Abstract
Experimental data suggest that the B-cell antigen CD20 may play a significant role in the pathogenesis of many diseases including glomerular diseases. These and other findings underpin the central concept of B-cell-depleting therapies that target CD20 antigen as treatments for lupus nephritis, idiopathic membranous nephropathy, focal segmental glomerulosclerosis, cryglobulinemic glomerulonephritis, antibody mediated renal allograft rejection and recurrent glomerulonephritis in renal allograft. Use of rituximab as a B-cell depleting therapy has been associated with clinical improvement and has emerged as a possible adjunct or alternative treatment option in this field of nephrology.
Keywords: rituximab, glomerular diseases, transplant, rejection
Introduction
Substantial progress in the field of clinical immunology has been achieved with the use of genetic and immunologic tools. Given the role of B cells in the pathogenesis of many immunological processes, attention has been focused on the development of monoclonal antibodies (mAbs) that target these cells. Central to the concept of B-cell targeted therapy in these diseases is the evidence that B-cell clones and their autoantibody products are engaged in immunological cascades that do not require T-cell auto-reactivity. It is plausible that the interruption of such cycles would restore immune tolerance and might allow sustained benefit. Recently, there has been tremendous interest specifically in B-cell depletion therapy in auto-immune diseases. Such therapy has been associated with clinical improvement in many conditions associated with autoantibody production such as rheumatoid arthritis, systemic lupus erythematosus (SLE), idiopathic thrombocytopenia, anti-neutrophil cytoplasmic antibody-associated vasculitis (AAV) and kidney diseases such as minimal change disease (MCD), focal segmental glomerulosclerosis (FSGS), idiopathic membranous nephropathy (MN), cryglobulinemic glomerulonephritis, antibody mediated renal allograft rejection and recurrent glomerular diseases in renal allograft.1–10 The most widely used B-cell depleting biologic agent is rituximab (Rituxan®), which was first approved by the United States (US) Food and Drug Administration for the treatment of B-cell non-Hodgkin lymphoma in 1997. Recently, rituximab has emerged as an additional therapeutic agent in the armamentarium for the treatment of glomerular disease in both native and transplant kidneys. In this review, we discuss the pharmaco-therapeutic role of rituximab in various glomerular conditions and have analyzed the published literatures in this field of nephrology.
Targeting B Cells with Rituximab
Rituximab is a B-cell depleting mAb that targets CD20, a transmembrane protein expressed on virtually all B cells (mature and immature) except when B cells differentiate into antibody secreting plasma cells. It was the first mAb to be approved for clinical use in the therapy of non-Hodgkin lymphoma resistant to other chemotherapy agents. In the US, it has also been approved as the second line therapy (for those with poor response to anti-tumor necrosis factor) in combination with methotrexate for treatment of rheumatoid arthritis. More recently the drug has been used off-label to treat multiple sclerosis, SLE, autoimmune hemolytic anemia, pure red cell aplasia, idiopathic thrombocytopenia, Evans syndrome, bullous skin disorders, type 1 diabetes mellitus, Sjogren's syndrome and several primary and recurrent glomerular diseases in renal allograft.
Rituximab, originally developed by IDEC Pharmaceuticals, is currently co-marketed by Biogen Idec and Genentech in the US, by Roche in Canada and the European Union, and by Chugai Pharmaceutical and Zenyaku Kogyo in Japan. Rituximab, sold under the trade names Rituxan® and MabThera®, is a chimeric mAb. Structurally, the binding regions from the original murine anti-human CD20, consisting of variable regions of immunoglobulin heavy and light chains, are fused to human IgG1 heavy chain and human kappa light-chain constant regions. Thus, rituximab retains the murine CD20-binding Fab regions, but uses a human Fc portion. The structure allows rituximab to be less immunogenic, i.e., it induces less human anti-mouse antibody response in patients than the murine version. The Fc portion contains the effector aspects of the molecule, e.g., complement activation and attraction of cytotoxic cells.
Evidence for multiple mechanisms of rituximab action has been reported. The events that lead to cell killing following antibody binding to CD20 are multifactorial. These events influence both the cytotoxicity of rituximab and development of resistance against rituximab.11 CD20 also acts as a calcium channel,12 either directly or by activating calcium channel, and is associated with a number of protein kinases, including lyn, fyn, lck and p75/85 kinases.13 CD20 engagement leads to activation of phospholipase Cγ via src-family kinases and other downstream events, including MAP kinase activation, viz., JNK, ERK and p38MAPK.14 Binding by rituximab initiates a cascade of intracellular signals, which may play a role in rituximab-mediated apoptosis, complement dependent cytotoxicity (CDC) and antibody-dependent cell-mediated cytotoxicity (ADCC). Rituximab can also act in a B-cell-independent manner, specifically targeting podocytes. Rituximab has been shown to bind sphingomyelin phosphodiesterase acid-like 3b protein and regulate acid sphingomyelinase activity to prevent disruption of the actin cytoskeleton and podocyte apoptosis.15
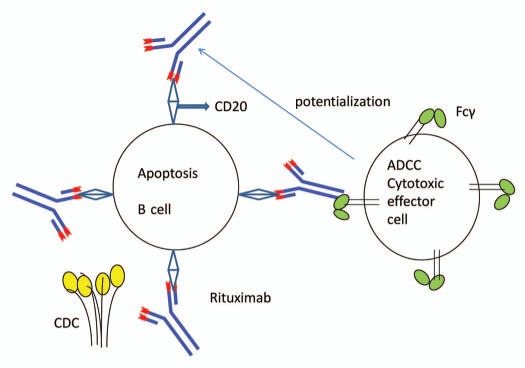
Rituximab-mediated apoptosis is thought to be a consequence of caspase-3 activation, whereas the FAS ligand/FAS death pathway may or may not be important.16 Complement activation by the Fc portion of the antibody leading to cell lysis is another postulated mechanism of rituximab. Complement lysis is controlled not only by the degree of activation, but also regulated by a series of complement inhibitory proteins especially complement receptor type 1, CR1 (CD35), membrane cofactor protein (CD46), decay accelerating factor (CD55) and membrane inhibitor of reactive lysis (protectin or CD59). These later steps are also important in the development of acquired resistance against rituximab.17 Rituximab induces ADCC mediated by a variety of effector cells (Fig. 1), including natural killer (NK) cells, granulocytes and macrophages. ADCC in the presence of rituximab represent killing of B cells by effector cells that are activated by binding to the Fc portion of the chimeric anti-CD20 molecule.18
Figure 1.
Rituximab mode of action: apoptosis and ADCC. Rituximab mediated apoptosis is thought to be a consequence of caspase-3 activation. Complement activation by the Fc portion of the antibody leading to cell lysis is another mode of action of rituximab. Rituximab also induces ADCC mediated by a variety of effector cells (natural killer cells, granulocytes and macrophages). ADCC in the presence of rituximab represent killing of B cells by effector cells that are activated by binding to the Fc portion of the chimeric anti-CD20 molecule.
Rituximab in Glomerular Diseases
Innate immunity, Toll-like receptors, dendritic cells and complement pathways, B cells and antibody networks are involved in the development of glomerular injury. The central role of B cells in the pathogenesis of autoimmunity has made it a therapeutic target for the treatment of glomerular diseases. Accordingly, rituximab is gaining popularity in the treatment of glomerular diseases, albeit its efficacy and safety remains to be established. Controlled randomized studies are required to develop treatment guidelines, evaluate the therapeutic and economical efficacy, and to define criteria for the selection of patients.
Steroid-Resistant and Steroid-Dependent Nephrotic Syndromes
Steroid resistance and steroid dependence constitute a major problem in the treatment of minimal-change disease (MCD) and FSGS. MCD has been postulated to result from a circulating T-cell factor that causes podocyte cytoskeleton disorganization. Recently, persistent CD80 expression in podocytes, induced by Toll-like receptors, has been suggested to be a causative factor.19 In addition to T cells, B cells also may play an important role in the pathogenesis of idiopathic nephrotic syndrome. Altered expression of B lymphocyte surface immunoglobulins in minimal change nephrotic syndrome and focal glomerulosclerosis have been reported.20 Case series have suggested that rituximab may maintain disease remission in steroid-resistant and steroid-dependent nephrotic syndrome patients. In a multicenter study of 22 cases of severe steroid- or cyclosporine-dependent nephrotic syndrome (16 MCD, others FSGS), treatment with rituximab resulted in the withdrawal of immunosuppressive therapies in 85% of the patients without relapse of proteinuria or increasing other immunosuppression therapies.21 Others have reported that a single dose of rituximab may be effective for refractory steroid-dependent nephrotic syndrome in reducing frequency of relapses and increasing the steroid-free period; however, the effects were transient in most of the patients.22 While rituximab was safe and effective in inducing and maintaining remission in a significant proportion of adult23 and children24 with steroid-dependent nephrotic syndrome, a second treatment was necessary in most children to maintain long-term (up to 18 mo) B-cell depletion. A small number suffered from reversible cytokine shock. Recently, a randomized controlled trial reported that a strategy based on rituximab and lower doses of prednisone and calcineurin inhibitors was noninferior to standard doses of these agents in maintaining 3-mo proteinuria as low as baseline or up to 1 g/d greater in children with steroid- and calcineurin-dependent nephrotic syndrome (MCD 19, FSGS 17, other 18).25 In children with difficult-to-treat steroid-dependent nephrotic syndrome who had previously received levamisole, cyclophosphamide or mycophenolate mofetil, and then were treated with either rituximab or tacrolimus and followed for 12 mo, treatment with 2–3 doses of rituximab was as effective as 12 mo of therapy with tacrolimus in reducing relapsed rates and steroid use.26
FSGS, which may require repeated courses of steroid therapy or additional long-term immunosuppression, represents another difficult therapeutic challenge. Only 50% of steroid resistant FSGS patients achieve long-term remission even with intensive immunosuppression and plasma exchange (PEX).27–29 A number of case reports and observational studies point to the beneficial effects of rituximab as a rescue therapy in children with steroid/cyclosporine dependent or steroid resistant FSGS. In renal transplant patients who are at high risk for recurrent FSGS, treatment with rituximab was able to prevent disruption of the actin cytoskeleton and podocyte apoptosis induced by patient sera, suggesting that modulation of sphingolipid-related proteins (independent of B-cell mechanism) plays a role in FSGS.15 Pediatric patients with primary FSGS seem to have positive responses to treatment with rituximab in achieving complete or partial remission of nephrotic remission.30–34 On the contrary, Spanish Group for the Study of Glomerular Diseases (GLOSEN) reported that rituximab was successful in improving renal function and achieving a sustained reduction in proteinuria in two of eight adult patients with FSGS.35 No differences were observed in CD20+ B lymphocyte count among the responder and non-responders. It is therefore plausible that rituximab may induce anti-proteinuric effect through mechanisms independent of B-cell depletion.
Idiopathic Membranous Nephropathy
Idiopathic membranous nephropathy (MN) is an immune-mediated disease that results from the deposition of IgG and complement components on the sub-epithelial layer of the glomerular basement membrane. The discovery that M-type phospholipase A2 (PLA2) receptor is a target antigen in idiopathic MN has provided therapeutic opportunities for the use of rituximab.36 Treatment of MN with rituximab has been associated with a fall in PLA2-receptor antibody levels followed by decreases in proteinuria, whereas an increase in PLA2-receptor antibody levels was associated with an increase in proteinuria.37,38 In a systematic review of 21 case reports or case series without controls where 50 of 85 published cases were from one center where rituximab was used as primary immunosuppression for idiopathic MN, the use of rituximab was associated with a 15–20% rate of complete remission and a 35–40% rate of partial remission with minimal adverse events.3 Rituximab-induced remission has been demonstrated to be associated with restoration of sodium homeostasis and kidney hemodynamics, and regression of the glomerular changes underlying proteinuria.39 Rituximab appears to promote sustained disease remission in patients otherwise predicted to progress to end stage renal disease.40,41 Rituximab can also be an effective tool to overcome dependence on calcineurin inhibitors in idiopathic MN patients with long-term calcineurin inhibitor dependence.42 Use of rituximab must be carefully considered because spontaneous remission is common among patients with nephrotic syndrome from idiopathic MN and carries a favorable long-term outcome with a low incidence of relapse.43
Lupus Nephritis
The rationale to target B cells in lupus lies in its production of antibodies targeting self-antigens to form immune complexes that deposit to tissues and activate the inflammatory process. Rituximab has been used in SLE, both with or without renal involvement. In moderate-to-severely active SLE without renal involvement, the effect of rituximab was not different than placebo in achieving and maintaining the primary endpoints of a major clinical response, a partial clinical response, or no clinical response at week 52 assessed using the British Isles Lupus Asssessment (BILAG) index organ system scores.44 This randomized, double-blind, placebo-controlled, multicenter North American study (Exploratory Phase II/III SLE Evaluation of Rituximab-EXPLORER trial) demonstrated that African-Americans and Hispanics achieved a significantly higher major clinical responses compared with other groups, and that rituximab effected a sustained depletion of CD20-positive peripheral B lymphocytes and anti-double-stranded DNA (ds DNA) and improvement in complement factor levels throughout the study period. Others have reported a decrease in CD21, CD40 and BR3 on the residual B cells, percentage of CD69+ CD4+ T cells and serum tumor necrosis factor levels and a shift in the Th1/Th2 balance of CD4+ T cells gradually toward a Th1 type with rituximab therapy.45
Data on the effectiveness of rituximab for remission induction in lupus nephritis (LN) is scarce. Complete remission was successfully induced with rituximab alone in several small studies with favorable response at 24 mo.46,47 In another series of patients with class III/IV/V LN who received rituximab induction therapy and mycophenolate mofetil (MMF) maintenance therapy, complete or partial remission (78%) with a sustained response (67%) was achieved at one year; a decrease in proteinuria and reduction in maintenance dose of steroids and an increase in serum albumin were also observed.48 Rituximab monotherapy appeared to be effective as induction therapy in LN and had similar effects on clinical, laboratory and renal histological parameters and duration of B-cell depletion at 48 weeks in combination therapy with cyclophosphamide.49 A recently published exploratory analysis, which was performed to determine the frequencies of disease flares of varying intensity in patients who achieved low disease activity (BILAG C or better in all organs) at some point during the above-mentioned EXPLORER study and to evaluate the incidence of prednisone rescue when flares occurred, failed to show significant additional effects of rituximab compared with standard induction treatment with azathioprine/MMF and steroids.50 Rituximab appears to work as a rescue therapy for some patients with relapsing or refractory LN. The combination of cyclophosphamide with rituximab has been reported to achieve clinical remission and lasting depletion of CD20+ cells and anti-ds DNA titers.51,52 The combination of rituximab and MMF also appears to be beneficial in relapsing LN with subnephrotic proteinuria.53 In an open-label, multicenter study of 15 patients with active and refractory SLE, 64% of the patients achieved complete or partial clinical response of BILAG scores.54 The clinical response was also associated with improvement in histopathologic class of nephritis with decrease in renal activity index (from 6 to 3) and reduction in the number of CD3, CD4 and CD20 cells in the renal interstitium on repeat biopsy.55 In a study of safety and efficacy of off-label use of rituximab in patients with severe, refractory systemic autoimmune diseases including SLE (54.6% of participants), complete and partial response were achieved in 51 and 26%, respectively.56 SLE was one of diseases that responded most favorably to rituximab. Rituximab in such cases must be used with other therapies, although maintenance steroids are not necessary. However, others have cautioned that good response to rituximab as induction therapy for refractory LN has not been associated with long-term favorable results. Furthermore, patients with rapidly progressive glomerular diseases, black ethnicity or hypoalbuminemia did not respond to rituximab in these studies.57 The achievement of B-cell depletion 1 mo after rituximab appears to be a strong predictor of renal response. Of cautionary note is the development of serum sickness with an elevated level of human anti-chimeric antibody (HACA) following treatment with rituximab, occurring in 1–20% of patients and more common with autoimmune conditions.58 Other have reported that approximately a third of the patients receiving rituximab may develop HACA titers, which correlate with poor B-cell depletion and may explain the variability of responses in patients with LN.59
Anti-Neutrophilic Cytoplasmic Antibody-Associated Vasculitis
AAVs comprise different forms of small vessel vasculitis characterized by B-cell driven autoimmune processes and endothelial cell activation. In vitro studies have demonstrated that anti-neutrophilic cytoplasmic antibodies specifically cause the release of B lymphocyte stimulator (BlyS) from activated neutrophils, which can support B-cell survival.60 BlyS protein regulates B-cell activation and differentiation. Uncontrolled studies had suggested for years that rituximab may be an effective remission induction therapy for refractory AAV.56,61–64 Two prospective randomized trials have since confirmed that rituximab is not less effective than cyclophosphamide for induction therapy for active AAV. Rituximab-based regimen was not superior to standard intravenous cyclophosphamide in the rituximab vs. cyclophosphamide in AAV (RITUXVAS) trial,65 nor was it inferior to daily oral cyclophosphamide for induction in the rituximab in AAV (RAVE) trial.66 In the RITUXVAS trial, newly diagnosed AAV with renal involvement patients were assigned via 3:1 randomization to a standard glucocorticoid regimen plus either rituximab vs. intravenous cyclophosphamide for 3 to 6 mo followed by azathioprine. Sustained remission rates of 76% and 82% (p = 0.77) at 12 mo were achieved in the rituximab vs. control group. For the primary safety endpoint, severe adverse events occurred at a similar rate; 42% in the rituximab vs. 36% in control group (p = 0.77). In the RAVE trial, rituximab was compared with standard cytotoxic therapy with oral cyclophosphamide for the induction of complete remission by 6 mo in patients with severe AAV. Complete remission was achieved in 63% in the rituximab group vs. 53% in the control group, a result that met the criterion for non-inferiority (p < 0.001). Remission was achieved with similar frequency in patients with newly diagnosed disease (rituximab 60% and control 65%) and in relapsing disease (rituximab 67%). Rituximab was equally effective as cyclophosphamide in the treatment of patients with major renal disease or alveolar hemorrhage, and there was no consistent pattern to organ-system involvement in those who failed to achieve remission. One-year mortality was high in elderly patients with advanced renal failure in the RITUXVAS trial and a third of patients did not achieve remission. The adverse events were similar in both studies; however, the potential for rituximab as a standard of care may have to be tempered until long-term adverse effects relating to fertility and malignant conditions are clarified. Rituximab has been successfully used as maintenance therapy in AAV in a small series of patients,67 but the RITUXVAS and RAVE trials did not assess this issue. Response to rituximab may also be affected by the disease modifying effect of long-term low-dose steroids. Of concern is that the most severe adverse events and deaths occurred in the first 12 weeks after the onset of treatment in the RITUXVAS trial. The investigators have suggested that strategies to reduce the early high dose of steroids may improve the safety of vasculitis treatment. There was also a nonsignificant yet higher rate of malignant conditions that may or may not be attributable to rituximab because malignant conditions are frequently associated with vasculitis at the time of diagnosis, most patients have been exposed to multiple drugs known to be associated with an increased risk of cancer, and rates of cancer occurrence is similar to that observed with other treatments for AAV. It is not known whether patients who are refractory to cyclophosphamide would benefit from rituximab as these patients were excluded from study. However, previous response to cyclophosphamide did not preclude response to rituximab. Recent retrospective data suggest that in patients with refractory granulomatous AAV, granulomatous manifestations such as orbital granuloma and pachymeningitis may be responsible for failure of response/progress.68 Rituximab appears to have fewer fertility-related adverse events, but long-term data are not available. Prolonged disease-free remission following rituximab and low-dose cyclophosphamide therapy for renal AAV has been reported;69 however, its necessity except in life threatening AAV has been questioned. An unresolved issue is the need for maintenance therapy and the timing of retreatment with rituximab to prevent relapses.
Cryoglobulin-Mediated Glomerular Diseases
Rituximab reduces antibody levels that drive cryoglobulin formation and thereby reduces clinical symptoms of vasculitis. A review of 119 cases of cryoglobulinemic vasculitis where rituximab was applied mostly after failure of other treatments, significant reduction in levels of rheumatoid factor, cryoglobulins and IgM were reported. Complete, partial and no responses were noted in 60%, 23% and 17% respectively. The incidence of adverse effects was 19%, mostly associated with high baseline levels of cryoglobulins, dose of rituximab infusion (1 g) and complement activation.70 In a prospective trial, rituximab plus peg-interferonα/ribavirin was more effective than peg-interferon-α/ribavirin in hepatitis C-related mixed cryoglobulinemia.71 Rituximab has also reported to be efficacious in a single case report of hepatitis C-negative therapy-resistant essential mixed cryoglobulinemia with renal and cardiac failure.72 Retrospective data from a large multicenter cohort study also demonstrated improvement in renal function and proteinuria in mixed cryoglobulinemic patients treated with rituximab irrespective of hepatitis C virus (HCV) status.73 Rituximab has been effectively used in cryoglobulin-related renal dysfunction in renal transplant patients, albeit at the expense of infectious complications due to chronic immunosuppression.74 Cryoglobulinemic glomerulonephritis derived from Waldenström's macroglobulinemia was successfully treated by rituximab-CHOP (cytoxan, vincristrine, adriamycin and prednisone) and tandem high-dose chemotherapy with autologous peripheral blood stem cell transplantation.75 Of interest, acute renal failure can also occur secondary to severe type I cryoglobulinemia following rituximab therapy for Waldenström's macroglobulinemia and post treatment monitoring of cryoglobulin levels are prudent. Another entity that responds well to rituximab is non-cryoglobulinemic glomerulonephritis with monoclonal Ig deposits associated with membranoproliferative glomerulonephritis (MPGN) and MN.76 Treatment of hepatitis C-related cryoglobulinemia is associated with transiently increased expression of HCV RNA and may be followed by hepatic flare, possibly immune-mediated, which can be life threatening in cirrhotic patients.77 Direct and indirect roles for B cells have been suggested to explain these observations. Results from in vitro models suggest that HCV is released from B cells in the presence of rituximab and may contribute to the increased viral load observed in patients.78 Further elucidation of the mechanisms involved and effect on clinical outcomes need clarification.
C1q Glomerulopathy, Nonamyloid Fibrillary and Immunotactoid Glomerulopathies
C1q nephropathy is characterized by mesangial deposition of immunoglobulin and complement with C1q dominance or co-dominance with IgG or IgM, absence of clinical and laboratory evidence of systemic lupus erythematosus and inconsistent response to immunotherapy. C1q nephropathy is also associated with B- and T-cell-rich tubulointerstitial infiltrates. Anecdotal cases of resolution of clinical and pathologic features of C1q nephropathy has been reported after rituximab therapy, but controlled trials are not available.79 A single case series of 3 patients with fibrillary glomerulonephritis who were treated with rituximab for nephrotic-range proteinuria have been reported. Treatment with rituximab and standard antiproteinuric therapy with renin-angiotensin system blockade affected a decrease in proteinuria and preservation of kidney function throughout the 27-mo follow-up period.80
Thrombotic Thrombocytopenic Purpura
Thrombotic thrombocytopenic purpura (TTP) is associated with excessive systemic platelet aggregation due to accumulation of ultralarge multimers of von Willebrand factor related to severe deficiency of the cleaving protease, a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13 (ADAMTS13). The rationale for immunosuppressive therapy of TTP was established by observations that TTP may be caused by autoantibodies to ADAMTS13. Patients with high-titer autoantibodies to ADAMTS13 may have a higher mortality, and survivors may require prolonged PEX therapy in spite of adjunctive glucocorticoid treatment.81–84 A Phase 2 study of the safety and efficacy of rituximab given within 3 d of acute TTP admission with standard therapy (PEXs and steroids) suggests a benefit of rituximab therapy given at acute presentation even in patients presenting with the severest disease phenotype. Significant reduction in the number of inpatient days, mean number of PEX treatments until remission, and decrease in relapse rates were noted compared with historical controls who did not receive rituximab.85 However, the observed benefits were lower in nonwhites compared with white patients, which requires further investigation. The efficacy and safety of rituximab in 23 adults responding poorly to standard therapy (PEX plus glucocorticoids) for severe autoimmune TTP were compared with 53 historical controls by the French Thrombotic Microangiopathies reference center group in an open-label prospective study.86 Treatment with rituximab was associated with a shorter time to durable remission, early recovery of ADAMTS13 activity and trend toward fewer exacerbations within 1 y. Complete B-cell depletion was achieved within 4–8 d, but the onset of rituximab effect (sustained normalization of platelets) was evident only at 12 ± 6.7 d, suggesting persistent anti-ADAMTS13 antibody or that rituximab affects precursor B cells and not antibody-forming cells. The time to rituximab effect was 12 d in the above-mentioned rituximab in acute TTP study that demonstrated benefit with rituximab. Nevertheless, these observations highlight the possibility that rituximab may fail to prevent early death or deterioration in patients with severe initial presentation at diagnosis.
Kidney Transplantation: Antibody-Mediated Rejection
B cells play important roles in antigen presentation, T-cell activation and alloantibody production that drive transplant rejection. A variety of new treatments that include rituximab are currently being explored to deplete B-cell populations in the hopes of reducing acute rejection episodes and preserving long-term graft survival.87
CD20+ cells were shown to be present in large numbers in renal allograft with steroid-resistant rejection episodes. Becker et al. reported that a single dose of rituximab was successful in reversing steroid-resistant in 24 of 27 kidney transplant patients.88 Shortly thereafter, Genberg et al. showed that, in ABO-incompatible kidney transplants, single-dose rituximab obviated the need for splenectomy; the overall graft survival was 86.7% at 3 y (comparable to the ABO-compatible control group).89 In a prospective, double-blinded, multicenter randomized trial comparing rituximab (n = 68) and placebo (n = 68) in kidney transplant recipients, the authors also showed a trend toward numerically fewer rejection episodes in rituximab arm (rituximab vs. placebo: 8 vs. 12; p = 0.317). All patients also received tacrolimus, MMF and steroids.90 A recent study attempted to compare single-dose rituximab with daclizumab, in non-sensitized kidney transplant patients (n = 120) but the study was halted due to high acute rejection episodes in the rituximab arm (rituximab vs. daclizumab: 83% vs. 14%, p = 0.01).91 The current data, therefore, suggest that rituximab may be useful as an induction agent in high-risk patients who are ABO-incompatible recipients or who experience steroid-resistant rejection episodes.
Antibody-mediated rejection (AMR) is a unique and often severe form of allograft rejection. The pathophysiology of AMR suggests a prime role of antibodies, B cells and plasma cells, and other effector molecules including complement systems. The immediate loss of allograft from hyperacute AMR is rare because of advancement in anti-HLA antibody detection and ABO matching. However, new-onset AMR related to increases in donor-specific antibody (DSA) can occur within hours of transplantation resulting in rapid allograft dysfunction. The established pathological criteria for diagnosing AMR include complement fragment C4d deposition in peritubular capillaries, pathological features of inflammation and allograft dysfunction.92 Recently, Vo et al. reported their experience with 123 highly sensitized patients who received kidney transplant after desensitization.93 Twenty-two patients who developed AMR were treated with combination of steroids, intravenous immunoglobulin and rituximab. The authors reported 73% survival rate in this high risk group (6/22 patients lost their allograft due to severe AMR). Kaposztas et al. retrospectively compared PEX with rituximab (n = 26) and PEX without rituximab (n = 28) in patients with AMR. Two year graft survival was significantly better in rituximab treated group (90% vs. 60%; p = 0.05).94 Others have reported complete reversal of AMR in all patients (7/7) with single low dose of rituximab (500 mg).95
Recurrent Glomerular Diseases in Renal Allograft
Glomerular diseases are the cause of end stage renal disease (ESRD) in 30% of kidney transplant recipients. In these patients, the rate of clinical recurrence of glomerular diseases ranges between 0–100%96–98 and recurrent glomerular diseases are considered to be the third most common cause of progressive renal allograft failure. All forms of primary glomerular diseases may recur after kidney transplantation and may negatively impact of the allograft survival. In a study that included almost 5,000 transplant recipients, the mean time to recurrence was 475, 594, 664 and 846 d for FSGS, MPGN, MGN and IgA nephropathy (IgAN), respectively.99 Steroids, PEX and various other immunosuppressive agents have been tried to halt the progression of recurrent glomerular diseases. As in the cases of native kidneys affected by glomerular diseases, rituximab, when administered with other immune modulating therapies, has also been shown to reduce proteinuria and prevent progression of recurrent GN. However, because of the lack of prospective, randomized trials in these diseases in renal allograft, recommendations come only from case reports and single center experiences. Six consecutive patients who developed recurrent glomerular diseases (FSGS 3, one of each with MPGN type I, MGN and IgAN) 3–72 mo post-transplant were treated with rituximab, pulse steroids or maximizing mycophenolate mofetil and calcineurin inhibitor therapy. After a follow-up of 9 mo, there were significant decrease in proteinuria (7.2 ± 4.4 g to 1.4 ± 1.5 g; p = 0.04) and improvement in graft function as measured by glomerular filtration rate (31.2 ± 13.1 to 42.5 ± 21.7 ml/min; p = 0.07).100
Among all glomerular diseases, FSGS has a high chance of recurrence especially when a first transplant fails due to recurrent disease; the risk of recurrence in the second transplant approaches 80–100%.101 No controlled studies have been performed yet to address the management of recurrent FSGS. Mixed reports of the efficacy of rituximab in renal transplantation patients with recurrent FSGS have been reported in reference 102–105 of the different approaches, only PEX with rituximab was associated with prolonged remission.15,105–107 In a recent study of 41 patients at high risk for recurrent FSGS, 27 of whom were treated with rituximab at time of kidney transplant, rituximab at time of kidney transplant was shown to prevent recurrent focal segmental glomerulosclerosis by modulating podocyte function in a sphingomyelin phosphodiesterase acid-like 3b-dependent manner.15 Despite the paucity of data, combination of PEX and rituximab remains an acceptable treatment in patients with posttransplantation recurrent FSGS in many instances. Successful treatment of collapsing FSGS in two children with a combination of rituximab, steroids and cyclosporine has been reported in reference 108.
The incidence of biopsy proven de novo or recurrent MGN has been reported to occur in 10–30% of patients in various series. Graft loss in those who develop recurrent or de novo GN is variable. Hariharan et al. reported a multi-center review of 4,913 patients and showed that MGN occurred in 16 cases with overall graft loss of 44% at 5 y and a kidney half-life of 1,193 d.99 Because of recent reports demonstrating beneficial effects of rituximab in idiopathic MGN in native kidneys (vide supra), similar approach has been utilized in the setting of recurrent MGN. Sprangers et al. reported their experience in four patients with recurrent MGN who received different doses of rituximab.109 After the first course of rituximab, proteinuria worsened (n = 1), stabilized (n = 1) and improved (n = 2). Subsequent courses of rituximab resulted in improvement in proteinuria in non-responders. All patients continued to have stable renal function 16 mo after the last administration of rituximab. In another report, eight patients with recurrent MGN with progressive increase in proteinuria (211 mg/day to 4,489 mg/day) were treated with rituximab. Twelve months post-rituximab, 75% of patients had either partial or complete remission.7
A case of recurrent immunotactoid glomerulopathy after transplantation, a glomerular disorder typified by hollow cylindrical and sometimes spherical microtubular deposits, has been reported to respond to rituximab with reduction and stabilization of serum creatinine level but with persistent proteinuria.110
From the point of adverse events, rituximab is generally well-tolerated. Most of the reported side effects have been typically mild, transient and felt to be due to infusion reaction. However, a number of recent case reports and studies have raised concerns regarding potential complications of rituximab usage in transplant setting. Although the rate of infection was similarly high in both groups (45.5% rituximab-treated vs. 53.9% controls), the mortality rate was significantly higher in the rituximab group (9.1% vs. 1.6%).111,112 Others have reported cases of lateonset Pneumocystis jiroveci pneumonia, cryptogenic organizing pneumonia and °C virus associated progressive multifocal leukoencephalopathy in patients treated with rituximab in posttransplant period.113,114
Conclusions
As one of a newer class of immune modulating and immunosuppressive agents, i.e., antibody-based therapeutics, rituximab offers an important option for treatment of refractory and relapsing glomerular diseases. However, systematic review has shown that its use is still at an early stage (Table 1). Rituximab has been effective in PLA2-associated membranous glomerulonephritis, but has not been uniformly effective in inducing remission in lupus nephritis and other glomerular diseases, highlighting the multiplicity of mechanisms involved in glomerular diseases. Mechanisms of glomerular diseases include damage to an essentially impermeable glomerular barrier; alterations in the charge and size selectivity of glomerular permeability; dysfunction of retrieval pathway of filtered protein by tubular cells; podocyte injury associated with disruption of signaling from any of the podocyte's specialized membrane domains, including slit diaphragm, apical and basal membranes, or originating at the level of the actin cytoskeleton; and single-gene defects. TRPC6 encoding gene, a non-selective cation channel of the TRP family expressed in podocyte foot processes, APOL1 (apolipoprotein L-1) and MHY9 (non-muscle myosin heavy chain 9 gene have been linked with glomerular diseases. Mutations in nephrin, podocin and α-actinin-4 gene alleles have been demonstrated to lead to congenital nephrosis. Cytokine overproduction, T cell and B cell activation, defective T-regulatory functions, circulating auto-antibodies interacting with antigens native to or planted in the glomerular capillary wall at the podocyte cell membrane-basement membrane interface are also involved. It is therefore not surprising that one agent is not suitable for all in the treatment of glomerular diseases. The role of rituximab in the treatment of glomerular diseases continues to expand with renewed understanding of the pathomechanism of the diseases involved. Finally, the potential complications associated with this agent dictate that rituximab use in renal diseases must be further elucidated through larger-scale randomized trials that compare rituximab with current gold standard treatments.
Table 1.
Proposed role of rituximab in glomerular diseases
| Lupus nephritis |
| Induction of remission |
| Relapsing or refractory disease |
| Inadequate data on role as maintenance therapy |
| ANCA-associated vasculitides |
| Induction of remission |
| Combination therapy with cytoxan for induction of remission |
| Poor response in granulomatosis manifestations (orbital/pachymeningitis) |
| Inadequate data on role as maintenance therapy or timing of retreatment to prevent relapse |
| Cryoglobulinemic vasculitis |
| Hepatitis C-related |
| Non-Hepatitis C-related |
| Non-cryoglobulinemic glomerulonephritis |
| Idiopathic membranous nephropathy |
| Anti-PLA2-related diseases |
| Overcome dependency on calcineurin inhibitors |
| Autoimmune thrombotic thrombocytopenic purpura |
| Beneficial in patients with poor response to standard therapy |
| Suggestion of benefit as first-line therapy in acute TTP |
| Focal segmental glomerulosclerosis |
| Recurrent FSGS in transplanted kidney |
| Uncertain benefit in primary FSGS |
References
- 1.Chinen J, Shearer WT. Advances in basic and clinical immunology in 2010. J Allergy Clin Immunol. 2011;127:336–341. doi: 10.1016/j.jaci.2010.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SL, Ballow M. Monoclonal antibodies and fusion proteins and their complications: targeting B cells in autoimmune diseases. J Allergy Clin Immunol. 2010;125:814–820. doi: 10.1016/j.jaci.2010.02.025. [DOI] [PubMed] [Google Scholar]
- 3.Bomback AS, Derebail VK, McGregor JG, Kshirsagar AV, Falk RJ, Nachman PH. Rituximab therapy for membranous nephropathy: a systematic review. Clin J Am Soc Nephrol. 2009;4:734–744. doi: 10.2215/CJN.05231008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruggenenti P, Chiurchiu C, Abbate M, Perna A, Cravedi P, Bontempelli M, et al. Rituximab for idiopathic membranous nephropathy: who can benefit? Clin J Am Soc Nephrol. 2006;1:738–748. doi: 10.2215/CJN.01080905. [DOI] [PubMed] [Google Scholar]
- 5.Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350:2572–2581. doi: 10.1056/NEJMoa032534. [DOI] [PubMed] [Google Scholar]
- 6.Smith KG, Jones RB, Burns SM, Jayne DR. Long-term comparison of rituximab treatment for refractory systemic lupus erythematosus and vasculitis: Remission, relapse and re-treatment. Arthritis Rheum. 2006;54:2970–2982. doi: 10.1002/art.22046. [DOI] [PubMed] [Google Scholar]
- 7.El-Zoghby ZM, Grande JP, Fraile MG, Norby SM, Fervenza FC, Cosio FG. Recurrent idiopathic membranous nephropathy: early diagnosis by protocol biopsies and treatment with anti-CD20 monoclonal antibodies. Am J Transplant. 2009;9:2800–2807. doi: 10.1111/j.1600-6143.2009.02851.x. [DOI] [PubMed] [Google Scholar]
- 8.Ponticelli C, Glassock RJ. Posttransplant recurrence of primary glomerulonephritis. Clin J Am Soc Nephrol. 2010;5:2363–2372. doi: 10.2215/CJN.06720810. [DOI] [PubMed] [Google Scholar]
- 9.Stegall MD, Gloor JM. Deciphering antibody-mediated reje ction: new insights into mechanisms and treatmen. Curr Opin Organ Transplant. 2010;15:8–10. doi: 10.1097/MOT.0b013e3283342712. [DOI] [PubMed] [Google Scholar]
- 10.Crew RJ, Ratner LE. ABO-incompatible kidney transplantation: current practice and the decade ahead. Curr Opin Organ Transplant. 2010;15:526–530. doi: 10.1097/MOT.0b013e32833bfbba. [DOI] [PubMed] [Google Scholar]
- 11.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22:7359–7368. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 12.Bubien JK, Zhou LJ, Bell PD, Frizzell RA, Tedder TF. Transfection of the CD20 cell surface molecule into ectopic cell types generates a Ca2+ conductance found constitutively in B lymphocytes. J Cell Biol. 1993;121:1121–1132. doi: 10.1083/jcb.121.5.1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deans JP, Schieven GL, Shu GL, Valentine MA, Gilliland LA, Aruffo A, et al. Association of tyrosine and serine kinases with the B cell surface antigen CD20. Induction via CD20 of tyrosine phosphorylation and activation of phospholipase Cgamma1 and PLC phospholipase Cgamma2. J Immunol. 1993;151:4494–4504. [PubMed] [Google Scholar]
- 14.Pedersen IM, Buhl AM, Klausen P, Geisler CH, Jurlander J. Latent sensitivity to Fas-mediated apoptosis after CD40 ligation may explain activity of CD154 gene therapy in chronic lymphocytic leukemia. Blood. 2002;99:1314–1319. doi: 10.1182/blood.V99.4.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fornoni A, Sageshima J, Wei C, Merscher-Gomez S, Aguillon-Prada R, Jauregui AN, et al. Rituximab targets podocytes in recurrent focal segmental glomerulosclerosis. Sci Transl Med. 2011;3:85. doi: 10.1126/scitranslmed.3002231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrd JC, Kitada S, Flinn IW, Aron JL, Pearson M, Lucas D, et al. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood. 2002;99:1038–1043. doi: 10.1182/blood.V99.3.1038. [DOI] [PubMed] [Google Scholar]
- 17.Treon SP, Mitisiades C, Mitisiades N, Young G, Doss D, Schlossman SF, et al. Tumor cell expression of CD59 is associated with resistance to CD20 serotherapy in patients with B-cell malignancies. J Immunother. 2001;24:263–271. doi: 10.1097/00002371-200105000-00011. [DOI] [PubMed] [Google Scholar]
- 18.Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6:443–446. doi: 10.1038/74704. [DOI] [PubMed] [Google Scholar]
- 19.Shimada M, Ishimoto T, Lee PY, Lanaspa MA, Rivard CJ, Roncal-Jimenez CA, et al. Toll-like receptor 3 ligands induce CD80 expression in human podocytes via an NF{kappa}B-dependent pathw. Nephrol Dial Transplant. 2012;27:81–89. doi: 10.1093/ndt/gfr271. [DOI] [PubMed] [Google Scholar]
- 20.Dall'Aglio P, Meroni PL, Barcellini W, Brigati C, Chizzolini C, De Bartolo G, et al. Altered expression of B lymphocyte surface immunoglobulins in minimal change nephrotic syndrome and focal glomerulosclerosis. Nephron. 1984;37:224–228. doi: 10.1159/000183253. [DOI] [PubMed] [Google Scholar]
- 21.Guigonis V, Dallocchio A, Baudouin V, Dehennault M, Hachon-Le Camus C, Afanetti M, et al. Rituximab treatment for severe steroid- or cyclosporine-dependent nephrotic syndrome: a multicentric series of 22 cases. Pediatr Nephrol. 2008;23:1269–1279. doi: 10.1007/s00467-008-0814-1. [DOI] [PubMed] [Google Scholar]
- 22.Kamei K, Ito S, Nozu K, Fujinaga S, Nakayama M, Sako M, et al. Single dose of rituximab for refractory steroid-dependent nephrotic syndrome in children. Pediatr Nephrol. 2009;24:1321–1328. doi: 10.1007/s00467-009-1191-0. [DOI] [PubMed] [Google Scholar]
- 23.Gulati A, Sinha A, Jordan SC, Hari P, Dinda AK, Sharma S, et al. Efficacy and safety of treatment with rituximab for difficult steroid-resistant and -dependent nephrotic syndrome: multicentric report. Clin J Am Soc Nephrol. 2010;5:2207–2212. doi: 10.2215/CJN.03470410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sellier-Leclerc AL, Macher MA, Loirat C, Guérin V, Watier H, Peuchmaur M, et al. Rituximab efficiency in children with steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2010;25:1109–1115. doi: 10.1007/s00467-010-1465-6. [DOI] [PubMed] [Google Scholar]
- 25.Ravani P, Magnasco A, Edefonti A, Murer L, Rossi R, Ghio L, et al. Short-term effects of rituximab in children with steroid- and calcineurin-dependent nephrotic syndrome: a randomized controlled trial. Clin J Am Soc Nephrol. 2011;6:1308–1315. doi: 10.2215/CJN.09421010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sinha A, Bagga A, Gulati A, Hari P. Short-term efficacy of rituximab versus tacrolimus in steroid-dependent nephrotic syndrome. Pediatr Nephrol. 2012;27:235–241. doi: 10.1007/s00467-011-1997-4. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Haffner D, Fischer DC. Nephrotic syndrome and rituximab: facts and perspectives. Pediatr Nephrol. 2009;24:1433–1438. doi: 10.1007/s00467-009-1226-6. [DOI] [PubMed] [Google Scholar]
- 28.Meyrier AY. Treatment of focal segmental glomerulosclerosis with immunophilin modulation: when did we stop thinking about pathogenesis? Kidney Int. 2009;76:487–491. doi: 10.1038/ki.2009.204. [DOI] [PubMed] [Google Scholar]
- 29.van Husen M, Kemper MJ. New therapies in steroidsensitive and steroid-resistant idiopathic nephrotic syndrome. Pediatr Nephrol. 2011;26:881–892. doi: 10.1007/s00467-010-1717-5. [DOI] [PubMed] [Google Scholar]
- 30.Benz K, Dötsch J, Rascher W, Stachel D. Change of the course of steroid-dependent nephrotic syndrome after rituximab therapy. Pediatr Nephrol. 2004;19:794–797. doi: 10.1007/s00467-004-1434-z. [DOI] [PubMed] [Google Scholar]
- 31.Gilbert RD, Hulse E, Rigden S. Rituximab therapy for steroid-dependent minimal change nephrotic syndrome. Pediatr Nephrol. 2006;21:1698–1700. doi: 10.1007/s00467-006-0228-x. [DOI] [PubMed] [Google Scholar]
- 32.Bagga A, Sinha A, Moudgil A. Rituximab in patients with the steroid-resistant nephrotic syndrome. N Engl J Med. 2007;356:2751–2752. doi: 10.1056/NEJMc063706. [DOI] [PubMed] [Google Scholar]
- 33.Nakayama M, Kamei K, Nozu K, Matsuoka K, Nakagawa A, Sako M, et al. Rituximab for refractory focal segmental glomerulosclerosis. Pediatr Nephrol. 2008;23:481–485. doi: 10.1007/s00467-007-0640-x. [DOI] [PubMed] [Google Scholar]
- 34.Suri M, Tran K, Sharma AP, Filler G, Grimmer J. Remission of steroid-resistant nephrotic syndrome due to focal and segmental glomerulosclerosis using rituximab. Int Urol Nephrol. 2008;40:807–810. doi: 10.1007/s11255-008-9393-0. [DOI] [PubMed] [Google Scholar]
- 35.Fernandez-Fresnedo G, Segarra A, González E, Alexandru S, Delgado R, Ramos N, et al. Trabajo de Enfermedades Glomerulares de la Sociedad Española de Nefrología (GLOSEN). Rituximab treatment of adult patients with steroid-resistant focal segmental glomerulosclerosis. Clin J Am Soc Nephrol. 2009;4:1317–1323. doi: 10.2215/CJN.00570109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoxha E, Harendza S, Zahner G, Panzer U, Steinmetz O, Fechner K, et al. An immunofluorescence test for phospholipase-A2-receptor antibodies and its clinical usefulness in patients with membranous glomerulonephritis. Nephrol Dial Transplant. 2011;26:2526–2532. doi: 10.1093/ndt/gfr247. [DOI] [PubMed] [Google Scholar]
- 38.Beck LH, Jr, Fervenza FC, Beck DM, Bonegio RG, Malik FA, Erickson SB, et al. Rituximab-induced depletion of anti-PLA2R autoantibodies predicts response in membranous nephropathy. J Am Soc Nephrol. 2011;22:1543–1550. doi: 10.1681/ASN.2010111125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruggenenti P, Cravedi P, Sghirlanzoni MC, Gagliardini E, Conti S, Gaspari F, et al. Effects of rituximab on morphofunctional abnormalities of membranous glomerulopathy. Clin J Am Soc Nephrol. 2008;3:1652–1659. doi: 10.2215/CJN.01730408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fervenza FC, Cosio FG, Erickson SB, Specks U, Herzenberg AM, Dillon JJ, et al. Rituximab treatment of idiopathic membranous nephropathy. Kidney Int. 2008;73:117–125. doi: 10.1038/sj.ki.5002628. [DOI] [PubMed] [Google Scholar]
- 41.Ruggenenti P, Chiurchiu C, Brusegan V, Abbate M, Perna A, Filippi C, et al. Rituximab in idiopathic membranous nephropathy: a one-year prospective study. J Am Soc Nephrol. 2003;14:1851–1857. doi: 10.1097/01.ASN.0000071511.35221.B3. [DOI] [PubMed] [Google Scholar]
- 42.Segarra A, Praga M, Ramos N, Polanco N, Cargol I, Gutierrez-Solis E, et al. Successful treatment of membranous glomerulonephritis with rituximab in calcineurin inhibitor-dependent patients. Clin J Am Soc Nephrol. 2009;4:1083–1088. doi: 10.2215/CJN.06041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Polanco N, Gutiérrez E, Covarsí A, Ariza F, Carreño A, Vigil A, et al. Grupo de Estudio de las Enfermedades Glomerulares de la Sociedad Española de Nefrología, author. Spontaneous remission of nephrotic syndrome in idiopathic membranous nephropathy. J Am Soc Nephrol. 2010;21:697–704. doi: 10.1681/ASN.2009080861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Merrill JT, Neuwelt CM, Wallace DJ, Shanahan JC, Latinis KM, Oates JC, et al. Efficacy and safety of rituximab in moderately-to-severely active systemic lupus erythematosus: the randomized, double-blind, phase II/III systemic lupus erythematosus evaluation of rituximab trial. Arthritis Rheum. 2010;62:222–233. doi: 10.1002/art.27233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tamimoto Y, Horiuchi T, Tsukamoto H, Otsuka J, Mitoma H, Kimoto Y, et al. A dose-escalation study of rituximab for treatment of systemic lupus erythematosus and Evans' syndrome: immunological analysis of B cells, T cells and cytokines. Rheumatology (Oxford) 2008;47:821–827. doi: 10.1093/rheumatology/ken071. [DOI] [PubMed] [Google Scholar]
- 46.Camous L, Melander C, Vallet M, Squalli T, Knebelmann B, Noël LH, et al. Complete remission of lupus nephritis with rituximab and steroids for induction and rituximab alone for maintenance therapy. Am J Kidney Dis. 2008;52:346–352. doi: 10.1053/j.ajkd.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 47.Moroni G, Gallelli B, Banfi G, Leoni A, Messa P. Rituximab monotherapy for remission induction of proliferative lupus nephritis flares: description of 3 cases. J Nephrol. 2010;23:357–361. [PubMed] [Google Scholar]
- 48.Pepper R, Griffith M, Kirwan C, Levy J, Taube D, Pusey C, et al. Rituximab is an effective treatment for lupus nephritis and allows a reduction in maintenance steroids. Nephrol Dial Transplant. 2009;24:3717–3723. doi: 10.1093/ndt/gfp336. [DOI] [PubMed] [Google Scholar]
- 49.Li EK, Tam LS, Zhu TY, Li M, Kwok CL, Li TK, et al. Is combination rituximab with cyclophosphamide better than rituximab alone in the treatment of lupus nephritis? Rheumatology (Oxford) 2009;48:892–898. doi: 10.1093/rheumatology/kep124. [DOI] [PubMed] [Google Scholar]
- 50.Merrill J, Buyon J, Furie R, Latinis K, Gordon C, Hsieh HJ, et al. Assessment of flares in lupus patients enrolled in a phase II/III study of rituximab (EXPLORER) Lupus. 2011;20:709–716. doi: 10.1177/0961203310395802. [DOI] [PubMed] [Google Scholar]
- 51.Fra GP, Avanzi GC, Bartoli E. Remission of refractory lupus nephritis with a protocol including rituximab. Lupus. 2003;12:783–787. doi: 10.1191/0961203303lu453cr. [DOI] [PubMed] [Google Scholar]
- 52.Jónsdóttir T, Gunnarsson I, Risselada A, Henriksson EW, Klareskog L, van Vollenhoven RF. Treatment of refractory SLE with rituximab plus cyclophosphamide: clinical effects, serological changes and predictors of response. Ann Rheum Dis. 2008;67:330–334. doi: 10.1136/ard.2007.079095. [DOI] [PubMed] [Google Scholar]
- 53.Boletis JN, Marinaki S, Skalioti C, Lionaki SS, Iniotaki A, Sfikakis PP. Rituximab and mycophenolate mofetil for relapsing proliferative lupus nephritis: a longterm prospective study. Nephrol Dial Transplant. 2009;24:2157–2160. doi: 10.1093/ndt/gfp002. [DOI] [PubMed] [Google Scholar]
- 54.Tanaka Y, Yamamoto K, Takeuchi T, Nishimoto N, Miyasaka N, Sumida T, et al. A multicenter phase I/II trial of rituximab for refractory systemic lupus erythematosus. Mod Rheumatol. 2007;17:191–197. doi: 10.1007/s10165-007-0565-z. [DOI] [PubMed] [Google Scholar]
- 55.Gunnarsson I, Sundelin B, Jónsdóttir T, Jacobson SH, Henriksson EW, van Vollenhoven RF. Histopathologic and clinical outcome of rituximab treatment in patients with cyclophosphamide-resistant proliferative lupus nephritis. Arthritis Rheum. 2007;56:1263–1272. doi: 10.1002/art.22505. [DOI] [PubMed] [Google Scholar]
- 56.Roccatello D, Baldovino S, Alpa M, Rossi D, Napoli F, Naretto C, et al. Effects of anti-CD20 monoclonal antibody as a rescue treatment for ANCA-associated idiopathic systemic vasculitis with or without overt renal involvement. Clin Exp Rheumatol. 2008;26:67–71. [PubMed] [Google Scholar]
- 57.Melander C, Sallée M, Trolliet P, Candon S, Belenfant X, Daugas E, et al. Rituximab in severe lupus nephritis: early B-cell depletion affects long-term renal outcome. Clin J Am Soc Nephrol. 2009;4:579–587. doi: 10.2215/CJN.04030808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Goto S, Goto H, Tanoshima R, Kato H, Takahashi H, Sekiguchi O, et al. Serum sickness with an elevated level of human anti-chimeric antibody following treatment with rituximab in a child with chronic immune thrombocytopenic purpura. Int J Hematol. 2009;89:305–309. doi: 10.1007/s12185-009-0269-6. [DOI] [PubMed] [Google Scholar]
- 59.Albert D, Dunham J, Khan S, Stansberry J, Kolasinski S, Tsai D, et al. Variability in the biological response to anti-CD20 B cell depletion in systemic lupus erythaematosus. Ann Rheum Dis. 2008;67:1724–1731. doi: 10.1136/ard.2007.083162. [DOI] [PubMed] [Google Scholar]
- 60.Holden NJ, Williams JM, Morgan MD, Challa A, Gordon J, Pepper RJ, et al. ANCA-stimulated neutrophils release BLyS and promote B cell survival: a clinically relevant cellular process. Ann Rheum Dis. 2011;70:2229–2233. doi: 10.1136/ard.2011.153890. [DOI] [PubMed] [Google Scholar]
- 61.Jones RB, Ferraro AJ, Chaudhry AN, Brogan P, Salama AD, Smith KG, et al. A multicenter survey of rituximab therapy for refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2009;60:2156–2168. doi: 10.1002/art.24637. [DOI] [PubMed] [Google Scholar]
- 62.Stasi R, Stipa E, Del Poeta G, Amadori S, Newland AC, Provan D. Long-term observation of patients with anti-neutrophil cytoplasmic antibody-associated vasculitis treated with rituximab. Rheumatology (Oxford) 2006;45:1432–1436. doi: 10.1093/rheumatology/kel098. [DOI] [PubMed] [Google Scholar]
- 63.Keogh KA, Wylam ME, Stone JH, Specks U. Induction of remission by B lymphocyte depletion in eleven patients with refractory antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2005;52:262–268. doi: 10.1002/art.20718. [DOI] [PubMed] [Google Scholar]
- 64.Keogh KA, Ytterberg SR, Fervenza FC, Carlson KA, Schroeder DR, Specks U. Rituximab for refractory Wegener's granulomatosis: report of a prospective, open-label pilot trial. Am J Respir Crit Care Med. 2006;173:180–187. doi: 10.1164/rccm.200507-1144OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jones RB, Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. European Vasculitis Study Group, author. Rituximab versus cyclophosphamide in ANCA-associated renal vasculit. N Engl J Med. 2010;363:211–220. doi: 10.1056/NEJMoa0909169. [DOI] [PubMed] [Google Scholar]
- 66.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. RAVE-ITN Research Group, author. Rituximab versus cyclophosphamide for ANCAassociated vasculitis. N Engl J Med. 2010;363:221–232. doi: 10.1056/NEJMoa0909905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rhee EP, Laliberte KA, Niles JL. Rituximab as maintenance therapy for anti-neutrophil cytoplasmic antibody-associated vasculitis. Clin J Am Soc Nephrol. 2010;5:1394–1400. doi: 10.2215/CJN.08821209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holle JU, Dubrau C, Herlyn K, Heller M, Ambrosch P, Noelle B, et al. Rituximab for refractory granulomatosis with polyangiitis (Wegener's granulomatosis): comparison of efficacy in granulomatous versus vasculitic manifestations. [Epub ahead of print] Ann Rheum Dis. 2011 doi: 10.1136/ard.2011.153601. [DOI] [PubMed] [Google Scholar]
- 69.Mansfield N, Hamour S, Habib AM, Tarzi R, Levy J, Griffith M, et al. Prolonged disease-free remission following rituximab and low-dose cyclophosphamide therapy for renal ANCA-associated vasculitis. Nephrol Dial Transplant. 2011;26:3280–3286. doi: 10.1093/ndt/gfr127. [DOI] [PubMed] [Google Scholar]
- 70.Wink F, Houtman PM, Jansen TL. Rituximab in cryoglobulinaemic vasculitis, evidence for its effectivity: a case report and review of literature. Clin Rheumatol. 2011;30:293–300. doi: 10.1007/s10067-010-1612-2. [DOI] [PubMed] [Google Scholar]
- 71.Saadoun D, Resche Rigon M, Sene D, Terrier B, Karras A, Perard L, et al. Rituximab plus Peg-interferonalpha/ribavirin compared with Peg-interferon-alpha/ribavirin in hepatitis C-related mixed cryoglobulinemia. Blood. 2010;116:326–334. doi: 10.1182/blood-2009-10-248518. [DOI] [PubMed] [Google Scholar]
- 72.Ghijsels E, Lerut E, Vanrenterghem Y, Kuypers D. Anti-CD20 monoclonal antibody (rituximab) treatment for hepatitis C-negative therapy-resistant essential mixed cryoglobulinemia with renal and cardiac failure. Am J Kidney Dis. 2004;43:34–38. doi: 10.1053/j.ajkd.2003.12.057. [DOI] [PubMed] [Google Scholar]
- 73.Ferri C, Cacoub P, Mazzaro C, Roccatello D, Scaini P, Sebastiani M, et al. Treatment with rituximab in patients with mixed cryoglobulinemia syndrome: results of multicenter cohort study and review of the literature. Autoimmun Rev. 2011;11:48–55. doi: 10.1016/j.autrev.2011.07.005. [DOI] [PubMed] [Google Scholar]
- 74.Basse G, Ribes D, Kamar N, Mehrenberger M, Sallusto F, Esposito L, et al. Rituximab therapy for mixed cryoglobulinemia in seven renal transplant patients. Transplant Proc. 2006;38:2308–2310. doi: 10.1016/j.transproceed.2006.06.131. [DOI] [PubMed] [Google Scholar]
- 75.Kawano N, Ikeda N, Yoshida S, Sugio Y, Yamashita K, Uezono S, et al. Successful treatment of cryoglobulinemic glomerulonephritis derived from Waldenström's macroglobulinemia by rituximab-CHOP and tandem high-dose chemotherapy with autologous peripheral blood stem cell transplantation. Int J Hematol. 2010;92:391–397. doi: 10.1007/s12185-010-0638-1. [DOI] [PubMed] [Google Scholar]
- 76.Guiard E, Karras A, Plaisier E, Duong Van Huyen JP, Fakhouri F, Rougier JP, et al. Patterns of noncryoglobulinemic glomerulonephritis with monoclonal Ig deposits: correlation with IgG subclass and response to rituximab. Clin J Am Soc Nephrol. 2011;6:1609–1616. doi: 10.2215/CJN.10611110. [DOI] [PubMed] [Google Scholar]
- 77.Coppola N, Pisaturo M, Guastafierro S, Tonziello G, Sica A, Iodice V, et al. Increased hepatitis C viral load and reactivation of liver disease in HCV RNApositive patients with onco-haematological disease undergoing chemotherapy. Dig Liver Dis. 2012;44:49–54. doi: 10.1016/j.dld.2011.07.016. [DOI] [PubMed] [Google Scholar]
- 78.Stamataki Z, Tilakaratne S, Adams DH, McKeating JA. Rituximab treatment in hepatitis C infection: an in vitro model to study the impact of B cell depletion on virus infectivity. PLoS One. 2011;6:25789. doi: 10.1371/journal.pone.0025789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sinha A, Nast CC, Hristea I, Vo AA, Jordan SC. Resolution of clinical and pathologic features of C1q nephropathy after rituximab therapy. Clin Exp Nephrol. 2011;15:164–170. doi: 10.1007/s10157-010-0377-x. [DOI] [PubMed] [Google Scholar]
- 80.Collins M, Navaneethan SD, Chung M, Sloand J, Goldman B, Appel G, et al. Rituximab treatment of fibrillary glomerulonephritis. Am J Kidney Dis. 2008;52:1158–1162. doi: 10.1053/j.ajkd.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 81.Gallerani E, Lerch E, Romagnani E, Stathis A, Giardelli G, Zwhalen H, et al. Thrombotic thrombocytopenic purpura associated with renal failure after autologous transplantation for multiple myeloma successfully treated with rituximab. Eur J Haematol. 2006;77:527–529. doi: 10.1111/j.1600-0609.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 82.Chemnitz J, Draube A, Scheid C, Staib P, Schulz A, Diehl V, et al. Successful treatment of severe thrombotic thrombocytopenic purpura with the monoclonal antibody rituximab. Am J Hematol. 2002;71:105–108. doi: 10.1002/ajh.10204. [DOI] [PubMed] [Google Scholar]
- 83.Gutterman LA, Kloster B, Tsai HM. Rituximab therapy for refractory thrombotic thrombocytopenic purpura. Blood Cells Mol Dis. 2002;28:385–391. doi: 10.1006/bcmd.2002.0522. [DOI] [PubMed] [Google Scholar]
- 84.Lombardi AM, de Marinis GB, Scandellari R, Magalini F, Sansoni P, Ballerini PF, et al. Clinical biological remission induced by rituximab in acute refractory chronic relapsing TTP. Thromb Res. 2010;126:154–156. doi: 10.1016/j.thromres.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 85.Scully M, McDonald V, Cavenagh J, Hunt BJ, Longair I, Cohen H, et al. A phase 2 study of the safety and efficacy of rituximab with plasma exchange in acute acquired thrombotic thrombocytopenic purpura. Blood. 2011;118:1746–1753. doi: 10.1182/blood-2011-03-341131. [DOI] [PubMed] [Google Scholar]
- 86.Froissart A, Buffet M, Veyradier A, Poullin P, Provôt F, Malot S, et al. French Thrombotic Microangiopathies Reference Center; Experience of the French Thrombotic Microangiopathies Reference Center, author. Efficacy and safety of first-line rituximab in severe, acquired thrombotic thrombocytopenic purpura with a suboptimal response to plasma exchange. Crit Care Med. 2012;40:104–111. doi: 10.1097/CCM.0b013e31822e9d66. [DOI] [PubMed] [Google Scholar]
- 87.Klipa D, Mahmud N, Ahsan N. Antibody immunosuppressive therapy in solid organ transplant: Part II. MAbs. 2010;2:607–612. doi: 10.4161/mabs.2.6.13586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Becker YT, Becker BN, Pirsch JD, Sollinger HW. Rituximab as treatment for refractory kidney transplant rejection. Am J Transplant. 2004;4:996–1001. doi: 10.1111/j.1600-6143.2004.00454.x. [DOI] [PubMed] [Google Scholar]
- 89.Genberg H, Kumlien G, Wennberg L, Berg U, Tydén G. ABO-incompatible kidney transplantation using antigen-specific immunoadsorption and rituximab: a 3-year follow-up. Transplantation. 2008;85:1745–1754. doi: 10.1097/TP.0b013e3181726849. [DOI] [PubMed] [Google Scholar]
- 90.Tydén G, Genberg H, Tollemar J, Ekberg H, Persson NH, Tufveson G, et al. A randomized, doubleblind, placebo-controlled, study of single-dose rituximab as induction in renal transplantation. Transplantation. 2009;87:1325–1329. doi: 10.1097/TP.0b013e3181a235fd. [DOI] [PubMed] [Google Scholar]
- 91.Clatworthy MR, Watson CJ, Plotnek G, Bardsley V, Chaudhry AN, Bradley JA, et al. B-cell-depleting induction therapy and acute cellular rejection. N Engl J Med. 2009;360:2683–2685. doi: 10.1056/NEJMc0808481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Solez K, Colvin RB, Racusen LC, Haas M, Sis B, Mengel M, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–760. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 93.Vo AA, Lukovsky M, Toyoda M, Wang J, Reinsmoen NL, Lai CH, et al. Rituximab and intravenous immunoglobulin for desensitization during renal transplantation: a paradigm ready for broad implementation. Transplantation. 2009;87:1246–1255. [Google Scholar]
- 94.Kaposztas Z, Podder H, Mauiyyedi S, Illoh O, Kerman R, Reyes M, et al. Impact of rituximab therapy for treatment of acute humoral rejection. Clin Transplant. 2009;23:63–73. doi: 10.1111/j.1399-0012.2008.00902.x. [DOI] [PubMed] [Google Scholar]
- 95.Mulley WR, Hudson FJ, Tait BD, Skene AM, Dowling JP, Kerr PG, et al. A single low-fixed dose of rituximab to salvage renal transplants from refractory antibodymediated rejection. Transplantation. 2009;87:286–289. doi: 10.1097/TP.0b013e31819389cc. [DOI] [PubMed] [Google Scholar]
- 96.Ivanyi B. A primer on recurrent and de novo glomerulonephritis in renal allografts. Nat Clin Pract Nephrol. 2008;4:446–457. doi: 10.1038/ncpneph0854. [DOI] [PubMed] [Google Scholar]
- 97.Briganti EM, Russ GR, McNeil JJ, Atkins RC, Chadban SJ. Risk of renal allograft loss from recurrent glomerulonephritis. N Engl J Med. 2002;347:103–109. doi: 10.1056/NEJMoa013036. [DOI] [PubMed] [Google Scholar]
- 98.Wadei HM, Geiger XJ, Mai ML. Recurrent glomerular diseases in the allograft: risk factors and management. In: Ahsan N, editor. Chronic allograft failure: natural history, pathogenesis, diagnosis and management. Austin TX: Landes Bioscience; 2008. pp. 200–221. [Google Scholar]
- 99.Hariharan S, Adams MB, Brennan DC, Davis CL, First MR, Johnson CP, et al. Recurrent and de novo glomerular disease after renal transplantation: a report from Renal Allograft Disease Registry (RADR) Transplantation. 1999;68:635–641. doi: 10.1097/00007890-199909150-00007. [DOI] [PubMed] [Google Scholar]
- 100.Damodar A, Mustafa R, Bhatnagar J, Panesar M, Gundroo A, Zachariah M, et al. Use of anti-CD20 antibody in the treatment of post-transplant glomerulonephritis. Clin Transplant. 2011;25:375–379. doi: 10.1111/j.1399-0012.2010.01245.x. [DOI] [PubMed] [Google Scholar]
- 101.Dall'Amico R, Ghiggeri G, Carraro M, Artero M, Ghio L, Zamorani E, et al. Prediction and treatment of recurrent focal segmental glomerulosclerosis after renal transplantation in children. Am J Kidney Dis. 1999;34:1048–1055. doi: 10.1016/S0272-6386(99)70010-7. [DOI] [PubMed] [Google Scholar]
- 102.Rodríguez-Ferrero M, Ampuero J, Anaya F. Rituximab and chronic plasmapheresis therapy of nephrotic syndrome in renal transplantation patients with recurrent focal segmental glomerulosclerosis. Transplant Proc. 2009;41:2406–2408. doi: 10.1016/j.transproceed.2009.06.044. [DOI] [PubMed] [Google Scholar]
- 103.Westphal S, Hansson S, Mjörnstedt L, Mölne J, Swerkersson S, Friman S. Early recurrence of nephrotic syndrome (immunoglobulin m nephropathy) after renal transplantation successfully treated with combinations of plasma exchanges, immunoglobulin and rituximab. Transplant Proc. 2006;38:2659–2660. doi: 10.1016/j.transproceed.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 104.Hickson LJ, Gera M, Amer H, Iqbal CW, Moore TB, Milliner DS, et al. Kidney transplantation for primary focal segmental glomerulosclerosis: outcomes and response to therapy for recurrence. Transplantation. 2009;87:1232–1239. doi: 10.1097/TP.0b013e31819f12be. [DOI] [PubMed] [Google Scholar]
- 105.Dello Strologo L, Guzzo I, Laurenzi C, Vivarelli M, Parodi A, Barbano G, et al. Use of rituximab in focal glomerulosclerosis relapses after renal transplantation. Transplantation. 2009;88:417–420. doi: 10.1097/TP.0b013e3181aed9d7. [DOI] [PubMed] [Google Scholar]
- 106.Hristea D, Hadaya K, Marangon N, Buhler L, Villard J, Morel P, et al. Successful treatment of recurrent focal segmental glomerulosclerosis after kidney transplantation by plasmapheresis and rituximab. Transpl Int. 2007;20:102–105. doi: 10.1111/j.1432-2277.2006.00395.x. [DOI] [PubMed] [Google Scholar]
- 107.Sakai K, Takasu J, Nihei H, Yonekura T, Aoki Y, Kawamura T, et al. Protocol biopsies for focal segmental glomerulosclerosis treated with plasma exchange and rituximab in a renal transplant patient. Clin Transplant. 2010;24:60–65. doi: 10.1111/j.1399-0012.2010.01279.x. [DOI] [PubMed] [Google Scholar]
- 108.Kaito H, Kamei K, Kikuchi E, Ogura M, Matsuoka K, Nagata M, et al. Successful treatment of collapsing focal segmental glomerulosclerosis with a combination of rituximab, steroids and ciclosporin. Pediatr Nephrol. 2010;25:957–959. doi: 10.1007/s00467-009-1410-8. [DOI] [PubMed] [Google Scholar]
- 109.Sprangers B, Lefkowitz GI, Cohen SD, Stokes MB, Valeri A, Appel GB, et al. Beneficial effect of rituximab in the treatment of recurrent idiopathic membranous nephropathy after kidney transplantation. Clin J Am Soc Nephrol. 2010;5:790–797. doi: 10.2215/CJN.04120609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sathyan S, Khan FN, Ranga KV. A case of recurrent immunotactoid glomerulopathy in an allograft treated with rituximab. Transplant Proc. 2009;41:3953–3955. doi: 10.1016/j.transproceed.2009.03.100. [DOI] [PubMed] [Google Scholar]
- 111.Kamar N, Milioto O, Puissant-Lubrano B, Esposito L, Pierre MC, Mohamed AO, et al. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant. 2010;10:89–98. doi: 10.1111/j.1600-6143.2009.02785.x. [DOI] [PubMed] [Google Scholar]
- 112.Shelton E, Yong M, Cohney S. Late onset Pneumocystis pneumonia in patients receiving rituximab for humoral renal transplant rejection. Nephrology (Carlton) 2009;14:696–699. doi: 10.1111/j.1440-797.2009.01168.x. [DOI] [PubMed] [Google Scholar]
- 113.Bitzan M, Ouahed JD, Carpineta L, Bernard C, Bell LE. Cryptogenic organizing pneumonia after rituximab therapy for presumed post-kidney transplant lymphoproliferative disease. Pediatr Nephrol. 2010;25:1163–1167. doi: 10.1007/s00467-010-1447-8. [DOI] [PubMed] [Google Scholar]
- 114.Kamar N, Mengelle C, Rostaing L. Incidence of JC-virus replication after rituximab therapy in solidorgan transplant patients. Am J Transplant. 2009;9:244–245. doi: 10.1111/j.1600-6143.2008.02499.x. [DOI] [PubMed] [Google Scholar]