Abstract
Damage to the hippocampal and frontostriatal systems can occur across the adult life span. As these 2systems are involved in learning processes, mild impairments of learning and generalization might be observed even in healthy aging. In this study, we examined both learning and generalization performance in 3 groups of older adults: young-older (ages 45–60), middle-older (ages 61–75), and oldest-older (ages 76–90).We used a simple computerized concurrent discrimination task in which the learning phase has demonstrated sensitivity to frontostriatal dysfunction, and the generalization phase to hippocampal damage. We found that age significantly affected initial learning performance, but generalization was spared in all but the oldest group, with some individuals still generalizing very well. This finding suggests that (a) learning abilities are affected in healthy aging (consistent with earlier reports of frontostriatal dysfunction in healthy aging) and (b) generalization deficit does not necessarily occur in early older age. We hypothesize that generalization deficits in some in the oldest group may be related to hippocampal pathology. Our data shed light on possible neural system dysfunction in healthy aging and Alzheimer disease.
Keywords: learning, generalization, basal ganglia, hippocampus, Alzheimer disease
Introduction
Behavioral and Neural Correlates of Healthy Aging
Human aging is associated with differing degrees of cognitive deficits (Raz et al., 1998; Collie et al., 2001). Learning and memory functions in the healthy aging population have been studied extensively from both behavioral and neural perspectives. Behaviorally, aging studies have tested various forms of learning processes (Frank & Kong, 2008; Simon, Howard, Howard, 2010, 2011; Simon, Vaidya, Howard, and Howard, 2011; Head & Isom, 2010), including weather prediction (Fera et al., 2005) and probabilistic classification (Frank & Kong, 2008; Simon et al., 2010).
At the neural level, volume in many brain areas decreases with increasing age (Golomb et al., 1993; Small et al., 2002). Normal aging can bring hippocampal volume reductions along with functional abnormalities (de Leon et al., 1997; Mu et al., 1999; Small et al., 2002; Lister & Barnes, 2009). Accelerated volume reduction in areas such as the hippocampus and other medial temporal structures may predict risk for cognitive decline and development of Alzheimer disease (AD) (de Leon et al., 1989; Golomb et al., 1996; de Toledo-Morrell et al., 2000; Rodrigue & Raz, 2004).The striatum also shows volume reduction in the course of normal aging (eg, Gunning-Dixon et al., 1998; Morgan, May & Finch, 1987; (Wang, Xu, & Zhang, 2010),a decrease that may translate into behavioral deficits and cognitive changes (Frank and Kong, 2008; Marschner et al., 2005).
Although the literature on the effects of aging on the hippocampal and frontostriatal systems is divided (for a review, see Hedden & Gabrieli, 2004), some argue that any meaningful deficit in medial temporal lobe functions might be caused by pathology (as in AD) rather than healthy aging per se (Hedden & Gabrieli, 2005; Raz & Rodrigue, 2006; Rand- Giovanetti et al, 2006). Importantly, the 2-stage theory of cognitive decline in aging (see Gabrieli et al., 1996; Hedden & Gabrieli, 2004, 2005; Rosen et al., 2005) suggests that the frontostriatal system changes first, decreasing dopamine levels and reducing prefrontal volume, and hippocampal dysfunction follows. In this study, we tested groups of healthy older adults (belonging to the comparatively younger ages of 45–60 y through the older ages of 76–90 y) on a concurrent discrimination learning and transfer generalization task(which recruits striatal and medial temporal structures) to examine whether aging has differential effects on learning and generalization.
Studying Learning and Generalization in Healthy Aging
Studies have shown that the striatum and the basal ganglia play different roles in learning and generalization. Specifically, the hippocampus and associated medial temporal structures are particularly implicated in learning that supports generalization when familiar stimulus features are recombined at testing (Eichenbaum et al., 1989; Eichenbaum et al., 1994; Myers & Gluck, 1994; Gluck & Myers, 2001). Humans with medial temporal damage are similarly impaired at retrieving studied information when study and test conditions are varied (eg, Schacter, 1985; Myers et al., 2003; Myers et al., 2008a). The striatal areas are also critically implicated in learning. For example, patients with striatal dysfunction caused by Parkinson disease are impaired at discrimination learning (Shohamy et al., 2002). Functional magnetic resonance imaging studies have also suggested that the striatal regions are especially active during learning of stimulus-response associations (Vandenberghe et al., 1999; Poldrack et al., 1999, 2001).
Some studies have shown an age-related deficit in ability to shift or generalize learning along a previously relevant stimulus dimension, such as shape or color (eg, Coppinger & Nehrke, 1972; Nehrke, 1973; Nehrke&Coppinger, 1971); others have found no age-related deficit in interdimensional shifting (eg, Robbins et al., 1998; Owen et al., 1991; Rabbitt& Lowe, 2000). Most of these studies have considered generalization tasks that involve presenting novel exemplars along a previously relevant dimension, rather than keeping the previously relevant features constant. To avoid the potential confound of introducing novel relevant features, we have developed an 8-pair concurrent visual discrimination task, in which each pair contains 2 objects that differ in 1relevant feature but share 1irrelevant feature.
In a 2002 study, Myers et al studied learning and generalization using the same task in nondemented elderly with or without mild hippocampal atrophy. The presence or absence of hippocampal atrophy did not affect the initial discrimination learning; however, individuals with mild hippocampal atrophy were significantly more impaired than nonatrophied controls on the generalization phase (Myers et al., 2002). By contrast, performance on other memory measures, such as a paragraph recall task, was not impaired in the atrophy group. In a later longitudinal study, Myers et al showed that performance on the generalization test among nondemented elderly was correlated with cognitive status assessed 2 years later; thus, generalization may be a useful predictor of risk for short-term cognitive deficit (Myers et al., 2008b). Consistent with these results, amnesic patients with bilateral hippocampal damage caused by hypoxic brain injury were not impaired at initial learning, but were impaired at transfer generalization (Myers et al., 2008a). Together, these results suggest that the transfer generalization portion of this task depends on hippocampal function.
The same task has also shown sensitivity to frontostriatal function. Specifically, patients with damage to the orbitofrontal cortex showed impairments in the learning phase of the task but performed better in the generalization phase, indicating the role of frontal cortex in learning and the role of intact medial temporal lobe structures in generalization (Chase et al., 2008). Patients with hypoxic brain injury and anterior communicating artery aneurysm rupture as well as various frontal lesions were slow at initial learning, but completed the generalization phase as well as controls. In contrast, the hypoxic patients were normal at initial learning, but impaired at transfer generalization (Myers et al, 2008a).
Learning and generalization were also studied in patients with bipolar disorder or schizophrenia and in first-degree relatives of patients with schizophrenia (Krause et al, 2009). All of these groups showed significant impairment in generalization compared with controls. Unlike the other groups, the schizophrenia group showed difficulty in the learning phase itself. The task has also been found to discriminate learning and generalization deficits in patients with Parkinson disease, tested on and off dopaminergic medication (L-dopa) (Shohamy et al., 2006). Patients tested off L-dopa performed better than patients on L-dopa in the learning phase, but not in the generalization phase.
The findings in these studies point to the possibility that disrupted frontostriatal circuits may produce impairments in the learning phase of this task, but that additional hippocampal disruptions may be required to show impairments in the generalization phase.
Because it had shown considerable sensitivity and moderate specificity to brain damage in the clinical population, we decided to use the same concurrent discrimination task to study possible learning and memory deficits in healthy normal individuals. To our knowledge, this is the first cross-sectional study to use this task to investigate age differences in initial acquisition versus generalization. Although previous studies have compared learning across different age groups using incrementally acquired learning tasks and tasks such as the Wisconsin Card Sorting Test, comparisons are usually between younger adults and older adults (Fera et al., 2005; Daigneault, Braun, Whitaker 1992; Head et al., 2009; Haaland, Vranes, Goodwin Garry 1987; Boone, Ghaffarian, Lesser, and Hill-Gutierrez (1993). Again, earlier studies of older adults have focused mainly on those older than 60. By contrast, our study of learning and generalization processes included healthy individuals aged 45–90.
We expected to see age-related deficits in initial learning, consistent with our prior studies (Myers et al., 2002; Shohamy et al., 2006). In our previous study, we used the same task in a small sample of 34 people with mild cognitive impairment and hippocampal atrophy (Myers, Kluger, et al., 2002). In the current study, we tested the performance on the learning and generalization task in a larger sample of healthy older participants, none of whom had mild cognitive impairment. The critical question was whether we would find a pattern of age differences between the younger and older groups for learning, but not generalization.
METHODS
Participants
We recruited 61 volunteers from a group of healthy adults taking a continuing education class in memory at the Academy of Lifelong Learning, University of Delaware, Newark. We recruited an additional 19 volunteers from 2 sources: the Memory Disorders Project at Rutgers-Newark and Eastern Mennonite University. We paid the Delaware and Rutgers volunteers $20 per hour for their time, and covered their transportation costs; the Eastern Mennonite University volunteers received class credit for participating. All 80 participants were between 45 and 90 y old and were fluent English speakers.
We screened the participants on the basis of self-reports of their medical and psychiatric history, including depression or affective disorder, alcohol abuse, diabetes, neuropsychological disorders (eg, multiple sclerosis, aphasia, seizure/epilepsy), and surgery with general anesthesia within the previous 6 months. We also screened the participants for drugs that could affect memory, particularly anticholinergic medications, and for colorblindness.
Before testing began, all participants signed statements of informed consent. Research conformed to guidelines for protection of human subjects established by Rutgers University and the federal government.
Neuropsychological Assessment
In an attempt to exclude anyone who showed evidence of cognitive impairment consistent with early dementia or another age-related disorder, we gave the participants a short battery of neuropsychological tests. We used the North American Adult Reading Test (Blair & Spreen, 1989) to index cognition; this test involves pronouncing a list of 61 orthographically irregular words. Scores can be used to generate estimates of verbal intelligence quotient (VIQ) (Spreen & Strauss, 1998, p. 78). Normal median VIQ score is defined as 100 (SD 15); no participants scored below normal.
We gave the Controlled Oral Word Association test (COWA) to index executive function. In this test, participants are given 1minute to generate as many words as possible beginning with a particular letter; scores are summed across trials with 3letters (F, A, S). COWA performance has been shown to correlate with frontal function (eg, Janowsky, Shimamura, Kritchevsky, & Squire, 1989). Using norms presented in Spreen and Strauss (1998, p. 455), we computed the age- and education-adjusted expected mean and standard deviation for each individual and used the data to compute a z-score (expected mean 0.0, SD 1.0). We excluded from further testing the 5 participants who scored more than 1 standard deviation below age-appropriate norms (z < −1.0).
We used the Logical Memory (LM) subtest of the Wechsler Memory Scale-Revised (Wechsler, 1987) to assess verbal memory; this test involves repeating 2 short paragraphs from memory immediately and after a delay of several minutes. Participants are scored according to the total items recalled from both paragraphs. Performance on delayed paragraph recall is especially sensitive as an indicator of hippocampal atrophy and a predictor of cognitive deficit in the nondemented elderly (eg, Golomb et al., 1994, 1996). In our testing, 20 minutes elapsed between the initial and delay trials; during the delay, the participant completed other tests. Five participants scored below the 25th percentile on either the immediate or the delay portion of the LM, according to the age-appropriate percentile scores provided in Wechsler (1987). These 5 volunteers were excluded from further testing.
Of the original 80 volunteers, the final sample consisted of the 70 who scored at or above age-appropriate norms on the standardized neuropsychological tests. Results for these 70 participants are shown in Table 1. Preliminary analysis using age as a continuous variable did not yield any statistically significant differences. Because our purpose was to examine both age-related deficits in performance and individual performance deficits in the main task, we divided our sample into 3 age groups: young-older (45–60 y), middle-older (61–75 y), and oldest-older (76–90 y). Education level did not differ among these groups (ANOVA, F[2,67] =1.51, P= 0.228), but there were more women than men in the youngest and middle groups, and more men than women in the oldest group (χ2 test, χ2=6.09, df=2, P= 0.043).
Table 1.
Demographic and Neuropsychological Test Data for the 70 Study Participants
| Young-Older | Middle-Older | Oldest-Older | All Subjects | ||
|---|---|---|---|---|---|
| N | 22 | 30 | 18 | 70 | |
| Age range (y) Mean age |
45–60 51.6 (4.7) |
61–75 68.7 (3.5) |
76–90 79.4 (3.6) |
45–90 66.1 (11.4) |
|
| Education (mean) (y) | 14.9 (2.4) | 15.9 (1.9) | 15.3 (2.5) | 15.4 (2.2) | F = 1.51, P = 0.228 |
| Men/women | 5/17 | 13/17 | 11/7 | 29/41 | |
| VIQ (mean) | 114.7 (8.7) | 116.9 (5.6) | 115.5 (7.1) | 115.9 (7.0) | F = 0.62, P > 0.500 |
| COWA (mean) | 0.04 (0.86) | 0.53 (0.96) | 0.39 (0.88) | 0.31 (0.93) | F = 2.56, P = 0.085 |
| LM immediate (mean) | 28.2 (8.2) | 28.4 (5.7) | 26.4 (4.5) | 27.8 (6.3) | F = 0.57, P > 0.500 |
| LM delay (mean) | 21.8 (5.1) | 25.6 (6.8) | 22.9 (7.2) | 23.7 (6.6) | F = 2.37, P = 0.102 |
VIQ indicates Verbal Intelligence Quotient on the North American Adult Reading Test; COWA, age-adjusted z-score on Controlled Oral Word Association Test; LM, recall scores for the immediate and delay portions of the Wechsler Memory Scale-Revised Logical Memory subtest.
Within each group, the mean neuropsychological scores shown in Table 1 are slightly higher than the expected level in a group of unselected normal people because we excluded our lowest-performing participants. ANOVAs revealed no significant differences among the groups on estimated VIQ (F[2,67] =0.62, P>0.500), COWA z-score (F[2,67] =2.56, P= 0.085), LM immediate (F[2,67] =0.57, P>0.500), or LM delay (F[2,67] =2.37, P= 0.102).
The 70 participants who scored at or above age-appropriate levels on neuropsychological testing were given the concurrent discrimination-and-generalization task.
Discrimination-and-Generalization Task
The discrimination-and-generalization task has been described in Myers et al (2002). In brief, participants learn a series of visual discriminations and are then challenged to generalize when stimulus information changes. The testing took place in a quiet room, with the participant seated in front of a laptop computer with a color screen. The keyboard was masked except for 2 keys, labeled LEFT and RIGHT, that the participant used to enter responses.
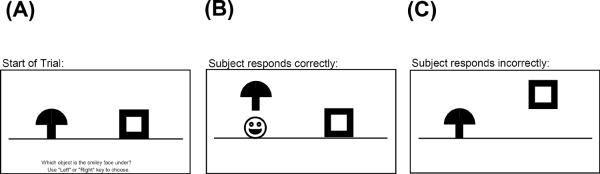
The discrimination-and-generalization task had 2 phases. Phase 1 was an 8-pair concurrent discrimination. On each trial, 2 colored shapes appeared, approximately 1 inch high on the screen and set about 3 inches apart (approximately 1.5 degrees of visual angle, at normal viewing distance). The participant was instructed to press the left or right key to choose 1 object. The chosen object rose onscreen and, if the choice was correct, a smiley face was revealed underneath (see Figure 1A). There was no limit on response time, and there was an interval of approximately 1 second between participant response and start of the next trial, allowing the participant to view the discrimination pair and feedback (presence or absence of the desired smiley face icon).
Figure 1.
Screen events on a sample trial of phase 1 (learning). (A) At the start of the trial, 2 objects appear, differing in color or shape, but not both. The printed instruction is, “Which object is the smiley face under? Use `Left' or `Right' key to choose.” (B) The participant chooses 1 object, and that object rises onscreen; if the choice was correct, a smiley face appears below it. C) If incorrect, no smiley face appears. Figure adapted from Myers, Kluger et al, 2002.
This was an incrementally acquired, feedback-based learning task in which participants were to learn which object was correct. They were given no information about the correct object ahead of time. Within each object pair, the same object was always rewarded. For 4 of the discrimination pairs, objects differed in shape but not color (eg, brown mushroom vs brown frame); for the other 4 pairs, objects differed in color but not shape (eg, red cat's-eye vs yellow cat's-eye). Thus, within each pair, 1 dimension (shape or color) was relevant to predicting the location of the smiley face, and 1 dimension was irrelevant.
Trials were organized into blocks, each containing 16 trials: 1 presentation of each discrimination pair in each possible left-right ordering. Trials in a block occurred in a pseudo-random but fixed order. Phase 1 continued until the participant reached a criterion of 16 consecutive correct responses, or for a maximum of 96 trials (6 blocks).
As soon as phase 1 ended, phase 2 began without any warning to the participant. The screen events were identical to phase 1 except that the discrimination pairs were altered so that the relevant features remained constant but the irrelevant features were altered. For example, the phase 1 discrimination in which a brown mushroom was rewarded over a brown frame became in phase 2 a discrimination in which a green mushroom was rewarded over a green frame. Similarly, the phase 1 discrimination in which a red cat's-eye was rewarded over a yellow cat's-eye became in phase 2 a red/yellow discrimination involving a new shape. Individuals who had solved phase 1 by basing associations on the relevant features (mushroom beats frame and red beats yellow) could perform perfectly in phase 2, since the relevant features were still predictive. By contrast, individuals who had approached phase 1 by learning to respond to whole objects (brown mushroom beats brown frame) should perform poorly in phase 2, which presented novel objects (green mushroom and green frame).
Phase 2 was also organized into blocks of 16 trials, 1 trial with each discrimination pair in each possible left-right ordering, in a pseudo-random but fixed order. Phase 2 continued until the participant reached a criterion of 16 consecutive correct responses, or a maximum of 48 trials (3 blocks).
The entire procedure took about15–20 minutes to complete.
RESULTS
Phase 1: Initial Learning
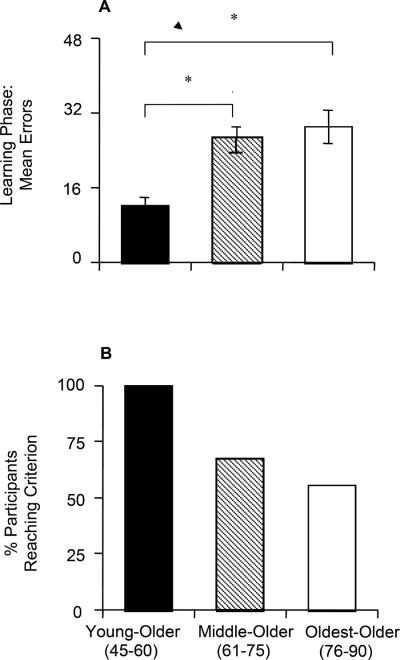
The mean number of phase 1 errors for the 3 age groups is shown in Figure 2A. A 1-way ANOVA revealed a significant effect of group (F [1,63]=9.53, P<0.001), with no effect of VIQ, COWA z-score, LM immediate, or LM delay scores (all P>0.100). Scheffé pairwise comparisons confirmed that the youngest group made significantly fewer errors than either the middle (P= 0.001) or oldest group (P<0.001); the middle and oldest groups did not differ in the number of errors (P>0.500). Individual bivariate correlations showed a moderately significant correlation between phase 1 errors and LM immediate and LM delayed scores for the middle group (r = −0.49, P= 0.01, and r = −0.45, P= 0.05), but there was no significant correlation in either of the other groups.
Figure 2.
(A) Phase 1 (learning) results: mean total errors. (B) Percentage of participants reaching criterion performance in phase 1 within the maximum 96 trials.
Fifty-two participants (74%) reached criterion performance (ie, 16 consecutive correct responses) in phase 1. Figure 2B shows that the distribution of these 52 participants was not equal across groups: All in the youngest group reached criterion, but only about half in the oldest group did so. This group effect was highly significant (χ2 test, χ2 =11.83, df=2, P= 0.003).
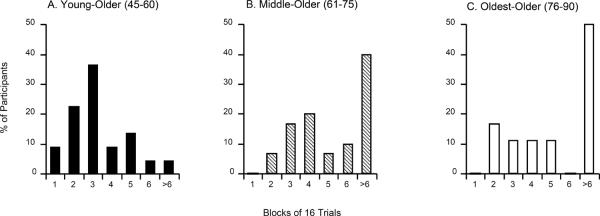
Figure 3 shows that a majority of participants aged 45–60 (youngest group) reached criterion within the first few blocks of training (fewer than 48 trials); among those in the middle and oldest groups, about half of participants failed to reach criterion at all, although many of those who succeeded, reached criterion about as quickly as the youngest participants.
Figure 3.
Distribution of trial blocks needed to reach phase 1 criterion in each age group. Participants who did not reach criterion within the maximum 6 blocks (96 trials) were scored as requiring >6 blocks.
In summary, there was an age effect on acquisition of the concurrent discrimination, with older participants making more errors and being less likely overall to reach criterion performance within the maximum 96 trials.
Phase 2: Generalization
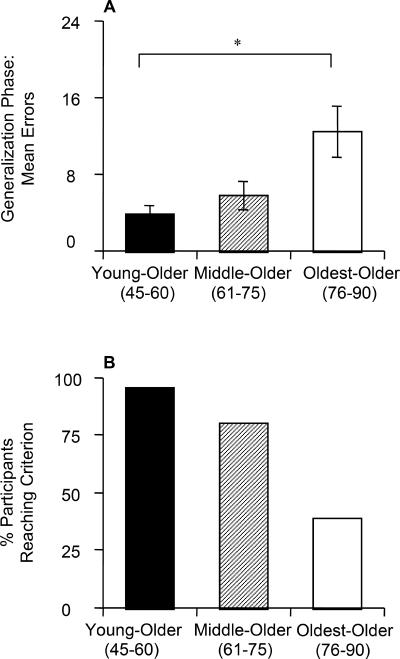
The mean number of phase 2 errors for the 3 age groups is shown in Figure 4A. ANOVA revealed a significant effect of group (F[1,62] =6.29, P= 0.003), with no effect of VIQ, COWA z-score, LM immediate, or LM delay scores (all F<2.20, all P> 0.100) but with a significant effect of phase 1 errors on phase 2 performance (F[1,62] =13.14, P= 0.001).
Figure 4.
(A) Phase 2 (generalization) results: mean total errors. (B) Percentage of participants reaching criterion performance in phase 2 within the maximum 48 trials.
Scheffé pairwise comparisons confirmed that the youngest group made significantly fewer errors than the oldest group (P= 0.004); the middle group did not differ significantly from either the youngest (P= 0.348) or the oldest group (P= 0.070). Individual bivariate correlations did not show any significant effect in any of the age groups.
Meeting the criterion of 16 consecutive correct responses, 52 participants (74%) solved phase 2. Figure 4B shows the distribution of participants who reached criterion in each age group; again, younger participants were more likely to reach criterion than older participants (χ2 test, χ2=20.63, df=2, P< 0.001). Just as in phase 1, most participants either solved phase 2 quickly (fewer than 16 trials) or not at all (more than 48 trials).
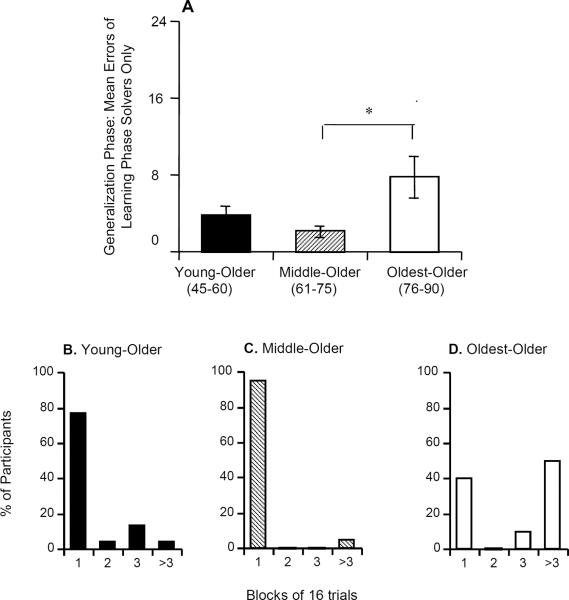
Because about 25% of the participants failed to reach criterion in phase 1 and because these nonsolvers were disproportionately older people, we did a second analysis of phase 2 errors for only those 52 people who had reached criterion in phase 1. Figure 5A shows phase 2 data for these participants. There was again a significant effect of group (F[1,44] =4.40, P= 0.018), but no significant effect of VIQ, COWA z-score, LM immediate, LM delay, or phase 1 errors (all P> 0.100). Scheffé pairwise comparisons revealed a significant difference between the middle and oldest (P= 0.031) groups; the differences between the youngest and middle (P> 0.500) and the youngest and oldest (P= 0.065) groups fell short of significance.
Figure 5.
(A) Phase 2 performance by participants who had reached criterion in phase 1. (B–D) Distribution of trials needed to reach phase 2 criterion. Participants who did not reach criterion within the maximum 3 blocks (48 trials) were scored as requiring >3 blocks.
Figure 5B shows that, in both the youngest and middle groups, most participants reached phase 1 criterion within a single block; by contrast, in the oldest group, about half of the participants did not reach criterion within the maximum 48 trials, although most of those who reached criterion, did so as quickly as the younger participants.
DISCUSSION
To learn about possible age-related cognitive differences in healthy aging, we assessed the performance of a group of active, healthy adults who had no history of psychiatric or medical conditions that might affect memory, in a computerized concurrent discrimination-and-generalization task. The results showed a significant age effect in both the learning and generalization phases of the experiment. Most important, with the cross-sectional design, we found that age affected learning more than generalization.
In the learning phase, although the 3 age groups performed at or better than age-appropriate norms on several neuropsychological measures, they nevertheless showed an age-related impairment on concurrent discrimination learning. This impairment was evident as a deficit in performance in the middle (age 61–75) and oldest (age 76–90) groups relative to the youngest group (age 45–60). Our finding is consistent with prior studies that likewise demonstrated age-related impairments on 2-choice discrimination (eg, Nehrke, 1973; Roger, Keyes, & Fuller, 1976) and with studies suggesting age-related impairments in components of associative learning, such as retaining information about previously correct choices (eg, Fisk & Warr, 1998; Salthouse, 1994; Salthouse & Dunlosky, 1995).
Some authors have suggested that humans seem to use declarative or explicit memory strategies to learn concurrent discriminations (Squire, Zola-Morgan & Chen, 1988; Hood, Postle & Corkin, 1999). In the context of our study, this idea might imply that our elderly participants had some difficulty with declarative learning. However, we did not find any consistent relationship between learning phase performance and declarative memory, as assessed by the Wechsler Memory Scale-Revised Logical Memory test. Similarly, in our prior study (Myers, Kluger et al., 2002), there was no relationship between learning phase and a related paragraph recall test derived from the Guild Memory Test (Crook, Gilbert, & Ferris, 1980).
It is possible that paragraph recall tests alone are not sufficiently sensitive to detect a subtle declarative memory deficit, which negatively affects concurrent discrimination learning. A paradigm such as the Word-List Learning Task, which is sensitive to mild memory impairments and has been shown to be related to both hippocampal and temporal lobe function (Koylu et al., 2008), would have been more appropriate for the current study. The current data cannot support the view that declarative memory deficits contribute to concurrent discrimination deficits.
In the generalization phase of the experimental task, irrelevant features were altered, but relevant features remained unchanged. Once again, there was a trend for impaired performance with increasing age. Although only the difference between the middle and oldest groups was significant (Figure 5A), our findings that those in the youngest group were more impaired at learning, but not at generalization performance than middle group, fit with the existing hypothesis that striatal deterioration is normal in healthy aging, but that hippocampal atrophy may be associated with severe pathology (Hedden & Gabrieli, 2004, 2005). In short, the data suggest that the frontostriatal and hippocampal systems age differently across the adult lifespan, in agreement with the 2-stage theory (Hedden & Gabrieli, 2004, 2005).
To rule out the possibility that learning phase performance affects generalization phase performance, we specifically tested the performance of those participants who had previously reached criterion in the learning phase. We then found that the difference in the generalization phase performance between the youngest and the middle groups was eliminated, but the performance was bimodal in the oldest group, with almost half of them reaching the phase 2 criterion (completing phase 2 within 48 trials) and the other half not reaching criterion at all (Figure 5D).
The current data showing half the oldest group reaching criterion are generally consistent with a prior study (Owen et al., 1991) that found elderly individuals (age 70–79) to be unimpaired at shifting to new exemplars of a previously relevant dimension. As for the half not reaching criterion despite learning adequately, their performance may suggest a nonspecific age effect among healthy people, such as functional hippocampal damage, impairing feature-irrelevant generalization in the oldest age group.
Our previous study (Myers et al., 2002) found that generalization phase impairment was associated with hippocampal atrophy in the nondemented elderly. In that study, we suggested that a threshold of 7 phase 2 errors maximized specificity versus selectivity in differentiating between participants with and those without hippocampal atrophy. As shown in Figure 4A, the mean generalization phase errors for both the youngest and middle groups were below that threshold, and the average for the oldest group was higher. As shown in Figure 5D, the relatively poor generalization performance in the oldest group reflected bimodality, with about half the group reaching criterion within 48 trials (some making 0 or 1 error) and about half the group failing to reach criterion (and making 10 or more errors). However, the high proportion of participants aged 45–75 who scored below this generalization performance threshold suggests that healthy aging is not associated with decreased generalization performance, since generalization performance appears strong in all but the oldest group.
Several experimental studies have reported frontostriatal and dopaminergic dysfunction and cognitive alterations in healthy aging people (Frank & Kong, 2008; Wang et al., 2010; Bäckman et al, 2010; Cherubini et al., 2009). For example, Bäckman et al (2010) reviewed evidence clearly supporting the findings that some of the behavioral deficits reported in healthy aging people are likely to be caused by dopaminergic dysfunction. Furthermore, in magnetic resonance imaging studies, Cherubini et al (2009) found structural changes in the frontostriatal system (but not the hippocampus) across the adult lifespan. It is possible that hippocampal dysfunction appears in late stages of aging, which would agree with our findings of generalization deficits only in the oldest group.
Although it is known that hippocampal volume decreases in healthy aging people (de Leon et al., 1997; Mu et al., 1999), the rate of this reduction is relatively slow—on the order of 1%–2% shrinkage per year, even in persons aged 70–89 (Jack et al., 1998). This fact may explain why we found generalization deficits only in the oldest group. It is reasonable to assume that small amounts of age-related hippocampal reduction, as in our middle group, do not affect generalization, but that accumulated reduction, as in our oldest group, can produce generalization deficits. The rate of hippocampal volume reduction is significantly higher in patients with AD than in nondemented same-aged peers (Jack et al., 1998).
Because our participants were nondemented and performed somewhat above normal age-appropriate levels on several neuropsychological tests, it could be assumed that those in the youngest and middle groups did not have significant hippocampal atrophy. At the same time, it is possible that some of those in our oldest group had hippocampal/medial temporal atrophy, resulting in poor generalization performance, and that the others, who did well on the generalization phase, may have had little or no atrophy. We could not assess this possibility in our participants because neuroimaging was not available, but it remains a question for future research.
Our data showing greater learning deficits in older than in younger age groupsis consistent with previous reports that healthy aging is mainly associated with atrophy in striatal regions and that a pathology such as dementia or AD is more likely to be associated with hippocampal dysfunction. For example, Head et al (2005) argue that pathological, but not normal, aging is associated with hippocampal dysfunction. Computational models have linked behavioral changes in healthy aging to dopamine reduction in the prefrontal cortex and basal ganglia (Braver & Barch, 2002; Li & Sikstrom, 2002). Clinical or neuroimaging data of our study's oldest adults who had difficulty in generalization might have confirmed those associations.
AD is believed to involve pathology that can begin years or decades before onset of symptoms (Alzheimer's Association, 2009). It is possible, then, that some people in our oldest group, especially those who showed impairments in the generalization phase, were in the prodromal stages of AD. A follow-up of these people on clinical and neuropsychological measures would help verify whether they have prodromal AD.
Apart from following our study sample to look for AD features at a later time, the chief implication of this study for further research is that even in older adults who do not report symptoms of pathology, there might be cognitive difficulties suggesting a functional deficit in the hippocampal and frontostriatal structures. More research, combining neuroimaging techniques with clinical data, could advance understanding of these age-related cognitive changes.
Despite some of the limitations of the current study, we can perhaps conclude that deficits in cognitive functions in older age are not necessarily a sign of ”normal” aging. Such signs warrant further investigation and caution to rule out possibilities of pathological structural and functional brain processes.
ACKNOWLEDGMENTS
For their assistance in data collection and analysis, the authors wish to thank Vincent P. Houck, Kimberly Berlepsch, Geoffrey Schnirman, David Engler, and Steven Grossman.
Partially supported by the NSF/NIH Collaborative Research in Computational Neuroscience (CRCNS) program and by NIAAA (RO1 AA018737-01).
Footnotes
Rakhee Krishna and Ahmed Moustafa contributed equally to the study.
Disclosures: The authors have no conflicts of interest or funding to disclose.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Alzheimer's Association Alzheimer's disease facts and figures. 2009 doi: 10.1016/j.jalz.2009.03.001. Report retrieved 5/3/2009 from Alzheimer's Association website: http://www.alz.org/national/documents/report_alzfactsfigures2009.pdf. [DOI] [PubMed]
- 2.Backman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: recent data and future avenues. Neuroscience and Biobehavioral Reviews. 2010;34(5):670–677. doi: 10.1016/j.neubiorev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Blair J, Spreen O. Predicting premorbid IQ: A revision of the National Adult Reading Test. The Clinical Neuropsychologist. 1989;3:129–136. [Google Scholar]
- 4.Boone KB, Ghaffarian S, Lesser IM, Hill-Gutierrez E. Wisconsin Card Sorting Test performance in healthy, older adults: Relationship to age, sex, education, and IQ. Journal of Clinical Psychology. 1993;49:54–60. doi: 10.1002/1097-4679(199301)49:1<54::aid-jclp2270490108>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 5.Braver TS, Barch DM. A theory of cognitive control, aging cognition, and neuromodulation. Neuroscience and Biobehavioral Reviews. 2002;26(7):809–817. doi: 10.1016/s0149-7634(02)00067-2. [DOI] [PubMed] [Google Scholar]
- 6.Chase HW, Clark L, Myers CE, Gluck MA, Sahakian BJ, Bullmore ET, et al. The role of the orbitofrontal cortex in human discrimination learning. Neuropsychologia. 2008 doi: 10.1016/j.neuropsychologia.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 7.Cherubini A, Peran P, Caltagirone C, Sabatini U, Spalletta G. Aging of subcortical nuclei: microstructural, mineralization and atrophy modifications measured in vivo using MRI. Neuroimage. 2009;48(1):29–36. doi: 10.1016/j.neuroimage.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 8.Collie A, Maruff P. The neuropsychology of preclinical Alzheimer's disease and mild cognitive impairment. Neuroscience and Biobehavioral Reviews. 2000;24:365–374. doi: 10.1016/s0149-7634(00)00012-9. [DOI] [PubMed] [Google Scholar]
- 9.Collie A, Maruff P, Shafiq-Antonacci R, Smith M, Hallup M, Schofield P, Masters C, Currie J. Memory decline in healthy older people: Implications for identifying mild cognitive impairment. Neurology. 2001;56:1533–1538. doi: 10.1212/wnl.56.11.1533. [DOI] [PubMed] [Google Scholar]
- 10.Coppinger N, Nehrke M. Discrimination learning and transfer of training in the aged. Journal of Genetic Psychology. 1972;120:93–102. doi: 10.1080/00221325.1972.10532221. [DOI] [PubMed] [Google Scholar]
- 11.Crook T, Gilbert J, Ferris S. Operationalizing memory impairment for elderly persons: The Guild Memory Test. Psychological Reports. 1980;47:1315–1318. doi: 10.2466/pr0.1980.47.3f.1315. [DOI] [PubMed] [Google Scholar]
- 12.Daigneault S, Braun CMJ, Whitaker HA. Early effects of normal aging in perseverative and non-perseverative prefrontal measures. Developmental Neuropsychology. 1992;8:99–114. [Google Scholar]
- 13.de Leon M, George A, Stylopoulos L, Smith G, Miller D. Early marker for Alzheimer's disease: The atrophic hippocampus. The Lancet. 1989;2(8664):672–673. doi: 10.1016/s0140-6736(89)90911-2. [DOI] [PubMed] [Google Scholar]
- 14.de Leon M, George A, Golomb J, Tarshish C, Convit A, Kluger A, de Santi S, McRae T, Ferris S, Reisberg B, Ince C, Rusinek H, Bobinski M, Quinn B, Miller D, Wisniewski H. Frequency of hippocampal formation atrophy in normal aging and Alzheimer's disease. Neurobiology of Aging. 1997;18(1):1–11. doi: 10.1016/s0197-4580(96)00213-8. [DOI] [PubMed] [Google Scholar]
- 15.de Toledo-Morrell L, Goncharova I, Dickerson B, Wilson R, Bennett D. From healthy aging to early Alzheimer's disease: In vivo detection of entorhinal cortex atrophy. Annals of the New York Academy of Sciences. 2000;19:240–253. doi: 10.1111/j.1749-6632.2000.tb06730.x. [DOI] [PubMed] [Google Scholar]
- 16.Eichenbaum H. The Hippocampal system and declarative memory in humans and animals: Experimental analysis and historical origins. In: Schacter DL, Tulving E, editors. Memory Systems 1994. MIT Press; Cambridge MA: 1994. pp. 147–201. [Google Scholar]
- 17.Eichenbaum H, Mathews P, Cohen NJ. Further studies of hippocampal representation during odor discrimination learning. Behavioral Neuroscience. 1989;103(6):1207–1216. doi: 10.1037//0735-7044.103.6.1207. [DOI] [PubMed] [Google Scholar]
- 18.Fera F, Weickert TW, Goldberg TE, Tessitore A, Hariri A, Das S, Lee S, Zoltick B, Meeter M, Myers CE, Gluck MA, Weinberger DR, Mattay VS. Neural mechanisms underlying probabilistic category learning in normal aging. Journal of Neuroscience, 7. 2005;25(49):11340–11348. doi: 10.1523/JNEUROSCI.2736-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fisk J, Warr P. Associative learning and short-term forgetting as a function of age, perceptual speed, and central executive functioning. Journals of Gerontology, Series B, Psychological Sciences and Social Sciences. 1998;53:P112–121. doi: 10.1093/geronb/53b.2.p112. [DOI] [PubMed] [Google Scholar]
- 20.Frank MJ, Kong L. Learning to avoid in older age. Psychology and. Aging. 2008;23:392–398. doi: 10.1037/0882-7974.23.2.392. [DOI] [PubMed] [Google Scholar]
- 21.Gabrieli JD. Memory systems analyses of mnemonic disorders in aging and age-related diseases. ProcNatlAcadSci U S A. 1996;93(24):13534–13540. doi: 10.1073/pnas.93.24.13534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gluck M, Myers C. Gateway to Memory: An Introduction to Neural Network Modeling of the Hippocampus in Learning and Memory. MIT Press; Cambridge, MA: 2001. [Google Scholar]
- 23.Golomb J, de Leon M, Kluger A, George A, Tarshish C, Ferris S. Hippocampal atrophy in normal aging: An association with recent memory impairment. Archives of Neurology. 1993;50(9):967–973. doi: 10.1001/archneur.1993.00540090066012. [DOI] [PubMed] [Google Scholar]
- 24.Golomb J, Kluger A, de Leon M, Ferris S, Convit A, Mittelman M, Cohen J, Rusinek H, de Santi S, George A. Hippocampal formation size in normal human aging: A correlate of delayed secondary memory performance. Learning and Memory. 1994;1:45–54. [PubMed] [Google Scholar]
- 25.Golomb J, Kluger A, de Leon M, Ferris S, Mittelman M, Cohen J, George A. Hippocampal formation size predicts declining memory performance in normal aging. Neurology. 1996;47:810–813. doi: 10.1212/wnl.47.3.810. [DOI] [PubMed] [Google Scholar]
- 26.Gunning-Dixon F, Head D, McQuain J, Acker J, Raz N. Differential aging of the human striatum: A prospective MR imaging study. AJNR American Journal of Neuroradiology. 1998;19:1501–1507. [PMC free article] [PubMed] [Google Scholar]
- 27.Haaland KY, Vranes LF, Goodwin JS, Garry PJ. Wisconsin Card Sort Test performance in a healthy elderly population. Journal of Gerontology. 1987;42:345–346. doi: 10.1093/geronj/42.3.345. [DOI] [PubMed] [Google Scholar]
- 28.Hasher L, Zacks RT. Working memory, comprehension, and aging: A review and a new view. In: Bower GH, editor. The psychology of learning and motivation: Advances in research and theory. Vol. 22. Academic Press; San Diego, CA: 1988. pp. 193–225. [Google Scholar]
- 29.Head D, Buckner RL, Shimony JS, Williams LE, Akbudak E, Conturo TE, et al. Differential vulnerability of anterior white matter in nondemented aging with minimal acceleration in dementia of the Alzheimer type: evidence from diffusion tensor imaging. Cereb Cortex. 2004;14(4):410–423. doi: 10.1093/cercor/bhh003. [DOI] [PubMed] [Google Scholar]
- 30.Head D, Isom M. Age effects on wayfinding and route learning skills. Behav Brain Res. 2010;209(1):49–58. doi: 10.1016/j.bbr.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Head D, Kennedy KM, Rodrigue KM, Raz N. Age differences in perseveration: cognitive and neuroanatomical mediators of performance on the Wisconsin Card Sorting Test. Neuropsychologia. 2009;47(4):1200–1203. doi: 10.1016/j.neuropsychologia.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Head D, Snyder AZ, Girton LE, Morris JC, Buckner RL. Frontal-hippocampal double dissociation between normal aging and Alzheimer's disease. Cereb Cortex. 2005;15(6):732–739. doi: 10.1093/cercor/bhh174. [DOI] [PubMed] [Google Scholar]
- 33.Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5(2):87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- 34.Hedden T, Gabrieli JD. Healthy and pathological processes in adult development: new evidence from neuroimaging of the aging brain. Current Opinion in Neurology. 2005;18:740–747. doi: 10.1097/01.wco.0000189875.29852.48. [DOI] [PubMed] [Google Scholar]
- 35.Hood K, Postle B, Corkin S. An evaluation of the concurrent discrimination task as a measure of habit learning: performance of amnesic subjects. Neuropsychologia. 1999;37:1375–1386. doi: 10.1016/s0028-3932(99)00048-2. [DOI] [PubMed] [Google Scholar]
- 36.Jack C, Petersen R, Xu Y, O'Brien P, Smith G, Ivnik R, Tangalos E, Kokmen E. Rate of medial temporal atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koylu B, Walser G, Ischebeck A, Ortler M, Benke T. Functional imaging of semantic memory predicts postoperative episodic memory functions in chronic temporal lobe epilepsy. Brain Research. 2008;1223:73–8. doi: 10.1016/j.brainres.2008.05.075. [DOI] [PubMed] [Google Scholar]
- 38.Krause V, Krastoshevsky O, Myers CE, Gluck MA, Mendell NR, Shin S, Titone D, Levy DL. Transfer Generalization in Schizophrenia: Specificity and Co-Familiality. Schizophrenia Bulletin. 2009);35:262. [Google Scholar]
- 39.Li SC, Sikstrom S. Integrative neurocomputational perspectives on cognitive aging, neuromodulation, and representation. NeurosciBiobehav Rev. 2002;26(7):795–808. doi: 10.1016/s0149-7634(02)00066-0. [DOI] [PubMed] [Google Scholar]
- 40.Lister JP, Barnes CA. Neurobiological changes in the hippocampus during normative aging. Archives of Neurology. 2009;66(7):829–33. doi: 10.1001/archneurol.2009.125. [DOI] [PubMed] [Google Scholar]
- 41.Marschner A, Mell T, Wartenburger I, Villringer A, Reischies FM, Heekeren HR. Reward-based decision-making and aging. Brain Research Bulletin. 2005;67(5):382–390. doi: 10.1016/j.brainresbull.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 42.Morgan D, May P, Finch C. Dopamine and serotonin systems in human and rodent brain: effects of age and neurodegenerative disease. Journal of the American Geriatric Society. 1987;35:334–345. doi: 10.1111/j.1532-5415.1987.tb04641.x. [DOI] [PubMed] [Google Scholar]
- 43.Mu Q, Xie J, Wen Z, Weng Y, Shuyun Z. A quantitative MR study of the hippocampal formation, the amygdala, and the temporal horn of the lateral ventricle in healthy subjects 40 to 90 years of age. AJNR American Journal of Neuroradiology. 1999;20:207–211. [PMC free article] [PubMed] [Google Scholar]
- 44.Myers C, Bryant D, DeLuca J, Gluck M. Dissociating basal forebrain and medial temporal amnesic syndromes: Insights from classical conditioning. Integrative Physiological and Behavioral Science. 2002;37(2):85–102. doi: 10.1007/BF02688822. [DOI] [PubMed] [Google Scholar]
- 45.Myers C, Gluck M. Context, conditioning and hippocampal re-representation. Behavioral Neuroscience. 1994;108(5):835–847. doi: 10.1037//0735-7044.108.5.835. [DOI] [PubMed] [Google Scholar]
- 46.Myers C, Kluger A, Golomb J, Ferris S, de Leon M, Schnirman G, Gluck M. Hippocampal atrophy disrupts transfer generalization in non-demented elderly. Journal of Geriatric Psychiatry and Neurology. 2002;15(2):82–90. doi: 10.1177/089198870201500206. [DOI] [PubMed] [Google Scholar]
- 47.Myers CE, Hopkins RO, DeLuca J, Moore NB, Wolansky LJ, Gluck MA. Learning and generalization deficits in patients with memory impairments due to anterior communicating artery aneurysm rupture or hypoxic brain injury. Neuropsychology. 2008a;22(5):681–686. doi: 10.1037/0894-4105.22.5.681. [DOI] [PubMed] [Google Scholar]
- 48.Myers CE, Kluger A, Golomb J, Gluck MA, Ferris S. Learning and generalization tasks predict short-term cognitive outcome in non-demented elderly. Journal of Geriatric Psychiatry and Neurology. 2008b;21(2):93–103. doi: 10.1177/0891988708316858. [DOI] [PubMed] [Google Scholar]
- 49.Myers CE, Shohamy D, Gluck MA, Grossman S, Kluger A, Ferris S, et al. Dissociating hippocampal versus basal ganglia contributions to learning and transfer. JCognNeurosci. 2003;15(2):185–193. doi: 10.1162/089892903321208123. [DOI] [PubMed] [Google Scholar]
- 50.Nehrke M. Age and sex differences in discrimination learning and transfer of training. Journal of Gerontology. 1973;28:320–327. doi: 10.1093/geronj/28.3.320. [DOI] [PubMed] [Google Scholar]
- 51.Nehrke M, Coppinger N. The effect of task dimensionality on discrimination learning and transfer of training in the aged. Journal of Gerontology. 1971;26:151–156. doi: 10.1093/geronj/26.2.151. [DOI] [PubMed] [Google Scholar]
- 52.Owen A, Roberts A, Polkey C, Sahakian B, Robbins T. Extra-dimensional versus intradimensional set shifting performance following frontal lobe excisions, temporal lobe excisions or amygdalo-hippocampectomy in man. Neuropsychologia. 1991;29:993–1006. doi: 10.1016/0028-3932(91)90063-e. [DOI] [PubMed] [Google Scholar]
- 53.Poldrack R, Clark J, Pare-Blagoev J, Shohamy D, CresoMoyano J, Myers C, Gluck M. Interactive memory systems in the brain. Nature. 2001;414:546–550. doi: 10.1038/35107080. [DOI] [PubMed] [Google Scholar]
- 54.Poldrack R, Prabakharan V, Seger C, Gabrieli J. Striatal activation during cognitive skill learning. Neuropsychology. 1999;13:564–574. doi: 10.1037//0894-4105.13.4.564. [DOI] [PubMed] [Google Scholar]
- 55.Rabbitt P, Lowe C. Patterns of cognitive aging. Psychological Research. 2000;63:308–316. doi: 10.1007/s004269900009. [DOI] [PubMed] [Google Scholar]
- 56.Radvansky GA, Zacks RT, Hasher L. Age and inhibition: the retrieval of situation models. Journals of Gerontology: Series B: Psychological Sciences and Social Sciences. 2005;60B(5):276–P278. doi: 10.1093/geronb/60.5.p276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA. Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiology of Aging. 2006;27:173–182. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 58.Raz N, Gunning-Dixon F, Head D, Dupuis J, Acker J. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12(1):95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 59.Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neuroscience and Biobehavioral Reviews. 2006;30:730–748. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Raz N, Williamson A, Gunning-Dixon F, Head D, Acker JD. Neuroanatomical and cognitive correlates of adult age differences in acquisition of a perceptual-motor skill. Microsc Res Tech. 2000;51(1):85–93. doi: 10.1002/1097-0029(20001001)51:1<85::AID-JEMT9>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 61.Robbins T, James M, Owen A, Sahakian B, Lawrence A, McInnes L, Rabbitt P. A study of performance on tests from the CANTAB battery sensitive to frontal lobe dysfunction in a large sample of normal volunteers: Implications for theories of executive functionoing and cognitive aging. Journal of the International Neuropsychological Society. 1998;4(5):474–490. doi: 10.1017/s1355617798455073. [DOI] [PubMed] [Google Scholar]
- 62.Rodrigue K, Raz N. Shrinkage of the entorhinal cortex over five years predicts memory performance in healthy adults. Journal of Neuroscience. 2004;24(4):956–963. doi: 10.1523/JNEUROSCI.4166-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rogers C, Keyes B, Fuller B. Solution shift performance in the elderly. Journal of Gerontology. 1976;31:670–675. doi: 10.1093/geronj/31.6.670. [DOI] [PubMed] [Google Scholar]
- 64.Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. ArchNeurol. 1999;56(3):338–344. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- 65.Salthouse T. Aging associations: influence of speed on adult age differences is associative learning. Journal of Experimental Psychology: Learning, Memory and Cognition. 1994;20:1486–1503. doi: 10.1037//0278-7393.20.6.1486. [DOI] [PubMed] [Google Scholar]
- 66.Salthouse T, Dunlosky J. Analyses of adult age differences in associative learning. Zeitschrift fur Pscyhologie Mit Zeitschrift fur Angewandte Psychologie. 1995;203(4):351–360. [PubMed] [Google Scholar]
- 67.Schacter D. Multiple forms of memory in humans and animals. In: Weinberger N, McGaugh J, Lynch G, editors. Memory Systems of the Brain: Animal and Human Cognitive Processes. Guildford Press; New York: 1985. pp. 351–379. [Google Scholar]
- 68.Shohamy D, Myers C, Onlaor S, Gluck M. Cognitive Neuroscience Society Annual Meeting Abstracts 2002. 2002. L-Dopa impairs learning of a simultaneous discrimination task in Parkinson's disease; p. 84. abstract only. [Google Scholar]
- 69.Shohamy D, Myers CE, Geghman KD, Sage J, Gluck MA. L-dopa impairs learning, but spares generalization, in Parkinson's disease. Neuropsychologia. 2006;44(5):774–784. doi: 10.1016/j.neuropsychologia.2005.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Simon JR, Howard JH, Howard DV. Adult age differences in learning from positive and negative probabilistic feedback. Neuropsychology. 2010;24(4):534–541. doi: 10.1037/a0018652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simon JR, Howard JH, Jr., Howard DV. Age differences in implicit learning of probabilistic unstructured sequences. JGerontol B PsycholSciSocSci. 2011;66(1):32–38. doi: 10.1093/geronb/gbq066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Simon JR, Vaidya CJ, Howard JH, Howard DV. The Effects of Aging on the Neural Basis of Implicit Associative Learning in a Probabilistic Triplets Learning Task. JCognNeurosci. 2011 doi: 10.1162/jocn_a_00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Small S, Tsai W, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human lifespan: Is memory decline normal or not? Annals of Neurology. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- 74.Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms and Commentary. Oxford University Press; New York: 1998. [Google Scholar]
- 75.Squire L, Zola-Morgan S, Chen K. Human amnesia and animal models of amnesia: performance of amnesic patients on tests designed for the monkey. Behavioral Neuroscience. 1988;102:210–221. doi: 10.1037//0735-7044.102.2.210. [DOI] [PubMed] [Google Scholar]
- 76.Stuss DT, Craik FIM, Sayer L, Franchi D, Alexander MP. Comparison of older people and patients with frontal lesions: Evidence from word list learning. Psychology and Aging. 1996;11:387–395. doi: 10.1037//0882-7974.11.3.387. [DOI] [PubMed] [Google Scholar]
- 77.Vandenberghe R, Dupont P, Bormans G, Mortelmans L, Orban G. Brain activity underlying sterotyped and non-stereotyped retrieval of learned stimulus-response associations. European Journal of Neuroscience. 1999;11(11):4037–50. doi: 10.1046/j.1460-9568.1999.00813.x. [DOI] [PubMed] [Google Scholar]
- 78.Wang Q, Xu X, Zhang M. Normal aging in the basal ganglia evaluated by eigenvalues of diffusion tensor imaging. AJNR American Journal of Neuroradiology. 2010;31(3):516–520. doi: 10.3174/ajnr.A1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wechsler D. Wechsler Memory Scale-Revised Manual. The Psychological Corporation; San Antonio, TX: 1987. [Google Scholar]