Summary
Neuronal excitation can be substantially modulated by alterations in metabolism, as evident from the anticonvulsant effect of diets that reduce glucose utilization and promote ketone body metabolism. We provide genetic evidence that BAD, a protein with dual functions in apoptosis and glucose metabolism, imparts reciprocal effects on metabolism of glucose and ketone bodies in brain cells. These effects involve phospho-regulation of BAD and are independent of its apoptotic function. BAD modifications that reduce glucose metabolism produce a marked increase in the activity of metabolically sensitive KATP channels in neurons, as well as resistance to behavioral and electrographic seizures in vivo. Seizure resistance is reversed by genetic ablation of the KATP channel, implicating the BAD-KATP axis in metabolic control of neuronal excitation and seizure responses.
INTRODUCTION
Neuronal electrical activity is tightly integrated with mitochondrial nutrient and energy metabolism in the brain. In addition to buffering intracellular calcium and generating ATP for proper maintenance of ion homeostasis, mitochondria can influence neuronal function by changing redox balance and availability of intermediary metabolites for biosynthetic processes (MacAskill et al., 2010; Nicholls, 2009). While glucose is the predominant mitochondrial fuel utilized by the brain, neural cells can metabolize alternative carbon substrates such as ketone bodies under conditions of glucose limitation or dietary restrictions (Zielke et al., 2009). The capacity of mitochondria to process alternate energy substrates such as carbohydrates and ketone bodies may influence neuronal excitability. For example, the ketogenic diet (KD), which reduces glucose metabolism and promotes the breakdown of fatty acids to generate ketone bodies, has shown efficacy in many cases of pharmacoresistant epilepsy (Hartman et al., 2007; Neal et al., 2009; Thiele, 2003)
The potent effect of increased ketone body metabolism on epilepsy in humans points to a link between mitochondrial fuel utilization and neuronal excitability. However, the molecular underpinnings of this link are not fully understood. Numerous mechanisms have been proposed (Schwartzkroin, 1999), including alterations in gene expression (Garriga-Canut et al., 2006) and changes in the levels of metabolic products and byproducts, such as ATP (DeVivo et al., 1978), amino acids (Dahlin et al., 2005; Yudkoff et al., 2001), reactive oxygen species, and glutathione (Jarrett et al., 2008). Moreover, ketone bodies can alter the open probability of the metabolically responsive ATP-sensitive potassium (KATP) channels (Ma et al., 2007; Schwartzkroin, 1999; Tanner et al., 2011). This can lead to reduced firing of neurons and reduced excitability during seizures. Ketone bodies have also been reported to suppress the vesicular release of glutamate, suggesting a link between metabolism and excitatory synaptic transmission (Juge et al., 2010). Other proposed mechanisms for the anticonvulsant effect of KD include elevation of inhibitory neurotransmitter γ-aminobutyric acid (GABA) levels (Wang et al., 2003; Yudkoff et al., 2001), acidosis-induced changes in acid-sensing ion channel 1a (ASIC1a; Ziemann et al., 2008), and changes in the activity of A1 purinergic receptors (Masino et al., 2011).
While these mechanisms have provided valuable insights into metabolic regulation of seizure responses, progress in dissecting the link between metabolism and seizure sensitivity has been difficult due to the complex systemic effects of dietary alterations and the relatively modest effect of KD in rodent models of epilepsy. Identification of cell-intrinsic mechanisms and molecular modulators that can simultaneously attenuate glucose metabolism and enhance ketone body consumption without any dietary manipulation may provide a useful molecular handle on understanding the metabolic control of seizure sensitivity.
BAD (BCL-2-associated Agonist of Cell Death) is a member of the BCL-2 family of cell death/survival proteins (Chipuk et al., 2010; Danial and Korsmeyer, 2004; Youle and Strasser, 2008). Separate from its well established role in apoptosis, BAD modulates glucose metabolism in multiple cell types, including hepatocytes, islet β-cells and fibroblasts (reviewed in Danial, 2008). BAD’s dual modalities in apoptosis and metabolism are mediated through a phospho-regulatory mechanism that modifies serine 155 (aa enumeration based on the murine sequence of BAD) located within an alpha helical segment known as the BCL-2 homology (BH)-3 domain (Danial et al., 2008; Datta et al., 2000). In hepatocytes and β-cells, Serine 155 phosphorylation is required for mitochondrial metabolism of glucose (reviewed in Danial, 2008). In the presence of apoptotic signals, including irreversible cellular damage, dephosphorylated BAD engages the mitochondrial apoptosis machinery through a BH3 domain-dependent mechanism that sensitizes cells to apoptosis.
Reduced glucose metabolism associated with BAD modification is reminiscent of reduced glycolysis and changes in carbon substrate utilization in response to low carbohydrate diet that promote ketone body metabolism. This prompted us to investigate potential BAD-dependent changes in seizure responses. Using a combination of genetic models, mitochondrial respirometry, electrophysiology, as well as electrographic and behavioral studies, we have examined the role of BAD in regulating the preferred carbon substrate utilized by neural cell types and its relevance to neuronal excitability and seizure sensitivity.
RESULTS
Blunted Mitochondrial Metabolism of Glucose in Primary Bad−/− and Bad S155A Neurons and Astrocytes
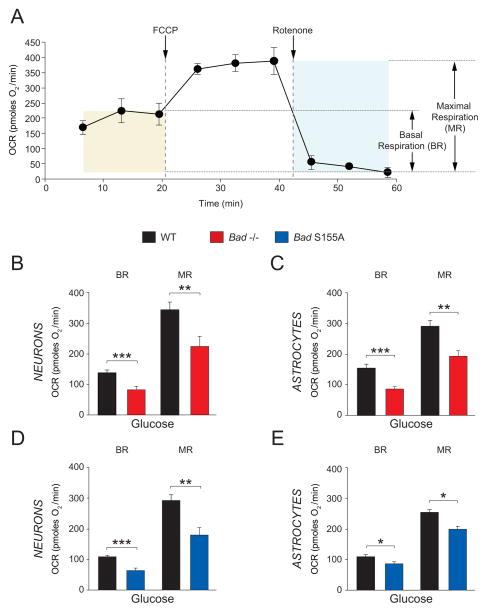
Our previous findings that BAD is required for mitochondrial utilization of glucose in hepatocytes and islet β-cells (Danial et al., 2003; Danial et al., 2008) prompted us to examine how BAD modification may affect glucose metabolism in the brain, where glucose is the predominant carbon and energy substrate. We assessed mitochondrial metabolism of glucose in primary cultures of cortical neurons and astrocytes (Figure S1) by real time measurement of mitochondrial oxygen consumption rates (OCR). We focused predominantly on two respiratory parameters; glucose-associated basal (steady state) and maximal respiratory rates (BR and MR, Figure 1A). Mitochondrial MR is an important indicator of a cell’s bioenergetic fitness for accommodating any potential rise in metabolic demand (Brand and Nicholls, 2011; Fern, 2003). MR is defined by the fraction of maximal achievable rate of oxygen consumption that is sensitive to the inhibition of mitochondrial respiratory chain activity. To measure MR, neural cultures were first treated with the proton ionophore FCCP to induce maximal OCR prior to treatment with the mitochondrial respiratory chain inhibitor rotenone, and the difference between respiratory rates in response to FCCP and rotenone was measured (Brand and Nicholls, 2011) (Figure 1A). When supplied with glucose, neurons and astrocytes derived from Bad−/− mice displayed diminished basal OCR and MR compared with wild-type counterparts (Figures 1B and C), providing genetic evidence for BAD’s relevance in mitochondrial handling of glucose in these cell types.
Figure 1. Blunted Mitochondrial Metabolism of Glucose in Primary Bad−/− and Bad S155A Neurons and Astrocytes.
(A) Representative trace of oxygen consumption rate (OCR) measured in real time indicating basal and maximal respiration rates (BR and MR, respectively). (B and C) BR and MR in the presence of glucose in primary Bad−/− cortical neurons (B) and astrocytes (C). (D and E) BR and MR in the presence of glucose in primary Bad S155A cortical neurons (D) and astrocytes (E). Data in B through E are presented as mean ± SEM; n=4–6 independent neuron or astrocyte cultures. * p < 0.05; **p < 0.01; *** p < 0.001; wild-type vs. Bad−/−, or Bad S155A; two-tailed Student’s t-test. See also Figure S1.
We have previously shown that phosphorylation of BAD on Ser 155 within its BH3 domain mediates its effect on glucose metabolism (Danial et al., 2008). Consistent with this idea, we found that in primary neurons and astrocytes from mice bearing a non-phosphorylatable knock-in allele of BAD (Bad S155A), glucose-associated BR and MR were significantly blunted, analogous to Bad−/−cells (Figures 1D and E). Importantly, as the Bad-null and S155A alleles have opposite effects on apoptosis (Danial et al., 2008), the comparable diminution of glucose-associated MR in neural cell types derived from both genetic models suggests that modulation of mitochondrial fuel handling stems from metabolic modulation by BAD rather than its effect on apoptosis.
BAD-Dependent Increase in Ketone Body Consumption in Face of Diminished Glucose Oxidation
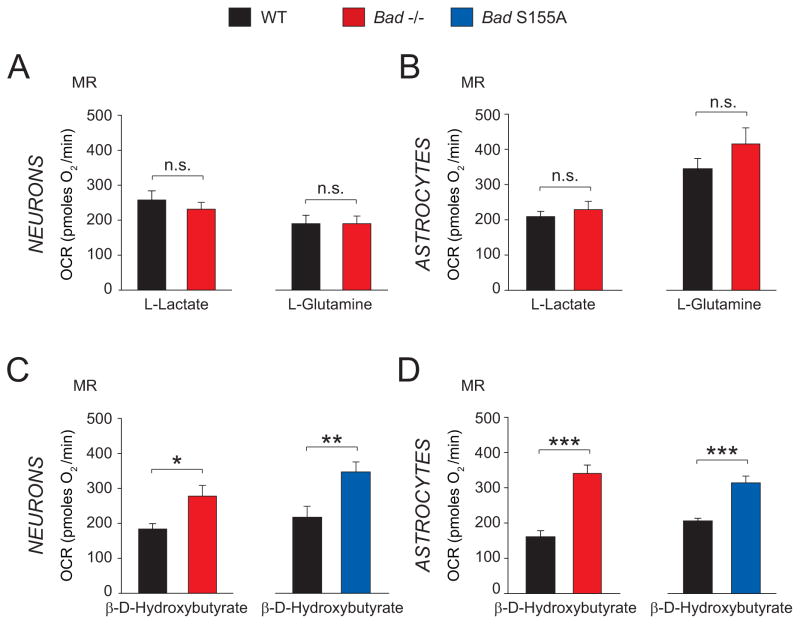
Diminished mitochondrial oxidation of glucose warranted investigation whether BAD modification alters consumption of non-glucose fuels in neurons and astrocytes. To test this possibility, we focused on three main physiologic non-glucose carbon substrates that can be utilized by the brain, namely L-glutamine, L-lactate and the ketone body β-D-hydroxybutyrate (Zielke et al., 2009). We used mitochondrial MR as an indicator of mitochondrial fuel handling in response to each of these non-glucose fuels.
Bad ablation did not alter mitochondrial handling of carbon substrates such as L-lactate and L-glutamine in neurons or astrocytes (Figures 2A and B). On the other hand, mitochondrial utilization of β-D-hydroxybutyrate was significantly higher in Bad−/− cortical neurons and astrocytes compared with wild-type cells (Figures 2C and D), indicating that Bad ablation is associated with a selective switch in fuel preference from glucose to ketone body consumption rather than pleiotropic changes in mitochondrial carbon substrate utilization. In addition, a preferential shift to ketone body consumption was observed in cortical neurons and astrocytes derived from Bad S155A mice (Figures 2C and D), suggesting that BAD phosphorylation on S155 may normally inhibit ketone body utilization. Taken together, these observations are consistent with a BAD-dependent reciprocal programming of mitochondrial glucose versus ketone body consumption that is regulated by the phosphorylation status of its BH3 domain.
Figure 2. Mitochondrial Metabolism of Non-Glucose Fuels in Bad−/− and Bad S155A Neurons and Astrocytes.
(A and B) Mitochondrial maximal respiration (MR) in wild-type and Bad−/− neurons (A) and astrocytes (B) supplied with 5 mM L-glutamine or 5 mM L-lactate. (C and D) MR in the presence of 5 mM β-D-hydroxybutyrate in primary neurons (C) and astrocytes (D) derived from Bad−/− and Bad S155A mice. Data in A through D are presented as mean ± SEM; n=4–6 independent neuron or astrocyte cultures. * p < 0.05; ** p < 0.01; *** p < 0.001; n.s. in A and B, non-significant; wild-type vs. Bad−/− or Bad S155A; two-tailed Student’s t-test
Altered Sensitivity of Bad−/− and Bad S155A Mice to Behavioral Seizures
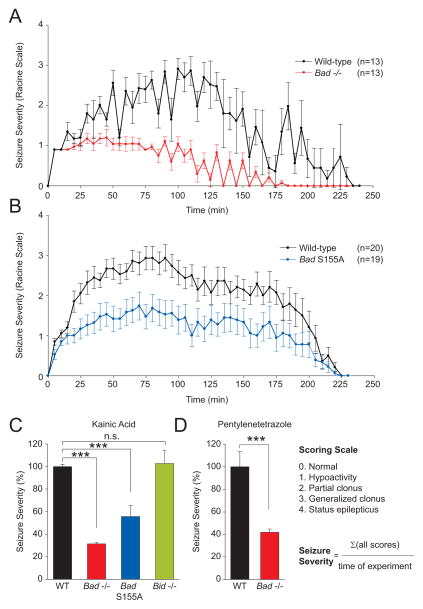
Metabolic manipulations such as a high-fat, low-carbohydrate ketogenic diet (KD) can prevent seizures in many cases of pharmacoresistant human epilepsy, as well as in certain rodent models of epilepsy (Stafstrom and Rho, 2004). The reprogramming of carbon substrate metabolism in Bad−/− and Bad S155A neurons and astrocytes is analogous to ketogenic diet-induced changes in brain metabolism, namely reduced glucose metabolism and elevated ketone body consumption. However, these BAD-dependent changes occur in the absence of any dietary manipulations. This led us to hypothesize that metabolic control by BAD may also influence seizure sensitivity in vivo, similar to the KD (Stafstrom and Rho, 2004). To test this hypothesis, we used the chemical proconvulsant kainic acid (KA), which induces acute seizures by stimulating excitatory glutamate receptors (Ben-Ari et al., 1980). Published studies in mice indicate that KD can delay the onset of KA-induced seizures (Jeong et al., 2011; Noh et al., 2003). The seizure responses in BAD mutant and control mice treated with KA were scored using a modified Racine scale as previously described (Ferraro et al., 1997).
Wild-type mice experienced an acute yet transient series of seizures that peaked on average between 50–120 min after KA administration and then slowly decayed (Figures 3A and B). The seizure diaries for individual mice are shown in Figure S2. The majority of wild-type mice (>80%) underwent status epilepticus with very severe tonic-clonic seizures. In striking contrast, Bad−/− mice did not progress in severity to the extent of wild-type animals and were significantly protected from status epilepticus (Figure 3A). Bad S155A mice were similarly resistant to behavioral seizures in this experimental model (Figure 3B). In addition to raw seizure scores based on the modified Racine scale, we integrated individual scores per mouse over the duration of the experiment in order to better account for seizure severity in mice that died during the experiment (Figure 3C). Seizure severity was significantly diminished in both Bad-null and S155A knock-in mice (Figure 3C). Moreover, seizure resistance in the absence of BAD is not limited to the kainate model. Bad-null mice were also protected against status epilepticus triggered by pentylenetetrazole (PTZ), which antagonizes GABAergic inhibitory receptors (Bough and Eagles, 1999; Ferraro et al., 1999) (Figure 3D).
Figure 3. Resistance of Bad Genetic Models to Kainic Acid- and Pentylenetetrazole-Induced Acute Seizures.
(A and B) Raw seizure scores in 8–10-wk old male Bad−/− (A) and Bad S155A (B) mice compared with wild-type control animals over a 4-hour period after a single i.p. injection of kainic acid (KA) at 30 mg/kg. See also Figure S2. (C) Integrated seizure severity of the above experimental cohorts and Bid−/− mice similarly treated with KA. The scoring scale and the formula used to derive seizure severity are provided. Wild-type, n=42; Bad−/−, n=13; Bad S155A, n=20; Bid−/−; n=9. (D) Integrated seizure severity in Bad−/− and wild-type mice (n=16) subjected to a single s.c. injection of pentylenetetrazole (PTZ) at 80 mg/kg monitored over a 70 minute-period. See also Figures S3 and S4. Data in A through D are presented as mean ± SEM. *** p < 0.001; n.s., non-significant; two-tailed Student’s t-test.
To determine whether seizure resistance in Bad−/− and Bad S155A mice is accompanied by neurobehavioral abnormalities, we conducted a detailed battery of cognitive and motor function tests. These studies did not reveal any significant differences in Bad genetic models compared with control mice (Figure S3). It therefore appears unlikely that these models produce major circuit-level effects that impair normal brain function and might also disrupt seizure mechanisms.
As the Bad-null and S155A alleles have opposite effects on BAD’s apoptotic function (Danial, 2008), the comparable resistance to seizures observed in Bad-null and S155A mice cannot be explained by changes in apoptosis. To further test for the specificity and selectivity of BAD in this paradigm, BID-deficient mice were examined. BID (BH3-Interacting domain Death agonist) is another BH3-only pro-apoptotic member of the BCL-2 family similar to BAD but does not affect glucose metabolism (Danial, 2008). Bid-null mice displayed comparable sensitivity to seizures as that seen in wild-type littermates (Figure 3C). In addition, targeted deletion of another BH3-only pro-apoptotic molecule BIM (BCL2-Interacting Mediator of cell death) in the brain did not confer resistance against acute seizures (Figure S4). Thus, it appears that the seizure resistance phenotype of BAD mutant mice is neither related to BAD’s apoptotic role nor universally shared among other BCL-2 family members.
Changes in the preferential ability to utilize ketone bodies in the absence of BAD and the attendant resistance to acute seizures may derive from local metabolic alterations in the brain and/or from altered metabolism in the liver, which is the body’s main source of ketone body production. Two lines of investigation suggest that seizure protection in the absence of BAD cannot be explained by systemic alterations in ketone body metabolism. First, we have not observed any differences in the serum levels of ketone bodies in these animals (data not shown). Second, liver-specific knock-down of Bad does not produce seizure protection in mice (Figure S5) despite fully mimicking the hepatic phenotype of Bad−/− mice (data not shown). These results are especially relevant as liver is the chief source of ketone bodies for systemic supply to other tissues. Our observations suggest that local metabolic alterations in the brain of Bad−/− animals, rather than systemic changes in ketone body metabolism, most likely contribute to seizure protection in the absence of BAD.
Altered Electrographic Seizures in Bad−/− Mice
Seizures produced by kainic acid, as for several other convulsant treatments in rodents, appear first as hypoactivity and focal “limbic seizures” involving automatisms, facial and forelimb clonus, and rearing; these seizures can progress to generalized tonic-clonic seizures and death (Velíšková, 2005). The former are attributed to forebrain or limbic activity, while the generalized seizures are thought to be mediated by brainstem or midbrain reticular systems (Browning, 1994). In several rodent seizure models that follow this pattern, clinically-useful anticonvulsants such as phenytoin (Browning et al., 1990), levetiracetam (Klitgaard et al., 1998) and topiramate (Haugvicova et al., 2000) have little effect on the focal seizures but disrupt progression to generalized motor seizures.
A similar protection against generalized seizures in the intraperitoneal (i.p.) kainate model was seen in the behavioral experiments on mice with alteration of BAD. Bad−/− mice or Bad S155A knock-in mice rarely exhibited generalized motor seizures (and when they occurred they were very brief), while most control animals had severe generalized seizures, and many control animals died during status epilepticus (Figure 3 and Figure S2).
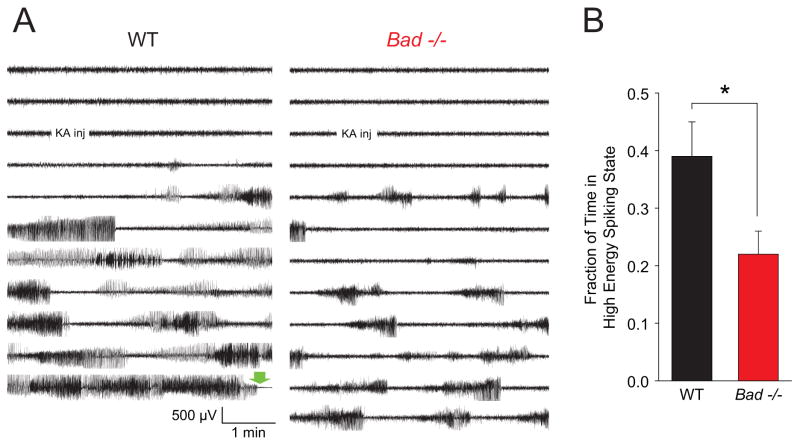
In addition to noting this marked difference in behavioral seizure response, we performed video-EEG analysis of the behavioral and electrographic seizures in cohorts of wild-type and Bad−/− mice subjected to i.p. kainate injection (Figure 4). As expected from the hypothesized deep-brain localization of the generalized motor seizures, the frontal and parietal screw electrodes did not report a clear electrographic correlate to the more severe seizures. They did, however, record high amplitude spiking activity that was usually correlated with the limbic seizures - as the electrographic activity intensified, a typical progression was seen from head movement to rearing with unilateral or bilateral forelimb clonus, which ceased upon the usually sudden cessation of spiking. Overall comparison of electrographic activity was complicated by the death of wild-type animals, often seen ~30 minutes after KA injection in this cohort; we therefore restricted the analysis to the first 30 minutes after injection. For the Bad−/− mice, the fraction of time spent in a spiking state was significantly reduced compared to the wild-type mice (Figure 4B), indicating a reduction in forebrain seizure activity in addition to the marked reduction in generalized seizures and death during status epilepticus.
Figure 4. EEGs of Kainic Acid-Induced Seizure Activity in Wild-Type and Bad−/− Animals.
(A) A single representative EEG channel recording of approximately 1 hour for each genotype (right frontoparietal electrode). A single i.p. injection of kainic acid (30 mg/kg of body weight) was administered at the indicated time. The green block arrow near the end of the wild-type recording indicates the time of death during generalized tonic-clonic status epilepticus. (B) The average fraction of time spent in a high energy spiking period for each genotype during a 30 minute period beginning 3 minutes after injection. EEGs were scored by an investigator blind to the genotype, guided by both the raw EEG traces and a trace of power in the 20–70 Hz band to identify onset and offset of high energy spiking (typically seen as rapid increases or decreases of ≥5 dB in power). Wild-type, n=17; Bad−/−, n=20; mean ± SEM, * p < 0.05 by two-tailed Student’s t-test. In these cohorts, 9 of 17 wild-type and 0 of 20 Bad−/− animals died during status epilepticus.
BAD manipulation increases KATP channel activity
ATP-sensitive K+ (KATP) channels are a well-known link between metabolism and cellular electrical activity. They are octamers comprised of four pore-forming Kir6.2 subunits and four modulatory SUR subunits, and are inhibited by intracellular ATP (Nichols, 2006; Proks and Ashcroft, 2009; Yellen, 2008). These channels are expressed in many brain regions associated with epileptiform activity, such as the hippocampus (Dunn-Meynell et al., 1998; Karschin et al., 1997; Zawar et al., 1999). Additionally, KATP channel activity is increased when ketone bodies are applied to central neurons (Ma et al., 2007; Tanner et al., 2011).
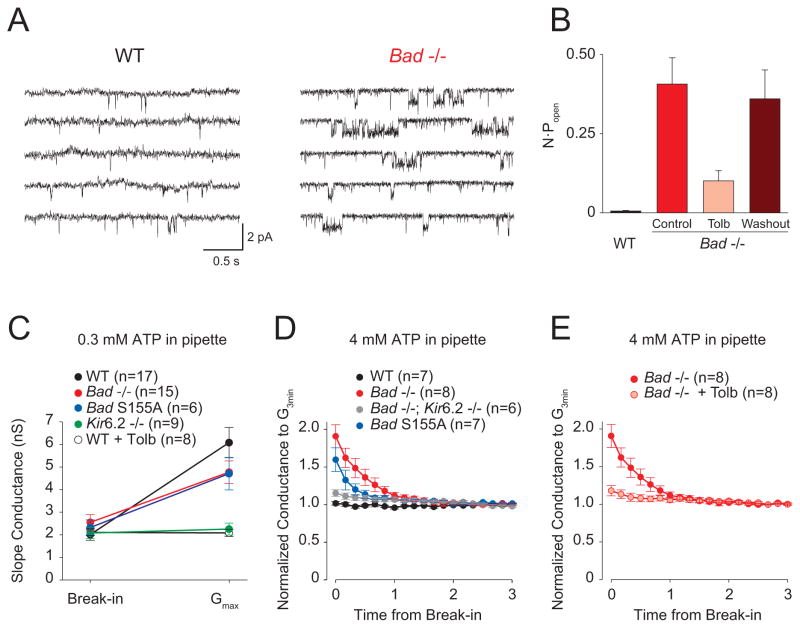
To investigate a possible role of the KATP channel in mediating the seizure resistance of Bad mutant mice, we performed patch clamp recordings in the cell-attached configuration from dentate granule neurons (DGNs) in hippocampal slices, under recording conditions that block activity from most other channels (Tanner et al., 2011). The open probability (Popen) of single KATP channels was substantially increased in BAD-deficient neurons (Figures 5A and B). The highly active single channels measured in Bad−/− neurons were reversibly inhibited in the presence of 200 μM tolbutamide, a KATP channel inhibitor (Figure 5B), thus confirming their identity (Figure 5B). These results indicate that, at the single channel level, the Popen of KATP channels is increased in Bad-null mice.
Figure 5. BAD Manipulations Increase KATP Channel Activity in Dentate Granule Neurons.
(A) Continuous recordings of wild-type (left) or Bad−/− (right) single-channel currents in cell-attached patches with zero applied voltage. Single-channel openings are seen as downward deflections of the current trace. (B) N·Popen values for WT and Bad−/− single channel recordings. For the Bad−/− recordings, the KATP blocker tolbutamide was added (Tolb; 200 μM) and then washed out (Washout). n=6 for each genotype. (C) Maximum activatable KATP conductance determined from the difference between the slope conductance at the time of break-in and after conductance run-up (Gmax), with low ATP (0.3 mM) in the recording pipette, for the indicated genotypes and for WT in the presence of 200 μM tolbutamide (WT + Tolb). (D) “Washdown” of an initially high KATP conductance, with high ATP (4 mM) in the recording pipette, seen in Bad−/− and Bad S155A but not in WT or in Bad−/−; Kir6.2−/−. The time course of slope conductance measured during whole-cell recording was normalized to the value 3 min after break-in. (E) “Washdown” (measured as in D) prevented by preincubation in 200 μM tolbutamide. Data in B through E are presented as mean ± SEM.
We also tested the effect of BAD alteration on the total number of functional channels present in the cell membrane by measuring whole-cell KATP currents in DGNs. To measure the total activatable KATP conductance, we performed whole-cell recordings with reduced ATP (0.3 mM) in the pipette solution; in such experiments, KATP channels are disinhibited as the cellular contents exchange with the pipette. In wild-type, Bad−/−, or Bad S155A neurons, the cellular slope conductance increased to a maximum total activatable (“run-up”) value within 10 minutes (Figure 5C) and the values were very similar for these three genotypes. As expected for a KATP-mediated current, current “run-up” was absent in Kir6.2−/− neurons or in wild-type neurons that were incubated in 200 μM tolbutamide prior to recording (Figure 5C).
To test for a whole-cell correlate of the high resting single channel Popen observed in cell-attached patches, we performed experiments with high ATP (4 mM) in the pipette, with the idea that as the ATP washes into the cell it might inhibit any initially active KATP channels. Indeed, we found that conductance in Bad−/− neurons decreased within the first minute after break-in with high ATP, and then remained constant (Figure 5D). This “washdown” was not seen in wild-type cells (Figure 5D). It was also eliminated in Bad−/− neurons if they were preincubated with tolbutamide (Figure 5E), or in neurons from animals that lacked both BAD and Kir6.2 (Figure 5D), confirming that this initial high conductance was due to KATP channels. Current washdown also occurred in neurons from Bad S155A mice (Figure 5D), showing that it is BAD’s metabolic rather than apoptotic function that is responsible for increasing KATP conductance.
BAD’s Effect on KA-induced Seizures is Dependent on the KATP Channel
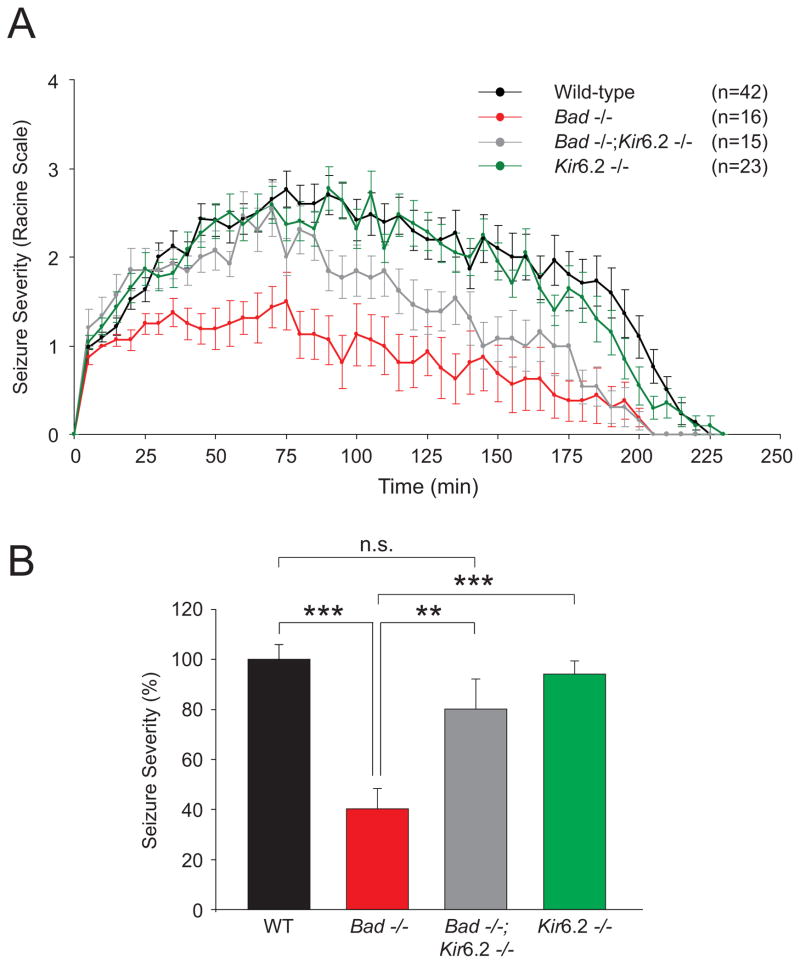
The marked increase in KATP channel open probability in Bad−/− DGNs suggests a link between KATP channel conductance and seizure protection in these mice and predicts that BAD’s effect on seizure sensitivity may be mediated by the KATP channel. To test this prediction, we generated Bad−/−; Kir6.2−/− double mutant mice and tested their sensitivity to KA in parallel with single Bad−/− or Kir6.2−/− mutants. Ablation of the Kir6.2 subunit in the Bad-null genetic background substantially diminished the seizure resistance phenotype of Bad−/− mice (Figures 6A and B). Single deletion of the Kir6.2 subunit did not sensitize mice to acute seizures, arguing against an orthogonal or additive effect on seizures. These findings provide genetic evidence that the KATP channel is required for mediating BAD’s effect on neuronal excitability.
Figure 6. BAD Modulation of Seizure Resistance is Dependent on the KATP Channel.
(A and B) Raw scores (A) and seizure severity (B) in Bad−/− and Kir6.2−/− single mutants and Bad−/−; Kir6.2−/− double mutant mice compared with wild-type mice after a single i.p. injection of kainic acid monitored and analyzed as in Figure 3. Data are presented as mean ± SEM. ** p < 0.01; *** p < 0.001; n.s., non-significant; two-tailed Student’s t-test.
DISCUSSION
Using a combination of genetic models and multiple experimental approaches ranging from mitochondrial respirometry in primary neural cultures and slice electrophysiology to behavioral and electrographic seizure monitoring in vivo, we provide a vertical analysis of BAD’s effect on neural carbon substrate utilization, neuronal excitability, and seizure susceptibility. Our observations suggest that BAD imparts reciprocal effects on glucose and ketone body consumption through a phospho-regulatory mechanism that modifies S155 within its BH3 domain. Specifically, BAD deficiency or interference with its phosphorylation is associated with diminished mitochondrial metabolism of glucose and a concomitant metabolic preference for ketone bodies. An electrophysiologic consequence of this metabolic shift is a marked increase in the open probability of the metabolically sensitive KATP channel. Moreover, genetic studies indicate that this link between BAD-dependent programming of neural metabolism and neuronal excitability extends to reductions in electrographic and behavioral seizures, including the near absence of generalized tonic-clonic seizures. Importantly, BAD-dependent changes in seizure sensitivity are reversed by genetic modification of the Kir6.2 pore-forming subunit of the KATP channel, indicating that the KATP channel is a necessary downstream mediator of BAD’s effect on neuronal excitation and seizure responses.
Several lines of evidence indicate that BAD modulation of sensitivity to acute seizures is distinct from alterations in the apoptotic pathway or a mere change in neuronal populations that might be expected from modification of a pro-apoptotic molecule. We have not found any evidence of neuronal loss in wild-type mice or Bad genetic models within the time course of acute seizures induced by i.p. delivery of KA (data not shown). Importantly, the shared seizure phenotype of Bad-null and Bad S155A alleles that otherwise have opposite effects on BAD’s apoptotic activity is consistent with the predominance of BAD’s non-apoptotic properties in this setting. However, our findings do not argue against a role for apoptosis in epileptogenesis (Engel et al., 2011). Indeed, our experimental system of acute seizures is distinct from a chronic model of seizures induced by hippocampal damage 1–12 days after stereotactic delivery of KA in the amygdala (Engel et al., 2010; Murphy et al., 2010). In this model, loss of certain pro-apoptotic members of the BCL-2 family such as BIM and PUMA is protective against neuronal loss and brain damage associated with status epilepticus. However, ablation of Bim or Puma did not protect against acute seizures immediately after KA administration (Engel et al., 2010; Murphy et al., 2010). These observations are in agreement with our results that tissue-specific deletion of Bim in the brain does not alter the sensitivity to acute seizures (Figure S4).
Our findings are also distinct from previous reports suggesting a role for BAD in regulating synaptic transmission through modified recruitment/activation of proteins with known function in the regulation of the core apoptotic machinery, such as BAX, caspase-3, BCL-XL, and VDAC (Hickman et al., 2008; Jiao and Li, 2011). Based on these studies, Bad-null and Bad S155A non-phosphorylatable mutants are predicted to exert opposite effects on the activity of these proteins. This is different from the shared phenotype of these BAD modifications in neuronal activity we report here. Our results are instead consistent with involvement of BAD-dependent changes in metabolism rather than modified components of the apoptotic machinery.
Metabolic changes similar to those produced by BAD manipulation have been found effective against epileptic seizures, notably in the case of therapeutic diets such as the KD. Reduced carbohydrate diets such as the KD (Hartman et al., 2007; Neal et al., 2009; Thiele, 2003) and the low glycemic index treatment (Pfeifer et al., 2008; Pfeifer and Thiele, 2005) are effective against human epileptic seizures that are refractory to anticonvulsant drugs. These diets mimic fasting by reducing the carbohydrate supply and forcing the breakdown of fatty acids and utilization of ketone bodies as predominant carbon substrates. Whether it is reduced glucose metabolism per se, increased ketone body metabolism, or a combination of both that mediates seizure protection is under active investigation. However, this metabolic shift clearly reduces the incidence of seizures. In experimental animals, glycolytic inhibition can alter gene regulation and reduce epileptogenesis in a kindling model (Garriga-Canut et al., 2006; Stafstrom et al., 2009).
The reduced capacity to metabolize glucose and a simultaneous increase in the propensity to metabolize ketone bodies upon BAD modification is consistent with fuel competition (Hue and Taegtmeyer, 2009) and recapitulates the actual change in fuel consumption by the brain in fasting (Owen et al., 1967) or on KD (DeVivo et al., 1978). However, these BAD-dependent changes occur in the absence of dietary manipulation. Compared with systemic effects of dietary alterations, the seizure resistance in Bad-null and S155A mice appears likely to arise from alterations in brain cell metabolism rather than systemic changes. In support of this idea, liver knock-down of Bad is not sufficient to produce seizure resistance (Figure S5) while it mimics the metabolic phenotype of the Bad-null allele in the liver (data not shown). In addition, serum levels of circulating ketone bodies are not elevated in BAD-deficient mice under steady state conditions (data not shown), thus it seems unlikely that changes in brain metabolism are driven by systemic changes.
The BAD-dependent metabolic shift can be demonstrated at the cellular level with changes in carbon substrate consumption in primary neuron or astrocyte cultures, consistent with cell-autonomous metabolic effects of BAD. These metabolic changes have the consequence of elevating the open probability of KATP channels, as seen in both whole cell and cell attached recordings from DGNs in brain slices. Either glucose deprivation or ketone body metabolism can produce elevated KATP channel activity, and these effects can be augmented by increased neuronal firing (Ma et al., 2007; Tanner et al., 2011), as seen during seizures. The exact mechanism of KATP channel activation by BAD-dependent metabolic changes is not known; changes in ATP and ADP are a possible mechanism, though other metabolites such as PIP2 are also known to regulate the activity of KATP channels (Nichols, 2006), and we cannot rule out changes in the properties of the channel through some unknown signal resulting from genetic alteration of Bad. Total cellular ATP levels and the ATP/ADP ratio in whole brain under steady state conditions are comparable in WT and Bad−/− brains (Figures S6A and B). However, these measurements may not reflect the dynamic fluctuations that occur during seizures or local changes that could contribute to the increased activity of the KATP channel. Remarkably, the whole brain content of β-hydroxybutyrate is significantly higher in Bad−/− mice compared with controls (Figure S6C). As ketone bodies can increase the activity of the KATP channel in central neurons (Ma et al., 2007; Tanner et al., 2011), their higher content in Bad−/− brain may provide, at least in part, a link to the observed increase in KATP channel activity. Interestingly, while the whole brain content of ketone bodies in Bad−/− mice is elevated, the serum level of these metabolites are unaltered (data not shown), suggesting local changes in ketone body generation and handling.
The observed increase in KATP activity in dentate granule neurons indicates one way in which BAD induced metabolic changes might lead to seizure resistance. The dentate gyrus, where DGNs are located, has been hypothesized to be one of the regions of the brain capable of functioning as a “seizure gate” (Brenner et al., 2005; Coulter, 1999; Heinemann et al., 1992; Hsu, 2007). However, these neurons are only one of the many types of central neurons that express KATP channels (Dunn-Meynell et al., 1998; Inagaki et al., 1996; Karschin et al., 1997; Zawar et al., 1999), and important effects may also arise elsewhere.
In addition to the observation that KATP activity is augmented in the Bad genetic models, the near complete reversal of the seizure resistance in Bad-null mice with genetic ablation of the Kir6.2 subunit of the KATP channel provides strong genetic evidence for the BAD-KATP axis in modulation of seizure susceptibility. While the genetic data underscore the KATP channel as a mechanistic component of BAD’s effect in this setting, they do not rule out contribution from additional consequences of a glucose to ketone body metabolic shift.
Our findings reveal BAD as a novel molecular player in the metabolic control of neuronal excitation that imparts robust changes in susceptibility to both behavioral and electrographic seizures. BAD’s capacity to modulate the neural choice of carbon substrate and energy metabolism in the brain, independent of dietary manipulation, makes it an attractive candidate for metabolic manipulation of seizure responses. As such, small molecules modeled after BAD variants that promote ketone body catabolism over glucose metabolism may help uncover new therapeutic targets to treat epileptic disorders.
EXPERIMENTAL PROCEDURES
Animals
Bad−/−, and S155A knock-in mice have been previously described (Danial et al., 2008). Bad genetic models used in this study were bred into the C57BL/6J genetic background for at least 14 generations and were validated by genome scanning to be 99.9% congenic with C57BL/6J. Bid−/−, Bim F/F and Kir6.2−/−, mice have been previously described (Miki et al., 1998; Takeuchi et al., 2005; Yin et al., 1999). Nestin-CRE mice were purchased from JAX laboratories. Mice received a standard chow diet, and were housed in a barrier facility with 12 hr light and dark cycles. All animal procedures were approved by the Institutional Animal Care and Use Committee of Dana-Farber Cancer Institute and Harvard Medical School. Cohorts of 8–10 week old male mice were used in all in vivo studies.
Kainic Acid Treatment and Monitoring of Behavioral Seizures
Kainic acid (KA) (Tocris Bioscience, MO, US) was dissolved in saline (Sigma) and injected intraperitoneally (i.p.) at a dose of 30 mg/kg of body weight. Behavioral seizures in mice were scored every 5 minutes for up to 4 hrs according to a modified version of the Racine scale as previously described (Ferraro et al., 1997). Briefly, the modified Racine scale includes 4 stages:
-
Stage 1
Hypoactivity: rigidity, immobility or crawling, fixed gaze, and postural abnormalities, including hunched posture.
-
Stage 2
Partial clonus: behavioral seizure activity affecting mostly the head (grimacing, twitching, head nodding) and/or forelimbs(s) (“paddling”). Rigidity.
-
Stage 3
Generalized clonus: loss of upright posture, whole body clonus, rearing, wild running and jumping, and autonomic signs. Forelimb and hindlimb extension.
-
Stage 4
Status epilepticus: Severe clonic-tonic convulsions. Complete loss of balance and control.
Seizure severity was scored by an investigator blind to the genotype. In addition to recording raw seizure scores, seizure severity was determined by integrating individual scores per mouse over the duration of the experiment using the following formula:
All scores for a single mouse were added and then divided by the total time of the experiment for each animal. The mean of the seizure severity values from wild-type mice was assigned a value of “100”. This value was then used to normalize the severity of the other tested genotypes within the same scale. This formula provided better accounting for seizure severity in mice that died during the experiment.
Pentylenetetrazole Treatment and Monitoring of Behavioral Seizures
Mice were injected subcutaneously (s.c.) with pentylenetetrazole (PTZ; Sigma) dissolved in saline at a final dose of 80 mg/kg of body weight as previously described (Ferraro et al., 1999). Behavioral seizures were scored every 2.5 minutes up to 80 minutes according to a modified version of the Racine scale as detailed above.
Electroencephalographic (EEG) Recording
Mice were anesthetized with a mixture of ketamine/xylazine at a dose of 120 and 12 mg/kg of body weight, respectively. Headmounts for EEG recordings (8200 Series, 3 channel-2 EEG/1 EMG for mice, Pinnacle Technology, Inc, KS, US) were then placed by stereotactic surgery per manufacturer’s instructions. Mice were allowed to recover for 5–7 days. After recovery, a Pinnacle preamplifier was plugged in the headmount, and the mouse was then placed in an open plexiglas recording cage with wires connected via a swivel to the digitizer. Data were acquired using the PAL 8200 software (Pinnacle Technology, Inc.) at a sample rate of 400 Hz.
EEG data were analyzed in MATLAB using the BIOSIG-toolbox (http://biosig.sf.net) and specially written browsing and analysis software. In addition to displaying the raw EEG and EMG traces, power in the 20–70 Hz band was calculated using a fifth-order Butterworth bandpass filter (Lehmkuhle et al., 2009), and measured relative to the baseline period, to help identify onset and offset of high energy spiking. EEGs were scored by an investigator blind to the genotype, and the fraction of time spent in a high energy spiking state was determined for a 30 min period beginning 3 min after KA injection.
Primary Cultures of Neurons and Astrocytes
Primary cortical neurons were cultured according to an established protocol with some modifications (Banker and Goslin, 1998). Briefly, 17-day-old embryos were dissected in pre-chilled Hank’s buffered salt solution (HBSS). After removal of the meninges, the striatum, and hippocampus, the intact cortices were washed in Ca2+ and Mg2+-free HBSS, cut into small pieces and incubated in a Ca2+ and Mg2+-free HBSS solution containing 0.25% trypsin (Sigma), 1 mg/ml DNaseI (Roche Diagnostics) for 15 minutes at 37 °C with gentle shaking every 3–4 minutes. The dissociated cells were then re-suspended and plated in minimum essential medium supplemented with 0.6% glucose and 10% horse serum (Invitrogen). The medium was changed after 4 hrs to Neurobasal culture medium supplemented with B-27, 2 mM GlutaMaxI (all from Invitrogen), and a mix of penicillin and streptomycin (100 U/ml and 100 μg/ml, respectively). The cells were plated at 3×105 cells/cm2 on poly-L-lysine pre-coated plates and fed every 3 days by replacing one third of the medium with fresh media. Cells were cultured 5–6 days prior to experiments
Primary cortical astrocytes were obtained from 1-day-old newborn mouse pups and processed as above. The cells were seeded on poly-L-lysine-coated plates in a mixture of DMEM+HAMs F-12 nutrient mixture (1:1) supplemented with 10% Fetal Bovine Serum (FBS; Invitrogen), 2 mM GlutaMaxI and a mix of penicillin and streptomycin (100 U/ml and 100 μg/ml, respectively) and cultured for 2–3 weeks but no more than two passages. This ensured homogeneity of the primary cultures prior to mitochondrial respirometry assays.
Assessment of Oxygen Consumption Rate (OCR)
Real time measurement of mitochondrial oxygen consumption rate (OCR) and data processing were carried out using the XF24 extracellular flux analyzer instrument and the AKOS algorithm built in the XF24 v1.7.0.74 software (Seahorse Bioscience, Inc., Billerica MA) (Wu et al., 2007). Briefly, primary neurons were seeded on poly-L-lysine-coated XF24 V7 plates at 1×105 cells/well and incubated for 5–6 days before OCR measurements. Primary astrocytes were seeded on poly-L-lysine-coated XF24 V7 plates at 4×104 cells/well and allowed to recover overnight. On the day of the experiment, the cells were rinsed once in DMEM without sodium bicarbonate (Sigma) and pre-incubated for 1 hr in sodium bicarbonate-free DMEM supplemented with the carbon substrate to be tested (10 mM D-glucose, 5 mM β-D-hydroxybutyrate, 5 mM L-lactate or 5 mM L-glutamine). For neurons, the media was additionally supplemented with B-27.
After baseline measurements, maximal mitochondrial maximal respiration (MR) in response to a given substrate was determined following the addition of the uncoupler carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone (FCCP, Sigma) at 1 and 0.5 μM final concentration to neurons and astrocytes, respectively. OCR was then recorded until it reached a plateau. The fraction of OCR that is due to mitochondrial respiration was determined by subsequent addition of the mitochondrial complex I inhibitor rotenone (Sigma) at 1 μM. MR was derived from the difference between maximal respiratory rate induced by FCCP and the lowest respiratory rate after rotenone treatment.
Hippocampal slice preparation for patch-clamp recordings
Acute hippocampal slices 400 μm thick were cut in the transverse plane from the brains of 12- to 18- day-old mice, using a vibrating tissue slicer (Vibratome). Mice were anesthetized by isoflurane inhalation and decapitated into an ice-cold slurry containing the following (in mM): 87 NaCl, 25 NaHCO3, 25 D-glucose, 75 sucrose, 2.5 KCl, 1.25 NaH2PO4, 0.5 CaCl2, 7 MgCl2 (osmolality 340 mmol/kg; pH 7.4 with NaOH). Slices were incubated at 37°C for 20 min in the slicing solution and then for 40 min in recording solution, then stored at room temperature for 0–3 h before use. During recording, ACSF was delivered to the bath using a peristaltic pump at a flow rate of 1 ml/min. All ACSF solutions for whole-cell and single-channel recordings contained the following (in mM): 120 NaCl, 26 NaHCO3, 10 D-glucose, 2.5 KCl, 2 CaCl2, 1.25 NaH2PO4, and 1 MgCl2 (osmolality 300 mmol/kg; pH 7.4). In addition, all recording solutions contained 100 μM picrotoxin and 1 mM kynurenic acid to block fast synaptic transmission. All bath solutions were bubbled continuously with 95% O2 and 5% CO2. All chemicals were purchased from Sigma.
Patch-clamp recordings
Cell-attached patch recordings from individual dentate granule neurons identified under infrared differential interference contrast (IR-DIC) visual guidance were made in voltage-clamp mode following establishment of high-resistance (multi-GΩ) seals. Data were collected with a HEKA EPC 10 patch-clamp amplifier (HEKA Instruments Inc.). Macroscopic and single-channel currents were filtered at 1 kHz and sampled at 10 kHz. Patchmaster v2x43 software (HEKA Instruments Inc.) was used for amplifier control and data acquisition. All recordings were obtained at room temperature. Patch pipettes were pulled from borosilicate glass (VWR Scientific or Dagan LG16 for macroscopic or single-channel current recordings, respectively) using a Sutter Instruments P-97 puller. Pipette tips were fire polished to resistances of 2–3 MΩ when filled with recording solutions.
For cell-attached recordings, the pipette solution contained (in mM): 140 potassium methanesulfonate (KMeS), 10 HEPES, 2 CaCl2, and 10 TEA-Cl, 2 CsCl, 1 4-aminopyridine (4-AP), 100 nM charybdotoxin (in 0.001% BSA; Alomone Labs) and 100 nM apamin (including 40 μM acetic acid; Calbiochem) to block non-KATP potassium channels (osmolality 310 mmol/kg; pH to 7.4 with KOH). Peptide blockers were stored as stocks at −20°C and diluted prior to recording.
For whole-cell recordings, the pipette solution contained (in mM): 140 KMeS, 10 NaCl, 10 HEPES, 1 MgCl2, 0.1 EGTA (osmolality 295 mmol/kg; pH 7.4), supplemented with 0.3 mM Na3GTP and either 0.3 mM or 4 mM MgATP. A voltage ramp protocol was used to measure input resistance and slope conductance: from the −90 mV holding potential followed two 100 ms steps first to −100 mV then to −120 mV before initiation of a 1 s ramp from −120 to −65 mV. The prepulses were used to calculate the cell’s input resistance. Voltage ramps were applied every 10 s and fitted with a straight line from −120 to −80 mV to provide a running assessment of whole-cell slope conductance.
Tolbutamide was diluted in ACSF from a 200 mM stock solution in ethanol. Ethanol as a vehicle control was added to ACSF at 0.1%. All chemicals from Sigma unless otherwise noted.
Patch-clamp data analysis
Data and statistical analysis were performed with Patchmaster v2x43 (HEKA Instruments Inc.) and Origin 8 (OriginLab). Single-channel analysis was performed using QuB (www.qub.buffalo.edu). Open and closed levels for channel activity were detected using a 50% threshold criterion. Raw datasets featuring excessive baseline shift, sufficient high- or low-frequency noise to preclude effective idealization of channel openings, or too few data traces (<3 min of recording) were discarded without idealization or further analysis. The number of independent experiments (n, Figure 5) corresponds to the number of patches or whole-cell experiments each from a different hippocampal slice. In all cases, each experimental condition was tested in slices from at least three different animals.
Statistical Analysis
In all cases, mean ± SEM are presented. The number of independent experiments is indicated in the figure or figure legend in each case. Statistical significance was determined using two-tailed Student’s t-test.
Supplementary Material
Acknowledgments
We thank Marina Godes and Juan Quijada for technical assistance and animal husbandry, Dr. Gregory Holmes for advice on EEG acquisition and analysis, Rosalind Segal, Bernardo Sabatini, Qiufu Ma, S. Robert Datta and members of the Danial and Yellen laboratories for critical reading of this manuscript and valuable discussions, and E. Smith for manuscript preparation. A.G.C. was supported by a post doctoral fellowship from the Ministerio de Educación y Ciencia (MEC, Spain). N.N.D. is a recipient of the Burroughs Wellcome Fund Career Award in Biomedical Sciences. This work was supported by US National Institute of Health grants K01CA106596 (N.N.D.), R01 NS055031 (G.Y.), R56 NS072142 (N.N.D. and G.Y.), Harvard Catalyst Pilot Award (based on NIH UL1 RR025758; N.N.D. and G.Y.) and a CURE Epilepsy Challenge Award (G.Y. and N.N.D.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Banker G, Goslin K. Culturing nerve cells. 2. Cambridge, Mass: MIT Press; 1998. [Google Scholar]
- Ben-Ari Y, Tremblay E, Ottersen OP, Meldrum BS. The role of epileptic activity in hippocampal and “remote” cerebral lesions induced by kainic acid. Brain Res. 1980;191:79–97. doi: 10.1016/0006-8993(80)90316-9. [DOI] [PubMed] [Google Scholar]
- Bough KJ, Eagles DA. A ketogenic diet increases the resistance to pentylenetetrazole-induced seizures in the rat. Epilepsia. 1999;40:138–143. doi: 10.1111/j.1528-1157.1999.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J. 2011;435:297–312. doi: 10.1042/BJ20110162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner R, Chen QH, Vilaythong A, Toney GM, Noebels JL, Aldrich RW. BK channel beta4 subunit reduces dentate gyrus excitability and protects against temporal lobe seizures. Nat Neurosci. 2005;8:1752–1759. doi: 10.1038/nn1573. [DOI] [PubMed] [Google Scholar]
- Browning RA. Anatomy of Generalized Convulsive Seizures. In: Malafosse A, Genton P, Hirsch E, Marescaux C, Broglin D, Bernasconi R, editors. Idiopathic Generalized Epilepsies: Clinical, Experimental and Genetic Aspects. London: Libbey; 1994. pp. 399–414. [Google Scholar]
- Browning RA, Wang C, Lanker ML, Jobe PC. Electroshock- and pentylenetetrazol-induced seizures in genetically epilepsy-prone rats (GEPRs): differences in threshold and pattern. Epilepsy Res. 1990;6:1–11. doi: 10.1016/0920-1211(90)90002-d. [DOI] [PubMed] [Google Scholar]
- Coulter DA. Chronic epileptogenic cellular alterations in the limbic system after status epilepticus. Epilepsia. 1999;40(Suppl 1):S23–33. doi: 10.1111/j.1528-1157.1999.tb00875.x. discussion S40–21. [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37:299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlin M, Elfving A, Ungerstedt U, Amark P. The ketogenic diet influences the levels of excitatory and inhibitory amino acids in the CSF in children with refractory epilepsy. Epilepsy Res. 2005;64:115–125. doi: 10.1016/j.eplepsyres.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Danial NN. BAD: undertaker by night, candyman by day. Oncogene. 2008;27(Suppl 1):S53–70. doi: 10.1038/onc.2009.44. [DOI] [PubMed] [Google Scholar]
- Danial NN, Gramm CF, Scorrano L, Zhang CY, Krauss S, Ranger AM, Datta SR, Greenberg ME, Licklider LJ, Lowell BB, et al. BAD and glucokinase reside in a mitochondrial complex that integrates glycolysis and apoptosis. Nature. 2003;424:952–956. doi: 10.1038/nature01825. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Danial NN, Walensky LD, Zhang CY, Choi CS, Fisher JK, Molina AJ, Datta SR, Pitter KL, Bird GH, Wikstrom JD, et al. Dual role of proapoptotic BAD in insulin secretion and beta cell survival. Nat Med. 2008;14:144–153. doi: 10.1038/nm1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta SR, Katsov A, Hu L, Petros A, Fesik SW, Yaffe MB, Greenberg ME. 14–3–3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- DeVivo DC, Leckie MP, Ferrendelli JS, McDougal DB., Jr Chronic ketosis and cerebral metabolism. Ann Neurol. 1978;3:331–337. doi: 10.1002/ana.410030410. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Rawson NE, Levin BE. Distribution and phenotype of neurons containing the ATP-sensitive K+ channel in rat brain. Brain Res. 1998;814:41–54. doi: 10.1016/s0006-8993(98)00956-1. [DOI] [PubMed] [Google Scholar]
- Engel T, Hatazaki S, Tanaka K, Prehn JH, Henshall DC. Deletion of Puma protects hippocampal neurons in a model of severe status epilepticus. Neuroscience. 2010;168:443–450. doi: 10.1016/j.neuroscience.2010.03.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel T, Plesnila N, Prehn JH, Henshall DC. In vivo contributions of BH3-only proteins to neuronal death following seizures, ischemia, and traumatic brain injury. J Cereb Blood Flow Metab. 2011;31:1196–1210. doi: 10.1038/jcbfm.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fern R. Variations in spare electron transport chain capacity: The answer to an old riddle? Journal of neuroscience research. 2003;71:759–762. doi: 10.1002/jnr.10553. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GG, Schork NJ, St Jean P, Ballas C, Choi H, Berrettini WH. Mapping murine loci for seizure response to kainic acid. Mamm Genome. 1997;8:200–208. doi: 10.1007/s003359900389. [DOI] [PubMed] [Google Scholar]
- Ferraro TN, Golden GT, Smith GG, St Jean P, Schork NJ, Mulholland N, Ballas C, Schill J, Buono RJ, Berrettini WH. Mapping loci for pentylenetetrazol-induced seizure susceptibility in mice. J Neurosci. 1999;19:6733–6739. doi: 10.1523/JNEUROSCI.19-16-06733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garriga-Canut M, Schoenike B, Qazi R, Bergendahl K, Daley TJ, Pfender RM, Morrison JF, Ockuly J, Stafstrom C, Sutula T, Roopra A. 2-Deoxy-D-glucose reduces epilepsy progression by NRSF-CtBP-dependent metabolic regulation of chromatin structure. Nat Neurosci. 2006;9:1382–1387. doi: 10.1038/nn1791. [DOI] [PubMed] [Google Scholar]
- Hartman AL, Gasior M, Vining EP, Rogawski MA. The neuropharmacology of the ketogenic diet. Pediatr Neurol. 2007;36:281–292. doi: 10.1016/j.pediatrneurol.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haugvicova R, Kubova H, Skutova M, Mares P. Anticonvulsant action of topiramate against motor seizures in developing rats. Epilepsia. 2000;41:1235–1240. doi: 10.1111/j.1528-1157.2000.tb04600.x. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res Suppl. 1992;7:273–280. [PubMed] [Google Scholar]
- Hickman JA, Hardwick JM, Kaczmarek LK, Jonas EA. Bcl-xL inhibitor ABT-737 reveals a dual role for Bcl-xL in synaptic transmission. J Neurophysiol. 2008;99:1515–1522. doi: 10.1152/jn.00598.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu D. The dentate gyrus as a filter or gate: a look back and a look ahead. Prog Brain Res. 2007;163:601–613. doi: 10.1016/S0079-6123(07)63032-5. [DOI] [PubMed] [Google Scholar]
- Hue L, Taegtmeyer H. The Randle cycle revisited: a new head for an old hat. Am J Physiol Endocrinol Metab. 2009;297:E578–591. doi: 10.1152/ajpendo.00093.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement JP, Wang CZ, Aguilar-Bryan L, Bryan J, Seino S. A family of sulfonylurea receptors determines the pharmacological properties of ATP-sensitive K+ channels. Neuron. 1996;16:1011–1017. doi: 10.1016/s0896-6273(00)80124-5. [DOI] [PubMed] [Google Scholar]
- Jarrett SG, Milder JB, Liang LP, Patel M. The ketogenic diet increases mitochondrial glutathione levels. J Neurochem. 2008;106:1044–1051. doi: 10.1111/j.1471-4159.2008.05460.x. [DOI] [PubMed] [Google Scholar]
- Jeong EA, Jeon BT, Shin HJ, Kim N, Lee DH, Kim HJ, Kang SS, Cho GJ, Choi WS, Roh GS. Ketogenic diet-induced peroxisome proliferator-activated receptor-gamma activation decreases neuroinflammation in the mouse hippocampus after kainic acid-induced seizures. Exp Neurol. 2011;232:195–202. doi: 10.1016/j.expneurol.2011.09.001. [DOI] [PubMed] [Google Scholar]
- Jiao S, Li Z. Nonapoptotic function of BAD and BAX in long-term depression of synaptic transmission. Neuron. 2011;70:758–772. doi: 10.1016/j.neuron.2011.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juge N, Gray JA, Omote H, Miyaji T, Inoue T, Hara C, Uneyama H, Edwards RH, Nicoll RA, Moriyama Y. Metabolic control of vesicular glutamate transport and release. Neuron. 2010;68:99–112. doi: 10.1016/j.neuron.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karschin C, Ecke C, Ashcroft FM, Karschin A. Overlapping distribution of KATP channel-forming Kir6.2 subunit and the sulfonylurea receptor SUR1 in rodent brain. FEBS Lett. 1997;401:59–64. doi: 10.1016/s0014-5793(96)01438-x. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Gobert J, Wulfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- Lehmkuhle MJ, Thomson KE, Scheerlinck P, Pouliot W, Greger B, Dudek FE. A simple quantitative method for analyzing electrographic status epilepticus in rats. J Neurophysiol. 2009;101:1660–1670. doi: 10.1152/jn.91062.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Berg J, Yellen G. Ketogenic diet metabolites reduce firing in central neurons by opening KATP channels. J Neurosci. 2007;27:3618–3625. doi: 10.1523/JNEUROSCI.0132-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAskill AF, Atkin TA, Kittler JT. Mitochondrial trafficking and the provision of energy and calcium buffering at excitatory synapses. Eur J Neurosci. 2010;32:231–240. doi: 10.1111/j.1460-9568.2010.07345.x. [DOI] [PubMed] [Google Scholar]
- Masino SA, Li T, Theofilas P, Sandau US, Ruskin DN, Fredholm BB, Geiger JD, Aronica E, Boison D. A ketogenic diet suppresses seizures in mice through adenosine A1 receptors. J Clin Invest. 2011;121:2679–2683. doi: 10.1172/JCI57813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel-deficient mice. Proc Natl Acad Sci USA. 1998;95:10402–10406. doi: 10.1073/pnas.95.18.10402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy BM, Engel T, Paucard A, Hatazaki S, Mouri G, Tanaka K, Tuffy LP, Jimenez-Mateos EM, Woods I, Dunleavy M, et al. Contrasting patterns of Bim induction and neuroprotection in Bim-deficient mice between hippocampus and neocortex after status epilepticus. Cell Death Differ. 2010;17:459–468. doi: 10.1038/cdd.2009.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. A randomized trial of classical and medium-chain triglyceride ketogenic diets in the treatment of childhood epilepsy. Epilepsia. 2009;50:1109–1117. doi: 10.1111/j.1528-1167.2008.01870.x. [DOI] [PubMed] [Google Scholar]
- Nichols CG. KATP channels as molecular sensors of cellular metabolism. Nature. 2006;440:470–476. doi: 10.1038/nature04711. [DOI] [PubMed] [Google Scholar]
- Nicholls DG. Mitochondrial calcium function and dysfunction in the central nervous system. Biochim Biophys Acta. 2009;1787:1416–1424. doi: 10.1016/j.bbabio.2009.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh HS, Kim YS, Lee HP, Chung KM, Kim DW, Kang SS, Cho GJ, Choi WS. The protective effect of a ketogenic diet on kainic acid-induced hippocampal cell death in the male ICR mice. Epilepsy Res. 2003;53:119–128. doi: 10.1016/s0920-1211(02)00262-0. [DOI] [PubMed] [Google Scholar]
- Owen OE, Morgan AP, Kemp HG, Sullivan JM, Herrera MG, Cahill GF., Jr Brain metabolism during fasting. J Clin Invest. 1967;46:1589–1595. doi: 10.1172/JCI105650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer HH, Lyczkowski DA, Thiele EA. Low glycemic index treatment: implementation and new insights into efficacy. Epilepsia. 2008;49(Suppl 8):42–45. doi: 10.1111/j.1528-1167.2008.01832.x. [DOI] [PubMed] [Google Scholar]
- Pfeifer HH, Thiele EA. Low-glycemic-index treatment: a liberalized ketogenic diet for treatment of intractable epilepsy. Neurology. 2005;65:1810–1812. doi: 10.1212/01.wnl.0000187071.24292.9e. [DOI] [PubMed] [Google Scholar]
- Proks P, Ashcroft FM. Modeling KATP channel gating and its regulation. Prog Biophys Mol Biol. 2009;99:7–19. doi: 10.1016/j.pbiomolbio.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Schwartzkroin PA. Mechanisms underlying the anti-epileptic efficacy of the ketogenic diet. Epilepsy Res. 1999;37:171–180. doi: 10.1016/s0920-1211(99)00069-8. [DOI] [PubMed] [Google Scholar]
- Stafstrom CE, Ockuly JC, Murphree L, Valley MT, Roopra A, Sutula TP. Anticonvulsant and antiepileptic actions of 2-deoxy-D-glucose in epilepsy models. Ann Neurol. 2009;65:435–447. doi: 10.1002/ana.21603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stafstrom CE, Rho JM. Epilepsy and the Ketogenic Diet. Humana Press; Totowa, NJ, USA: 2004. [Google Scholar]
- Takeuchi O, Fisher J, Suh H, Harada H, Malynn BA, Korsmeyer SJ. Essential role of BAX,BAK in B cell homeostasis and prevention of autoimmune disease. Proc Natl Acad Sci USA. 2005;102:11272–11277. doi: 10.1073/pnas.0504783102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner GR, Lutas A, Martínez-François JR, Yellen G. Single KATP channel opening in response to action potential firing in mouse dentate granule neurons. J Neurosci. 2011;31:8689–8696. doi: 10.1523/JNEUROSCI.5951-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele EA. Assessing the efficacy of antiepileptic treatments: the ketogenic diet. Epilepsia. 2003;44(Suppl 7):26–29. doi: 10.1046/j.1528-1157.44.s7.4.x. [DOI] [PubMed] [Google Scholar]
- Velíšková J. Behavioral Characterization of Seizures in Rats. In: Pitkänen A, Schwartzkroin PA, Moshé S, editors. Models of Seizures and Epilepsy. Burlington, MA: Elsevier Academic Press; 2005. pp. 601–611. [Google Scholar]
- Wang ZJ, Bergqvist C, Hunter JV, Jin D, Wang DJ, Wehrli S, Zimmerman RA. In vivo measurement of brain metabolites using two-dimensional double-quantum MR spectroscopy--exploration of GABA levels in a ketogenic diet. Magn Reson Med. 2003;49:615–619. doi: 10.1002/mrm.10429. [DOI] [PubMed] [Google Scholar]
- Wu M, Neilson A, Swift AL, Moran R, Tamagnine J, Parslow D, Armistead S, Lemire K, Orrell J, Teich J, et al. Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am J Physiol Cell Physiol. 2007;292:C125–136. doi: 10.1152/ajpcell.00247.2006. [DOI] [PubMed] [Google Scholar]
- Yellen G. Ketone bodies, glycolysis, and KATP channels in the mechanism of the ketogenic diet. Epilepsia. 2008;49(Suppl 8):80–82. doi: 10.1111/j.1528-1167.2008.01843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin XM, Wang K, Gross A, Zhao Y, Zinkel S, Klocke B, Roth KA, Korsmeyer SJ. Bid-deficient mice are resistant to Fas-induced hepatocellular apoptosis. Nature. 1999;400:886–891. doi: 10.1038/23730. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. doi: 10.1038/nrm2308. [DOI] [PubMed] [Google Scholar]
- Yudkoff M, Daikhin Y, Nissim I, Lazarow A, Nissim I. Ketogenic diet, amino acid metabolism, and seizure control. J Neurosci Res. 2001;66:931–940. doi: 10.1002/jnr.10083. [DOI] [PubMed] [Google Scholar]
- Zawar C, Plant TD, Schirra C, Konnerth A, Neumcke B. Cell-type specific expression of ATP-sensitive potassium channels in the rat hippocampus. J Physiol. 1999;514(Pt 2):327–341. doi: 10.1111/j.1469-7793.1999.315ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke HR, Zielke CL, Baab PJ. Direct measurement of oxidative metabolism in the living brain by microdialysis: a review. J Neurochem. 2009;109(Suppl 1):24–29. doi: 10.1111/j.1471-4159.2009.05941.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemann AE, Schnizler MK, Albert GW, Severson MA, Howard MA, 3rd, Welsh MJ, Wemmie JA. Seizure termination by acidosis depends on ASIC1a. Nat Neurosci. 2008;11:816–822. doi: 10.1038/nn.2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.