SUMMARY
Numerous studies in humans link a nonsynonymous genetic polymorphism (I148M) in adiponutrin (ADPN) to various forms of fatty liver disease and liver cirrhosis. Despite its high clinical relevance, the molecular function of ADPN and the mechanism by which I148M variant affects hepatic metabolism are unclear. Here we show that ADPN promotes cellular lipid synthesis by converting lysophosphatidic acid (LPA) into phosphatidic acid. The ADPN-catalyzed LPA acyltransferase (LPAAT) reaction is specific for LPA and long-chain acyl-CoAs. Wild-type mice receiving a high-sucrose diet exhibit substantial upregulation of Adpn in the liver and a concomitant increase in LPAAT activity. In Adpn-deficient mice, this diet-induced increase in hepatic LPAAT activity is reduced. Notably, the I148M variant of human ADPN exhibits increased LPAAT activity leading to increased cellular lipid accumulation. This gain of function provides a plausible biochemical mechanism for the development of liver steatosis in subjects carrying the I148M variant.
INTRODUCTION
Excessive hepatic triacylglycerol (TG) deposition (hepatosteatosis) is the earliest hallmark of nonalcoholic fatty liver disease (NAFLD), which can progress to nonalcoholic steatohepatitis (NASH) and end-stage liver disease, i.e., cirrhosis. The high prevalence of fatty liver disease in developed countries and the concomitant risk for morbidity and mortality underline the importance of a detailed characterization of the processes that regulate hepatic TG content. Generally, TGs provide efficient storage for surplus fatty acids (FAs). They are synthesized through the stepwise acylation of glycerol-3-phosphate by acyltransferases and phosphatidic acid phosphohydrolases (Coleman, 2007; Reue and Brindley, 2008; Yen et al., 2008). Subsequent to their synthesis, TGs form lipid droplets (LDs), which develop in variable numbers and sizes in essentially all cell types of multicellular organisms. Upon demand, FAs are released from TGs by enzymatic hydrolysis. This process, commonly named lipolysis, is catalyzed by TG hydrolases (“lipases”) (Zechner et al., 2012). In the liver, a precisely regulated balance between lipid synthesis and lipolysis, lipoprotein uptake and secretion, and lipid remodeling and oxidation regulates the cellular TG content (Adams et al., 2005; Marchesini et al., 2001). Perturbation of this balance disrupts metabolic homeostasis, leading to lipodystrophies or lipid overload. Excessive lipid storage as observed in hepatosteatosis results in lipotoxicity, a condition associated with insulin resistance and type 2 diabetes (Schaffer, 2003).
In a landmark study, Hobbs and colleagues reported a strong association of a sequence variant within the human patatin-like phospholipase domain containing A3 (PNPLA3) gene with NAFLD (Romeo et al., 2008). The PNPLA3 gene encodes a 481 amino acid protein named adiponutrin (ADPN). The sequence variant in PNPLA3 (rs738409 C/G) leads to an Ile to Met exchange at amino acid position 148 of ADPN (I148M). Numerous subsequent studies confirmed this association and showed that the I148M polymorphism also associates with other liver pathologies, including alcoholic liver disease and hepatic injury (Johansson et al., 2008; Kantartzis et al., 2009; Kotronen et al., 2009; Romeo et al., 2010b; Yuan et al., 2008). However, the mechanisms by which the I148M variant of ADPN contributes to these hepatic pathologies remain unknown.
ADPN belongs to a protein family defined by the presence of a patatin domain, which was originally discovered in patatin, the most abundant protein of the potato tuber. Due to this structural feature and the fact that some members exhibit phospholipase activity, the protein family was named patatin-like phospholipase domain containing proteins (PNPLAs) (Wilson et al., 2006). The human and murine PNPLA families consist of nine and eight members, respectively, and exhibit diverse enzymatic functions, including phospholipase, TG hydrolase, retinylester hydrolase, and acyl-CoA-dependent or -independent transacylase activities (Kienesberger et al., 2009). ADPN is officially named PNPLA3 and was originally identified as an adipose tissue-specific protein that is upregulated in genetically obese rodents and by feeding a high-carbohydrate diet (Baulande et al., 2001; Polson and Thompson, 2003b). Recent studies revealed that ADPN is also expressed in hepatocytes when mice are fed a high-sucrose diet (HSD) or with increase in the liver fat content (Hoekstra et al., 2010; Huang et al., 2010). In humans, hepatic expression of ADPN is higher than in mice and exceeds ADPN expression in adipose tissue (Huang et al., 2010; Wilson et al., 2006). ADPN shares the highest sequence homology (56% amino acid identity in the patatin domain) with adipose triglyceride lipase (ATGL, also designated PNPLA2) (Zimmermann et al., 2004), the rate-limiting enzyme for TG hydrolysis in adipose and nonadipose tissues. Consistent with the structural similarity to ATGL, ADPN was also reported to exhibit TG hydrolase activity (He et al., 2010; Huang et al., 2011; Jenkins et al., 2004; Lake et al., 2005). The physiological relevance of this activity was questioned when Adpn deficiency in mice did not significantly affect TG hydrolysis or TG content in adipose tissue and liver (Basantani et al., 2011; Chen et al., 2010). Various nutritional (e.g., sucrose, fructose) (Baulande et al., 2001; Polson and Thompson, 2003b), hormonal (e.g., insulin, glucose, tri-iodothyronine) (Calvo and Obregon, 2009; Kershaw et al., 2006; Moldes et al., 2006), and pharmacological factors (e.g., troglitazone, lipoic acid, liver X receptor [LXR] agonists) (Huang et al., 2010; Huong and Ide, 2008; Polson and Thompson, 2003a) regulate ADPN and ATGL expression, but in most cases in opposite directions. For example, lipogenic stimuli dramatically increase ADPN expression in adipocytes (Kershaw et al., 2006; Polson and Thompson, 2003b) and in the liver (Hoekstra et al., 2010), whereas lipolytic stimuli induce ATGL and inhibit ADPN expression. Furthermore, overexpression of wild-type ADPN or the I148M variant induced TG accumulation in cultured hepatocytes in vitro (He et al., 2010; Qiao et al., 2011), and adenovirus-mediated overexpression of I148M in mice liver increased the hepatic TG content. Taken together, these findings suggested that ADPN is involved in lipid synthesis rather than in lipolysis, but the biochemical activity promoting lipid synthesis is unknown.
Here we show that human and murine ADPN acts as nutritionally regulated acyl-CoA-dependent lysophosphatidic acid acyltransferase (LPAAT) and that the I148M variant of ADPN exhibits elevated LPAAT activity in comparison to the wild-type protein. We conclude that I148M promotes hepatic lipid synthesis due to a gain of function.
RESULTS
Mouse Adpn Increases Intracellular Lipid Synthesis In Vitro
To investigate the metabolic role of Adpn, we overexpressed the murine protein in various cell lines (see Figure S1 online) and determined the incorporation of radiolabeled oleic acid into TG (Figure 1). Cells expressing β-galactosidase (LacZ) were used as control. Expression of Adpn substantially enhanced the incorporation of oleic acid into TG stores of HepG2 (human hepatoma), CHO (Chinese hamster ovary), and Cos-7 (monkey embryonic kidney) cells, indicating that Adpn promotes lipid synthesis independent of the cell type used.
Figure 1. Overexpression of Adpn Results in Increased TG Synthesis.
Accumulation of [1-14C] oleic acid into TGs was assessed in HepG2, CHO, and Cos-7 cells. HepG2 and CHO cells were infected with murine Adpn expressing adenovirus, and Cos-7 cells were transfected with an expression vector encoding murine Adpn. Adenovirus or vector DNA encoding β-galactosidase (LacZ) was used as a control. After 36 hr of infection or transfection, cells were incubated with 1 μCi/ml [1-14C] oleic acid and 400 μM oleic acid complexed to BSA for 6 hr. Subsequently, lipids were extracted and separated by TLC. Radioactivity comigrating with the TG band was excised and quantified by scintillation counting. Data are presented as mean ± SD and represent two independent experiments with each sample in triplicate. Statistical significance was determined by a two-tailed Student’s t test, **p < 0.01, ***p < 0.001.
Purified Adpn Exhibits LPAAT Activity
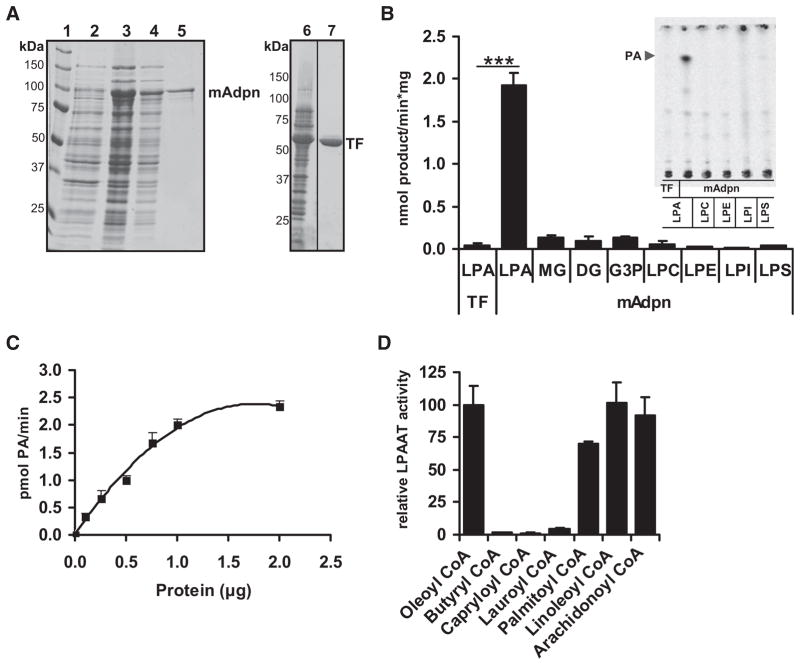
To identify potential enzymatic activities of Adpn in lipid synthesis, the murine cDNA was cloned into pCold vector, and the recombinant protein (mAdpn) was expressed in Escherichia (E.) coli. pCold encodes a fusion protein containing an N-terminal His tag and a 48 kDa trigger factor (TF) for cytosolic localization of the expressed protein. This expression system enabled us to purify mAdpn by metal-ion affinity chromatography without use of detergents that may interfere with potential enzymatic activities. mAdpn fusion protein exhibited a MW of ~95 kDa (Figure 2A). His-tagged TF (MW ~53.5 kDa) was isolated under identical conditions and used as a negative control in all in vitro experiments. To investigate whether Adpn is involved in acyl-CoA-dependent lipid synthesis, we incubated purified mAdpn with radiolabeled oleoyl-CoA as an acyl donor and monoacylglycerol (MG), diacylglycerol (DG), glycerol-3-phosphate, or various lysophospholipids as acceptor molecules. Thin-layer chromatography (TLC) analysis revealed that mAdpn efficiently catalyzes the acyl-CoA-dependent acylation of LPA to generate phosphatidic acid (PA) (Figure 2B). No significant acyltransferase reactions were detected when MG, DG, glycerol-3-phosphate, lysophosphatidyl (LP)-choline, LP-ethanolamine, LP-inositol, and LP-serine were used as acyl-group acceptors (Figure 2B). mAdpn enhanced the formation of PA in a dose-dependent manner with a specific activity of ~2 pmol/min·μg (2 mU/mg; Figures 2B and 2C). Concerning FA specificity, we found that oleoyl-CoA, linoleoyl-CoA, and arachidonoyl-CoA were the preferred acyl donors, followed by palmitoyl-CoA. Essentially no LPAAT activity was observed for acyl-CoAs with chain lengths below 16 carbons (Figure 2D). mAdpn was unable to utilize unactivated FAs for the LPAAT reaction (data not shown). Thrombin cleavage to facilitate tag removal from mAdpn fusion protein resulted in a 30% increase in LPAAT activity (Figure S2), indicating that the presence of TF in recombinant mAdpn moderately interferes with its enzymatic activity. Taken together, these results show that mouse Adpn is a highly substrate-specific LPAAT.
Figure 2. Recombinant mAdpn Possesses Lysophosphatidic Acid Acyltransferase Activity.
(A) Purification of recombinant murine Adpn (mAdpn) and TF by metal-ion affinity chromatography. Lane 1, protein standard; lane 2, uninduced E. coli lysate; lane 3, IPTG-induced lysate (E. coli BL21 pCold-mAdpn); lane 4, flowthrough of Ni-NTA column; lane 5, eluate of Ni-NTA column containing mAdpn; lane 6, IPTG-induced lysate (E. coli BL21 pCold); lane 7, eluate of Ni-NTA column containing TF. (B) Purified mAdpn or TF (1 μg protein) was incubated with 20 μM [1-14C] oleoyl-CoA as acyl donor in the presence of 100 μM of various acyl acceptors (lysophosphatidic acid [LPA], MG, DG, glycerol-3-phosphate [G3P], lysophosphatidyl choline [LPC], lysophosphatidyl ethanolamine [LPE], lysophosphatidyl inositol [LPI], or lysophosphatidyl serine [LPS]) for 10 min at 37°C. Lipids were solvent extracted and separated by TLC. Enzymatic activity was calculated from the radioactivity associated with the product band. The inset represents a phosphorimager scan of the TLC plate showing that mAdpn is active only in the presence of oleoyl-CoA and LPA but not with other lysophospholipids. PA, phosphatidic acid. Purified TF incubated with oleoyl-CoA and LPA was used as a negative control (represented as TF in the inset). LPAAT activity of purified mAdpn was measured (C) at various protein concentrations and (D) in the presence of acyl-CoAs with varying fatty acid chain length. Relative LPAAT activity was determined by normalizing the specific activity of mAdpn (2.4 nmol/min*mg) in presence of [3H] LPA and oleoyl-CoA to 100%. Data are presented as mean ± SD and are representative of three independent experiments with each sample in duplicate. Statistical significance was determined by a two-tailed Student’s t test, ***p < 0.001.
The I148M Genetic Variants of ADPN Exhibit Increased LPAAT Activity
Previous studies demonstrated that the I148M variant of human ADPN is associated with fatty liver in humans (Romeo et al., 2010a; Valenti et al., 2010). The isoleucine residue at position 148 is conserved between human and mouse enzymes. To investigate the effect of this mutation on LPAAT activity, we generated I148M variant of the murine enzyme replacing Ile-148 to Met by site-directed mutagenesis, and purified the overexpressed TF fusion protein in E. coli (mI148M) as described above (Figure 3A). The specific LPAAT activity of mI148M was 2.0-fold higher than for mAdpn (4 mU/mg; Figure 3B). Additionally, mI148M retained the same substrate specificity as the wild-type enzyme with regard to acyl-CoAs as FA donors (Figure S3A).
Figure 3. LPAAT Activity of Purified Murine and Human I148M Variants.
(A) Coomassie blue stain showing purified TF and recombinant mAdpn, mI148M, hADPN, and hI148M. I148M mutants were generated by site-directed mutagenesis, purified, and analyzed for LPAAT activity in comparison to wild-type proteins. Purified proteins were detected by western blotting using anti-His monoclonal antibody.
(B and C) (B) LPAAT activity of mAdpn and mI148M and (C) hADPN and hI148M using 1 μg of purified protein in a reaction with 20 μM [1-14C] oleoyl-CoA and 100 μM LPA. Purified TF was used as negative control with negligible LPAAT activity (<0.1 nmol PA/min*mg).
(D) Substrate specificity of recombinant hADPN and hI148M for acyl donors was measured by incubating 2 μg of partially purified proteins with 25 μM [3H] LPA (600,000 cpm/assay) and 20 μM of various acyl-CoAs. Relative LPAAT activity was determined by normalizing the specific activity of hADPN and hI148M (4.2 and 8.1 nmol/min*mg, respectively) in the presence of [3H] LPA and oleoyl-CoA to 100%.
(E) Substrate specificity of recombinant hADPN and hI148M for acyl acceptors was measured by incubating 2 μg of partially purified proteins with 20 μM [1-14C] oleoyl-CoA as acyl donor in the presence of 100 μM of various acyl acceptors (LPA, lysophosphatidyl choline [LPC], lysophosphatidyl ethanolamine [LPE], lysophosphatidyl inositol [LPI], or lysophosphatidyl serine [LPS]). Relative LPAAT activity was determined by normalizing the specific activity of hADPN and hI148M (as shown in Figure 3C) in the presence of LPA and [1-14C] oleoyl-CoA to 100%.
(F) Acylation of glycerol-3-phosphate (G3P) was measured by incubating 2 μg purified TF, hADPN, and hI148M with 300 μM G3P and 20 μM [1-14C] oleoyl-CoA. Data are presented as mean ± SD and are representative of at least two independent experiments with each sample in duplicates. Statistical significance was determined by a two-tailed Student’s t test, **p < 0.01, ***p < 0.001.
Next, we tested whether human ADPN also exhibits LPAAT activity. We expressed both the human TF fusion protein (hADPN) and the mutated version of this protein (hI148M) in E. coli. LPAAT activity of both hADPN and hI148M was detected in bacterial lysates overexpressing these enzymes without purification (Figure S3B). Purified hADPN and hI148M exhibited a higher molecular mass (MW ~106 KDa) than mAdpn, which is because the human ADPN is 97 amino acids longer than the murine ortholog (Figure 3A). Both human protein versions were more prone to proteolytic degradation than their mouse counterparts. However, despite some degradation, the specific LPAAT activity of hADPN was higher (3 mU/mg) than for mAdpn (2 mU/mg, p ≤ 0.05), and the specific activity of hI148M mutant was again 2.1-fold higher than that of the wild-type enzyme (Figure 3C). This indicated a gain of function in hI148M as well as in mI148M. The substrate specificity of hADPN and hI148M was similar to that of the murine enzymes (Figures 3D and 3E). Notably, however, hI148M exhibited a moderate but consistent acyltransferase activity when glycerol-3-phosphate was used as acyl acceptor (Figure 3F).
Steady-state kinetic analysis of LPAAT activity using purified mAdpn revealed a KM value at 12.46 μM and a Vmax of 3.41 nmol/min*mg. KM and Vmax values of the mI148M mutant were increased 2.6- and 2.8-fold, respectively (Figure 4A). This indicates that the I148M mutation affects both turnover rate and substrate binding. Since no experimental 3D structure is available for ADPN, residues mediating LPAAT activity of ADPN are unknown. A 3D homology model, however, revealed that Ile148 resides in very close spatial proximity of a GXSXG-consensus sequence containing the active serine of many hydrolases/esterases (~2.5 Å from side chain Asp166, Figure 4B). Ser-47 located within the GXSXG sequence of ADPN has been shown to be essential for the TG hydrolase activity of the protein (He et al., 2010). To test whether this domain is also relevant for LPAAT activity of human ADPN, we generated two additional mutants of ADPN: Ser-47 to Ala (hS47A) and Cys-15, located within the predicted oxyanion hole, to Ser (hC15S). Interestingly, Ser-47 was not essential for LPAAT activity, whereas mutation of Cys-15 led to a 65% loss of the activity (Figure 4C).
Figure 4. Enzyme Properties of Murine and Human ADPN Variants.
(A) LPAAT activity of mAdpn and mI148M was monitored as a function of [1-14C] oleoyl-CoA concentration and kinetic parameters were calculated by Michaelis-Menten plot using GraphPad Prism software.
(B) Structure around the putative “catalytic dyad” (Ser-47 and Asp-166) involved in the proposed lipase activity of ADPN. The structure was deduced from an alignment of Leu10-Phe175 of ADPN to Leu32-Ala225 of Pat17 (PDB ID 1OWX), the best-scoring template with known 3D structure using the Phyre2 server (Kelley and Sternberg, 2009), and PyMOL (The PyMOL Molecular Graphics System, Version 1.4.1, Schrödinger, LLC). The model reveals close spatial proximity of Ile148 (highlighted in yellow) and Cys15 (highlighted in green) to the proposed active site (highlighted in red).
(C) LPAAT activity of hADPN and hADPN mutants with point mutations at Cys-15 to Ser (hC15S), Ser-47 to Ala (hS47A), Asp-206 to Ala (hD206A), and Pro-311 to Gly (hP311G). Mutants were generated by site-directed mutagenesis and purified under conditions identical to those of the wild-type protein. The inset shows a Coomassie blue-stained SDS-PAGE gel with various purified recombinant ADPN variants (2 μg protein/lane). LPAAT activity was measured using 2 μg of purified proteins or TF as described in the Experimental Procedures.
(D) LPAAT activity was determined in bacterial total lysates (10 μg lysate protein) overexpressing TF, hADPN, or hADPN mutants. Protein overexpression was confirmed by western blotting in bacterial total lysates using anti-His monoclonal antibody (inset). Experiments were done in duplicates and repeated twice. Data are presented as mean ± SD. Statistical significance was determined by a two-tailed Student’s t test, **p < 0.01, ***p < 0.001.
The reaction mechanism proposed for classical O-acyl- and O-acetyltransferases implies an essential role of several motifs (motifs I, II, III, and IV as defined by Takeuchi and Reue, 2009). Sequence analysis of human ADPN revealed only very weak homology to LPAATα and LPAATβ (Figure S3C). Nevertheless, we mutated two conserved amino acids in the putative motifs I and IV: Asp-206 to Ala (hD206A) and Pro-311 to Gly (hP311G). The hP311G mutation resulted in 67% loss of activity, whereas the hD206A point mutation had no effect on LPAAT activity (Figure 4C). Additionally, LPAAT activity of hADPN, hC15S, hS47A, hD206A, and hP311G was determined in crude bacterial lysates. hADPN overexpression increased LPAAT activity by 95% over the endogenous LPAAT activity observed in TF-expressing lysates (Figure 4D). The hADPN-mediated increase in LPAAT activity was 40% and 42% lower in hC15S- and hP311G-expressing lysates, respectively. hS47A- and hP311G-expressing lysates exhibited LPAAT activity similar to that of hADPN lysates.
ADPN Possesses Very Low TG Hydrolase Activity and Is Not Activated by CGI-58
TG hydrolysis is initiated by ATGL (Zimmermann et al., 2004), which is activated by comparative gene identification-58 (CGI-58; also named α/β hydrolase domain-containing 5, ABHD5) (Lass et al., 2006). Since ADPN exhibits high sequence identity to ATGL and was reported to hydrolyze TG (He et al., 2010; Jenkins et al., 2004; Lake et al., 2005), we performed TG hydrolase assays with purified ADPN and lysates of ADPN-expressing E. coli in the presence or absence of CGI-58. As shown in Figure 5A, purified mAdpn and hADPN exhibited detectable but very low TG hydrolase activity (specific activity, 8 and 10 μU/mg, respectively) in comparison to LPAAT activity (~2 mU/mg). The addition of purified murine CGI-58 (mCGI-58) did not increase ADPN-catalyzed TG hydrolysis. Overexpression of ADPN in E. coli or in Cos-7 cells did not result in increased TG hydrolase activity, whereas ATGL was active and stimulated by CGI-58 under identical conditions (Figure 5B, Figures S4A and S4B).
Figure 5. TG Hydrolase Activity of ADPN.
(A) TG hydrolase activity assay using radiolabeled triolein as substrate in the presence of 5 μg purified TF, mAdpn, mI148M, hADPN, hI48M, and (B) bacterial total lysates (40 μg lysate protein) overexpressing TF, mAdpn, mI148M, hADPN, hI148M, and strep-tagged mATGL. Assays were performed in the absence or presence of purified mCGI-58. Data are shown as mean ± SD and represent three independent experiments. Statistical significance was determined by a two-tailed Student’s t test, *p < 0.05, ***p < 0.001. FFA, free fatty acid.
ADPN Promotes TG and Phospholipid Synthesis in Mammalian Cells
Next, we overexpressed mAdpn and hADPN in Cos-7 cells (Figure 6A) and determined cytosolic, membrane-associated, and LD-associated LPAAT activity. mAdpn and hADPN predominantly localized to the membrane- and LD- associated cellular fractions, but not to the cytosolic fraction (Figure 6A), confirming previous localization studies (He et al., 2010). Consistent with this finding, membrane-associated LPAAT activity was increased ~2-fold with overexpression of mAdpn and hADPN (Figure 6B), and LD-associated LPAAT activity of mAdpn and hADPN was increased ~2.2- and 2.8-fold, respectively (Figure 6C). LPAAT activity generates PA, which is an important metabolic intermediate and precursor for both TGs and glycerophospholipids (PLs). In accordance to this function in lipid synthesis, we found that the overexpression of mAdpn and hADPN significantly increased the incorporation of radiolabeled oleic acid into TGs and PLs by 1.5- and 1.6-fold, respectively, in Cos-7 cells cultivated in the presence of high concentration of glucose in the medium (Figures 6D and 6E and Figure S5A). Overexpression of mI148M or hI148M in Cos-7 cells led to even higher incorporation of oleic acid into TGs and PLs than overexpression of wild-type enzymes. Separation of PLs by TLC revealed that most of the oleic acid was incorporated into phosphatidylcholine (Figure 6E and Figure S5B). Thus, ADPN promotes cellular TG and PL synthesis (presumably by increased generation of PA), and the I148M variant displays a gain of function resulting in increased lipid synthesis.
Figure 6. Overexpression of ADPN in Mammalian Cells Increases LPAAT Activity and Lipid Synthesis.
Cos-7 cells were transfected with His-tagged pcDNA4/HisMax expression vector encoding mouse or human ADPN and LacZ as a control. After 24 hr, cells were loaded with 400 μM oleic acid complexed to FA-free BSA (3:1) in DMEM containing 10% FBS for 20 hr. LDs, membrane, and cytosol fractions were isolated using density gradient centrifugation.
(A) Expression of recombinant proteins was determined by immunoblotting using anti-His monoclonal antibody. Marker proteins for LDs and total membrane fractions were detected by anti-ADRP and anti-IRE1α antibody, respectively.
(B and C) (B) LPAAT activity assay using 20 μM [1-14C] oleoyl-CoA as acyl donor and 100 μM LPA as acyl acceptor in total membrane fractions (2 μg of total membrane protein) and (C) isolated LD fractions (20 μg of LD protein). Experiments were done in triplicate and repeated twice. Data are shown as mean ± SD. Statistical significance was determined by a two-tailed Student’s t test, *p < 0.05 and **p < 0.01.
(D and E) (D) Incorporation of [1-14C] oleic acid into TGs and total PLs in Cos-7 cells overexpressing mouse or human ADPN or their respective I148M mutant proteins, in the presence of high-glucose medium. Lipids were separated by TLC and radioactivity comigrating with TGs and total PLs, and (E) phosphatidylcholine (PC) was quantified by liquid scintillation counting. Experiments were done in duplicate and repeated thrice. The graph depicts the mean ± SD of two experiments. Statistical significance was determined by a two-tailed Student’s t test, *p < 0.05 (compared to LacZ), #p < 0.05 (compared to mouse/human WT ADPN).
Adpn Deficiency in Mice Results in Decreased Hepatic LPAAT Activity but Normal TG Hydrolase Activity
In accordance with previous studies (Dubuquoy et al., 2011; Rae-Whitcombe et al., 2010), we observed a strong increase in Adpn mRNA expression (25-fold) in response to a HSD (Figure 7A and Figure S6A). To investigate whether this increase affects liver LPAAT activity, we compared acyltransferase activities in liver homogenates of chow- and HSD-fed wild-type and Adpn-KO mice. In chow-fed mice, Adpn deficiency was not associated with a change in hepatic LPAAT activity in total tissue homogenates (Figure 7B). Feeding wild-type mice a HSD resulted in an ~3-fold increase in total LPAAT activity in liver homogenates. Importantly, hepatic LPAAT activity of Adpn-KO mice on HSD was 41% lower than in HSD-fed wild-type mice. Even more impressively, LD-associated LPAAT activity in the liver was reduced by 42% on chow diet and by 80% on HSD in Adpn-KO compared to wild-type mice (Figure 7C and Figure S6B).
Figure 7. Hepatic LPAAT Activity of Wild-Type and Adiponutrin-Deficient Mice.
Five hours postfeeding, male wild-type (WT) and Adpn-KO mice fed a chow diet or HSD were sacrificed, and the liver was excised.
(A) Relative liver Adpn mRNA expression as determined by RT-qPCR was normalized to β-actin as a reference gene and compared to WT mice fed chow diet, n = 6.
(B) LPAAT activity was determined in liver tissue homogenates (20 μg total protein), n = 5.
(C) LD-associated LPAAT activity was determined in freshly prepared liver LDs (10 μg LD protein). Data are representative of three independent diet studies, n = 4 or 5. Data are shown as mean ± SD. Statistical significance was determined by a two-tailed student’s t test, **p < 0.01, ***p < 0.001.
(D) Phosphatidic acid (PA) to lysophosphatidic acid (LPA) ratio in WT and Adpn-KO mice liver lipid extracts. Liver samples were collected from chow diet- and HSD-fed WT and Adpn-KO mice after 2 min organ perfusion with Krebs-Henseleit Buffer 2. For the determination of LPA and PA concentrations, hepatic glycerophospholipids were extracted using a modified Bligh and Dyer method (Bligh and Dyer, 1959). Lipids were quantified by LC/MS/MS (details described in the Supplemental Experimental Procedures), n = 6. Data are shown as mean ± SEM. Statistical significance was determined by post hoc analysis, ***p < 0.001.
Next, we determined whether reduced LPAAT activity affects hepatic PA and LPA concentrations. A trend toward lower PA and increased LPA concentrations (Figures S6C and S6D) led to a reduction of the PA/LPA ratio in total liver lipid extracts of Adpn-deficient mice on chow diet (p < 0.001) and HSD (p < 0.1) (Figure 7D). Total cellular DG levels were similar in wild-type and Adpn-deficient mice (Figure S6E). However, when specific DG species were analyzed, we noted a marked decrease in 34:1 DG by 40% (Figure S6F). Assuming that the de novo lipid synthesis predominantly generates 34:1 DG, these data suggested that Adpn deficiency may affect the de novo synthesis of TGs and PLs due to reduced provision of PA precursor. In line with previous studies (Basantani et al., 2011; Chen et al., 2010), Adpn deficiency in mice did not alter total liver TG content (Figure S6G), TG hydrolase activities in WAT or liver lysates (Figures S7A–S7D), or plasma parameters (Table S1).
DISCUSSION
Numerous independent studies reported a strong association between a common human ADPN variant (I148M) and the pathogenesis of fatty liver disease (Johansson et al., 2008; Kotronen et al., 2009; Romeo et al., 2010b). Yet, the underlying mechanisms for this association are still elusive. Experiments in the present study demonstrate that purified recombinant mouse and human ADPN exhibit acyl-CoA-dependent LPAAT activity. The fact that increased LPAAT activity is found in crude lysates of both bacterial (E. coli) and eukaryotic (Cos-7) expression systems suggests that the activity is intrinsic to ADPN and not caused by alternative proteins. This conclusion is further supported by the facts that single-point mutations in human ADPN drastically reduced LPAAT activity and that extensive sequence analysis of purified ADPN variants revealed no evidence for the presence of other contaminating acyltransferases than ADPN (data not shown). In accordance with an anabolic function, overexpression of ADPN promotes TG and PL synthesis in cultured cells. Together with the previously shown dietary effects on ADPN expression in adipose tissue and liver (Baulande et al., 2001; Hoekstra et al., 2010; Polson and Thompson, 2003b), these results imply that ADPN is a nutritionally regulated LPAAT participating in hepatic lipid synthesis. Importantly, the respective I148M variants of mouse and human ADPN exhibit higher LPAAT activities than the wild-type enzymes. This gain of function provides a potential explanation for the increased susceptibility of patients carrying the I148M variant to the development of liver steatosis and NAFLD.
Previous studies assigned other biochemical activities to ADPN, including a function as TG hydrolase (He et al., 2010; Huang et al., 2011; Jenkins et al., 2004; Lake et al., 2005). We confirmed this activity for mouse and human ADPN but found that the specific TG hydrolase activity is much lower than that for ATGL. The rather low TG hydrolase activity and the fact that ADPN overexpression failed to decrease cellular TG levels in vitro (Kershaw et al., 2006; Lake et al., 2005) or hepatic TG content in mice in vivo (He et al., 2010; Qiao et al., 2011) argue against a physiological function of ADPN as a catabolic TG lipase.
ADPN is the second member of the mammalian PNPLA family of proteins with acyl-CoA-dependent acyltransferase activity. Gao et al. (Gao et al., 2009; Gao and Simon, 2007) reported that a structurally related protein, GS2 (PNPLA4), catalyzes acyl-CoA-dependent and -independent acylation of retinol. In the yeast Saccharomyces cerevisiae, the patatin domain-containing yeast proteins TGL3, TGL4, and TGL5 were also shown to possess LPAAT activity in addition to lipolytic activities (Rajakumari and Daum, 2010). Other members of the PNPLA family are established TG hydrolases and phospholipases. Phospholipases of the family consistently hydrolyze the secondary ester at the sn-2 position of PLs. ADPN also acts at the sn-2 position of the glycerol backbone, but instead of ester hydrolysis, it catalyzes ester formation at this position. Notably, the enzyme is highly substrate specific for LPA and long-chain fatty acyl-CoAs, which is similar to the substrate preference reported for acyl-CoA acylglycerol-3 phosphate acyltransferase 2 (AGPAT2) (Takeuchi and Reue, 2009). It does not esterify other lysophospholipids or utilize acyl-CoAs shorter than C16.
ADPN adds to a long list of known LPAAT enzymes. The majority of the established LPAATs belong to the AGPAT family of enzymes (Takeuchi and Reue, 2009). At least seven AGPAT members (AGPAT1– AGPAT5, AGPAT8, and AGPAT9) mediate acylation of lysophospholipids in an acyl-CoA-dependent manner. The enzymes differ in substrate specificity and tissue-specific expression profile (Agarwal et al., 2002; Beigneux et al., 2006; Chen et al., 2008; Cortes et al., 2009; Vergnes et al., 2006). The physiological relevance of most AGPATs remains poorly defined, with one notable exception, AGPAT2 (LPAATβ). Deficiency of this enzyme causes congenital generalized lipid dystrophy (CGL) in humans (Agarwal et al., 2002). CGL is an autosomal recessive disorder characterized by pronounced lack of adipose tissue, severe hypertriglyceridemia, hepatic steatosis, insulin resistance, and diabetes. A similar phenotype is observed in AGPAT2-deficient mice (Cortes et al., 2009). In these animals, severe hepatic steatosis is apparently not caused by the compensatory action of alternative AGPATs but by a metabolic diversion that involves the dephosphorylation of LPA, the acylation of the resulting MG to DG by MGAT1, and the subsequent formation of TG by acyl-CoA:diacylglycerol acyltransferase 2 (DGAT2). In addition to AGPATs, CGI-58 can also act as an LPAAT independent to its ATGL coactivator function (Ghosh et al., 2008; Gruber et al., 2010; Montero-Moran et al., 2010). CGI-58 has no clearly defined acyltransferase domain and is structurally not related to members of the AGPAT family (Oberer et al., 2011). Accordingly, the enzymatic mechanism for the acyltransferase activity is unknown.
Unexpectedly, Adpn deficiency is not associated with a detectable metabolic phenotype in mice (Basantani et al., 2011; Chen et al., 2010). Adpn gene knockout does not affect TG hydrolysis, energy/glucose/lipid homeostasis, or hepatic lipid content in mice kept on chow, high-fat, or HSDs. Considering these resounding negative results, it was concluded that loss of ADPN does not contribute to the pathogenesis of fatty liver disease, at least in mice. Our finding that ADPN has significant LPAAT activity is consistent with this conclusion and suggests that liver steatosis in patients carrying the I148M mutation is more likely to be caused by a gain-of-function effect leading to increased TG synthesis and deposition. It is also consonant with the observation that adenoviral overexpression of the I148M mutant ADPN causes fatty liver in mice (He et al., 2010). However, why the adenoviral overexpression of wild-type ADPN does not affect hepatic TG levels (He et al., 2010) remains unclear.
The absence of changes in the cellular lipid composition or energy metabolism in Adpn-deficient mice (Basantani et al., 2011; Chen et al., 2010) indicates that the Adpn-catalyzed LPAAT activity is not essential under various dietary conditions. We noticed a clear difference in enzyme activities as well as a reduction in the hepatic PA/LPA ratio of Adpn-deficient mice. That these changes did not translate into a more severe phenotype in Adpn-deficient mice suggests that alternative pathways of lipid synthesis such as the MGAT1 pathway in AGPAT2-deficient mice (Cortes et al., 2009) can compensate for the reduced LPAAT reaction. Alternatively, the reduced LPAAT activity in the liver of Adpn-deficient mice may still suffice to ensure normal TG and PL synthesis.
The observed reduction in LD-associated LPAAT activity in Adpn-KO mice also suggests that Adpn may facilitate PA synthesis on LDs. Previous studies showed that other enzymes in the biosynthetic pathway of TGs and PLs are also present on LDs, including lipins (Valdearcos et al., 2011; Wang et al., 2011), DGAT2 (Kuerschner et al., 2008; Stone et al., 2009), and CTP:phosphocholine cytidylyltransferase (Krahmer et al., 2011). This indicates that lipid synthesis may not be restricted to the ER membrane but may also occur at least partially on LDs. The spatial separation of synthetic pathways for identical lipids suggests different functions for distinct lipid pools in different organelles. The importance of LDs and lipolysis as a platform for the generation of products participating in cardiac peroxisome proliferator-activated receptor (PPAR) signaling was recently shown (Haemmerle et al., 2011), and it is conceivable that ADPN-generated PA on LDs may also affect cellular signaling processes such as PPAR (Moolenaar et al., 2004), mammalian target of rapamycin (TOR) (Foster, 2009), or mitogen-activated protein kinase (MAPK) signaling (Wang et al., 2006).
In summary, we identified a biochemical function for ADPN. The protein acts as a LD-associated, nutritionally regulated LPAAT. In this function, the enzyme may contribute to local lipid synthesis and/or affect the generation of potential signaling lipids. The I148M variant of ADPN is more active than the wild-type enzyme, suggesting that hepatosteatosis in humans carrying this mutation results from a gain of function and increased hepatic TG synthesis.
EXPERIMENTAL PROCEDURES
Cloning, Expression, and Purification of mAdpn and hADPN
The coding sequences for mAdpn and hADPN were amplified by PCR using Phusion DNA polymerase and primers as given in Table S2. The purified amplicon was ligated into the bacterial expression vector pCold or the eukaryotic expression vector pcDNA4/HisMax C. Site-directed mutagenesis was performed using the GeneTailor Site-Directed Mutagenesis System (Invitrogen, Carlsbad, CA, USA). Wild-type and mutant murine and human ADPN proteins overexpressed in E. coli were purified by metal-ion affinity column.
[1-14C] Oleic Acid Incorporation in Cellular Lipids
HepG2, CHO, and Cos-7 cells were used to determine [1-14C] oleic acid accumulation in cellular TGs. HepG2 and CHO cells were infected with adenovirus expressing Adpn. Cos-7 cells were transfected with expression vector pcDNA4/HisMax C encoding Adpn. Cells were incubated with 1 μCi/ml [1-14C] oleic acid and 400 μM oleic acid complexed to BSA for 6 hr, lipids were extracted, and radioactivity comigrating with the TG band was quantified by scintillation counting. Cells were lysed in 0.3 N NaOH containing 0.1% SDS to determine the protein concentrations.
Animal Studies
Adpn-KO mice were generated and bred as described previously (Basantani et al., 2011). Plasma parameters were determined using commercially available kits. Isolation of tissue RNA and quantification by RT-qPCR followed standard laboratory procedures.
For HSD studies, male Adpn-KO mice (10 weeks old) were housed individually at 25°C using a 12 hr light/dark “switch cycle.” After 1 week acclimatization, mice were fed a semisynthetic diet consisting of 60% sucrose, 20% casein, 5% cellulose, 6% mixture of minerals, 5% vitamins, cysteine, choline chloride, and soybean oil (Ssniff, Soest, Germany) for 3 weeks (Polson and Thompson, 2003b). Mice were killed by cervical dislocation 5 hr after the feeding period (postmeal). Freshly dissected liver samples (~0.8 g) were used for LD isolation. Other tissues were dissected, weighed, immediately frozen in liquid nitrogen, and stored at −80°C until used. All animal procedures were carried out according to the guidelines of the Federation of European Laboratory Animal Science and were approved by Austrian government authorities.
Enzymatic Activity Assays
LPAAT activity was measured by acylation of LPA using [1-14C] oleoyl-CoA. Formation of radioactively labeled PA was determined in lipid extracts that were separated by TLC (Ghosh et al., 2008). Spots comigrating with PA standard were cut and quantified by scintillation counting.
TG hydrolase activity was determined in the purified protein fractions, bacterial total lysates, and membrane and total lysates fraction of Cos-7 cells as described previously (Lass et al., 2006).
Membrane- and LD-Associated LPAAT Assay in Cos-7 Cells
Cos-7 cells were transiently transfected with expression plasmids encoding His-tagged mAdpn or hADPN. After 24 hr of transfection, cells were incubated with 400 μM oleic acid for further 20 hr. Cells were harvested to isolate total membrane and LD fractions. LPAAT activity was measured in the LD, cytosolic, and membrane fractions as described above.
Western Blotting
Cell lysates (10 μg protein), purified proteins (2 μg protein), or cell fractions (6 μg protein) were separated by 10% SDS PAGE and transferred to polyvinylidene fluoride membranes (Carl Roth GmbH, Karlsruhe, Germany). The blots were incubated with anti-His (N-terminal) monoclonal antibody (1:5,000; GE Healthcare), anti-His (C-terminal) polyclonal antibody (1:1,000; ReliaTech, Germany), anti-adiponutrin polyclonal antibody (1:10,000; Everest Biotech), anti-adipophilin (ADRP) antibody (1:3,000; Progen Biotechnik), or anti-Inositol-requiring 1α (IRE1α) monoclonal antibody (1:1,000; Cell Signaling). For all immunoblots, appropriate horseradish peroxidase-conjugated secondary antibodies were used. Detection was performed with enhanced chemiluminescence (ECL plus, GE Healthcare) and exposure to X-ray film (Hyperfilm ECL, GE Healthcare).
Lipid Extraction and Mass Spectrometry Analysis
Liver samples were collected from Adpn-KO mice on chow or HSD after 2 min perfusion with Krebs-Henseleit Buffer 2. Lipids were extracted using a modified Bligh and Dyer procedure (Bligh and Dyer, 1959) and individual lipid species were identified by LC/MS/MS based on their chromatographic, and mass spectral characteristic.
Statistical Analyses
Data are presented as mean ± standard deviation (SD). Measurements in wild-type and Adpn-KO mice were compared by Student’s unpaired t tests (two tailed) or post hoc analysis. Data were considered statistically significant at *p < 0.05, **p < 0.01, ***p < 0.001.
Supplementary Material
Acknowledgments
We thank A. Hermann, Mag. B. Juritsch, Mag. C. Schober-Trummler, and Mag. C. Lanschuetzer for technical and administrative assistance. We thank D.S. Myers and Dr. S.B. Milne for assistance with the lipid identification and analysis. This work was supported by the Austrian Science Foundation (FWF) grants SFB LIPOTOX F30 (R.Z., M.O.), DK Molecular Enzymology W009 (R.Z., R.Zi., M.O.), Wittgenstein Z136 (R.Z.), P22170 (M.O.), and P21296 (R.Zi.) as well as by “GOLD - Genomics of Lipid-associated Disorders” within the framework of GEN-AU Genome Research in Austria by FFG and the Austrian Federal Ministry of Science and Research (R.Z., R.Zi, M.O.). Additional support for this work was provided by the European Regional Development Fund, the Province of Styria (R.Z.); National Institutes of Health (NIH) P30 DK-036836, Howard Hughes Medical Institute Early Career Award, and University of Pittsburgh Department of Medicine Junior Scholar Award (E.E.K.); and NIH Grant U54 GM069338 award (H.A.B.).
Footnotes
Supplemental Information includes seven figures, three tables, Supplemental Experimental Procedures, and Supplemental References and can be found with this article online at doi:10.1016/j.cmet.2012.04.008.
References
- Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899–905. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agarwal AK, Arioglu E, De Almeida S, Akkoc N, Taylor SI, Bowcock AM, Barnes RI, Garg A. AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet. 2002;31:21–23. doi: 10.1038/ng880. [DOI] [PubMed] [Google Scholar]
- Basantani MK, Sitnick MT, Cai L, Brenner DS, Gardner NP, Li JZ, Schoiswohl G, Yang K, Kumari M, Gross RW, et al. Pnpla3/adipo-nutrin deficiency in mice does not contribute to fatty liver disease or metabolic syndrome. J Lipid Res. 2011;52:318–329. doi: 10.1194/jlr.M011205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baulande S, Lasnier F, Lucas M, Pairault J. Adiponutrin, a transmembrane protein corresponding to a novel dietary- and obesity-linked mRNA specifically expressed in the adipose lineage. J Biol Chem. 2001;276:33336–33344. doi: 10.1074/jbc.M105193200. [DOI] [PubMed] [Google Scholar]
- Beigneux AP, Vergnes L, Qiao X, Quatela S, Davis R, Watkins SM, Coleman RA, Walzem RL, Philips M, Reue K, Young SG. Agpat6—a novel lipid biosynthetic gene required for triacylglycerol production in mammary epithelium. J Lipid Res. 2006;47:734–744. doi: 10.1194/jlr.M500556-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Calvo RM, Obregon MJ. Tri-iodothyronine upregulates adiponutrin mRNA expression in rat and human adipocytes. Mol Cell Endocrinol. 2009;311:39–46. doi: 10.1016/j.mce.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Chen YQ, Kuo MS, Li S, Bui HH, Peake DA, Sanders PE, Thibodeaux SJ, Chu S, Qian YW, Zhao Y, et al. AGPAT6 is a novel microsomal glycerol-3-phosphate acyltransferase. J Biol Chem. 2008;283:10048–10057. doi: 10.1074/jbc.M708151200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W, Chang B, Li L, Chan L. Patatin-like phospholipase domain-containing 3/adiponutrin deficiency in mice is not associated with fatty liver disease. Hepatology. 2010;52:1134–1142. doi: 10.1002/hep.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman RA. How do I fatten thee? Let me count the ways. Cell Metab. 2007;5:87–89. doi: 10.1016/j.cmet.2007.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes VA, Curtis DE, Sukumaran S, Shao X, Parameswara V, Rashid S, Smith AR, Ren J, Esser V, Hammer RE, et al. Molecular mechanisms of hepatic steatosis and insulin resistance in the AGPAT2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 2009;9:165–176. doi: 10.1016/j.cmet.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubuquoy C, Robichon C, Lasnier F, Langlois C, Dugail I, Foufelle F, Girard J, Burnol AF, Postic C, Moldes M. Distinct regulation of adiponutrin/PNPLA3 gene expression by the transcription factors ChREBP and SREBP1c in mouse and human hepatocytes. J Hepatol. 2011;55:145–153. doi: 10.1016/j.jhep.2010.10.024. [DOI] [PubMed] [Google Scholar]
- Foster DA. Phosphatidic acid signaling to mTOR: signals for the survival of human cancer cells. Biochim Biophys Acta. 2009;1791:949–955. doi: 10.1016/j.bbalip.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao JG, Simon M. A comparative study of human GS2, its paralogues, and its rat orthologue. Biochem Biophys Res Commun. 2007;360:501–506. doi: 10.1016/j.bbrc.2007.06.089. [DOI] [PubMed] [Google Scholar]
- Gao JG, Shih A, Gruber R, Schmuth M, Simon M. GS2 as a retinol transacylase and as a catalytic dyad independent regulator of retinylester accretion. Mol Genet Metab. 2009;96:253–260. doi: 10.1016/j.ymgme.2008.12.007. [DOI] [PubMed] [Google Scholar]
- Ghosh AK, Ramakrishnan G, Chandramohan C, Rajasekharan R. CGI-58, the causative gene for Chanarin-Dorfman syndrome, mediates acylation of lysophosphatidic acid. J Biol Chem. 2008;283:24525–24533. doi: 10.1074/jbc.M801783200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber A, Cornaciu I, Lass A, Schweiger M, Poeschl M, Eder C, Kumari M, Schoiswohl G, Wolinski H, Kohlwein SD, et al. The N-terminal region of comparative gene identification-58 (CGI-58) is important for lipid droplet binding and activation of adipose triglyceride lipase. J Biol Chem. 2010;285:12289–12298. doi: 10.1074/jbc.M109.064469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerle G, Moustafa T, Woelkart G, Buttner S, Schmidt A, van de Weijer T, Hesselink M, Jaeger D, Kienesberger PC, Zierler K, et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-alpha and PGC-1. Nat Med. 2011;17:1076–1085. doi: 10.1038/nm.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He S, McPhaul C, Li JZ, Garuti R, Kinch L, Grishin NV, Cohen JC, Hobbs HH. A sequence variation (I148M) in PNPLA3 associated with nonalcoholic fatty liver disease disrupts triglyceride hydrolysis. J Biol Chem. 2010;285:6706–6715. doi: 10.1074/jbc.M109.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra M, Li Z, Kruijt JK, Van Eck M, Van Berkel TJ, Kuiper J. The expression level of non-alcoholic fatty liver disease-related gene PNPLA3 in hepatocytes is highly influenced by hepatic lipid status. J Hepatol. 2010;52:244–251. doi: 10.1016/j.jhep.2009.11.004. [DOI] [PubMed] [Google Scholar]
- Huang Y, He S, Li JZ, Seo YK, Osborne TF, Cohen JC, Hobbs HH. A feed-forward loop amplifies nutritional regulation of PNPLA3. Proc Natl Acad Sci USA. 2010;107:7892–7897. doi: 10.1073/pnas.1003585107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Cohen JC, Hobbs HH. Expression and characterization of a PNPLA3 protein isoform (I148M) associated with nonalcoholic fatty liver disease. J Biol Chem. 2011;286:37085–37093. doi: 10.1074/jbc.M111.290114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huong DT, Ide T. Dietary lipoic acid-dependent changes in the activity and mRNA levels of hepatic lipogenic enzymes in rats. Br J Nutr. 2008;100:79–87. doi: 10.1017/S0007114507876227. [DOI] [PubMed] [Google Scholar]
- Jenkins CM, Mancuso DJ, Yan W, Sims HF, Gibson B, Gross RW. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J Biol Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- Johansson LE, Lindblad U, Larsson CA, Rastam L, Ridderstrale M. Polymorphisms in the adiponutrin gene are associated with increased insulin secretion and obesity. Eur J Endocrinol. 2008;159:577–583. doi: 10.1530/EJE-08-0426. [DOI] [PubMed] [Google Scholar]
- Kantartzis K, Peter A, Machicao F, Machann J, Wagner S, Konigsrainer I, Konigsrainer A, Schick F, Fritsche A, Haring HU, Stefan N. Dissociation between fatty liver and insulin resistance in humans carrying a variant of the patatin-like phospholipase 3 gene. Diabetes. 2009;58:2616–2623. doi: 10.2337/db09-0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ. Protein structure prediction on the Web: a case study using the Phyre server. Nat Protoc. 2009;4:363–371. doi: 10.1038/nprot.2009.2. [DOI] [PubMed] [Google Scholar]
- Kershaw EE, Hamm JK, Verhagen LA, Peroni O, Katic M, Flier JS. Adipose triglyceride lipase: function, regulation by insulin, and comparison with adiponutrin. Diabetes. 2006;55:148–157. [PMC free article] [PubMed] [Google Scholar]
- Kienesberger PC, Oberer M, Lass A, Zechner R. Mammalian patatin domain containing proteins: a family with diverse lipolytic activities involved in multiple biological functions. J Lipid Res Suppl. 2009;50:S63–S68. doi: 10.1194/jlr.R800082-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotronen A, Johansson LE, Johansson LM, Roos C, Westerbacka J, Hamsten A, Bergholm R, Arkkila P, Arola J, Kiviluoto T, et al. A common variant in PNPLA3, which encodes adiponutrin, is associated with liver fat content in humans. Diabetologia. 2009;52:1056–1060. doi: 10.1007/s00125-009-1285-z. [DOI] [PubMed] [Google Scholar]
- Krahmer N, Guo Y, Wilfling F, Hilger M, Lingrell S, Heger K, Newman HW, Schmidt-Supprian M, Vance DE, Mann M, et al. Phosphatidylcholine synthesis for lipid droplet expansion is mediated by localized activation of CTP:phosphocholine cytidylyltransferase. Cell Metab. 2011;14:504–515. doi: 10.1016/j.cmet.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuerschner L, Moessinger C, Thiele C. Imaging of lipid biosynthesis: how a neutral lipid enters lipid droplets. Traffic. 2008;9:338–352. doi: 10.1111/j.1600-0854.2007.00689.x. [DOI] [PubMed] [Google Scholar]
- Lake AC, Sun Y, Li JL, Kim JE, Johnson JW, Li D, Revett T, Shih HH, Liu W, Paulsen JE, Gimeno RE. Expression, regulation, and triglyceride hydrolase activity of Adiponutrin family members. J Lipid Res. 2005;46:2477–2487. doi: 10.1194/jlr.M500290-JLR200. [DOI] [PubMed] [Google Scholar]
- Lass A, Zimmermann R, Haemmerle G, Riederer M, Schoiswohl G, Schweiger M, Kienesberger P, Strauss JG, Gorkiewicz G, Zechner R. Adipose triglyceride lipase-mediated lipolysis of cellular fat stores is activated by CGI-58 and defective in Chanarin-Dorfman Syndrome. Cell Metab. 2006;3:309–319. doi: 10.1016/j.cmet.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Marchesini G, Brizi M, Bianchi G, Tomassetti S, Bugianesi E, Lenzi M, McCullough AJ, Natale S, Forlani G, Melchionda N. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- Moldes M, Beauregard G, Faraj M, Peretti N, Ducluzeau PH, Laville M, Rabasa-Lhoret R, Vidal H, Clement K. Adiponutrin gene is regulated by insulin and glucose in human adipose tissue. Eur J Endocrinol. 2006;155:461–468. doi: 10.1530/eje.1.02229. [DOI] [PubMed] [Google Scholar]
- Montero-Moran G, Caviglia JM, McMahon D, Rothenberg A, Subramanian V, Xu Z, Lara-Gonzalez S, Storch J, Carman GM, Brasaemle DL. CGI-58/ABHD5 is a coenzyme A-dependent lysophosphatidic acid acyltransferase. J Lipid Res. 2010;51:709–719. doi: 10.1194/jlr.M001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moolenaar WH, van Meeteren LA, Giepmans BN. The ins and outs of lysophosphatidic acid signaling. Bioessays. 2004;26:870–881. doi: 10.1002/bies.20081. [DOI] [PubMed] [Google Scholar]
- Oberer M, Boeszoermenyi A, Nagy HM, Zechner R. Recent insights into the structure and function of comparative gene identification-58. Curr Opin Lipidol. 2011;22:149–158. doi: 10.1097/MOL.0b013e328346230e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polson D, Thompson M. Adiponutrin gene expression in 3T3-L1 adipocytes is downregulated by troglitazone. Horm Metab Res. 2003a;35:508–510. doi: 10.1055/s-2003-41811. [DOI] [PubMed] [Google Scholar]
- Polson DA, Thompson MP. Adiponutrin mRNA expression in white adipose tissue is rapidly induced by meal-feeding a high-sucrose diet. Biochem Biophys Res Commun. 2003b;301:261–266. doi: 10.1016/s0006-291x(02)03027-9. [DOI] [PubMed] [Google Scholar]
- Qiao A, Liang J, Ke Y, Li C, Cui Y, Shen L, Zhang H, Cui A, Liu X, Liu C, et al. Mouse PNPLA3 influences systemic lipid and glucose homeostasis. Hepatology. 2011;54:509–521. doi: 10.1002/hep.24402. [DOI] [PubMed] [Google Scholar]
- Rae-Whitcombe SM, Kennedy D, Voyles M, Thompson MP. Regulation of the promoter region of the human adiponutrin/PNPLA3 gene by glucose and insulin. Biochem Biophys Res Commun. 2010;402:767–772. doi: 10.1016/j.bbrc.2010.10.106. [DOI] [PubMed] [Google Scholar]
- Rajakumari S, Daum G. Multiple functions as lipase, steryl ester hydrolase, phospholipase, and acyltransferase of Tgl4p from the yeast Saccharomyces cerevisiae. J Biol Chem. 2010;285:15769–15776. doi: 10.1074/jbc.M109.076331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reue K, Brindley DN. Thematic Review Series: Glycerolipids. Multiple roles for lipins/phosphatidate phosphatase enzymes in lipid metabolism. J Lipid Res. 2008;49:2493–2503. doi: 10.1194/jlr.R800019-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, Boerwinkle E, Cohen JC, Hobbs HH. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo S, Sentinelli F, Cambuli VM, Incani M, Congiu T, Matta V, Pilia S, Huang-Doran I, Cossu E, Loche S, Baroni MG. The 148M allele of the PNPLA3 gene is associated with indices of liver damage early in life. J Hepatol. 2010a;53:335–338. doi: 10.1016/j.jhep.2010.02.034. [DOI] [PubMed] [Google Scholar]
- Romeo S, Sentinelli F, Dash S, Yeo GS, Savage DB, Leonetti F, Capoccia D, Incani M, Maglio C, Iacovino M, et al. Morbid obesity exposes the association between PNPLA3 I148M (rs738409) and indices of hepatic injury in individuals of European descent. Int J Obes. 2010b;34:190–194. doi: 10.1038/ijo.2009.216. [DOI] [PubMed] [Google Scholar]
- Schaffer JE. Lipotoxicity: when tissues overeat. Curr Opin Lipidol. 2003;14:281–287. doi: 10.1097/00041433-200306000-00008. [DOI] [PubMed] [Google Scholar]
- Stone SJ, Levin MC, Zhou P, Han J, Walther TC, Farese RV., Jr The endoplasmic reticulum enzyme DGAT2 is found in mitochondria-associated membranes and has a mitochondrial targeting signal that promotes its association with mitochondria. J Biol Chem. 2009;284:5352–5361. doi: 10.1074/jbc.M805768200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Reue K. Biochemistry, physiology, and genetics of GPAT, AGPAT, and lipin enzymes in triglyceride synthesis. Am J Physiol Endocrinol Metab. 2009;296:E1195–E1209. doi: 10.1152/ajpendo.90958.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdearcos M, Esquinas E, Meana C, Gilde-Gomez L, Guijas C, Balsinde J, Balboa MA. Subcellular localization and role of lipin-1 in human macrophages. J Immunol. 2011;186:6004–6013. doi: 10.4049/jimmunol.1003279. [DOI] [PubMed] [Google Scholar]
- Valenti L, Al-Serri A, Daly AK, Galmozzi E, Rametta R, Dongiovanni P, Nobili V, Mozzi E, Roviaro G, Vanni E, et al. Homozygosity for the patatin-like phospholipase-3/adiponutrin I148M polymorphism influences liver fibrosis in patients with nonalcoholic fatty liver disease. Hepatology. 2010;51:1209–1217. doi: 10.1002/hep.23622. [DOI] [PubMed] [Google Scholar]
- Vergnes L, Beigneux AP, Davis R, Watkins SM, Young SG, Reue K. Agpat6 deficiency causes subdermal lipodystrophy and resistance to obesity. J Lipid Res. 2006;47:745–754. doi: 10.1194/jlr.M500553-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Devaiah SP, Zhang W, Welti R. Signaling functions of phosphatidic acid. Prog Lipid Res. 2006;45:250–278. doi: 10.1016/j.plipres.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang J, Qiu W, Han GS, Carman GM, Adeli K. Lipin-1gamma isoform is a novel lipid droplet-associated protein highly expressed in the brain. FEBS Lett. 2011;585:1979–1984. doi: 10.1016/j.febslet.2011.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PA, Gardner SD, Lambie NM, Commans SA, Crowther DJ. Characterization of the human patatin-like phospholipase family. J Lipid Res. 2006;47:1940–1949. doi: 10.1194/jlr.M600185-JLR200. [DOI] [PubMed] [Google Scholar]
- Yen CL, Stone SJ, Koliwad S, Harris C, Farese RV., Jr Thematic Review Series: glycerolipids. DGAT enzymes and triacylglycerol biosynthesis. J Lipid Res. 2008;49:2283–2301. doi: 10.1194/jlr.R800018-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Waterworth D, Perry JR, Lim N, Song K, Chambers JC, Zhang W, Vollenweider P, Stirnadel H, Johnson T, et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. 2008;83:520–528. doi: 10.1016/j.ajhg.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner R, Zimmermann R, Eichmann TO, Kohlwein SD, Haemmerle G, Lass A, Madeo F. Fat signals—lipases and lipolysis in lipid metabolism and signaling. Cell Metab. 2012;15:279–291. doi: 10.1016/j.cmet.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, Zechner R. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.