Abstract
The resting state amplitude of low frequency fluctuations (ALFF) has been shown to be reliable in healthy subjects, and to correlate with antipsychotic treatment response in antipsychotic–naïve schizophrenia patients. We found moderate to high test-retest stability of ALFF in chronically-treated schizophrenia patients assessed twice over a median interval of 2.5 months.
Keywords: ALFF, schizophrenia, stability
1. Introduction
Resting state functional magnetic resonance imaging (fMRI) studies of schizophrenia have typically focused on functional connectivity, i.e., the degree of temporal correlation of the BOLD signal among anatomically distributed brain regions. Different connectivity patterns within and between several functionally distinct networks have been reported in healthy and schizophrenia groups (Liang et al., 2006; Liu et al., 2006; Jafri et al., 2008; Lui et al., 2009a).
The amplitude of low frequency fluctuations (ALFF) can also be measured from resting fMRI data. These low frequency fluctuations, slower than cardiac or respiratory fluctuations, are considered to be related to spontaneous neural activity within a region, and contribute strongly to the correlations seen in BOLD signal time courses across regions (Cordes et al., 2001; Yang et al., 2007; Lui et al., 2009b). ALFF has recently been shown to be reduced in schizophrenia in cross-sectional studies, albeit with some regional inconsistencies (Hoptman et al., 2010; Huang et al., 2010). A promising longitudinal study, however, reported an increase (normalization) of frontal and parietal ALFF in antipsychotic–naïve schizophrenia patients after 6 weeks of antipsychotic drug treatment (Lui et al., 2010). Hence, ALFF may provide a relatively easy and safe method to track antipsychotic response in clinical populations and may shed light on the mechanism of action of these agents. However, the test-retest reliability of ALFF has only been examined in healthy volunteers to date (Zuo et al., 2010), and it is unclear whether ALFF is reliable and stable over the short-term illness course in chronically treated persons with schizophrenia. A related measure, fractional ALFF or fALFF, measures the amount of power in the lower frequency spectrum relative to the total power in the detectable range; however, it is slightly less reliable than ALFF in healthy controls(Zuo et al., 2010). Thus, in the present study we examined the stability of ALFF, with a median test-retest interval of 2.5 months in subjects with chronic schizophrenia.
2. Materials and Methods
2.1. Subjects
The study was approved by the UNM Institutional Review Board (IRB). Data represent a convenience sample, aggregated across studies that collected the same resting state scan on the same scanner. Twenty-one outpatients with DSM-IV-TR schizophrenia (diagnosed based on the Structured Clinical Interview for DSM-IV (First et al., 2002) provided informed consent and participated in the multiple studies. Subjects were outpatients ranging in age from 23 to 62 years (mean 42 years). Positive, negative, and general symptom severity as measured by the Positive and Negative Symptoms Scales (PANSS; (Kay et al., 1987)) were assessed for each subject within one week of each scan. All were treated with antipsychotic medication with clinically equivalent-olanzapine dosages ranging from 2.5 to 70 milligrams/day (Gardner et al., 2010).
2.2. Scanning methods
Patients were scanned twice in a Siemens 3T Tim Trio. Each scanning session included an echo-planar BOLD-weighted resting state scan (33 slices, 165 measurements, 3.8×3.8×3.5 mm voxels, 64 × 64 resolution, FOV 240 mm, axial, TR = 2000 ms, TE = 29 ms, flip angle 75 degrees, 5 min 34 second duration, eyes open). Interscan interval ranged from 5 to 326 days, with a median interval of 2.5 months (mean interval = 112 days, stdev = 97 days).
2.3. ALFF Analysis
Resting state data were pre-processed using SPM5 (Wellcome Department of Imaging Neuroscience, London, England, http://www.fil.ion.ucl.ac.uk). The first two images of each scan were discarded at the scanner to remove T1 equilibration effects; the images were realigned using INRIalign, included in SPM5 (Freire et al., 2002); and slice-timing correction was applied using the middle slice as the reference frame. Data were then spatially normalized to the Montreal Neurological Institute (MNI) echo-planar template, and resliced to 3×3×3mm voxels. Maximal head movement within a scan was less than 5 mm.
Voxelwise ALFF maps were calculated for each patient and scan using the Lui et al (2010) methods implemented in the REST software (http://www.restfmri.net/forum/rest). The ALFF calculation consists of linearly detrending each voxel’s time-series, bandpass filtering (0.01–0.08 Hz), then extracting the power spectra via the fast Fourier transform. The ALFF is the averaged square root of the power in the 0.01 to 0.08 Hz window. ALFF maps are normalized by the mean within-brain ALFF value for each subject to account for differences in scan intensity units, then smoothed with an 8 mm FWHM Gaussian kernel, as done in Lui et al., 2010.
To assess the test-retest stability of the ALFF measures, voxelwise intraclass correlation coefficients (ICC) were calculated as in Zuo et al. (2010). In order to assess the effect of the interscan interval and medication changes on ALFF stability, the relative mean difference in ALFF across scans (the absolute difference of the first and second images scaled by their average) was regressed on the interscan interval and on medication changes in separate analyses.
3. Results
3.1 Clinical stability
On average, the positive, negative, and general symptoms from the PANSS did not differ significantly between scanning sessions (p < .05 in each case by a paired t-test). The median scores at the first scans were 15, 15, and 30, respectively; at the 2nd scan, they were 15, 12, and 28. The median absolute differences at the second scan were 3, 2, and 4 points, respectively, with an interquartile range of 3, 3, and 4 points, respectively. Antipsychotic dosages expressed in olanzapine equivalents also did not change significantly; 13 subjects had no change, and the average non-zero change was 1.6 milligrams/day of olanzapine equivalents.
3.2 ALFF stability
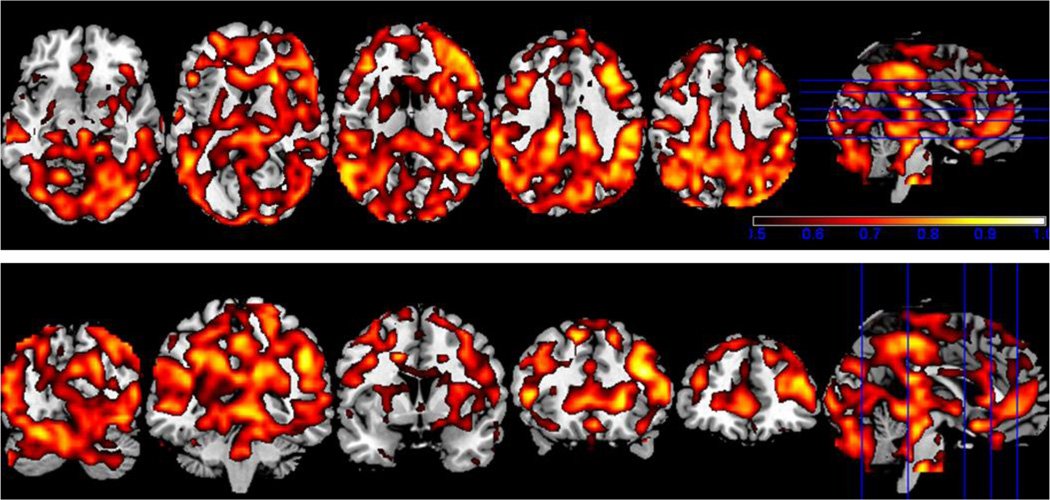
A view of the ICC results are shown in Figure 1, overlaid on a structural template. Similar to Zuo et al. (2010), who examined healthy controls, most of the brain voxels showed an ICC of 0.5 or greater; the exceptions included the orbital frontal cortex, and the lateral, frontal parts of the temporal lobe, which showed only localized regions of high stability.
Figure 1.
The ICC image thresholded at 0.5 or greater overlaid on the MNI template brain, in both axial and coronal views. The color bar covers from red (ICC = 0.5) to white (ICC =1).
Clusters of at least 50 contiguous voxels with an ICC ≥ 0.80 were found throughout the cortex. The largest cluster, 165 voxels with a maximum ICC of 0.94, was in the left hemisphere, in the inferior parietal cortex into the posterior superior and middle temporal gyrus. The remaining clusters were medial and in the right hemisphere, as follows:
154 voxels spanning midline BA6 and 4A, left and right precuneus and mid cingulate cortex, (max ICC = 0.88);
154 voxels in right BA 45, middle and inferior frontal gyrus (ICC = 0.9);
63 voxels also in the right middle and inferior frontal gyrus but in BA 44/45 (ICC = 0.88);
76 voxels in the right inferior parietal cortex and supramarginal gyrus (ICC = 0.95);
94 voxels in the right inferior parietal cortex into the middle and superior occipital gyrus, angular gyrus, and precuneus (ICC = 0.87);
and a 64 voxel cluster from right BA 18 into the midline cuneus and superior parietal lobule (ICC=0.86).
Clusters of less than 50 voxels’ extent with ICC ≥ 0.80 were seen in matching areas in the contralateral hemisphere, specifically in left BA 45/44/middle and inferior frontal gyrus, inferior parietal cortex and supramarginal gyrus, and the right posterior superior and middle temporal gyrus.
The effect of interscan interval on ALFF variation was not significant when FWE-corrected for whole-brain analysis. Using a gray matter mask (created using WFUPickatlas (Maldjian et al., 2003) by dilating the gray matter mask by 2 mm in 2D), and correcting for the restricted number of voxels, a single cluster of 13 voxels in the head and body of the left caudate was significant at the cluster level (p < 0.03, FWE corrected), though not at the voxel level (p < 0.057, FWE corrected). The average value within these 13 voxels for each subject was extracted using Marsbar (Brett et al., 2002), and regression analysis showed that larger changes in ALFF in this cluster were associated with increasing interscan intervals (R2 = 0.76). The effect of medication changes showed no signification relationship to ALFF variation when corrected for whole-brain analysis (FWE or FDR < 0.05).
4. Discussion
In clinically stable persons with schizophrenia, the measurements of the amplitude of low frequency fluctuations during the resting state are quite reliable over time, similar to what has been found in healthy subjects (Zuo et al., 2010), even though our patients were scanned over longer and more variable interscan intervals. These relatively high stability coefficients over such long durations provide conservative estimates of the likely test-retest reliability of ALFF in patients assessed over a shorter interscan interval.
The stability of the ALFF measures showed some regional variation. Parietal and posterior temporal cortex and the middle and inferior frontal areas may be slightly stable than other regions. In all the regions identified by Lui et al. (2010) as showing changes with treatment, there were areas of moderate to high reliability (ICCs ranging from 0.65 to almost 0.9). The exception was the superior temporal gyrus/BA38 in the temporal pole (max ICC of 0.60 in left BA 38). There may be regional increase in the variation in ALFF with increasing interscan interval, but this relationship was not significant at the voxel level in our sample.
Only one study has investigated whether changes in ALFF track antipsychotic treatment response, and the results were encouraging (Lui et al., 2010). The focus of the Lui et al. 2010 study was only first-episode, drug naïve patients; it is unclear if ALFF can similarly track treatment response in medicated chronic patients undergoing changes in their treatment regimens. However, the present findings of moderate to high test-retest reliability in a sample of chronic, clinically stable, schizophrenia patients lays a foundation for examining the use of ALFF measures to track treatment response in this population.
Acknowledgements
NIMH R01MH084898-01A1 to J. Bustillo; UNM CTSC 1 KL2 RR 31976-1 to C. Abbott; COBRE NIMH 5P20RR021938 to V. Calhoun; NIMH R01 MH076989 to D. Mathalon.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Brett M, Anton J-L, Valabregue R, Poline JB. Region of interest analysis using an spm toolbox; 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Cordes D, Haughton VM, Arfanakis K, Carew JD, Turski PA, Moritz CH, Quigley MA, Meyerand ME. Frequencies contributing to functional connectivity in the cerebral cortex in "resting-state" data. American Journal of Neuroradiology. 2001;22:1326–1333. [PMC free article] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I disorders, research version, patient edition. New York: (SCID-I/P) Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Freire L, Roche A, Mangin JF. What is the best similarity measure for motion correction in fMRI time series? IEEE Transactions in Medical Imaging. 2002;21:470–484. doi: 10.1109/TMI.2002.1009383. [DOI] [PubMed] [Google Scholar]
- Gardner DM, Murphy AL, O'Donnell H, Centorrino F, Baldessarini RJ. International consensus study of antipsychotic dosing. American Journal of Psychiatry. 2010;167:686–693. doi: 10.1176/appi.ajp.2009.09060802. [DOI] [PubMed] [Google Scholar]
- Hoptman MJ, Zuo XN, Butler PD, Javitt DC, D'Angelo D, Mauro CJ, Milham MP. Amplitude of low-frequency oscillations in schizophrenia: A resting state fMRI study. Schizophrenia Research. 2010;117:13–20. doi: 10.1016/j.schres.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XQ, Lui S, Deng W, Chan RC, Wu QZ, Jiang LJ, Zhang JR, Jia ZY, Li XL, Li F, Chen L, Li T, Gong QY. Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. Neuroimage. 2010;49:2901–2906. doi: 10.1016/j.neuroimage.2009.11.072. [DOI] [PubMed] [Google Scholar]
- Jafri MJ, Pearlson GD, Stevens M, Calhoun VD. A method for functional network connectivity among spatially independent resting-state components in schizophrenia. Neuroimage. 2008;39:1666–1681. doi: 10.1016/j.neuroimage.2007.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophrenia Bulletin. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Liang M, Zhou Y, Jiang T, Liu Z, Tian L, Liu H, Hao Y. Widespread functional disconnectivity in schizophrenia with resting-state functional magnetic resonance imaging. Neuroreport. 2006;17:209–213. doi: 10.1097/01.wnr.0000198434.06518.b8. [DOI] [PubMed] [Google Scholar]
- Liu H, Liu Z, Liang M, Hao Y, Tan L, Kuang F, Yi Y, Xu L, Jiang T. Decreased regional homogeneity in schizophrenia: A resting state functional magnetic resonance imaging study. Neuroreport. 2006;17:19–22. doi: 10.1097/01.wnr.0000195666.22714.35. [DOI] [PubMed] [Google Scholar]
- Lui S, Deng W, Huang X, Jiang L, Ma X, Chen H, Zhang T, Li X, Li D, Zou L, Tang H, Zhou XJ, Mechelli A, Collier DA, Sweeney JA, Li T, Gong Q. Association of cerebral deficits with clinical symptoms in antipsychotic-naive first-episode schizophrenia: An optimized voxel-based morphometry and resting state functional connectivity study. American Journal of Psychiatry. 2009a;166:196–205. doi: 10.1176/appi.ajp.2008.08020183. [DOI] [PubMed] [Google Scholar]
- Lui S, Huang X, Chen L, Tang H, Zhang T, Li X, Li D, Kuang W, Chan RC, Mechelli A, Sweeney JA, Gong Q. High-field MRI reveals an acute impact on brain function in survivors of the magnitude 8.0 earthquake in China. Proceedings of the National Academy of Science, U S A. 2009b;106:15412–15417. doi: 10.1073/pnas.0812751106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lui S, Li T, Deng W, Jiang L, Wu Q, Tang H, Yue Q, Huang X, Chan RC, Collier DA, Meda SA, Pearlson G, Mechelli A, Sweeney JA, Gong Q. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by "resting state" functional magnetic resonance imaging. Archives of General Psychiatry. 2010;67:783–792. doi: 10.1001/archgenpsychiatry.2010.84. [DOI] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Yang H, Long XY, Yang Y, Yan H, Zhu CZ, Zhou XP, Zang YF, Gong QY. Amplitude of low frequency fluctuation within visual areas revealed by resting-state functional MRI. Neuroimage. 2007;36:144–152. doi: 10.1016/j.neuroimage.2007.01.054. [DOI] [PubMed] [Google Scholar]
- Zuo XN, Di Martino A, Kelly C, Shehzad ZE, Gee DG, Klein DF, Castellanos FX, Biswal BB, Milham MP. The oscillating brain: Complex and reliable. Neuroimage. 2010;49:1432–1445. doi: 10.1016/j.neuroimage.2009.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]