Abstract
Neuropathic pain is a chronic debilitating disease characterized by mechanical allodynia and spontaneous pain. Because symptoms are often unresponsive to conventional methods of pain treatment, new therapeutic approaches are essential. Here, we describe a strategy that not only ameliorates symptoms of neuropathic pain, but is also potentially disease modifying. We show that transplantation of immature telencephalic GABAergic interneurons from the mouse medial ganglionic eminence (MGE) into the adult mouse spinal cord completely reverses the mechanical hypersensitivity produced by peripheral nerve injury. Underlying this improvement is a remarkable integration of the MGE transplants into the host spinal cord circuitry, in which the transplanted cells make functional connections with both primary afferent and spinal cord neurons. By contrast, MGE transplants were not effective against inflammatory pain. Our findings suggest that MGE-derived GABAergic interneurons overcome the spinal cord hyperexcitability that is a hallmark of nerve-injury induced neuropathic pain.
INTRODUCTION
Loss of spinal cord dorsal horn inhibitory circuits, many of which involve interneurons that express gamma aminobutyric acid (GABA), is one of the major contributors to the persistent neuropathic pain that can follow nerve injury. The loss of inhibition contributes not only to the development of spontaneous pain, but also to the hyperexcitability that underlies the mechanical hypersensitivity (allodynia) and exacerbated pain (hyperalgesia). For example, partial sciatic nerve injury reduces spinal GABA release and expression of the GABA synthesizing enzyme, glutamic acid decarboxylase (GAD) (Moore et al., 2002). The consequence of this is a loss of inhibitory tone in the dorsal horn. It is not clear, however, whether the reduced GABAergic inhibition results from injury-induced degeneration of GABAergic interneurons (Scholz et al., 2005; Sugimoto et al., 1990), reduced primary afferent input to these interneurons (Kohno et al., 2003; Polgar and Todd, 2008), decreased release of GABA (Lever et al., 2003) or down-regulation of GABA, GAD or pre and postsynaptic GABA receptors (Castro-Lopes et al., 1993; Eaton et al., 1998; Fukuoka et al., 1998; Ibuki et al., 1997; Polgar et al., 2004).
Not surprisingly, many pharmacological approaches to managing nerve injury-induced neuropathic pain enhance inhibitory controls. Indeed, significant analgesia can be achieved by activating spinal GABAA or GABAB receptors, in various models of inflammatory and neuropathic pain (Asiedu et al., 2010; Knabl et al., 2008; Munro et al., 2009). The pharmacological regulation of GABA controls, however, is not straightforward. For instance, some patients do not respond to these therapies and adverse side effects that result from systemic drug administration, are dose limiting. Here we describe a potentially disease-modifying therapeutic approach designed to restore the inhibitory tone in the spinal cord. This approach consists of transplanting embryonic GABAergic neuronal precursors in the dorsal horn of the spinal cord.
Previous studies reported that embryonic GABAergic cortical interneuron precursors from the medial ganglionic eminence (MGE) grafted into adult forebrain disperse and synaptically integrate into functional circuits (Baraban et al., 2009; Southwell et al., 2010; Wichterle et al., 1999). These grafts are effective in different neurological disorders associated with neuronal hyperexcitability, e.g., animal models of epilepsy (Alvarez-Dolado et al., 2006; Baraban et al., 2009; Calcagnotto et al., 2010; Martinez-Cerdeno et al., 2010). Here we asked whether MGE transplants are also viable in the spinal cord, which is outside of their natural environment. We then investigated whether MGE cells can receive and form connections within local circuits of the host dorsal horn. Finally, we assessed the behavioral consequences of transplanting MGE cells in mouse models of inflammatory and nerve injury-induced pain. We report that MGE cells survive outside of the forebrain, retain features of cortical interneurons, integrate into host spinal cord circuitry and promote an almost complete reversal of the mechanical hypersensitivity generated by the nerve, but not tissue injury.
RESULTS
Cortical inhibitory precursor cells grafted into the adult spinal cord differentiate into GABAergic interneurons
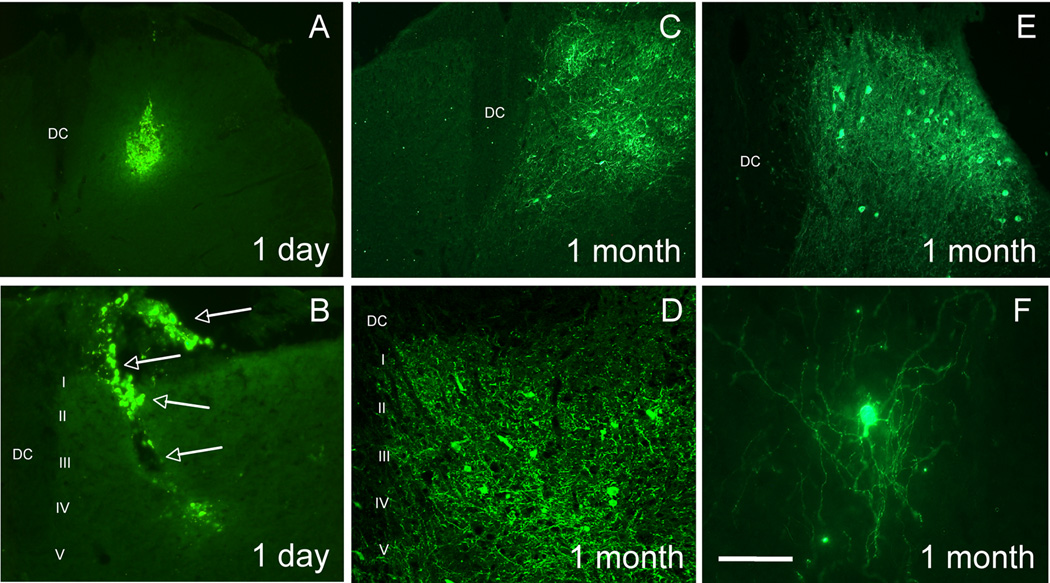
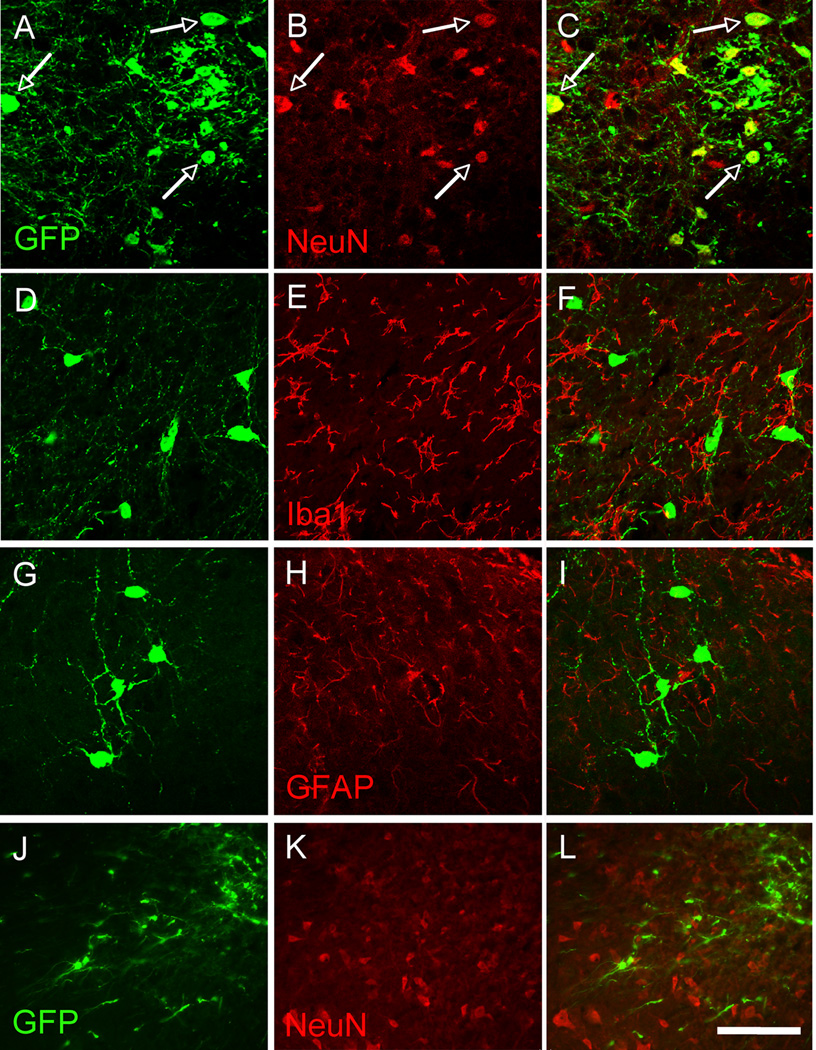
We first asked whether the spinal cord environment was sufficient to promote survival of the MGE cells transplants. To this end, we used MGE cells that express green fluorescent protein (GFP) under the control of the Gad1 (GAD67) promoter (Tamamaki et al., 2003). In these mice, GAD+/GABAergic MGE cells constitutively express GFP. Figures 1A–B illustrate expression of GFP in the spinal cord of naïve, non-injured adult mice, one day after transplantation of MGE cells into the dorsal horn. Most transplanted GFP+ cells formed an aggregate at or near the injection site, with some cells dispersed along the needle track. Isolated cells were occasionally detected at a distance from the heart of the injection site. In contrast, one month after transplantation, we observed GFP+ cells in both the dorsal and ventral horns of the spinal cord. The transplanted cells had extensive and ramified processes (Figures 1C–F). We estimate that ~2.7% of transplanted MGE cells survived one month after transplantation (1380 ± 478 cells per animal, n=5). However, given the likelihood that many cells were lost in the course of injection (cells remaining in the injection pipette, cell death in the course of the injection, cells trapped in the pia, etc.), it is likely that the MGE survival rate is significantly underestimated. The majority of the GFP+ (~71%) were located in the deep dorsal horn of the spinal cord (laminae III–V), over ~2.5mm of the rostro-caudal lumbar enlargement, all ipsilateral to the injection side. We did not detect any GFP+ cells in thoracic or cervical spinal cord. Most GFP+ cells co-labeled for NeuN, a marker of neurons (89.4 ± 2.7%, Figure 2A–C) but none for Iba1, a marker of microglia (Figures 2D–F), or glial fibrillary acid protein (GFAP), a marker of astrocytes (Figures 2G–I), indicating that the vast majority of MGE-derived cells differentiated into neurons. By following the grafted cells from 1 to 5 weeks after transplantation we conclude that it takes at least 2 weeks for the MGE-derived cells to acquire a neuronal (NeuN+) phenotype (Figures 2J–L).
Figure 1. MGE-derived transplants are viable in the spinal cord of naïve, adult mice.
MGE-derived cells constitutively express GFP (green). (A–B) One day after transplantation, the transplant consisted of an aggregate of GFP+ MGE cells, at or near the injection site. Occasionally we observed isolated cells that migrated from the injection site as well as cells at the surface of the cord (arrows in B). The transplants were injected unilaterally and we never observed GFP+ cells that migrated to the other side of the cord. (C–E) The majority of GFP+ MGE cells were located in deep laminae (III–V). (F) MGE cells extended long processes throughout the spinal cord. Scale bar equals 200 µm in A and C, 50 µm in B, D and E and 25 µm in F.
Figure 2. MGE-derived cells differentiate into neurons.
(A–C) One month after transplantation, most GFP+ (green) MGE cells expressed NeuN (red), a marker of neurons. (D–F) GFP+ (green) MGE cells did not express Iba1 (red), a marker of microglia, or GFAP (G–I; red), a marker of astrocytes. (J–K) In contrast, one week after transplantation, MGE cells did not co-stain for NeuN (red). Arrows point to examples of NeuN-GFP+, double-labeled cells. Scale bar equals 50 µm.
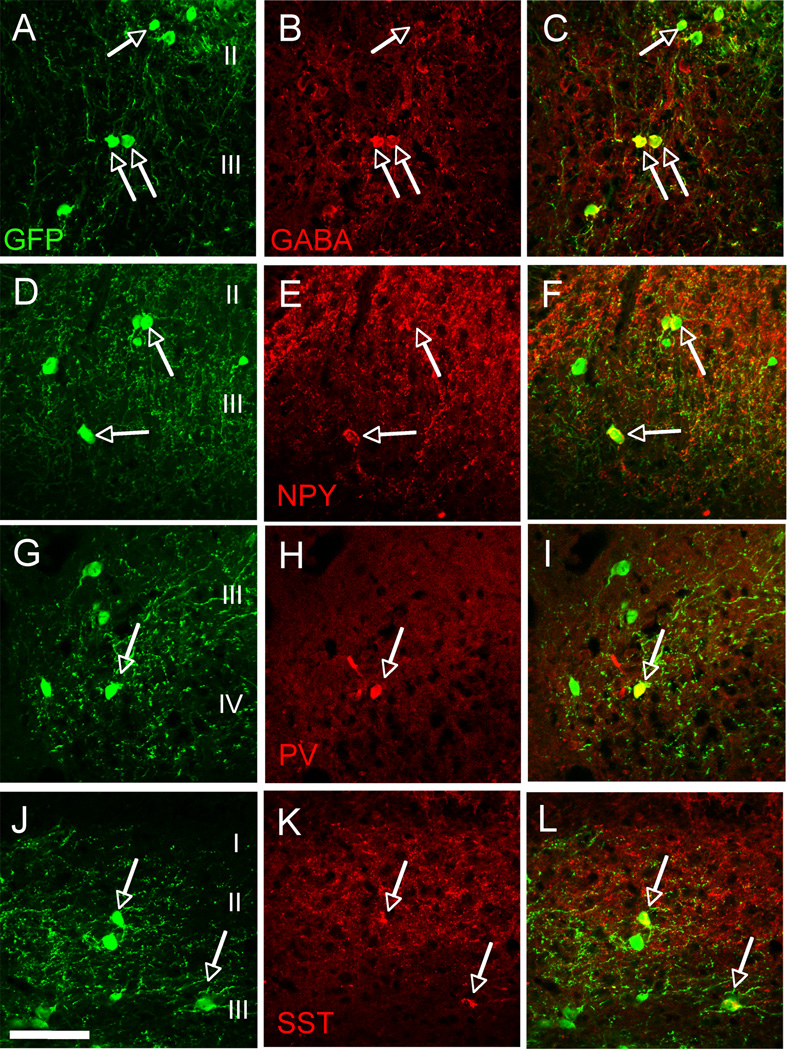
The majority of the GFP+ MGE cells expressed markers of subpopulations of cortical GABAergic interneurons, including GABA (75.1 ± 9.6%, Figures 3A–C), neuropeptide Y (NPY, 33.4 ± 9.1%, Figures 3D–F), parvalbumin (PV, 22.2 ± 2.3%, Figures 3G–I) and somatostatin (40.1 ± 4.1%, Figures 3J–L). The presence of somatostain (SST)-GFP positive neurons is of particular interest as this neurochemical phenotype is characteristic of a large percentage (~40%) of MGE-derived cortical GABAergic interneurons (Alvarez-Dolado et al., 2006). By contrast, GABA does not colocalize with SST in spinal cord interneurons; SST in fact marks a subpopulation of excitatory interneurons (Yasaka et al., 2010). These results provide evidence that the environment of the spinal cord does not alter the differentiation of MGE-derived cells into phenotypes similar to those observed in cortex. Taken together, these results indicate that MGE transplants are viable in a foreign tissue environment (spinal cord versus cortex), and that the majority of grafted cells differentiate into GABAergic neurons that recapitulate the normal heterogeneity of cortical (but not spinal) GABAergic interneurons.
Figure 3. MGE-derived neurons assume a GABAergic phenotype.
MGE-derived cells (green) co-expressed markers (red) of cortical GABAergic interneurons, including GABA (A–C), neuropeptide Y (NPY, D–F), parvalbumin (PV, G–I) and somatostatin (SST, J–L). Arrows point to examples of double-labeled cells. Scale bar equals 50 µm.
As peripheral nerve injury induces a plethora of changes in the dorsal horn, including a profound activation of potentially phagocytic microglia, we next compared MGE graft survival in uninjured mice and others that underwent partial nerve injury (spared nerve injury, SNI). Survival rate of MGE cells in SNI animals (~1.3%; 667 ± 267 cells per animal, n=5) was, in fact significantly lower (50% less) than in naïve animals. However, we found there were no significant differences in the neurochemical phenotype of surviving cells in intact and injured animals (data not shown). To determine whether the peripheral nerve injury contributed to the lower recovery of transplanted neurons, we used TUNEL staining to monitor apoptosis of transplanted neurons at 1 and 4 weeks after transplantation. Consistent with the report of Moore et al (2002), although we recorded a few TUNEL positive neurons at these time points, none were GFP-positive (Figure S1). We conclude that cell death, at least via apoptosis, is neither the major contributor to the limited recovery of transplanted neurons, nor to the further reduction recorded in nerve-injured animals.
Integration of cortical inhibitory precursor cells into host circuitry: presynaptic inputs
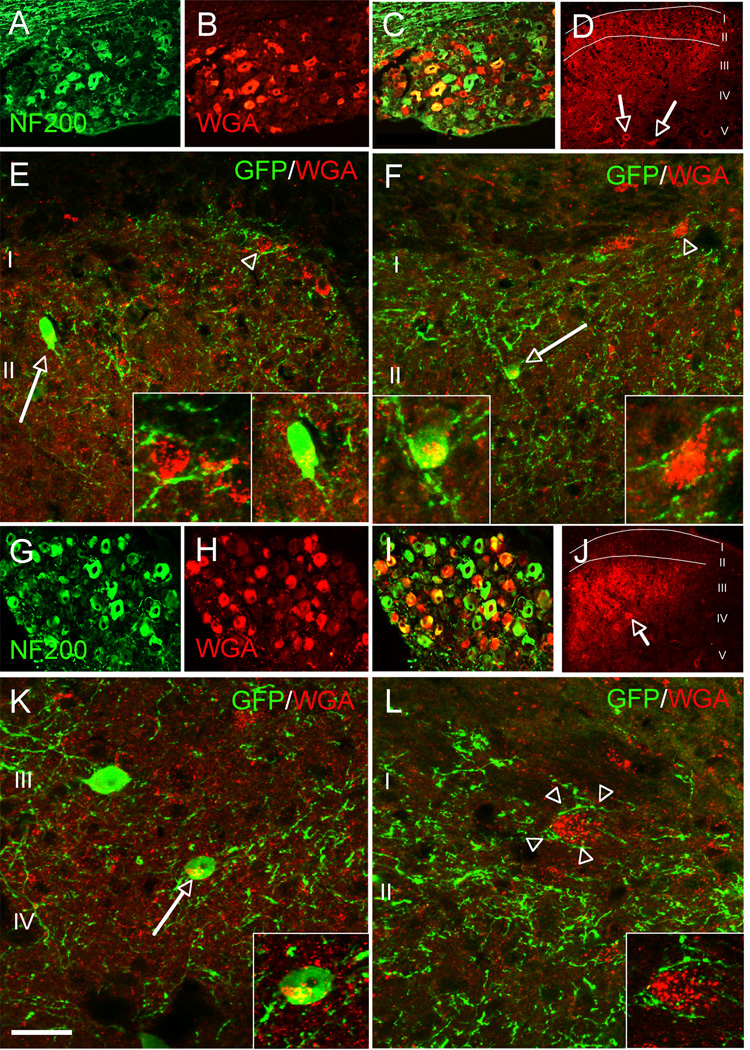
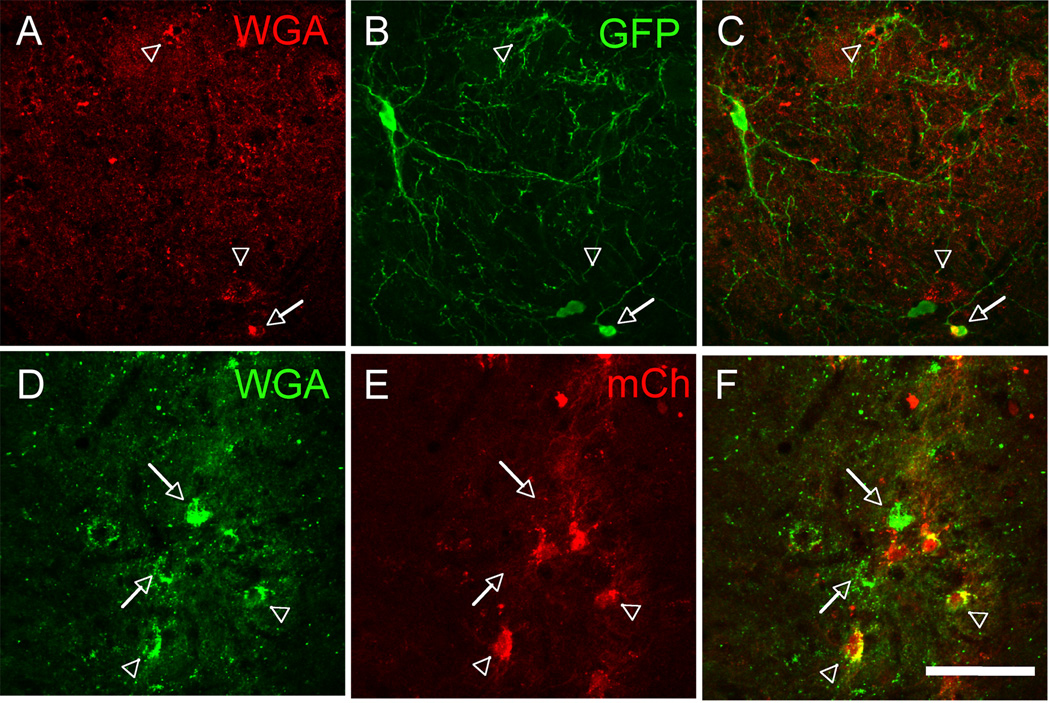
We next addressed the extent to which the MGE cells integrate into and make connections with the spinal cord host circuitry. To determine whether the transplanted cells are the targets of primary sensory neurons, we transplanted the MGE cells into the spinal cord of our ZW transgenic mouse line. In ZW mice, expression of the transneuronal tracer wheat germ agglutinin (WGA) can be induced by Cre recombination in sensory neurons (Braz and Basbaum, 2008; Braz et al., 2005; Braz et al., 2002). We crossed the ZW mice with peripherin-Cre mice (ZW-Per) to induce WGA expression in a mixed (large and small diameter) afferent population of dorsal root ganglion (DRG) neurons (Zhou et al., 2002). If the transplanted MGE cells receive inputs from primary sensory neurons, then we should detect transneuronal transfer of WGA from the sensory neurons to the MGE transplanted cells (Figures S2A–B). Figure 4 illustrates that this is indeed the case. The WGA is produced in DRG neurons and transported into postsynaptic spinal cord neurons (Figures 4A–D). Double labeling experiments with antibodies against GFP and WGA revealed that MGE-derived cells (arrows, Figures 4E–F) were among the WGA-positive spinal cord neurons.
Figure 4. Transplanted MGE-derived neurons receive inputs from both myelinated and unmyelinated primary afferent neurons.
(A–F) We transplanted MGE cells into the spinal cord of mice that express the inducible transneuronal tracer WGA (red) in a mixed, unmyelinated and myelinated, NF200+ (green) population of DRG neurons (A,C). In these mice WGA is released throughout the dorsal horn of the spinal cord (laminae I–V), where it was taken up by local neurons (arrows in D), including the MGE-derived cells (green, E–F). Insets show high magnifications of neurons from E and F. (G–L) When we transplanted MGE cells into the spinal cord of ZWX mice that expressed the WGA selectively in myelinated DRG neurons (NF200+, G–I), we also detected transneuronal transfer of WGA (arrows in J), including the MGE-derived cells (green, K). Here, most WGA labeling was concentrated in deeper laminae (III–V), where cutaneous myelinated primary afferents terminate (compare WGA-labeling patterns in D and J). We also observed WGA+, but GFP− neurons that were enveloped by terminals that derive from the MGE cells (L). Insets show high magnifications of neurons from K and L. Scale bars equals 200 µm in A–C and G–I; 100 µm in D and J; 30 µm in E, F, K and L.
To determine whether a particular subpopulation of sensory neurons communicates with the MGE transplants, we used our recently described ZWX line (Braz and Basbaum, 2009) in which it is possible, in the adult, to target expression of the tracer to subsets of afferents, by peripheral nerve injury. To target expression of the tracer to DRG neurons with myelinated axons, we took advantage of the fact that NPY is expressed in these neurons, but only after peripheral nerve section (Noguchi et al., 1993). In these studies, we made injections of MGE cells into the spinal cord of ZWX mice crossed with NPY-Cre mice (DeFalco et al., 2001). One month after transplantation we transected the sciatic nerve, so as to induce WGA expression in the NPY subset of sensory neurons. Figures 4G–I show that transection of the sciatic nerve in the adult indeed induced high levels of WGA expression in NF200+ (a marker of myelinated axons) DRG neurons (1 week post-transection). As expected, subsequent transport of the WGA tracer to the spinal cord predominated in deeper laminae III–V of the dorsal horn (Figure 4J), a major termination zone for cutaneous NPY-expressing DRG neurons. As for the ZW-Per crosses, the MGE transplants were also among the spinal neurons that had taken up the WGA (arrow, Figure 4K). We estimate that 32.5 ± 3.8% of MGE cells contained the WGA tracer. We also observed WGA+, but GFP−, neurons that were enveloped by terminals that derive from the MGE cells (arrowhead, Figure 4L). These studies demonstrate that myelinated afferents, the great majority of which respond to innocuous peripheral stimulation, make connections with transplanted MGE cells and that MGE cells also target circuits engaged by primary afferents.
Functionality of the MGE cell transplants
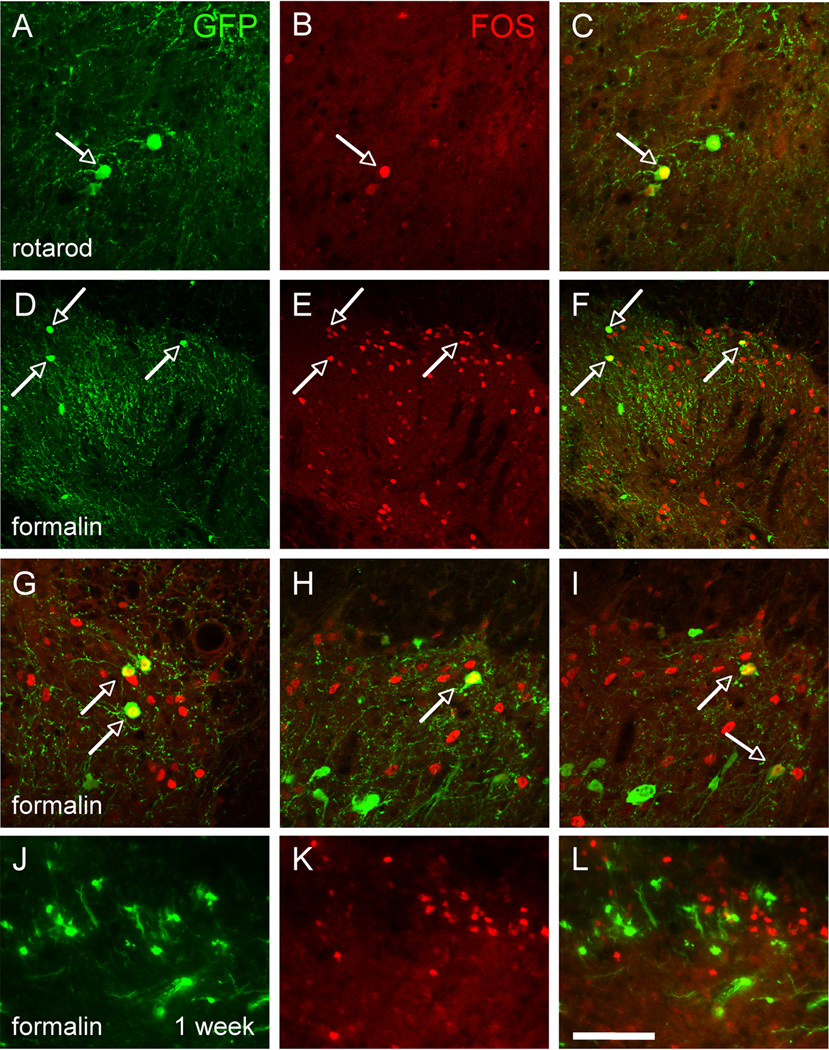
If the connections established between DRG neurons and MGE grafts are, in fact, functional, then a peripheral stimulus should “activate” the MGE transplants. Because the transneuronal WGA studies revealed a myelinated afferent fiber connection with MGE transplants, we asked whether innocuous peripheral inputs could activate grafted neurons. One month after transplantation, MGE transplanted mice walked on a rotarod for 90 min, a condition that predominantly engages low threshold mechanoreceptive (myelinated) afferents (Neumann et al., 2008) (Figure S3). Consistent with the results from the tracing studies, we found that this non-noxious peripheral input induced expression of Fos in 18.6 ± 6.3% MGE cells (Figures 5A–C). The majority of Fos+ MGE cells predominated in deeper laminae (III–V) and around the central canal, a region that receives input from myelinated muscle spindle and joint afferents.
Figure 5. Transplanted MGE-derived neurons are activated by innocuous and noxious peripheral stimulation.
(A–C) Walking for 90’ on a rotarod induced expression of Fos (red) in MGE cells, indicating that innocuous peripheral stimuli (in this case conveyed by myelinated primary afferents) activate the MGE transplants. (D–L) Hindpaw injection of formalin (1%) also induces expression of Fos (red) in a large number of spinal cord neurons, including GFP+ (green) MGE-derived neurons, 1 month after transplantation (D–I) but not at 1 week post-transplantation (J–L). Arrows point to examples of Fos and GFP+ double-labeled cells. Scale bars equals 50 µm in A–C and G–L, 100 µm in D–F.
We next asked whether a noxious chemical stimulus (hindpaw injection of 1% formalin), which engages both unmyelinated and myelinated axons (Braz and Basbaum, 2010; Shields et al., 2010) can activate the MGE cells. Figure 5B illustrates that injection of formalin evoked Fos in both host and GFP+ transplanted neurons in the dorsal horn, ipsilateral to the noxious stimulus (1 month post-transplantation; Figures 5D–I and Figure S3). In the superficial dorsal horn, where Fos+ neurons predominate, we found that 34.6 ± 13.4% of MGE neurons expressed Fos. On the other hand, injection of formalin in the hindpaw of one week-transplanted mice, despite inducing significant Fos in host neurons, did not evoke Fos in the transplanted neurons (Figures 5J–L). In fact, by following the grafted cells from 1 to 4 weeks after transplantation, we conclude that it takes at least 2 weeks for the MGE-derived cells to respond to a peripheral noxious input, which coincides with the time when they acquire a neuronal phenotype. Taken together, these functional assays indicate that 1 month after transplantation, presumptive GABAergic MGE neurons are “activated” by non-noxious as well as noxious inputs and confirm the existence of functional connections between primary afferent neurons and transplants.
Integration of cortical inhibitory precursor cells into host circuitry: targets of the transplanted neurons
We next asked whether grafted cells made connections with host spinal cord neurons. To this end, we genetically modified the MGE cells to express both the WGA tracer and a reporter gene, mCherry (mCh). We hypothesized that WGA-expressing transplanted MGE cells will release the WGA tracer in the spinal cord, and that the tracer, in turn, will be taken up by neurons that are connected with the grafted cells (Figures S2C–D). In these studies, we generated a lentiviral vector that expresses both WGA and mCh (Lenti-WmCh; Figures S4A–D) under the control of the CMV enhancer. We infected the MGE cells with Lenti-WmCh and transplanted them into the spinal cord of naïve, non-injured adult mice. Animals were killed 1 month after transplantation, which provided sufficient time for MGE integration, expression and release of the WGA by the transplanted cells, and uptake of the tracer into cells of the host spinal cord.
Figure 6 illustrates that transplanted Lenti-WmCh-infected MGE cells were viable in the spinal cord. We estimate that 35.2 ± 4.4% of MGE, GFP+ cells expressed the mCh marker and among these, most (95.1 ± 2.1%) expressed the WGA, indicating that almost all infected MGE cells synthesized the WGA tracer. Within 2 weeks of transplantation, we also detected many WGA+, but GFP− spinal neurons (Figures 6A–C and Figure S4), indicating that the WGA was released and taken up by host (GFP−) neurons. Importantly, 23.4 ± 2.1% of these WGA+ neurons were mCh− (Figures 6D–F). Given that mCh labels infected neurons, WGA+ and mCh− neurons correspond to host spinal neurons that took up the WGA tracer after its release from transplanted cells, not because they express the WGA. These connections always remained on the side of the cord ipsilateral to the transplant. When the transplanted cells were located in both dorsal and ventral horn, we also found WGA transneuronal transfer to presumptive motoneurons (Figure S4). Taken together, we conclude that MGE cells integrate into host spinal cord circuitry. They receive inputs from different categories of primary afferents and establish connections with neurons of the host spinal cord.
Figure 6. Transplanted MGE-derived neurons establish connections with host spinal cord neurons.
(A–C) We infected MGE cells with a lentivirus that expresses the tracer WGA and the reporter mCherry and transplanted them in naïve, adult mice. Two weeks after transplantation, ~30% of GFP+ (green, I) MGE cells expressed WGA (red). Importantly, we detected WGA+/GFP− (arrowheads in A–C) but also WGA+/mCh− (arrows in D–F) neurons in the spinal cord of transplanted mice indicating that spinal cord neurons had taken up the tracer after its release from MGE cells (see also Figure S3). Arrowheads in D–F point to examples of WGA+/mCh+ double labeled cells. Scale bars equals 100 µm in A–C and 50 µm in D–F.
MGE transplants target spinal cord projection neurons
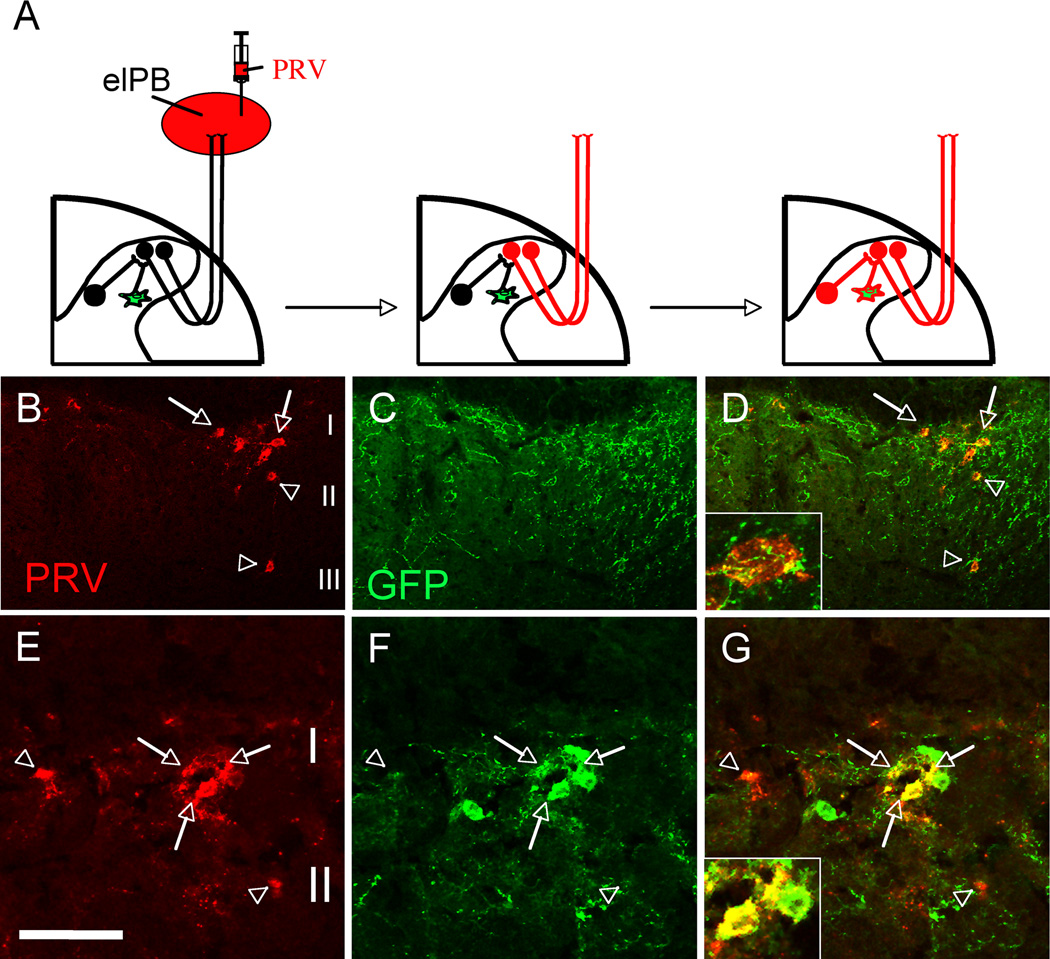
Spinal cord projection neurons receive inputs from nociceptors and transmit this information to the brain. It is thus of interest to ask whether these projection neurons are targeted by transplanted MGE cells. In this regard, it is significant that many transneuronally labeled WGA+ cells in lamina I, which contains projection neurons, were enveloped by axon terminals that derived from the transplanted cells (Figure 4L). To assess directly the connectivity between transplanted cells and projection neurons, we examined whether the transplanted cells could be labeled after retrograde transneuronal transport of pseudorabies virus (PRV) (Jasmin et al., 1997) from a major brainstem target of spinal cord projection neurons.
In naïve adult mice, we injected PRV into a known target of spinal cord projection neurons, namely the external lateral parabrachial nucleus (elPB), one month after they received an MGE transplant. We then followed the retrograde transneuronal spread of the PRV. If MGE cells contact projection neurons that project to the elPB, then the PRV should not only retrogradely infect projection neurons of lamina I, but also the MGE cells that are upstream of the projection neurons (Figure 7A). Indeed, 3 days after PRV infection, we detected a large number of PRV-infected spinal cord neurons (Figures 7B–D and Figure S5). These cells were concentrated in laminae I and II. We presume that the cells in lamina II correspond to interneurons that targeted the projection cells of lamina I (Jasmin et al., 1997). Furthermore, virtually all PRV+ neurons in lamina I were extensively enveloped by GFP+ processes, indicating that projection neurons of lamina I receive inputs from MGE transplanted cells. Of particular interest, however, was the observation of a small number of double-labeled GFP+/PRV+ cells (5.2 ± 3.1%; arrows in Figure 7E–G) in lamina II, which we hypothesize correspond to MGE cells that have engaged a circuit targeting the projection neurons. As we previously showed, PRV only “travels” between interconnected neurons (Braz et al., 2009) indicating that the MGE transplanted cells can influence lamina I projection neurons and possibly modulate the transmission of “pain” messages to the brain.
Figure 7. Transplanted MGE-derived neurons integrate into spinal cord circuits that engage lamina I projection neurons.
(A) Injection of the retrograde transneuronal viral tracer pseudorabies (PRV, red) in the external lateral parabrachial nucleus (elPB; left panel) results in infection of spinal cord projection neurons that target the elPB (projection neurons “become” red; middle panel). Subsequent retrograde transfer of PRV labels spinal cord neurons that are upstream of the infected projection neurons (spinal neurons, including GFP+ (green) MGE cell “become” red; right panel). (B–D) PRV+ (red) projection neurons of spinal cord lamina I (arrows) and PRV+ interneurons of laminae II–III (arrowheads). Note the extensive arborization of GFP-positive terminals around retrogradely PRV-labeled neurons (D, inset; see also Supplementary Figure 5). (E–G) MGE transplanted cells (green) are among the cells that are retrogradely infected by PRV (red), indicating that MGE cells have integrated into a neuronal circuit that influences projection neurons of lamina I. Arrowheads in E point to retrogradely infected PRV+/GFP− spinal cord interneurons. Arrows point to examples of PRV+/GFP+ double-labeled cells. Arrowheads Scale bars equals 100 µm in B–D and 50 µm in E–G.
Grafted MGE cells reverse the persistent pain produced by peripheral nerve injury
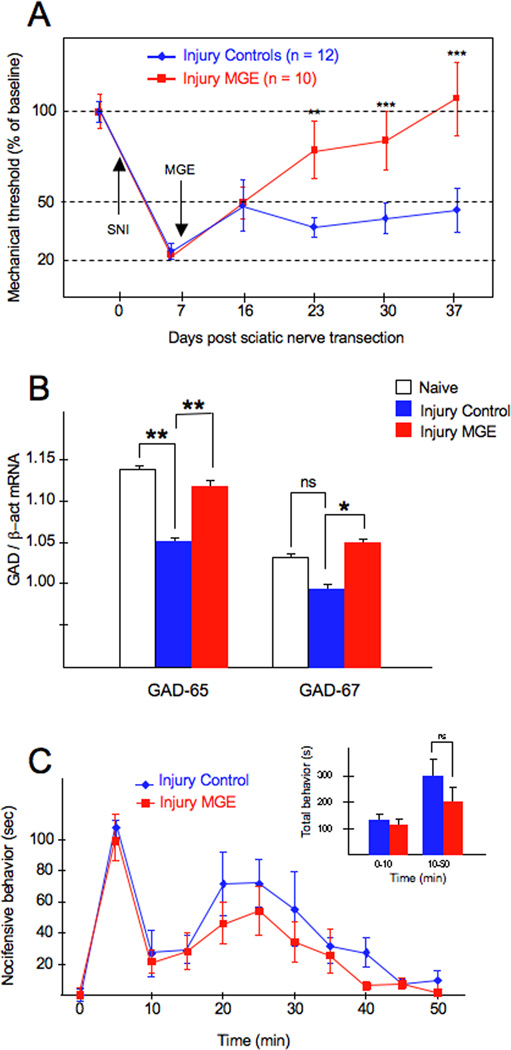
We next assessed the behavioral consequences of transplanting MGE GABAergic neuronal precursors into the spinal cord of adult mice, in a standard model of nerve injury-induced neuropathic pain. The spared nerve injury (SNI) model is produced by transection of two of the three branches of the sciatic nerve resulting in prolonged mechanical hypersensitivity (Shields et al., 2003). One week after SNI, mice received a suspension of MGE cells (transplanted group) or medium alone (no cells, control group), ipsilateral to the injury side. Mechanical thresholds were recorded before (baseline) and once a week (for 4 weeks) after transplantation. Figure 8A illustrates that 1 day after SNI, there is a dramatic reduction of the mechanical threshold (von Frey) ipsilateral to the injury side. In the control group (SNI animals that received an injection of medium alone), the marked mechanical allodynia persisted for the one month observation period (blue line in Figure 8). By contrast, we observed a gradual reduction of the SNI-induced mechanical allodynia in MGE-transplanted animals (red line, Figure 8), with a complete reversal by one month. A significant difference between control and MGE-transplanted groups was first detected two weeks post-transplantation (23 days post-SNI), similar to the time necessary for the MGE cells to differentiate into neurons (Figure 2), and presumably integrate into the host circuitry. However, the magnitude of the recovery continued to improve and thresholds returned to pre-injury baseline levels 4 weeks after transplantation.
Figure 8. MGE-derived neurons reverse the mechanical allodynia produced by partial nerve injury but do not affect inflammatory pain.
(A) Changes of mechanical threshold (allodynia; von Frey test) after partial sciatic nerve injury (SNI) and after MGE transplantation are reported as percent of pre-SNI baseline ± SEM. One day after SNI, there is a dramatic reduction of the mechanical threshold of the hindpaw ipsilateral to the injury. In SNI animals that only received cell medium, the marked mechanical allodynia persisted throughout the 4 week observation period (blue line). In contrast, there was a gradual reduction of the mechanical allodynia in MGE-transplanted animals (red line), with a complete reversal at one month (day 37 post SNI). The difference between mechanical thresholds of the 2 groups was statistically significant 2 weeks after transplantation. (B) Compared to naïve animals, we found that after SNI, there is a small but significant decrease in the spinal cord levels of GAD65, but not of GAD67 mRNA, ipsilateral to the nerve injury. In the MGE transplanted group, both GAD65 and GAD67 levels were significantly greater than those in medium-injected nerve-injured mice. However, the levels of GAD mRNA in the MGE transplanted and naïve mice did not differ. Asterisks (*) indicate statistically significant differences between groups, with * = p < 0.05 and ** = p < 0.01. (C) Transplanting MGE cells did not reduce pain behaviors in the formalin test. Although there was a slight decrease throughout the second phase of testing, the effect was not statistically significant.
In another group of animals, we recorded the baseline thresholds of naïve, non-injured mice, before (0.97 ± 0.25) and after (1.01 ± 0.23) transplantation of MGE cells. With neither protocol did the MGE cell transplantation cells alter baseline mechanical thresholds in the absence of nerve injury.
Effects of MGE transplants on GABA signaling
Several studies demonstrated that a peripheral nerve injury reduces GABAergic signaling, including a reduction of the expression of GAD, the biosynthetic enzyme for GABA (Moore et al., 2002). Here we used quantitative PCR to determine whether MGE transplants alter GAD mRNA levels, relative to those in nerve-injured animals, where a decrease was predicted. For this analysis, we transplanted MGE cells 1 week after SNI and assayed both GAD65 and 67 levels 1 week later. We chose this time point as Moore et al (2002) reported that GAD protein levels were significantly reduced 2 weeks after nerve injury. Figure 8B demonstrates that compared to uninjured animals, nerve injury induced a small, but significant decrease in the spinal cord levels of GAD65 mRNA, ipsilateral to the injury; GAD67 levels did not differ. By contrast, in the MGE transplanted group, we recorded significantly greater GAD65 (6.7%) and GAD67 (7.1%) levels compared to levels in the medium-injected group. However, GAD mRNA levels in the MGE transplanted group did not differ from those in the naïve group. Taken together, these results indicate that MGE transplantation does not significantly raise basal GAD mRNA expression (in uninjured animals), but that the transplant can overcome the decrease of GAD mRNA (and presumptive inhibitory tone and consequent mechanical hypersensitivity) triggered by peripheral nerve injury.
Grafted MGE cells are not effective in a model of chemical nociception associated with inflammation
Finally, we asked whether transplantation of MGE GABAergic precursor neurons also has antinociceptive effects in a model of the other major class of chronic pain, namely a chemical nociception pain model associated with inflammation. Hindpaw injection of formalin produces significant inflammation of the paw and two distinct phases of pain behavior, characterized by flinching and/or licking of the paw. In this condition, GABA transmission is not compromised and, in fact, GABAergic tone may be increased in the dorsal horn in the setting of tissue injury (Hossaini et al., 2010). In our studies, we injected 1% formalin into the hindpaw, 4 weeks after the mice had received a dorsal horn transplant (ipsilateral to the transplant). Figure 8C illustrates that the transplant did not reduce pain behaviors in either the first or the second phases of this test. There was a slight, decrease at all time points during Phase II, but the reduction was not statistically significant. We conclude that mechanical allodynia in a standard model of neuropathic pain, but not the pain behavior evoked in a common chemical nociception model associated with inflammation, can be reversed by spinal cord transplantation of GABAergic precursor cells.
DISCUSSION
Here, we show that MGE GABAergic interneuron precursor cells transplanted into the spinal cord of adult mice with peripheral nerve injury integrate into the host circuitry and are functionally engaged by different modes of afferent stimulation. The transplants also significantly reduce the mechanical hypersensitivity produced in a model of neuropathic pain, without altering baseline thresholds (without nerve injury). On the other hand, MGE transplants do not affect inflammatory pain. These findings indicate that GABAergic precursor cells have the essential properties for a cell-based therapy, particularly when loss of inhibitory control is a major contributor to the clinical condition. This approach differs from traditional pharmacological approaches to pain therapy in two ways: 1) transplantation provides a continuous and local delivery of inhibitory neurotransmitters, which not only recapitulates more closely the endogenous condition, but also avoids many of the adverse side effects associated with systemically administered drugs; and 2) transplantation is potentially disease modifying (i.e. it is directed at the underlying etiology of the persistent pain, namely loss of GABAergic inhibitory controls).
Using the genetically engineered ZW and ZWX mice that express the anterograde transneuronal tracer WGA, as well as viral retrograde transneuronal tracers, we demonstrate that MGE cells integrate into the host spinal cord circuitry. MGE cells established connections with both primary afferent neurons and postsynaptic elements of the circuitry, throughout the dorsal horn, including projection neurons of lamina I. But perhaps more importantly, we showed that these connections are functional. MGE cells are activated (express Fos) by noxious as well as non-noxious peripheral stimuli. Although we cannot distinguish between mono and polysynaptic primary afferent activation of the transplanted neurons, our study provides the most direct assessment of the extent to which transplanted cells integrate into the host spinal cord circuitry, and begins to define the types of afferents that engage the transplanted neurons and the time course of the integration. It is of particular interest that some MGE-derived neurons were located postsynaptic to myelinated (presumably Aβ) DRG neurons. This connection likely drives a low threshold mediated inhibitory control, which is one of the major postulates of the Gate Control Theory of pain, which emphasized large fiber-mediated inhibition of the transmission of nociceptive message (Melzack and Wall, 1965).
The observation that MGE cells receive functional inputs in naïve non-injured adult animals is of particular interest. Indeed, integration of the MGE cells into local spinal circuits provides evidence that elements of the circuit (primary afferents and/or spinal neurons) have the ability to extend new processes (e.g. sprout) and form new synapses in the absence of peripheral nerve injury. It is, of course, possible that the transplantation itself produces an injury that induces sprouting of the afferents. It is also possible that the input to MGE cells is derived from regeneration of the central branch of sensory neurons that were injured during the transplant procedure. Indeed, in the setting of peripheral nerve injury, regeneration, particularly of large diameter axons, may be enhanced (Neumann et al., 2002).
We demonstrated that MGE transplanted cells make connections with a large number of spinal cord neurons. Importantly, even when the MGE cells were located ventral to lamina III, the entire mediolateral width of the superficial and dorsal horn was often encompassed by MGE derived axonal arbors. The latter enveloped many transneuronally labeled WGA+ cells, including projection neurons of lamina I. Hence, MGE cells target and can influence a large variety of spinal cord neurons, including many that respond to noxious stimuli. We conclude that MGE-derived transplants do not serve merely as “cell-based chemical pumps”, which is characteristic of other cell-based approaches (e.g. intrathecal injection of adrenal chromaffin cell or other precursor cells). Rather, by integrating into functional circuits, MGE cells overcome a functional deficit that reverses a critical etiology (i.e. defects in endogenous inhibition) of the persistent pain (Eaton et al., 1999; Hao et al., 2005; Liu et al., 2004; Sagen et al., 1990; Winnie et al., 1993; Yu et al., 1998).
Our results go considerably beyond previous efforts to overcome the loss of GABAergic inhibition. For example, both trigeminal injection of an adenoviral vector expressing the GABA synthesizing enzyme, GAD65, or peripheral injection of an HSV vector expressing GAD67 had antinociceptive effects in models of facial pain (Vit et al., 2009) and spinal nerve ligation (Hao et al., 2005), respectively. Intrathecal (Vaysse et al., 2011) and intraspinal (Mukhida et al., 2007) injection of human cell lines engineered in vitro to express GABA also attenuated nerve-injury induced mechanical allodynia in the rat. However, in none of these cases was there evidence for integration of the GABAergic cells into the host. Furthermore, embryonic human progenitor cells, whether immortalized or not, require expansion in vitro. With increasing time in culture, i.e., after multiple passages, the properties of the cells can change, which reduces the likelihood of their differentiating into neurons (Jain et al., 2003). Furthermore, as many neural stem cells maintain their proliferative potential after transplantation (Mukhida et al., 2007), the potential for tumor development cannot be ignored. Finally, and of particular importance to long-term pain management, is that transplants reported to date have a relatively short survival, which reduces their clinical utility. In contrast, we show that MGE cells have the essential properties for a cell-based therapy: long survival rate, stability and safety, differentiation into functionally integrated mature interneurons, and presumptive rescue of GABAergic inhibitory control.
We demonstrate that the spinal cord environment is sufficient to promote survival (even in the context of a peripheral nerve injury) and, perhaps more importantly, integration of transplanted precursor cells. Interestingly, transplanted MGE cells recapitulated the normal heterogeneity of cortical (but not spinal) GABAergic neurons, indicating that the phenotype of the MGE cells is predetermined. Apparently, MGE cells are not influenced by the local environment, at least with respect to their neurochemical make-up. On the other hand, despite their rather rigid differentiation program, these cortically-derived cells clearly adapt and thrive in a novel environment. The time course analyses showed that it takes at least 2 weeks for the MGE cells to acquire a neuronal (NeuN+) phenotype and to respond to a peripheral stimulus (i.e., express Fos). This time point corresponds remarkably well with the time where we first recorded a significant difference in the mechanical thresholds between control and MGE transplanted groups. This tight temporal correlation between integration of the transplanted cells and reduction of the mechanical allodynia indicates that integration is essential for the recovery. Interestingly, although we recorded a reduction of mechanical allodynia only in animals in which MGE cells were detected, there was no correlation between the number of surviving MGE cells and their anti-allodynic effect, i.e. animals with the highest number of MGE cells did not always have the greatest recovery of mechanical threshold. This finding suggests that there may be a threshold above which the number of transplanted MGE cells may be less relevant to achieve a functional improvement.
Importantly, despite the fact that systemic or direct spinal administration of GABA agonists is antinociceptive in various inflammatory pain models, including the formalin test (Knabl et al., 2008; Vit et al., 2009), transplantation of GABAergic precursor neurons did not reduce pain behaviors induced by hindpaw injection of formalin. This differential effect of MGE transplantation on nerve vs tissue injury-induced pain suggests that the transplants recapitulate the GABAergic circuits that were altered by nerve injury. In other words, the transplants are disease, rather than symptom modifying.
Recent studies reported that nerve injury-induced activation of microglia can lead to a BDNF-mediated shift in the chloride gradient of projection neurons in lamina I (and likely in deep dorsal horn), such that GABAergic inputs now become excitatory (Coull et al., 2003; De Koninck, 2007; Price et al., 2009). However, our findings provide evidence that enhancing GABAergic function by transplantation is clearly anti-, not pronociceptive in the setting of nerve injury. Thus, any changes that result in a GABAergic excitatory action secondary to changes in chloride gradients (which likely occur only in a subset of neurons) can clearly be overcome by the transplant. Again, this points to the transplants reestablishing normal GABAergic circuitry, rather than serving as a generalized source of GABA, as would occur with a pump.
Of course, we cannot be certain that the antinociceptive effect of the transplants is GABA-mediated. However, because almost 75% of MGE cells differentiated into GABAergic neurons, it is likely that this is the case. The fact that the MGE transplants normalized GAD65 mRNA levels, which decrease after peripheral nerve injury, and increased GAD67 mRNA levels, is consistent with our proposal that the therapeutic effect of MGE transplants is GABA-mediated. It is, however, also possible that release of neurotransmitters/neuromodulators other than GABA contribute to the observed behaviors. For example, the inhibitory neurotransmitter glycine, which co-occurs in some spinal cord (but not cortical) GABAergic neurons (Mackie et al., 2003; Todd et al., 1996), could provide a significant source of inhibition. As the transplanted cells appear to retain their cortical make-up (e.g. they continue to express somatostatin, which is not found in spinal cord GABAergic interneurons), we favor the hypothesis that the inhibition derives from GABAergic, rather than glycinergic control.
As previous studies found that pharmacological treatment with GABAergic agonists can produce long-term amelioration of nerve-injury induced pain conditions (Knabl et al., 2009) in animals as well as humans, it will be of interest to follow the MGE-transplanted animals for longer periods of time. Such studies will determine whether there is further improvement or deterioration/tolerance, whether some animals show delayed anti-allodynic effects and whether animals eventually develop an analgesic phenotype (i.e. have mechanical thresholds greater than baseline).
Finally, it is of interest to ask whether transplants such as these might have clinical utility. Unquestionably, there are many neuropathic pain conditions where the nerve damage is limited and the pain presumably arises from pathophysiological changes (including GABAergic dysfunction) in localized regions of the cord (as in complex regional pain syndrome and even phantom limb pain) or in the trigeminal nucleus caudalis (as in trigeminal neuralgia and other facial pain conditions). On the other hand, in individuals with diabetic neuropathy or chemotherapy-induced polyneuropathy, the nerve damage is likely widespread. Nevertheless, even in the majority of these individuals the most debilitating pains occur in the extremities (hands and feet). Thus, a focal (lumbar or cervical enlargement transplant) that can overcome a GABAergic circuit abnormality may also be beneficial. A great advantage of this approach, of course, is that the adverse and typically dose-limiting side effects associated with systemic drug administration can be avoided.
EXPERIMENTAL PROCEDURES
Mouse lines
All experiments were reviewed and approved by the Institutional Care and Animal Use Committee at the University of California San Francisco. MGE cells were dissected from transgenic mice that express GFP under the control of the GAD67 promoter [Gad1tm1.1Tama ; (Tamamaki et al., 2003)]. All transplants were performed on male mice (6–8 weeks old) with the same genetic background as the MGE donors (CD1xC57BL6/J). The ZW and ZWX mice were described previously (Braz and Basbaum, 2009; Braz et al., 2002). To generate double transgenic ZWX-NPY mice, we crossed the ZWX mouse with mice that express Cre recombinase in NPY expressing neurons ((DeFalco et al., 2001); gift of Dr. Jeffrey Friedman). To generate Per-ZW mice, we crossed the ZW mice with Peripherin-Cre mice (Jackson; (Zhou et al., 2002)).
Sciatic nerve injury
To produce mechanical hypersensitivity in a model that mimics a neuropathic pain condition, we used the spared nerve injury (SNI) model as described previously (Shields et al., 2003). In a different series of transplanted mice, we induced expression of the WGA tracer in sensory neurons of ZWX-NPY mice as described previously (Braz et al., 2009), 1 month after transplantation.
Transplantation of medial ganglionic eminence (MGE) cells
The methods used to transplant MGE cells have been described previously (Alvarez-Dolado et al., 2006). For transplantation, 6 to 8 week old mice (naïve or one week after SNI) were anesthetized by an intraperitoneal injection of ketamine (60 mg/kg)/xylazine (8 mg/kg). We then made a dorsal hemilaminectomy at the level of the lumbar enlargement to expose 2 segments (~1.5–2 mm) of lumbar spinal cord, after which the dura mater was incised and reflected. A cell suspension containing 5×104 MGE cells was loaded into a glass micropipette (prefilled with mineral oil). The micropippete was connected to a microinjector mounted on a stereotactic apparatus. The cell suspension injections were targeted to the dorsal horn, ipsilateral to the nerve injury. The control groups were injected with an equivalent volume of DMEM medium. The wound was closed and the animals were allowed to recover before they were returned to their home cages. Animals were killed at different times post-transplantation (from 1 to 5 weeks). Importantly, none of the transplanted animals exhibited signs of motor impairment. Furthermore, mice in both groups walked on a rotating rod for the 90 min observation period.
For some anatomical studies, naïve mice were transplanted with MGE cells that were genetically modified so as to express the WGA tracer. In these experiments, freshly dissociated MGE cells were incubated with a Lenti-WGAmCh vector (see below: multiplicity of infection of 2; ~45 min to 1h, 37°C). After several washes, the cells were pelleted, resuspended in DMEM and kept on ice until transplantation.
Antibodies
Rabbit anti-WGA (1:50,000, Sigma), mouse anti-NF200 (1:10,000, Sigma), rabbit anti-GFP (1:2000, Molecular Probe), chicken anti-GFP (1:2000, Abcam), rabbit anti-PRV (1:20,000, gift from Dr. Lynn Enquist), mouse anti-PV (1:2000, Sigma), rabbit anti-Fos (1:5000, Oncogene), rabbit anti-GFAP (1:20,000, DAKO), rabbit anti-Iba1 (1:1000, Wako), rabbit anti-dsRed (1:500, Clontech), rat anti-SST (1:400, Millipore), mouse anti-GABA (1:4000, Sigma), mouse anti-NPY (1:2000, gift from Dr. J.M. Allen, London, UK), mouse anti-NeuN (1:2000, Chemicon).
Immunohistochemistry
Immunohistochemistry was performed as previously described (Braz et al., 2008). Sections were viewed with a Nikon Eclipse fluorescence microscope and images were collected with a Zeiss camera (Axiocam). High resolution confocal images taken on a Zeiss confocal confirmed that we are examining intracellular label (0.8mm optical sections). Brightness and contrast were adjusted using Adobe Photoshop, v. 6.0 (San Jose, CA).
Cell counts and quantification
Labeled cell bodies were counted from digitized images. The percentage of surviving MGE cells was determined by counting all GFP+ cell bodies in 10 spinal cord sections (separated by 100 µm). The average number of GFP+ cells per section was then extrapolated to the total number of spinal cord sections that contained GFP+ cells, using the formula: Total GFP = A × B/2 (where A is the average number of GFP+ cells per section and B the total number of spinal cord sections containing GFP+ cells; given the thickness of the spinal cord sections and the size of the MGE cells, we only included every other section so that cells were not counted twice). For example, if in one transplanted animal, the average number of GFP+ cells per section was 15 and we detected GFP+ cells over 100 serial spinal cord sections, then the total number of GFP+ cells per animal would be 15 × (100/2) = 750. The percentage of cell survival was then estimated as 100 × (totalGFP)/(number of transplanted cells). Five animals per group were counted. We estimate that 1 month after transplantation, naïve animals had on average 22 GFP+ cells per spinal cord section, whereas SNI transplanted animals had only 12.
The percentage of transplanted GFP+ MGE cells expressing a second marker (NeuN, Iba1, GFAP, Fos, WGA, dsRed, GABA, PV, SST, or NPY) after transplantation was calculated from 10 coronal spinal cord sections (separated by 100 µm). At least 100 GFP+ MGE cells were analyzed for each marker, in each animal (n=3). Spinal cords were analyzed at 1 and 2 weeks after transplantation for the NeuN, Iba1 and GFAP markers and at 1 month after transplantation for all other markers. The percentage of double-labeled neurons (marker+ and GFP+) was calculated by dividing the number of double-labeled neurons by the number of single GFP-labeled neuron × 100. Values are given as mean ± standard deviation (STD).
Quantitative PCR
Mice were transplanted with medium (n=6) or MGE cells (n=5) 1 week after SNI, and killed 1 week after transplantation (i.e. 2 weeks after the nerve injury). Naïve (uninjured) mice (n=3) were also used as controls. The lumbar spinal cord that contained the transplant was rapidly dissected and a comparable region was dissected from naïve animals after which the cord was divided into ipsilateral and contralateral halves (containing both dorsal horn ventral tissue). We used the RNeasy Minikit from Qiagen to extract mRNA after which 200 ng of purified mRNA was reverse-transcribed into cDNA using Superscript II (Invitrogen). The mRNA levels for GAD65, GAD67 and β-actin were quantified with a Realplex2 real-time PCR system (Eppendorf) using SYBR Green PCR Master Mix (Applied Biosystems, Warrington, UK). Ratios of GAD65 and GDA67 to β-actin mRNA were compared and analyzed by a two-way ANOVA followed by a Bonferroni post hoc test. Asterisks (*) indicate statistically significant differences between groups, with * = p < 0.05 and ** = p < 0.01.
Behavioral analyses
Mechanical sensitivity was assessed by placing animals on an elevated wire mesh grid and stimulating the hindpaw with von Frey hairs. We used the up-down paradigm (Chaplan et al., 1994) to define threshold. Animals were tested 3 times, once every other day before surgery to determine baseline threshold, and once 2 days after surgery, to assess the magnitude of the mechanical allodynia. Only animals that displayed at least a 50% drop of the mechanical withdrawal threshold were included in the MGE transplantation (transplanted group) or the medium injection (control group) groups. Behavioral testing took place on days 7, 14, 21 and 28 after MGE/medium injections. For the behavioral tests, the investigator was blind to treatment (cell medium or MGE injection). Prior to analyzing the behavioral results, MGE transplanted animals were killed, and then the spinal cord was immunostained for the presence of GFP+ cells in the spinal cord. Only successfully transplanted animals (defined as containing at least 1 GFP+ cell per section) were included in the MGE transplanted group and their behavior subsequently analyzed. Importantly, the investigator performing this anatomical analysis was not the investigator who performed the behavior analysis and thus was blind to the behavior results. Transplanted animals from which no GFP-immunoreactive cells were detected were included in a “failed transplant” group. As the mechanical thresholds of the “failed transplant” and the medium-injected control groups were not significantly different, results from the “failed transplant” animals were not included in the statistical analysis.
In another set of experiments, we injected 10 µl of a 1% formalin solution (Sigma, St. Louis) into the hindpaw of medium or MGE cell transplanted mice, ipsilateral to the transplanted side. We scored the mice for the total time spent flinching or licking the injected hindpaw (in 5 min bins). The behavioral scores were made by an experimenter blinded to treatment group. Only after the animals were defined as belonging to the MGE transplant, “failed transplant” or medium transplant group were the behavioral results analyzed.
Statistical analyses
Behavioral data are expressed as mean +/− SEM (standard error of the mean), where n represents the number of mice tested. Raw data of the mechanical withdrawal thresholds obtained in the course of the study were analyzed by a two-way ANOVA followed by a Tukey post hoc test. Asterisks (*) indicate statistically significant differences between groups, with * = p < 0.05; ** = p < 0.01 and *** = p < 0.001.
Highlights.
Transplanted embryonic cortical inhibitory precursors thrive in adult spinal cord
Transplanted cells differentiate into GABAergic interneurons
Transplanted GABAergic cells make functional connections in the spinal cord
Transplanted GABAergic cells reverse nerve injury persistent pain
Supplementary Material
ACKNOWLEDGMENTS
Supported by the NIH (NS14627) and fellowships to RSN from the International Association for the Study of Pain, funded by the Scan|Design Foundation by INGER and JENS BRUUN and the Canadian Institutes of Health Research. The authors have a patent pending on the treatment outlined in this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Alvarez-Dolado M, Calcagnotto ME, Karkar KM, Southwell DG, Jones-Davis DM, Estrada RC, Rubenstein JL, Alvarez-Buylla A, Baraban SC. Cortical inhibition modified by embryonic neural precursors grafted into the postnatal brain. J Neurosci. 2006;26:7380–7389. doi: 10.1523/JNEUROSCI.1540-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asiedu M, Ossipov MH, Kaila K, Price TJ. Acetazolamide and midazolam act synergistically to inhibit neuropathic pain. Pain. 2010;148:302–308. doi: 10.1016/j.pain.2009.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraban SC, Southwell DG, Estrada RC, Jones DL, Sebe JY, Alfaro-Cervello C, Garcia-Verdugo JM, Rubenstein JL, Alvarez-Buylla A. Reduction of seizures by transplantation of cortical GABAergic interneuron precursors into Kv1.1 mutant mice. Proc Natl Acad Sci U S A. 2009;106:15472–15477. doi: 10.1073/pnas.0900141106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Basbaum AI. Genetically expressed transneuronal tracer reveals direct and indirect serotonergic descending control circuits. J Comp Neurol. 2008;507:1990–2003. doi: 10.1002/cne.21665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Basbaum AI. Triggering genetically-expressed transneuronal tracers by peripheral axotomy reveals convergent and segregated sensory neuron-spinal cord connectivity. Neuroscience. 2009;163:1220–1232. doi: 10.1016/j.neuroscience.2009.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Basbaum AI. Differential ATF3 expression in dorsal root ganglion neurons reveals the profile of primary afferents engaged by diverse noxious chemical stimuli. Pain. 2010;150:290–301. doi: 10.1016/j.pain.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Enquist LW, Basbaum AI. Inputs to serotonergic neurons revealed by conditional viral transneuronal tracing. J Comp Neurol. 2009;514:145–160. doi: 10.1002/cne.22003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel "pain" pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. doi: 10.1016/j.neuron.2005.08.015. [DOI] [PubMed] [Google Scholar]
- Braz JM, Rico B, Basbaum AI. Transneuronal tracing of diverse CNS circuits by Cre-mediated induction of wheat germ agglutinin in transgenic mice. Proc Natl Acad Sci U S A. 2002;99:15148–15153. doi: 10.1073/pnas.222546999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calcagnotto ME, Zipancic I, Piquer-Gil M, Mello LE, Alvarez-Dolado M. Grafting of GABAergic precursors rescues deficits in hippocampal inhibition. Epilepsia. 2010;51(Suppl 3):66–70. doi: 10.1111/j.1528-1167.2010.02613.x. [DOI] [PubMed] [Google Scholar]
- Castro-Lopes JM, Tavares I, Coimbra A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 1993;620:287–291. doi: 10.1016/0006-8993(93)90167-l. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Coull JA, Boudreau D, Bachand K, Prescott SA, Nault F, Sik A, De Koninck P, De Koninck Y. Trans-synaptic shift in anion gradient in spinal lamina I neurons as a mechanism of neuropathic pain. Nature. 2003;424:938–942. doi: 10.1038/nature01868. [DOI] [PubMed] [Google Scholar]
- De Koninck Y. Altered chloride homeostasis in neurological disorders: a new target. Curr Opin Pharmacol. 2007;7:93–99. doi: 10.1016/j.coph.2006.11.005. [DOI] [PubMed] [Google Scholar]
- DeFalco J, Tomishima M, Liu H, Zhao C, Cai X, Marth JD, Enquist L, Friedman JM. Virus-assisted mapping of neural inputs to a feeding center in the hypothalamus. Science. 2001;291:2608–2613. doi: 10.1126/science.1056602. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Plunkett JA, Karmally S, Martinez MA, Montanez K. Changes in GAD− and GABA− immunoreactivity in the spinal dorsal horn after peripheral nerve injury and promotion of recovery by lumbar transplant of immortalized serotonergic precursors. J Chem Neuroanat. 1998;16:57–72. doi: 10.1016/s0891-0618(98)00062-3. [DOI] [PubMed] [Google Scholar]
- Eaton MJ, Plunkett JA, Martinez MA, Lopez T, Karmally S, Cejas P, Whittemore SR. Transplants of neuronal cells bioengineered to synthesize GABA alleviate chronic neuropathic pain. Cell Transplant. 1999;8:87–101. doi: 10.1177/096368979900800102. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Tokunaga A, Kondo E, Miki K, Tachibana T, Noguchi K. Change in mRNAs for neuropeptides and the GABA(A) receptor in dorsal root ganglion neurons in a rat experimental neuropathic pain model. Pain. 1998;78:13–26. doi: 10.1016/S0304-3959(98)00111-0. [DOI] [PubMed] [Google Scholar]
- Hao S, Mata M, Wolfe D, Huang S, Glorioso JC, Fink DJ. Gene transfer of glutamic acid decarboxylase reduces neuropathic pain. Ann Neurol. 2005;57:914–918. doi: 10.1002/ana.20483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossaini M, Duraku LS, Sarac C, Jongen JL, Holstege JC. Differential distribution of activated spinal neurons containing glycine and/or GABA and expressing c-fos in acute and chronic pain models. Pain. 2010;151:356–365. doi: 10.1016/j.pain.2010.07.023. [DOI] [PubMed] [Google Scholar]
- Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- Jain M, Armstrong RJ, Tyers P, Barker RA, Rosser AE. GABAergic immunoreactivity is predominant in neurons derived from expanded human neural precursor cells in vitro. Exp Neurol. 2003;182:113–123. doi: 10.1016/s0014-4886(03)00055-4. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Burkey AR, Card JP, Basbaum AI. Transneuronal labeling of a nociceptive pathway, the spino-(trigemino-)parabrachio-amygdaloid, in the rat. J Neurosci. 1997;17:3751–3765. doi: 10.1523/JNEUROSCI.17-10-03751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knabl J, Witschi R, Hosl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, et al. Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature. 2008;451:330–334. doi: 10.1038/nature06493. [DOI] [PubMed] [Google Scholar]
- Knabl J, Zeilhofer UB, Crestani F, Rudolph U, Zeilhofer HU. Genuine antihyperalgesia by systemic diazepam revealed by experiments in GABAA receptor point-mutated mice. Pain. 2009;141:233–238. doi: 10.1016/j.pain.2008.10.015. [DOI] [PubMed] [Google Scholar]
- Kohno T, Moore KA, Baba H, Woolf CJ. Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J Physiol. 2003;548:131–138. doi: 10.1113/jphysiol.2002.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lever I, Cunningham J, Grist J, Yip PK, Malcangio M. Release of BDNF and GABA in the dorsal horn of neuropathic rats. Eur J Neurosci. 2003;18:1169–1174. doi: 10.1046/j.1460-9568.2003.02848.x. [DOI] [PubMed] [Google Scholar]
- Liu J, Wolfe D, Hao S, Huang S, Glorioso JC, Mata M, Fink DJ. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther. 2004;10:57–66. doi: 10.1016/j.ymthe.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Mackie M, Hughes DI, Maxwell DJ, Tillakaratne NJ, Todd AJ. Distribution and colocalisation of glutamate decarboxylase isoforms in the rat spinal cord. Neuroscience. 2003;119:461–472. doi: 10.1016/s0306-4522(03)00174-x. [DOI] [PubMed] [Google Scholar]
- Martinez-Cerdeno V, Noctor SC, Espinosa A, Ariza J, Parker P, Orasji S, Daadi MM, Bankiewicz K, Alvarez-Buylla A, Kriegstein AR. Embryonic MGE precursor cells grafted into adult rat striatum integrate and ameliorate motor symptoms in 6-OHDA-lesioned rats. Cell Stem Cell. 2010;6:238–250. doi: 10.1016/j.stem.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhida K, Mendez I, McLeod M, Kobayashi N, Haughn C, Milne B, Baghbaderani B, Sen A, Behie LA, Hong M. Spinal GABAergic transplants attenuate mechanical allodynia in a rat model of neuropathic pain. Stem Cells. 2007;25:2874–2885. doi: 10.1634/stemcells.2007-0326. [DOI] [PubMed] [Google Scholar]
- Munro G, Ahring PK, Mirza NR. Developing analgesics by enhancing spinal inhibition after injury: GABAA receptor subtypes as novel targets. Trends Pharmacol Sci. 2009;30:453–459. doi: 10.1016/j.tips.2009.06.004. [DOI] [PubMed] [Google Scholar]
- Neumann S, Bradke F, Tessier-Lavigne M, Basbaum AI. Regeneration of sensory axons within the injured spinal cord induced by intraganglionic cAMP elevation. Neuron. 2002;34:885–893. doi: 10.1016/s0896-6273(02)00702-x. [DOI] [PubMed] [Google Scholar]
- Neumann S, Braz JM, Skinner K, Llewellyn-Smith IJ, Basbaum AI. Innocuous, not noxious, input activates PKCgamma interneurons of the spinal dorsal horn via myelinated afferent fibers. J Neurosci. 2008;28:7936–7944. doi: 10.1523/JNEUROSCI.1259-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, De Leon M, Nahin RL, Senba E, Ruda MA. Quantification of axotomy-induced alteration of neuropeptide mRNAs in dorsal root ganglion neurons with special reference to neuropeptide Y mRNA and the effects of neonatal capsaicin treatment. J Neurosci Res. 1993;35:54–66. doi: 10.1002/jnr.490350108. [DOI] [PubMed] [Google Scholar]
- Polgar E, Gray S, Riddell JS, Todd AJ. Lack of evidence for significant neuronal loss in laminae I–III of the spinal dorsal horn of the rat in the chronic constriction injury model. Pain. 2004;111:144–150. doi: 10.1016/j.pain.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Polgar E, Todd AJ. Tactile allodynia can occur in the spared nerve injury model in the rat without selective loss of GABA or GABA(A) receptors from synapses in laminae I–II of the ipsilateral spinal dorsal horn. Neuroscience. 2008;156:193–202. doi: 10.1016/j.neuroscience.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Cervero F, Gold MS, Hammond DL, Prescott SA. Chloride regulation in the pain pathway. Brain Res Rev. 2009;60:149–170. doi: 10.1016/j.brainresrev.2008.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagen J, Wang H, Pappas GD. Adrenal medullary implants in the rat spinal cord reduce nociception in a chronic pain model. Pain. 1990;42:69–79. doi: 10.1016/0304-3959(90)91093-X. [DOI] [PubMed] [Google Scholar]
- Scholz J, Broom DC, Youn DH, Mills CD, Kohno T, Suter MR, Moore KA, Decosterd I, Coggeshall RE, Woolf CJ. Blocking caspase activity prevents transsynaptic neuronal apoptosis and the loss of inhibition in lamina II of the dorsal horn after peripheral nerve injury. J Neurosci. 2005;25:7317–7323. doi: 10.1523/JNEUROSCI.1526-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Cavanaugh DJ, Lee H, Anderson DJ, Basbaum AI. Pain behavior in the formalin test persists after ablation of the great majority of C-fiber nociceptors. Pain. 2010;151:422–429. doi: 10.1016/j.pain.2010.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Eckert WA, 3rd, Basbaum AI. Spared nerve injury model of neuropathic pain in the mouse: a behavioral and anatomic analysis. J Pain. 2003;4:465–470. doi: 10.1067/s1526-5900(03)00781-8. [DOI] [PubMed] [Google Scholar]
- Southwell DG, Froemke RC, Alvarez-Buylla A, Stryker MP, Gandhi SP. Cortical plasticity induced by inhibitory neuron transplantation. Science. 2010;327:1145–1148. doi: 10.1126/science.1183962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T, Bennett GJ, Kajander KC. Transsynaptic degeneration in the superficial dorsal horn after sciatic nerve injury: effects of a chronic constriction injury, transection, and strychnine. Pain. 1990;42:205–213. doi: 10.1016/0304-3959(90)91164-E. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Yanagawa Y, Tomioka R, Miyazaki J, Obata K, Kaneko T. Green fluorescent protein expression and colocalization with calretinin, parvalbumin, and somatostatin in the GAD67-GFP knock-in mouse. J Comp Neurol. 2003;467:60–79. doi: 10.1002/cne.10905. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Watt C, Spike RC, Sieghart W. Colocalization of GABA, glycine, and their receptors at synapses in the rat spinal cord. J Neurosci. 1996;16:974–982. doi: 10.1523/JNEUROSCI.16-03-00974.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaysse L, Sol JC, Lazorthes Y, Courtade-Saidi M, Eaton MJ, Jozan S. GABAergic pathway in a rat model of chronic neuropathic pain: modulation after intrathecal transplantation of a human neuronal cell line. Neurosci Res. 2011;69:111–120. doi: 10.1016/j.neures.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Vit JP, Ohara PT, Sundberg C, Rubi B, Maechler P, Liu C, Puntel M, Lowenstein P, Castro M, Jasmin L. Adenovector GAD65 gene delivery into the rat trigeminal ganglion produces orofacial analgesia. Mol Pain. 2009;5:42. doi: 10.1186/1744-8069-5-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Winnie AP, Pappas GD, Das Gupta TK, Wang H, Ortega JD, Sagen J. Subarachnoid adrenal medullary transplants for terminal cancer pain. A report of preliminary studies. Anesthesiology. 1993;79:644–653. doi: 10.1097/00000542-199310000-00004. [DOI] [PubMed] [Google Scholar]
- Yasaka T, Tiong SY, Hughes DI, Riddell JS, Todd AJ. Populations of inhibitory and excitatory interneurons in lamina II of the adult rat spinal dorsal horn revealed by a combined electrophysiological and anatomical approach. Pain. 2010;151:475–488. doi: 10.1016/j.pain.2010.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Hao JX, Xu XJ, Saydoff J, Haegerstrand A, Hokfelt T, Wiesenfeld-Hallin Z. Long-term alleviation of allodynia-like behaviors by intrathecal implantation of bovine chromaffin cells in rats with spinal cord injury. Pain. 1998;74:115–122. doi: 10.1016/s0304-3959(97)00204-2. [DOI] [PubMed] [Google Scholar]
- Zhou L, Nepote V, Rowley DL, Levacher B, Zvara A, Santha M, Mi QS, Simonneau M, Donovan DM. Murine peripherin gene sequences direct Cre recombinase expression to peripheral neurons in transgenic mice. FEBS Lett. 2002;523:68–72. doi: 10.1016/s0014-5793(02)02936-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.