and female offspring, the ratio of AT1aR/AT2R followed a similar pattern as that of AT1aR. The females’ subtle and delayed increase in AT1aR expression or AT1aR/AT2R ratio relative to male protein-restricted offspring is also similar to that observed in female and male spontaneously hypertensive rats,28 and the presence of estrogens in the females is proposed to attenuate AT1R29 and promote AT2R expression.30 Thus, the present study demonstrates that prenatal protein restriction causes a gender-specific reprogramming of the AT1R expression pattern, resulting in significantly increased arterial AT1R levels that relate to the onset and magnitude of hypertension in prenatally protein-restricted animals.
In this study, losartan abolished hypertension induced by prenatal protein restriction in adult male and female rats, suggesting that RAS is the primary factor in the development of hypertension in rats exposed to protein restriction during prenatal life. Previous studies show that RAS blockade at an early age (2 to 4 week of age) by use of the angiotensin converting enzyme inhibitor7 or by AT1R blocker16 prevented development of hypertension in adult prenatally protein-restricted rats. This supports a role for RAS in both the development and maintenance of established hypertension induced by prenatal protein restriction.
Consistent with increases in mesenteric vascular AT1aR expression, other studies also show AT1R increase in kidneys. To dissect out the contribution of the vasculature to contractile responses and to blood pressure increase, we examined vascular reactivity to ANG II. In the present study, ANG II-induced contractile responses were significantly increased in prenatally protein-restricted male and female offspring. The ED50 and Emax were greater in the male than female offspring in proportion to the AT1aR expression levels and blood pressure increases. The inhibition of ANG II-induced arterial contractions by losartan in both control and prenatally protein-restricted animals indicated a primary role of AT1R in vasoconstrictions. Increased vasomotor response to ANG II can be observed due to the presence of vascular remodeling. However, previous reports show no difference in lumen diameter, media cross-sectional area, media thickness, and media-to-lumen ratio of the mesenteric arteries of adult males subjected to prenatal protein restriction.31;32 Because prenatal protein restriction caused increases in ANG II-induced contractions in the absence of functional endothelium, we suggest the enhanced arterial sensitivity to ANG II primarily occurred in the vascular smooth muscle cells. Other studies in prenatally protein-restricted rats have also reported exaggerated vasomotor responses to ANG II in other vascular beds like the aorta, femoral, and carotid arteries.26;33 Interestingly, the vasomotor response to other potent constrictors, such as phenylephrine and serotonin, was not enhanced in either male or female protein-restricted offspring. Other studies of developmentally programmed hypertension have also found no modification in Emax response to U-46619 or to phenylephrine in pial microvessels and mesenteric arteries.31;34 Thus, it is likely that the effect of fetal programming on vasoconstrictors is agonist dependent. In addition, these findings suggest that prenatal protein-restriction-mediated programming occurs at the agonist-specific level rather than at common intracellular signaling pathways. Intriguingly, the contractile responses to serotonin were reduced in the prenatally protein-restricted females, the reason for which is unclear.
Although the mRNA transcripts of AT2R were unaltered in both male and female protein-restricted offspring, we cannot completely exclude a role for AT2R in the resulting hypertension in this model of programmed hypertension. Future studies in male and female vessels are needed to dissect out the specific role of AT2R in contributing to increased vasoconstriction and blood pressure. In conclusion, temporal alterations in vascular AT1R expression or AT1R/AT2R ratio appear to correlate with the progression and degree of hypertension in the male and female offspring of protein-restricted dams. The exaggerated vasoconstriction in the protein-restricted male and female offspring appears to be specific to ANG II. This suggests that vascular RAS, in addition to central and intrarenal RAS, may play an important role in the development and maintenance of hypertension in intrauterine growth-restricted offspring.
Acknowledgments
Financial support: The findings reported in this manuscript result from the National Institutes of Health (NIH) grant numbers HL102866 and HL 58144.
Footnotes
Presentation at Meeting: Developmental Origins of Health and Diseases (DOHaD), Portland, Oregon, September 18–21, 2011
Disclosure: None of the authors has a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jackson AA. Nutrients, growth, and the development of programmed metabolic function. Adv Exp Med Biol. 2000;478:41–55. doi: 10.1007/0-306-46830-1_4. [DOI] [PubMed] [Google Scholar]
- 2.Bassan H, Trejo LL, Kariv N, Bassan M, Berger E, Fattal A, et al. Experimental intrauterine growth retardation alters renal development. Pediatr Nephrol. 2000;15(3–4):192–195. doi: 10.1007/s004670000457. [DOI] [PubMed] [Google Scholar]
- 3.Gangula PR, Reed L, Yallampalli C. Antihypertensive effects of flutamide in rats that are exposed to a low-protein diet in utero. Am J Obstet Gynecol. 2005;192(3):952–960. doi: 10.1016/j.ajog.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 4.Longo LD, Pearce WJ. Fetal cerebrovascular acclimatization responses to high-altitude, long-term hypoxia: a model for prenatal programming of adult disease? Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R16–R24. doi: 10.1152/ajpregu.00462.2004. [DOI] [PubMed] [Google Scholar]
- 5.Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein Restriction during Pregnancy Induces Hypertension and Impairs Endothelium-Dependent Vascular Function in Adult Female Offspring. J Vasc Res. 2008;46(3):229–239. doi: 10.1159/000166390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sathishkumar K, Elkins R, Yallampalli U, Yallampalli C. Protein restriction during pregnancy induces hypertension in adult female rat offspring - influence of oestradiol. Br J Nutr. 2011:1–9. doi: 10.1017/S0007114511003448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Langley-Evans SC, Jackson AA. Captopril normalises systolic blood pressure in rats with hypertension induced by fetal exposure to maternal low protein diets. Comp Biochem Physiol A Physiol. 1995;110(3):223–228. doi: 10.1016/0300-9629(94)00177-u. [DOI] [PubMed] [Google Scholar]
- 8.Sherman RC, Langley-Evans SC. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin Sci (Lond) 2000;98(3):269–275. [PubMed] [Google Scholar]
- 9.Mao C, Shi L, Xu F, Zhang L, Xu Z. Development of fetal brain renin-angiotensin system and hypertension programmed in fetal origins. Prog Neurobiol. 2009;87(4):252–263. doi: 10.1016/j.pneurobio.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guron G, Friberg P. An intact renin-angiotensin system is a prerequisite for normal renal development. J Hypertens. 2000;18(2):123–137. doi: 10.1097/00004872-200018020-00001. [DOI] [PubMed] [Google Scholar]
- 11.Hilgers KF. Genetic variation of the renin system--effects on blood pressure and the kidney. Kidney Blood Press Res. 2000;23(3–5):185–187. [PubMed] [Google Scholar]
- 12.Pladys P, Lahaie I, Cambonie G, Thibault G, Le NL, Abran D, et al. Role of brain and peripheral angiotensin II in hypertension and altered arterial baroreflex programmed during fetal life in rat. Pediatr Res. 2004;55(6):1042–1049. doi: 10.1203/01.PDR.0000127012.37315.36. [DOI] [PubMed] [Google Scholar]
- 13.McMullen S, Gardner DS, Langley-Evans SC. Prenatal programming of angiotensin II type 2 receptor expression in the rat. Br J Nutr. 2004;91(1):133–140. doi: 10.1079/bjn20031029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vehaskari VM, Stewart T, Lafont D, Soyez C, Seth D, Manning J. Kidney angiotensin and angiotensin receptor expression in prenatally programmed hypertension. Am J Physiol Renal Physiol. 2004;287(2):F262–F267. doi: 10.1152/ajprenal.00055.2004. [DOI] [PubMed] [Google Scholar]
- 15.Mesquita FF, Gontijo JA, Boer PA. Maternal undernutrition and the offspring kidney: from fetal to adult life. Braz J Med Biol Res. 2010;43(11):1010–1018. doi: 10.1590/s0100-879x2010007500113. [DOI] [PubMed] [Google Scholar]
- 16.Sahajpal V, Ashton N. Renal function and angiotensin AT1 receptor expression in young rats following intrauterine exposure to a maternal low-protein diet. Clin Sci (Lond) 2003;104(6):607–614. doi: 10.1042/CS20020355. [DOI] [PubMed] [Google Scholar]
- 17.Sahajpal V, Ashton N. Increased glomerular angiotensin II binding in rats exposed to a maternal low protein diet in utero. J Physiol. 2005;563(Pt 1):193–201. doi: 10.1113/jphysiol.2004.078642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vehaskari VM, Aviles DH, Manning J. Prenatal programming of adult hypertension in the rat. Kidney Int. 2001;59(1):238–245. doi: 10.1046/j.1523-1755.2001.00484.x. [DOI] [PubMed] [Google Scholar]
- 19.Woods LL, Ingelfinger JR, Rasch R. Modest maternal protein restriction fails to program adult hypertension in female rats. Am J Physiol Regul Integr Comp Physiol. 2005;289(4):R1131–R1136. doi: 10.1152/ajpregu.00037.2003. [DOI] [PubMed] [Google Scholar]
- 20.Kalk P, Sharkovska Y, Kashina E, von WK, Relle K, Pfab T, et al. Endothelin-converting enzyme/neutral endopeptidase inhibitor SLV338 prevents hypertensive cardiac remodeling in a blood pressure-independent manner. Hypertension. 2011;57(4):755–763. doi: 10.1161/HYPERTENSIONAHA.110.163972. [DOI] [PubMed] [Google Scholar]
- 21.Lee S, Ji H, Wu Z, Zheng W, Hassan A, Sandberg K. Translational regulation of ANG II type 1 receptors in proliferating vascular smooth muscle cells. Am J Physiol Regul Integr Comp Physiol. 2006;290(1):R50–R56. doi: 10.1152/ajpregu.00448.2005. [DOI] [PubMed] [Google Scholar]
- 22.McMullen S, Langley-Evans SC. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. Am J Physiol Regul Integr Comp Physiol. 2005;288(1):R85–R90. doi: 10.1152/ajpregu.00435.2004. [DOI] [PubMed] [Google Scholar]
- 23.Alexander BT. Placental insufficiency leads to development of hypertension in growth-restricted offspring. Hypertension. 2003;41(3):457–462. doi: 10.1161/01.HYP.0000053448.95913.3D. [DOI] [PubMed] [Google Scholar]
- 24.Grigore D, Ojeda NB, Alexander BT. Sex differences in the fetal programming of hypertension. Gend Med. 2008;5 (Suppl A):S121–S132. doi: 10.1016/j.genm.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilbert JS, Nijland MJ. Sex differences in the developmental origins of hypertension and cardiorenal disease. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R1941–R1952. doi: 10.1152/ajpregu.90724.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530(Pt 1):141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurley SB, Le TH, Coffman TM. Gene-targeting studies of the renin-angiotensin system: mechanisms of hypertension and cardiovascular disease. Cold Spring Harb Symp Quant Biol. 2002;67:451–457. doi: 10.1101/sqb.2002.67.451. [DOI] [PubMed] [Google Scholar]
- 28.Silva-Antonialli MM, Tostes RC, Fernandes L, Fior-Chadi DR, Akamine EH, Carvalho MH, et al. A lower ratio of AT1/AT2 receptors of angiotensin II is found in female than in male spontaneously hypertensive rats. Cardiovasc Res. 2004;62(3):587–593. doi: 10.1016/j.cardiores.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 29.Roesch DM, Tian Y, Zheng W, Shi M, Verbalis JG, Sandberg K. Estradiol attenuates angiotensin-induced aldosterone secretion in ovariectomized rats. Endocrinology. 2000;141(12):4629–4636. doi: 10.1210/endo.141.12.7822. [DOI] [PubMed] [Google Scholar]
- 30.Baiardi G, Macova M, Armando I, Ando H, Tyurmin D, Saavedra JM. Estrogen upregulates renal angiotensin II AT1 and AT2 receptors in the rat. Regul Pept. 2005;124(1–3):7–17. doi: 10.1016/j.regpep.2004.06.021. [DOI] [PubMed] [Google Scholar]
- 31.Brawley L, Itoh S, Torrens C, Barker A, Bertram C, Poston L, et al. Dietary protein restriction in pregnancy induces hypertension and vascular defects in rat male offspring. Pediatr Res. 2003;54(1):83–90. doi: 10.1203/01.PDR.0000065731.00639.02. [DOI] [PubMed] [Google Scholar]
- 32.Pladys P, Sennlaub F, Brault S, Checchin D, Lahaie I, Le NL, et al. Microvascular rarefaction and decreased angiogenesis in rats with fetal programming of hypertension associated with exposure to a low-protein diet in utero. Am J Physiol Regul Integr Comp Physiol. 2005;289(6):R1580–R1588. doi: 10.1152/ajpregu.00031.2005. [DOI] [PubMed] [Google Scholar]
- 33.Yzydorczyk C, Gobeil F, Jr, Cambonie G, Lahaie I, Le NL, Samarani S, et al. Exaggerated vasomotor response to ANG II in rats with fetal programming of hypertension associated with exposure to a low-protein diet during gestation. Am J Physiol Regul Integr Comp Physiol. 2006;291(4):R1060–R1068. doi: 10.1152/ajpregu.00798.2005. [DOI] [PubMed] [Google Scholar]
- 34.Lamireau D, Nuyt AM, Hou X, Bernier S, Beauchamp M, Gobeil F, Jr, et al. Altered vascular function in fetal programming of hypertension. Stroke. 2002;33(12):2992–2998. doi: 10.1161/01.str.0000039340.62995.f2. [DOI] [PubMed] [Google Scholar]