Abstract
Objectives
Inflammation may directly impair HDL functions, in particular reverse cholesterol transport (RCT), but limited data support this concept in humans.
Methods and Results
We employed low-dose human endotoxemia to assess the effects of inflammation on HDL and RCT-related parameters in vivo. Endotoxemia induced remodelling of HDL with depletion of pre-β1a HDL particles determined by 2-D gel electrophoresis (-32.2 ± 9.3% at 24h, p<0.05) as well as small (-23.0 ± 5.1%, p<0.01, at 24h) and medium (-57.6 ± 8.0% at 16h, p<0.001) HDL estimated by nuclear magnetic resonance (NMR). This was associated with induction of class II secretory phospholipase A2 (~36 fold increase) and suppression of lecithin:cholesterol acyltransferase activity (-20.8 ± 3.4% at 24h, p<0.01) and cholesterol ester transfer protein mass (-22.2 ± 6.8% at 24h, p<0.001). The HDL fraction, isolated following endotoxemia, had reduced capacity to efflux cholesterol in vitro from SR-BI and ABCA1, but not ABCG1 transporter cell models.
Conclusions
These data support the concept that “atherogenic-HDL dysfunction” and impaired RCT occur in human inflammatory syndromes, largely independent of changes in plasma HDL-C and ApoA-I levels.
Keywords: inflammation, atherosclerosis, cholesterol, lipoproteins, macrophages
Introduction
Reverse cholesterol transport (RCT) is considered a major anti-atherogenic function of high density lipoprotein (HDL)1. Loss of HDL RCT functions may contribute to atherosclerosis in chronic inflammatory states including obesity and insulin resistance. In fact, inflammation may directly impair HDL function and RCT2, 3, but limited data support this concept in humans4, 5.
Experimental studies in rodents and primates suggest that acute inflammation alters HDL composition6, 7, with increased catabolism of HDL particles8, enrichment of acute phase HDL with serum amyloid-A (SAA)9, and loss of apolipoprotein (apo) A-I2. These changes combined with inflammatory down-regulation of the hepatic scavenger receptor BI (SR-BI)10, and reduced transport of cholesterol to bile and feces3, 11, provide indirect support for an integrated effect in vivo of inflammation on RCT. A few human studies have characterized inflammatory changes in plasma lipoproteins4, 5, 12, 13.
We published the first direct in vivo evidence in rodents that inflammation impairs multiple steps of the RCT pathway11, which was recently validated by others14, 15. We also provided preliminary proof of this concept in humans11 and found that human endotoxemia induced a loss of HDL cholesterol efflux functions coincident with HDL depletion of phospholipid and enrichment with SAA11.
In this paper, we extend our human endotoxemia studies11 to examine (1) inflammatory change in HDL-particle size and distribution of ApoA-I-containing HDL particles at nuclear magnetic resonance (NMR) and 2D gel electrophoresis, (2) change in HDL modulating enzymes and proteins, and (3) relation of change in HDL parameters with loss of HDL cholesterol efflux functions in vitro in ABCA1, SR-BI and ABCG1 model systems. We define specific inflammatory changes in HDL particles as well as key proteins in HDL metabolism and RCT that may drive selective modulation of cholesterol efflux functions. These studies suggest potential for development of specific HDL functional parameters, distinct from HDL-C and ApoA-I, for prognostic and therapeutic applications.
METHODS
Descriptions of the human study protocol, laboratory methods and statistical analysis are provided in the online supplement. The University of Pennsylvania Institutional Review Board (IRB) approved the human study and written informed consent was obtained.
RESULTS
Baseline Characteristics and Inflammatory Responses
Participants’ baseline characteristics have been published previously16. Briefly, subjects were young healthy adults (N=20, 50% male, 80% Caucasian, age 25.7±3.9) with normal blood pressure, plasma lipoproteins, and BMI (Supplement Table 1). As expected16 endotoxemia induced an acute febrile illness largely resolving in 8–12 hrs (Supplement Table 2).
Endotoxemia modulates HDL composition and size
Previously, we demonstrated that human endotoxemia reduced HDL phospholipids (~25%) while coincidentally increasing HDL-SAA (~80 fold) but with small impact on HDL cholesterol (+2.6%) and apoA-I (-11%)11. Indeed, relative to apoA-I, there was a much greater loss in HDL phospholipids than change in cholesterol and apoA-II. Conversely, the increase in HDL SAA was out of proportion to the reduction in apoA-I (Supplement Figure 1A-D).
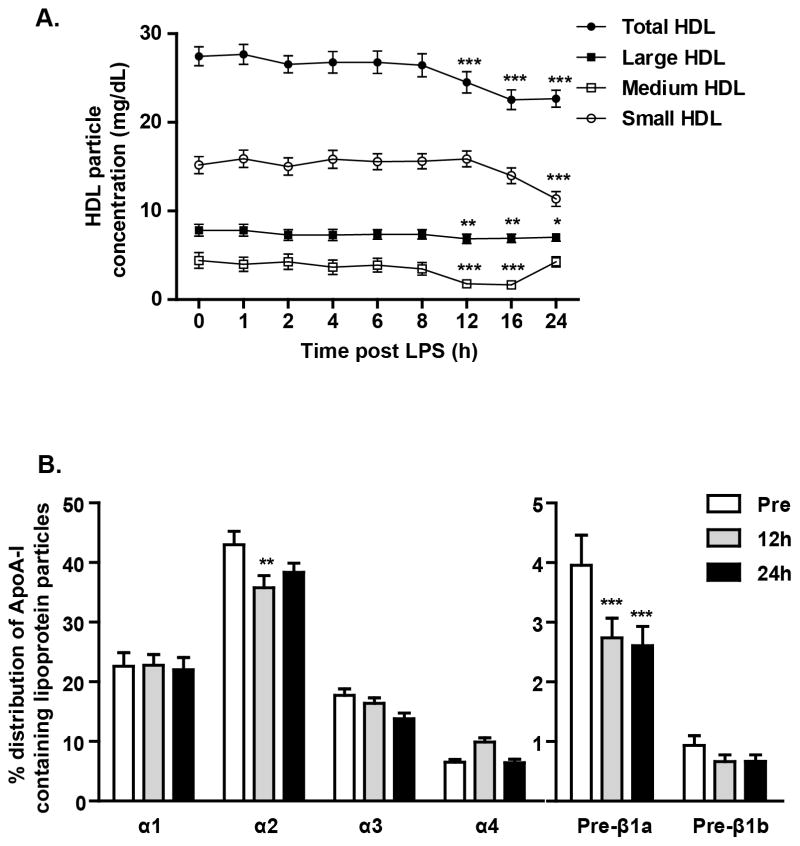
Endotoxemia-induced change in mean HDL-particle size at NMR and in the distribution of apoA-I -containing HDL particles by 2D gel electrophoresis (Figure 1 and Supplement Table 3). Although the total HDL particle number at NMR did not change during endotoxemia, we observed significant decreases in the numbers of small and medium sized particles (Figure 1A). HDL separation by 2D gel electrophoresis also showed significant reductions in the small pre-β1a and α3 as well as in the medium-sized α2 HDL subpopulations but no change in the large α1 particles (Figure 1B).
Figure 1.
Effects of endotoxin (3ng/kg, iv) on (A) HDL sub-populations by nuclear magnetic resonance spectroscopy (*p<0.05, **p<0.01, ***p<0.001 vs time 0, analyzed by repeated measures one-way ANOVA) and (B) selective ApoA-I containing subpopulations of HDL particles by 2D gel electrophoresis (**p<0.01, ***p<0.001 vs pre-LPS, analyzed by repeated measures one-way ANOVA). Data presented as mean ± SEM (n=20).
Potential mechanisms of HDL particle remodeling by inflammation
We examined HDL-related lipases, transfer proteins and regulatory enzymes that may contribute to HDL remodeling 3, 4, 17, 18. Previously, our group has shown increased plasma endothelial lipase (EL) levels during endotoxemia17 and now we also demonstrate a marked increase in circulating class II secretory phospholipase A2 (sPLA2-IIA) (Figure 2A), lipases known to selectively modulate HDL phospholipids. Indeed, increases in both plasma EL (Spearman rho -0.50, p=0.03) and sPLA2-IIA (Spearman rho -0.43, p=0.2) tended to correlate with reductions in HDL phospholipids. In addition, modulation of the cholesterol content of HDL particles during endotoxemia is suggested by a reduction in plasma CETP levels (Figure 2B) and plasma LCAT (Figure 2C).
Figure 2.
Plasma levels of (A) secretory phospholipase A2 and endothelial lipase (***p<0.001 vs. time 0, assessed by repeated measures one-way ANOVA), (B) plasma cholesterol esterification rate (nmols/h/ml plasma), an index of lecithin:cholesterol acyltransferase (LCAT) activity, and (C) cholesterol ester transport protein levels (**p<0.01 vs pre-LPS; assessed by t-test). Data presented as mean ± SEM (n=20).
Endotoxemia impairs the capacity of HDL to efflux cellular cholesterol
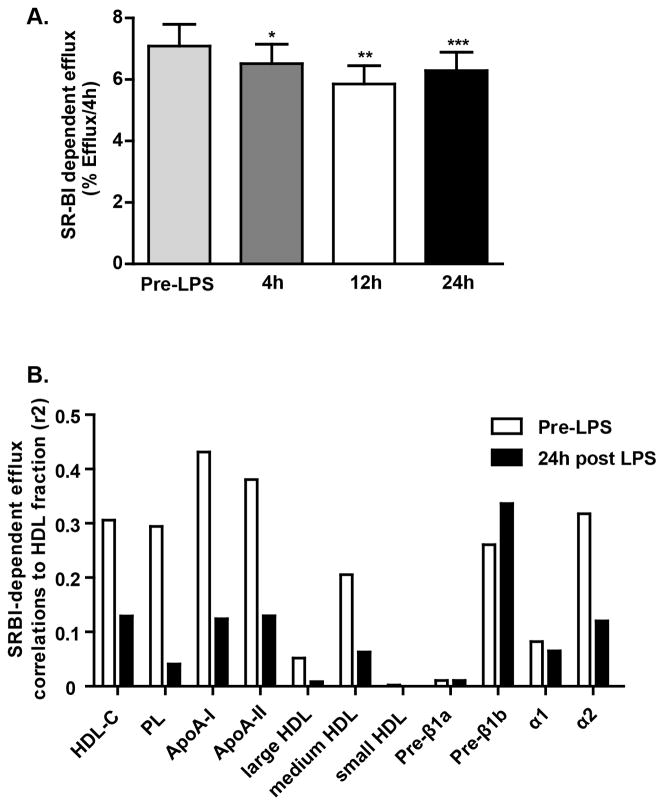
Inflammatory remodeling of human HDL may impair its anti-atherogenic functions, in particular the capacity to remove cholesterol from peripheral cells, a critical step in the RCT process. We reported previously that endotoxemia induced a progressive reduction in the capacity of mouse and human HDL to promote 3H-cholesterol efflux ex vivo from J774 macrophages, an ABCA1-dependent efflux model (~60% reduction in efflux at 24h, Figure 3A)11. We now demonstrate a specific loss (~20% reduction in efflux at 12 hrs) in the capacity of inflammatory HDL to remove cholesterol in an SR-BI dependent model (Figure 4A) but no change in an ABCG1 over-expressing BHK-1 cell model (e.g., pre-LPS ABCG1 efflux of 2.60 ± 0.25% vs. 12h post-LPS efflux of 2.58 ± 0.25%; mean ± SEM, n=6 sera).
Figure 3.
(A) ABCA1-dependent cholesterol efflux (over 4h) to HDL fraction during endotoxemia (**p<0.01 vs. pre-LPS efflux, n=12, assessed by repeated measures one-way ANOVA). (B) ABCA-1 efflux correlations (r2) with lipid parameters pre-LPS and 24h post LPS. Figure 3A data, published previously14, is presented for comparative interpretation with Figure 3B and Figure 4.
Figure 4.
(A) SR-BI-dependent cholesterol efflux (over 4h) to HDL fraction during endotoxemia (*p<0.05, **p<0.01, ***p<0.001 vs. pre-LPS, n=20, assessed by repeated measures one-way ANOVA). (B) SR-BI efflux correlations (r2) with lipid parameters pre-LPS and 24h post LPS.
We explored the relationship of HDL particles with efflux in ABCA1 and SR-BI model systems. Pre-endotoxemia ABCA1-mediated efflux correlated with HDL-C, phospholipids, apoA-I, small HDL and pre-β1a and pre-β1b particles but these correlations were markedly reduced 24h following LPS coincident with reduced efflux (Figure 3B). These data are consistent with a loss of HDL particles that interact with ABCA1. Pre-endotoxemia SR-BI-mediated efflux correlated with HDL-C, apoA-I, phospholipids and mostly medium sized particles; these correlations were also attenuated following LPS (Figure 4B) consistent with remodeling of particles that are known to interact with SR-BI.
Although post-endotoxemia human HDL was significantly enriched in SAA, we found that these HDL fractions had reduced cholesterol efflux capacity in both ABCA1 and SR-BI model systems. In fact, the SAA content of post-LPS inflammatory human HDL was neither correlated with ABCA1 nor SR-BI dependent efflux.
DISCUSSION
Although epidemiological studies suggest an inverse relation between circulating HDL-C and cardiovascular disease (CVD), whether low HDL-C is merely a bystander or is causative remains controversial. Unlike LDL-C, genetic factors that influence HDL-C levels are not associated with coronary artery disease (CAD) in genome wide association studies (GWAS)19. Failure of the cholesterol ester transfer protein (CETP) inhibitor torcetrapib and more recently niacin to reduce CVD despite their HDL-C raising effects, has raised doubts about the therapeutic potential of raising HDL-C20. However, HDL is the most complex lipoprotein family and its function and metabolism are not well understood as yet. Global measures such as HDL-C or apoA-I likely fail to capture functionality that may reside in HDL sub-fractions. We present novel data demonstrating that inflammation in humans modulates HDL composition and function in the absence of substantial change in HDL-C or apoA-I thereby reinforcing the concept that alternative measures of HDL function need to be applied in order to address rigorously whether HDL is causally involved in atherosclerosis.
Previously, we reported the first in vivo functional evidence that acute inflammation retards RCT in rodents and provided proof-of-principle for similar effect in humans11. In the current paper we demonstrate that human endotoxemia (a) remodels HDL with a characteristic decrease in lipid-poor pre-β1 and other small and medium sized HDL particles, (b) induces plasma sPLA2-IIA and suppresses CETP mass and LCAT activity, biochemical consequences known to remodel HDL phospholipid and cholesterol, and (c) reduces HDL capacity to efflux cholesterol from SR-BI and ABCA1, but not ABCG1, model systems.
Low-dose experimental endotoxin, which transduces toll-like receptor 4 (TLR-4) signaling in vivo, may be an informative model of cardio-metabolic pathologies in humans4, 21. Sepsis and chronic infections12, 22 in humans induce insulin resistance, glucose intolerance and lipoprotein changes similar to the metabolic profile in obesity, type-2 diabetes and CAD. We and others have shown that experimental endotoxemia induces insulin resistance16, 21, adipose inflammation23 and lipoprotein changes4, 6, 7, 11, 24 that resemble those in CVD risk states. A role for TLR4 is specifically suggested by work demonstrating reduced diet induced obesity25 and atherosclerosis in TLR4 deficient mouse models. In fact, TLR4 may be activated by endogenous ligands including fatty-acids that are increased in diet induced obesity and insulin resistance25. Finally several groups, including ours11, have defined mechanisms of altered HDL function and impaired reverse cholesterol transport during inflammation in rodent models14, 15.
Hudgins et al. were the first to examine diverse lipoprotein changes during placebo-controlled, experimental endotoxemia in humans4. In contrast to studies in rodents 6, 26, they found no change in HDL-C and apoA-I levels, but did observe increased SAA and loss of HDL phospholipids. The functional consequences of HDL remodelling were not directly addressed. In our published work, we have confirmed marked increase in HDL-SAA with reduction in HDL phospholipids and a relative preservation of HDL apoA-I. Here, we present novel data on the effects of innate inflammatory responses on the HDL subpopulation profiles determined by NMR and 2D gel analyses and how those changes influence HDL-mediated cellular cholesterol efflux.
We observed a marked increase of circulating sPLA2-IIA during human endotoxemia. Our prior finding of increased plasma EL after LPS administration17 and others’ work showing suppressed hepatic lipase (HL) during inflammation27 support a role for these lipases in HDL phospholipid turnover in human inflammatory syndromes18, 28, 29. While changes in HDL-C, apoA-I and apoA-II were minor, HDL phospholipids and small HDL particles decreased substantially suggesting impaired HDL maturation and increased catabolism of the small, less lipidated particles during acute inflammation.
Characterization of HDL particle size and the distribution apoA-I containing HDL particles during human inflammation may provide insight into specific changes in HDL that reduce HDL function and RCT leading to increased CVD risk. The majority of apoA-I in plasma has α mobility. These HDL particles have been classed as α1, α2, α3, and α4 , with median sizes of 11.0 , 9.2 , 8.05, and 7.43 nm, respectively30. Smaller amounts of HDL particles contain apoA-I with pre-β mobility (pre-β1 and pre-β2). Four distinct particles have pre-α mobility (pre-α 1–4). Prior to LPS treatment, we have found only modest correlations of selective NMR HDL measures and 2D-gel parameters (e.g., medium HDL at NMR with α2 particles on 2D gels, r2=0.35, p<0.01) perhaps because these two methods provide distinct information regarding HDL particles. NMR estimates lipid content while non-denaturing 2D gel analyses measure apoA-I content. During endotoxemia, there was a substantial loss of small and medium sized HDL particles without significant reduction in levels of large HDL at NMR while 2D-gel electrophoresis suggested a reduction in smaller pre-β1 and α3 HDL while large α1 HDL particle levels were not decreased. These selective changes are consistent with induction of inflammatory lipases coincident with the suppression of LCAT5, PLTP4 and CETP 4, 5, 31 (and Figure 2).
We determined the functional impact of endotoxemia on human HDL by measuring the ex vivo capacity of the isolated HDL fraction to efflux cholesterol. We and others have reported an inflammation-induced loss of HDL’s capacity to efflux cholesterol from an ABCA1 macrophage cell model11, 14. This is consistent with our finding of reduced levels of pre-β1 particles, major ABCA1 interacting HDL particles. We also report a significant loss of HDL cholesterol efflux from a SR-BI cell model. This loss is likely driven by reduced HDL phospholipids and an unfavorable remodeling of α2 HDL particles which are preferential substrates of SR-BI32. Endotoxemia increased HDL-associated SAA by almost 100-fold. Our observation of reduced ABCA1 and SR-BI efflux despite in vivo increases in HDL SAA, coupled to our recent findings of reduced efflux and RCT during inflammation in rodents11, suggest that inflammatory increases in HDL-SAA do not enhance HDL acceptor function in vivo33. Notably, we did not observe any loss of HDL acceptor capacity in an ABCG1-model arguing against a non-specific effect on all HDL efflux pathways. The reduction in HDL efflux from ABCA1 and SR-BI systems was remarkable also because it was associated with only minimal change in HDL-C and apoA-I suggesting that these traditional HDL parameters may be poor markers of important HDL functions in humans.
Recently Khera et al., demonstrated that measurement of the capacity of plasma to induce cholesterol efflux (in an ABCA1 model) was a stronger and independent inverse predictor of CAD than HDL-C levels34, supporting the argument that measures of HDL function may be more useful than HDL-C levels in CVD risk prediction. It remains to be established in prospective studies whether any measures of HDL function and genetic correlates of these measures predict incident CVD and if therapies that modulate these HDL functions reduce CVD. That none of the CAD genes discovered to date through GWAS relate to circulating HDL-C levels should not be surprising if HDL-C is truly a poor surrogate for HDL function in atherosclerosis. Notably, no GWAS studies have been published yet using measures of HDL function. Our experimental data reinforces the notion that alternative measures of HDL function need to be applied in order to address more rigorously whether HDL is causally involved in atherosclerosis.
This study has several limitations. It is correlative and does not permit causal inference. Although we demonstrate alterations in HDL composition and function during induced inflammation, we have not examined the effect of inflammation on HDL biogenesis in vivo nor on ABCA1, SR-BI, or ABCG1 expression and function within the liver, peripheral tissues or macrophages. Induction of acute inflammation, even low grade, may not reflect accurately the chronic inflammatory pathophysiology that is a feature of obesity, insulin resistance, and CAD. However, several lines of data suggest that low-dose experimental endotoxin may be an informative model of cardio-metabolic disease in humans4, 21. A major additional advantage is that the model allows a direct assessment of the directional impact of induced inflammation on metabolic parameters including HDL composition and function. This avoids confounding and reverse causation that are features of observational studies where inflammatory changes may result from risk factors and disease rather than be causal.
In summary, we present evidence that human acute inflammation can induce selective remodelling of HDL with induction of specific HDL lipases, suppression of CETP and LCAT activity, HDL enrichment with SAA, loss of specific small-medium sized HDL particles, and reduction in selective HDL cholesterol efflux functions. These data support the concept that atherogenic HDL dysfunction and impaired RCT occur in human inflammatory syndromes, independent of significant change in plasma HDL-C levels. Overall, our work suggests that experimental human inflammation induces HDL remodeling and loss of HDL atheroprotective functions in a model that is broadly relevant to diverse human inflammatory disorders.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by a Clinical and Translational Science Award (UL1RR024134) from the National Center for Research Resources (NCRR) to the University of Pennsylvania and by R01 HL-073278 and P50 HL-083799-SCCOR from the National Institute of Health (to MPR). MM is supported by R01 HL-22633 from National Heart, Lung and Blood Institute. FMcG is jointly funded by Science Foundation Ireland, the Health Research Board and the Wellcome Trust (097311/Z/11/Z) under the SFI-HRB-Wellcome Trust Biomedical Research Partnership.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wang X, Rader DJ. Molecular regulation of macrophage reverse cholesterol transport. Curr Opin Cardiol. 2007;22:368–372. doi: 10.1097/HCO.0b013e3281ec5113. [DOI] [PubMed] [Google Scholar]
- 2.van der Westhuyzen DR, de Beer FC, Webb NR. HDL cholesterol transport during inflammation. Curr Opin Lipidol. 2007;18:147–151. doi: 10.1097/MOL.0b013e328051b4fe. [DOI] [PubMed] [Google Scholar]
- 3.Khovidhunkit W, Kim MS, Memon RA, Shigenaga JK, Moser AH, Feingold KR, Grunfeld C. Effects of infection and inflammation on lipid and lipoprotein metabolism: mechanisms and consequences to the host. J Lipid Res. 2004;45:1169–1196. doi: 10.1194/jlr.R300019-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Hudgins LC, Parker TS, Levine DM, Gordon BR, Saal SD, Jiang XC, Seidman CE, Tremaroli JD, Lai J, Rubin AL. A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. J Lipid Res. 2003;44:1489–1498. doi: 10.1194/jlr.M200440-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Levels JH, Pajkrt D, Schultz M, Hoek FJ, van Tol A, Meijers JC, van Deventer SJ. Alterations in lipoprotein homeostasis during human experimental endotoxemia and clinical sepsis. Biochim Biophys Acta. 2007;1771:1429–1438. doi: 10.1016/j.bbalip.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 6.Memon RA, Grunfeld C, Moser AH, Feingold KR. Tumor necrosis factor mediates the effects of endotoxin on cholesterol and triglyceride metabolism in mice. Endocrinology. 1993;132:2246–2253. doi: 10.1210/endo.132.5.8477669. [DOI] [PubMed] [Google Scholar]
- 7.Paradis ME, Badellino KO, Rader DJ, Deshaies Y, Couture P, Archer WR, Bergeron N, Lamarche B. Endothelial lipase is associated with inflammation in humans. J Lipid Res. 2006;47:2808–2813. doi: 10.1194/jlr.P600002-JLR200. [DOI] [PubMed] [Google Scholar]
- 8.de Beer FC, Connell PM, Yu J, de Beer MC, Webb NR, van der Westhuyzen DR. HDL modification by secretory phospholipase A(2) promotes scavenger receptor class B type I interaction and accelerates HDL catabolism. J Lipid Res. 2000;41:1849–1857. [PubMed] [Google Scholar]
- 9.Jahangiri A, de Beer MC, Noffsinger V, Tannock LR, Ramaiah C, Webb NR, van der Westhuyzen DR, de Beer FC. HDL remodeling during the acute phase response. Arterioscler Thromb Vasc Biol. 2009;29:261–267. doi: 10.1161/ATVBAHA.108.178681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khovidhunkit W, Moser AH, Shigenaga JK, Grunfeld C, Feingold KR. Regulation of scavenger receptor class B type I in hamster liver and Hep3B cells by endotoxin and cytokines. J Lipid Res. 2001;42:1636–1644. [PubMed] [Google Scholar]
- 11.McGillicuddy FC, de la Llera Moya M, Hinkle CC, Joshi MR, Chiquoine EH, Billheimer JT, Rothblat GH, Reilly MP. Inflammation impairs reverse cholesterol transport in vivo. Circulation. 2009;119:1135–1145. doi: 10.1161/CIRCULATIONAHA.108.810721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grunfeld C, Pang M, Doerrler W, Shigenaga JK, Jensen P, Feingold KR. Lipids, lipoproteins, triglyceride clearance, and cytokines in human immunodeficiency virus infection and the acquired immunodeficiency syndrome. J Clin Endocrinol Metab. 1992;74:1045–1052. doi: 10.1210/jcem.74.5.1373735. [DOI] [PubMed] [Google Scholar]
- 13.Fong YM, Marano MA, Barber A, He W, Moldawer LL, Bushman ED, Coyle SM, Shires GT, Lowry SF. Total parenteral nutrition and bowel rest modify the metabolic response to endotoxin in humans. Ann Surg. 1989;210:449–456. doi: 10.1097/00000658-198910000-00005. discussion 456–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malik P, Berisha SZ, Santore J, Agatisa-Boyle C, Brubaker G, Smith JD. Zymosan-mediated inflammation impairs in vivo reverse cholesterol transport. J Lipid Res. 2011;52:951–957. doi: 10.1194/jlr.M011122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smoak KA, Aloor JJ, Madenspacher J, Merrick BA, Collins JB, Zhu X, Cavigiolio G, Oda MN, Parks JS, Fessler MB. Myeloid differentiation primary response protein 88 couples reverse cholesterol transport to inflammation. Cell metabolism. 2010;11:493–502. doi: 10.1016/j.cmet.2010.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, Tabita-Martinez J, Sellers KF, Rickels MR, Reilly MP. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes. 2010;59:172–181. doi: 10.2337/db09-0367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Badellino KO, Wolfe ML, Reilly MP, Rader DJ. Endothelial lipase is increased in vivo by inflammation in humans. Circulation. 2008;117:678–685. doi: 10.1161/CIRCULATIONAHA.107.707349. [DOI] [PubMed] [Google Scholar]
- 18.Tietge UJ, Maugeais C, Lund-Katz S, Grass D, deBeer FC, Rader DJ. Human secretory phospholipase A2 mediates decreased plasma levels of HDL cholesterol and apoA-I in response to inflammation in human apoA-I transgenic mice. Arterioscler.Thromb.Vasc. Biol. 2002;22:1213–1218. doi: 10.1161/01.atv.0000023228.90866.29. [DOI] [PubMed] [Google Scholar]
- 19.Schunkert H, Konig IR, Kathiresan S, Reilly MP, Assimes TL, Holm H, Preuss M, Stewart AF, Barbalic M, Gieger C, Absher D, Aherrahrou Z, Allayee H, Altshuler D, Anand SS, Andersen K, Anderson JL, Ardissino D, Ball SG, Balmforth AJ, Barnes TA, Becker DM, Becker LC, Berger K, Bis JC, Boekholdt SM, Boerwinkle E, Braund PS, Brown MJ, Burnett MS, Buysschaert I, Carlquist JF, Chen L, Cichon S, Codd V, Davies RW, Dedoussis G, Dehghan A, Demissie S, Devaney JM, Diemert P, Do R, Doering A, Eifert S, Mokhtari NE, Ellis SG, Elosua R, Engert JC, Epstein SE, de Faire U, Fischer M, Folsom AR, Freyer J, Gigante B, Girelli D, Gretarsdottir S, Gudnason V, Gulcher JR, Halperin E, Hammond N, Hazen SL, Hofman A, Horne BD, Illig T, Iribarren C, Jones GT, Jukema JW, Kaiser MA, Kaplan LM, Kastelein JJ, Khaw KT, Knowles JW, Kolovou G, Kong A, Laaksonen R, Lambrechts D, Leander K, Lettre G, Li M, Lieb W, Loley C, Lotery AJ, Mannucci PM, Maouche S, Martinelli N, McKeown PP, Meisinger C, Meitinger T, Melander O, Merlini PA, Mooser V, Morgan T, Muhleisen TW, Muhlestein JB, Munzel T, Musunuru K, Nahrstaedt J, Nelson CP, Nothen MM, Olivieri O, Patel RS, Patterson CC, Peters A, Peyvandi F, Qu L, Quyyumi AA, Rader DJ, Rallidis LS, Rice C, Rosendaal FR, Rubin D, Salomaa V, Sampietro ML, Sandhu MS, Schadt E, Schafer A, Schillert A, Schreiber S, Schrezenmeir J, Schwartz SM, Siscovick DS, Sivananthan M, Sivapalaratnam S, Smith A, Smith TB, Snoep JD, Soranzo N, Spertus JA, Stark K, Stirrups K, Stoll M, Tang WH, Tennstedt S, Thorgeirsson G, Thorleifsson G, Tomaszewski M, Uitterlinden AG, van Rij AM, Voight BF, Wareham NJ, Wells GA, Wichmann HE, Wild PS, Willenborg C, Witteman JC, Wright BJ, Ye S, Zeller T, Ziegler A, Cambien F, Goodall AH, Cupples LA, Quertermous T, Marz W, Hengstenberg C, Blankenberg S, Ouwehand WH, Hall AS, Deloukas P, Thompson JR, Stefansson K, Roberts R, Thorsteinsdottir U, O'Donnell CJ, McPherson R, Erdmann J, Samani NJ. Large-scale association analysis identifies 13 new susceptibility loci for coronary artery disease. Nature genetics. 2011;43:333–338. doi: 10.1038/ng.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, Lopez-Sendon J, Mosca L, Tardif JC, Waters DD, Shear CL, Revkin JH, Buhr KA, Fisher MR, Tall AR, Brewer B. Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 21.Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. Insulin resistance and substrate utilization in human endotoxemia. The Journal of clinical endocrinology and metabolism. 2000;85:3770–3778. doi: 10.1210/jcem.85.10.6914. [DOI] [PubMed] [Google Scholar]
- 22.Copeland S, Warren HS, Lowry SF, Calvano SE, Remick D. Acute inflammatory response to endotoxin in mice and humans. Clin Diagn Lab Immunol. 2005;12:60–67. doi: 10.1128/CDLI.12.1.60-67.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shah R, Lu Y, Hinkle CC, McGillicuddy FC, Kim R, Hannenhalli S, Cappola TP, Heffron S, Wang X, Mehta NN, Putt M, Reilly MP. Gene profiling of human adipose tissue during evoked inflammation in vivo. Diabetes. 2009;58:2211–2219. doi: 10.2337/db09-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Suffredini AF, Fantuzzi G, Badolato R, Oppenheim JJ, O'Grady NP. New insights into the biology of the acute phase response. Journal of clinical immunology. 1999;19:203–214. doi: 10.1023/a:1020563913045. [DOI] [PubMed] [Google Scholar]
- 25.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. The Journal of clinical investigation. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feingold KR, Hardardottir I, Memon R, Krul EJ, Moser AH, Taylor JM, Grunfeld C. Effect of endotoxin on cholesterol biosynthesis and distribution in serum lipoproteins in Syrian hamsters. J Lipid Res. 1993;34:2147–2158. [PubMed] [Google Scholar]
- 27.Feingold KR, Memon RA, Moser AH, Shigenaga JK, Grunfeld C. Endotoxin and interleukin-1 decrease hepatic lipase mRNA levels. Atherosclerosis. 1999;142:379–387. doi: 10.1016/s0021-9150(98)00265-2. [DOI] [PubMed] [Google Scholar]
- 28.Pruzanski W, Stefanski E, de beer FC, de Beer MC, Vadas P, Ravandi A, Kuksis A. Lipoproteins are substrates for human secretory group IIA phospholipase A2: preferential hydrolysis of acute phase HDL. Journal of Lipid Research. 1998;39:2150–2160. [PubMed] [Google Scholar]
- 29.Duong M, Psaltis M, Rader DJ, Marchadier D, Barter PJ, Rye KA. Evidence that hepatic lipase and endothelial lipase have different substrate specificities for high-density lipoprotein phospholipids. Biochemistry. 2003;42:13778–13785. doi: 10.1021/bi034990n. [DOI] [PubMed] [Google Scholar]
- 30.Asztalos BF, Sloop CH, Wong L, Roheim PS. Two-dimensional electrophoresis of plasma lipoproteins: recognition of new apo A-I-containing subpopulations. Biochim Biophys Acta. 1993;1169:291–300. doi: 10.1016/0005-2760(93)90253-6. [DOI] [PubMed] [Google Scholar]
- 31.Asztalos BF, Schaefer EJ, Horvath KV, Yamashita S, Miller M, Franceschini G, Calabresi L. Role of LCAT in HDL remodeling: investigation of LCAT deficiency states. J Lipid Res. 2007;48:592–599. doi: 10.1194/jlr.M600403-JLR200. [DOI] [PubMed] [Google Scholar]
- 32.Asztalos BF, de la Llera-Moya M, Dallal GE, Horvath KV, Schaefer EJ, Rothblat GH. Differential effects of HDL subpopulations on cellular ABCA1- and SR-BI-mediated cholesterol efflux. J Lipid Res. 2005;46:2246–2253. doi: 10.1194/jlr.M500187-JLR200. [DOI] [PubMed] [Google Scholar]
- 33.de Beer MC, Ji A, Jahangiri A, Vaughan AM, de Beer FC, van der Westhuyzen DR, Webb NR. ATP binding cassette G1-dependent cholesterol efflux during inflammation. J Lipid Res. 2010;52:345–353. doi: 10.1194/jlr.M012328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Khera AV, Cuchel M, de la Llera-Moya M, Rodrigues A, Burke MF, Jafri K, French BC, Phillips JA, Mucksavage ML, Wilensky RL, Mohler ER, Rothblat GH, Rader DJ. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. The New England journal of medicine. 2011;364:127–135. doi: 10.1056/NEJMoa1001689. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.