Abstract
The safety and pharmacokinetics assessment of antibodies targeting CD22 (e.g., epratuzumab) have been established in western Caucasian populations, but there are no reports of the effects in Chinese populations. This dose-escalation study examines the safety, pharmacokinetics and biologic effects of multiple doses of anti-CD22 human-murine chimeric monoclonal antibody SM03 in 21 Chinese patients with CD22-positive non-Hodgkin lymphoma. Most of drug-related adverse events (AEs) were mild and reversible. Two patients experienced serious AEs (hemorrhage); one patient had grade 4 neutropenia; one patient had asymptomatic grade III prolongation of activated partial thromboplastin time (APTT). Major AEs included fever (71%), prolongation of APTT (42.8%), leukocytopenia (44.4%), alanine transaminase elevation (28.6%), elevated serum creatinine (23.8%) and injection site skin redness (14.3%). Circulating B cells transiently decreased without significant effects on T cells or immunoglobulin levels. Pharmacokinetic data revealed that mean maximum observed SM03 concentration and mean AUC from time zero to infinity increased in a dose-dependent manner up to 360 mg/m2 SM03. Mean clearance was similar at doses ≤360 mg/m2 and decreased significantly at dose 480 mg/m2, supporting saturation of B-cell binding at 360 mg/m2. Across all dose levels and histologies, one patient achieved partial response at 480 mg/m2 dose; 14 patients had stable disease as best response and four patients progressed. Overall, SM03 was tolerated at doses ranging from 60–480 mg/m2 and had potential efficacy in Chinese patients with follicular lymphoma.
Keywords: anti-CD22 monoclonal antibody, tolerance, pharmacokinetics
Introduction
Non-Hodgkin lymphomas (NHLs), a heterogeneous group of cancers principally arising from B lymphocytes, represent approximately 4% of newly diagnosed cancers, and are characterized by expression of lineage-specific B cell antigens, such as CD19, CD20 and CD22.1 Aggressive NHL comprises approximately 30–40% of adult NHL, and indolent (or low-grade) B-cell lymphomas represent approximately 40% of NHLs.2 Effective treatments for indolent lymphomas include radiotherapy, single-agent therapy or combination chemotherapy and the response rate is in the range of 60–80% for first-line therapy.3 Patients with aggressive NHL have high response rates to the front-line combination chemotherapy regimen of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP),4–6 but disease recurs and becomes resistant to treatment in most patients.
The successful use of monoclonal antibodies (mAbs) in the treatment of human disease has increased steadily in the past two decades. Rituximab, a human-mouse chimeric anti-CD20 immunoglobulin (Ig) G1, was approved in 1997 in the United States as the first mAb used in combination with chemotherapy for first and subsequent lines of treatment for lymphoma.7,8 However, a subgroup of patients does not respond, and early relapses occur in patients with initial response. There is thus a clear need to explore alternative antibodies that are non-cross resistant to rituximab as therapy for B-NHL. One alternative is to explore new antibodies targeting B-cell antigens such as CD22; two such mAbs, epratuzumab (a humanized anti-CD22 IgG1), and inotuzumab ozogamicin (a humanized anti-CD22 IgG4 conjugated to calicheamicin), are currently in clinical studies.9–12
CD22 is a 135-kDa B lymphocyte restricted type-I transmembrane sialoglycoprotein of the immunoglobulin superfamily, with seven Ig-like domains and three cytoplasmic immunoreceptor tyrosine-based inhibition motifs.12 CD22 is expressed in mature B cells, but not in their precursor or memory B cells.13,14 It is strongly expressed in follicular, mantle and marginal-zone B cells, but is weakly present in germinal B cells, thus indicting its potential therapeutic target for B-NHL.15 The function of CD22 has not been entirely clarified; it acts as a homing receptor for recirculating B cells through the affinity of the lectin-like domains for 2,6-linked sialic acid-bearing glycans and as a B-cell antigen receptor (BCR) down-modulating co-receptor.16
In early clinical studies of epratuzumab (Immunomedics, Inc.,), single-agent activity was shown in patients with aggressive NHL and indolent NHL. Monotherapy was found to be well-tolerated, and there was evidence of clinical activity when western Caucasian patients with NHL were administered doses from 120–1,000 mg/m2 and, specifically, at the 360 mg/m2 dose used for a Phase 2 clinical trial.17 It is suggested that biological agents do not necessary have optimal activity at the maximally tolerated dose. If toxic effects are mediated by a mechanism distinct from that of antitumor action, increasing doses to toxic levels would not be necessary to achieve efficacy. This is especially important for relatively nontoxic agents such as antibodies because the ultimate goal of early clinical studies is to identify an optimal dose. Based on preclinical studies, the optimal dose was defined as the dose at which B cells were completely saturated such that maximal inhibition of tumor growth would be achieved. It has been suggested that complete saturation of the B-cell binding might be associated with a plateau of systemic clearance of antibody, but the published pharmacokinetic data describing the behavior of antibodies targeted to CD22 in man are limited.17,18
However, no antibodies targeted to CD22 have been evaluated in Chinese patients with NHL as yet. SM03 is a recombinant, human/mouse chimeric IgG1 mAb directed against CD22 antigen on human B lymphocytes. It is composed of Fv regions of a murine anti-CD22 antibody with human Ig G1 heavy, with molecular weight of 148 KD, produced by Shenzhen Lonn Ryonn Pharmaceutical Co., Ltd., SM03 is expressed in ST20 cell, which is stably transfected murine myeloma cell line (SP2/0). We compared the affinity the in vitro and in vivo biological activities of these two antibodies. The vitro assays showed SM03 had the same high affinity (0.82 nM) against CD22 antigen as epratuzumab, and also demonstrated reactivity antibody with 50 of 51 B cell NHL specimens tested, but not with other malignancies or normal non-lymphoid tissues CD19 and CD20 antigens (data not shown).
We describe here the safety, clinical activity, pharmacokinetics and immunogenicity of SM03 in Chinese patients with NHL. Effects on blood mononuclear cells, Ig levels, aminotransferase and other laboratory parameters were assessed. SM03 was administered as four once-weekly infusions at doses from 60–480 mg/m2 to patients with NHL and, based on the results, an optimal dose was chosen for further research. These data demonstrate that, although SM03 showed more adverse effects occurrence and higher incidence rate of side effects in Chinese patients compared with those observed in western Caucasian patients treated with epratuzumab, SM03 had an acceptable safety profile, exhibited clinical benefits and offers promise as a new agent with potential utility in the treatment of NHL.
Results
Demographics and baseline disease characteristics.
From April 2007 to September 2008, a total of 21 patients were enrolled in the study. First, one patient was administered SM03 at a dose of 60 mg/m2/week and monitored for infusion reactions. Dose escalation was conducted with two patients who received 120 mg/m2 in the initial cohort. Subsequently, six patients per group were treated at each of the 240, 360 and 480 mg/m2/week dose levels. Demographic and other baseline characteristics for all patients are summarized in Table 1. There were ten males and 11 females, with median age of 54 y (range, 24–67 y). All patients had CD22-positive B-cell non-Hodgkin lymphoma and were categorized in stage II (9.5%), stage III (42.9%) and stage IV (47.6%) according to clinical staging.
Table 1.
Baseline demographic characteristics for patients receiving at least 1 dose of SM03
| Baseline characteristic | Patients receiving at least 1 dose of SM03 (n = 21) |
| Median age (range) (years) | 52 (24–67) |
| No. of females (%) | 52 |
| Median no. prior therapies (range) | 3 (0–6) |
| No. with prior rituximab treatment (%) | 38 |
| No. with prior high-dose chemotherapy and stem cell transplant (%) | 9 |
| Pathology of lymphoma: Follicular (n) | 8 |
| Diffuse large B-cell lymphoma | 10 |
| Others | 3 |
| No. with ECOG performance scale = 0–1 | 18 |
| No. with bulky disease at least 5 cm (%) | 14 |
| SPD range (cm) | 1.4–68 |
| No. with LDH >234 units/liter (%) | 9 |
| Median peripheral blood B-cell counts | 11.54 |
| CD20, cells/µl (range) | 0–33.29 |
| No. with FLIPI score = 1∼2 (%) | 7 |
Dose escalation. In dose escalation, one patient of six cohorts at the dose of 360 mg/m2/week had dose limiting toxicity (DLT) and experienced grade 4 neutropenia for 7 d. We did not observe a maximum tolerated dose (MTD) in the dose range of 60–480 mg/m2/week. Estimates of systemic clearance of SM03 were indicative of possible nonlinear elimination at doses >360 mg/m2. Thus, we defined 480 mg/m2/week as the maximum dose without dose escalation. Additional patients were treated at dose levels (≥240 mg/m2) to define a more effective dose for additional clinical trials.
Safety.
All patients experienced one or more adverse event, with at least 85% of patients experiencing one treatment-related adverse effect. Most of treatment-related events were mild to moderate in severity. The major toxicities for all adverse effects were fever (71%), prolongation of activated partial thromboplastin time (PT) (42.8%), leukocytopenia (24%), neutropenia (19%), alanine transaminase elevation (24%), elevated serum creatinine (19%), injection site skin redness (14.3%). Other toxicities, regardless of whether or not they were infusion/treatment-related, included fatigue, nausea, vomiting, tumor pain, skin rash, hypertension, hypotension, fatigue, occult blood (Table 2).
Table 2.
All adverse events with at least 5% incidence or grade ≤2 in severity over all treatment groups combined
| Preferred term | Weekly dose group (mg/m2) | |||||
| 60(n = 1) | 120(n = 2) | 240(n = 6) | 360(n = 6) | 480(n = 6) | Total* | |
| No. of patients with adverse events | ||||||
| Fever | 1(100%) | 2(100%) | 5(83%) | 2(33%) | 4(67%) | 14(67%) |
| Fatigue | 2(100%) | 1(17%) | 0(0%) | 1(17%) | 4(9%) | |
| Leukocytopenia | 1(100%) | 2(33%) | 1(17%) | 1(17%) | 5(24%) | |
| Neutropenia | 1(100%) | 1(17%) | 1(17%) | 1(17%) | 4(19%) | |
| ALT elevation | 1(17%) | 2(33%) | 1(17%) | 5(24%) | ||
| Injection site skin redness | 3(50%) | 3(14%) | ||||
| Elevated serum creatinine | 1(50%) | 1(17%) | 1(17%) | 1(17%) | 4(19%) | |
| APTT | 1(100%) | 1(50%) | 2(33%) | 3(50%) | 7(33%) | |
Six patients developed grade I prolongation of activated partial thromboplastin time (APTT) and one developed asymptomatic grade III prolongation treated at 360 mg/m2, and needed to be supplied with intravenous coagulation factors; in all cases the APTT returned to baseline on discontinuation of SM03. Elevated transaminase (grade 1–2) occurred in six patients, three with hepatitis B virus (HBV) infection who needed orally administered heptodin to inhibit HBV replication, whereas no significant changes were seen in the number of copies of HBV. All six patients recovered during observation without supportive medication. Hematologic toxicity was usually mild and reversible. Six of 21 patients experienced leukopenia that was mild and reversible, of which five had grade I toxicity and one had grade II toxicity.
One patient experienced serious adverse effects (SAE) of gastrointestinal hemorrhage (about 300 ml blood) after 3 doses. Intravenous blood transfusion (400 ml) and fluid replacement were administered to the patient, who subsequently recovered. Another patient with a dental ulcer also experienced SAE of hemorrhage (about 5 ml) in the follow-up period. One patient discontinued treatment due to progression.
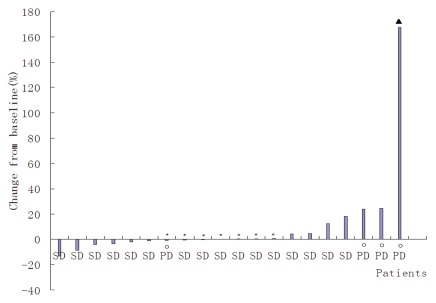
Response. When all dosing groups and histologies are considered, 15 patients (75%) of the 20 patients (excluding those who discontinued treatment) achieved stable disease as best progress, and four (20%) progressed by study day 42. However, one patient had a PR on study day 142 without interim therapy. Efficacy evaluations based on pathologic classification are detailed in Table 3. Some patients (all histologies) derived clinical benefit in the form of some amount of tumor mass reduction (Fig. 1). The average decrease in lesion size was 56.1% in patients achieving a PR and 2.3–48.6% in patients with stable disease (SD). Tumor size was reduced by at least 2.3% in 9 patients. Patients reported symptoms or other disease-related signs and symptoms at baseline, and symptoms resolved completely or were relived transiently in two of eight patients.
Table 3.
Analysis of efficacy by different pathology classification
| Pathology | CR/PR | SD | PD | NE | TOTAL |
| Follicular lymphoma | 1 | 7 | 0 | 0 | 8 |
| Diffuse large B | 0 | 3 | 4 | 1 | 8 |
| Follicular transformed to diffuse | 0 | 2 | 0 | 0 | 2 |
| Small lymphocytic lymphoma/CLL | 0 | 2 | 0 | 0 | 2 |
| Birkitt's lymphoma | 0 | 1 | 0 | 0 | 1 |
| Total | 0 | 16 | 4 | 1 | 21 |
Figure 1.
Maximum percent change from baseline in SPD for each assessable treated patient with baseline and post-baseline SPD measurements (n = 20). *Patients with absolute value of maximum percentage change ≤1. Filled triangle, SPD value >100%; open circle, patients with progressive disease as best response; no post-baseline SPD measurement available.
A female patient who was diagnosed with stage IV A follicular grade I lymphoma (FLIPI score 3) received treatment at a dose of 480 mg/m2 SM03 for 4 consecutive cycles. On day 35, the tumor had shrunk by 23.4%, and decreased by 48.53% and 56.1% on day 72 and 142, respectively; efficacy rated as PR. A male patient, who was diagnosed with stage IEA follicular grade II lymphoma and progressed after having received anti-CD20 mAb treatment for almost 10 mo, was enrolled in the SM03 clinical trial at the dosage level of 360 mg/m2 SM03. The SPD of target lesion of 24.75 cm2 at base line; after he received 4 administrations of SM03, SPD decreased to 16.00 cm2 and 14.00 cm2 at day 42 and 95, respectively, which represents shrinkage by 35.35% and 43.43% in SPD.
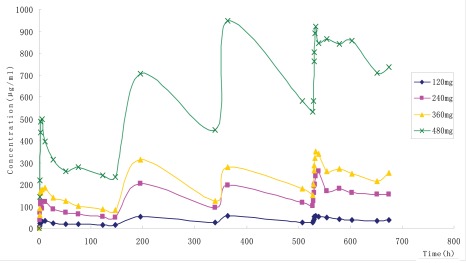
Pharmacokinetic results. Pharmacokinetic data after four-week dosing were obtained for 19 of the 21 patients. Pharmacokinetic data for one patient who discontinued treatment during the third-dose phase due to disease progression and one patient who received at dose of 60 mg/m2 were not included. A summary of the SM03 pharmacokinetic variables are presented in Figure 2 and Table 4. Following infusion of a single dose of SM03 at 120 mg/m2 (n = 2), 240 mg/m2 (n = 6), 360 mg/m2 (n = 6) or 480 mg/m2 (n = 6), levels of SM03 in serum reached a maximum at ∼3 h and then declined slowly (Fig. 2). The observed maximum serum concentration after each dose increased slightly from dose 1 through dose 4 at all dose levels, consistent with an accumulation ratio of 3 for area under the curve (AUC). Cmax varied greatly across individuals and ranged from 2.16% to 93.35% and related to tumor burden, circulating tumor cells and CD22 antigen in the circulation of the patients.
Figure 2.
Mean (SD) serum concentrations of total SM03 after 120, 240, 360, 480 mg/m2 infusion of SM03 once-weekly for 4 consecutive weeks. Filled diamond, 120 mg/m2; filled square, 240 mg/m2; filled triangle, 360 mg/m2; X, 480 mg/m2.
Table 4.
Pharmacokinetic Parameter Summary (120–480 mg/m2 Dosage)
| Dosage | 120 mg | 240 mg | 360 mg | 480 mg | ||||
| Infusion | C1 | C4 | C1 | C4 | C1 | C4 | C1 | C4 |
| Tmax (h) | 7.00 ± 5.66 | 8.00 ± 4.24 | 6.50 ± 4.11 | 7.10 ± 3.61 | 6.17 ± 3.80 | 7.67 ± 3.72 | 3.92 ± 0.86 | 23.83 ± 31.29 |
| Cmax µ (ug/ml) | 34.7 ± 0.99 | 29.97 ± 11.59 | 126.81 ± 23.34 | 200.22 ± 60.26 | 196.03 ± 30.98 | 215.92 ± 47.42 | 569.05 ± 156.98 | 562.72 ± 229.35 |
| ^ | 0.004 ± 0.0071 | 0.07 ± 0.0016 | 0.0054 ± 0.0015 | 0.0064 ± 0.004 | 0.0051 ± 0.0018 | 0.0069 ± 0.0029 | 0.0043 ± 0.0024 | 0.012 ± 0.0061 |
| t1/2 (h) | 175.85 ± 32.44 | 102.33 ± 23.96 | 135.98 ± 38.85 | 137.32 ± 64.67 | 152.58 ± 55.04 | 112.9 ± 37.15 | 215.47 ± 128.58 | 70.90 ± 29.31 |
| AUC0®t (ug·ml−1·h) | 2860.69 ± 105.56 | 1983.34 ± 631.56 | 10077.99 ± 3119.52 | 10396.4 ± 3614.36 | 16805.39 ± 2003.02 | 15168.16 ± 4537.81 | 41523.43 ± 10,506.78 | 40424.01 ± 25,580.46 |
| AUC0®?? (ug·ml−1·h) | 6305.79 ± 610.75 | 3409.92 ± 1213.31 | 19774.96 ± 8298.86 | 22157.73 ± 15,551.01 | 35434.66 ± 9175.52 | 32180.2 ± 14,130.49 | 129409.83 ± 97,073.82 | 62655.61 ± 49,517.93 |
| Vz (L) | 4.81 ± 0.42 | 5.78 ± 3.27 | 2.57 ± 0.76 | 2.40 ± 0.92 | 2.21 ± 0.39 | 1.93 ± 0.50 | 1.24 ± 0.16 | 1.02 ± 0.48 |
| CL (L/h·m2) | 0.019 ± 0.00184 | 0.04 ± 0.01 | 0.014 ± 0.00567 | 0.01 ± 0.01 | 0.011 ± 0.0028 | 0.013 ± 0.005 | 0.0055 ± 0.0035 | 0.01 ± 0.01 |
| MRT (h) | 246.9 ± 45.76 | 152.71 ± 8.43 | 195.92 ± 49.96 | 184.46 ± 91.48 | 222.12 ± 73.58 | 187.96 ± 52.62 | 316.92 ± 188.21 | 58.64 ± 12.42 |
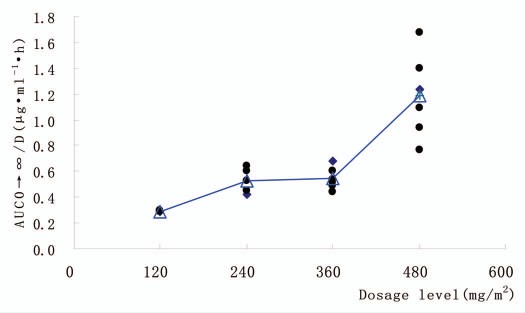
Mean maximum observed SM03 concentrations (Cmax) and mean area under the concentration-time curve from time zero to infinity (AUC0→t) increased in a generally dose-proportional manner up to 360 mg/m2. The data for Cmax and AUC0→t were in keeping with this observation in that the Cmax and AUC0→t increased by an order of magnitude between patients treated at 360 and 480 mg/m2, an indication that at doses of SM03 up to 360 mg/m2, the B cells in circulation may be saturated with SM03. Mean Cmax values of 196.03 and 569.05 µg/ml and AUC0→t values of 16.81 and 41.52 mg·h/ml were distinguishable for the 360 and 480 mg/m2 dose group, respectively (Fig. 3).
Figure 3.
Dose-normalized area under the serum-concentration time curve (AUC AUC0→∞, ng·ml−1h) as function of SM03 doses ranging from 120 to 480 mg/m2 (n = 19). Filled circle, individuals; open triangle, mean.
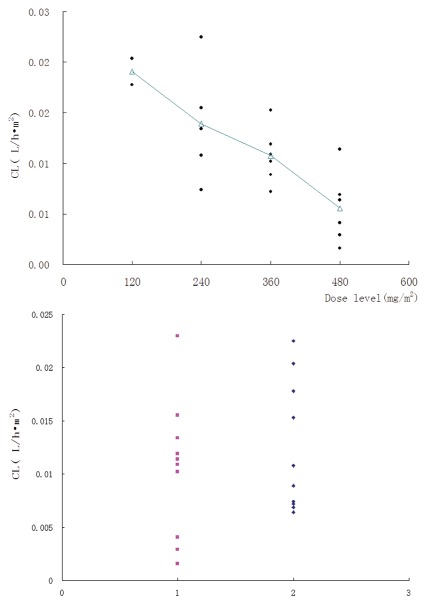
Mean terminal half-life (t1/2) ranged from (175.85 ± 32.44) to (215.47 ± 128.58) h across the 120 to 480 mg/m2 doses. The mean total body clearance based on body surface area for SM03 was similar following doses from 120 mg/m2 to 360 mg/m2 (range, 0.01–0.013 L/h/m2), but smaller in the 480 mg/m2 dose group (0.0055 L/h/m2). Mean estimates of the volume of distribution at steady-state based on body surface area ranged from 4.81, 2.57, 2.21 and 1.24 L/m2 across the dose range (120, 240, 360 and 480 mg/m2 SM03) and were consistent with minimal distribution of SM03 into the extravascular space.
Effects on peripheral blood lymphocytes and Ig levels.
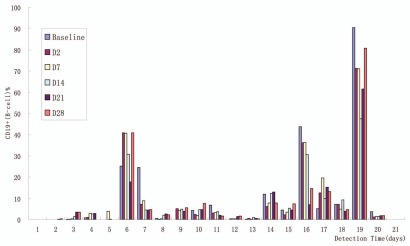
SM03 treatment did not affect T-cell levels as measured by CD3+ and CD3+/CD8+, and CD4+/CD8+ cell count, and also did not have effect on IgA, IgM, IgG levels, but did decrease B-cell levels (30–70% decrease from baseline for 360 and 480 mg/m2/week) for up to 42 d as measured by CD19+ cell count (Fig. 4). In this study, some patients had high levels of peripheral blood B cells because of their disease and others had below-normal levels as a consequence of previous treatment, and the number of patients at each dose level was small; thus, it is not possible to draw meaningful conclusions regarding correlations between specific SM03 dose levels and peripheral blood B-cell counts. No patient developed anti-drug antibodies.
Figure 4.
Post-treatment changes in peripheral B-cell counts (n = 21). B-cell levels as measured by CD19+ cell count decreased by 30–70% from baseline up to 42 d at dose 360 and 480 mg/m2/week.
Mean serum Ig levels remained stable; no significant differences were found in IgA and IgM on Day 7, 14, 21 and 28 after dosing compared with baseline. IgG decreased on Day 7 after treatment started but returned to normal level on Day 14. No significant differences were detected on Day 21 and 28 compared with baseline (Table 5).
Table 5.
Changes in IgA, IgG and IgM before and after treatment in 21 patients
| Baseline (g/l) | Day 7 (g/l) | Day 14 (g/l) | Day 21 (g/l) | Day 28 (g/l) | |
| IgA | 1.20 ± 0.70 | 1.18 ± 0.64 | 1.23 ± 0.71 | 1.21 ± 0.67 | 1.22 ± 0.79 |
| IgG | 10.53 ± 4.60 | 9.10 ± 3.55 | 9.65 ± 4.02 | 9.52 ± 3.84 | 9.67 ± 3.79 |
| IgM | 0.82 ± 0.50 | 0.72 ± 0.49 | 0.76 ± 0.49 | 0.74 ± 0.50 | 0.71 ± 0.41 |
Discussion
In 1980, Nadler et al. demonstrated that a murine mAb could target human lymphoma cells and induce death in these cells.20 This result stimulated research that led to the development of chimeric anti-CD20 rituximab, a marketed treatment for NHL. Despite the availability of mAbs that target CD20, patients with NHL relapse or become refractory to treatment, indicating that new therapeutic agents are needed. One strategy is the development of non-cross-reactive antibodies targeting other B-cell antigens that may have different and non-overlapping mechanisms of antitumor activity. Combination therapy may result in higher antitumor activity than treatment with one drug alone. For oncology treatments, the first demonstration of combination therapy that suggested the possibility of improved efficacy involved combining epratuzumab and rituximab.23–25 Leonard et al. demonstrated that combination therapy with 360 mg/m2 epratuzumab and 375 mg/m2 rituximab was well tolerated and effective (with CR/Cru rates and durable remissions that appeared to exceed those reported for rituximab alone) when administered to 41 patients with indolent NHL once weekly for 4 consecutive weeks.24 The results were confirmed by two other studies of the combination of rituximab with epratuzumab, totaling over 130 patients with NHL. The results showed that, in indolent lymphoma, this combined therapy is effective, ORs are durable and, most importantly, the report provided encouraging evidence that patients who achieved CRs experienced durable remission that appears to exceed those reported in other trials in patients with FL who were given the standard 4-week rituximab schedule.25 In addition, clinical trials performed in DLBCL also showed encouraging results; a study reported that an OR rate of 47% (33% CR/CRu) was observed in 15 patients with DLBCL. Results from another trial combining these two antibodies with conventional CHOP therapy treating 15 previously untreated patients with DLBLC indicated an OR of 87% (67% CR, 20% PR), a 2-y PFS, and an overall survival of 86.26 Thus, targeting CD22 antigen might be a better choice to improve the efficacy in patients with NHL.
The safety and pharmacokinetics assessment of epratuzumab have been established in western Caucasian population,26,27 but not in Chinese patients with NHL. This study is the first clinical evaluation of the safety and pharmacokinetics of the anti-CD22 chimeric IgG1 SM03 administered to Chinese patients with NHL. It was designed to assess the tolerance and pharmacokinetics of weekly administration of SM03 for 4 weeks, and to define dose regimens for further clinical development. Our findings indicate that the safety profile of SM03 in this study was unfavorable compared with that observed for epratuzumab, i.e., more adverse effects occurrence and higher incidence rate of side effects were observed in patients administered SM03. Apart from infusion reactions, epratuzumab is well tolerated when given at doses of 120 to 1,000 mg/m2/week for four doses each over 1 h. In the present Phase 1 escalation study in Chinese patients with NHL, the side effects experienced in patients included fever, prolonged prothrombin time, leukocytopenia, alanine transaminase elevation, elevated serum creatinine, injection site skin change, skin rash, hypertension, hypotension, fatigue, occult blood. However, most treatment-related toxicities were mild to moderate in severity excluding grade 4 lymphopenia and serious PT and APTT. The most frequent toxicity for all adverse events was infusion reactions (72%) that occurred predominately during the first infusion, with low-grade (mild-moderate) chills, rigors, fever to those observed previously in epratuzumab.
Patients treated with SM03 developed mild and generally reversible hematologic toxicity; 8 patients experienced grade 1/2 leukopenia and 4 patients developed grade 1/2 neutropenia, and all resolved without any therapy. However, a patient experienced grade 4 neutropenia 5 d, and resolved rapidly without G-CSF therapy, and etiology could not be found. An interesting result was observed in a patient who developed severe neutropenia 1 mo after each of two courses of rituximab therapy; the condition resolved rapidly after G-CSF therapy.28 The connection between B cells and myeloid maturation is not yet known. It has been noted that white cell disorders associated with rituximab were mainly classified as severe, the incidence of cytopenias of 25∼40% in lymphopenia, 2∼6% neutropenia, 4% leucopenia, 1.3% anemia and 1∼2% thrombocytopenia.29 It is strange that myleosuppresion toxicities were not observed in 103 patients treated with epratuzumab.17,26
Elevated levels of aminotransferase were observed in six patients of 21 patients after SM03 administration; the severity was mild (grade 1 and 2) and reversible, with no correlation to administered dose. We did not observe hepatitis B virus (HBV) reactivation induced by SM03 in 3 patients who had been anti-HBs-positive and were treated with anti-viral drugs such as heptodin, although their aminotransferase levels were elevated (1–2 times), and they resolved with no need for administration of hepatinica. According to literature reports, liver dysfunction was not observed in patients who were administered epratuzumab. However, 55% of patients who received rituximab therapy experienced liver failure, while the associated mortality rate was 48%.30 There were some reports showing that rituximab can induce HBV reactivation, in part relating to reduction of anti-HBV antibodies caused by rituximab and the associated host immunity balance. Further research will be done to monitor the anti-HBV surface antibody titers with SM03 therapy.31
It is surprising that we observed prolonged prothrombin time in 9 patients because this effect was reported as rare after rituximab and epratuzumab therapy. Specially, a patient developed severe PT and APTT and showed low coagulation factors levels. The patient resolved rapidly after intravenous administration of coagulation factors. There is no known connection between B cells and prothrombin. Further research is needed to determine whether there are correlations between SM03 and disordered coagulative function, which may be induced by lymphoma itself or concomitant treatment (such as chemotherapy).
Side effects were not related to protein dose amount. No effects were seen on Ig levels or T cell counts. Because of relatively low baseline levels (likely related to previous therapy), the direct effect of SM03 on circulating B-cell levels could not be clearly determined in this study, which is similar to the results observed in patients given epratuzumab.17
The clearance of SM03 is controlled by non-specific IgG clearance and irreversible binding to free B cells, and it is protected from endocytosis by recycling through the IgG salvage receptor FcRn.32 Target-mediated drug disposition (TMDD) is the main clearance pathway reported for therapeutic mAbs and is intimately linked to their high affinity and high specificity. The nonlinear kinetic caused by TMDD has substantial consequences on both preclinical and clinical study design, in particular on dose selection and dosing scheme.33 Thus, a common PK model for mAbs is the two-compartment model in which a saturable clearance is added to the linear clearance in the case of nonlinear kinetics.34,35 A recent review showed that nonlinear kinetics is reported for about half of the currently marketed mAbs.32 For example, cetuximab shows nonlinear elimination at doses ≥250 mg/m2. At this dose, the binding of antibody to the epidermal growth factor receptor (EGFR) is saturated, and the dose of 250 mg/m2 is recommended for clinical administration.36 It is generally accepted that target binding is closely linked to efficacy, and the saturation of the clearance implies the saturation of the target.
As a consequence of the saturable binding of SM03 to its target, a dose-dependent (nonlinear) elimination is observed. The pharmacokinetic profiles of SM03 indicated that disposition was nonlinear and was associated with an increase in drug exposure with increasing dose or number doses. The AUC of SM03 in Chinese patients was 16.81 mg·h/L and 41.52 mg·h/L at doses of 360 and 480 mg/m2, respectively, which is lower than that of non-Chinese patients administered epratuzumab (AUC of 46.5 and 72.5 mg·h/L respectively at the doses of 360 and 480 mg/m2).19 In the study presented here, clearance appeared to decrease by 3.4 times with doses from 120 to 480 mg/m2/week, indicating the saturation of clearance at 480 mg/m2 dose. It is widely believed that antibodies accumulate primarily in the circulation system and only to a low level in extracellular fluid and tissue with their distribution volume of about 4–5 L. The theoretical calculation of Cmax (according to the formula Cmax = amount of drug/distribution volume) was 38.4, 76.8, 153.6, 230.4, 307.2 µg/L at respective doses after the first administration, which was in accordance with the measured peak concentration excluding the 480 mg/m2 dose. After administration of 4 doses, the ratio of the amount of SM03 binding to CD22 antigen and the total amount of SM03 were 80, 70, 60, 50, ∼0% at different dosage, a result demonstrating the saturation binding of antigen at 480 mg/m2 dose, and therefore the maximum anti-tumor effect would be expected to be reached at the 480 mg/m2 dose. The extended half-life of SM03 also indicates that less frequent dosing schedules may be feasible. Tumor burden was observed to relate to clearance, i.e., clearance of patients carrying less tumor burden was lower than that of patients carrying moderate or huge tumor burden.
A previous clinical study of epratuzumab showed a significant number of objective tumor responses, with a total of nine of 51 assessable patients achieving OR after 4 once-weekly infusions of epratuzumab.26 In another Phase 1/2 clinical study of epratuzumab in NHL, five of 52 evaluable NHL patients achieved an OR (three CR and two PR), and 12 patients had stable disease and 35 progressed. Across all histologies, 20% of patients experienced anti-lymphoma activity as measured by reduction in tumor mass.18 However, in the study presented here, no objective treatment response was observed at day 28, and only one patient with FL achieved PR at day 178 with one cycle of SM03 and no follow-up chemotherapy, which may be due to short treatment cycles. This was a relatively small Phase 1 study designed to test the safety and pharmacokinetics profile of SM03 in NHL (eight patients with FL, eight with DLBCL); as such, tumor responses of CD22-targeted therapy in the treatment of FL were not confirmed in our preliminary result, but six of eight patients with FL derived clinical benefit in the form of some amount of tumor mass reduction. It is notable that at the end of SM03 treatment, without any therapy, three patients (two at 360 mg/m2 dose and one at 480 mg/m2) with FL remained with lower tumor mass, and one response is ongoing at .10 mo and two sustained at ≥4 mo. Research has shown that patients with follicular morphology, lower tumor burden and normal LDH tended to be more likely to respond to antibodies targeting CD22, and these characteristics are also associated with other anti-lymphoma agents.
In conclusion, the results from this Phase 1 study suggest that SM03 is safe, acceptably tolerated, and shows potential efficacy in Chinese patients with FL. When this is analyzed in conjunction with the pharmacokinetic data, which showed evidence of saturation of clearance at the 480 mg/m2 dose, the implication was that further studies could focus on doses of 480 mg/m2/week. Phase 2 clinical trials evaluating SM03 combined with CHOP standard regiments in treating Chinese patients with NHL are now in progress.
Materials and Methods
SM03.
SM03 is a recombinant, human/mouse chimeric IgG1 mAb directed against the extracellular domain of CD22 on human B lymphocytes. It is different from epratuzumab, a humanized IgG1, in amino acid sequence. However, SM03 targets the same epitope domain of CD22 as epratuzumab. Preclinical trial studies show that SM03 effectively removes B cells in the peripheral blood of macaque and cynomolgus, and does not induce any anti-SM03 antibodies in cynomolgus after weekly administration of SM03 at doses of 373.1 mg/m2, 1,119.4 mg/m2 or 3,358.2 mg/m2 consecutively for 12 weeks (data not shown). SM03 is produced in ST20 cells, which is a stably transfected murine myeloma cell line (SP2/0). The chimeric antibody is encoded from the variable region (Fab domain) cDNAs of the murine anti-human CD22 mAb and the cDNAs for human kappa and gamma 1 constant regions (Fc domain). SM03 is produced by cell culture in bioreactors, using serum-free media, at Lonn Ryonn Pharma Ltd., (Shenzhen, China). After clarification, the concentrated solution was purified though a series of chromatographic steps, resolved in potassium phosphate buffer solution (pH 7.2) containing polysorbate 80, vialed under asceptic conditions and formulated as a colorless, sterile product without any preservatives.
Study design.
This study was an open-label, Phase 1 study conducted at Sun Yat-sen University Cancer Center and designed to evaluate the safety, pharmacokinetic and antitumor effects of SM03 therapy. All subjects gave written informed consent before participating in study-related procedures. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki, the ethical and quality standards of good clinical practice, and all applicable regulatory requirements and laws. Infusions were administered once weekly for 4 consecutive weeks at doses from 60 mg/m2 to 480 mg/m2. The study consisted of a screening period (within 21 d before day 1), an inpatient treatment period (days-1 to 28), and an outpatient period (days 29 to 42).
Patients. Patients were eligible for enrollment if they had a diagnosis of CD22-positive B NHL, according to the World Health Organization (WHO) classification, version 2. Patients were included if they had progressed after at least one or two chemotherapy regimens, which included anthracycline or anthraquinone for aggressive B NHL. Other inclusion criteria were age ≥18 and ≤70 y, a performance status of one or better on the eastern Cooperative Oncology Group Scale, life expectancy ≥12 weeks, an absolute neutrophil count (ANC) ≥1.5 × 109/L and platelet count ≥100 × 109/L, serum creatinine ≤1.5x upper limit of normal (ULN), urine protein-to-creatinine ratio of ≤0.2, total bilirubin ≤1.5x ULN, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) ≤2.5x ULN and at least one measurable lesion ≥1.5 cm in at least one dimension by computer tomography (CT) at inclusion, in an area of no prior radiation therapy, or clear progression in an area that had been previously irradiated.
Study drug administration.
SM03 (chimeric IgG1 anti-CD22 mAb) was produced and quality-controlled at Shenzhen Lonn Ryonn Pharmaceutical Co., Ltd., (Shenzhen, Guangdong province, China) and administered by intravenous infusion at doses of 60, 120, 240, 360, 480 mg/m2/week for 4 consecutive weeks with 42 d cycle. Each dose of SM03 in 250 ml 0.9% sterile NaCl was prepared by the hospital pharmacy (Sun Yat-sen University Cancer Hospital, Guangzhou, Guangdong province, China) and was to be administered throughout a 3 h period. To minimize hypersensitivity, patients were premedicated with acetaminophen (0.5–1 g) and antihistamine (25–50 mg per os or intravenous polaramine). Because clinical activity of epratuzumab in the treatment of NHL was at doses of ≥240 mg/m2 without any DLT at doses from 120 to 1,000 mg/m2 and the recommended dose was 360 mg/m2 for further evaluation, SM03 dose escalation was stopped if two patients experienced dose limited toxicity (DLT) and dose escalation to next dose if one patient experienced DLT 19. Dose escalation was halted at 480 mg/m2 according to the clinical trial instruction of SM03 informed by State Food and drug Administration of China and additional patients were treated at dose level of ≥240 mg/m2 to further assess efficacy.
A DLT was defined as any of the following that were at least possibly related to SM03 during the first 42 d after the first dose: any grade 3 or 4 [National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTC), version 3] nonhematologic toxicity (except grade 3 alopecia, nausea or vomiting unless the patient was receiving optimal medical therapy); febrile neutropenia (grade 4 ANC ≥3-d duration and temperature ≥38); grade 4 ANC ≥7-d duration; grade 4 thrombocytopenia ≥3-d duration, or any bleeding episode requiring platelet transfusion.19 Patients who experienced a DLT were not to receive the additional cycles of SM03 at the same dose. The maximum number of doses of SM03 was 6 for 240 mg/m2, 360 mg/m2, 480 mg/m2.
Clinical assessment and safety.
Clinical assessment consisted of vital pathological signs monitoring (before and every 15 min during infusion); general laboratory examination (screening, every week and at restaging); hematology tests, hepatic function tests, renal tests and immunoglobulin tests (screening, every week and at restaging), T-cell amounts, B-cell amounts, Ig levels (screening, every week and at restaging) Version 3.0 of the NCI Common Terminology Criteria for Adverse Events (AE) was used for toxicity and AE reporting. AEs were recorded throughout the study and graded according to the NCI CTC, and were considered to be treatment emergent if onset occurred between the first and the last dose, plus a lag of 42 d provided the following criteria were: (i) the AE was not present before the start of the first dose and did not occur in the patients as a chronic condition; (ii) the AE was present before the start of the first dose or as part of the patient's medical history, but the severity or frequency increased after the start of the first dose. Patients were evaluated for response 3 weeks after the last infusion.
Pharmacokinetics. Blood samples were taken for determination of serum SM03 concentrations on days 1 and 22 before dosing, and at 1, 2, 3 (2 min before the end of the SM03 infusion), 2.5, 4, 8, 24, 48, 72, 120 and 144 h after dosing, and on day 8 and 15 before dosing and at 3, 24, 72, 144 h after dosing. SM03 concentration was used an anti-SM03 idiotype LRID03 adsorbed onto a microtiter plate to capture SM03 in 0.01% serum. The captured SM03 was detected using a peroxidase-conjugated AffiniPure horseradish polyclonal anti-human IgG Fcγ fragment reagent. The assay has a calibration range of 0.19 to 200 ng/ml in 0.01% serum; the lower limit of quantification was determined to be 0.19 ng/ml. Deviations of the predicted concentrations from the nominal values for the quality control samples were within 12.3%. The inter-run and intra-run variability estimates were within 5.0–9.6% and 8.0–11.0% coefficient of variation (CV), respectively.
The non-compartmental pharmacokinetic parameters of SM03 were estimated using the WinNonlin (version 5.0) program. The parameters determined included the following: end-of peak concentration (Cmax), area under steady-state volume of distribution (Vss) and the terminal-phase elimination half-life (t1/2).
HAHA assay.
Blood samples were collected within 1 week before study entry, 24 h after the first and the last infusion and at restaging to test for the presence of human antihuman antibodies (HAHAs). This assay was developed by Shenzhen Lonn Ryonn Pharmaceutical Co., Ltd., using an enzyme-linked immunosorbent assay with a lower level of detection of 50 ng/ml.
Efficacy.
Patients were evaluable for efficacy at day 42 with CT scan. Tumor response was assessed according to the International Workshop Response Criteria for non-Hodgkin Lymphoma. The overall response rate (ORR) was defined as the percentage of patients meeting the criteria for complete response (CR), unconfirmed complete (CRu) or partial response (PR). Stable disease (SD) was measured from the start of the treatment until the criteria for PD were met, taking as the reference the smallest measurements recorded since the initiation of the treatment.
Statistical analysis.
In general, discrete variables, including responder rates and AEs, were summarized using frequency counts and percentages. Percentage changes in individual efficacy parameters, B- and T-cell counts, Igs, duration of infusion times, and other continuous numerical variables were summarized using descriptive statistics. The Wilcoxon signed rank test was used to assess the statistical significance of changes in the subjective B cells, T cells and Igs, compared with their baseline values. All statistical tests used a significance level of ≤0.05.
Figure 5.
(A) Total body clearance based on body surface area (CLpBSA) as function of SM03 doses ranging 120 to 480 mg/m2 (n = 19). Filled circle, individuals; open triangle, mean. (B) Total body clearance based on body surface area as function of SM03 in female and male patients. Filled square, male; filled diamond, female.
Acknowledgments
The authors would like to thank Dongshen Zhang (Sun Yat-sen University, Cancer Center) for editing the manuscript and for logistics of submission and Dr. Wenqi Jiang (Sun Yat-sen University, Cancer Center) for discussions.
Abbreviations
- NHLs
non-Hodgkin lymphomas
- CHOP
cyclophosphamide, doxorubicin, vincristine and prednisone
- Aes
drug-related adverse events
- mAbs
monoclonal antibodies
- Ig
immunoglobulin
- BCR
B-cell antigen receptor
- MTD
a maximum tolerated dose
- PT
partial thromboplastin time
- APTT
activated partial thromboplastin time
- HBV
hepatitis B virus
- SAE
serious adverse effects
- FLIPI
follicular grade I lymphoma
- Cmax
mean maximum concentrations
- AUC0→t
area under the concentration-time curve from time zero to infinity
- t1/2
terminal half-life
- FcRn
IgG salvage receptor
- TMDD
target-mediated drug disposition
- EGFR
epidermal growth factor receptor
- WHO
World Health Organization
- ANC
absolute neutrophil count
- ULN
upper limit of normal
- AST
aspartate aminotransferase
- ALT
alanine aminotransferase
- CT
computer tomography
- DLT
dose limited toxicity
- NCI CTC
National Cancer Institute Common Terminology Criteria for Adverse Events
- CV
coefficient of variation
- Vss
area under steady-state volume of distribution
- HAHAs
human antihuman antibodies
- ORR
overall response rate
- CR
complete response
- Cru
unconfirmed complete response
- PR
partial response
- SD
stable disease
- LDH
lactate dehydrogenase
- ECOG
Eastern Cooperative Oncology Group
- SPD
sum of products of greatest diameter
Disclosure of Potential Conflicts of Interest
Jian Sun, Zhiming Li, Liting Deng, Jing Zhan and Benyan Zou are all full-time employees of Sun Yat-sen University, Cancer Center. The study was sponsored in full by Zhendong Li (Shenzhen Lonn Ryonn Pharmaceutical Co., Ltd., Shenzhen, Guangdong province, China).
References
- 1.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics 2002. CA Cancer J Clin. 2002;52:23–47. doi: 10.3322/canjclin.52.1.23. [DOI] [PubMed] [Google Scholar]
- 2.Harris NL, Jaffe ES, Diebold J, Flandrin G, Muller-Hermelink HK, Vardiman J, et al. The World Health Organization classification of neoplasms of the hematopoietic and lymphoid tissues: report of the Clinical Advisory Committee meeting—Airlie House, Virginia, November 1997. Hematol J. 2000;1:53–66. doi: 10.1038/sj.thj.6200013. [DOI] [PubMed] [Google Scholar]
- 3.Multani P, White CA, Grillo-López A, Grillo-Lopez A. Non-Hodgkin's lymphoma: review of conventional treatments. Curr Pharm Biotechnol. 2001;2:279–291. doi: 10.2174/1389201013378581. [DOI] [PubMed] [Google Scholar]
- 4.Fisher RI. Current therapeutic paradigm for the treatment of non-Hodgkin's lymphoma. Semin Oncol. 2000;27:2–8. [PubMed] [Google Scholar]
- 5.Gordon LI, Harrington D, Andersen J, Colgan J, Glick J, Neiman R, et al. Comparison of a second-generation combination chemotherapeutic regimen (m-BACOD) with a standard regimen (CHOP) for advanced diffuse non-Hodgkin's lymphoma. N Engl J Med. 1992;327:1342–1349. doi: 10.1056/NEJM199211053271903. [DOI] [PubMed] [Google Scholar]
- 6.McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Haioun C, Ketterer N, Engert A, Tilly H, Ma D, et al. Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92:1927–1932. [PubMed] [Google Scholar]
- 8.Turner A. Antibodies. In: Roitt I, Brostoff J, Male D, editors. Immunology. New York: Mosby; 2001. pp. 65–86. [Google Scholar]
- 9.Lundin J, Osterborg A, Brittinger G, Crowther D, Dombret H, Engert A, et al. CAMPATH-1H monoclonal antibody in therapy for previously treated low-grade non-Hodgkin's lymphomas: a phase II multicenter study. European Study Group of CAMPATH-1H Treatment in Low-Grade Non-Hodgkin's Lymphoma. J Clin Oncol. 1998;16:3257–3263. doi: 10.1200/JCO.1998.16.10.3257. [DOI] [PubMed] [Google Scholar]
- 10.Fayad L, Patel H, Verhoef G. Clinical activity of the immunoconjugate CMC-544 in B-cell malignancies: preliminary report of the expanded maximum tolerated dose (MTD) cohort of a phase I study. Blood. 2006;108:2711. [Google Scholar]
- 11.DiJoseph JF, Armellino DC, Boghaert ER, Khandke K, Dougher MM, Sridharan L, et al. Antibody-targeted chemotherapy with CMC-544: a CD22-targeted immunoconjugate of calicheamicin for the treatment of B-lymphoid malignancies. Blood. 2004;103:1807–1814. doi: 10.1182/blood-2003-07-2466. [DOI] [PubMed] [Google Scholar]
- 12.Clark EA. CD22, a B cell-specific receptor, mediates adhesion and signal transduction. J Immunol. 1993;150:4715–4718. [PubMed] [Google Scholar]
- 13.Dörken B, Moldenhauer G, Pezzutto A, Schwartz R, Feller A, Kiesel S, et al. HD39 (B3), a B lineage-restricted antigen whose cell surface expression is limited to resting and activated human B lymphocytes. J Immunol. 1986;136:4470–4479. [PubMed] [Google Scholar]
- 14.Cesano A, Gayko U, Brannan C. Differential expression of CD22 by indolent and aggressive NHLs: implications for targeted immunotherapy. Blood. 2002;100:350. [Google Scholar]
- 15.Otipoby KL, Andersson KB, Draves KE, Klaus SJ, Farr AG, Kerner JD, et al. CD22 regulates thymus-independent responses and the lifespan of B cells. Nature. 1996;384:634–637. doi: 10.1038/384634a0. [DOI] [PubMed] [Google Scholar]
- 16.Sato S, Tuscano JM, Inaoki M, Tedder TF. CD22 negatively and positively regulates signal transduction through the B lymphocyte antigen receptor. Semin Immunol. 1998;10:287–297. doi: 10.1006/smim.1998.0121. [DOI] [PubMed] [Google Scholar]
- 17.Leonard JP, Coleman M, Ketas JC, Chadburn A, Furman R, Schuster MW, et al. Epratuzumab, a humanized anti-CD22 antibody, in aggressive non-Hodgkin's lymphoma: phase I/II clinical trial results. Clin Cancer Res. 2004;10:5327–5334. doi: 10.1158/1078-0432.CCR-04-0294. [DOI] [PubMed] [Google Scholar]
- 18.Carnahan J, Wang P, Kendall R, Chen C, Hu S, Boone T, et al. Epratuzumab, a humanized monoclonal antibody targeting CD22: characterization of in vitro properties. Clin Cancer Res. 2003;9:3982–3990. [PubMed] [Google Scholar]
- 19.National Cancer Institute Common Toxicity Criteria. Version 3.0. http://home earthlink.net/∼johnres/ncitox/ncitox.html. [PubMed]
- 20.Nadler LM, Stashenko P, Hardy R, Kaplan WD, Button LN, Kufe DW, et al. Serotherapy of a patient with a monoclonal antibody directed against a human lymphoma-associated antigen. Cancer Res. 1980;40:3147–3154. [PubMed] [Google Scholar]
- 21.Nadler LM, Stashenko P, Hardy R, Schlossman SF. A monoclonal antibody defining a lymphoma-associated antigen in man. J Immunol. 1980;125:570–577. [PubMed] [Google Scholar]
- 22.Stein R, Qu Z, Chen S, Rosario A, Shi V, Hayes M, et al. Characterization of a new humanized anti-CD20 monoclonal antibody, IMMU-106, and its use in combination with the humanized anti-CD22 antibody, epratuzumab, for the therapy of non-Hodgkin's lymphoma. Clin Cancer Res. 2004;10:2868–2878. doi: 10.1158/1078-0432.CCR-03-0493. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez-Ilizaliturri F, Gada P, Repasky EA. Enhancement in anti-tumor activity of rituximab when combined with epratuzumab or apolizumab (Hu1D10) in a B-cell lymphoma severe combined immunodeficiency (SCID) mouse model. Blood. 2002;100:158. [Google Scholar]
- 24.Leonard JP, Schuster SJ, Emmanouilides C, Couture F, Teoh N, Wegener WA, et al. Durable complete responses from therapy with combined epratuzumab and rituximab: final results from an international multicenter, phase 2 study in recurrent, indolent, non-Hodgkin lymphoma. Cancer. 2008;113:2714–2723. doi: 10.1002/cncr.23890. [DOI] [PubMed] [Google Scholar]
- 25.Micallef IN, Kahl BS, Maurer MJ, Dogan A, Ansell SM, Colgan JP, et al. A pilot study of epratuzumab and rituximab in combination with cyclophosphamide, doxorubicin, vincristine and prednisone chemotherapy in patients with previously untreated, diffuse large B-cell lymphoma. Cancer. 2006;107:2826–2832. doi: 10.1002/cncr.22342. [DOI] [PubMed] [Google Scholar]
- 26.Leonard JP, Coleman M, Ketas JC, Chadburn A, Ely S, Furman RR, et al. Phase I/II trial of epratuzumab (humanized anti-CD22 antibody) in indolent non-Hodgkin's lymphoma. J Clin Oncol. 2003;21:3051–3059. doi: 10.1200/OCO.2003.01.082. [DOI] [PubMed] [Google Scholar]
- 27.Wang W, Lu P, Fang Y, Hamuro L, Pittman T, Carr B, et al. Monoclonal antibodies with identical Fc sequences can bind to FcRn differentially with pharmacokinetic consequences. Drug Metab Dispos. 2011;39:1469–1477. doi: 10.1124/dmd.111.039453. [DOI] [PubMed] [Google Scholar]
- 28.Davis TA, Grillo-López AJ, White CA, McLaughlin P, Czuczman MS, Link BK, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin's lymphoma: safety and efficacy of re-treatment. J Clin Oncol. 2000;18:3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 29.Plosker GL, Figgitt DP. Rituximab: a review of its use in non-Hodgkin's lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63:803–843. doi: 10.2165/00003495-200363080-00005. [DOI] [PubMed] [Google Scholar]
- 30.Evens AM, Jovanovic BD, Su YC, Raisch DW, Ganger D, Belknap SM, et al. Rituximab-associated hepatitis B virus (HBV) reactivation in lymphoproliferative diseases: meta-analysis and examination of FDA safety reports. Ann Oncol. 2011;22:1170–1180. doi: 10.1093/annonc/mdq583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tsutsumi Y, Ogasawara R, Kamihara Y, Ito S, Yamamoto Y, Tanaka J, et al. Rituximab administration and reactivation of HBV. Hepatitis Res Treat. 2010;2010:182067. doi: 10.1155/2010/182067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hans PG. Gaining insights into the consequences of target-mediated drug disposition of monoclonal antibodies using quasi-steady-state approximations. J Pharcokinetic Pharmacodyn. 2009;36:407–420. doi: 10.1007/s10928-009-9129-5. [DOI] [PubMed] [Google Scholar]
- 33.Ng CM, Bruno R, Combs D, Davies B. Population pharmacokinetics of rituximab (anti-CD20 monoclonal antibody) in rheumatoid arthritis patients during a phase II clinical trial. J Clin Pharmacol. 2005;45:792–801. doi: 10.1177/0091270005277075. [DOI] [PubMed] [Google Scholar]
- 34.Nolting A, Fox FE, Kovar A. Clinical drug development of cetuximab, a monoclonal antibody. In: Meibohm B, editor. Pharmacokinetics and pharmacodynamics of biotech drugs: principles and case studies in drug development. Wiley; 2007. pp. 353–370. [Google Scholar]
- 35.Mould DR, Sweeney KR. The pharmacokinetics and pharmacodynamics of monoclonal antibodies—mechanistic modeling applied to drug development. Curr Opin Drug Discov Devel. 2007;10:84–96. [PubMed] [Google Scholar]
- 36.Fracasso PM, Burris H, 3rd, Arquette MA, Govindan R, Gao F, Wright LP, et al. A phase 1 escalating single-dose and weekly fixed-dose study of cetuximab: pharmacokinetic and pharmacodynamic rationale for dosing. Clin Cancer Res. 2007;13:986–993. doi: 10.1158/1078-0432.CCR-06-1542. [DOI] [PubMed] [Google Scholar]