Abstract
Maintenance of immune homeostasis requires regulatory T (Treg) cells. Here we show that Treg-specific ablation of Ubc13, a lysine 63-specific ubiquitin-conjugating enzyme, caused aberrant T cell activation and autoimmunity. Although Ubc13 deficiency did not affect Treg cell survival or Foxp3 expression, it impaired the in vivo suppressive function of Treg cells and rendered them sensitive for acquiring T helper (TH) 1- and TH17-like effector T cell phenotypes. This function of Ubc13 involved its downstream target, IκB kinase (IKK). The Ubc13-IKK signaling axis controlled the expression specific Treg effector molecules, including interleukin 10 (IL-10) and SOCS1. Collectively, these findings suggest that the Ubc13-IKK signaling axis regulates the molecular program that maintains Treg function and prevents Treg cells from acquiring inflammatory phenotypes.
Keywords: Ubc13, ubiquitination, Treg, IKK, NF-κB
Regulatory T (Treg) cells represent a subset of CD4+ T cells with a pivotal role in maintaining peripheral immune tolerance and, thereby, preventing autoimmunity and chronic inflammations1. A hallmark of Treg cells is expression of forkhead box P3 (Foxp3), a master transcription factor required for the differentiation, maintenance, and suppressive functions of Treg cells2. Foxp3 genetic deficiency causes aberrant activation and homeostasis of T cells, leading to multiorgan inflammation2. The suppressive function of Treg cells is mediated through both cell-cell contact and secretion of immunosuppressive cytokines, the latter of which include transforming growth factor-β (TGF-β), interleukin 10 (IL-10) and IL-351,3.
Treg cells are generally stable; however, a fraction of them may lose Foxp3 expression and undergo phenotypic changes to acquire diverse effector functions under lymphopenic and/or inflammatory conditions4–6. Studies using different in vivo models have demonstrated the conversion of Treg cells into TH1-, TH17-, or TFH-like effector T cells, which appears to contribute to uncontrolled chronic inflammation and autoimmunity7–10. The acquisition of inflammatory effector functions by Treg cells may even occur without losing Foxp3 expression7,11–13. Although the mechanisms mediating the phenotypic conversion of Treg cells are still poorly understood, proinflammatory cytokines were shown to play a role7,14. Moreover, the stability and suppressive function of Treg cells rely on expression of SOCS1 (suppressor of cytokine signaling 1), a molecule that negatively regulates the signaling function of several cytokine receptors13,15. SOCS1 inhibits the activation of both STAT1 and STAT3, thereby restraining Treg cells from being converting into TH1- and TH17-like effector T cells13.
The T cell receptors (TCRs) of Treg cells recognize both self and non-self antigens and appear to be constantly stimulated in vivo16. However, how the TCR-mediated signaling pathways regulate the stability and suppressive function of Treg cells remains poorly understood. One major TCR-elicited pathway involves activation of a signaling complex composed of Carma1, Bcl-10, Molt1, and the E2 ubiquitin-conjugating enzyme Ubc1317. Although mammalian cells express multiple E2 ubiquitin-conjugating enzymes, Ubc13 is unique in that it specifically conjugates lysine 63 (K63)-linked polyubiquitin chains known to be critical for activation of IκB kinase (IKK) and its downstream transcription factor NF-κB17. Thus, a major function of Ubc13 in conventional T cells is to mediate TCR-stimulated IKK-NF-κB activation and peripheral T cell homeostasis18,19. Recent studies have demonstrated an important role for the NF-κB family member c-Rel in the regulation of Treg cell differentiation20–23; however, whether the Ubc13-IKK-regulated signaling pathway has a role in regulating the homeostasis, stability, or suppressive function of committed Treg cells is unknown.
Here we studied the role of Ubc13 by conditional ablation of the Ubc13-coding gene Ube2n. We found that Ubc13 is dispensable for the survival and homeostasis of Treg cells as well as their in vitro suppressive activity. Ubc13 however had a pivotal role in maintaining the in vivo immunosuppressive function of Treg cells and in preventing the conversion of Treg cells into TH1- and TH17-like effector T cells in a manner dependent on its downstream target IKK. The Ubc13-IKK signaling axis is dispensable for expression of Treg signature genes but is required for expression of specific Treg functional factors, including IL-10 and SOCS1. These findings suggest that the Ubc13-IKK signaling axis is an important part of the signaling program that maintains the stability and immunosuppressive function of Treg cells.
RESULTS
Multiorgan inflammation by Treg-specific ablation of Ubc13
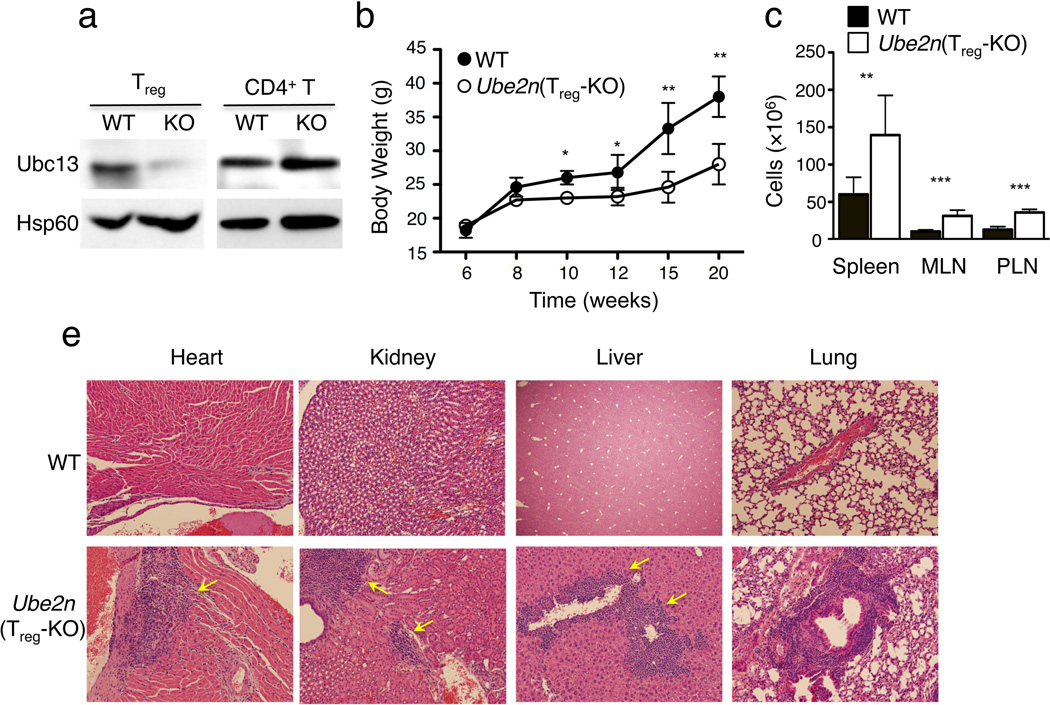
To examine the Treg-specific function of Ubc13, we generated Treg-specific conditional Ube2n-deficient mice by crossing Ube2n-floxed (Ube2nfl/fl) mice24 with the Foxp3-GFP-hCre mice25. The resulting Ube2nfl/flFoxp3GFP-hCre (hereafter called Ube2nTreg-KO) mice and their age-matched Ube2n+/+Foxp3GFP-hCre wild-type controls were used for experiments. Specific deletion of Ubc13 in Treg, but not in CD4+ conventional T cells, was confirmed by Ubc13 immunoblotting (Fig. 1a). Ube2nTreg-KO mice were born at expected Mendelian ratios and appeared to be grossly normal at young ages. However, starting from 8 weeks of age, they displayed weight reduction compared to the age-matched wild-type controls, a phenotype that became more prominent at 12 weeks of age or older (Fig. 1b). This phenotype of the Ube2nTreg-KO mice was associated with autoimmune symptoms, including increased size and cellularity of peripheral lymphoid organs (Fig. 1c and data not shown) and lymphocytic infiltration into multiple nonlymphoid organs (Fig. 1d). These pathological phenotypes were reminiscent of those reported in Treg-deficient mice2. However, in general, the disease severity in Ube2nTreg-KO mice was milder than that of Treg-deficient mice and was not associated with fatality. These findings suggest that Ubc13 may regulate certain aspects of the Treg cell function.
Figure 1. Treg-specific ablation of Ubc13 causes systemic autoimmunity.
(a) immunoblot of Ubc13 expression in CD4+ T cells and Treg cells sorted from Ube2nfl/flFoxp3GFP-Cre (KO) and Ube2n+/+Foxp3GFP-Cre (WT) mice. Loading control: protein Hsp60. Data are representative of two independent experiments. (b) Bodyweight of Ube2nTreg-KO and Ube2n+/+Foxp3GFP-Cre (WT) measured at the indicated times and presented as mean±SD. Results were from a total of three independent experiments (5–7 mice/genotype in each experiment). *p=0.05; **p=0.01 (two-tailed unpaired t-test). (c) Number of total cells from spleen, mesenteric lymph node (MLN) and peripheral lymph node (PLN) of age- and sex-matched Ube2nTreg-KO and Ube2n+/+Foxp3GFP-Cre (WT) 10 week old mice presented as the mean±SD value. **p=0.01 and ***p=0.001 (two-tailed unpaired t-test). Data are representative of two independent experiments. (d) H&E staining of various non-lymphoid tissue sections from 20 weeks old Ube2nTreg-KO and Ube2n+/+Foxp3GFP-Cre (WT) mice. Data are representative of 3–4 mice/genotype.
Abnormal conventional T cell activation in Ube2nTreg-KO mice
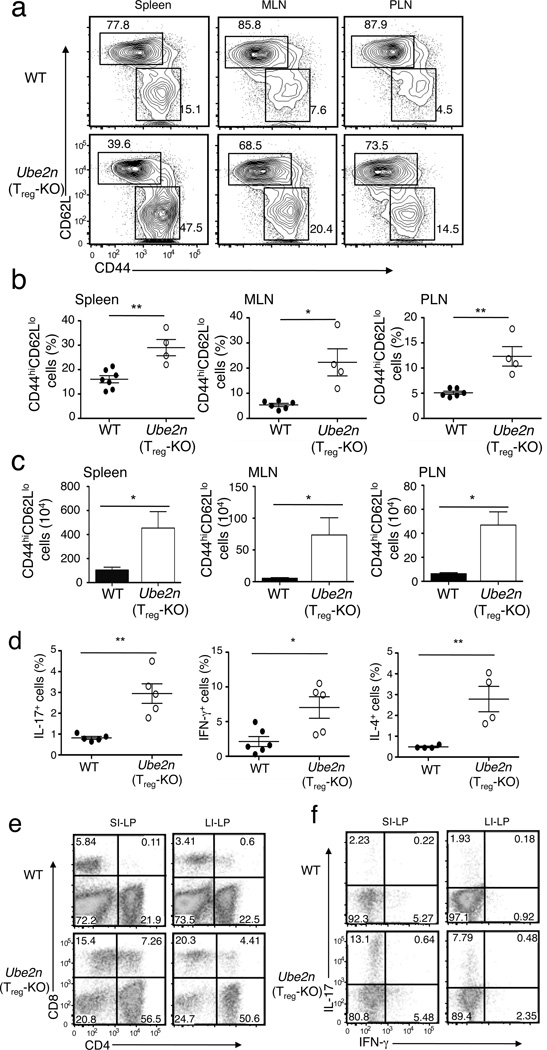
An important function of Treg cells is to prevent abnormal T cell activation and maintain immune cell homeostasis. We found that the percentage of CD4+ and CD8+ T cells was lower in the spleen and lymph nodes (LNs) of the Ube2nTreg-KO mice (Supplementary Fig. 1a). However, this was not due to a reduction in the number of T cells (Supplementary Fig. 1b) but apparently resulted from an increase in the population of non-T cells (CD4−CD8−) associated with the splenomegaly and lymphadenopsy (Supplementary Fig. 1a). This abnormality was not detected in younger mice (4 week old) that had no autoimmune phenotypes (Supplementary Fig. 1c, d). The non-T cell population in the older Ube2nTreg-KO mice included predominantly B220+ B cells in the mesenteric LNs (MLNs) (Supplementary Fig. 2a), but also B220−CD3− cells, such as CD11c+ and F4/80+ myeloid cells and NK1.1+ NK cells, in the spleen (Supplementary Fig. 2b). To look at T cell homeostasis in Ube2nTreg-KO mice, we quantified the memory and naïve (Ubc13-sufficient) T cells based on surface expression of CD44 and CD62L. In both the spleen and LNs, the Ube2nTreg-KO mice had a significant increase in the frequency of memory and effector-like (CD44hiCD62Llo) CD4+ T cells with concomitant reduction in the frequency of naïve (CD44loCD62Lhi) CD4+ T cells (Fig. 2a,b). The absolute number of memory and effector-like CD4+ T cells was also significantly increased in the Ube2nTreg-KO mice (Fig. 2c). Consistent with these findings, the Ube2nTreg-KO mice had a significantly increased frequency of TH1, TH2, and TH17 CD4+ effector T cells in the spleen (Fig. 2d).
Figure 2. Treg-specific ablation of Ubc13 impairs T-cell homeostasis.
(a) Flow cytometric analysis of T cells derived from the indicated lymphoid organs of WT and Ube2nTreg-KO mice (8 weeks old), showing the percentage of naïve (CD44loCD62Lhi) and memory-like (CD44hiCD62Llo) CD4+ T cells. Data are representative of five experiments with three mice per group. (b, c) Summary (mean ± SD value) of the frequency (b) and absolute numbers (c) of memory-like CD4+ T cells in the indicated lymphoid organs of WT and Ube2nTreg-KO mice (8 weeks old), determined by flow cytometry. *p=0.05 and **p=0.01 (two-tailed unpaired t-test). (d) ICS measuring the frequency of IL-17-, IFN-γ, and IL-4-producing CD4+ T cells (gated on CD3+CD4+ cells) within the spleen of WT and Ube2nTreg-KO mice (8–10 weeks old). *p=0.05 and **p=0.01 (two-tailed unpaired t-test). Data are representative of three independent experiments (each circle represents one mouse). (e, f) Flow cytometric analyses of CD4+ or CD8+ (e) and IL-17- and IFN-γ-producing CD4+ T cells (f) from small intestine lamina propria (SI-LP) and large intestine lamina propria (LI-LP) of WT and Ube2nTreg-KO mice (8 weeks old). Numbers in quadrants indicate percentage of cells. Data are representative of two experiments with three mice per group.
Because the intestine is an organ that is often affected by impaired Treg cell function, we examined the effect of Treg-specific Ubc13 ablation on the frequency of T cells in the lamina propria of this organ. Indeed, the lamina propria of both the small and the large intestines of Ube2nTreg-KO mice had a marked increase in the frequency of CD4+ and CD8+ T cells (Fig. 2e). Within the CD4+ T cell population, there was a significant increase in the frequency of TH17 cells (Fig. 2f). The large intestine, although not the small intestine, also had a moderate increase in TH1 cell frequency (Fig. 2f). Thus, loss of Ubc13 in Treg cells impaired the homeostasis of the Ubc13-sufficient conventional T cells, which further indicates a role for Ubc13 in regulating the immunosuppressive function of Treg cells.
Ubc13 is dispensable for Treg survival or in vitro function
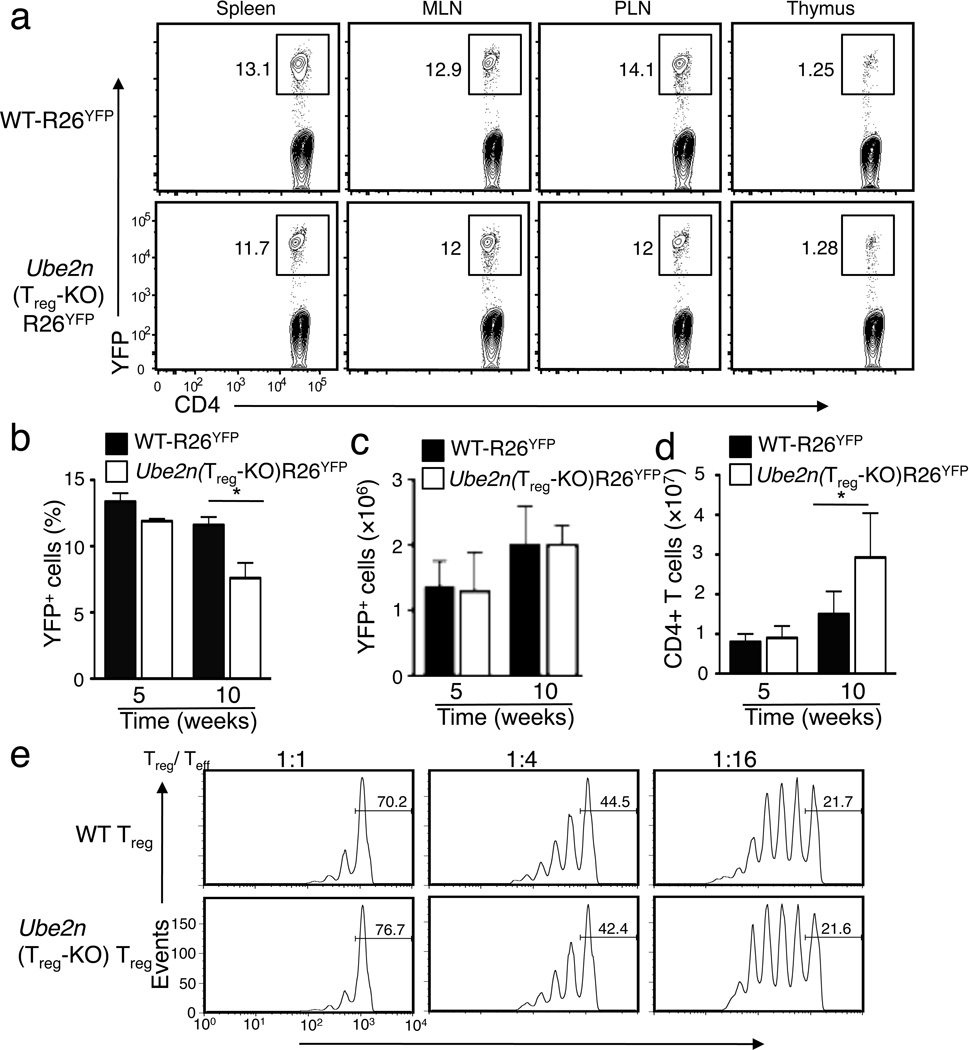
Wild-type and Ube2nTreg-KO mice contained comparable frequency of Foxp3+ Treg cells in both the thymus and the peripheral lymphoid organs (Supplementary Fig. 3a,b), suggesting that Ubc13 is dispensable for Treg homeostasis. To further confirm this conclusion, we used Rosa26-YFP Cre reporter (hereafter called R26YFP) mice that express YFP in a Cre-dependent manner26. The progeny of R26YFP mice crossed with Foxp3Cre mice express YFP specifically in Treg cells25. Because GFP is less bright compared to YFP (Supplementary Fig. 4), so GFP should not interfere with the YFP signal during analysis, and because all GFP+ cells were YFP+ (Supplementary Fig. 4), we used either YFP or Foxp3 as a marker for Treg cells in our subsequent analyses. As seen in the Foxp3 staining experiments, young R26YFP and Ube2nTreg-KOR26YFP mice had comparable YFP+ Treg frequencies (Fig. 3a,b) and numbers (Fig. 3c) in all of the immune organs analyzed. At an older age (10 week), Ube2nTreg-KOR26YFP mice had a reduced Treg percentage in the spleen (Fig. 3b), which was not due to the reduction in Treg numbers (Fig. 3c) but was apparently caused by the uncontrolled expansion of the Ubc13-sufficient conventional T cells (Fig. 3d).
Figure 3. Ubc13 is dispensable for Treg homeostasis and in vitro suppressive activity.
(a) Flow cytometric analysis of the frequency of YFP+ Treg cells (among CD3+CD4+ cells) in the indicated lymphoid organs of 6 weeks old mice. Data are representative of five experiments with three mice per group. (b–d) Frequency (b) and absolute number (c) of YFP+ cells (among CD3+CD4+ cells) and absolute number of total CD4+ T cells (d) in the spleen of the indicated ages of mice, measured by flow cytometry and shown as the mean±SD value. *p=0.05 (n=5) (two-tailed unpaired t-test). (e) In vitro suppressive activity of Treg cells, measured based on the proliferation (CFSE dilution) of naïve CD4+ T cells activated by anti-CD3 plus antigen-presenting cells (irradiated CD3-depleted splenocytes from WT mice) in the presence of the indicated ratios of sorted Treg cells derived from WT or Ube2nTreg-KO mice (6 weeks old). The percentage of undivided cells is indicated.
In vitro Treg assays revealed that the wild-type and Ubc13-deficient Treg cells displayed comparable ability to suppress the activation of CD4+ naïve T cells (Fig. 3e). Thus, unlike Foxp3 deficiency, the loss of Ubc13 did not compromise the overall suppressive capacity of Treg cells.
Ubc13 is required for Treg function in vivo
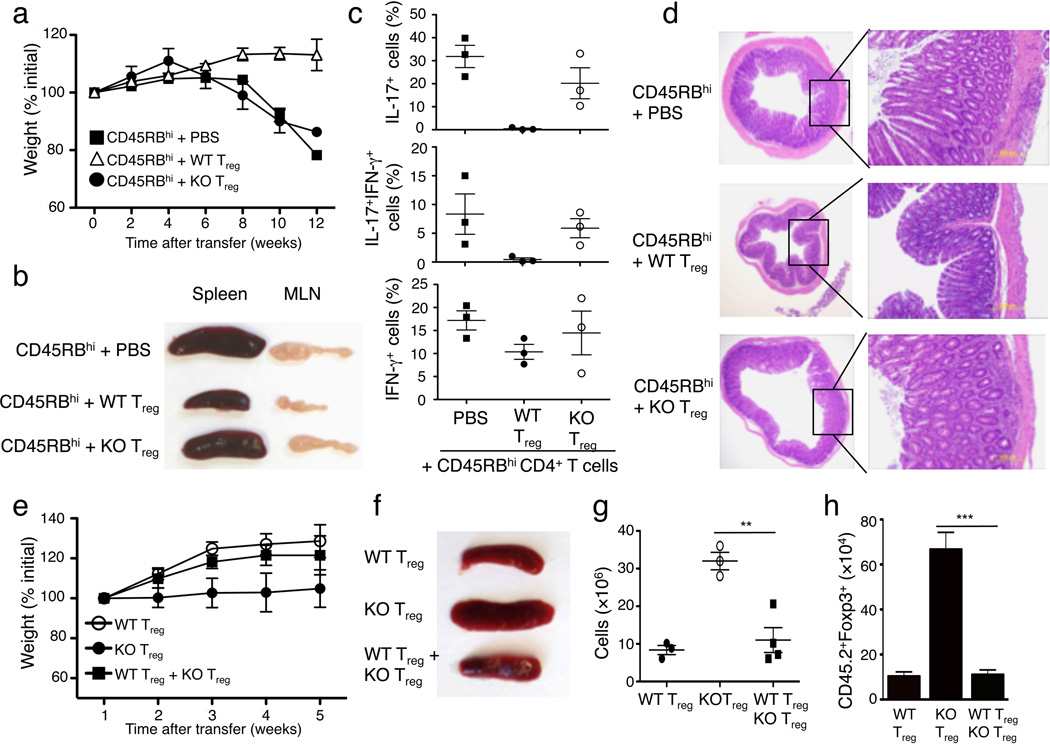
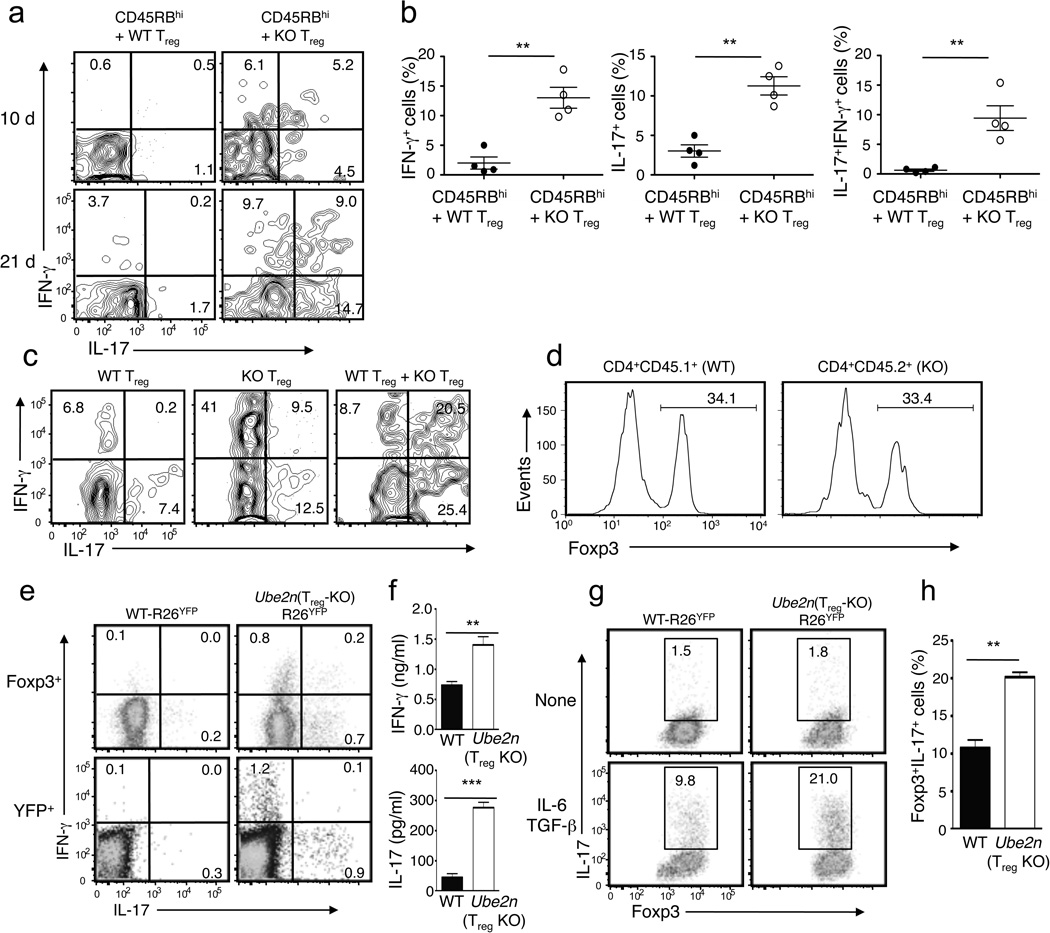
To examine the in vivo function of Treg cells, we employed a well-characterized adoptive transfer approach27. Transfer of CD45RBhi naïve CD4+ T cells to Rag1−/− mice induced overt autoimmunity, defined by gradual weight loss (Fig. 4a), splenomegaly and lymphadenopasy (Fig. 4b), increased frequency of memory and effector-like T cells (Fig. 4c) and hyperplasia of the colonic mucosa (Fig. 4d). Co-transfer of wild-type Treg cells, but not Ubc13-deficient Treg cells, effectively suppressed the autoimmune phenotypes of the naïve CD4+ T cells (Fig. 4a-d), suggesting a marked attenuation of the suppressive activity of Ubc13-deficient Treg cells. Thus, although Ubc13 is dispensable for the homeostasis and in vitro activity of Treg cells, Ubc13 is required for the immunosuppressive function of Treg cells in vivo, a finding that explains the autoimmune phenotype of the Ube2nTreg-KO mice.
Figure 4. Treg-specific Ubc13 ablation impairs the in vivo immunosuppressive function of Treg cells.
(a–d) Disease phenotypes of Rag1 KO mice (6 weeks old) adoptively transferred with WT CD45.1+ congenic CD45RBhi naïve CD4+ T cells together with either PBS or sorted Treg cells derived from WT or Ube2nTreg-KO mice (6 weeks old). Bodyweight was measured at the indicated times and presented as percentage of initial weight (mean±SD) (a). A representative lymphoid organ picture (b), frequency of the indicated cytokine-producing effector CD4+ T cells in the MLN (measured by flow cytometry and gated on CD45.1+ cells) (c), and H&E staining of colon (d) were obtained from recipient mice at 12 weeks after adoptive transfer. Data are from a total of three independent experiments (3 recipient mice per group in each experiment). (e–h) Disease phenotypes of Rag1 KO mice (5 weeks old) adoptively transferred with CD45.1+Foxp3+ WT Treg (from B6.SJL congenic mice) and/or CD45.2+Foxp3+ Ube2nTreg-KO (KO) Treg cells. Bodyweight was measured weekly after transfer (e). A representative picture of spleen (f), absolute number of total splenocytes (g, presented as mean±SD value), and absolute number of CD45.2+Foxp3+ cells from the spleen (h, presented as the mean±SD value) were determined 5 weeks after transfer. **p=0.01 (two-tailed unpaired t-test).
When Treg cells were transferred in the absence of CD45RBhi naïve CD4+ T cells, they did not induce severe loss of bodyweight; however, in contrast to recipients of wild-type Treg cells, the recipients of Ubc13-deficient Treg cells failed to gain weight during the 5-week post-transfer period, indicative of a disease phenotype (Fig. 4e), had splenomegaly coupled with increased spleen cellularity (Fig. 4f,g) as well as a markedly higher number of transferred Treg cells, suggesting their abnormal expansion (Fig. 4h). Abnormal expansion of Ubc13-deficient Treg cells was also detected when they were cotransferred with CD45RBhi naïve CD4+ T cells (Supplementary Fig. 5a). Since the Ubc13-deficient Treg cells displayed only a moderate reduction in apoptosis (Supplementary Fig. 5b), transferred Ubc13-deficient Treg cells might possess increased expansion ability. We found that the pathological phenotypes of Ubc13-deficient Treg cells were efficiently suppressed when they were cotransferred with wild-type Treg cells (Fig. 4e–h). Collectively, these results indicate that the Ubc13-deficient Treg cells may acquire certain inflammatory functions under lymphopenic conditions that can be controlled by wild-type Treg cells.
Effector function acquistion by Ubc13-deficient Treg cells
Recent studies suggest that Treg cells may acquire the pathological ability to produce proinflammatory cytokines when functionally perturbed by genetic alterations or environmental conditions5, 6, 13. Because of the pathological phenotype of the Ubc13-deficient Treg cells, we asked whether Ubc13 had a role in maintaining the stability of Treg cells under lymphopenic or inflammatory conditions. YFP+ Treg cells purified from young (6 week) R26YFP and Ube2nTreg-KOR26YFP mice were almost exclusively Foxp3+, suggesting stable Foxp3 expression (Supplementary Fig. 6a). In agreement with previous studies5,6,10, when YFP+ Treg cells were adoptively transferred into Rag1-deficient mice together with wild-type CD45RBhi naïve CD4+ T cells, a fraction of the Treg cells lost Foxp3, and this happened at comparable levels in Ubc13-sufficient and the Ubc13-deficient Treg cells (Supplementary Fig. 6b).
Because Treg cell conversion into effector-like T cells often occurs without losing Foxp3 expression7,11–13, we next examined the possible acquisition of effector functions in the transferred Treg cells in the MLNs. Consistent with the idea that the majority of Treg cells are stable6, only a small fraction of the Ubc13-sufficient Treg cells acquired the ability to produce interferon-γ (IFN-γ) and IL-17 10 and 21 days after transfer (Fig. 5a). However, a substantially higher frequency of Ubc13-deficient Treg cells displayed a TH1- and TH17-like effector phenotype (Fig. 5a,b). Some of the converted Ubc13-deficient Treg cells were IFN-γ-IL-17 double positive.
Figure 5. Ubc13-deficient Treg cells are sensitive to lymphopenic and inflammatory conditions for acquiring effector functions.
(a, b) Flow cytometry analysis of IL-17- and IFN-γ-producing MLN Treg cells (gated on CD45.2+Foxp3+CD4+ cells) derived from Rag1 KO mice (6 weeks old) adoptively transferred, for 10 or 21 days, with WT CD45.1+CD4+CD25−CD45RBhi T cells plus CD4+YFP+ Treg cells purified from WT-R26YFP (WT) or Ube2nTreg-KOR26YFP (KO) mice (6 weeks old, CD45.2+). Data are representative (a) or summary (b, day 21 data only) of two independent experiments (n=3). *p=0.05. (c) Flow cytometry analysis of IL-17- and IFN-γ-producing MLN Treg cells (gated on CD45.2+Foxp3+ cells) from Rag1 KO mice (5 weeks old) adoptively transferred, for 5 weeks, with CD45.1+Foxp3+ WT and/or CD45.2+Foxp3+ Ube2nTreg-KO (KO) Treg cells. (d) Flow cytometric analysis of Foxp3 expression in CD45.1+Foxp3+ and CD45.2+Foxp3+ Treg cells from the Rag1 KO recipients of WT plus KO Treg cells described in d. (e) Flow cytometry measuring the frequency of IL-17- and IFN-γ-expressing Treg cells in the spleen of 10 weeks old mice, gating on Foxp3+ or YFP+ cells. (f) ELISA quantifying the secreted IFN-γ and IL-17 by YFP+CD4+ Treg cells (isolated from 10 weeks old mice), stimulated with PMA and ionomycin for 24 h. Data are presented as means±SD of two independent experiments. (g, h) Flow cytometric analysis of the frequency of Foxp3+IL-17+ cells in sorted YFP+CD4+ Treg cells, derived from the indicated mice (6 weeks old) and activated in the absence or presence of TGF-β and IL-6. Data are representative (e) or summary (f) of four independent experiments (n=3). **p=0.01.
We next examined whether the effector phenotype acquisition by the Ubc13-deficient Treg cells depended on the cotransferred naïve CD4+ T cells. Interestingly, even when transferred alone, Ubc13-deficient Treg cells acquired effector T cell phenotype (Fig. 5c), with a large proportion of these mutant Treg cells displaying an IFN-γ+ TH1-like effector phenotype, although the frequency of IL-17+ and IL-17+IFN-γ+ cells was also increased compared to wild-type Treg cells (Fig. 5c). The effector T cell phenotype of Ubc13-deficient Treg cells was not suppressed by the cotransfer of wild-type Treg cells. Instead, wild-type Treg cells promoted the shift of Ubc13-deficient Treg cells from an IFN-γ+ to an IL-17+ and IL-17+IFN-γ+ effector phenotype (Fig. 5c). We also analyzed Foxp3 expression in the transferred Treg cells. Consistent with a recent study13, we found that in the absence of naïve T cells, transferred Treg cells had a profound loss of Foxp3. This was similarly seen in wild-type Treg and Ubc13-deficient Treg cells (Fig. 5d).
We next examined whether Ubc13-deficient Treg cells acquired an effector phenotypes under non-transfer conditions, in Ube2nTreg-KO mice, at the time of overt autoimmune symptoms (Fig. 5e). Gating on either Foxp3+ (Treg) or YFP+ (Treg and ex-Foxp3) cells, we detected a fraction of Treg cells expressing IFN-γ or IL-17 in the Ube2nTreg-KOR26YFP, but not the R26YFP mice (Fig. 5e). Consistently, ELISA revealed that the Ubc13-deficient Treg cells secreted significantly more IFN-γ and IL-17 than wild-type Treg cells (Fig. 5f). Thus, Ubc13 maintains the integrity of Treg cells at steady-state.
Treg conversion into TH17-like cells can be induced in vitro by the proinflammatory cytokine IL-6 or by IL-6 in combination with other cytokines7,14. We found that YFP+ Treg cells purified from 6 week old R26YFP and Ube2nTreg-KOR26YFP mice were largely inert in the production of effector cytokines (data not shown). Furthermore, ex vivo activation of purified Treg cells under neutral conditions led to the induction of a small percentage of IL-17-producing cells, which was not substantially different between the control and Ubc13-deficient Treg cells (Fig. 5g). However, upon activation in the presence of IL-6 and TGF-β, Ubc13-deficient Treg cells produced a substantially higher percentage of TH17-like cells than wild-type Treg cells (Fig. 5g,h). Acquisition of an effector T cell phenotype was not associated with loss of Foxp3. Collectively, these results suggest that Ubc13 deficiency rendered Treg cells sensitive to acquiring TH1- and TH17-like effector activity under lymphopenic and inflammatory conditions.
IKK mediates the function of Ubc13 in Treg cells
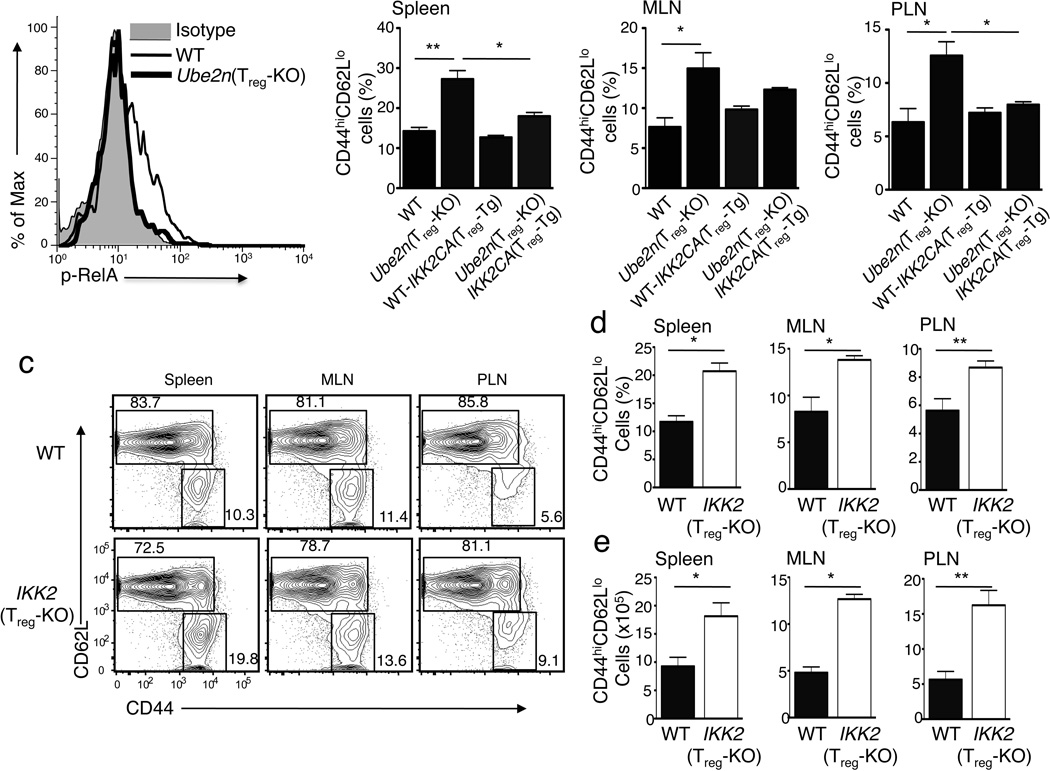
A major signaling function of Ubc13 in T cells is to mediate TCR-CD28-stimulated activation of IKK-NF-κB signaling pathway18,19. Consistently, we found that crosslinking of TCR and CD28 in wild-type, but not in Ubc13-deficient, Treg cells induced the phosphorylation of RelA (Fig. 6a), a molecular event known to be mediated by IKK2 (also called IKKβ)28. To examine whether impaired IKK-NF-κB signaling is responsible for the immunosuppressive defect of Ubc13-deficient Treg cells, we rescued this pathway by employing a transgenic mouse carrying a constitutively active IKK2 (IKK2CA) transgene under the control of a loxP-flanked Stop cassette. In this transgenic system, the expression of IKK2CA occurs conditionally when crossed with cell-specific Cre mice29. By crossing this IKK2CA-floxed transgenic (Tg) mouse with the Ube2nf/fFoxp3GFP-Cre mice, we generated age-matched Ube2n+/+IKK2CATg/+Foxp3GFP-Cre (hereafter named WT-IKK2CATreg-Tg) and Ube2nfl/flIKK2CATg/+Foxp3GFP-Cre (hereafter named Ube2nTreg-KOIKK2CATreg-Tg) mice.
Figure 6. The Treg-specific function of Ubc13 involves its downstream target IKK.
(a) Flow cytometric analysis of RelA phosphorylation in sorted Treg cells from WT or Ube2nTreg-KO mice, stimulated with anti-CD3 and anti-CD28 for 15 min using a crosslinking method45. (b) Flow cytometric analysis of CD44hiCD62Llo memory-like T cells (gated on CD3+CD4+ cells) within different lymphoid organs of the indicated mice (6–8 weeks old). Data are presented as mean ± SD value and representative of three independent experiments. *p=0.05 and **p=0.01 (two-tailed unpaired t-test). (c) Flow cytometric analysis of CD4+ T cells derived from the indicated lymphoid organs of WT or IKK2Treg-KO mice (7 weeks old), measuring the percentage (numbers in quadrangles) of naïve (CD44loCD62Lhi) and memory-like (CD44hiCD62Llo) T cells. Data are representative of three experiments. (d, e) Flow cytometric analysis of the frequency (d) and absolute number (e) of CD44hiCD62lo memory-like CD4+ T cells in the indicated lymphoid organs of WT and IKK2Treg-KO littermates (7 weeks old). Data are representative of two independent experiments.
Treg-specific expression of the IKK2CA transgene, in either WT or Ube2nTreg-KO background, did not substantially alter the homeostasis of Treg cells (Supplementary Fig. 7). However, IKK2CA rescued the suppressive function of Ubc13-deficient Treg cells, as indicated by the reduction of memory and effector-like CD4+ T cell in Ube2nTreg-KOIKK2CATreg-Tg mice (Fig. 6b). Treg-specific expression of IKK2CA did not appreciably alter the frequency of memory-effector-like T cells in wild-type mice (Fig. 6b). To examine whether IKK2 was required for Treg function, we generated Treg-conditional IKK2-deficient mice by crossing IKK2-floxed mice30 with the Foxp3-GFP-hCre mice25, resulting in IKK2fl/flFoxp3GFP-hCre (hereafter called IKK2Treg-KO) and age-matched IKK2+/+Foxp3GFP-hCre littermate control (hereafter called wild-type) mice. As seen in Ube2nTreg-KO mice, IKK2Treg-KO mice had a marked increase in the frequency (Fig. 6c, d) and number (Fig. 6e) of memory and effector-like T cells in peripheral lymphoid organs. Furthermore, the frequency of effector CD4+ T cells producing IL-17 and IFN-γ was greatly increased (Supplementary Fig. 8a,b). However, like in the case of Ubc13 deficiency (Fig. 3a-d), the loss of IKK2 in Treg cells did not substantially alter Treg homeostasis (Supplementary Fig. 9a). The moderate reduction in the frequency of Treg cells observed in IKK2Treg-KO mice might be due to the expansion of IKK2-sufficient effector T cells, because the absolute number of Treg cells was comparable in IKK2-deficient and wild-type mice (Supplementary Fig. 9b). Moreover, the IKK2-deficient Treg cells resembled the Ubc13-deficient Treg cells in that they were vulnerable to acquisition of TH17- and TH1-like effector functions in vivo (Supplementary Fig. 10a,b). These findings suggest an important role for the Ubc13-IKK signaling axis in maintaining the function and stability of Treg cells.
Ubc13 regulates Treg expression of SOCS1 and IL-10
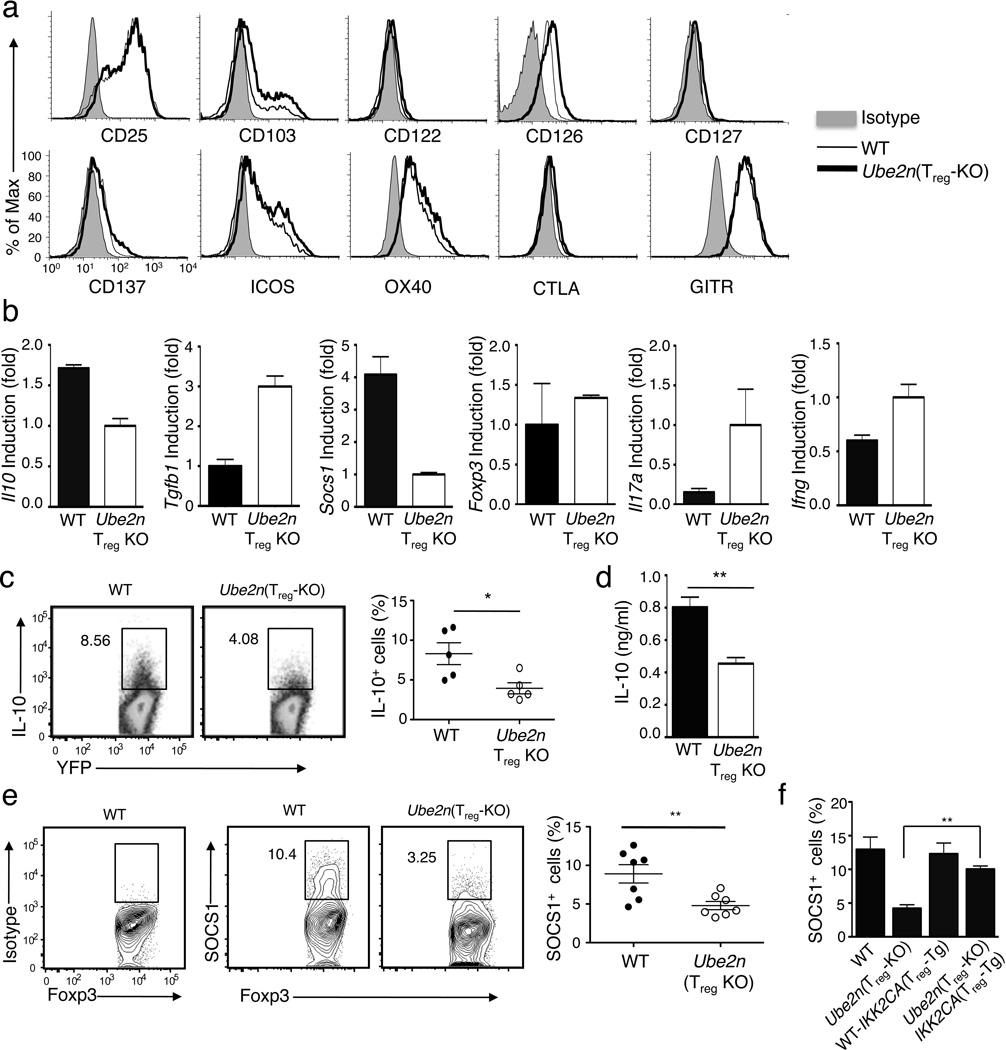
To further explore the molecular mechanism by which Ubc13 regulates the function of Treg cells, we examined the effect of Treg-specific Ubc13 deletion on the expression of various Treg signature genes. Ubc13 deficiency did not inhibit the expression of a panel of well-characterized Treg markers in young mice (5 weeks old) (Fig. 7a). In older (8 weeks) Ube2nTreg-KO mice, Treg cells displayed moderately increased expression of several Treg markers, including ICOS, OX40, CTLA-4, and GITR (data not shown), probably due to the inflammatory environment in these mice. Because Foxp3 is largely responsible for the expression of these Treg markers, this result was consistent with the dispensable role of Ubc13 in the expression of Foxp3.
Figure 7. Ubc13 is dispensable for expression of Treg signature genes but regulates expression SOCS1 and IL-10.
(a) Flow cytometric analysis of splenocytes derived from WT-R26YFP and Ube2nTreg-KOR26YFP mice (5 weeks old), measuring the expression of surface markers on Treg cells (gated on Foxp3+CD4+). Data are representative of two independent experiments. (b) Real-time RT-PCR analysis of the relative mRNA expression level of the indicated genes in YFP+CD4+ Treg cells, sorted (based on YFP) from 5-week old WT-R26YFP and Ube2nTreg-KOR26YFP mice. Data were normalized to a reference gene, β-actin. (c) Flow cytometry measuring the frequency of IL-10-producing cells among the YFP+CD4+ Treg cells from MLN of WT-R26YFP and Ube2nTreg-KOR26YFP mice (5 weeks old). Data are representative (left) and summary (right) of three independent experiments (n=5/genotype). *p=0.05 (two-tailed unpaired t-test). (d) ELISA determining IL-10 production by purified YFP+CD4+ Treg cells stimulated with PMA and ionomycin for 24 h. Data are mean±SD of three independent experiments. (e) Flow cytometric analysis of SOCS1-expressing cells in Foxp3+CD4+ Treg cells from MLN of WT-R26YFP and Ube2nTreg-KOR26YFP mice (6 weeks old). Data are representative (left) and summary (right) of three independent experiments (n=4–7/genotype in each experiment). **p=0.01 (two-tailed unpaired t-test). (f) Flow cytometric analysis of SOCS1-expressing Treg cells in the indicated littermates (6 weeks old), presented as a summary graph. **p=0.01 (two-tailed unpaired t-test).
We next performed real-time RT-PCR to measure the expression of various cytokine genes involved in the suppressive function and effector conversion of Treg cells. The Ubc13-deficient Treg cells competently expressed Tgfb1; however, the expression of Il10 was attenuated (Fig. 7b). This result was further confirmed by flow cytometry (Fig. 7c) and ELISA (Fig. 7d). The IL-10 expression defect of Ubc13-deficient Treg cells was largely rescued by expression of the IKK2CA transgene, suggesting the involvement of IKK signaling axis (Supplementary Fig. 11). Because the IL-10 receptor signaling plays an important role in Treg function, particularly in the control of TH17-cell responses31–33, this finding partially explains the functional impairment of Ubc13-deficient Treg cells.
Another gene that was substantially affected by Ubc13 deficiency was Socs1 (Fig. 7b), which is known to regulate Treg stability and prevent Treg conversion into TH1- and TH17-like effector T cells13. SOCS1, as well as its homologue SOCS3, negatively regulate signal transduction stimulated by different cytokines, including IL-6, which stimulates Treg conversion. Consistent with a prior report34, SOCS3 expression was extremely low in both control and Ubc13-deficient Treg cells (data not shown), while SOCS1 was abundantly expression in the wild-type but not in Ubc13-deficient Treg cells (Fig. 7b). Flow cytometry also showed strong expression of SOCS1 protein in wild-type Treg cells and substantially reduced expression in Ubc13-deficient Treg cells (Fig. 7e). Furthermore, the expression of Il10 and Socs1 was also reduced in IKK2-deficient Treg cells (Supplementary Fig. 12).
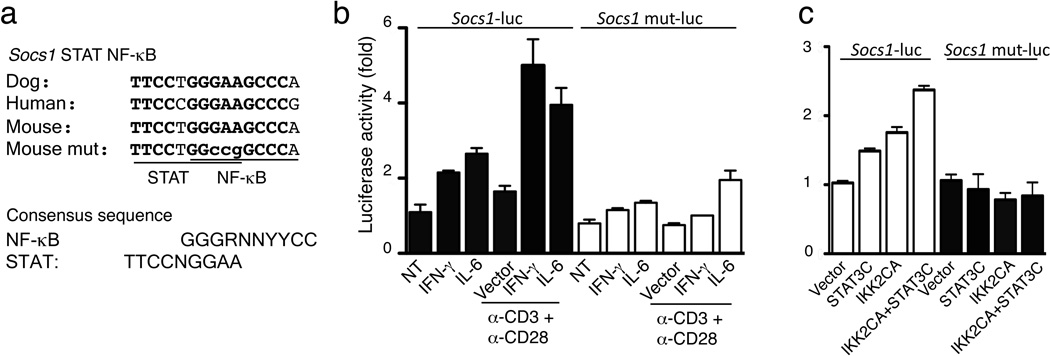
The Il10 promoter is known to contain functional binding sites for NF-κB and STAT35,36, while the role of NF-κB in Socs1 gene regulation is less well defined. The Socs1 promoter contains STAT-binding sites and responds to stimulation by IFN-γ and IL-637. However, optimal induction of SOCS1 in T cells requires the synergy of cytokine and TCR signals38. SOCS1 is also induced by human T-cell leukemia virus type I, which appears to involve IKK-NF-κB activation39. To examine the role of the IKK-NF-κB pathway in SOCS1 expression, we tested if the Treg-specific IKK2CA transgene can rescue SOCS1 expression in Ubc13-deficient Treg cells. SOCS1 expression was largely rescued in Treg cells from Ube2nTreg-KOIKK2CATreg-Tg (Fig. 7f,). By analyzing the conserved noncoding sequences of the Socs1 gene, we identified a putative NF-κB-binding site that is highly conserved among different species and overlaps with a previously identified STAT-binding site to form a putative STAT-NF-κB composite (Fig. 8a). In luciferase reporter assays, a wild-type Socs1 promoter was activated by TCR-CD28 stimuli in synergy with IFN-γ or IL-6, while activation of a STAT-NF-κB binding site mutant Socs1 promoter was impaired (Fig. 8b). In similar reporter assays, a constitutive form of STAT3 (STAT3C) synergized with IKK2CA to activate the Socs1 promoter, in a manner dependent on the STAT-NF-κB composite site as well (Fig. 8c). These findings emphasize an important role for the Ubc13-IKK axis in maintaining expression of IL-10 and SOCS1 in Treg cells and suggest the direct involvement of NF-κB in mediating Socs1 gene induction.
Figure 8. Synergistic activation of Socs1 promoter by IKK2 and STAT3 via a STAT-NF-κB composite.
(a) A putative NF-κB-binding sequence with an overlapping STAT-binding site. Mutated nucleotides in Socs1 mut-luc are shown in lower-case letters. STAT- and NF-κB-binding consensus sites are shown below. (b) Luciferase assays determining the activation of Socs1-luciferase and Socs1-mut luciferase reporters in EL-4 cells stimulated by the indicated inducers. Luciferase activity was normalized to Renilla luciferase and presented as fold of induction compared to the non-treated (NT) cells. Data are representative of three independent experiments with consistent results. (c) Luciferase assays measuring the activation of Socs1-luciferase and Socs1-mut luciferase reporters in EL-4 cells by the constitutively active STAT3 (STAT3C) and IKK2 (IKK2CA). Luciferase activity was determined as in b and presented as fold of induction compared to the vector-transfected cells. Data are representative of three independent experiments with consistent results.
SOCS1 plays a role in modulating Treg stability
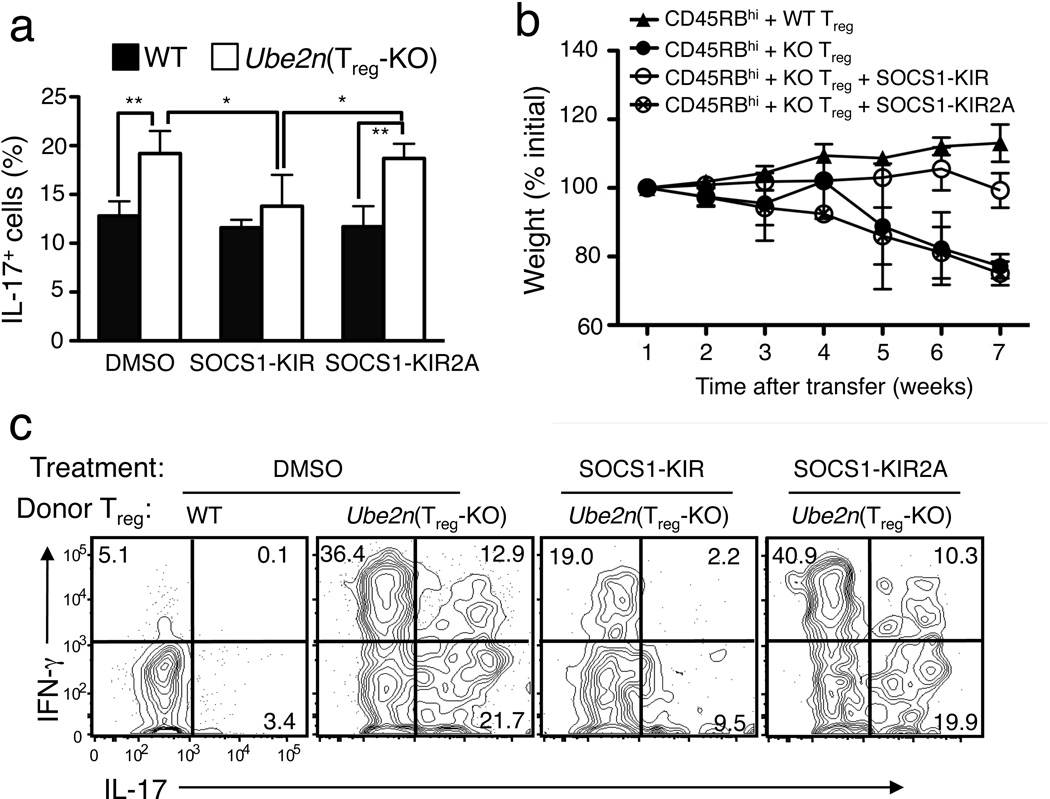
To determine whether the reduced SOCS1 expression in Ubc13-deficient Treg cells contributed to their functional abnormality, we used a SOCS1 mimetic peptide that spans the kinase inhibitory region (KIR) of SOCS1 and is known to effectively inhibit IL-6 and IFN-γ signaling in vitro and partially rescue SOCS1 function in vivo15,40–42. SOCS1-KIR peptide, but not the control peptide SOCS1-KIR2A, significantly inhibited the conversion of Ubc13-deficient Treg cells to TH17-like cells in vitro (Fig. 9a). At the dose used, SOCS1-KIR did not significantly inhibit the conversion of wild-type Treg cells, probably due to the already high levels of endogenous SOCS1.
Figure 9. A SOCS1 mimetic peptide partially rescues the functional defect of Ubc13-deficient Treg cells both in vitro and in vivo.
(a) Flow cytometry measuring the frequency of IL-17-producting cells in sorted YFP+CD4+ Treg cells derived from WT-R26YFP and Ube2nTreg-KOR26YFP mice, activated under TH17 polarizing conditions in the presence of DMSO, the SOCS1 mimetic peptide SOCS1-KIR, or the negative control peptide SOCS1-KIR2A. *p=0.05, **p=0.01 (two-tailed unpaired t-test). Data are representative of two independent experiments. (b,c) Disease phenotypes of Rag1 KO mice (5 weeks old) adoptively transferred with WT CD45.1+CD4+CD25−CD45RBhi naïve T cells together with CD4+YFP+ Treg cells purified from WT-R26YFP or Ube2nTreg-KOR26YFP mice (6 weeks old). The recipient mice were either not treated or injected (i.p.) with SOCS1-KIR or SOCS1-KIR2A every other day. Bodyweight of recipient mice was measured at different times and presented as percentage of initial weight (b), and the frequency of IL-17+ or IFN-γ+ cells among the transferred CD4+YFP+ Treg cells was determined by flow cytometry (gated on CD45.2+ cells) after 7 weeks of transfer (c).
We next examined whether SOCS1-KIR could rescue the functional defect of Ubc13-deficient Treg cells in vivo using the CD45RBhi T-cell transfer model. The Rag1 KO mice adoptively transferred with CD45RBhi naïve CD4+ T cells plus Ubc13-deficient Treg cells experienced severe weight loss (Fig. 9b). Repeated injection of SOCS1-KIR, but not the control SOCS1-KIR2A, into these recipient mice substantially, although not completely, prevented their weight loss (Fig. 9b). Furthermore, injection of SOCS1-KIR, but not SOCS1-KIR2A, also partially inhibited the conversion of Ubc13-deficient Treg cells into inflammatory effector T cells in vivo (Fig. 9c). Although direct suppression of effector T cells may account for the in vivo effect of SOCS1-KIR treatment, these results indicate a role for Ubc13-mediated SOCS1 expression in the maintenance of Treg stability.
DISCUSSION
Here we identify the ubiquitin-conjugating enzyme (E2) Ubc13 as a mediator of Treg-intrinsic function in immune tolerance. Because Ubc13 is the predominant E2 that conjugates K63-linked ubiquitin chains, this finding further emphasizes a role for K63 ubiquitination in Treg function. We found that Treg-specific ablation of Ubc13 impaired the in vivo immunosuppressive function of Treg cells, causing aberrant activation and homeostasis of conventional T cells and multiorgan inflammation. Unlike most other known Treg regulators, Ubc13 was dispensable for maintaining the expression of Foxp3 and various Treg signature genes. Consistently, the autoimmune disease of the Ube2nTreg-KO mice was relatively milder than that of the Scurfy mice or Foxp3-deficient mice that completely lack Treg cells2. This phenotype suggests that Ubc13 regulates specific aspects of Treg functions, rather than controls the overall suppressive activity of Treg cells.
Our data suggest that Ubc13 has an important role in preventing the conversion of Treg cells into TH1- and TH17-like effector T cells under lymphopenic conditions. Transfer of Treg cells into Rag1-deficient mice, either alone or along with CD45RBhi naïve CD4+ T cells, induced a large portion of the Ubc13-deficient Treg cells to acquire the ability to produce the proinflammatory cytokines IL-17 and IFN-γ, in contrast to only a small fraction of transferred wild-type cells. Moreover, the acquisition of effector-like functions by Ubc13-deficient Treg cells occurred without losing the expression of Foxp3, thus distinguishing Ubc13 from the known Foxp3-regulatory factors. We believe that the Ubc13 signaling pathway maintains Treg stability by reducing the sensitivity of Treg cells to environmental factors. Under inflammatory conditions, a fraction of Foxp3+ Treg cells can simultaneously express effector T cell cytokines, particularly IL-177,11,12,43. These findings suggest that proinflammatory cytokines may partially override the inhibitory function of Foxp3. In support of this idea, we found that activation of Treg cells in vitro in the presence of IL-6 and TGF-β induces the production of Foxp3+IL-17+ T cells. Thus, Ubc13-deficient Treg cells were more sensitive to cytokine-induced conversion in vitro.
A major downstream signaling molecule of Ubc13 is IKK17–19. It is possible that MAP kinases, known to be regulated by Ubc1319, also play a role, but both gain-of-function and loss-of-function studies suggest an important role of the IKK pathway downstream Ubc13. Although previous studies suggest a role for IKK-NF-κB in regulating Foxp3 expression and Treg development, they did not address the role of IKK-NF-κB in regulating the stability and functionality of committed Treg cells20–23. Our data suggest that the Ubc13-IKK signaling axis induces specific genes involved in the regulation of Treg stability and function. We have identified Il10 and Socs1 as two important genes regulated by this pathway.
Our findings suggest Socs1 to be a direct target gene of the Ubc13-IKK signaling axis that is synergistically transactivated by STAT and NF-κB in Treg cells. Strong evidence suggests that Il10 may also be a gene that is cooperatively regulated by STAT and NF-κB. A prior study has identified an NF-κB-binding sequence (−54 TGCCAGGAAGGCCCC −43) in the murine Il10 promoter35. We noticed that this NF-κB site is overlapping with a previously identified STAT-binding site (−54 TGCCAGGAA −46)36. These studies suggest the involvement of both NF-κB and STAT3 in the induction of Il10 gene expression, although it remains to be examined whether this putative STAT-NF-κB composite is required for Il10 induction by TCR-CD28 or TCR-CD28 in combination with cytokines35, 36. Given the important role of Treg-derived IL-10 in mediating the in vivo function of Treg cells, especially in the suppression of TH17 cells31,33, the reduced IL-10 expression may contribute to the impaired in vivo function of Ubc13-deficient Treg cells. Future studies need to address whether the Ubc13-IKK signaling pathway mediates expression of additional genes involved in the stability and function of Treg cells.
In conclusion, we present genetic evidence for the involvement of Ubc13-IKK signaling axis in the control of Treg cell stability and function. We propose that Ubc13-IKK regulates the expression of specific molecules involved in the suppressive function and phenotypic stabilization of Treg cells. These findings provide novel insight into the signaling mechanisms that mediate Treg function and also suggest Ubc13 as a possible therapeutic target for the pharmacological manipulation of Treg function.
METHODS
Mice
IKK2-floxed and Ube2n-floxed mice were as described24, 30. Foxp3-GFP-hCre BAC transgenic mice25 (Jackson Lab) were backcrossed for 9 generations to the C57BL/6 background and then crossed with Ube2n-floxed mice to produce age-matched Ube2n+/+Foxp3GFP-hCre (termed WT) and Ube2nfl/flFoxp3 GFP-hCre (termed Ube2nTreg-KO) mice. The WT and IKK2Treg-KO mice were similarly generated. In some experiments, these mice were further crossed with the Rosa26-YFP Cre reporter (termed R26YFP)26 (Jackson Lab) to generate WT and Ube2nTreg-KO mice that express YFP in their Treg cells (termed WT-R26YFP and Ube2nTreg-KOR26YFP mice, respectively). IKK2CA transgenic mice (IKK2CATg; Jackson Lab) carry a constitutively active IKK2 (IKK2CA) transgene under the control of a loxP-flanked stop cassette29. These mice were crossed with the Foxp3-GFP-hCre mice to generate IKK2CATg/+Foxp3 GFP-hCre (termed IKK2CATreg-Tg) mice. These mice were further crossed with the Ube2nTreg-KO mice to generate age-matched Ube2n+/+IKK2CATg/+Foxp3-GFP-hCre (WT-IKK2CATreg-Tg), and Ube2nfl/flIKK2CATg/+ Foxp3-GFP-hCre (Ube2nTreg-KOIKK2CATreg-Tg) mice. B6.SJL mice expressing the CD45.1 congenic marker and Rag1 KO mice (B6 background) were from Jackson Lab. Mice were maintained in specific pathogen-free facility of The University of Texas MD Anderson Cancer Center, and all animal experiments were in accordance with protocols approved by the Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center.
Plasmids, antibodies, and reagents
The expression vectors encoding a FLAG-tagged active STAT3 (STAT3-C Flag pRc-CMV) and an HA-tagged active IKKβ (S177,181E; named IKK2CA in this study) were from Addgene and Dr. Michael Karin, respectively. RelA vector was described previously44. Socs1-luc (previously named pSI2) is a firefly luciferase reporter driven by an 835-base pair murine Socs1 promoter37 (provided by Dr. Tadamitsu Kishimoto). Socs1 mut-luc was created by site-directed mutagenesis to substitute three nucleotides (GAA to CCG) in the STAT-NF-κB composite of the Socs1-luc (see Fig. 8a).
Agonistic anti-CD3 and anti-CD28 antibodies were from eBioscience, anti-UBC13 (Zymed) anti-Hsp60 (H-1) were from Santa Cruz, and other antibodies are listed in the section of flow cytometric analysis. The SOCS1 mimetic peptide SOCS1-KIR and its negative control SOCS1-KIR2A were as described previously42. PMA (phorbol 12-myristate 13-acetate) and ionomycin were from Sigma; IL-6, TGF-β, and IFN-γ were from PeproTech.
Cell isolation from intestinal lamina propria
Intestines were treated twice with PBS containing 10% FBS, 20 mM HEPES, 100 U/ml penicillin, 100 µg/ml streptomycin, and 10 mM EDTA for 20 min at 37°C, to remove epithelial cells, and then digested with 400 Mandl U/ml collagenase D (Roche Applied Science) and 50 µg/ml DNase I (Sigma-Aldrich), to isolate mononuclear cells from the lamina propria. Cells were then enriched by a discontinuous density gradient containing 40 and 75% Percoll (Amersham Bioscience).
Flow cytometric analysis
Flow cytometric analyses and cell sorting were performed45 using a FACSCalibur (BD Bioscience) and FACSAria (BD bioscience), respectively. For RelA phospho-flow, sorted YFP+CD4+ Treg cells were preincubated on ice with anti-CD3 plus anti-CD28 and then stimulated at 37°C by crosslinking using an anti-immunoglobulin antibody45. Cells were stained with the phospho-NF-κB p65(Ser536) antibody. For intracellular cytokine staining (ICS), cells were stimulated with PMA (50 ng/ml) plus ionomycine (750 ng/ml) for 4–6 hrs in the presence of monensin (10 µg/ml), and the fixed cells were incubated with the indicated antibodies and subjected to flow cytometry. Fluorescence-labeled antibodies used include phycoerythrin (PE)-conjugated anti-IL-17, anti-IL-10, anti-CD8, anti-CD25, anti-CD127, anti-CD137, anti-ICOS (eBioscience); FITC-conjugated anti-Foxp3 (eBioscience) and anti-CD62L (BD Bioscience); APC-conjugated anti-Foxp3, anti-CD44, anti-IFN-γ and anti-CD4 (eBioscience); PE-conjugated anti-CD103, anti-CD122, anti-CD126, anti-OX40, anti-GITR (BD Bioscience); Biotin-conjugated anti-SOCS1 (MBL); anti-phospho-NF-κB p65(Ser536) and APC-conjugated anti-Rabbit IgG (Cell Signaling).
In vitro Treg suppression assay
CFSE-labeled naïve CD4+CD25- T cells (1×105) were co-cultured with an increasing ratio of sorted Treg cells in the presence of anti-CD3 (1µg/ml) plus irradiated T-cell-depleted spenocytes (5×104) in 96-well plates for 4 days. The suppressive function of Treg cells was determined by measuring the proliferation of activated CD4 effector T cells based on CFSE dilution45.
In vitro Treg conversion assay
Purified YFP+ Treg cells from WT and Ube2nTreg-KO were stimulated with anti-CD3 Ab and irradiated antigen-presenting cells (CD3-depleted splenocytes) plus IL-6 (20 ng/ml) and TGF-β (1 ng/ml). In some experiments, SOCS1-KIR (30 µM) or SOCS1-KIR2A (30 µM) was used for providing mimetic function of SOCS1. Cells were cultured for 4 days and then subjected to flow cytometric analysis.
Histology
Organs were removed from sacrificed mice, fixed in 10% neutral buffered formalin, embedded in paraffin, and sectioned for hematoxylin-eosin staining.
T-cell adoptive transfer
CD4+CD25−CD45RBhi cells from the B6.SJL congenic mice (carrying the CD45.1 marker) and YFP+ Treg cells from WT or Ube2nTreg-KO mice were prepared by FACS sorting. Rag1−/− mice were injected i.p. with 4 × 105 syngeneic CD4+CD25−CD45RBhi T cells either alone or together with WT Treg or Ube2n KO Treg cells (2 × 105). Mice were observed daily, and any mice showing clinical signs of severe disease were sacrificed. In some experiments, mice were administered, via i.p. injection (in 100 µl final volume), with SOCS1-KIR (60 µg/mouse), SOCS1-KIR2A (60 µg/mouse), or PBS every other day.
IB
Purified Treg and CD4+ T cells were lysed in RIPA buffer with proteasome inhibitors. The cell lysates were fractionated by SDS-PAGE and subjected to IB with the indicated antibodies.
Real-time quantitative RT-PCR
RNA isolation and real-time quantitative RT-PCR were performed as previously described45. The gene-specific primer sets are listed in Supplementary Table 1.
Luciferase reporter assay
Murine EL-4 T cells (2 × 105) were transfected, using lipofectamine (Invitrogen), with Socs1-Luc or Socs1 mut-luc together with a control Renilla luciferase reporter. In some experiments, the cells were also cotransfected with the indicated cDNA expression vectors. After 30–40 h, the cells were either not treated or stimulated as indicated and subjected to dual luciferase assay (Promega). Specific luciferase activity was normalized based on the internal control Renilla luciferase activity in each sample.
ELISA
Immuno Plate Maxisorp plates (NUNC) were coated with 1µg/ml capture Abs for the indicated cytokines in 50 mM sodium bicarbonate and incubated overnight at 4°C. After blocking with 1% BSA in PBS, the plates were incubated with serially diluted standard or samples and then with HRP-conjugated detection antibodies. Following colorimetric reaction (tetramethylbnzidine; Moss), the absorbance at 450 nm was measured using an ELISA plate reader.
Statistical analysis
Prism software was used for two-tailed unpaired t-tests. P values of less than 0.05 or 0.01 were considered significant and very significant, respectively.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Drs. M. Karin and T. Kishimoto for reagents. We also thank the personnel from the flow cytometry, the DNA analysis, and the histology core facilities at MD Anderson Cancer Center for technical assistance. This study was supported by grants from National Institutes of Health (AI057555, AI064639, GM84459) and the G. S. Hogan Gastrointestinal Cancer Research Fund.
Footnotes
AUTHOR CONTRIBUTIONS
J.-H.C. designed and did the research, prepared the figures, and wrote part of the manuscript; Y.X. did the luciferase assays; X.Z. provided technical help in adoptive transfer; H.H. did the Ubc13 IB; J.J. constructed the Socs1 mut-luc plasmid; J.Y., X.H.C., and X.W. contributed to the generation of mouse models; H.M.J., S.A., and M.P contributed reagents; and S.-C.S. designed the research and wrote the manuscript.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
REFERENCES
- 1.Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell. 2008;133:775–787. doi: 10.1016/j.cell.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 2.Rudensky AY. Regulatory T cells and Foxp3. Immunol. Rev. 2011;241:260–268. doi: 10.1111/j.1600-065X.2011.01018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shevach EM. Mechanisms of foxp3+ T regulatory cell-mediated suppression. Immunity. 2009;30:636–645. doi: 10.1016/j.immuni.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 4.Rubtsov YP, et al. Stability of the regulatory T cell lineage in vivo. Science. 2010;329:1667–1671. doi: 10.1126/science.1191996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey-Bucktrout SL, Bluestone JA. Regulatory T cells: stability revisited. Trends Immunol. 2011;32:301–306. doi: 10.1016/j.it.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hori S. Regulatory T cell plasticity: beyond the controversies. Trends Immunol. 2011;32:295–300. doi: 10.1016/j.it.2011.04.004. [DOI] [PubMed] [Google Scholar]
- 7.Yang XO, et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity. 2008;29:44–56. doi: 10.1016/j.immuni.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duarte JH, Zelenay S, Bergman ML, Martins AC, Demengeot J. Natural Treg cells spontaneously differentiate into pathogenic helper cells in lymphopenic conditions. Eur. J. Immunol. 2009;39:948–955. doi: 10.1002/eji.200839196. [DOI] [PubMed] [Google Scholar]
- 9.Tsuji M, et al. Preferential generation of follicular B helper T cells from Foxp3+ T cells in gut Peyer's patches. Science. 323:1488–1492. doi: 10.1126/science.1169152. (323) [DOI] [PubMed] [Google Scholar]
- 10.Zhou X, et al. Instability of the transcription factor Foxp3 leads to the generation of pathogenic memory T cells in vivo. Nat. Immunol. 2009;10:1000–1007. doi: 10.1038/ni.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ayyoub M, et al. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc. Natl. Acad. Sci. USA. 2009;106:8635–8640. doi: 10.1073/pnas.0900621106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Voo KS, et al. Identification of IL-17-producing FOXP3+ regulatory T cells in humans. Proc. Natl. Acad. Sci. USA. 2009;106:4793–4798. doi: 10.1073/pnas.0900408106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takahashi R, et al. SOCS1 is essential for regulatory T cell functions by preventing loss of Foxp3 expression as well as IFN-{gamma} and IL-17A production. J. Exp. Med. 2011;208:2055–2067. doi: 10.1084/jem.20110428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu L, Kitani A, Fuss I, Strober W. Cutting edge: regulatory T cells induce CD4+CD25-Foxp3- T cells or are self-induced to become TH17 cells in the absence of exogenous TGF-beta. J. Immunol. 2007;178:6725–6729. doi: 10.4049/jimmunol.178.11.6725. [DOI] [PubMed] [Google Scholar]
- 15.Collins EL, et al. Inhibition of SOCS1−/− lethal autoinflammatory disease correlated to enhanced peripheral Foxp3+ regulatory T cell homeostasis. J. Immunol. 2011;187:2666–2676. doi: 10.4049/jimmunol.1003819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 17.Bhoj VG, Chen ZJ. Ubiquitylation in innate and adaptive immunity. Nature. 2009;458:430–437. doi: 10.1038/nature07959. [DOI] [PubMed] [Google Scholar]
- 18.Sun L, Deng L, Ea C-K, Xia Z-P, Chen ZJ. The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell. 2004;14:289–301. doi: 10.1016/s1097-2765(04)00236-9. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto M, et al. Cutting Edge: Pivotal function of Ubc13 in thymocyte TCR signaling. J. Immunol. 2006;177:7520–7524. doi: 10.4049/jimmunol.177.11.7520. [DOI] [PubMed] [Google Scholar]
- 20.Isomura I, et al. c-Rel is required for the development of thymic Foxp3+ CD4 regulatory T cells. J. Exp. Med. 2009;206:3001–3014. doi: 10.1084/jem.20091411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Long M, Park SG, Strickland I, Hayden MS, Ghosh S. Nuclear factor-kappaB modulates regulatory T cell development by directly regulating expression of Foxp3 transcription factor. Immunity. 2009;31:921–931. doi: 10.1016/j.immuni.2009.09.022. [DOI] [PubMed] [Google Scholar]
- 22.Ruan Q, et al. Development of Foxp3(+) regulatory t cells is driven by the c-Rel enhanceosome. Immunity. 2009;31:932–940. doi: 10.1016/j.immuni.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng Y, et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature. 2010;463:808–812. doi: 10.1038/nature08750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto M, et al. Key function for the Ubc13 E2 ubiquitin-conjugating enzyme in immune receptor signaling. Nat. Immunol. 2006;7:962–970. doi: 10.1038/ni1367. [DOI] [PubMed] [Google Scholar]
- 25.Zhou X, et al. Selective miRNA disruption in T reg cells leads to uncontrolled autoimmunity. J. Exp. Med. 2008;205:1983–1991. doi: 10.1084/jem.20080707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morrissey PJ, Charrier K, Braddy S, Liggitt D, Watson JD. CD4+ T cells that express high levels of CD45RB induce wasting disease when transferred into congenic severe combined immunodeficient mice. Disease development is prevented by cotransfer of purified CD4+ T cells. J. Exp. Med. 1993;178:237–244. doi: 10.1084/jem.178.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mattioli I, et al. Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. J. Immunol. 2004;172:6336–6344. doi: 10.4049/jimmunol.172.10.6336. [DOI] [PubMed] [Google Scholar]
- 29.Sasaki Y, et al. Canonical NF-kappaB activity, dispensable for B cell development, replaces BAFF-receptor signals and promotes B cell proliferation upon activation. Immunity. 2006;24:729–739. doi: 10.1016/j.immuni.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 30.Pasparakis M, et al. TNF-mediated inflammatory skin disease in mice with epidermis-specific deletion of IKK2. Nature. 2002;417:861–866. doi: 10.1038/nature00820. [DOI] [PubMed] [Google Scholar]
- 31.Rubtsov YP, et al. Regulatory T cell-derived interleukin-10 limits inflammation at environmental interfaces. Immunity. 2008;28:546–558. doi: 10.1016/j.immuni.2008.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Chaudhry A, et al. Interleukin-10 signaling in regulatory T cells is required for suppression of TH17 cell-mediated inflammation. Immunity. 2011;34:566–578. doi: 10.1016/j.immuni.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huber S, et al. TH17 cells express interleukin-10 receptor and are controlled by Foxp3− and Foxp3+ regulatory CD4+ T cells in an interleukin-10-dependent manner. Immunity. 2011;34:554–565. doi: 10.1016/j.immuni.2011.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pillemer BB, Xu H, Oriss TB, Qi Z, Ray A. Deficient SOCS3 expression in CD4+CD25+FoxP3+ regulatory T cells and SOCS3-mediated suppression of Treg function. Eur. J. Immunol. 2007;37:2082–2089. doi: 10.1002/eji.200737193. [DOI] [PubMed] [Google Scholar]
- 35.Cao S, Zhang X, Edwards JP, Mosser DM. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. J. Biol. Chem. 2006;281:26041–26050. doi: 10.1074/jbc.M602222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Benkhart EM, Siedlar M, Wedel A, Werner T, Ziegler-Heitbrock HW. Role of Stat3 in lipopolysaccharide-induced IL-10 gene expression. J. Immunol. 2000;165:1612–1617. doi: 10.4049/jimmunol.165.3.1612. [DOI] [PubMed] [Google Scholar]
- 37.Saito H, et al. IFN regulatory factor-1-mediated transcriptional activation of mouse STAT-induced STAT inhibitor-1 gene promoter by IFN-gamma. J Immunol. 2000;164:5833–5843. doi: 10.4049/jimmunol.164.11.5833. [DOI] [PubMed] [Google Scholar]
- 38.Diehl S, et al. Inhibition of TH1 differentiation by IL-6 is mediated by SOCS1. Immunity. 2000;13:805–815. doi: 10.1016/s1074-7613(00)00078-9. [DOI] [PubMed] [Google Scholar]
- 39.Charoenthongtrakul S, Zhou Q, Shembade N, Harhaj NS, Harhaj EW. Human T cell leukemia virus type 1 Tax inhibits innate antiviral signaling via NF-kappaB-dependent induction of SOCS1. J. Virol. 2011;85:6955–6962. doi: 10.1128/JVI.00007-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flowers LO, et al. Characterization of a peptide inhibitor of Janus kinase 2 that mimics suppressor of cytokine signaling 1 function. J. Immunol. 2004;172:7510–7518. doi: 10.4049/jimmunol.172.12.7510. [DOI] [PubMed] [Google Scholar]
- 41.Flowers LO, Subramaniam PS, Johnson HM. A SOCS-1 peptide mimetic inhibits both constitutive and IL-6 induced activation of STAT3 in prostate cancer cells. Oncogene. 2005;24:2114–2120. doi: 10.1038/sj.onc.1208437. [DOI] [PubMed] [Google Scholar]
- 42.Jager LD, et al. The kinase inhibitory region of SOCS-1 is sufficient to inhibit T-helper 17 and other immune functions in experimental allergic encephalomyelitis. J. Neuroimmunol. 2011;232:108–118. doi: 10.1016/j.jneuroim.2010.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kryczek I, et al. IL-17+ regulatory T cells in the microenvironments of chronic inflammation and cancer. J. Immunol. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 44.Harhaj EW, Maggirwar SB, Sun S-C. Inhibition of p105 processing by NF-κB proteins in transiently transfected cells. Oncogene. 1996;12:2385–2392. [PubMed] [Google Scholar]
- 45.Chang M, et al. The ubiquitin ligase Peli1 negatively regulates T cell activation and prevents autoimmunity. Nat. Immunol. 2011;12:1002–1009. doi: 10.1038/ni.2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.