Abstract
Objective
The purpose of this review is to correlate the clinical finding that patients receiving parenteral nutrition with a fish oil-based lipid emulsion do not develop essential fatty acid deficiency (EFAD) with an experimental murine model, thus showing that arachidonic acid (AA) and docosahexaenoic acid (DHA) are likely to be the essential fatty acids.
Background
Conventional belief is that linoleic acid (LA, omega-6) and alpha-linolenic acid (ALA, omega-3) are the essential fatty acids (EFAs). We have shown that a fish oil-based lipid emulsion containing AA (omega-6) and docosahexaenoic acid (DHA, omega-3) and insignificant quantities of LA and ALA is efficacious in the treatment of parenteral nutrition-associated liver disease (PNALD), a major cause of liver-related morbidity and mortality. The prospect of using a fish oil-based lipid emulsion as monotherapy has raised concerns of EFAD development, hindering its adoption into clinical practice.
Design
Data from patients in our institution who received PN with a fish oil-based lipid emulsion was reviewed for clinical and biochemical evidence of EFAD, defined as an elevated triene-tetraene ratio (Mead acid/AA >0.2). We also investigated the minimum amount of fish oil required to prevent EFAD in a murine model and determined whether DHA and AA alone can prevent EFAD.
Results
No patients receiving PN with a fish oil-based lipid emulsion in our institution have developed biochemical or clinical evidence of EFAD such as an elevated triene-tetraene ratio, growth retardation or dermatitis. This observation parallels our previously published animal studies, which demonstrated prevention of EFAD when thirteen percent of total calories were from fish oil. Moreover, current work in our laboratory shows that AA and DHA provision alone is sufficient to prevent biochemical and physiologic evidence of EFAD in a murine model.
Conclusions
When dosed appropriately, fish oil-based lipid emulsions contain sufficient EFAs to prevent EFAD. Furthermore, AA and DHA alone may be the true EFAs.
Introduction
Essential fatty acids are termed as such because they are the “essential” nutrients for growth, development, and function. EFAs must be acquired through dietary sources because mammals cannot synthesize them from simple carbon precursors. Traditionally, α-linolenic acid (ALA, omega-3) and linoleic acid (LA, omega-6) are considered the only EFAs. This view is supported in textbooks on biochemistry and in the current literature (1, 2). LA and ALA are considered essential because all downstream fatty acids (FAs) can be synthesized from these two 18-carbon precursors. Among these downstream products are the highly physiologically relevant arachidonic acid (AA, omega-6), docosahexaenoic acid (DHA, omega-3), and eicosapentaenoic acid (EPA, omega-3). These are considered critical metabolites as they are important precursors to both eicosanoids and prostanoids, mediating numerous physiological and biochemical processes. Recent evidence regarding fish oils, which contain very low concentrations of LA and ALA, are rich in DHA and EPA, and have small amounts of AA, may necessitate a re-evaluation of what is truly an EFA. We review the evidence supporting the premise that other polyunsaturated fatty acids (PUFAs), particularly AA and DHA, may be considered EFAs.
Essential fatty acids and their synthesis
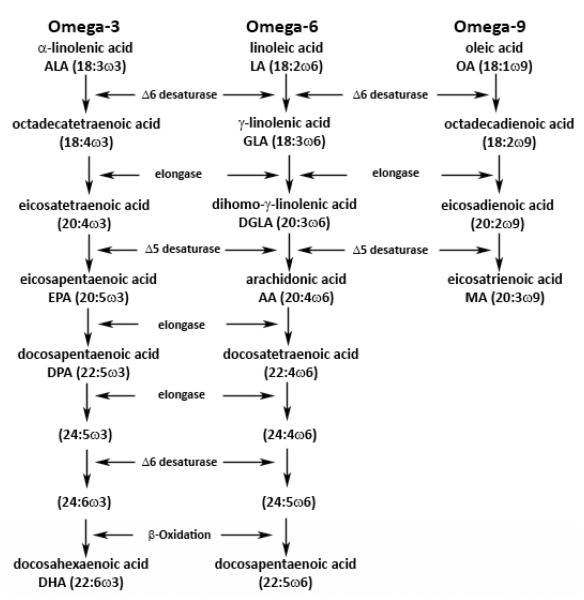
The term EFA was first coined by Burr and Burr in 1929 when these FAs were used to reverse the symptoms of EFA deficiency (EFAD) in young rodents fed with a fat-free diet (3). In a series of experiments, rats were maintained on a fat-free diet until they developed signs of EFAD such as dry, scaly skin. Various doses of LA, ALA, oleic acid (OA) or medium chain triglyceride (MCT) oil (as hydrogenated coconut oil) were then provided, leading to the discovery that only LA and ALA were able to reverse the skin lesions and facilitate growth (4, 5). Hence they were termed EFAs. LA and ALA are members of two PUFA families, the omega-6 and omega-3 FAs, respectively (Figure 1). PUFAs are an integral part of the cell membrane, significantly impacting its fluidity and function. Within the plasma lipoprotein particles, PUFAs serve as the major constituents of phospholipids, triglycerides, and cholesterol esters. There are three major families of PUFAs: omega-3 (e.g. ALA, EPA, DHA), omega-6 (e.g. LA, AA), and omega-9 (e.g. oleic acid (OA), Mead acid (MA)), all of which are metabolized using the same group of enzymes. For example, omega-3 FAs compete with LA in the AA pathway, thereby reducing the metabolism of AA to prostaglandin E2 and thromboxane A2 (Figure 2). These PUFAs produce numerous bioactive metabolites that mediate inflammatory responses, serve as secondary messengers, and regulate various functions such as platelet aggregation, vascular tone (6).
Figure 1.
Pathway of metabolism and synthesis of omega-3, omega-6, and omega-9 PUFAs (adapted and modified from Lee et. al.) (52)
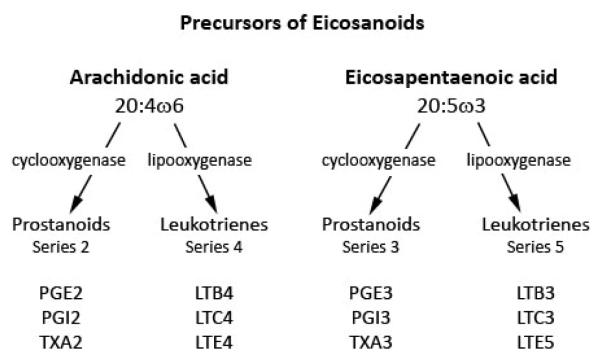
Figure 2.
Eicosanoids synthesis from arachidonic acid and docosahexaenoic acid (adapted from Lee et al) (52)
Among these 3 families of PUFAs, only OA, a parent omega-9 FA, can be synthesized by mammals from simple carbon precursors. Both the parent omega-3 and omega-6 FAs must be acquired from the diet, as mammals cannot insert double bonds at position-3 and position-6 to produce ALA and LA, respectively. Rodent studies have shown that ALA and LA can be synthesized from 14-carbon omega-3 and omega-6 PUFAs, homologues of the 18-carbon ALA and LA, respectively (7). However, 14-carbon omega-3 and omega-6 PUFAs are not present in sufficient quantities in the human diet. Humans can also convert the 16-carbon omega-3 and omega-6 PUFAs, hexadecatrienoate and hexadecadienoate (found in green vegetables), to ALA and LA at 3.5% and 0.2%, respectively, but these precursors are still inadequate in the human diet. (8). Thus, ALA and LA are considered the EFAs. Sometimes, AA, EPA, and DHA are considered conditional EFAs because their production may be inadequate in certain conditions such as prematurity and periods of growth, thus requiring exogenous supplementation.
In vivo, LA is desaturated by the action of enzyme Δ6 desaturase to γ-linolenic acid (GLA, omega-6) by inserting a double bond between the sixth and seventh carbon. GLA is then converted to dihomo-γ-linolenic acid (DGLA) by the action of an enzyme called elongase, by the addition of two carbons. DGLA is then transformed to AA by Δ5 desaturase, by the addition of a double bond between the fifth and sixth carbons. AA is very important for the synthesis of eicosanoids as it is the primary substrate for the synthesis of the 4-series leukotrienes (leukotriene B4, C4, E4), the 2-series prostaglandins (prostaglandin E2, prostagcyclin I2), and thromboxane A2. These eicosanoids are mediated by cyclooxygenase (COX) and 5-lipooxygenase and play important roles in cellular signaling, inflammation, and vasomodulation. An intact pathway between LA and AA is therefore important to maintain adequate levels of AA and its downstream eicosanoids (Figure 2).
Similar to the omega-6 pathway, the omega-3 pathway is mediated by the same set of enzymes that convert LA to AA. In the omega-3 pathway, ALA is transformed by Δ6 desaturase, then elongated and desaturated to EPA. EPA serves as the main precursor for the production of the 5-series leukotrienes (leukotriene B5, C5, D5), the 3-series prostaglandins (prostaglandin E3, prostagcyclin I3), and thromboxane A3. Eicosanoid synthesis is also mediated by the COX and 5-lipooxyganase pathways.
Essential fatty acid deficiency
EFAD typically occurs when less than 1-2% of total calories are provided from EFAs. In the general population, EFAD is extremely rare. Holman described the symptoms of EFAD in rats and other mammalian species that included primarily impaired growth and dermatitis, and secondarily hepatic steatosis, renal toxicity, pulmonary abnormalities, and an increased metabolic rate (9).
In humans, biochemical changes consistent with EFAD can occur in as little as a few days in infants, and within several weeks in older children and adults. Clinical symptoms of EFAD may take 4-6 weeks to appear in older patients. Physiological and pathological signs of EFAD include growth retardation, hair loss, infertility, coagulopathies, dry and scaly skin. These clinical signs were initially used as ”biological” markers for determining dietary deficiency of LA (10).
Due to their limited fat stores, premature infants may develop EFAD in less than a week when EFAs are less than 4-5% of their total calories. It may also occur in patients with chronic malnutrition, malabsorption, and in patients receiving prolonged courses of parenteral nutrition (PN) without adequate fat calories.
With the advent of gas chromatography and standardization of fatty acid analysis, a biochemical indicator of EFAD has been established. This biochemical evidence of EFAD is a state of decreased AA levels and increased MA levels (Figure 1). In times of deficiency, omega-3 and omega-6 FA stores are depleted. As discussed above, LA is normally converted to AA, a tetraene. Desaturase enzymes display differential activity in the following order of preference: omega-3 > omega-6 > omega-9. As a result, conversion of OA to MA (omega-9), a triene, only occurs when there are low dietary levels of both ALA and LA. This metabolic switch is seen as a compensatory mechanism to maintain the number of double bonds in FAs incorporated in cell membranes. Therefore, an elevated MA level in conjunction with a diminished AA level has been associated with the EFAD. A determination of the relative amount of MA to AA, called the triene-tetraene ratio, can be made.
Through multiple, extensive experiments in the 1950-1960s, Holman et al showed that the dietary requirement of LA in male rats required to prevent symptoms of EFAD was approximately 1% of the total calories. In this classic experiment, Holman demonstrated that the triene-tetraene ratio in plasma, erythrocytes, and heart tissues was less than 0.4 when the above criteria for LA was met (11). However, due to the nature of his experiment, in which rats were deprived of all exogenous fats, this ratio reflected a deficiency of both omega-6 and omega-3 FAs. Moreover, more recent studies using more sensitive age-based range criteria for plasma EFA status suggest that a triene-tetraene ratio of greater than 0.2 is diagnostic of EFAD as the average ratio in normal individuals was found to be less than 0.1 ±0.08 (standard deviation) (12-15). The minimum recommended dietary requirement of ALA is 0.2-1% of total caloric intake in adults and 0.5% in infants and young children (16).
Essentiality of arachidonic acid
Because LA is abundant in the human diet, the amount of AA available almost always exceeds the level needed to maintain a triene-tetraene ratio below 0.2. It was only upon the introduction of parenteral nutrition (PN) that EFAD became more common. It was first reported in patients who received PN without dietary fat supplementation (17). In rodents, AA alone has been shown to be as effective as LA in preventing symptoms of EFAD such as skin lesions and growth retardation (18). AA supplementation has also been shown to prevent epidermal water loss as it is retroconverted to LA (19). This is not totally unexpected, as LA, a precursor for both DGLA and AA, is incorporated into epidermal phospholipids that function as the water-permeability barrier of the skin (20). AA has also been shown to play an important role in closure of the ductus arteriosus in a rodent model via its eicosanoid pathways (21). These studies demonstrate that AA can replace LA as the sole source of dietary omega-6 FAs.
Essentiality of eicosapentaenoic acid and docosahexaenoic acid
Omega-3 derived eicosanoids have been shown to be either less-proinflammatory or “anti-inflammatory” while omega-6 derived eicosanoids are believed to be proinflammatory (22-24). More recently, EPA has been found to be the precursor for resolvin E1. Resolvin E1 is a lipid mediator that plays a major role in reducing the inflammatory response by regulating leukocyte extravasation through cell surface adhesion, reducing leukocyte rolling, and by reducing platelet aggregation (25-27). EPA is also the precursor of another potent omega-3 FA, DHA, which itself is the precursor of neuroprotectin D1, a lipid mediator that has been shown to protect neurons and retinal cells from oxidative stress induced apoptosis (28).
To date, there have not been any dose response studies in animals or humans investigating the ability of ALA, EPA, or DHA to reverse omega-3 FA deficiency. Perhaps this is because symptoms of omega-3 FA deficiency are difficult to diagnose and monitor. Typically, symptoms attributed to omega-3 FA deficiency include visual impairment and learning disabilities (29). However, there is no effect on growth rate, and the dermatologic abnormalities that characterize omega-6 FA deficiency do not occur. Similarly, the lack of ALA in a person’s diet will not raise the triene-tetraene ratio, as adequate LA intake can mask this deficiency. There is currently no biochemical marker for omega-3 FA deficiency as there is for omega-6 FA deficiency. Omega-3 FA deficiency was first reported in a patient receiving PN containing fat emulsions rich in LA but containing very little ALA (30). Omega-3 FA deficiency was also reported in patient receiving gastric feeding with formula low in omega-3 FA (31). In these subjects, both total plasma and tissue omega-3 concentrations were lower than controls. Unlike their omega-6 FA counterparts, there are no accepted values for plasma or tissue EPA and DHA levels below which symptoms of omega-3 FA deficiency may occur. In rodent studies, omega-3 FA deficiency has very little impact on weight gain. However, supplemental ALA given to rats on a diet deficient in PUFAs did produce significant weight gain (16, 32). Similar findings were seen in humans. In adult males, the conversion of ALA from dietary intake to EPA and DHA is limited, approximately 8% and 0.1%, respectively. The conversion rate of ALA to DHA is higher in females and is even further up-regulated during pregnancy. Perhaps this is due to lower beta-oxidation or up-regulation of conversion due to estrogen (33). Interestingly, supplementation of pregnant women with ALA did not result in increased umbilical blood levels of DHA (34). Maternal levels of DHA decreased during pregnancy, starting at 18 weeks of pregnancy while levels of LA stayed constant (35). However, supplementation of pregnant women with fish oil rich in DHA, increased umbilical blood levels and infant plasma levels of both DHA and AA at birth (36). These findings indicate that ALA can be substituted by the more potent and efficient EPA and DHA.
Parenteral nutrition and essential fatty acid deficiency
PN is a life-saving therapy for patients who are unable to absorb enteral nutrients secondary to insufficient intestinal length or function. Before the development of PN, patients with insufficient gastrointestinal absorptive capability commonly died of starvation and subsequent complications of malnutrition. Today, more than 30,000 US patients are permanently dependent on PN for survival (37). Long term use of PN is associated with many complications, including septicemia and metabolic abnormalities (38). The most serious complication associated with long term PN use is PN-associated liver disease (PNALD). Despite years of extensive study, the etiology of PNALD remains unclear. Recent findings suggest that the composition of the intravenous lipid emulsion administered with the PN may contribute to this liver injury. Lipid emulsions derived from soybean oils have been shown to cause liver injury both in vitro and in vivo in rodent models (39-41). This has prompted us to investigate an emulsion derived from an alternative fat source (42). The current FDA approved lipid emulsions in the United States are comprised of safflower or soybean oils, both rich in omega-6 FAs. As shown in our murine model, fish oil-based lipid emulsions are capable of both preventing and reversing PN induced hepatic steatosis, likely via improved triglyceride clearance coupled with the anti-inflammatory properties of omega-3 FAs (42).
The use of fish oil-based fat emulsions as the sole source of fat energy is not recommended by its manufacturer because of concerns surrounding the low levels of LA present in fish oils could potentially lead to EFAD. Omegaven® (Fresenius Kabi, Bad Homburg, Germany) is the only lipid emulsion that contains solely fish oils. There is 0.1-0.7% LA in 10% Omegaven®emulsion (Table 1). In 2005, Gura et al reported the first use of Omegaven® to treat a patient who developed EFAD while receiving fat-free PN secondary to a soy allergy (43). The patient’s triene-tetraene ratio normalized from 0.231 prior to treatment to 0.047 after 17 days of Omegaven®therapy at the goal dose of 0.67g/kg/day. The total LA intake of that patient was 45mg/kg/day, which corresponded to 0.7% of total energy. Although this is below the 1% that Holman et al reported, the patient did experience reversal of both his biochemical and physical signs and symptoms of EFAD. As previously reported, ALA, EPA, and DHA might play a role in reducing the amount of LA required to prevent EFAD (32). Since that case report, Omegaven® monotherapy has been used as a treatment for PN-dependent patients with cholestasis. In an initial case series, 2 pediatric patients with cholestasis due to PNALD were treated with the fish oil-based lipid emulsion. Within 60 days their serum bilirubin levels normalized and remained normal (44). The children tolerated this therapy well, and no adverse reactions attributed to its use were observed. Based on these results, we have treated more than 80 children with PNALD at Children’s Hospital Boston with a fish oil-based lipid emulsion under a compassionate use protocol (45). Outcomes of the first 18 patients were compared to a group of historical controls studied by Andorsky et al (46). Findings demonstrated that patients who received the fish oil-based lipid emulsion (Omegaven®) experienced reversal of cholestasis 4.8 times faster than the historical control cohort, who received a soybean oil-based lipid emulsion. When adjusted for baseline bilirubin concentration, gestational age, and the diagnosis of necrotizing enterocolitis, the time to reversal was 6.8 times faster. Only 2 deaths were recorded in the fish oil cohort, compared to 7 deaths in the historical cohort. There were no liver transplants in the fish-oil treated patients, whereas 2 transplants occurred in the patients receiving soy bean oil emulsions. Moreover, the provision of the fish oil-based lipid emulsion was not associated with EFAD, hypertriglyceridemia, coagulopathy, infections, or growth delay.
Table 1.
Comparison and characteristics of parenteral lipid emulsions
| Product | Intralipid® 20% | Liposyn II® 20% | SMOF lipid® 20% | Omegaven® 10% |
|---|---|---|---|---|
| Manufacturer | Baxter Healthcare / Fresenius Kabi |
Hospira | Fresenius Kabi | Fresenius Kabi |
| Oil source (%) | ||||
| Soy bean | 100 | 50 | 30 | 0 |
| Safflower | 0 | 50 | 0 | 0 |
| MCT | 0 | 0 | 30 | 0 |
| Olive oil | 0 | 0 | 25 | 0 |
| Fish | 0 | 0 | 15 | 100 |
| Fat composition (%) | ||||
| Linoleic | 50 | 65 | 18.6 | 0.1-0.7 |
| α-Linolenic | 9 | 4 | 2.35 | <0.2 |
| EPA | 0 | 0 | 2.35 | 1.28-2.82 |
| DHA | 0 | 0 | 2.2 | 1.44-3.09 |
| Oleic | 26 | 17.7 | 27.65 | 0.6-1.3 |
| Palmitic | 10 | 8.8 | 9.1 | 0.25-1 |
| Stearic | 3.5 | 3.4 | 2.75 | 0.05-0.2 |
| Arachidonic | 0 | 0 | 0.5 | 0.1 -0.4 |
MCT indicates medium chain triglyceride; EPA: eicosapentaenoic acid; DHA: docosahexaenoic acid.
Fish oil prevents essential fatty acid deficiency
The aforementioned studies using fish oil as the sole source of fat in a subset of pediatric patients dependent on PN showed that their EFAD reversed, liver function improved, and growth was maintained (47). However, there is still skepticism regarding the ability of a fish oil-based diet, when used as monotherapy, to prevent EFAD and maintain adequate growth rate (48-50).
In a recent study by Strijbosch et al, a fish oil-based diet was shown to prevent EFAD and to maintain growth in a murine model (51). Mice were fed isocaloric diets that had 2.6%, 13%, or 26% of their total calories as fish oil, or a control diet that had 13% of total calories derived from soybean oil. During the nine-week intervention period, only mice receiving 2.6% of their total calories as fish oil failed to gain adequate weight. When compared to the group receiving soybean oil, both the 13% and 26% fish oil-based diets actually enhanced growth, which is in contrast to earlier reports about the negative effects of fish oil on infant growth (48-50). When examining the triene-tetraene ratios, only the 2.6% fish oil group exceeded the EFAD threshold, whereas the 13% fish oil-based diet clearly prevented EFAD (Table 2).
Table 2.
Mean triene-tetraene ratios in nanomoles per milliliter of relevant plasma triglycerides and phospholipids at baseline and at 3-, 6-, and 9-week time points for pair-fed groups (n=5) and different diets (adapted and modified from Strijbosch, et. al.) (51).
| Triglycerides | Phospholipids | |||||||
|---|---|---|---|---|---|---|---|---|
| 2.6% FO | 13% FO | 26% FO | 13% SB | 2.6% FO | 13% FO | 26% FO | 13% SB | |
| Baseline | 0.005 | 0.008 | 0.033 | 0.059 | 0.000 | 0.002 | 0.008 | 0.009 |
| 3 wk | 0.105 2,3,4 | 0.014 1 | 0.026 1 | 0.010 1 | 0.020 2,3,4 | 0.018 1 | 0.026 1 | 0.000 1 |
| 6 wk | 0.093 2,3,4 | 0.038 1 | 0.011 1 | 0.000 1 | 0.081 2,3,4 | 0.033 1 | 0.011 1 | 0.004 1 |
| 9 wk | 0.227 2,3,4 | 0.089 1,4 | 0.030 1 | 0.000 1,2 | 0.131 2,3,4 | 0.007 1 | 0.030 1 | 0.000 1 |
FO indicates fish oil; SB: soy bean.
Value is significantly different from 1% fish oil group within the same time point and ratio group, P < .05
Value is significantly different from 5% fish oil group within the same time point and ratio group, P < .05
Value is significantly different from 10% fish oil group within the same time point and ratio group, P < .05
Value is significantly different from 5% soy bean group within the same time point and ratio group, P < .05
New directions
Lipid emulsions currently available in the United States such as Intralipid® (Baxter Pharmaceuticals, Deerfield, IL) and Lipoysn® III (Hospira, Lake Forest, IL) are soybean oil-based. Since there is evidence that soybean oil-based lipid emulsions might contribute to PNALD (40), we hypothesize that lipid emulsions with soybean oil as their major component might lead to PNALD. Other fat emulsions such as SMOF® (Fresenius Kabi, Bad Homburg, Germany), an emulsion containing 30% soybean oils, 15% fish oils, 25% olive oils and 30% MCT oils, Liposyn® II (50% soybean oils, 50% safflower oils) and Clinoleic® (Clintec Parenteral S.A.,Cedex, France), emulsion containing 80% purified olive oils and 20% soybean oils, should be vigorously scrutinized as they also contain substantial quantities of soybean oils and could theoretically pose the same risk to patients. Importantly, unlike soybean, olive, safflower, and coconut oils, fish oil contains AA, EPA, and DHA, all of which are essential for very low birth weight infants who cannot convert LA and ALA to AA, EPA, and DHA efficiently. The requirements of very low birth weight infants and older children likely vary due to the fact that rapidly growing premature infants have higher requirements for EFAs. As a basis for developing new lipid emulsions specifically for children, we’ve sought to determine the appropriate amount of each fatty acid, as well as the essentiality of DHA and AA. The use of DHA and AA as the sole source of PUFAs has shown promise in preventing PNALD and EFAD (unpublished data).
Summary
Essential FAs are important for human development and various biological functions. Traditional EFAs such as LA and ALA play a major role in preventing EFAD, and are the main precursors for the production of vital metabolites such as AA, EPA, and DHA. While AA produces mostly pro-inflammatory eicosanoids, EPA and DHA produce anti-inflammatory eicosanoids, as well as other novel molecules such as Resolvin E1 and neuroprotectin D1 that are essential in cognitive and visual functions. LA and ALA are acquired in the human diet in which LA is abundant. Although pregnant women and infants have an increased AA and DHA requirements,the conversions of dietary LA and ALA to AA and DHA are insufficient. AA and DHA supplementation in the form of fish oil ameliorates this problem and prevents EFAD. This explains the effectives of treatment of children with EFAD secondary to fat-free PN with a fish oil-based lipid emulsion as evidenced by complete resolution of their biochemical and clinical evidence of EFAD. This emulsion also prevented and attenuated PN-induced cholestasis in both human and animal models. AA and DHA as the sole FAs demonstrated similar results. Taken together, there is preliminary evidence to suggest that DHA and AA are EFAs, although further investigation is required.
Acknowledgments
The authors are grateful to Danielle Arsenault and Katherine B. Novak for their editorial assistance.
The authors’ responsibilities were as follows – HLD: primarily reviewed the literature and wrote the majority of the manuscript. JAM, VED, KMG: wrote separate parts of the manuscript and provided critical inputs on the manuscript’s content. MP: generated the concept of the manuscript, provided critical inputs and approved the final version of the manuscript.
Omegaven® patent was filed by Children’s Hospital Boston on behalf of KMG and MP.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This paper was presented at the workshop “DHA as a Required Nutrient” in Baltimore, MD, June 20-21, 2008 by Dr. Puder.
References
- 1.Voet DV, JG . Biochemistry. 3rd ed J. Wiley & Sons; New York: 2004. [Google Scholar]
- 2.Das UN. Essential fatty acids: biochemistry, physiology and pathology. Biotechnol J. 2006;1:420–39. doi: 10.1002/biot.200600012. [DOI] [PubMed] [Google Scholar]
- 3.Burr GO, Burr MM. Nutrition classics from The Journal of Biological Chemistry 82:345-67, 1929. A new deficiency disease produced by the rigid exclusion of fat from the diet. Nutr Rev. 1973;31:248–9. doi: 10.1111/j.1753-4887.1973.tb06008.x. [DOI] [PubMed] [Google Scholar]
- 4.Burr GO, Burr MM. On the nature and role of the fatty acids essential in nutrition. The Journal of Biological Chemistry. 1930;86 [Google Scholar]
- 5.Burr GO, Burr MM, Miller ES. On the fatty acids essential in nutrition. III. The Journal of Biological Chemistry. 1932;97:9. [Google Scholar]
- 6.Hornstra G. The role of (n-6) fatty acids and their metabolites in arterial thrombus formation. Prog Lipid Res. 1981;20:407–13. doi: 10.1016/0163-7827(81)90072-2. [DOI] [PubMed] [Google Scholar]
- 7.Sprecher H. The synthesis and metabolism of hexadeca-4,7,10-trienoate, eicosa-8,11,14-trienoate, docosa-10,13,16-trienoate and docosa-6,9,12,15-tetraenoate in the rat. Biochim Biophys Acta. 1968;152:519–30. doi: 10.1016/0005-2760(68)90092-1. [DOI] [PubMed] [Google Scholar]
- 8.Cunnane SC, Ryan MA, Craig KS, et al. Synthesis of linoleate and alpha-linolenate by chain elongation in the rat. Lipids. 1995;30:781–3. doi: 10.1007/BF02537807. [DOI] [PubMed] [Google Scholar]
- 9.Aaes-Jorgensen E, Leppik EE, Hayes HW, Holman RT. Essential fatty acid deficiency. II. In adult rats. J Nutr. 1958;66:245–59. doi: 10.1093/jn/66.2.245. [DOI] [PubMed] [Google Scholar]
- 10.Alfin-Slater RB, Aftergood L. Essential fatty acids reinvestigated. Physiol Rev. 1968;48:758–84. doi: 10.1152/physrev.1968.48.4.758. [DOI] [PubMed] [Google Scholar]
- 11.Holman RT. The ratio of trienoic: tetraenoic acids in tissue lipids as a measure of essential fatty acid requirement. J Nutr. 1960;70:405–10. doi: 10.1093/jn/70.3.405. [DOI] [PubMed] [Google Scholar]
- 12.Holman RT, Smythe L, Johnson S. Effect of sex and age on fatty acid composition of human serum lipids. Am J Clin Nutr. 1979;32:2390–9. doi: 10.1093/ajcn/32.12.2390. [DOI] [PubMed] [Google Scholar]
- 13.Mascioli EA, Lopes SM, Champagne C, Driscoll DF. Essential fatty acid deficiency and home total parenteral nutrition patients. Nutrition. 1996;12:245–9. doi: 10.1016/s0899-9007(96)90850-3. [DOI] [PubMed] [Google Scholar]
- 14.Jeppesen PB, Hoy CE, Mortensen PB. Essential fatty acid deficiency in patients receiving home parenteral nutrition. Am J Clin Nutr. 1998;68:126–33. doi: 10.1093/ajcn/68.1.126. [DOI] [PubMed] [Google Scholar]
- 15.Siguel EN, Chee KM, Gong JX, Schaefer EJ. Criteria for essential fatty acid deficiency in plasma as assessed by capillary column gas-liquid chromatography. Clin Chem. 1987;33:1869–73. [PubMed] [Google Scholar]
- 16.Mohrhauer H, Holman RT. The Effect of Dose Level of Essential Fatty Acids Upon Fatty Acid Composition of the Rat Liver. J Lipid Res. 1963;4:151–9. [PubMed] [Google Scholar]
- 17.Wene JD, Connor WE, DenBesten L. The development of essential fatty acid deficiency in healthy men fed fat-free diets intravenously and orally. J Clin Invest. 1975;56:127–34. doi: 10.1172/JCI108061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomasson HJ. Essential fatty acids. Nature. 1962;194:973. doi: 10.1038/194973a0. [DOI] [PubMed] [Google Scholar]
- 19.Hansen HS, Jensen B, von Wettstein-Knowles P. Apparent in vivo retroconversion of dietary arachidonic to linoleic acid in essential fatty acid-deficient rats. Biochim Biophys Acta. 1986;878:284–7. doi: 10.1016/0005-2760(86)90158-x. [DOI] [PubMed] [Google Scholar]
- 20.Hansen HS, Jensen B. Essential function of linoleic acid esterified in acylglucosylceramide and acylceramide in maintaining the epidermal water permeability barrier. Evidence from feeding studies with oleate, linoleate, arachidonate, columbinate and alpha-linolenate. Biochim Biophys Acta. 1985;834:357–63. doi: 10.1016/0005-2760(85)90009-8. [DOI] [PubMed] [Google Scholar]
- 21.Segi E, Sugimoto Y, Yamasaki A, et al. Patent ductus arteriosus and neonatal death in prostaglandin receptor EP4-deficient mice. Biochem Biophys Res Commun. 1998;246:7–12. doi: 10.1006/bbrc.1998.8461. [DOI] [PubMed] [Google Scholar]
- 22.Grimminger F, Wahn H, Mayer K, Kiss L, Walmrath D, Seeger W. Impact of arachidonic versus eicosapentaenoic acid on exotonin-induced lung vascular leakage: relation to 4-series versus 5-series leukotriene generation. Am J Respir Crit Care Med. 1997;155:513–9. doi: 10.1164/ajrccm.155.2.9032187. [DOI] [PubMed] [Google Scholar]
- 23.Grimminger F, Mayer K, Kiss L, Wahn H, Walmrath D, Seeger W. Synthesis of 4- and 5-series leukotrienes in the lung microvasculature challenged with Escherichia coli hemolysin: critical dependence on exogenous free fatty acid supply. Am J Respir Cell Mol Biol. 1997;16:317–24. doi: 10.1165/ajrcmb.16.3.9070617. [DOI] [PubMed] [Google Scholar]
- 24.Lee TH, Mencia-Huerta JM, Shih C, Corey EJ, Lewis RA, Austen KF. Effects of exogenous arachidonic, eicosapentaenoic, and docosahexaenoic acids on the generation of 5-lipoxygenase pathway products by ionophore-activated human neutrophils. J Clin Invest. 1984;74:1922–33. doi: 10.1172/JCI111612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dona M, Fredman G, Schwab JM, et al. Resolvin E1, an EPA-derived mediator in whole blood, selectively counterregulates leukocytes and platelets. Blood. 2008 doi: 10.1182/blood-2007-11-122598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Serhan CN, Hong S, Gronert K, et al. Resolvins: a family of bioactive products of omega-3 fatty acid transformation circuits initiated by aspirin treatment that counter proinflammation signals. J Exp Med. 2002;196:1025–37. doi: 10.1084/jem.20020760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arita M, Bianchini F, Aliberti J, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med. 2005;201:713–22. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc Natl Acad Sci U S A. 2004;101:8491–6. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lauritzen L, Hansen HS, Jorgensen MH, Michaelsen KF. The essentiality of long chain n-3 fatty acids in relation to development and function of the brain and retina. Prog Lipid Res. 2001;40:1–94. doi: 10.1016/s0163-7827(00)00017-5. [DOI] [PubMed] [Google Scholar]
- 30.Holman RT, Johnson SB, Hatch TF. A case of human linolenic acid deficiency involving neurological abnormalities. Am J Clin Nutr. 1982;35:617–23. doi: 10.1093/ajcn/35.3.617. [DOI] [PubMed] [Google Scholar]
- 31.Bjerve KS, Mostad IL, Thoresen L. Alpha-linolenic acid deficiency in patients on long-term gastric-tube feeding: estimation of linolenic acid and long-chain unsaturated n-3 fatty acid requirement in man. Am J Clin Nutr. 1987;45:66–77. doi: 10.1093/ajcn/45.1.66. [DOI] [PubMed] [Google Scholar]
- 32.Greenberg SM, Calbert CE, Savage EE, Deuel HJ., Jr The effect of fat level of the diet on general nutrition. VI. The interrelation of linoleate and linolenate in supplying the essential fatty acid requirement in the rat. J Nutr. 1950;41:473–86. doi: 10.1093/jn/41.3.473. [DOI] [PubMed] [Google Scholar]
- 33.Williams CM, Burdge G. Long-chain n-3 PUFA: plant v. marine sources. Proc Nutr Soc. 2006;65:42–50. doi: 10.1079/pns2005473. [DOI] [PubMed] [Google Scholar]
- 34.de Groot RH, Hornstra G, van Houwelingen AC, Roumen F. Effect of alpha-linolenic acid supplementation during pregnancy on maternal and neonatal polyunsaturated fatty acid status and pregnancy outcome. Am J Clin Nutr. 2004;79:251–60. doi: 10.1093/ajcn/79.2.251. [DOI] [PubMed] [Google Scholar]
- 35.Al MD, van Houwelingen AC, Kester AD, Hasaart TH, de Jong AE, Hornstra G. Maternal essential fatty acid patterns during normal pregnancy and their relationship to the neonatal essential fatty acid status. Br J Nutr. 1995;74:55–68. doi: 10.1079/bjn19950106. [DOI] [PubMed] [Google Scholar]
- 36.van Houwelingen AC, Sorensen JD, Hornstra G, et al. Essential fatty acid status in neonates after fish-oil supplementation during late pregnancy. Br J Nutr. 1995;74:723–31. doi: 10.1079/bjn19950175. [DOI] [PubMed] [Google Scholar]
- 37.Howard L, Ament M, Fleming CR, Shike M, Steiger E. Current use and clinical outcome of home parenteral and enteral nutrition therapies in the United States. Gastroenterology. 1995;109:355–65. doi: 10.1016/0016-5085(95)90321-6. [DOI] [PubMed] [Google Scholar]
- 38.Buchman AL. Complications of long-term home total parenteral nutrition: their identification, prevention and treatment. Dig Dis Sci. 2001;46:1–18. doi: 10.1023/a:1005628121546. [DOI] [PubMed] [Google Scholar]
- 39.Chen WJ, Yeh SL, Huang PC. Effects of fat emulsions with different fatty acid composition on plasma and hepatic lipids in rats receiving total parenteral nutrition. Clin Nutr. 1996;15:24–8. doi: 10.1016/s0261-5614(96)80257-3. [DOI] [PubMed] [Google Scholar]
- 40.Zaman N, Tam YK, Jewell LD, Coutts RT. Effects of intravenous lipid as a source of energy in parenteral nutrition associated hepatic dysfunction and lidocaine elimination: a study using isolated rat liver perfusion. Biopharm Drug Dispos. 1997;18:803–19. doi: 10.1002/(sici)1099-081x(199712)18:9<803::aid-bdd65>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 41.Aksnes J, Eide TJ, Nordstrand K. Lipid entrapment and cellular changes in the rat myocard, lung and liver after long-term parenteral nutrition with lipid emulsion. A light microscopic and ultrastructural study. Apmis. 1996;104:515–22. doi: 10.1111/j.1699-0463.1996.tb04906.x. [DOI] [PubMed] [Google Scholar]
- 42.Alwayn IP, Gura K, Nose V, et al. Omega-3 fatty acid supplementation prevents hepatic steatosis in a murine model of nonalcoholic fatty liver disease. Pediatr Res. 2005;57:445–52. doi: 10.1203/01.PDR.0000153672.43030.75. [DOI] [PubMed] [Google Scholar]
- 43.Gura KM, Parsons SK, Bechard LJ, et al. Use of a fish oil-based lipid emulsion to treat essential fatty acid deficiency in a soy allergic patient receiving parenteral nutrition. Clin Nutr. 2005;24:839–47. doi: 10.1016/j.clnu.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 44.Gura KM, Duggan CP, Collier SB, et al. Reversal of parenteral nutrition-associated liver disease in two infants with short bowel syndrome using parenteral fish oil: implications for future management. Pediatrics. 2006;118:e197–201. doi: 10.1542/peds.2005-2662. [DOI] [PubMed] [Google Scholar]
- 45.Gura KM, Lee S, Valim C, et al. Safety and efficacy of a fish-oil-based fat emulsion in the treatment of parenteral nutrition-associated liver disease. Pediatrics. 2008;121:e678–86. doi: 10.1542/peds.2007-2248. [DOI] [PubMed] [Google Scholar]
- 46.Andorsky DJ, Lund DP, Lillehei CW, et al. Nutritional and other postoperative management of neonates with short bowel syndrome correlates with clinical outcomes. J Pediatr. 2001;139:27–33. doi: 10.1067/mpd.2001.114481. [DOI] [PubMed] [Google Scholar]
- 47.Gura K, Strijbosch R, Arnold S, McPherson C, Puder M. The role of an intravenous fat emulsion composed of fish oil in a parenteral nutrition-dependent patient with hypertriglyceridemia. Nutr Clin Pract. 2007;22:664–72. doi: 10.1177/0115426507022006664. [DOI] [PubMed] [Google Scholar]
- 48.Carlson SE, Cooke RJ, Werkman SH, Tolley EA. First year growth of preterm infants fed standard compared to marine oil n-3 supplemented formula. Lipids. 1992;27:901–7. doi: 10.1007/BF02535870. [DOI] [PubMed] [Google Scholar]
- 49.Carlson SE, Werkman SH, Peeples JM, Cooke RJ, Tolley EA. Arachidonic acid status correlates with first year growth in preterm infants. Proc Natl Acad Sci U S A. 1993;90:1073–7. doi: 10.1073/pnas.90.3.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Carlson SE, Werkman SH, Tolley EA. Effect of long-chain n-3 fatty acid supplementation on visual acuity and growth of preterm infants with and without bronchopulmonary dysplasia. Am J Clin Nutr. 1996;63:687–97. doi: 10.1093/ajcn/63.5.687. [DOI] [PubMed] [Google Scholar]
- 51.Strijbosch RA, Lee S, Arsenault DA, et al. Fish oil prevents essential fatty acid deficiency and enhances growth: clinical and biochemical implications. Metabolism. 2008;57:698–707. doi: 10.1016/j.metabol.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee S, Gura KM, Kim S, Arsenault DA, Bistrian BR, Puder M. Current clinical applications of omega-6 and omega-3 fatty acids. Nutr Clin Pract. 2006;21:323–41. doi: 10.1177/0115426506021004323. [DOI] [PubMed] [Google Scholar]