Summary
Circadian rhythms offer an excellent opportunity to dissect the neural circuits underlying innate behavior because the genes and neurons involved are relatively well-understood. We first sought to understand how Drosophila clock neurons interact in the simple circuit that generates circadian rhythms in larval light avoidance. We used genetics to manipulate two groups of clock neurons, increasing or reducing excitability, stopping their molecular clocks and blocking neurotransmitter release and reception. Our results revealed that Lateral Neuron (LNv) clock neurons promote and Dorsal Neurons (DN1s) inhibit light avoidance, that these neurons probably signal at different times of day, and that both signals are required for rhythmic behavior. We found similar principles apply in the more complex adult circadian circuit that generates locomotor rhythms. Thus the changing balance in activity between clock neurons with opposing behavioral effects generates robust circadian behavior and likely helps organisms transition between discrete behavioral states such as sleep and wakefulness.
Introduction
A major goal of Neuroscience is to understand how the nervous system functions at multiple different levels (from genes to neural circuits) to generate behavior. Innate behaviors are particularly attractive to study since they are hardwired into the nervous system and are very similar between individual animals. The control of circadian (~24hr) rhythms offers an excellent opportunity to genetically dissect neural circuits since dedicated clock genes have been identified. This enabled the identification of pacemaker neurons where clock genes function to modulate multiple innate behaviors including sleep, courtship and drug sensitivity (reviewed by Allada and Chung, 2010).
Although recent studies have shown the importance of neuronal communication in synchronizing and strengthening molecular behavioral rhythms (Hogenesch and Herzog, 2011; Nitabach and Taghert, 2008), the nature of the signals between clock and neurons and their effects on neuronal activity are unclear. To address this, we utilized the “minimal” circadian network in Drosophila larvae, which has only 9 clock per brain that neurons lobe, with the idea general principles of circadian neural circuits in larvae would also apply to adult flies and perhaps even in mammals. Drosophila larvae show circadian rhythms light sensitivity, which is measured by assaying how well larvae avoid light on a half light-half dark agar plate (Mazzoni et al., 2005). This requires both the larval visual system (Bolwig's Organ) and clock neurons (Keene et al., 2011). Bolwig's Organ likely innervates the 5 larval Lateral neurons (LNvs) (Keene et al., 2011; Klarsfeld et al., 2011), including the 4 LNvs which express the neuropeptide Pigment Dispersing Factor (PDF). Consistent with direct innervation, light transmitted via Bolwig's Organ rapidly increases neuronal activity of the PDF-expressing LNvs (Yuan et al., 2011).
We used the spatial precision of the Gal4/UAS system (Brand and Perrimon, 1993) to target specific groups of clock neurons. This approach is extremely powerful when combined with transgenes that increase or decrease neuronal excitability. The specific neurotransmitters and neuropeptides produced by different neurons can also be manipulated relatively easily, as can the receptors that mediate the responses of downstream neurons. Armed with these genetic tools, we set out to decode the logic and function of the network interactions between clock neurons.
We found that LNvs and a group of dorsal larval clock neurons (DN1s) have opposite behavioral effects: LNvs promote whilst DN1s inhibit larval light avoidance. We also found that the similarly phased molecular clocks in LNvs and DN1s have opposite relationships to neuronal activity: low CLK/CYC activity, which normally occurs at dawn, makes LNvs highly excitable but decreases DN1 signaling. Thus the cells which become adult Morning cells (Grima et al., 2004; Stoleru et al., 2004) are most excitable in the morning, while the DN1s, which become the adult DN1as, a subset of adult Evening cells (Grima et al., 2004; Stoleru et al., 2004), seem most excitable in the evening. Our data also reveal that the morning peak of light avoidance requires that DN1s signal minimally at dawn. DN1s therefore seem to gate LNv activity, which could be a general mechanism for the dual oscillator model underlying circadian rhythms (Pittendrigh and Daan, 1976). Finally we show that rhythmic light avoidance requires glutamatergic inhibitory inputs from the two larval DN1s, received on LNvs via GluCl, a glutamate-gated chloride channel that inhibits LNv Our studies of the circuit activity.
Our studies of the circuit interactions between larval LNvs and DN1s lead to simple principles that hold true in adult flies: Signaling from non-LNv clock neurons promotes circadian rhythms by inhibiting the outputs of the master LNv pacemaker neurons. This presumably narrows the morning peak of locomotor activity and helps sharpen the behavioral transition from inactivity (sleep) to activity (wakefulness). These data add to the emerging concept that the precision and robustness of whole animal behavioral rhythms arise from network interactions between individual clock neurons and offer a cellular mechanism for how clock neurons are coupled.
Results
Pre- and post-synaptic DN1 terminals are located close to LNv axonal termini
Adult E cells are labeled by the cry13-Gal4 driver in combination with a Pdf-Gal80 transgene, and, along with LNvs, are required to generate normal behavioral rhythms in 12h Light: 12h Dark (LD) cycles (Stoleru et al., 2004). We found that this driver combination only labeled the two larval DN1s (Fig 1A and data not shown). Although expression of GFP was often difficult to detect simultaneously in both larval DN1s (as in Fig 1A), expression of UAS-Diphtheria toxin (UAS-Dti) always ablated both larval DN1s, while the PDF+ LNvs, the 5th PDF- LNv and the 2 DN2s were unaffected, as judged by clock protein staining (data not shown). This is consistent with larval DN1s becoming the adult DN1a neurons, a subset of adult E cells (Grima et al., 2004; Stoleru et al., 2004).
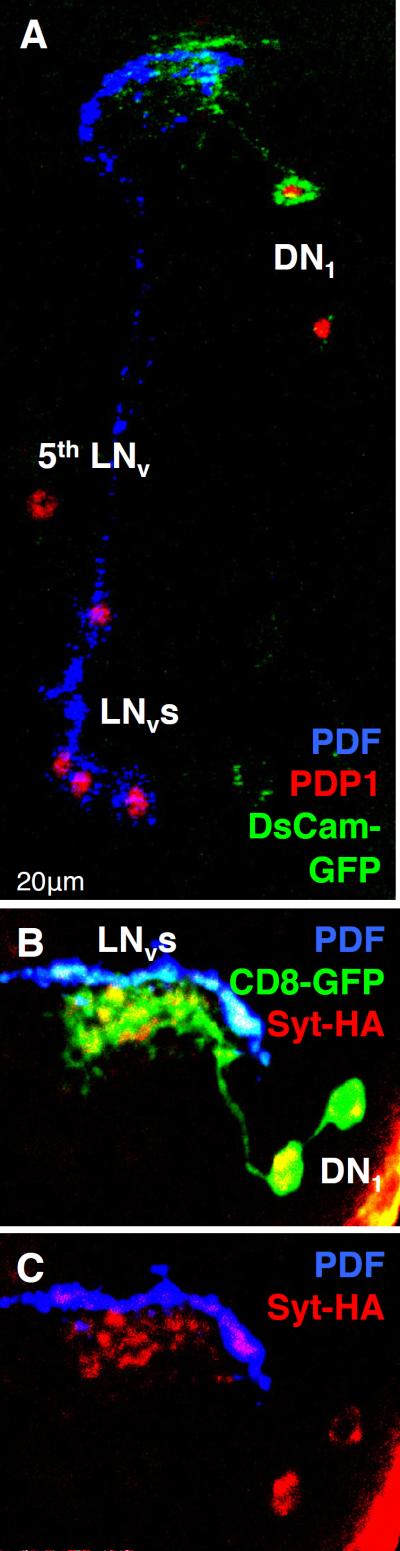
Figure 1. Pre- and post-synaptic DN1 terminals are located close to LNv axonal termini.
(A) The nuclei of larval clock neurons were marked with the circadian transcription factor Par Domain Protein 1 (PDP1, red). LNvs were co-labeled with PDF (blue). cry13-Gal4; Pdf-Gal80 driven expression of the Dscam17.1-GFP post-synaptic marker (green) labels DN1 projections very close to LNv axons. The 5th LNv was identified by lack of PDF and GFP staining and its location.
(B) cry16-Gal4; Pdf-Gal80 driven expression of UAS-CD8-GFP (green) and the pre-synaptic marker UAS-Syt-HA (anti-HA, red) co-localize to DN1 projections adjacent to LNv axons (labeled with PDF, blue).
(C) Same image as (B) with GFP channel removed to show Syt expression in DN1 projections adjacent to LNv axons. This image shows a 20μm stack but single 4μm sections also show DN1 projections adjacent to LNv axons.
GFP-labeled DN1 projections terminate in the vicinity of the PDF+ LNv axonal termini (Fig 1A). Since the GFP derivative used is a post-synaptic marker (Dscam17.1-GFP, (Wang et al., 2004), larval DN1 projections could receive inputs in this region, including from LNvs. To localize DN1 pre-synaptic termini, we used UAS-Synaptotagmin-HA (UAS-Syt-HA, (Robinson et al., 2002) expressed via the stronger cry16-Gal4 driver in combination with Pdf-Gal80 since cry13-Gal4 expression zof Syt-HA was undetectable. The two larval DN1s marked by CD8-GFP expression project to the LNv termini where Syt-HA is detectable in several foci, some of which are very close to LNv axons (Fig 1B–C). Thus DN1s could signal to LNvs and receive their inputs. This is consistent with electron microscopy studies of adult s-LNvs that revealed input synapses to s-LNv projections in the dorsal protocerebrum, the location of adult DNs (Yasuyama and Meinertzhagen, 2010).
We also detected low levels of CD8-GFP and Syt-HA expression in LNvs when expressed with the cry16-Gal4; Pdf-Gal80 combination, presumably because cry16-Gal4 is not completely repressed by Pdf-Gal80. Since cry16-Gal4 also labels a few non-clock neurons in the brain (data not shown), we did not use cry16-Gal4 in subsequent behavioral experiments.
LNs and DN1s have opposite roles in light avoidance
Given the possibility that DN1s signal to LNvs, we first characterized the contributions of these different groups of clock neurons to light larvae raised in 12:12 LD cycles at 25°C. In this assay, 15 larvae are placed on a half-covered Petri dish and the number of larvae on the dark side is counted after 15min. At 750lux, ~70% of wild-type larvae are in the dark at the end of the assay and this requires the clock genes period (per) and timeless (tim) (Gong, 2009; Keene et al., 2011; Mazzoni et al., 2005). In the Drosophila clock, per and tim expression is activated by the Clock (CLK) and Cycle (CYC) transcription factors. PER and TIM proteins then feedback to inhibit CLK/CYC activity (reviewed by (Hardin, 2011)). Strikingly, Clk and cyc mutant larvae have the opposite light avoidance phenotype to per and tim mutants: At 150lux, wild-type larvae cannot distinguish between light and dark but cyc and Clk mutant larvae display robust levels of light avoidance at this lower light intensity. Thus clock genes strongly modulate light avoidance (Mazzoni et al., 2005). At these light intensities, light avoidance is mediated by the Rh5-expressing subset of Bolwig's Organ photoreceptors (Keene et al., 2011) and is independent of the larval body wall photoreceptors (Xiang et al., 2010).
To test the role of LNvs and DN1s in light avoidance, we tested larvae at 150lux since starting from a basal level of light avoidance allowed us to identify manipulations that induce light avoidance and bypass redundancies in the system (Keene et al., 2011). Larvae were taken during the light phase of an LD cycle between Zeitgeber Times 3–6 (ZT, where ZT0=lights on and ZT12=lights off). We used Pdf-Gal4 (abbreviated as Pdf > hereafter) and cry-Gal4; Pdf-Gal80 (DN1 >) to target expression to larval LNvs and DN1s respectively. We first tested the effect of ablating LNvs or DN1s or altering their electrical excitability.
We found that hyperpolarizing LNvs through dORKΔC or ablation via Dti had no effect on light avoidance (Fig 2A) compared to Pdf > dORKΔNC control larvae, which express a non-conducting version of dORKΔC (Nitabach et al., 2002). However, LNv expression of NaChBac, a bacterial voltage-gated Na+ channel that increases adult LNv excitability (Nitabach et al., 2006; Sheeba et al., 2008a) and larval LNv responses to light (Yuan et al., 2011) increased light avoidance scores (Fig 2A). Since hyper-exciting LNvs increases light avoidance, we conclude that LNvs promote light avoidance.
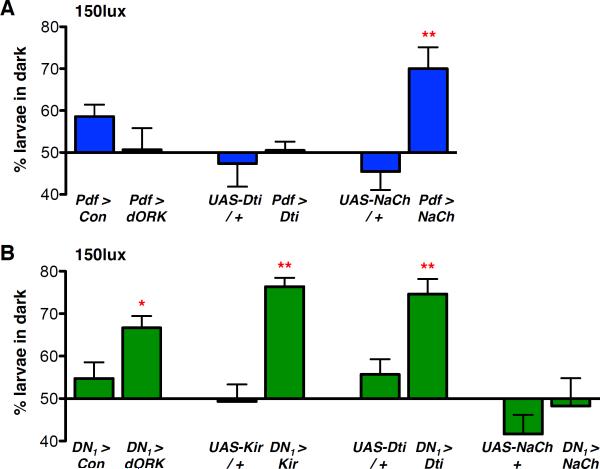
Figure 2. LNvs promote and DN1s inhibit larval light avoidance.
Larval light avoidance was measured by counting the number of larvae on the dark sides of a Petri dish after 15min. Transgenes were targeted to either LNvs using Pdf-Gal4 (Pdf >), or DN1s using cry-Gal4; Pdf-Gal80 (DN1 >). Control lines are either the Gal4 line crossed to the non-conducting UAS-dORKΔNC transgene (Con) or the relevant UAS-transgene crossed to y w (transgene / +). Error bars show SEM. All statistical comparisons to the relevant control line were made using the students t-test. *p<0.05, **p<0.01.
(A) Light avoidance was assayed between ZT 3–6 at 150lux. Hyperpolarizing LNvs with UAS-dORKΔC (Pdf > dORK) or ablating LNvs with UAS-Dti (Pdf > Dti) had no significant effect on light avoidance vs control larvae. Hyperexciting LNvs via NaChBac (Pdf > NaCh, p<0.005) increased larval light avoidance.
(B) Light avoidance was assayed as in (A). Hyperpolarizing DN1s with UAS-dORKΔC or UAS-mKir2.1 (DN1 > dORK, p<0.05 and DN1 > Kir, p<0.005), or DN1-ablation (DN1 > Dti, p<0.01) significantly increased larval light avoidance. Hyperexciting DN1s (DN1 > NaCh) had no significant effect on light avoidance.
Expression of these same transgenes in DN1s yielded opposite results (Fig 2B). Compared with DN1 > dORKΔNC control larvae, light avoidance levels increased significantly when DN1s were hyperpolarized with either dORKΔC or mKir2.1 or ablated with Dti. Thus, LNvs promote and DN1s inhibit light avoidance, with the difference between their excitability presumably determining overall levels of light avoidance.
Low CLK/CYC activity in LNvs or DN1s increases light avoidance
Larvae would be unlikely to avoid light if LNvs and DN1s released their conflicting signals simultaneously. Therefore we hypothesized that LNvs and DN1s signal at different times of day. Since the molecular clocks in LNvs and DN1s are similarly phased, we speculated that relationship between their molecular clocks and excitability must differ in LNvs and DN1s.
To test this, we used transgenes that encode dominant negative forms of CLK (UAS-ClkDN) or CYC (UAS-cycDN) that block CLK/CYC-activated transcription (Tanoue et al., 2004). We found that expressing ClkDN or cycDN in LNvs (Fig 3A) or DN1s (Fig 3B) significantly increased light avoidance compared to control larvae.
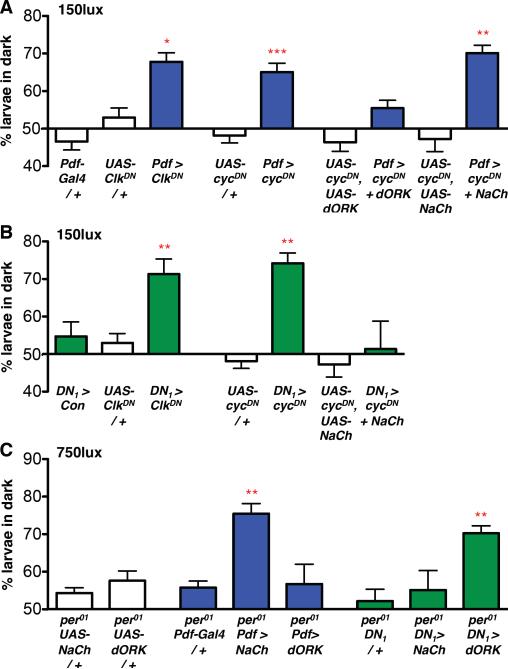
Figure 3. Altering CLK/CYC activity has opposite effects on LNv and DN1 excitability.
All Statistical comparisons were made by ANOVA with Tukey's post-hoc test. *p<0.05, **p<0.01, ***p<0.001
(A) Light avoidance was assayed between ZT 3–6 in LD at 150lux. Expressing ClkDN (p<0.05) or cycDN (p<0.001) in LNvs increased larval light avoidance at 150lux compared to controls (Pdf-Gal4 or UAS-transgene crossed to y w). Hyperpolarization of LNvs expressing cycDN (Pdf > cycDN + dORK) restored light avoidance to wild-type levels while hyperexcitation of LNvs expressing cycDN (Pdf > cycDN + NaCh) did not. See also Fig S1.
(B) Light avoidance was assayed as in (A). Expressing ClkDN (p<0.01) and cycDN (p<0.01) in DN1s increased larval light avoidance at 150lux compared to controls (DN1 > Con, reproduced from Fig 2B, or UAS-transgenes crossed to y w, reproduced from Fig 3A). Hyperexcitation of DN1s expressing cycDN (DN1 > cycDN + NaCh) restored light avoidance to wild-type levels.
(C) Light avoidance was assayed between ZT 3–6 in LD at 750lux. Hyperexciting LNvs (per01; Pdf > NaCh, p<0.01) rescued the low levels of light avoidance of per01 larvae whilst hyperpolarizing LNvs did not (per01; Pdf > dORK). Hyperpolarizing DN1s (per01; DN1 > dORK, p<0.01) also increased light avoidance whilst hyperexciting DN1s (per01; DN1 > NaCh) did not.
Since Pdf > ClkDN and Pdf > cycDN larvae had similar light avoidance phenotypes as hyperexciting LNvs via NaChBac, we infer that low CLK/CYC activity increases LNv excitability which in turn promotes light avoidance. Conversely, since expressing ClkDN or cycDN in DN1s has a similar light avoidance phenotype to hyperpolarizing DN1s via dORKΔC or Kir2.1, we infer that low CLK/CYC activity decreases DN1 excitability and consequently increases light avoidance by reducing DN1-mediated inhibition.
To test this further, we asked whether the increased light avoidance caused by expression of cycDN in LNvs or DN1s could be reduced by altering neuronal electrical excitability. We found that co-expressing dORKΔC with cycDN in LNvs (Fig 3A) or NaChBac with cycDN in DN1s (Fig 3B) rendered larvae as insensitive to light at 150lux as wild-type larvae. However, co-expressing NaChBac with cycDN in LNvs (Fig 3A) did not reverse the increased sensitivity caused by expressing cycDN. These results are consistent with low levels of CLK/CYC activity increasing LNv excitability and thus light avoidance levels – and this is rescued by hyperpolarizing LNvs. Conversely, low CLK/CYC activity seems to decrease DN1 excitability, which also increases light avoidance – and this is rescued by hyperexciting DN1s.
Since the phenotypes caused by cycDN can be rescued by altering the excitability of LNvs and DN1s, it seems unlikely that the behavioral phenotypes caused by cycDN arise from putative developmental defects caused by reduced CLK/CYC activity during development (Goda et al., 2011). Furthermore, we found that expressing cycDN in differentiated larval LNvs for only the 24hr immediately prior to assaying behavior still increased light avoidance (Fig S1).
High CLK/CYC activity likely increases DN1 and decreases LNv excitability
The per01 mutation stops the clock with constitutively high levels of CLK/CYC activity, allowing us to test how high levels of CLK/CYC activity affect LNv and DN1 excitability. Since per01 larvae display low levels of light avoidance at 750lux (Mazzoni et al., 2005), we tested whether light avoidance in per01 mutants could be restored to wild-type levels by manipulating LNv and DN1 excitability. We found that hyperexciting LNvs in a per01 background via NaChBac significantly increased levels of light avoidance, while hyperpolarizing LNvs through dORKΔC expression had no effect (Fig 3C), suggesting that per01 LNvs have reduced excitability. Conversely, dORKΔC expression in DN1s of per01 mutants significantly increased light avoidance, whereas NaChBac expression had no effect (Fig 3C), suggesting that per01 DN1s have increased excitability. From this, we conclude that per01 mutants display low levels of light avoidance because high CLK/CYC activity in per01 mutants simultaneously reduces LNv excitability and increases DN1 excitability.
These experiments indicate that CLK/CYC activity levels have opposite effects on LNv and DN1 excitability, with LNvs most excitable when CLK/CYC activity is low, and DN1s most excitable when CLK/CYC activity is high. The normal daily rhythm in CLK/CYC activity would then make LNvs and DN1s most likely to signal around dawn and dusk respectively. These conclusions for larval LNvs arrived at via genetic manipulations parallel electrophysiological recordings that reveal adult LNvs to be most excitable around dawn (Cao and Nitabach, 2008; Sheeba et al., 2008b), and are consistent with the role of adult s-LNvs in promoting morning locomotor activity (Grima et al., 2004; Stoleru et al., 2004). Although no recordings have been made from non-LNv clock neurons, increased excitability at dusk in larval DN1s is consistent with adult E cells promoting evening locomotor activity (Grima et al., 2004; Stoleru et al., 2004).
DN1s are essential for circadian rhythms in light avoidance
Larvae become more sensitive to light after several hours in darkness and wild-type larvae display circadian oscillations in avoiding 150lux light. This rhythm peaks at dawn (CT24, CT: circadian time, time in constant darkness) and is lowest at dusk (CT12) (Mazzoni et al., 2005). Our data from larvae taken from LD cycles suggest a mechanism for generating circadian rhythms in light avoidance: When CLK/CYC activity is low, around dawn, LNvs are most excitable and promote light avoidance with minimal inhibition by DN1s. Conversely, when CLK/CYC activity is high, around dusk, reduced LNv activity coupled with increased DN1 inhibition results in low levels of light avoidance.
To test this model, we first asked whether DN1s are required for rhythmic light avoidance. Larvae were entrained to at least 3 LD cycles before transfer to DD, with light avoidance assayed on days 2–3 in DD. Control (UAS-Dti / +) larvae displayed a rhythm in light avoidance at 150lux, with levels higher at subjective dawn than at subjective dusk (Fig 4A). However, no rhythm was detected in DN1-ablated (DN1 > Dti) larvae, with light avoidance levels constitutively high (Fig 4A). Since light avoidance levels were elevated when DN1s were ablated, we tested these larvae at a lower light intensity (50lux), but were still unable to detect any rhythm in light avoidance (Fig 4A). Therefore, we conclude that DN1s are necessary for circadian rhythms of light avoidance.
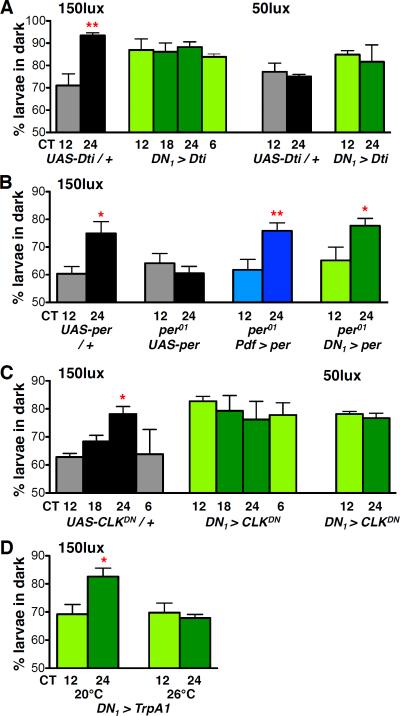
Figure 4. A signal from DN1s is necessary and sufficient for light avoidance rhythms.
All Statistical comparisons are as specified below. *p<0.05, **p<0.01, (A) Light avoidance was assayed on day 2 (CT12, 18, 24) or day 3 (CT6) of DD after prior LD entrainment. Control UAS-Dti / + larvae (grey) show time-dependent light avoidance at 150lux (CT12 vs CT24, t-test p<0.01). DN1-ablated larvae (green) show no time-dependent light avoidance, (ANOVA p=0.79). 2-Way ANOVA between control and DN1-ablated larvae for CT12 and 24 reveals a significant Genotype × Time interaction (F1,11=8.53, p<0.05). No time-dependent differences in light avoidance were observed in control or DN1-ablated larvae at 50lux (t-test).
(B) Light avoidance was assayed as in (A) at 150lux. All statistical comparisons by students t-test. Light avoidance scores were higher at CT24 than CT12 in control (per+ UAS-per, p<0.05) but not in per01UAS-per larvae. Rhythms were rescued by restoring per expression to LNvs (blue, p<0.005) or DN1s (green, p<0.05) in per01 mutants. See also Fig S2.
(C) Light avoidance was assayed as in (A). Light avoidance scores were lower at CT12 than at CT24 in control UAS-CLKDN / + larvae at 150lux (t-test p<0.001). DN1 > CLKDN increased light avoidance compared to controls (2 Way ANOVA F1,31=5.81, p<0.05), with no time-dependent differences in light avoidance observed at either 150lux (ANOVA) or 50lux (t-test).
(D) Light avoidance was assayed on day 2 in DD at 150lux using larvae reared at 20°C. Light avoidance scores were lower at CT12 than at CT24 in DN1 > TrpA1 larvae when assayed at 20°C (t-test p<0.01) but not 26°C. At 26°C, temperature-induced activation of DN1s via TrpA1 reduces light avoidance at CT24 to CT12 levels (2 Way ANOVA, Temperature × Time interaction F1,12=5.73, p<0.05). See also Fig S3
A functional LNv or DN1 clock is sufficient for light avoidance rhythms
To test whether a functional molecular clock in LNvs or DN1s is sufficient to generate circadian rhythms in light avoidance we used a UAS-per transgene (Yang and Sehgal, 2001) to restore per expression to either LNvs or DN1s in per01 mutant larvae (Fig 4B). We confirmed that these manipulations at least partly rescued molecular clock oscillations in the relevant cells (Fig S2). Control (per+ UAS-per) larvae showed higher light avoidance scores at CT24 than CT12, while per01 mutant larvae carrying the UAS-per transgene but no Gal4 driver displayed low levels of light avoidance at both CT12 and CT24 with no significant rhythm. We found that restoring per expression to either LNvs or DN1s rescued rhythmic light avoidance (Fig 4B).
We propose that a rhythmic molecular clock in the DN1s of per01; DN1 > per larvae drives rhythmic signals from DN1s, that regulate LNv neuronal activity. Since DN1s seem to be most active at dusk, this would allow LNvs to promote light avoidance at dawn even in the absence of their own functional clock. This result directly parallels observations from adult flies, where restoring per to only non-LNv clock neurons in per01 mutant flies restored the morning peak of locomotor activity (Stoleru et al., 2004). Conversely, we propose that larvae lacking per expression in DN1s (per01; Pdf > per, Fig 4B), remain rhythmic because high CLK/CYC activity in per01 DN1s (Fig 3C) renders them excitable and able to release their essential signal, whilst the functional LNv clock controls the timing of behavior. This contrasts with DN1 ablation, which prevents rhythms (Fig 4A). Therefore the DN1 signal is both necessary (ablated DN1s, Fig 4A) and sufficient (per+ DN1s with per mutant LNvs, Fig 4B) for light avoidance rhythms.
If CLK/CYC activity regulates DN1 excitability (Fig 3), low CLK/CYC activity should block release of the essential DN1 signal and be phenotypically similar to ablating DN1s. To test this, we assayed the effect of stopping the DN1 molecular clock with low CLK/CYC activity on light avoidance rhythms at 150lux (Fig 4C). We found that DN1 > ClkDN larvae lost light avoidance rhythms, with larvae constitutively sensitive to light at both 150lux and 50lux, similar to DN1 ablation. It should be noted that the experiments in Fig 4B and 4C are complementary rather than identical since expression of ClkDN or cycDN in a single neuronal group blocks the clock in those cells but leaves the other clock neurons wild-type, whereas restoration of per to a single neuronal group leaves the rest of the larva in a mutant per01 state. Overall, our LD and DD data suggest that the DN1 molecular clock regulates DN1 neuronal activity, with DN1s least active when CLK/CYC activity is lowest at dawn.
Transient activation of DN1s at dawn blocks light avoidance rhythms
Next we sought to directly test when DN1s normally signal using a transgene that expresses the heat-activated cation channel, TrpA1 (Hamada et al., 2008) Since TrpA1 is activated at temperatures >25°C, it can be used to transiently activate neurons in which it is expressed (Pulver et al., 2009). We used TrpA1 to transiently stimulate DN1s at CT12 and CT24 and measure the effect on light avoidance (Fig 4D). At 20°C, DN1 > TrpA1 larvae displayed normal light avoidance rhythms. However, activating DN1s via TrpA1 at 26°C blocked the rhythm, with levels of light avoidance constitutively low at both CT12 and CT24. No reduction in light avoidance at CT24 was observed between 20°C and 26°C for either UAS-TrpA1 / + or DN1 / + control larvae (Fig S3).
Since TrpA1 activation of DN1s did not affect light avoidance at CT12, we conclude that DN1s are already active at CT12. However, as DN1 activation reduces light avoidance at CT24, we conclude that DN1s are usually inactive at CT24. These data are consistent with the model that DN1s are much more active when CLK/CYC activity is high (CT12) than when CLK/CYC activity is low (CT24). Taking all these experiments together, we conclude that CLK/CYC activity regulates DN1 neuronal activity, peaking at dusk.
Glutamate is the inhibitory neurotransmitter produced by larval DN1s
One mechanism that could explain these data is that DN1s regulate light avoidance by inhibiting LNv neuronal activity. This is consistent with the inhibition of light avoidance at CT24 through TrpA1 activation of DN1s (Fig 4D) and with possible axo-axonal synapses between the DN1 projections and LNv axonal termini (Fig 1). Without the ability to conduct paired recordings between LNvs and DN1s, we sought to identify the relevant signal released by DN1s and its receptor on LNvs.
Larval DN1s produce the neuropeptide IPNamide (Shafer et al., 2006) and the vesicular glutamate transporter, suggesting that they are also glutamatergic (Hamasaka et al., 2007). Glutamate is a good candidate for the DN1 signal since larval LNv activity can be inhibited by directly applying glutamate to dissociated LNvs (Dahdal et al., 2010; Hamasaka et al., 2007).
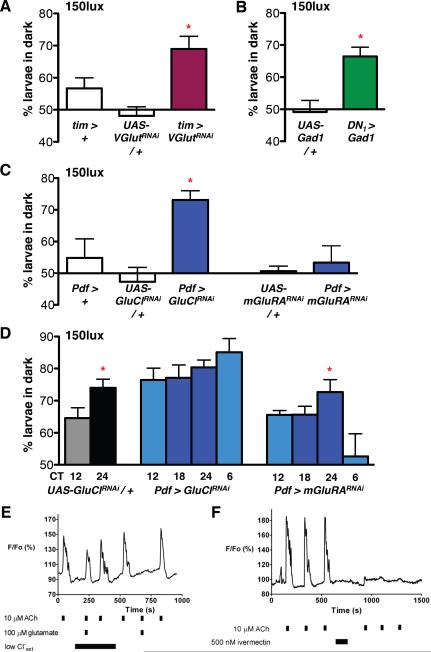
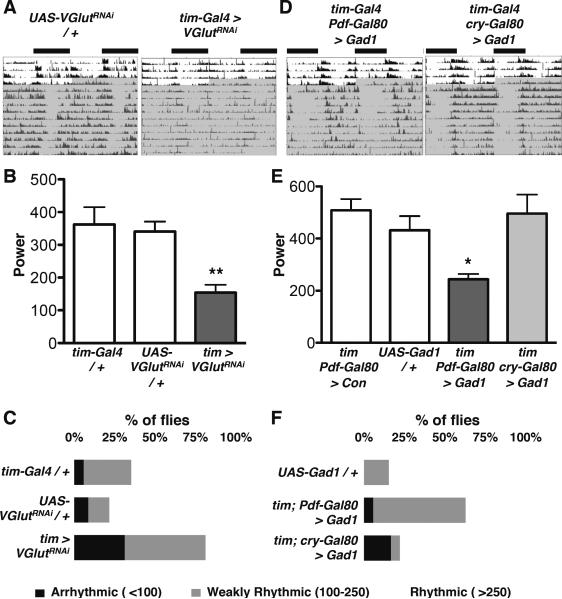
We used two independent methods to genetically alter glutamate signaling. First, we used RNAi to reduce expression of the vesicular glutamate transporter (VGlut), using the strong tim-Gal4 driver. (All RNAi experiments co-expressed UAS-dicer-2 (dcr-2), to increase RNAi efficacy, but this is omitted from written genotypes for simplicity.) Although tim-Gal4 is expressed in all clock neurons, DN1s are the only larval clock neurons expressing VGlut (Hamasaka et al., 2007). We found that tim > VGlutRNAi larvae displayed increased light avoidance in LD at 150lux (Fig 5A), as seen for hyperpolarizing or ablating DN1s (Fig 2) and also lost circadian rhythms in light avoidance (Fig S4A).
Figure 5. DN1s release glutamate to inhibit light avoidance.
For all RNAi experiments UAS-dcr-2 was co-expressed to improve efficacy. The Gal4 control lines shown also express UAS-dcr-2. Statistical comparisons are as stated below. *p<0.05
(A–C) Larval light avoidance was measured as in Fig 2.
(A) Expression of a VGlut-RNAi transgene (GD2574) in all clock neurons (tim > VGlutRNAi increased light avoidance at 150lux compared to control larvae. These data are significantly different (ANOVA p<0.005). Tukey's post-hoc comparison gives a significant difference only between tim > VGlutRNAi and UAS-VGlutRNAi / +. However, light avoidance in tim > VGlutRNAi is higher than tim > + by t-test (p<0.05) and tim > VGlutRNAi larvae also lose circadian rhythms in light avoidance (Fig S4A).
(B) Expression of Glutamate decarboxylase (UAS-Gad1) in DN1s (DN1 > Gad1) significantly increased light avoidance at 150lux compared to UAS-Gad1 / + control larvae (Gad1 / +, t-test p<0.05). See also Fig S4B.
(C) A GluCl-RNAi transgene expressed in LNvs (Pdf > GluClRNAi) significantly increased light avoidance at 150lux compared to control larvae (ANOVA p<0.05). An mGluRA-RNAi transgene expressed in LNvs (Pdf > mGluRARNAi) had no effect on light avoidance compared to controls (ANOVA). See also Fig S4C.
(D) Light avoidance was assayed in DD at 150lux as in Fig 4. Light avoidance is higher at CT24 than CT12 in control larvae (UAS-GluClRNAi / +, which also contain a UAS-dcr-2 transgene, t-test p<0.05). No rhythms in light avoidance were detectable when GluCl-RNAi was expressed in LNvs (Pdf > GluClRNAi, ANOVA). Rhythmic light avoidance was still detectable in larvae expressing mGluRA-RNAi in LNvs (Pdf > mGluRARNAi, ANOVA p<0.05). By 2 Way ANOVA comparison of CT12 and 24 time points, Pdf > GluClRNAi is different to control (F1,22=9.17, p<0.01) whilst Pdf > mGluRARNAi is not (F1,24=0.00, p=0.9547)
(E) Glutamate-mediated inhibition of ACh-stimulated Ca2+ transients in dissociated larval LNvs. Representative relative fluorescence (F/Fo) recordings are shown from dissociated larval LNvs expressing UAS-GCaMP1.6. Solution changes, including neurotransmitter applications, are indicated by black bars. Lowering extracellular Cl− to 13.6mM completely relieved glutamate-dependent inhibition. Glutamate completely blocked ACh-stimulated transients when physiological Cl− was restored.
(F) A 2min incubation of a larval LNv with 500nM ivermectin irreversibly blocks subsequent ACh-induced Ca2+ transients.
Next, we followed the method of Featherstone et al (2002) who ectopically expressed Glutamate decarboxylase 1 (Gad1) in glutamatergic neurons. Although Gad1 is normally used by GABAergic neurons to synthesize GABA from glutamate, Gad1 expression in a glutamatergic neuron phenocopies the effect of mutants defective in glutamate synthesis and reduces pre-synaptic glutamate levels (Featherstone et al., 2002). Since larval DN1s are not GABAergic (Hamasaka et al., 2005) and do not normally produce Gad1 (data not shown), they are unlikely to express the vesicular GABA transporter and so should be unable to load the GABA produced by Gad1 mis-expression into synaptic vesicles.
We found that DN1 > Gad1 larvae also showed increased levels of light avoidance in LD at 150lux (Fig 5B), again similar to DN1 hyper-polarization or ablation. DN1s in DN1 > Gad1 larvae still display normal TIM oscillations, indicating that Gad1 mis-expression does not affect DN1 viability or molecular clock function (Fig S4B). The identical phenotypes from these two independent manipulations of glutamatergic signaling lead us to conclude that glutamate is the inhibitory signal released by DN1s to modulate light avoidance.
DN1 glutamate regulates LNv activity via GluCl
Hamasaka et al. (2007) proposed that glutamate inhibits LNv activity via the metabotropic mGluRA glutamate receptor. They also showed that light avoidance levels are increased in mGluRA mutant larvae, although they did not determine the relevant cells (Hamasaka et al., 2007). However, our gene expression profiles from purified larval LNvs revealed that they also express the glutamate-gated chloride channel GluCl ~2.5-fold more highly than in Elav+ neurons (M. Ruben & JB, data not shown). Adult l-LNvs also have functional GluCl channels, although its behavioral role is unknown (McCarthy et al., 2011).
To test whether glutamate regulates light avoidance in LNvs via GluCl or mGluRA, we used RNAi to reduce expression of each receptor. Both transgenes reduce expression of their target (Hamasaka et al., 2007 and Fig S4C). We found that Pdf > GluClRNAi larvae had significantly increased light avoidance at 150lux, whereas Pdf > mGluRARNAi and control larvae did not avoid light (Fig 5C). Thus reducing GluCl in LNvs phenocopies reducing glutamate release from DN1s.
Next we tested the roles of GluCl and mGluRA in regulating circadian behavior. Our data show that Pdf > GluClRNAi larvae had no light avoidance rhythm, with levels of light avoidance constitutively high (Fig 5D), whilst Pdf > mGluRARNAi larvae display rhythmic light avoidance (Fig 5D). Thus GluCl is required in LNvs for rhythmic light avoidance. We propose that DN1s rhythmically release glutamate, which is perceived via GluCl in LNvs to mediate rhythmic inhibition of LNv neuronal activity. We have subsequently found that mGluRA helps synchronize LNv molecular oscillations (BC and JB in prep).
To directly test if GluCl can inhibit LNv activity, we measured the responses of dissociated larval LNvs expressing the intracellular Ca2+ sensor GCaMP1.6 (Reiff et al., 2005) to directly applied neurotransmitters. ACh produced by Bolwig's Organ is required for larval light avoidance (Keene et al., 2011). Applying ACh to dissociated LNvs increased intracellular Ca2+ levels, as previously reported (Dahdal et al., 2010; Wegener et al., 2004), measured by increased GCaMP fluorescence (Fig 5E–F). ACh increases intracellular Ca2+ in LNvs by activating nicotinic ACh receptors to produce excitatory post-synaptic potentials, eventually causing depolarization. In turn, this increases cytoplasmic Ca2+ via voltage-gated Ca2+ channels (Dahdal et al., 2010; Wegener et al., 2004), which is observed as increased GCaMP fluorescence. Given the relative insensitivity of GCaMP1.6 to single action potentials (Pologruto et al., 2004), these Ca2+ transients in LNvs likely reflect bursts of action potentials.
Co-applying 100μM glutamate completely blocked ACh-induced Ca2+ transients (see Fig 5E for a representative recording). We were unable obtain a narrowly defined IC50 value for glutamate, perhaps due to cell-to-cell variation in glutamate receptor content induced by dissociation. However, full inhibition of the response to 10μM ACh was produced with 10μM glutamate (n=6). To test if GluCl contributes to the inhibitory effects of glutamate on LNvs, we repeated these experiments in a low chloride buffer (Fig 5E). This reduced glutamate inhibition of LNv responses to ACh by 75% ± 13 (n=12 neurons). Therefore LNvs require extracellular Cl- for the majority of glutamate-induced inhibition. We also found that applying 500nM ivermectin, an irreversible GluCl activator (Cully et al., 1994), blocked the response of LNvs to ACh in the absence of glutamate (Fig 5F, n=4 neurons). These in vitro data parallel our in vivo data and support the idea that ACh released from the visual system can only fully activate LNvs in the absence of DN1 glutamatergic signals mediated via GluCl in LNvs.
Taking all the larval data in Figures 1 to 5 together, we propose the following model for rhythmic light avoidance (Fig S5). Around dawn, low CLK/CYC activity increases LNv excitability and reduces DN1 activity. With DN1s releasing minimal glutamate, the LNvs respond strongly to ACh from the visual system and promote the dawn peak in light avoidance. Around dusk, high CLK/CYC activity reduces LNv excitability but increases DN1 activity, causing glutamate release and inhibition of the response of the LNvs to ACh via GluCl, reducing light avoidance. Thus we propose a mechanism for the Morning and Evening dual oscillator model (Grima et al., 2004; Pittendrigh and Daan, 1976; Stoleru et al., 2004): Neuronal excitability peaks in antiphase between excitatory LNvs and inhibitory DN1s to generate robust behavioral rhythms.
Signals from non-LNvs are required for robust adult behavioral rhythms
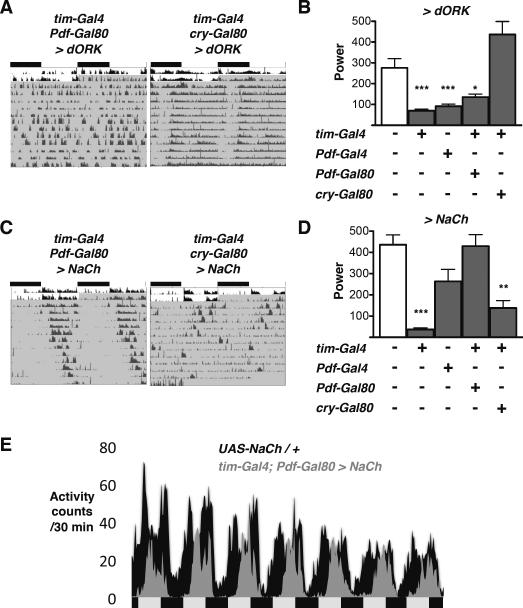
Although adult clock neurons are more numerous and control more behaviors than their larval counterparts, we sought to test if the principles we identified in larvae also operate in adult flies, focusing on locomotor activity rhythms in DD. Previous studies suggested that the neurons targeted by cry13-Gal4; Pdf-Gal80 are dispensable for adult DD rhythms since their ablation leaves flies rhythmic, possibly because sufficient CRY- non-LNvs remain to support rhythms (Stoleru et al., 2004). Therefore we used the tim-Gal4; Pdf-Gal80 combination to target strong transgene expression to all clock neurons except LNvs i.e. the dorsal Lateral Neurons (LNds) and the three groups of Dorsal Neurons. We also used the tim-Gal4;cry-Gal80 combination to target the non-CRY expressing subset of adult clock neurons (DN2s and subsets of LNds, DN1s and DN3s). tim-Gal4; Pdf-Gal80 and tim-Gal4; cry-Gal80 drivers both display robust rhythms when crossed to the dORKΔNC control transgene (Table 1; power > 500; see Experimental Procedures for a description of power).
To test the requirement for adult non-LNv clock neuron signals in circadian behavior, we first reduced neuronal excitability using the dORKΔC transgene. We found that tim-Gal4; Pdf-Gal80 > dORKΔC flies have as low power rhythms in DD as Pdf > dORKΔC flies, whereas tim-Gal4; cry-Gal80 > dORKΔC flies display robust rhythms (Fig 6A–B and Table 1). Thus strong adult locomotor rhythms require signals from the CRY-expressing non-LNv clock neurons. These include the DN1as which are descended from the larval DN1s (Klarsfeld et al., 2004; Shafer et al., 2006).
Figure 6. Adult CRY+ non-LNvs are required for robust locomotor activity rhythms.
Locomotor activity was recorded from flies entrained to 12:12 LD (white area of actogram), then transferred to DD (shaded). Comparisons to UAS-Control / + are by ANOVA with Tukey's post-hoc test. *p<0.05, **p<0.01, ***p<0.001
(A) Representative, double-plotted, normalized actograms are shown for tim-Gal4; Pdf-Gal80 > dORK and tim-Gal4; cry-Gal80 > dORK flies.
(B) The power of rhythms is plotted for flies expressing UAS-dORK under the control of tim-Gal4, Pdf-Gal4, tim-Gal4; Pdf-Gal80 and tim-Gal4; cry-Gal80 drivers. Power is significantly reduced in Pdf > dORK, tim > dORK and tim-Gal4; Pdf-Gal80 > dORK flies compared to UAS-dORK / + control flies.
(C) Representative, double-plotted, normalized actograms are shown for tim-Gal4; Pdf-Gal80 > NaCh and tim-Gal4; cry-Gal80 > NaCh flies.
(D) The power of rhythms is plotted for flies expressing UAS-NaCh under the control of tim-Gal4, Pdf-Gal4, tim-Gal4; Pdf-Gal80 and tim-Gal4; cry-Gal80 drivers. Power is significantly reduced in tim-Gal4; cry-Gal80 > NaCh flies compared to UAS-NaCh / + control flies.
(E) Average locomotor activity over the first 7 days in DD plotted for UAS-NaCh / + control (black) and tim-Gal4; Pdf-Gal80 > NaCh flies (grey). tim-Gal4; Pdf-Gal80 > NaCh fly activity is substantially reduced during the subjective morning. Each genotype shows the average of 16 flies from a single experiment.
Adult non-LNvs can inhibit morning activity
TrpA1 activation of larval DN1s at CT24 inhibited the morning peak of light avoidance (Fig 4D), suggesting that LNvs can only promote light avoidance in the absence of DN1 activity. Since the adult morning activity peak lasts for several hours, an equivalent experiment would require a prolonged temperature increase, which could complicate data interpretation because temperature is a potent zeitgeber (Glaser and Stanewsky, 2007). Instead, we analyzed the behavior of flies with hyper-excited non-LNvs. We noticed that although tim-Gal4;Pdf-Gal80 > NaChBac flies had robust rhythms, their activity becomes unimodal after several days in DD and morning activity is lost (Fig 6C–E, Table 1). We infer that NaChBac increases non-LNv excitability so that they now signal at the wrong time of day and block the morning peak of locomotor activity, normally promoted by LNvs. Thus cessation of inhibitory signaling by non-LNvs around dawn may be as important as excitatory signaling by LNvs in generating the morning activity peak and non-LNvs seem to gate LNv activity in both larvae and adult flies.
As with dORKΔ expression, this phenomenon requires the CRY-expressing non-LNv clock neurons since tim-Gal4; cry-Gal80 > NaChBac flies had reduced strength rhythms (Fig 6C, D, Table 1). Since this transgene combination targets a smaller subset of the non-LNV clock neurons than tim-Gal4;Pdf-Gal80, these data suggest that the CRY- clock neurons do not contribute to the specific inhibition of morning activity in tim-Gal4; Pdf-Gal80 > NaChBac flies.
Overall, our broad manipulations to non-LNv clock neurons indicate that, as in larvae, non-LNv signals are required for robust circadian behavior (Fig 6A–B) and probably gate LNv activity to refine the dawn peak of activity (Fig 6C–E).
Non-LNv glutamate signals are required for robust locomotor activity rhythms
Finally we tested whether glutamate released from adult non-LNv clock neurons is required for circadian behavior. Reducing VGlut expression in all clock neurons (tim > VGlutRNAi) significantly reduced the strength of locomotor activity rhythms compared to controls (Fig 7A–C, Table 1). A second insertion of the same transgene and an independent VGlutRNAi transgene gave similar reductions in rhythm strength (Table 1). This phenotype is likely due to glutamate released from non-LNv clock neurons because VGlut is only expressed in subsets of DN1 and DN3 neurons in the adult clock network (Hamasaka et al., 2007), and the strength of rhythms in Pdf > VGlutRNAi flies was not reduced (Table 1).
Figure 7. Glutamate signaling from Adult CRY+ non-LNvs is required for robust locomotor activity rhythms.
Locomotor activity was recorded from flies entrained to 12:12 LD (white area of actogram), then transferred to DD (shaded). Comparisons to UAS-Control / + by ANOVA with Tukey's post-hoc test. *p<0.05, **p<0.01
(A) Representative, double-plotted, normalized actograms are shown for UAS-VGlutRNAi/ + and tim > VGlutRNAi flies. UAS-dcr-2 was co-expressed to improve RNAi efficacy and the Gal4 control line also expresses UAS-dcr-2.
(B) The average power of rhythms is shown for tim > dcr2 + VGlutRNAi flies and tim > dcr2 and UAS-VGlutRNAi / + control flies. Power is significantly reduced in tim > VGlutRNAi flies compared to tim > dcr2 and UAS-VGlutRNAi control flies. Error bars represent SEM.
(C) Data from (B) plotted as % of flies that are “arrhythmic” (power >100) “weakly rhythmic” (power from 100–250) or “rhythmic” (power > 250).
(D) Representative, double-plotted, normalized actograms are shown for tim-Gal4; Pdf-Gal80 > Gad1 and tim-Gal4; cry-Gal80 > Gad1 flies.
(E) The average power of rhythms is shown for flies expressing UAS-Gad1 with the tim-Gal4; Pdf-Gal80 and tim-Gal4; cry-Gal80 drivers. Power is significantly reduced in tim-Gal4; Pdf-Gal80 > Gad1 flies compared to UAS-Gad1 / + control or tim-Gal4; Pdf-Gal80 crossed to the UAS-dORKΔNC control (con) flies. Error bars represent SEM.
(F) Data from (E) plotted as in (C)
To independently test a role for glutamate in the generation of adult rhythms in DD, we mis-expressed Gad1, as in larvae (Fig 5), to reduce pre-synaptic glutamate. This specifically affects glutamate levels since no adult clock neurons are GABAergic (Dahdal et al., 2010; Hamasaka et al., 2005). tim-Gal4; Pdf-Gal80 > Gad1 flies had lower power rhythms than control flies whereas tim-Gal4; cry-Gal80 > Gad1 flies had robust DD rhythms (Fig 7D–F and Table 1). Thus two independent manipulations of glutamate signaling indicate that glutamate released from CRY+ non-LNv clock neurons is required for robust locomotor activity rhythms. However, the rhythms of tim > + VGlutRNAi and tim-Gal4; Pdf-Gal80 > Gad1 flies are both stronger than tim-Gal4; Pdf-Gal80 > dORKΔC flies, suggesting that additional signals from non-LNvs contribute to rhythmic behavior. This interpretation makes sense given the diversity of Drosophila adult clock neurons and the incomplete arrhythmicity of even mutants in Pdf, the major circadian neuropeptide (Renn et al., 1999).
Taking all of the adult data together, we find evidence that the principles we identified in the larval circadian network may also operate in adult flies. Specifically our broad manipulations to adult non-LNv clock neurons indicate that non-LNv signals: (i) are important for strong adult rhythms; (ii) may gate LNv outputs to shape activity at dawn; and (iii) include glutamate.
Discussion
We identified some of the network logic that helps generate a simple rhythmic behavior through precise genetic manipulations of the larval circadian circuit and extended these findings to the more complex adult circadian network. Previous studies have shown that intercellular signaling in clock neuron networks promotes molecular clock synchrony (Lin et al., 2004; Maywood et al., 2006; Stoleru et al., 2005) and can strengthen genetically weak molecular clocks (Liu et al., 2007). Our study increases the importance of circadian neural networks by finding that non-LNv clock neurons are as important as the “master' pacemaker LNv clock neurons for rhythmic behavior in both larvae and adult flies. However, LNvs can still be considered pacemakers in DD since most manipulations to non-LNv clock neurons do not affect period length.
Non-LNv signals appear to gate pacemaker neuron activity. Why is this necessary when LNvs have their own intrinsic excitability rhythms? We propose that the interaction of two oscillators with opposite signs helps reduce the time when LNvs signal. Without signaling from non-LNvs, adult locomotor activity rhythms are weak and activity is distributed throughout the day and night as in tim-Gal4; Pdf-Gal80 > dORKΔC flies. In contrast, in tim-Gal4; Pdf-Gal80 > NaChBac flies, the timing of locomotor activity is narrowed. Thus the gating of LNv activity by non-LNvs may help turn gradual changes in the excitability of each neuronal group into thresholds that promote a switch in overall output and allow flies to abruptly transition from inactivity to activity.
This gating system can only function if LNvs and non-LNvs have differently phased neuronal activity. However, most Drosophila clock neurons have similarly phased molecular clocks. We propose that molecular clocks in different clock neurons regulate divergent sets of output genes to generate distinct phases of neuronal excitability. This would be analogous to the mammalian circadian system where molecular clocks in different tissues drive tissue-specific outputs (e.g. Storch et al., 2002). In summary, our genetic dissection of a circadian neural circuit reveals an unexpected and essential role for inhibitory signals from non-LNvs (E cells) in shaping activity profiles at dawn and a novel mechanism for how clock neurons couple together to promote robust rhythms.
Experimental procedures
Fly stocks
For a complete list of fly stocks used in this paper see Supplemental experimental procedures.
Larval light avoidance
For LD experiments, larvae were entrained to 5 days of 12:12 LD cycles at 25°C and tested on the 6th day as 3rd instar larvae. For DD experiments, larvae were entrained to 12:12 LD at 25°C for 3–4 days and tested on the second or third days in DD. Larvae were removed from LD or DD immediately prior to testing. ~15 larvae were placed in the middle of an 8.5 cm diameter agar-filled Petri dish and the number of larvae in the light and dark was recorded after 15 min as in Mazzoni et al (2005) with the following minor modifications: (i) To speed up scoring, any larvae visible through the lid of the plate were recorded as being on the light side even if crossing the midline; (ii) As larvae could be found on the walls lid on both the light and dark sides of the plate, they were included in the scoring; (iii) Light intensity was reduced by moving the light source away from the plate rather than adding filters; and (iv) The light source used was a circular fluorescent 22W GE Cool White bulb. Data are plotted as `% larvae in the dark'. Each data point is the average of 3 or more experiments, with each experiment consisting of ~45 larvae on 3 plates assayed simultaneously, except when insufficient larvae of the required genotype were obtained from individual crosses. In this case, data from separate experiments were added in chronological order to reach a total of ~45 larvae. All experiments on larvae in LD were carried out between ZT3-6, and in DD between CT11.5 and CT13 (“CT12”) and CT23.5 and CT1 (“CT24”). For TrpA1 experiments, larvae were entrained to LD cycles at 20°C for 7 days then moved to DD and tested on the second day in DD. Larvae were at 26°C for only the duration of the assay. Statistical comparisons were made using Student's t-test (for pairwise comparisons), ANOVA with Tukey's Post-hoc tests (for multiple comparisons within a single data set), or Two-way ANOVA (for comparisons between genotypes across multiple time points), as stated in the figure legends.
Dissociated larval LNv recordings
Recordings from dissociated LNvs Expressing GCaMP1.6 were carried out as in Dahdal et al (2010). Briefly, 30–60 larval brains were dissociated by treatment with 2 Units/mL dispase II and manual trituration. GCaMP fluorescence from individual neurons was imaged on an inverted epifluorescence microscope (TE2000U, Nikon) via a standard GFP filter set. Cells were continuously superfused at 2 ml/min with standard saline (in mM: NaCl 128, KCl 2, MgCl2 4, CaCl2 1.8, sucrose 36, HEPES 5, pH 7.1) to which compounds were added as indicated. For low chloride experiments, standard saline was modified to reduce Cl- to 13.6 mM by replacement of NaCl with sodium gluconate.
Adult locomotor activity
For locomotor activity experiments, adults were entrained to 12:12 LD cycles at 25°C for at least 3 days before transfer to DD. Locomotor activity was recorded using the DAM system (TriKinetics, Waltham, MA). We used χ2 analysis in ClockLab (Actimetrics, Wilmette, IL) to derive a power and significance for each rhythm over 10 days in DD. We subtracted the significance score from the power to calculate the strength of each rhythm (presented as `power' in the text). Using this analysis, control lines have average powers ranging from ~270–580 (“Rhythmic”, see Table 1) while classical clock mutants (per01, ClkJrk, Clkar) have powers from 10–40 (“Arrhythmic”). Pdf > dORKΔC flies, previously described as ~70% arrhythmic / 30% weakly rhythmic (Nitabach et al., 2002; Wu et al., 2008), have an average power of 91, establishing a baseline for the effect of manipulations of electrical excitability. All statistical comparisons were made by ANOVA.
Supplementary Material
Acknowledgments
We are very grateful to the following for their generous gifts of antibodies and flies: Ravi Allada, Patrick Emery, Paul Hardin, Rob Jackson, Michael Nitabach, Jae Park, Marie-Laure Parmentier, Michael Rosbash, Amita Sehgal and Mike Young. Additional fly stocks were obtained from the Vienna Drosophila RNAi and Bloomington Stock centers and PDF antisera from the DSHB. We also thank Afroditi Petsakou for advice on qPCR and Ravi Allada, Matthieu Cavey, Claude Desplan, Bambos Kyriacou and Afroditi Petsakou for helpful comments on the manuscript. Ben Collins was supported by The Robert Leet and Clara Guthrie Patterson Trust Postdoctoral Fellowship, Bank of America, Trustee. Elizabeth Kane was supported by a Deans Undergraduate Research Fellowship from NYU. This investigation was conducted in a facility constructed with support from Research Facilities Improvement Grant Number C06 RR-15518-01 from the National Center for Research Resources, National Institutes of Health. Confocal microscopy was performed in the NYU Center for Genomics & Systems Biology Core Facility. This work was supported by NIH grants NS030808 (MHA) and GM063911 (JB).
Findings LNv and DN1 clock neurons have opposite effects on light avoidance
LNv and DN1s likely signal at opposite times of day despite similarly-phased clocks
Larval rhythms need inhibitory glutamatergic DN1 signals that gate LNv activity
Non-LNv signals also required for circadian locomotor activity rhythms in adults
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Allada R, Chung BY. Circadian organization of behavior and physiology in Drosophila. Annu Rev Physiol. 2010;72:605–624. doi: 10.1146/annurev-physiol-021909-135815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Cao G, Nitabach MN. Circadian control of membrane excitability in Drosophila melanogaster lateral ventral clock neurons. J Neurosci. 2008;28:6493–6501. doi: 10.1523/JNEUROSCI.1503-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully DF, Vassilatis DK, Liu KK, Paress PS, Van der Ploeg LH, Schaeffer JM, Arena JP. Cloning of an avermectin-sensitive glutamate-gated chloride channel from Caenorhabditis elegans. Nature. 1994;371:707–711. doi: 10.1038/371707a0. [DOI] [PubMed] [Google Scholar]
- Dahdal D, Reeves DC, Ruben M, Akabas MH, Blau J. Drosophila pacemaker neurons require g protein signaling and GABAergic inputs to generate twenty-four hour behavioral rhythms. Neuron. 2010;68:964–977. doi: 10.1016/j.neuron.2010.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone DE, Rushton E, Broadie K. Developmental regulation of glutamate receptor field size by nonvesicular glutamate release. Nat Neurosci. 2002;5:141–146. doi: 10.1038/nn789. [DOI] [PubMed] [Google Scholar]
- Glaser FT, Stanewsky R. Synchronization of the Drosophila circadian clock by temperature cycles. Cold Spring Harb Symp Quant Biol. 2007;72:233–242. doi: 10.1101/sqb.2007.72.046. [DOI] [PubMed] [Google Scholar]
- Goda T, Mirowska K, Currie J, Kim MH, Rao NV, Bonilla G, Wijnen H. Adult circadian behavior in Drosophila requires developmental expression of cycle, but not period. PLoS Genet. 2011;7:e1002167. doi: 10.1371/journal.pgen.1002167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Z. Behavioral dissection of Drosophila larval phototaxis. Biochem Biophys Res Commun. 2009;382:395–399. doi: 10.1016/j.bbrc.2009.03.033. [DOI] [PubMed] [Google Scholar]
- Grima B, Chelot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- Hamada FN, Rosenzweig M, Kang K, Pulver SR, Ghezzi A, Jegla TJ, Garrity PA. An internal thermal sensor controlling temperature preference in Drosophila. Nature. 2008;454:217–220. doi: 10.1038/nature07001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamasaka Y, Rieger D, Parmentier ML, Grau Y, Helfrich-Forster C, Nassel DR. Glutamate and its metabotropic receptor in Drosophila clock neuron circuits. J Comp Neurol. 2007;505:32–45. doi: 10.1002/cne.21471. [DOI] [PubMed] [Google Scholar]
- Hamasaka Y, Wegener C, Nassel DR. GABA modulates Drosophila circadian clock neurons via GABAB receptors and decreases in calcium. J Neurobiol. 2005;65:225–240. doi: 10.1002/neu.20184. [DOI] [PubMed] [Google Scholar]
- Hardin PE. Molecular genetic analysis of circadian timekeeping in Drosophila. Adv Genet. 2011;74:141–173. doi: 10.1016/B978-0-12-387690-4.00005-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogenesch JB, Herzog ED. Intracellular and intercellular processes determine robustness of the circadian clock. FEBS Lett. 2011;585:1427–1434. doi: 10.1016/j.febslet.2011.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene AC, Mazzoni EO, Zhen J, Younger MA, Yamaguchi S, Blau J, Desplan C, Sprecher SG. Distinct visual pathways mediate Drosophila larval light avoidance and circadian clock entrainment. J Neurosci. 2011;31:6527–6534. doi: 10.1523/JNEUROSCI.6165-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A, Malpel S, Michard-Vanhee C, Picot M, Chelot E, Rouyer F. Novel features of cryptochrome-mediated photoreception in the brain circadian clock of Drosophila. J Neurosci. 2004;24:1468–1477. doi: 10.1523/JNEUROSCI.3661-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klarsfeld A, Picot M, Vias C, Chelot E, Rouyer F. Identifying specific light inputs for each subgroup of brain clock neurons in Drosophila larvae. J Neurosci. 2011;31:17406–17415. doi: 10.1523/JNEUROSCI.5159-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Stormo GD, Taghert PH. The neuropeptide pigment-dispersing factor coordinates pacemaker interactions in the Drosophila circadian system. J Neurosci. 2004;24:7951–7957. doi: 10.1523/JNEUROSCI.2370-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AC, Welsh DK, Ko CH, Tran HG, Zhang EE, Priest AA, Buhr ED, Singer O, Meeker K, Verma IM, et al. Intercellular coupling confers robustness against mutations in the SCN circadian clock network. Cell. 2007;129:605–616. doi: 10.1016/j.cell.2007.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maywood ES, Reddy AB, Wong GK, O'Neill JS, O'Brien JA, McMahon DG, Harmar AJ, Okamura H, Hastings MH. Synchronization and maintenance of timekeeping in suprachiasmatic circadian clock cells by neuropeptidergic signaling. Curr Biol. 2006;16:599–605. doi: 10.1016/j.cub.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Mazzoni EO, Desplan C, Blau J. Circadian pacemaker neurons transmit and modulate visual information to control a rapid behavioral response. Neuron. 2005;45:293–300. doi: 10.1016/j.neuron.2004.12.038. [DOI] [PubMed] [Google Scholar]
- McCarthy EV, Wu Y, Decarvalho T, Brandt C, Cao G, Nitabach MN. Synchronized Bilateral Synaptic Inputs to Drosophila melanogaster Neuropeptidergic Rest/Arousal Neurons. J Neurosci. 2011;31:8181–8193. doi: 10.1523/JNEUROSCI.2017-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitabach MN, Blau J, Holmes TC. Electrical silencing of Drosophila pacemaker neurons stops the free-running circadian clock. Cell. 2002;109:485–495. doi: 10.1016/s0092-8674(02)00737-7. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- Nitabach MN, Wu Y, Sheeba V, Lemon WC, Strumbos J, Zelensky PK, White BH, Holmes TC. Electrical hyperexcitation of lateral ventral pacemaker neurons desynchronizes downstream circadian oscillators in the fly circadian circuit and induces multiple behavioral periods. J Neurosci. 2006;26:479–489. doi: 10.1523/JNEUROSCI.3915-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh C, Daan S. A functional analysis of circadian pacemakers in nocturnal rodents. V. Pacemaker structure: a clock for all seasons. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1976:333–355. [Google Scholar]
- Pologruto TA, Yasuda R, Svoboda K. Monitoring neural activity and [Ca2+] with genetically encoded Ca2+ indicators. J Neurosci. 2004;24:9572–9579. doi: 10.1523/JNEUROSCI.2854-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulver SR, Pashkovski SL, Hornstein NJ, Garrity PA, Griffith LC. Temporal dynamics of neuronal activation by Channelrhodopsin-2 and TRPA1 determine behavioral output in Drosophila larvae. J Neurophysiol. 2009;101:3075–3088. doi: 10.1152/jn.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiff DF, Ihring A, Guerrero G, Isacoff EY, Joesch M, Nakai J, Borst A. In vivo performance of genetically encoded indicators of neural activity in flies. J Neurosci. 2005;25:4766–4778. doi: 10.1523/JNEUROSCI.4900-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A Pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- Robinson IM, Ranjan R, Schwarz TL. Synaptotagmins I and IV promote transmitter release independently of Ca2+ binding in the C2A domain. Nature. 2002;418:336–340. doi: 10.1038/nature00915. [DOI] [PubMed] [Google Scholar]
- Shafer OT, Helfrich-Forster C, Renn SC, Taghert PH. Reevaluation of Drosophila melanogaster's neuronal circadian pacemakers reveals new neuronal classes. J Comp Neurol. 2006;498:180–193. doi: 10.1002/cne.21021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Fogle KJ, Kaneko M, Rashid S, Chou YT, Sharma VK, Holmes TC. Large ventral lateral neurons modulate arousal and sleep in Drosophila. Curr Biol. 2008a;18:1537–1545. doi: 10.1016/j.cub.2008.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheeba V, Gu H, Sharma VK, O'Dowd DK, Holmes TC. Circadian- and light-dependent regulation of resting membrane potential and spontaneous action potential firing of Drosophila circadian pacemaker neurons. J Neurophysiol. 2008b;99:976–988. doi: 10.1152/jn.00930.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- Storch KF, Lipan O, Leykin I, Viswanathan N, Davis FC, Wong WH, Weitz CJ. Extensive and divergent circadian gene expression in liver and heart. Nature. 2002;417:78–83. doi: 10.1038/nature744. [DOI] [PubMed] [Google Scholar]
- Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE. Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr Biol. 2004;14:638–649. doi: 10.1016/j.cub.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Wang J, Ma XJ, Yang JS, Zheng XY, Zugates CT, Lee CHJ, Lee T. Transmembrane/juxtamembrane domain-dependent Dscam distribution and function mushroom body neuronal morphogenesis. Neuron. 2004;43:663–672. doi: 10.1016/j.neuron.2004.06.033. [DOI] [PubMed] [Google Scholar]
- Wegener C, Hamasaka Y, Nassel DR. Acetylcholine increases intracellular Ca2+ via nicotinic receptors in cultured PDF-containing clock neurons of Drosophila. J Neurophysiol. 2004;91:912–923. doi: 10.1152/jn.00678.2003. [DOI] [PubMed] [Google Scholar]
- Wu Y, Cao G, Nitabach MN. Electrical silencing of PDF neurons advances the phase of non-PDF clock neurons in Drosophila. J Biol Rhythms. 2008;23:117–128. doi: 10.1177/0748730407312984. [DOI] [PubMed] [Google Scholar]
- Xiang Y, Yuan Q, Vogt N, Looger LL, Jan LY, Jan YN. Light-avoidance-mediating photoreceptors tile the Drosophila larval body wall. Nature. 2010;468:921–926. doi: 10.1038/nature09576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Sehgal A. Role of molecular oscillations in generating behavioral rhythms in Drosophila. Neuron. 2001;29:453–467. doi: 10.1016/s0896-6273(01)00218-5. [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA. Synaptic connections of PDF-immunoreactive lateral neurons projecting to the dorsal protocerebrum of Drosophila melanogaster. J Comp Neurol. 2010;518:292–304. doi: 10.1002/cne.22210. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Xiang Y, Yan Z, Han C, Jan LY, Jan YN. Light-induced structural and functional plasticity in Drosophila larval visual system. Science. 2011;333:1458–1462. doi: 10.1126/science.1207121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.