Abstract
Repeated stress impacts emotion, and can induce mood and anxiety disorders. These disorders are characterized by imbalance of emotional responses. The amygdala is fundamental in expression of emotion, and is hyperactive in many patients with mood or anxiety disorders. Stress also leads to hyperactivity of the amygdala in humans. In rodent studies, repeated stress causes hyperactivity of the amygdala, and increases fear conditioning behavior that is mediated by the basolateral amygdala (BLA). Calcium-activated potassium (KCa) channels regulate BLA neuronal activity, and evidence suggests reduced small conductance KCa (SK) channel function in male rats exposed to repeated stress. Pharmacological enhancement of SK channels reverses the BLA neuronal hyperexcitability caused by repeated stress. However, it is not known if pharmacological targeting of SK channels can repair the effects of repeated stress on amygdala-dependent behaviors. The purpose of this study was to test whether enhancement of SK channel function reverses the effects of repeated restraint on BLA-dependent auditory fear conditioning. We found that repeated restraint stress increased the expression of cued conditioned fear in male rats. However, 1-EBIO (1 or 10 mg/kg) or CyPPA (5 mg/kg) administered 30 minutes prior to testing of fear expression brought conditioned freezing to control levels, with little impact on fear expression in control handled rats. These results demonstrate that enhancement of SK channel function can reduce the abnormalities of BLA-dependent fear memory caused by repeated stress. Furthermore, this indicates that pharmacological targeting of SK channels may provide a novel target for alleviation of psychiatric symptoms associated with amygdala hyperactivity.
Keywords: fear conditioning, calcium-activated potassium channel, stress, 1-EBIO, amygdala-dependent behavior
1. Introduction
Repeated stress leads to long term changes in brain regions that contribute to mood and cognition [1]. In humans, repeated stress can predispose individuals to later psychiatric illness, and can precipitate the recurrence and exacerbation of episodes of mood disorders [2–4]. The amygdala contributes to expression and interpretation of affect and displays functional and anatomical changes in patients with mood disorders [5–8]. Stressors and trauma have also been demonstrated to lead to prolonged hyperactivation of the amygdala [9–13]. In rodents, a similar pattern has emerged, where repeated stress leads to abnormalities in behavioral measures of anxiety and fear. These changes are paralleled by evidence of morphological changes and hyperactivity in the amygdala [14–16].
Pavlovian fear conditioning requires the lateral nucleus (LAT) of the basolateral amygdala (BLA), and activity of the BLA reflects the progression and expression of fear-related behaviors during this form of conditioning [17–19]. Fear conditioning is enhanced by many treatments known to increase BLA excitability, and dampened by treatments that suppress BLA excitability. Of interest here, repeated stress has been demonstrated to increase fear conditioning (e.g. [20, 21]), as well as increase the excitability of BLA neurons [16, 22]. This increased excitability has been reported to be caused by a reduction in the impact of a calcium-activated potassium (KCa) current in BLA neurons [16]. This current mediates several different aspects of afterhyperpolarization potentials (AHPs) following action potentials [23, 24], and regulates the excitability of BLA neurons. However, despite the role for KCa channels in regulation of BLA excitability, and the finding that overexpression of small conductance KCa channel (SK channel) subunits that contribute to the AHP suppress fear conditioning [25, 26], previous studies have demonstrated that drugs that enhance the KCa current do not suppress fear conditioning [27]. One possibility for this dissociation is that the AHPs in BLA neurons in vivo are already fairly large [16, 28, 29], and therefore perhaps not strongly modulated by drugs that enhance the KCa current. However, in conditions that display suboptimal AHPs, such as following repeated stress, these drugs may be effective [16]. The purpose of this study was to test whether drugs that enhance the KCa current by targeting SK channels can decrease the enhanced fear memory that is caused by repeated stress, a condition associated with a diminished slow and medium AHPs (sAHP and mAHP). To test this we randomly assigned adult rats to control or repeated restraint groups, and trained them with a BLA-dependent cued fear conditioning procedure. The following day, rats were injected with vehicle or drugs that enhance KCa currents, and then their freezing to the conditioned cue was measured.
2. Materials and methods
2.1 Animals
All methods were approved by the Institutional Animal Care and Use Committee at Rosalind Franklin University of Medicine and Science, and followed the Guidelines for the Care and Use of Laboratory Animals (National Research Council). Adult male Sprague-Dawley rats (Harlan, Indianapolis, IN; 63–70 days postnatal at arrival) were habituated to the animal facility for one week before inclusion in the study. Rats were housed in a 12:12, on:off light-dark cycle, with free access to food and water.
2.2 Drugs
1-Ethyl-2-benzimidazolinone (1-EBIO, 1 or 10 mg/kg) was purchased from Ascent Scientific, Cambridge, MA. N-Cyclohexyl-N-[2-(3,5-dimethyl-pyrazol-1-yl)-6-methyl-4-pyrimidinamine (CyPPA, 5 mg/kg) was purchased from Tocris Bioscience, Minneapolis, MN. Drugs were suspended in 2% Tween 80/0.5% methylcellulose.
2.3 Repeated restraint
Following one week of habituation, rats underwent a repeated stress or control procedures. These procedures took place in a separate room with bright overhead fluorescent lighting and a distinct odor cue (Lemon-scented Lysol). The repeated stress consisted of daily placement into a clear Plexiglas transport cage to the procedure room followed by a 20 minute session of restraint in a hemi-cylinder restraint tube. This occurred for 7 out of 9 consecutive days [16]. Control rats were exposed to the same procedure, except they remained in their transport cage, and were not placed in a restraint cylinder.
2.4 Cued fear conditioning
Twenty-four hours following the final restraint, rats were brought to a different room with dim fluorescent overhead lights for the fear conditioning procedure. Rats were run through these experiments with control and stress groups as cohorts. Drug or vehicle injections were counterbalanced across days. This room contained behavioral chambers (Scientific Design, Pittsburgh, PA and Ugo Basile, Collegeville, PA) with distinct features, including wall patterns and flooring, enclosed in sound attenuating cabinets with distinct odor cues (dilute acetic acid or Simple Green).
Fear conditioning was performed under low light conditions, with background white noise from a fan (60–70 dB). Fear conditioning consisted of a 3 minute habituation period followed by four pairings of a tone (2 kHz, 20 s, 70 dB) with a co-terminating footshock (1 s, intensity that produced forepaw withdrawal in the rat, typically 0.3 – 0.6 mA, depending on the chamber). This pairing was repeated with a 120 s inter-trial interval. Rats remained in the chamber for 2 minutes following the final trial, and were then returned to their home cages. Memory of fear was tested the following day. Thirty minutes prior to fear expression testing, rats were injected with vehicle (2% Tween 80/0.5% methylcellulose) or 1-EBIO (1 or 10 mg/kg), or CyPPA (5 mg/kg). Both of these drugs increase the activity of KCa channels, and in particular small coductance (SK) channels [30–32]. All drugs were administered at 1 mL/kg, subcutaneously. The half-lifes of 1-EBIO and CyPPA are not currently published. The 30 minute latency was chosen based on the observed actions on BLA neurons in vivo [16] and on BLA-mediated behaviors [27]. For testing of the memory of fear conditioning, rats were placed in a chamber that was contextually distinct from the conditioning chamber. Fear expression testing consisted of a 3 minute habituation period followed by 15 trials of the same tone (2 kHz, 30 s, 70 dB), with a 90 s intertrial interval.
2.5 Data acquisition and analysis
Behavior was video recorded and freezing was measured using AnyMaze software (Wood Dale, IL) based on detection of changes in pixel luminosity of the videos (video detection was enhanced using an IR-sensitive camera and an IR light source close to the chambers). Detection thresholds were set based upon >95% convergence with a manual rater. The total freezing time during trials (over the entire 90 s intertrial interval) was measured and compared across groups with two-way ANOVAs (GraphPad Prism, La Jolla, CA). Significance was set at p<0.05. If a significant result was obtained, groups were further compared with one-way ANOVAs (or t-tests if fewer than 3 groups) or individual post-hoc t-tests with Bonferroni corrections of the p value.
In a subset of rats that were not used for conditioning, the responsiveness to footshock was measured. This was quantified on an ordinal scale (0= no observable response, 1=flinch, 2=forepaw withdrawal, 3=scramble, 4=run in circle, 5=attempt to jump out of chamber). This assay was used to determine if there was an effect of stress on sensitivity to footshock. In a separate group of rats, footshock threshold was quantified by increasing footshock stimulation intensity until paw withdrawal was observed in response to the footshock. In addition, the amount of freezing in a 20 s period following a suprathreshold footshock (0.5 mA) was also measured. These measures were used to determine whether 1-EBIO had an effect on the responses to aversive stimuli. In an additional separate group of rats, the effect of 1-EBIO was examined on fear conditioning (as described above). Freezing during the tone was measured as the effect of 1-EBIO on the response to conditioned aversive stimuli during acquisition.
3. Results
3.1 Fear conditioning
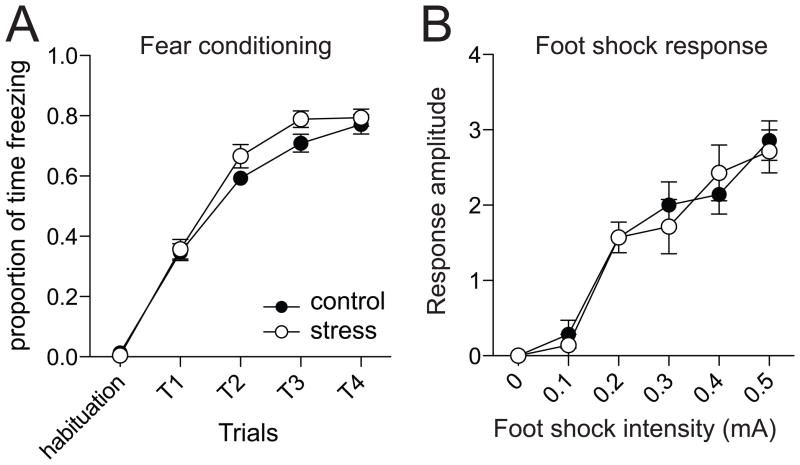
Fear conditioning was performed one day following the final restraint (range 20–26 hours). Freezing was measured over the course of the conditioning. All rats displayed a progressive increase in freezing over subsequent conditioning trials (Fig 1A; two-way repeated measures ANOVA, significant main effect of Trials, F(4,93)=370.2, p<0.001). There was no significant difference between the stress and control groups (two-way repeated measures ANOVA, no significant effect of Stress, F(4,93)=2.2, p=0.14).
Figure 1. Repeated stress does not significantly increase fear acquisition or response to unconditioned stimulus.
A) During the course of fear conditioning, a tone is paired with a footshock. The freezing response to the paired tone increases during the conditioning procedure. However, repeated restraint stress does not increase the freezing response observed during the conditioning procedure. B) The response to increasing amplitude footshock can be measured as an indication of the response to the unconditioned stimulus. Repeated restraint stress does not increase responsiveness to the footshock. Here, and in all plots, data points represent mean ± SEM.
A subset of rats was tested for their responsiveness to footshock. There was a dose-dependent increase in the response of rats to the footshock (Fig 1B; two-way repeated measures ANOVA, significant main effect of footshock intensity, F(5,12)=52.2, p<0.001, n=7 control, 7 stress), however, there was no significant difference between control and stress groups (two-way repeated measures ANOVA, no significant effect of Stress, F(5,12)=0.06, p=0.81).
3.2 Repeated stress enhances fear memory
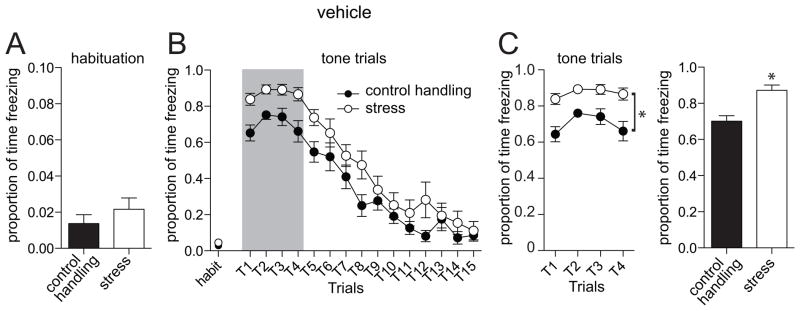
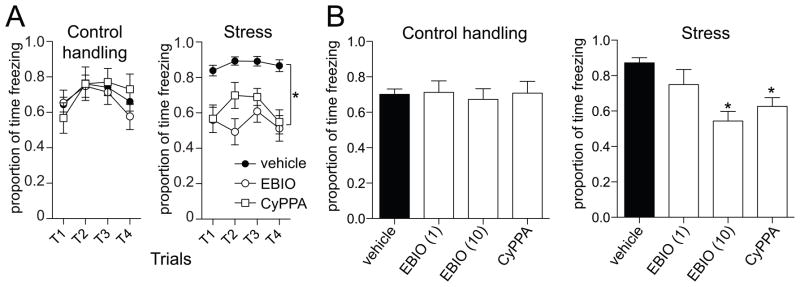
Testing of fear memory was performed one day following fear conditioning (range 19–25 hours). This session consisted of a habituation period followed by repeated presentation of the tone alone (Methods). The typical response pattern was an initial robust freezing response to the tone, followed by a gradual extinction with repeated trials (Fig 2). For the purpose of this study, we quantified the initial 4 trials as an index of fear memory (Fig 2B,C). There was a significant effect of stress on freezing (p=0.0002, F(1,21)=20.2, two-way repeated measures ANOVA, n=11 control handling, 12 stress), where repeated restraint stress increased freezing to the conditioned tone (control handling 70.2 ± 2.6 % time freezing, stress 87.2 ± 1.3 % time freezing; p=0.0011, t=5.88, df=6, two-tailed unpaired t-test). This difference was not caused by increased baseline freezing in the stress group, as there was not a significant difference in freezing during the habituation period before tones were presented (Fig 2A; control handling 2.5 ± 0.8 s freezing, stress 3.9 ± 1.0 s freezing; p=0.29, t=1.1, df=21, two-tailed unpaired t-test).
Figure 2. Repeated stress increases the memory of conditioned fear.
A) When animals are placed in a novel chamber, there is no observed difference in freezing during habituation. B) When the conditioned tone is repeatedly presented, there is an initial robust freezing response that extinguishes with repeated presentation. There is a greater freezing response to the conditioned tone in animals that were exposed to repeated restraint stress. C) For comparison of fear memory, the first four trials were used (grey box in panel B), before significant extinction is observed. There is a significantly more freezing to the conditioned tone in animals exposed to repeated restraint stress. For this, and remaining figures, the amount of time freezing in the four expression trials (left), as well as the proportion of time spent freezing will be displayed (right). * indicates p<0.05.
3.3 Pharmacological activation of SK channels reduces the effect of repeated stress on fear memory
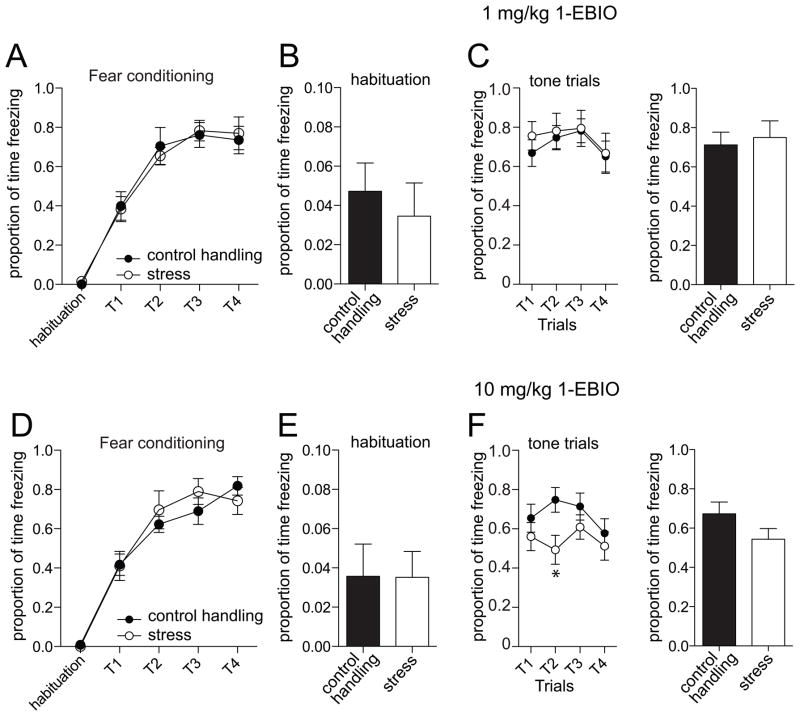
Previous studies have demonstrated that 1-EBIO normalizes neuronal excitability in the LAT after repeated stress [16]. Therefore, we tested the impact of this enhancer of SK channel activity on amygdala-dependent fear memory. There was no significant difference in freezing measured during fear acquisition (Day 1; 1 mg/kg 1-EBIO group, p=0.98, F(1,4)=0.001, two-way repeated measures ANOVA, stress condition x trial, n=10/group; 10 mg/kg 1-EBIO group, p=0.77, F(1,4)=0.09, two-way repeated measures ANOVA, stress condition x trial, n=10/group). The following day, 1-EBIO was administered 30 m before testing for fear memory (Day 2, tone alone), and freezing was measured. Doses of 1-EBIO (1 or 10 mg/kg) were selected based upon their electrophysiological impact and previous behavior studies [16, 27, 33]. There was no significant effect of 1-EBIO on freezing during the habituation period on the testing day in either control handling or stress groups (Fig 3A,C; p=0.14, F(2,57)=2.02, n=11 vehicle control, 12 vehicle stress, 10/group for 1 mg/kg 1-EBIO (n= 10 control handling, 10 stress), 10 mg/kg 1-EBIO (n=10 control, 10 stress), two-way repeated measures ANOVA, drug treatment x stress condition), and in all groups, the average amount of time spent freezing during the habituation period was less than 5%.
Figure 3. Administration of 1-EBIO normalizes fear memory.
A) Freezing during acquisition of fear conditioning on the first day was not significantly different in those rats that were to be tested for the effectiveness of 1-EBIO (1 mg/kg) on fear memory during the second day. B) During habituation on the testing day, there is no significant effect of repeated restraint stress on freezing when 1-EBIO (1 mg/kg) is administered. C) During testing of memory of conditioned fear there is no significant effect of repeated restraint stress on freezing to the conditioned tone if 1-EBIO is administered (1 mg/kg), in contrast to vehicle conditions (Fig 2). D) Freezing during acquisition of fear conditioning on the first day was not significantly different in those rats that were to be tested for the effectiveness of 1-EBIO (10 mg/kg) on fear memory during the second day. E) During habituation on the testing day, there is no significant effect of repeated restraint stress on freezing when 1-EBIO (10 mg/kg) is administered. F) During testing of memory of conditioned fear there is no significant effect of repeated restraint stress on freezing to the conditioned tone if 1-EBIO is administered (10 mg/kg), in contrast to vehicle conditions (Fig 2).
To test whether enhancement of the channels that contribute to the mAHP and sAHP may mitigate the impact of stress on fear expression, we measured the effects of 1-EBIO on freezing to the conditioned tone. After administration of 1-EBIO, animals still displayed conditioned fear responses to the tone (Fig 3B, D). We found that 1-EBIO had a dose-dependent effect on freezing in response to the conditioned tone in the stress group, with minimal impact on the control handling group. Specifically, after administration of 1-EBIO (1 mg/kg), there was no longer a significant effect of stress on conditioned freezing (p=0.73, F(1,54)=0.12, n=10/group, two-way repeated measures ANOVA), whereas a higher dose (10 mg/kg) led to a significant reduction of freezing in the stress group compared to the control handling at some time points (p=0.03, F(3,54)=3.10, n=10/group, significant stress x trial interaction, two-way repeated measures ANOVA; post-hoc t-tests with Bonferroni corrections indicate significant difference at Trial 2; no significant overall main effect of stress p=0.17, F(1,54)=2.09, n=10/group, two-way repeated measures ANOVA).
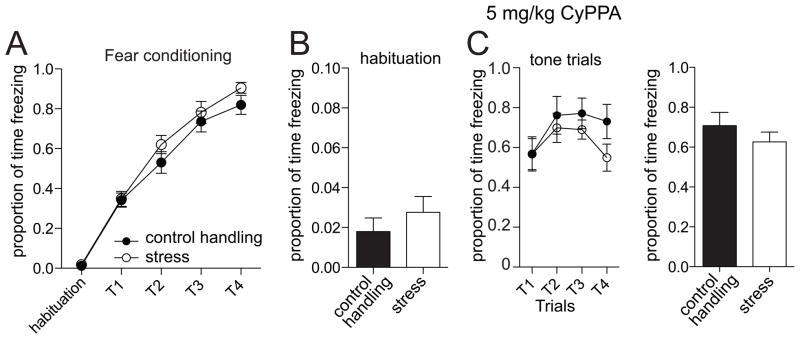
To further confirm and extend these findings, we tested the effects of a more specific pharmacological enhancer of SK2/3 channels, CyPPA [30]. There was no significant difference in freezing measured during fear acquisition (Day 1; p=0.16, F(1,4)=2.14, two-way repeated measures ANOVA, stress condition x trial, n=12 rats/group). Similar to 1-EBIO, CyPPA (5 mg/kg) was administered prior to fear testing the following day. CyPPA had no significant effect on freezing during the habituation period on the testing day (Fig 4A; p=0.46, F(1,43)=0.56, n=12 rats/group, two-way repeated measures ANOVA drug treatment x stress condition), and conditioned responding to the tone was observed (Fig 4B).
Figure 4. Administration of CyPPA normalizes fear memory.
A) Freezing during acquisition of fear conditioning on the first day was not significantly different in those rats that were to be tested for the effectiveness of CyPPA (5 mg/kg) on fear memory during the second day. B) During habituation on the testing day, there is no significant effect of repeated restraint stress on freezing when CyPPA is administered. C) During testing of memory of conditioned fear there is no significant effect of repeated restraint stress on freezing to the conditioned tone if CyPPA is administered (5 mg/kg), in contrast to vehicle conditions (Fig 2).
In the comparisons above, we found that administration of 1-EBIO or CyPPA led to absence of an effect of stress on freezing in response to a conditioned stimulus. To determine whether drug adminsitration decreased freezing compared to vehicle controls, we perfromed further analysis. There was a significant overall effect of 1-EBIO treatment on freezing in response to a conditioned tone (Fig 5A,B; p=0.008, F(2,57)=5.31, main effect of drug, two-way repeated measures ANOVA drug treatment x stress condition). However, in post-hoc comparisons, there was only a significant effect of 1-EBIO on the amount of conditioned freezing in the stress group (Fig 5A, B; p<0.001, F(2,9)=50.13, n=12 rats vehicle, 10 rats 1-EBIO (1 mg/kg), 10 rats 1-EBIO (10 mg/kg), one-way ANOVA), with significant reductions in freezing after 1 mg/kg (Fig 5B; t=3.70, p<0.05 after Bonferroni correction) and 10 mg/kg (Fig 5B; t=9.91, p<0.05 after Bonferroni correction) compared to vehicle. However, there was no significant effect of 1-EBIO on the amount of conditioned freezing in the control handling group (Fig 5B; p=0.69, F(2,9)=0.38, p>0.05, n=11 rats vehicle, 10 rats 1-EBIO (1 mg/kg), 10 rats 1-EBIO (10 mg/kg), one-way ANOVA). This is consistent with a reduction in conditioned freezing caused by 1-EBIO in animals exposed to repeated stress.
Figure 5. Pharmacological enhancement of SK channels decreases conditioned freezing in animals exposed to repeated stress.
A) When comparing across stress condition, it is observed that 1-EBIO and CyPPA decrease conditioned freezing time in animals exposed to repeated restraint stress, but not in controls. B) The proportion of time freezing in response to a conditioned tone is decreased in a dose-dependent manner by 1-EBIO and decreased by CyPPA compared to vehicle in animals exposed to repeated restraint stress (right), but not control animals (left). EBIO (1) = 1 mg/kg 1-EBIO; EBIO (10) = 10 mg/kg 1-EBIO; * indicates significant difference compared to vehicle.
Similar to 1-EBIO, administration of CyPPA decreased conditioned freezing in animals exposed to repeated stress. There was a significant effect of CyPPA treatment on freezing in response to a conditioned tone (Fig 5A, B; p=0.02, F(1,43)=5.72, n=11 vehicle control handling, 12 vehicle stress, 12 CyPPA control handling, 12 CyPPA stress, main effect of drug, two-way repeated measures ANOVA, drug treatment x stress condition). There was a significant effect of CyPPA on the amount of conditioned freezing in the stress group (p=0.001, t=5.92, df=6, Student’s t-test). However, there was no significant effect of CyPPA on the amount of conditioned freezing in the control handling group (Fig 5A, B; p=0.91, t=0.11, df=6, Student’s t-test). This is consistent with a reduction in conditioned freezing caused by CyPPA in animals exposed to repeated stress.
3.4 1-EBIO does not interfere with freezing
One potential concern is that 1-EBIO leads to a reduction of conditioned freezing by either inducing hyperlocomotion or otherwise impairing ability to freeze in response to aversive stimuli. To test whether 1-EBIO causes hyperlocomotion, we measured the total distance traveled and the average speed during the post-injection habituation period of vehicle and drug-treated rats (as described above). When compared across the vehicle and drug treatments, there was no significant difference in total distance traveled (Fig 6A; p=0.89, F(3,39)=0.21, one-way ANOVA), nor a significant difference in the average speed (Fig 6A; p=0.85, F(3,39)=0.26, one-way ANOVA). To test whether 1-EBIO impairs freezing, we examined the effects of 1-EBIO (10 mg/kg) on the amount of freezing following suprathreshold footshocks. To verify this as an appropriate measure, we first tested whether 1-EBIO leads to a change in the threshold of pain response to footshock. We found no significant difference in the footshock threshold after 1-EBIO compared to vehicle (Fig 6B; p=0.23, t=1.25, df=18, n=10 rats/group, Student’s t-test), nor in the amount of freezing after the series of footshocks (Fig 6B; p=0.65, t=0.46, df=18, n=10 rats/group, Student’s t-test). These data are consistent with an effect of 1-EBIO on the recall of fear, but not on the expression of freezing responses in general.
Figure 6. 1-EBIO does not increase locomotion or response to footshocks.
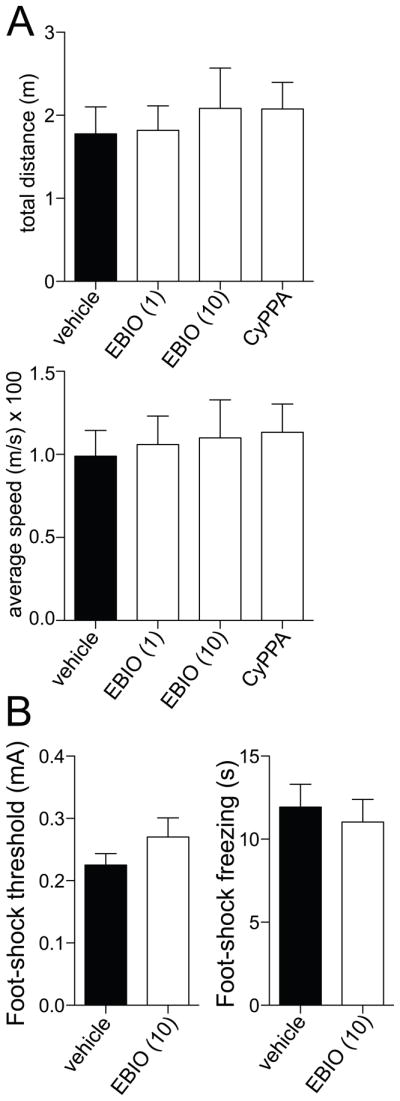
A) Administration of 1-EBIO or CyPPA does not significantly increase locomotion, measured during the pre-test habituation period as the total distance traveled (left) or the average speed (right). B) Administration of 1-EBIO (10 mg/kg) does not significantly decrease the response to aversive stimulation, measured as the threshold stimulation intensity to evoke a withdrawal response to a footshock (left) or the amount of time freezing following suprathreshold footshocks (0.5 mA).
4. Discussion
Repeated stress leads to enhancement of BLA-dependent fear memory [20, 21]. In parallel, repeated stress also increases the activity of BLA neurons [16, 22], in part through a reduction of the mAHP and sAHP. The current studies tested whether pharmacological enhancement of the mAHP and sAHP is able to reverse the effects of repeated stress on BLA-dependent fear conditioning. We found that repeated stress increased the memory of conditioned fear, and that administration of 1-EBIO or CyPPA reduced this enhanced memory of conditioned fear. The effectiveness of 1-EBIO and CyPPA on conditioned fear was limited to the stress group, and these pharmacological enhancers of the AHP had no significant effect on memory of fear conditioning in the control group. Importantly, the effects of these drugs on freezing in response to the conditioned tone did not appear to be the result of deficits in ability to freeze or hyperlocomotion, as 1-EBIO did not diminish freezing observed after an unconditioned aversive stimulus, vehicle and 1-EBIO groups displayed a similar amount of freezing during the habituation phase prior to fear testing, and a similar amount of locomotion was measured from the various groups. This is in agreement with a previous study that indicates that locomotive effects of 1-EBIO (at the doses used in this study) are similar to baseline levels within 30 m after injection [27]. These data are therefore consistent with a specific effect of 1-EBIO on freezing in response to the conditioned tone. However, it is not clear what component of fear memory is enhanced by repeated restraint stress (consolidation, retrieval or expression) and normalized by modulation of SK channels (retrieval or expression). Furthermore, it is possible that the effects of repeated restraint observed here are actually due to a long-lasting effect of the first restraint (e.g. [34, 35]) or the final restraint, though preliminary evidence suggests that one session of the shorter duration restraint utilized in the procedures described here does not cause long-lasting effects on fear memory [36].
Pharmacological enhancement of the mAHP and sAHP has little effect on acquisition of fear conditioning [27]. Here we demonstrate that similar pharmacological enhancement has little effect on memory of conditioned fear in control animals. It is unclear why pharmacological enhancement of the mAHP and sAHP has little effect on BLA-dependent conditioning in control groups. 1-EBIO (at the same doses used here) has little effect on the mAHP and sAHP in vivo in control groups, but greatly enhances the mAHP and sAHP after repeated stress [16]. This may indicate that these AHPs are near maximally active under control conditions during normal BLA neuronal function, perhaps accounting for the minimal effect of 1-EBIO under control conditions. However, in situations when the mAHP and sAHP are compromised, there is room for enhancement, and 1-EBIO has impact. Consistent for a role of mAHP and sAHP in BLA-dependent behavior, over-expression of SK channels reduces BLA-dependent cued fear conditioning [25], and BLA- and hippocampus-dependent contextual fear conditioning [25, 37]. In addition, over-expression of SK channels in the BLA reduces measures of anxiety in the elevated plus maze and open field [26].
A calcium-dependent mAHP and sAHP plays a prominent role in regulation of BLA neurons [28, 29, 38–41]. However, the molecular identity of the channels that comprise this AHP in BLA neurons is not entirely clear. Apamin, which blocks SK2/3 channels that may contribute to the mAHP, has been reported to either increase BLA neuronal excitability [29] or to have minimal effect [40]. However, SK channels in the BLA play a prominent role in regulation of the response to synaptic input [42], which can thereby influence the neuronal response to synaptic inputs. In addition, it is difficult to conclude where in the brain these drugs exerted their action. SK channels are observed in a wide range of brain areas [43], and a variety of brain regions display altered neuronal excitability after repeated stress (e.g. [44–47]), which may contribute to the effects of the treatments observed here.
5. Conclusions
This study examined the impact of enhancement of SK channel function on the memory of BLA-dependent cued fear, in a stress model that causes impaired function of SK channels in BLA neurons [16]. This study demonstrates the potential for the use of pharmacological enhancement of SK channels in stress-related mood disorders. In particular, pharmacological targeting of the mAHP and sAHP may be a viable option for normalizing behaviors associated with abnormally hyperactive amygdala following stress.
Highlights.
Repeated restraint stress increases the memory of cued conditioned fear
Administration of 1-EBIO reduced conditioned freezing in stressed rats
Administration of 1-EBIO did not decrease conditioned freezing in control rats
Administration of CyPPA mimicked these effects
Neither drug impaired freezing in response to footshock
Acknowledgments
The authors thank Mallika Padival for experimental assistance. Support provided by the U.S. National Institutes of Health (MH084970) and the Brain Research Foundation. Neither funding source had direct involvement in these studies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol Neurobiol. 2009;40:166–82. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- 2.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- 3.Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci. 2009;10:434–45. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- 4.Yehuda R. Post-traumatic stress disorder. N Engl J Med. 2002;346:108–14. doi: 10.1056/NEJMra012941. [DOI] [PubMed] [Google Scholar]
- 5.Wik G, Fredrikson M, Ericson K, Eriksson L, Stone-Elander S, Greitz T. A functional cerebral response to frightening visual stimulation. Psychiatry Res. 1993;50:15–24. doi: 10.1016/0925-4927(93)90020-i. [DOI] [PubMed] [Google Scholar]
- 6.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992;12:3628–41. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breiter HC, Rauch SL, Kwong KK, Baker JR, Weisskoff RM, Kennedy DN, et al. Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry. 1996;53:595–606. doi: 10.1001/archpsyc.1996.01830070041008. [DOI] [PubMed] [Google Scholar]
- 9.Armony JL, Corbo V, Clement MH, Brunet A. Amygdala response in patients with acute PTSD to masked and unmasked emotional facial expressions. Am J Psychiatry. 2005;162:1961–3. doi: 10.1176/appi.ajp.162.10.1961. [DOI] [PubMed] [Google Scholar]
- 10.Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S, et al. Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry. 1999;45:817–26. doi: 10.1016/s0006-3223(98)00246-7. [DOI] [PubMed] [Google Scholar]
- 11.Protopopescu X, Pan H, Tuescher O, Cloitre M, Goldstein M, Engelien W, et al. Differential time courses and specificity of amygdala activity in posttraumatic stress disorder subjects and normal control subjects. Biol Psychiatry. 2005;57:464–73. doi: 10.1016/j.biopsych.2004.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL, et al. Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Arch Gen Psychiatry. 1997;54:233–41. doi: 10.1001/archpsyc.1997.01830150057010. [DOI] [PubMed] [Google Scholar]
- 13.van Wingen GA, Geuze E, Vermetten E, Fernandez G. Perceived threat predicts the neural sequelae of combat stress. Mol Psychiatry. 2010;16:664–71. doi: 10.1038/mp.2010.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143:387–93. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Rosenkranz JA, Venheim ER, Padival M. Chronic stress causes amygdala hyperexcitability in rodents. Biol Psychiatry. 2010;67:1128–36. doi: 10.1016/j.biopsych.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Quirk GJ, Repa C, LeDoux JE. Fear conditioning enhances short-latency auditory responses of lateral amygdala neurons: parallel recordings in the freely behaving rat. Neuron. 1995;15:1029–39. doi: 10.1016/0896-6273(95)90092-6. [DOI] [PubMed] [Google Scholar]
- 18.Pare D, Collins DR. Neuronal correlates of fear in the lateral amygdala: multiple extracellular recordings in conscious cats. J Neurosci. 2000;20:2701–10. doi: 10.1523/JNEUROSCI.20-07-02701.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maren S, Poremba A, Gabriel M. Basolateral amygdaloid multi-unit neuronal correlates of discriminative avoidance learning in rabbits. Brain Res. 1991;549:311–6. doi: 10.1016/0006-8993(91)90473-9. [DOI] [PubMed] [Google Scholar]
- 20.Conrad CD, LeDoux JE, Magarinos AM, McEwen BS. Repeated restraint stress facilitates fear conditioning independently of causing hippocampal CA3 dendritic atrophy. Behav Neurosci. 1999;113:902–13. doi: 10.1037//0735-7044.113.5.902. [DOI] [PubMed] [Google Scholar]
- 21.Wood GE, Norris EH, Waters E, Stoldt JT, McEwen BS. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav Neurosci. 2008;122:282–92. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- 22.Correll CM, Rosenkranz JA, Grace AA. Chronic cold stress alters prefrontal cortical modulation of amygdala neuronal activity in rats. Biol Psychiatry. 2005;58:382–91. doi: 10.1016/j.biopsych.2005.04.009. [DOI] [PubMed] [Google Scholar]
- 23.Stocker M. Ca(2+)-activated K+ channels: molecular determinants and function of the SK family. Nat Rev Neurosci. 2004;5:758–70. doi: 10.1038/nrn1516. [DOI] [PubMed] [Google Scholar]
- 24.Faber ES, Sah P. Functions of SK channels in central neurons. Clin Exp Pharmacol Physiol. 2007;34:1077–83. doi: 10.1111/j.1440-1681.2007.04725.x. [DOI] [PubMed] [Google Scholar]
- 25.Hammond RS, Bond CT, Strassmaier T, Ngo-Anh TJ, Adelman JP, Maylie J, et al. Small-conductance Ca2+-activated K+ channel type 2 (SK2) modulates hippocampal learning, memory, and synaptic plasticity. J Neurosci. 2006;26:1844–53. doi: 10.1523/JNEUROSCI.4106-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mitra R, Ferguson D, Sapolsky RM. SK2 potassium channel overexpression in basolateral amygdala reduces anxiety, stress-induced corticosterone secretion and dendritic arborization. Mol Psychiatry. 2009 doi: 10.1038/mp.2009.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vick KAt, Guidi M, Stackman RW., Jr In vivo pharmacological manipulation of small conductance Ca(2+)-activated K(+) channels influences motor behavior, object memory and fear conditioning. Neuropharmacology. 2010;58:650–9. doi: 10.1016/j.neuropharm.2009.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lang EJ, Pare D. Synaptic and synaptically activated intrinsic conductances underlie inhibitory potentials in cat lateral amygdaloid projection neurons in vivo. J Neurophysiol. 1997;77:353–63. doi: 10.1152/jn.1997.77.1.353. [DOI] [PubMed] [Google Scholar]
- 29.Chen JC, Lang EJ. Inhibitory control of rat lateral amygdaloid projection cells. Neuroscience. 2003;121:155–66. doi: 10.1016/s0306-4522(03)00430-5. [DOI] [PubMed] [Google Scholar]
- 30.Hougaard C, Eriksen BL, Jorgensen S, Johansen TH, Dyhring T, Madsen LS, et al. Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol. 2007;151:655–65. doi: 10.1038/sj.bjp.0707281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hougaard C, Jensen ML, Dale TJ, Miller DD, Davies DJ, Eriksen BL, et al. Selective activation of the SK1 subtype of human small-conductance Ca2+-activated K+ channels by 4-(2-methoxyphenylcarbamoyloxymethyl)-piperidine-1-carboxylic acid tert-butyl ester (GW542573X) is dependent on serine 293 in the S5 segment. Mol Pharmacol. 2009;76:569–78. doi: 10.1124/mol.109.056663. [DOI] [PubMed] [Google Scholar]
- 32.Pedarzani P, McCutcheon JE, Rogge G, Jensen BS, Christophersen P, Hougaard C, et al. Specific enhancement of SK channel activity selectively potentiates the afterhyperpolarizing current I(AHP) and modulates the firing properties of hippocampal pyramidal neurons. J Biol Chem. 2005;280:41404–11. doi: 10.1074/jbc.M509610200. [DOI] [PubMed] [Google Scholar]
- 33.Anderson NJ, Slough S, Watson WP. In vivo characterisation of the small-conductance KCa (SK) channel activator 1-ethyl-2-benzimidazolinone (1-EBIO) as a potential anticonvulsant. Eur J Pharmacol. 2006;546:48–53. doi: 10.1016/j.ejphar.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 34.Chauveau F, Lange MD, Jungling K, Lesting J, Seidenbecher T, Pape HC. Prevention of Stress-Impaired Fear Extinction Through Neuropeptide S Action in the Lateral Amygdala. Neuropsychopharmacology. 2012 doi: 10.1038/npp.2012.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mitra R, Jadhav S, McEwen BS, Vyas A, Chattarji S. Stress duration modulates the spatiotemporal patterns of spine formation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2005;102:9371–6. doi: 10.1073/pnas.0504011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang W, Padival M, Rosenkranz JA. Chronic stress increases neuronal activity in the basolateral amygdala of adolescent rats. Society for Neuroscience; San Diego, CA: 2010. [Google Scholar]
- 37.Stackman RW, Jr, Bond CT, Adelman JP. Contextual memory deficits observed in mice overexpressing small conductance Ca2+-activated K+ type 2 (KCa2.2, SK2) channels are caused by an encoding deficit. Learn Mem. 2008;15:208–13. doi: 10.1101/lm.906808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Power JM, Bocklisch C, Curby P, Sah P. Location and function of the slow afterhyperpolarization channels in the basolateral amygdala. J Neurosci. 2011;31:526–37. doi: 10.1523/JNEUROSCI.1045-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McKinney BC, Sze W, Lee B, Murphy GG. Impaired long-term potentiation and enhanced neuronal excitability in the amygdala of Ca(V)1.3 knockout mice. Neurobiol Learn Mem. 2009;92:519–28. doi: 10.1016/j.nlm.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Faber ES, Sah P. Physiological role of calcium-activated potassium currents in the rat lateral amygdala. J Neurosci. 2002;22:1618–28. doi: 10.1523/JNEUROSCI.22-05-01618.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Womble MD, Moises HC. Muscarinic modulation of conductances underlying the afterhyperpolarization in neurons of the rat basolateral amygdala. Brain Res. 1993;621:87–96. doi: 10.1016/0006-8993(93)90301-3. [DOI] [PubMed] [Google Scholar]
- 42.Faber ES, Delaney AJ, Sah P. SK channels regulate excitatory synaptic transmission and plasticity in the lateral amygdala. Nat Neurosci. 2005;8:635–41. doi: 10.1038/nn1450. [DOI] [PubMed] [Google Scholar]
- 43.Stocker M, Pedarzani P. Differential distribution of three Ca(2+)-activated K(+) channel subunits, SK1, SK2, and SK3, in the adult rat central nervous system. Mol Cell Neurosci. 2000;15:476–93. doi: 10.1006/mcne.2000.0842. [DOI] [PubMed] [Google Scholar]
- 44.Joels M, Karst H, Alfarez D, Heine VM, Qin Y, van Riel E, et al. Effects of chronic stress on structure and cell function in rat hippocampus and hypothalamus. Stress. 2004;7:221–31. doi: 10.1080/10253890500070005. [DOI] [PubMed] [Google Scholar]
- 45.Verkuyl JM, Hemby SE, Joels M. Chronic stress attenuates GABAergic inhibition and alters gene expression of parvocellular neurons in rat hypothalamus. Eur J Neurosci. 2004;20:1665–73. doi: 10.1111/j.1460-9568.2004.03568.x. [DOI] [PubMed] [Google Scholar]
- 46.McDermott CM, LaHoste GJ, Chen C, Musto A, Bazan NG, Magee JC. Sleep deprivation causes behavioral, synaptic, and membrane excitability alterations in hippocampal neurons. J Neurosci. 2003;23:9687–95. doi: 10.1523/JNEUROSCI.23-29-09687.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kole MH, Czeh B, Fuchs E. Homeostatic maintenance in excitability of tree shrew hippocampal CA3 pyramidal neurons after chronic stress. Hippocampus. 2004;14:742–51. doi: 10.1002/hipo.10212. [DOI] [PubMed] [Google Scholar]