Abstract
Introduction
We developed models of Specialized Care for Bipolar Disorder (SCBD) and a psychosocial treatment [Enhanced Clinical Intervention (ECI)] that is delivered in combination with SCBD. We investigated whether SCBD and ECI + SCBD are able to improve outcomes and reduce health disparities for young and elderly individuals, African Americans, and rural residents with bipolar disorder.
Method
Subjects were 463 individuals with bipolar disorder, type I, II, or not otherwise specified, or schizoaffective disorder, bipolar type, randomly assigned to SCBD or ECI + SCBD and followed longitudinally for a period of one to three years at four clinical sites.
Results
Both treatment groups significantly improved over time, with no significant differences based on age, race, or place of residence, except for significantly greater improvement among elderly versus adult subjects. Improvement in quality of life was greater in the ECI + SCBD group. Of the 299 participants who were symptomatic at study entry, 213 achieved recovery within 24 months, during which 86 of the 213 subjects developed a new episode. No significant difference was found for race, place of residence, or age between the participants who experienced a recurrence and those who did not. However, the adolescent patients were less likely than the adult and elderly patients to experience a recurrence.
Conclusion
This study demonstrated the effectiveness of SCBD and the additional benefit of ECI independent of age, race, or place of residence. It also demonstrated that new mood episodes are frequent in individuals with bipolar disorder who achieve recovery and are likely to occur in spite of specialized, guideline-based treatments.
Keywords: bipolar disorder, health disparities, outcomes, randomized trial
While there do not appear to be disparities in those who are at risk for developing bipolar disorder, there are marked disparities in who is likely to be diagnosed and treated. Even when a diagnosis of bipolar disorder is made, there are equally marked disparities in treatment outcome. Children and adolescents (1–4), elderly individuals (5, 6), African Americans (7, 8), and rural residents (9, 10) with bipolar disorder are less likely than their midlife, white, urban counterparts to be diagnosed, to receive adequate treatment, to remain in treatment once identified, and to have favorable outcomes if they remain in treatment (11, 12). Under the auspices of the Commonwealth of Pennsylvania, Department of Health Research Program (ME-02385), we created the Bipolar Disorder Center for Pennsylvanians (BDCP) to study ways to reduce these serious health disparities. We formed an interdisciplinary group of clinicians, investigators, and educators working at the University of Pittsburgh and the DuBois Regional Medical Center (in rural western Pennsylvania) to develop specialized clinics with procedures designed to increase the probability of accurate diagnosis, increase adequacy of treatment, increase retention in treatment, and improve treatment outcomes for adolescent, elderly, and African American patients with bipolar disorder. Patients were randomly assigned to treatment with Specialized Care for Bipolar Disorder (SCBD) or to SCBD plus a psychosocial intervention that we called Enhanced Clinical Intervention (ECI). We hypothesized that our model of treatment would reduce the disparities in treatment outcome among patients of different age, race, and place of living. We also hypothesized that individuals assigned to SCBD + ECI would have better clinical, functional, and quality of life outcomes than subjects assigned to SCBD alone.
Methods
The BDCP is a longitudinal, randomized, controlled, multicenter trial evaluating the efficacy of SCBD alone or in combination with ECI. The aims of these interventions were to increase the probability of accurate diagnosis, increase adequacy of treatment, increase retention in treatment, and improve treatment outcomes for adolescent, elderly, and African American patients with bipolar disorder. The study inclusion criteria were age 12 years or older and a DSM-IV diagnosis of bipolar I disorder, bipolar II disorder, bipolar disorder not otherwise specified (NOS), or schizo-affective bipolar subtype disorder, as established via the Structured Clinical Interview for DSM-IV (SCID) (13, 14) for adults or the Schedule for Affective Disorders and Schizophrenia for School Age Children, Present and Lifetime version (KSADS-PL) (15) for adolescents between the ages of 12 and 18 years. Parents were also interviewed about their children. The study exclusion criteria were IQ ≤ 70, current substance or alcohol dependence, organic mental disorder, and unstable or severe medical illness or other medical contraindication to treatment with mood stabilizers, antidepressants or antipsychotic medications, including pregnancy or breast-feeding. All study participants had a baseline general physical examination, including an electrocardiogram (EKG), urinalysis, thyroid examination, medication levels as appropriate, blood studies, and pregnancy tests if female. In addition, a complete assessment of mood state, comorbid psychiatric disorders, treatment, social and role functioning, and care utilization was conducted. A more detailed description of the study procedures is provided elsewhere (16). The Institutional Review Board at the University of Pittsburgh (BDCP coordinating center) reviewed and approved all study procedures and all subjects gave written informed consent prior to participating in the study.
Randomization
Randomization to either SCBD or SCBD + ECI was site specific, using a single permutated block randomization design stratified on site to ensure that equal numbers of subjects were entered into each treatment arm for each site. From November 2003 to April 2005, participants were randomly assigned only when they met the DSM-IV criteria for manic, hypomanic, depressive, or mixed episodes or when they demonstrated a clinical worsening, defined as a Clinical Global Impressions Severity Scale (CGI) (17) ≥3 for two weeks following a score of CGI = 1 or 2. From April 2005 to October 2005, all new participants were randomly assigned independent of their clinical status to evaluate the prophylactic efficacy of the study procedures. Table 1 reports the demographic and clinical characteristics of the two study groups.
Table 1.
Demographic and clinical characteristics of the two study groups
| SCBD + ECI (n = 235) | SCBD (n = 228) | |
|---|---|---|
| Age group, n (%) | ||
| Adolescent | 38 (16) | 37 (16) |
| Adult | 176 (75) | 173 (76) |
| Late life | 21 (9) | 18 (8) |
| Age, mean (SD) | 41.7 (17.5) | 39.7 (17.8) |
| Gender, n (%) | ||
| Women | 149 (63) | 132 (58) |
| Race, n (%) | ||
| Caucasian | 189 (80) | 196 (86) |
| African American | 39 (17) | 29 (13) |
| Other | 6 (3) | 3 (1) |
| Diagnosis, n (%) | ||
| Bipolar I disorder | 158 (67) | 155 (68) |
| Bipolar II disorder | 44 (19) | 43 (19) |
| Bipolar disorder NOS | 27 (11) | 26 (11) |
| Schizoaffective | 6 (3) | 4 (2) |
| Marital status, n (%) | ||
| Never married | 104 (44) | 96 (42) |
| Married | 63 (27) | 80 (35) |
| Separated/divorced | 54 (23) | 43 (19) |
| Widowed | 13 (6) | 7 (3) |
| Education, n (%) | ||
| <High school | 43 (18) | 43 (19) |
| High school or GED | 41 (18) | 38 (17) |
| Some college | 70 (30) | 75 (32) |
| College degree | 43 (18) | 49 (22) |
| Graduate | 37 (16) | 21 (9) |
| Employment, n (%) | ||
| Full time/part time | 80 (34) | 79 (35) |
| Disabled/leave of absence | 47 (20) | 45 (20) |
| Unemployed | 64 (27) | 56 (25) |
| Retired | 18 (8) | 16 (7) |
| Annual household income ($US), n (%) | ||
| <$10,000 | 43 (19) | 36 (17) |
| $10,000–20,000 | 54 (23) | 44 (20) |
| $20,000–30,000 | 33 (14) | 31 (14) |
| $30,000–40,000 | 25 (11) | 29 (13) |
| $40,000–50,000 | 20 (9) | 20 (9) |
| $50,000–75,000 | 30 (13) | 32 (15) |
| $75,000–100,000 | 13 (6) | 13 (6) |
| >$100,000 | 12 (5) | 13 (6) |
| Illness severity, mean (SD) | ||
| CGI-Manic | 1.70 (1.04) | 1.82 (1.09) |
| CGI-Depressed | 2.54 (1.29) | 2.50 (1.25) |
| CGI-Overall | 2.86 (1.23) | 2.89 (1.20) |
| GAF past week | 60.3 (11.2) | 60.3 (10.3) |
| QLESQ | 43.8 (10.3) | 45.2 (10.4) |
| Place of residence, n (%) | ||
| Urban | 191 (81) | 185 (81) |
| Rural | 44 (19) | 43 (19) |
SCBD = Specialized Care for Bipolar Disorder; ECI = Enhanced Clinical Intervention; NOS = not otherwise specified; GED = General Educational Development; CGI = Clinical Global Impressions Severity Scale; GAF = Global Assessment of Functioning; QLESQ = Quality of Life Enjoyment and Satisfaction Questionnaire.
Treatment
Study participants had, on average, a treatment period of 19.7 ± 5.2 months (range 18–34 months). All treatment was provided at no cost to the patient, with the exception of medications prescribed to patients who did not respond to or tolerate the study medications (see below). Also, in the second part of the study, patients and their insurers were responsible for the cost of the standard of care laboratory assessments.
Specialized Care for Bipolar Disorder
SCBD is a manualized system of clinical management for patients with bipolar disorder consisting of: diagnosis via the SCID or KSADS-PL; standardized assessment of quality of life via the Quality of Life Enjoyment and Satisfaction Questionnaire (QLESQ) (18); standardized assessment of threshold and subthreshold symptoms for mood, obsessive-compulsive, social phobic, and panic agoraphobic spectra (via the mood and anxiety ‘spectrum’ instruments; see http://www.spectrum-project.org); comprehensive medical evaluations (extensive physical exam and EKG at least yearly, laboratory assessment at least quarterly); provision of appropriate referrals and follow-up for ongoing medical comorbidities; regular and relatively frequent visits with the treatment team (at least once every two weeks in the presence of acute symptoms and at least once every two months during the remission periods); standardized algorithm-driven pharmacological treatment provided by a psychiatrist specialized in the pharmacological treatment of bipolar disorder; and automatic tracking of visit frequency and proactive reminder and reschedule calls from the clinics’ office managers, all highly trained in the management of patients with bipolar disorder. Treatment was delivered by site psychiatrists who operated under the supervision of DJK (Primary Investigator), AF (Medical Director for the entire BDCP study), MET and ESF (the Pittsburgh Adult Unit), BB and DAA (the Pittsburgh Adolescent Unit), AGG (the Pittsburgh Late-Life Unit), and SRT (the DuBois Adult Unit). All patients were treated pharmacologically following specific algorithmic guidelines for the treatment of mania, mixed states, or depression that were based on the expert consensus guidelines of Sachs and colleagues (19), the algorithms developed by the Texas Medication Algorithm Project (TMAP) (20), and the standard of care provided in our research clinics at the time the grant was written in 2002. First-line antimanic agents were lithium and divalproex. Subjects in a major depressive episode also received sertraline or lamotrigine, whereas subjects with psychotic symptoms received adjunctive aripiprazole or olanzapine. Lorazepam was also permitted for marked anxiety, sleeplessness, or agitation. When lorazepam was not an appropriate clinical choice, gabapentin was used. Subjects who did not respond to/tolerate (or with a previous history of nonresponse/intolerance to) the medications above were offered alternative standard-of-care medications.
Psychiatrists received a pharmacotherapy manual and underwent training focused on the following: pharmacotherapy treatment of patients with bipolar disorder; standardizing the procedures to obtain past psychiatric, family, and medical history; discussion and documentation of the course of present and previous affective episodes; establishing target symptoms; assessment and management of medication side effects; fully assessing suicidal ideation and intent; providing an understandable model of how and why medications can be effective; instructing the subject to avoid all nonprescription medications and to immediately report to the psychiatrist before taking other medications (including over-the-counter compounds); and conferring with investigators regarding unimproved or deteriorating subjects. For the entire duration of the study, the pharmacological treatment provided to each study patient was reviewed by AF and the unit directors once every week.
Enhanced Clinical Intervention
ECI consisted of the same treatment provided as part of SCBD with the addition of a manualized intensive clinical and psychosocial management program provided by a nurse or master’s-level clinician consisting of 10 basic components, each of which can be adapted to the specific needs of the subpopulations being treated. For the purposes of our study, added to these 10 basic elements were specific intervention modules for young, elderly, and African American patients. The 10 components of the ECI can be divided into four educational components, five management components, and a support component. The educational components consisted of: (i) education about the mood disorder itself; (ii) education about medications used to treat the disorder; (iii) education about basic sleep and social rhythm hygiene; and (iv) education regarding the use of rescue medication. The management component consisted of: (i) a careful review of symptoms; (ii) a careful review of side effects; (iii) medical and behavioral management of side effects; (iv) discussion of early-warning signs of impending episodes; and (v) 24-hour on-call service. The support component consisted of nonspecific support that may be provided to the individual patient him / herself or to the patient’s family members or significant others. Clinicians at all sites were trained by EF and her colleagues from the Depression and Manic Depression Prevention Program at Western Psychiatric Institute and Clinic. Clinicians received the Enhanced Clinical Intervention Manual designed for this study and underwent intensive training to review the manual and standard procedures, as well as to provide a forum for an intensive discussion of questions, possible problems, and troubleshooting.
ECI clinicians met with the patient at each clinic visit for 20 to 30 minutes prior to the patient’s visit with the physician. Once the ECI session was complete, the physician joined the patient and ECI clinician for the medication management portion of the visit. Subjects received ECI weekly for the first 12 weeks of the trial, every other week for the subsequent eight weeks of the trial, and then monthly for their remaining time in the randomized intervention or until they experienced a recurrence of mania or depression. At that time, they returned to weekly visits until they restabilized.
This team approach to disease management was drawn primarily from two sources. The first is the randomized trial evaluating systematic care for bipolar disorder as developed by Simon and colleagues (21). Their intervention program is intended to address previously identified shortcomings in the care of bipolar disorder such as inadequate patient education, absence of structured psychotherapy programs, inadequate follow-up visit frequency, high rates of medication nonadherence, and poor adherence to laboratory monitoring guidelines (21). Their intervention program uses a nurse–care manager working in conjunction with the patient to develop a structured plan that includes assessment, feedback, and periodic recommendations. Other aspects of their program include telephone monitoring and structured group programs. The second source for the development of our ECI strategy was our study of maintenance therapies in bipolar disorder which included an Intensive Clinical Management condition (22).
Other care
Patients were not permitted to start psychotherapy during the study. However, patients who were already receiving psychotherapy at entry in the study were permitted to continue seeing their therapist regardless of their random assignment to ECI or SCBD.
Clinical Status
For the purposes of this study, we used the following definitions: (i) recovery: a period of at least eight weeks with a CGI Severity Scale–Bipolar version, depression (CGI-D) and a CGI Severity Scale–Bipolar version, mania (CGI-M) of ≤ 2; (ii) symptomatic: a patient with a CGI-D or a CGI-M of ≥ 3; and (iii) recurrence: a period of at least two weeks with a CGI-D of ≥ 4 or a period of at least four days with a CGI-M of ≥ 4 following a period of recovery. When recovery from the episode occurred, patients continued to receive their assigned treatment for the duration of the treatment trial. During the Recurrence Prevention Phase, patients were seen at least every two months for clinical visits and immediately (within 36 hours) if a recurrence was impending. Patients who experienced a recurrence continued to receive algorithm-guided treatment according to whichever randomized intervention (SCBD or SCBD + ECI) they were originally assigned.
Statistical analyses
Demographic and clinical measures at randomization were compared between SCBD and SCBD + ECI with Chi-square test for categorical data and group t-test for continuous measures. We fit nonparametric mixed-effects models (23) to determine temporal differences in 18 months (the minimum period of treatment offered to all study subjects) postbaseline across ECI and SCBD in CGI-Overall, CGI-D, CGI-M, Global Assessment of Functioning (GAF), and 14-item QLESQ scores. A nonlinear mixed model accommodates trajectories of these outcomes, which vary in a highly nonlinear fashion over time across both subjects and subgroups, allowing for data-driven estimates of outcome trajectories and predictor effects. All visits were used from baseline to a maximum of 18 months postrandomization. Group (ECI versus SCBD) was entered as a predictor in each model, as were baseline levels of the outcome in question.
To ensure comparability with previously published data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) trial (24), time to recovery and frequency of recurrence were calculated over a period of 24 months.
Results
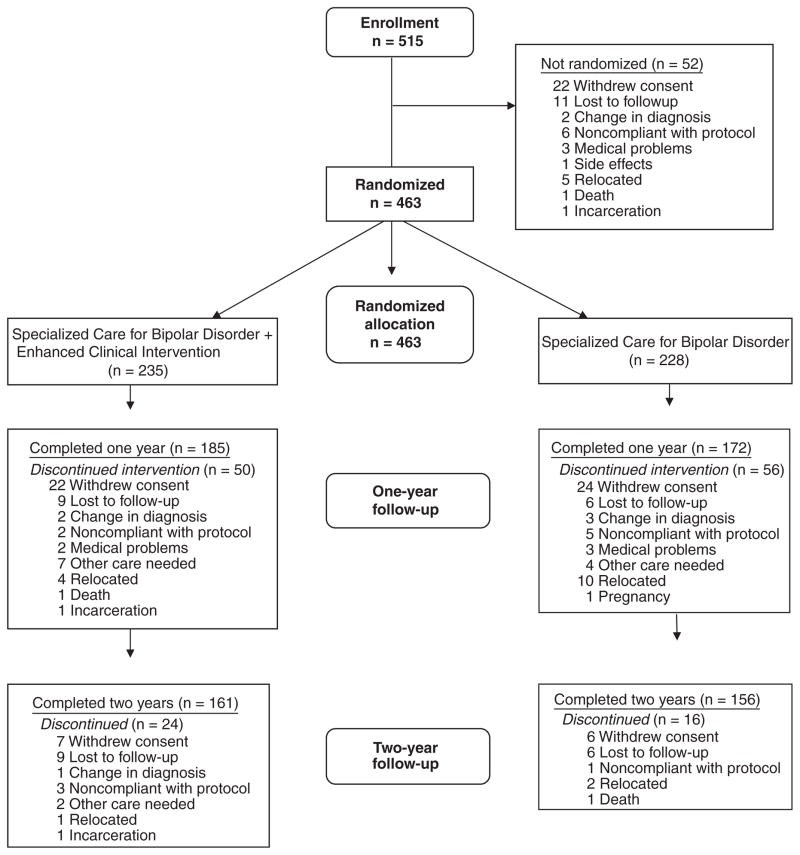
Study recruitment started on November 2003 and ended in October 2005. The study was completed on February 28, 2007. Of the 515 individuals who met inclusion and exclusion criteria and enrolled in the study, 463 participants with bipolar [I (n = 313), II (n = 87), or NOS (n = 53)], or schizoaffective, bipolar type (n = 10), disorders were randomly assigned to SCBD + ECI (n = 235) or SCBD alone (n = 228) and offered treatment for up to three years, depending on the date of enrollment. Of these randomized participants, 75 subjects were adolescent (age 12–18), 349 adult (age 19–64), and 39 late-life (age 65 or older) individuals. There were 68 African Americans (10 adolescents, 57 adults, and one late life). Of the non-African American subjects, 385 were Caucasian (65 adolescents, 283 adults, and 37 late life), one was Native American, three were Asian, one was Pacific Islander, and five were biracial. Of the adult subjects, 87 lived in a rural area and were treated at the DuBois site. The Pittsburgh site treated 376 individuals (with subsites for adolescent, adult, and late-life patients). The 52 subjects who entered the study but were not randomly assigned left the study for the reasons outlined in Fig. 1.
Fig. 1.
Patient flow.
No significant difference was found between the ECI + SCBD and the SCBD-alone groups for baseline demographic variables of interest (see Table 1) or for rate of study completion (see Table 2). Furthermore, neither a difference in degree of study completion nor a significant difference due to geographical location (rural versus nonrural) was noted among the age and race groups.
Table 2.
Study retention
| Entered n |
Completed n (%) |
Attrition (Year 1) n |
Attrition (Year 2) n |
|
|---|---|---|---|---|
| Entire sample | 463 | 317 (68) | 106 | 40 |
| SCBD + ECI | 235 | 161 (69) | 50 | 24 |
| SCBD alone | 228 | 156 (68) | 56 | 16 |
| Adolescents | 75 | 45 (60) | 16 | 14 |
| Adults | 349 | 245 (70) | 81 | 23 |
| Late life | 39 | 27 (69) | 9 | 3 |
| Rural residents | 87 | 62 (71) | 22 | 3 |
| African Americans | 67 | 40 (60) | 17 | 10 |
| Non-African Americans | 396 | 277 (70) | 89 | 30 |
SCBD = Specialized Care for Bipolar Disorder; ECI = Enhanced Clinical Intervention.
All outcome variables exhibited significant improvement over time (see Table 3), as shown by the mean difference between predicted values at 18 months and baseline levels. For CGI-Overall, the mean difference from baseline across both groups at 18 months is −0.78 (p < 0.01), meaning there was, on average, almost a full point improvement in CGI-Overall for both groups.
Table 3.
Outcomes
| Baseline | 18 Months | Mean difference | |
|---|---|---|---|
| CGI-Overall | 2.80 ± 1.20 | 2.02 ± 0.56 | −0.78 (p < 0.01) |
| CGI-Depressed | 2.48 ± 1.20 | 1.92 ± 0.56 | −0.56 (p < 0.01) |
| CGI-Mania | 1.74 ± 1.02 | 1.23 ± 0.32 | −0.51 (p < 0.01) |
| GAF | 60.88 ± 10.79 | 66.50 ± 6.57 | 5.63 (p < 0.01) |
| QLESQ | 44.55 ± 10.30 | 46.33 ± 6.90 | 1.78 (p = 0.07) |
Values expressed as mean ± SD.
CGI = Clinical Global Impressions Severity Scale; GAF = Global Assessment of Functioning; QLESQ = Quality of Life Enjoyment and Satisfaction Questionnaire.
For CGI-D, the estimated difference was −0.56 (p < 0.01) and for CGI-M the estimated difference was −0.51 (p < 0.01). The average change in GAF score was 6.63 points (p < 0.01) and in QLESQ score 1.78 (p = 0.07). There were no group effects on any of these outcomes with the exception of QLESQ. For QLESQ, the ECI + SCBD group showed significantly greater improvement over time than the SCBD-alone group. The mean difference from baseline at 18 months for the ECI group was 2.61 points, while the mean difference was 0.95 in the SCBD alone group (p = 0.04). No significant differences in treatment outcomes were found between patients of different age, race, and geographic location, except for a greater GAF improvement in late-life versus adult participants (t = 2.69, p = 0.008).
Of the 299 participants who were symptomatic at study entry (CGI > 2), 213 (71%) achieved ‘recovery’ clinical status (at least eight weeks with CGI of 1 or 2) within 24 months. Significant relationships were found between time to recovery and pretreatment assessments of quality of life (Chi square = 5.62, p = 0.027). No significant relationship was found between time to recovery and gender, age group, place of living, race, pretreatment assessments of global illness severity (CGI), functioning (GAF), polarity at intake, or random treatment assignment.
During this 24-month period, 86 (40%) of the 213 subjects who had achieved ‘recovery’ clinical status met criteria for a new episode (CGI-D ≥ 4 for two weeks or CGI-M ≥ 4 for four or more days). Of the 86 participants who experienced a recurrence, 69 met criteria for a depressive episode (mean time to recurrence was 58 weeks) and 17 met criteria for a manic or mixed episode (mean time to recurrence 68 weeks). The time from recovery until 25% of the individuals experienced a new depressive or manic episode was 21 weeks. The mean time to recurrence was 51 weeks. No significant difference was found for race, place of living, or age between the participants who experienced a recurrence and those who did not. However, when we looked at differences among the three age groups (12–17, 18–64, and ≥ 65 years), we observed that the 12–17-year-old patients were significantly less likely to develop a recurrence than the adult and late-life patients (Logrank = 10.32, df = 2, p = 0.006). No significant difference was found between the adult patients living in a rural area (the DuBois site recruited only adult patients) and the adult patients living in an urban area for the likelihood of recurrence. A significant relationship was found between time to recurrence and quality of life (Chi square = 18, p < 0.001) and CGI (Chi square = 5.97, p = 0.01) measured at the time of randomization.
Discussion
The results of this study indicate a favorable clinical impact for the model of specialized care for the treatment of bipolar disorder used in the BDCP. Both participants assigned to SCBD alone and those assigned to SCBD + ECI experienced significant improvement over time in CGI and functioning. No significant differences in treatment outcomes were found between participants of different race and place of residence, except for a higher GAF improvement in late-life versus adult subjects and a lower risk of recurrence in adolescent participants. Several factors may have contributed to the generally positive outcomes observed, many of which may be implemented in different settings and possibly become the standard of care for patients with bipolar disorder. Although it remains to be established which and to what degree each specific factor played a role, we posit that the following conditions contributed to study outcomes and should be among the first to consider among the strategies that may be exported to other settings: a well-trained team specialized in the care of patients with bipolar disorder; highly standardized and thorough diagnostic and clinical monitoring strategies; relatively frequent visits to the clinic; reminder calls and immediate availability when there are changes in clinical status in the period between scheduled visits; collaborative care among centers / clinics that provide a specialized treatment for bipolar disorder; and standardized, yet not overly rigid pharmacotherapy protocols. We believe that all patients benefited from attendance at clinics structured as above. However, it is possible that patients with certain demographic or clinical characteristics may benefit more than others. For instance, our study model seemed particularly effective for the improvement in functioning in late-life patients.
We demonstrated that adding ECI to SCBD confers the additional benefit of greater improvement in quality of life. Quality of life has gained increasing attention as an important outcome in patients with severe and persistent mental disorders, including bipolar disorder. The efficacy of ECI in the improvement of quality of life is of particular importance, given that patients with bipolar disorder experience lower quality of life than the general population even during euthymic periods (25). Several factors may have contributed to the efficacy of ECI, including its social and general support component. In fact, previous research has shown that the availability of social support plays an important role in enhancing quality of life in patients with bipolar disorder (25).
Seventy-seven percent of our study participants completed one year of follow-up and 68.5% completed two years of follow-up, with no difference between the SCBD and the SCBD + ECI group, possibly because of the adoption of similar retention strategies in both groups. For example, reminder and follow-up calls were made to patients assigned to both groups by the office manager, study coordinators, or treating ECI therapist. Remarkably, no significant differences for retention rate were observed based on age, race, and place of residence.
Although statistical comparisons of our outcomes with those reported in other published studies conducted with different methodologies are not appropriate, it is noteworthy that: (i) 71% [versus 58% in the STEP-BD study (24)] of the participants who were symptomatic at study entry (CGI > 2) achieved ‘recovery’ clinical status (at least eight weeks with CGI of 1 or 2) within 24 months; (ii) during this 24-month period, 40% [versus 48.5% of the STEP-BD study (24)] of the subjects who had achieved ‘recovery’ met criteria for a new episode (CGI-D ≥ 4 for two weeks or CGI-M ≥ 4 for four or more days); (iii) the time until 25% of the participants experienced a depressive episode was 21.3 weeks [versus 21.4 weeks in the STEP-BD study (24)], and less than 25% of our subjects experienced a manic recurrence.
In the present study, participants with bipolar I disorder were symptomatically ill (CGI ≥ 3) 33% of study weeks and those with bipolar II disorder were also symptomatically ill 33% of weeks. In a prospective follow-up of 146 patients with bipolar I disorder and 86 patients with bipolar II disorder who entered the National Institute of Mental Health Collaborative Depression Study, those with bipolar I disorder were symptomatically ill 47.3% of weeks and those with bipolar II disorder were symptomatically ill 53.9% of weeks (26). The Course and Outcome of Bipolar Youth (COBY) study with children and adolescents yielded similar results (2).
Although outcomes in the present study are somewhat better than those of the research mentioned above, the fact that 29% of our study participants were still symptomatic after two years of treatment and that 40% of those who recovered from an acute episode experienced a recurrence in a relatively short period of time clearly confirms that bipolar disorder remains a chronic, difficult-to-treat, and highly recurrent condition in a large number of affected individuals. Of interest, we found no significant difference for race or age between the patients who experienced a recurrence and those who did not. However, we found that patients living in a rural area were more likely to recur following the resolution of an acute episode, despite the absence of differences in the study protocol at the rural and urban sites.
Sixty-eight percent of our patients completed the study. Long-term studies conducted on patients with bipolar disorders have reported variable completion rates, strongly depending on the duration and type of treatment and follow-up that was provided, which makes it very difficult to perform any comparison. For instance, Miller and colleagues (27) reported a 69% completion rate in patients with bipolar disorder followed for about two years; Keck and colleagues (28) reported a much lower retention rate in a group of patients treated for 100 weeks under a very rigid protocol; and Judd and colleagues (26) reported a very high completion rate (93%) in a follow-up nontreatment study that required a much lower level of commitment. Two studies have employed a design similar to ours. In the first study (20), patients with bipolar disorder were randomized to usual treatment or to algorithm implementation and the retention rate was 81% after the first year of follow-up; two-year follow-up data were too sparse for reporting. The second study (29) compared the long-term effectiveness of a systematic care program versus usual care: 381 of 441 patients (86.4%) completed the 12-month follow-up, yet the number of completers decreased to 335 (76%) at 24 months.
The present study demonstrated the utility of a long-term, standardized treatment protocol based on specialized care for bipolar disorder in which the procedures were designed to increase the probability of accurate diagnosis, increase adequacy of treatment, increase retention in treatment, and improve treatment outcomes for all patients, including those at higher risk for a poorer outcome, such as adolescent, elderly, and African American individuals with bipolar disorder. Among the limitations of this study, we would like to acknowledge the exclusion of patients with substance dependence, who represent a non-negligible proportion of patients with bipolar disorder. This fact clearly limits the generalizability of our results and calls for more studies that integrate the interventions that were tested in our study with the specialized interventions that are currently provided to patients with co-occurring substance dependence and bipolar disorder. Regrettably, the study also confirms that bipolar disorder is a difficult-to-treat, highly recurrent condition even when individuals receive consistent, high-quality treatment. We hope that the present study may provide a model for establishing or re-establishing treatment teams or clinics specialized in the diagnosis, assessment, and treatment of patients with bipolar disorder.
Acknowledgments
Support for the research presented here has been provided by grants from the Commonwealth of Pennsylvania Department of Health (ME-02385) and the National Institute of Mental Health (MH030915).
Footnotes
Disclosures
AF has served as a consultant and on the speakers bureau for Bristol-Myers Squibb and Pfizer; and has served as a consultant for Novartis and Takeda. EF has received grant support from the Forest Research Institute, the Pittsburgh Foundation, and the Fine Foundation; serves as a member and Scientific Advisory Board Chair for the Depression and Bipolar Support Alliance (DBSA); serves as a consultant for Clinical Tools, Deborah Wood and Associates, Innovative Medical Education, Eli Lilly & Co., Pfizer, Servier, Physician’s Postgraduate Press, LLC, Medesta Associates, LLC, Universal CIT, Inc., Novartis, Cardinal Health, Central Services, and Lundbeck; and receives royalties from Guilford Publications. BB has participated in forums for JAZZ Pharmaceuticals, Solvay, and Abcomm, Inc.; and has received royalties from Random House. ESF has received grant/research support from AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Forest Specialty Sales, GlaxoSmithKline, Novartis, Roerig Division of Pfizer, Sanofi-aventis, and Wyeth-Ayerst Laboratories; has received financial/material support from Abbott Laboratories; has served as a consultant for Cephalon, GlaxoSmithKline, and Wyeth-Ayerst Laboratories; has served on the speakers bureau for Abbott Laboratories, AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Forest Specialty Sales, GlaxoSmithKline, Pfizer, and Wyeth-Ayerst Laboratories; and is a major stockholder for Cephalon. AGG has served on the advisory board for Shire Pharmaceuticals and has a custodial account with Eli Lilly & Co. MET has received grant/research support from Eli Lilly & Co. and Sepracor, Inc.; has served in an advisory/consultant capacity for AstraZeneca, Bristol-Myers Squibb, Cephalon, Cyberonics, Inc., Eli Lilly & Co., Forest Laboratories, GlaxoSmithKline, Janssen Pharmaceutica, MedAvante, Inc., Neuronetics, Inc., Novartis, Organon International, Sepracor, Inc., Shire US, Inc., Supernus Pharmaceuticals, and Wyeth Pharmaceuticals; has served on the speakers bureau for AstraZeneca, Bristol-Myers Squibb, Cyberonics, Inc., Eli Lilly & Co., GlaxoSmithKline, Sanofi-aventis, Schering Plough, and Wyeth; has provided expert testimony for Jones Day, Phillips Lyttle, and Pepper Hamilton, LLP; has equity holdings in MedAvante, Inc.; receives royalties from American Psychiatric Publishing, Guilford Publications, Herald House, and W.W. Norton & Company; and his wife is employed by Advogent (formerly Cardinal Health). DAA, YC, DEC, TG, VJG, PRH, MGS, WKT, SRT, and DJK have no conflicts of interest to report.
References
- 1.Berk M, Dodd S, Callaly P, et al. History of illness prior to a diagnosis of bipolar disorder or schizoaffective disorder. J Affect Disord. 2007;103:181–186. doi: 10.1016/j.jad.2007.01.027. [DOI] [PubMed] [Google Scholar]
- 2.Birmaher B, Axelson D. Course and outcome of bipolar spectrum disorder in children and adolescents: A review of the existing literature. Dev Psychopathol. 2006;18:1023–1035. doi: 10.1017/S0954579406060500. [DOI] [PubMed] [Google Scholar]
- 3.DelBello MP, Hanseman D, Adler CM, Fleck DE, Strakowski SM. Twelve-month outcome of adolescents with bipolar disorder following first hospitalization for a manic or mixed episode. Am J Psychiatry. 2007;164:582–590. doi: 10.1176/ajp.2007.164.4.582. [DOI] [PubMed] [Google Scholar]
- 4.Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM. The National Depressive and Manicdepressive Association (DMDA) survey of bipolar members. J Affect Disord. 1994;31:281–294. doi: 10.1016/0165-0327(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 5.Charney DS, Reynolds CF, Lewis L, et al. Depression and Bipolar Support Alliance Depression and Bipolar Support Alliance consensus statement on the unmet needs in diagnosis and treatment of mood disorders in late life. Arch Gen Psychiatry. 2003;60:664–672. doi: 10.1001/archpsyc.60.7.664. [DOI] [PubMed] [Google Scholar]
- 6.Young RC, Gyulai L, Mulsant BH, et al. Pharmacotherapy of bipolar disorder in old age: review and recommendations. Am J Geriatr Psychiatry. 2004;12:342–357. doi: 10.1176/appi.ajgp.12.4.342. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez JM, Thompson P, Escamilla M, et al. Treatment characteristics and illness burden among European Americans, African Americans, and Latinos in the first 2,000 patients of the systematic treatment enhancement program for bipolar disorder. Psychopharmacol Bull. 2007;40:31–46. [PubMed] [Google Scholar]
- 8.Kilbourne AM, Bauer MS, Han X, et al. Racial differences in the treatment of veterans with bipolar disorder. Psychiatr Serv. 2005;56:1549–1555. doi: 10.1176/appi.ps.56.12.1549. [DOI] [PubMed] [Google Scholar]
- 9.McCarthy JF, Blow FC. Older patients with serious mental illness: sensitivity to distance barriers for outpatient care. Med Care. 2004;42:1073–1080. doi: 10.1097/00005650-200411000-00006. [DOI] [PubMed] [Google Scholar]
- 10.Wallace AE, Weeks WB, Wang S, Lee AF, Kazis LE. Rural and urban disparities in health-related quality of life among veterans with psychiatric disorders. Psychiatr Serv. 2006;57:851–856. doi: 10.1176/ps.2006.57.6.851. [DOI] [PubMed] [Google Scholar]
- 11.Bauer MS, McBride L, Williford WO, et al. Cooperative Studies Program 430 Study Team. Collaborative care for bipolar disorder: Part 1: Intervention and implementation in a randomized effectiveness trial. Psychiatr Serv. 2006;57:927–936. doi: 10.1176/ps.2006.57.7.927. [DOI] [PubMed] [Google Scholar]
- 12.Simon NM, Otto MW, Wisniewski SR, et al. Anxiety disorder comorbidity in bipolar disorder patients: Data for the first 500 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD) Am J Psychiatry. 2004;161:2222–2229. doi: 10.1176/appi.ajp.161.12.2222. [DOI] [PubMed] [Google Scholar]
- 13.First MB, Gibbon ML, Spitzer RL, Williams JBW. Structured Clinical Interview for DSM-IV (SCID-I): User’s Guide and Interview, Research Version. New York: Biometrics Research Department, New York Psychiatric Institute; 1995. [Google Scholar]
- 14.American Psychiatric Association. Task Force for the Handbook of Psychiatric Measures: Handbook of Psychiatric Measures. 1. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 15.Kaufman J, Birmaher B, Brent D, et al. Schedule for Affective Disorders and Schizophrenia for School-Age Children – Present and Lifetime Version (K-SADS-PL): initial reliability and validity data. J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. doi: 10.1097/00004583-199707000-00021. [DOI] [PubMed] [Google Scholar]
- 16.Kupfer DJ, Axelson DA, Birmaher B, et al. The Bipolar Disorder Center for Pennsylvanians: implementing an effectiveness trial to reduce health disparities for patients at risk for poor outcomes. Psychiatri Serv. doi: 10.1176/appi.ps.60.7.888. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spearing MK, Post RM, Leverich GS, Brandt D, Nolen W. Modification of the Clinical Global Impressions (CGI) Scale for use in bipolar illness (BP): the CGI-BP. Psychiatry Res. 1997;73:159–171. doi: 10.1016/s0165-1781(97)00123-6. [DOI] [PubMed] [Google Scholar]
- 18.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol Bull. 1993;29:321–326. [PubMed] [Google Scholar]
- 19.Sachs GS, Printz DJ, Kahn DA, Carpenter D, Docherty JP. The Expert Consensus Guideline Series: medication treatment of bipolar disorder 2000. Postgrad Med J. 2000 Apr;(Spec No):1–104. [PubMed] [Google Scholar]
- 20.Suppes T, Rush AJ, Dennehy EB, et al. Texas Medication Algorithm Project, Phase 3 (TMAP-3): clinical results for patients with a history of mania. J Clin Psychiatry. 2003;64:370–382. doi: 10.4088/jcp.v64n0403. [DOI] [PubMed] [Google Scholar]
- 21.Simon GE, Ludman E, Unutzer J, Bauer MS. Design and implementation of a randomized trial evaluating systematic care for bipolar disorder. Bipolar Disord. 2002;4:226–236. doi: 10.1034/j.1399-5618.2002.01190.x. [DOI] [PubMed] [Google Scholar]
- 22.Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62:996–1004. doi: 10.1001/archpsyc.62.9.996. [DOI] [PubMed] [Google Scholar]
- 23.Guo W. Functional mixed effects models. Biometrics. 2002;58:121–128. doi: 10.1111/j.0006-341x.2002.00121.x. [DOI] [PubMed] [Google Scholar]
- 24.Perlis RH, Ostacher MJ, Patel JK, et al. Predictors of recurrence in bipolar disorder: primary outcomes from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Am J Psychiatry. 2006;163:217–224. doi: 10.1176/appi.ajp.163.2.217. [DOI] [PubMed] [Google Scholar]
- 25.Gutiérrez-Rojas L, Gurpegui M, Ayuso-Mateos JL, Gutiérrez-Ariza JA, Ruiz-Veguilla M, Jurado D. Quality of life in bipolar disorder patients: a comparison with a general population sample. Bipolar Disord. 2008;10:625–634. doi: 10.1111/j.1399-5618.2008.00604.x. [DOI] [PubMed] [Google Scholar]
- 26.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59:530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- 27.Miller IW, Uebelacker LA, Keitner GI, Ryan CE, Solomon DA. Longitudinal course of bipolar I disorder. Compr Psychiatry. 2004;45:431–440. doi: 10.1016/j.comppsych.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 28.Keck PE, Jr, Calabrese JR, McIntyre RS, et al. Aripiprazole monotherapy for maintenance therapy in bipolar I disorder: a 100-week, double-blind study versus placebo. J Clin Psychiatry. 2007;68:1480–1491. doi: 10.4088/jcp.v68n1003. [DOI] [PubMed] [Google Scholar]
- 29.Simon GE, Ludman EJ, Bauer MS, Unützer J, Operskalski B. Long-term effectiveness and cost of a systematic care program for bipolar disorder. Arch Gen Psychiatry. 2006;63:500–508. doi: 10.1001/archpsyc.63.5.500. [DOI] [PubMed] [Google Scholar]