Abstract
Background
Non-alcoholic fatty liver disease (NAFLD) results from over-consumption and is a significant and increasing cause of liver failure. The type of diet that is conducive to the development of this disease has not been established and evidence-based treatment options are currently lacking. We hypothesized that the onset of hepatic steatosis is linked to the consumption of a diet with a high fat content, rather than related to excess caloric intake. In addition, we also hypothesized that fully manifested hepatic steatosis could be reversed by reducing the fat percentage in the diet of obese mice.
Methods
C57Bl/6J male mice were either fed a purified rodent diet containing 10% fat or a diet with 60% of calories derived from fat. A pair-feeding design was used to distinguish the effects of dietary fat content and caloric intake on dietary-induced hepatic lipid accumulation and associated injury. Livers were analyzed by quantitative RT-PCR for lipid metabolism-related gene expression.
Results
After 9 weeks, mice on the 60% fat diet exhibited more weight gain, insulin resistance and hepatic steatosis, compared to mice on a 10% fat diet with equal caloric intake. Furthermore, mice with established metabolic syndrome at 9 weeks showed reversal of hepatic steatosis, insulin resistance and obesity when switched to a 10% fat diet for an additional 9 weeks, independent of caloric intake. Quantitative RT-PCR revealed that transcripts related to both de novo lipogenesis and increased uptake of free fatty acids were significantly upregulated in mice pair-fed a 60% fat diet, compared to 10% fat-fed animals.
Conclusion
Dietary fat content, independent from caloric intake, is a crucial factor in the development of hepatic steatosis, obesity and insulin resistance in the C57Bl/6J diet-induced obesity model caused by increased uptake of free fatty acids and de novo lipogenesis. In addition, once established, all these features of the metabolic syndrome can be successfully reversed after switching obese mice to a diet low in fat. Low fat diets deserve attention in the investigation of a potential treatment of patients with NAFLD.
Introduction
Non-alcoholic fatty liver disease (NAFLD) consists of a variety of pathologic states ranging from the simple build-up of fat in the liver (hepatic steatosis) to non-alcoholic steatohepatitis, cirrhosis, and ultimately liver failure (1-3). Mostly unrecognized before 1980, today NAFLD is the most common chronic liver disease in Western countries with a prevalence up to 30 percent (4, 5). Its prevalence in non-Western countries is also increasing, mainly due to globalization of the Western diet (6, 7). NAFLD is strongly associated with metabolic disorders such as obesity (8, 9) and diabetes mellitus (10), and is considered to be the hepatic manifestation of the metabolic syndrome (11).
C57Bl/6J male mice fed a high fat (HF) diet is the most commonly used model of diet-induced obesity (DIO) sharing many of the same obesity phenotypes as humans. When allowed ad libitum access to a HF diet, the animals gradually develop visceral adiposity, hyperglycemia, insulin and leptin resistance, as well as hepatic steatosis (12). In contrast, C57Bl/6J mice raised on regular, low fat (LF) chow are lean, euglycemic, have normal insulin and leptin levels and do not develop hepatic steatosis.
Although high fat feeding is associated with hyperphagia, the onset of obesity and insulin resistance in the DIO model is associated with an increased dietary fat content, rather than being simply a consequence of excess caloric consumption, or from decreased levels of physical activity (13, 14). Furthermore, in C57Bl/6J mice receiving a HF diet for 16 weeks, complete reversal of both diabetes and obesity has been demonstrated after switching to a LF diet (15). In human studies, reduction of dietary fat content produces weight loss for periods up to 7 years and is considered to be one of the cornerstones of obesity management (16, 17). It must be stated, however, that this remains controversial since other studies emphasize the importance of reduced caloric intake, regardless of diet macronutrient composition (18). The combination of reducing dietary fat consumption and increasing physical activity has been shown to reduce the incidence of diabetes by 58% in subjects with impaired glucose tolerance (19). The effect of dietary fat content reduction on the prevention and reversal of hepatic steatosis, however, remains to be established.
We hypothesized that the onset of hepatic steatosis in C57Bl/6J mice results primarily from consumption of a diet with a high fat content, rather than from excess caloric intake. In addition, we also hypothesized that fully manifested hepatic steatosis could be reversed by reducing the fat percentage in the diet of obese mice. To test these hypotheses, a pair-feeding design was used to distinguish the effects of dietary fat content and caloric intake on dietary-induced hepatic lipid accumulation and associated injury.
Methods
Animals
Male 5-week-old C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME) were housed five per cage on paper chip bedding in a barrier room with regulated temperature (21°C ± 1.6°C), humidity (45% ± 10%), and an alternating 12-hour light and dark cycle. The animals had free access to tap water and standard rodent chow pellets (Prolab Isopro, RMH 3000 #25; containing 14% fat, 26% protein, and 60% carbohydrate by calories; energy density 4.1 kcal/g; Prolabs Purina, Richmond, IN) for an acclimation period of 1 week prior to study initiation. During the study period, the animals were weighed twice a week and food intake was measured daily. Intake was measured per cage to avoid the stress of individual housing. Animal protocols complied with the National Institutes of Health Animal Research Advisory Committee guidelines and were approved by the Children’s Hospital Boston Animal Care and Use Committee (protocol no. A06-08-065R).
Study diets
Study diets were stored at −4°C and provided fresh each day. The LF diet is the standard American Institute of Nutrition (AIN) 93M (TD.94048; Harlan Teklad, Madison, WI) purified diet with 10.2% of calories derived from fat, and 13.8% and 76% calories from protein and carbohydrates, respectively (energy density of 3.6 kcal/g) (20). All fat in the LF purified rodent diet is comprised of polyunsaturated fatty acids (100% soybean oil). The HF diet (TD.97070; Harlan Teklad, Madison, WI) consists of 59.9% of fat calories, and has 18.8% and 21.3% of calories derived from protein and carbohydrates, respectively (energy density of 5.1 kcal/g) (21). The HF diet is comprised of 45% saturated, 24% trans, 24% monounsaturated, and 7% polyunsaturated fatty acids.
Study design
After the one week acclimation period, fifteen mice were randomized into three groups. The first group (LF) had ad libitum access to the LF diet, and a second group (HF) had the same ad libitum access to a HF diet. The third group (HF-P) was pair-fed with the LF group, and received a HF diet restricted to the amount of calories that the LF group had consumed the day before. When present, residual food in each cage was weighed, discarded, and replaced with fresh diet every 24 hours for the complete study period of 9 weeks.
In a separate study, fifteen mice were acclimatized for one week, and were fed a HF diet ad libitum in order to develop obesity, insulin resistance and hepatic steatosis. After 9 weeks, mice were anesthetized and blood was collected for baseline parameters. After allowing the animals to recover for an additional week, mice were randomized equally to remain on an ad libitum HF diet (RHF), an ad libitum LF purified rodent chow diet (RLF), or a HF diet restricted to the amount of calories that the LF group had consumed the day prior (RHF-P). Mice remained on the diets for another 9 weeks until sacrifice.
Chemistry
At the end of the feeding experiments, mice were fasted for 6 hours. Glucose concentration was determined from tail vein blood using an OneTouch UltraSmart Blood Glucose Monitoring System (LifeScan, Milpitas, CA). Mice were then anesthetized and blood was collected via retro-orbital sinus puncture and centrifuged at 14000 rpm at 4°C for 10 min to obtain plasma. Plasma was delivered to the Clinical Laboratory at Children’s Hospital Boston for analysis of alanine aminotransferase (ALT), total cholesterol and triglyceride levels. Insulin levels were measured using a rat/mouse insulin ELISA kit (Linco Research, St. Charles, MO).
Surrogate indexes of insulin sensitivity and resistance
Surrogate indexes for insulin sensitivity were calculated, including quantitative insulin-sensitivity check index (QUICKI), homeostasis model assessment (HOMA) and log(HOMA) (22). The calculations were performed as follows: QUICKI=1/[log(I0)+log(G0)], where I0 is the fasting insulin (μU/mL) and G0 is the fasting glucose (mg/dL); HOMA=(G0xI0)/22.5 (with glucose expressed as mmol/L and insulin expressed as μU/mL); and log(HOMA).
Histology
Paraffin-embedded sections of the liver were stained by hematoxylin and eosin and periodic acid Schiff’s/diastase to examine cellular architecture, glycogen deposition and lipid accumulation. Frozen tissue sections were stained with Oil Red-O to detect fat. A pathologist blinded to the treatment groups conducted a histological analysis of the liver sections.
Fat pad collection
White adipose tissue was dissected according to previously defined anatomic landmarks, weighed and snap-frozen in liquid nitrogen (15). Inguinal (all subcutaneous fat between the lower part of the rib cage and mid thigh), mesenteric (all fat along the mesentery from the lesser curvature of the stomach to the sigmoid colon), retroperitoneal and epidydimal fat pads were weighed and expressed relative to eviscerated body weight. A white adipose tissue fat-index was calculated using the sum of the individual fat pads as a percentage of the eviscerated body weight (15).
Magnetic resonance imaging
MR imaging was performed by the MR Laboratory at Beth Israel Deaconess Medical Center as described previously (23). Briefly, samples were thawed at room temperature for 1 hour prior to analysis, blotted free of excess water and connective tissue, and placed in 5 mm diameter glass tubes for MR spectroscopy. An 8.5 T vertical bore magnet (DRX system, Bruker Instruments, Billerica, MA) was used for spectroscopic measurements of fat and water resonances. Specifically, a point resolved echo spectroscopic acquisition was applied to homogenous regions of liver, as identified from fast low angle shot images of the liver specimen. Voxel volumes interrogated spectroscopically with the point resolved echo spectroscopic sequence were 2 mm3. The repetition and echo times were 8 s and 12 ms, respectively, and 16 signal averages were acquired per spectrum. The water resonance and the methylene/methyl resonances were numerically integrated using the manufacturer supplied Paravision 4.0 software (Bruker Instruments, Billerica, MA). The methylene/methyl area was divided by the sum of the methylene/ methyl area plus the water area to obtain the MR spectroscopy parameter representing hepatic fat fraction used for group comparisons.
Analysis of mRNA expression
Isolation of mRNA, reverse transcription and quantitative real-time RT-PCR was performed as described previously (24). Briefly, 200-300 mg snap-frozen liver tissue was homogenized and total RNA was extracted using Tri Reagent (Molecular Research Center, Cincinnati, OH). cDNA was obtained by reverse transcription of 1 mg of total RNA using Superscript II Reverse Transcriptase (Invitrogen, Karlsruhe, Germany), using 50 pmol random hexamer and 100 pmol oligo-dT primers (Promega, Mannheim, Germany). Relative transcript levels of sterol regulatory element-binding protein (SREBP)-1c, SREBP-2, elongation of long chain fatty acids family member(Elovl)-6, fatty acid synthase (Fasn), stearoyl-CoA desaturase (SCD)-1, and peroxisome proliferator activated receptor (PPAR)-α were quantified by real-time RT-PCR on a LightCycler 1.5 instrument (Roche, Mannheim, Germany) using the TaqMan methodology. TaqMan probes (dual-labeled with 5′-FAM and 3′-TAMRA) and primers were designed using the Primer Express software (Perkin Elmer, Waltham, MA), synthesized at MWG Biotech AG (Ebersberg, Germany), and are outlined in Table 1. The housekeeping gene beta-2 microglobulin (β2MG) was amplified in parallel reactions for normalization.
Table 1.
Primers and probes used in quantitative real-time RT-PCR
| Target gene | 5′-Primer | TaqMan probe | 3′-Primer |
|---|---|---|---|
| β2MG | CTGATACATACGCCTGCA | GACCGTCTACTGGGATC | ATGAATCTTCAGAGCATC |
| SREBP1c | GAGTTAA CCAGAGGGTGAGCCTGA |
GAGACATGTG CAATCAGGACCATGCCG |
ATGAT AGCCTCTGCAATTTCCAG |
| SREBP2 | CAA GCGGACAACACACAATAT CATTG |
ACCTCT AGCGCTACCGGTCCTCC |
ATCT TGACTAAGTCCTTCAACT |
| Fasn | CCTGGATAGCATTCCGAA | ATCA CCTGAGGGACCCTACCG |
CTATGATTTTG AGCACATCTCGAAGGCTA |
| SCD-1 | CCT CAACACCATGGCGTTCCA |
CATAGC TGACGTGTACGAATGGG |
CACA GGTGGGCGCGGTGAT |
| Elovl-6 | GCGCTGTACGCTGCCTTT | CCCGA TCGGCATCTGATGAACAA |
GCGGCTTCCGAAGTTCAA |
| PPAR-α | AT TGGTTCCTGGTGCCGATT |
GCGAGC TGGTGGTAGATGCCTGC |
A ACTAGCATCCCACTTAAT |
| TA | AACCCCA | TATGTATCTGAA |
Statistical analyses
Data are expressed as means ± standard error of the mean (SEM). Data sets involving more than two groups were assessed by analysis of variance (ANOVA). Repeated measures ANOVA was used to analyze body weight gain. Differences between two groups were assessed using the unpaired two-tailed Student’s t test, or if nonparametric, by using the Mann-Whitney U test. A paired t test was used when the difference was calculated between two paired observations. P<0.05 was considered statistically significant. All data were collected in a computerized Microsoft Excel database (Microsoft Inc., Redmond, WA). The analysis was performed with SPSS version 16.0 (SPSS Inc., Chicago, IL) statistical software, and figures were created using GraphPad Prism version 5.0 (GraphPad Software Inc., La Jolla, CA) software.
Results
Feeding C57Bl/6J mice a HF diet ad libitum for 9 weeks induces hyperphagia and increases body weight gain, liver weight and white adipose tissue, compared to mice fed a LF diet
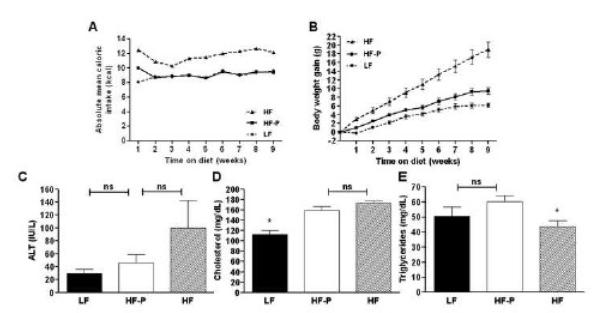
Mice fed a diet containing 60% of calories from fat for 9 weeks ad libitum (group HF) consumed more calories than did mice receiving a diet containing 10% of calories from fat ad libitum (group LF) (Table 1, Figure 1A). As a consequence of this hyperphagia, HF mice gained more weight and had larger livers compared to the mice with ad libitum access to the LF (Table 1; Figure 1B). HF mice showed an increased caloric efficiency compared to LF mice, determined by dividing the total weight gain per group by their total intake in kilocalories (Table 1).The white adipose tissue fat index, as a measure of obesity, was increased in HF mice (21.88±0.80 %) compared to LF mice (4.63±0.11 %, P=0.0079) (Table 1).
Figure 1.
Absolute mean caloric intake per mouse per week (A) was calculated per group on a daily base. Body weight gain (B) was calculated relative to the weight of each individual animal before initiation of the experiment. Plasma alanine aminotransferase (ALT; C), total cholesterol (D) and triglyceride (E) levels in the different groups. Values represent the mean ± SEM. Statistical significance is calculated between the HF-P animals and the difference between LF animals and HF animals (*, P<0.05; **, P<0.01).
Pair-feeding C57Bl/6J mice a HF diet increases body weight gain, liver and white adipose tissue, compared to mice on a LF diet with the same caloric intake
Mice receiving the HF diet restricted to the same amount of calories as the LF mice to rule out the effects of hyperphagia (group HF-P) gained 53% more weight (P=0.0097) and had 27% larger livers (P=0.0052) than did the pair-fed LF mice (Table 1; Figure 1A). When determining caloric efficiency, HF-P mice demonstrated more weight gain per consumed calorie compared to the LF control mice (Table 1). Although their caloric intake was similar, relative inguinal, mesenteric, retroperitoneal, and epidydimal fat masses, as well as the white adipose tissue fat index were significantly increased in HF-P mice, compared to the LF mice (Table 1).
Insulin sensitivity is impaired in C57Bl/6J mice fed a HF diet restricted to the caloric intake of mice on a LF diet
Insulin sensitivity was determined by calculating surrogate indexes for insulin sensitivity, including QUICKI, HOMA and log(HOMA). Although fasting plasma levels of glucose and insulin in HF-P and LF mice were not significantly different, HF-P mice had significantly impaired insulin sensitivity as determined by all surrogate indexes, compared to LF mice (Table 1).
Pair-feeding C57Bl/6J mice a HF diet aggravates plasma liver function tests, compared to mice on a LF diet with the same caloric intake
ALT is used as a marker for evaluation of hepatic injury and is increased in mice with hepatic steatosis (Figure 1). HF-P animals did not exhibit a significant elevation of plasma values for ALT (46±12 IU/L) than did LF animals (29±7 IU/L; P=0.2631). Compared to LF mice, HF mice demonstrated an elevated mean ALT level of 100±42 IU/L (P=0.0317), which was not statistically different to HF-P mice (P=0.2222). Compared to LF mice, pair-fed HF-P mice had increased mean plasma values for cholesterol (P=0.0027). Plasma triglyceride levels were not significantly elevated in HF-P mice, compared to LF mice (P=0.2202). These data suggest that the liver injury associated with the HF diet was not completely prevented by restricting the animals to the caloric intake of LF diet fed animals, but that cholesterol levels showed a similar increase as mice fed a HF diet ad libitum.
High dietary fat content, apart from excess caloric intake, stimulates hepatic steatosis in C57Bl/6J mice on histology
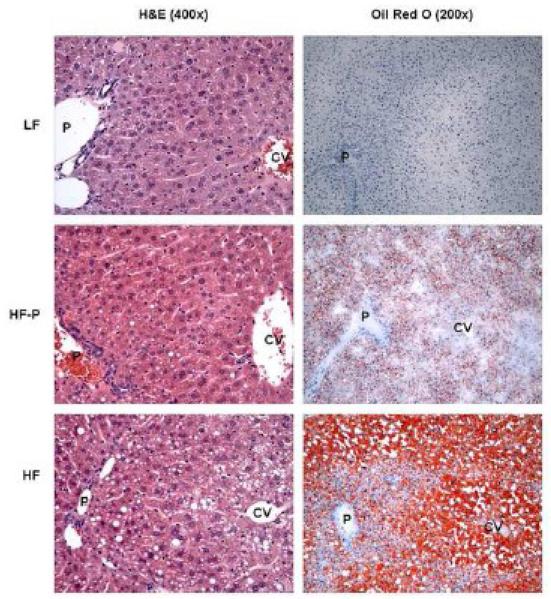
Hematoxylin and eosin and Oil Red-O stained liver sections from LF-fed mice exhibited hepatic architecture with no evidence of hepatic steatosis (Figure 2). In contrast, liver sections from HF-fed mice showed fat throughout the liver parenchyma, including both macro- and microvesicular steatosis. Microvesicular and macrovesicular steatosis was present predominantly in the periportal and midzone areas, whereas occasional ballooned hepatocytes and macrovesicular steatosis were present in the central vein area. HF-P mice that received the HF diet restricted to the caloric intake of the LF group, however, showed moderate steatosis, predominantly microvesicular. Occasional macrovesicular hepatocytes were observed in the periportal zone. Analysis of periodic acid Schiff’s/diastase-stained liver sections excluded glycogen deposition as a cause of microvesicular changes in hepatocytes (data not shown). Steatohepatitis and acute inflammation were not observed in any of the experimental groups.
Figure 2.
Representative liver sections stained with hematoxylin and eosin (H&E; left panels; original magnification 400x), and Oil Red O (right panels; original magnification 200x). LF livers exhibited normal hepatic architecture, whereas HF-P livers revealed moderate, microvesicular steatosis, and HF livers revealed extensive, microvesicular and macrovesicular steatosis. P indicates portal tract; CV, central vein.
High dietary fat content, apart from excess caloric intake, stimulates hepatic fat accumulation in C57Bl/6J mice determined by MR spectroscopy
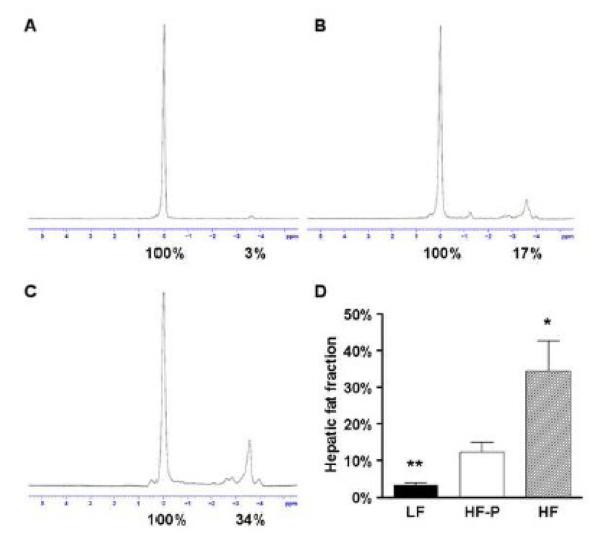
Hepatic fat content was quantified by using MR spectroscopy. Representative spectra of the different groups are shown (Figure 3). HF mice fed a 60% fat diet ad libitum exhibited an increase in liver fat content to 34.5±8.2% (P=0.0159) when compared to HF-P mice, demonstrating the aggravating effect of hyperphagia on hepatic fat accumulation (Figure 3D). Although both groups had a similar caloric intake, LF animals were found to have a liver fat content of 3.2±0.8%, whereas HF-P animals demonstrated increased fat content of 12.3±2.6% (P=0.0079). These data corroborate our histological findings that a HF diet, apart from hyperphagia-induced excess caloric intake, leads to the onset of hepatic steatosis.
Figure 3.
Magnetic resonance spectra for LF (A), HF-P (B) and HF (C) livers. Percent fat content was determined relative to water (100%) by numerical integration of the areas under the lipid and water peaks. Mean hepatic fat fraction as measured by magnetic resonance spectroscopy (D). Statistical significance is calculated between the HF-P animals and the difference between LF animals and HF animals (*, P<0.05; **, P<0.01). Variance statistic is SEM.
Reduction of fat percentage, but not caloric intake alone, decreased body, liver and white adipose tissue weights in C57Bl/6J mice with established metabolic syndrome
In mice that had been fed a HF diet ad libitum for 9 weeks, we investigated whether previously established obesity, insulin resistance and hepatic steatosis could be reversed by either switching to a LF diet (group RLF), or by pair-feeding the HF diet to reduce caloric intake (group RHF-P). A control group that had remained on the HF diet ad libitum for another 9 weeks (group RHF) demonstrated a significant increase in body, liver and white adipose tissue weights (Table 2). After switching mice from a HF diet to a LF diet ad libitum, we initially observed a decrease in mean caloric intake (Figure 4A). After 3 weeks, however, the mice on the LF diet consumed the same mean caloric intake as we had demonstrated before (Figure 1A). Consistent with a previous report, reducing the amount of fat in the diet significantly reversed obesity in the RLF mice that had been switched to an ad libitum LF diet (Figure 4B) (15). Although pair-feeding RHF-P animals to the caloric intake of the RLF mice resulted in significant less weight gain, smaller livers and less white adipose tissue compared to RHF mice, they gained significantly more weight, had larger livers and more adipose tissue than did the RLF group (Table 2). This indicates that even though both RLF and RHF-P groups consumed an equal amount of calories, the higher fat content in the diet of the RHF-P mice resulted in maintenance of the obese phenotype.
Table 2.
Body weights, tissue weights, caloric intake and surrogate markers for insulin resistance in mice assigned to the different dietary interventions after 9 weeks.
| LF (n=5) | HF-P (n=5) | HF (n=5) | |
|---|---|---|---|
| 9 weeks | 9 weeks | 9 weeks | |
| Body weight gain (g) | 6.2 ± 0.5 ** | 9.5 ± 0.8 | 19.0 ± 1.8 ** |
| Liver weight (g) | 0.89 ± 0.03 ** | 1.13 ± 0.05 | 1.72 ± 0.17 ** |
| Liver/body weight ratio | 0.034 ± 0.00 ** | 0.039 ± 0.00 | 0.045 ± 0.00 |
| Total caloric intake / mouse (kcal) | 559 | 569 | 727 |
| Caloric efficiency (g/kcal) | 0.0111 | 0.0167 | 0.0261 |
| Relative Inguinal fat mass (%) | 1.19 ± 0.17 ** | 2.60 ± 0.33 | 6.92 ± 0.38 *** |
| Mesenteric fat pad (%) | 0.58 ± 0.04 *** | 2.02 ± 0.12 | 3.55 ± 0.14 *** |
| Retroperitoneal fat pad (%) | 0.57 ± 0.08 ** | 1.34 ± 0.13 | 2.33 ± 0.16 ** |
| Epidydimal fat pad (%) | 2.29 ± 0.17 *** | 5.42 ± 0.31 | 9.09 ± 0.38 *** |
| White adipose tissue fat index (%) | 4.63 ± 0.11 *** | 11.39 ± 0.54 | 21.88 ± 0.80 *** |
| Fasting glucose (mg/dL) | 168 ± 32 | 227 ± 24 | 406 ± 40 ** |
| Fasting insulin (μU/mL) | 11.2 ± 1.3 | 30.1 ± 10.2 | 42.8 ± 3.2 |
| QUICKI | 0.31 ± 0.01 * | 0.27 ± 0.01 | 0.24 ± 0.00 * |
| HOMA | 4.3 ± 0.6 * | 18.1 ± 7.4 | 43.0 ± 6.8 * |
| Log(HOMA) | 0.62 ± 0.06 * | 1.12 ± 0.17 | 1.62 ± 0.06 * |
Values given are means ± SEM; HF-P group was used as reference;
P<0.05;
P<0.01;
P<0.001.
LF indicates low fat; HF-P, high fat pair-fed; HF, high fat; QUICKI, quantitative insulin-sensitivity check index; HOMA, homeostasis model assessment.
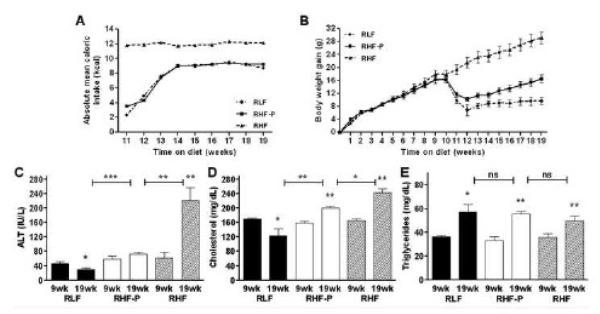
Figure 4.
Body weight gain (A) was calculated relative to the weight of each individual animal before initiation of the experiment. After 9 weeks, blood was collected via retro-orbital puncture. Mice were allowed one week to recover, before groups were randomly assigned to the study diets. Absolute mean caloric intake per mouse per week (B) was calculated per group on a daily base. Plasma alanine aminotransferase (ALT; C), total cholesterol (D) and triglyceride (E) levels in the different groups. Values represent the mean ± SEM. Statistical significance is calculated between the RHF-P animals and the difference between RLF animals and RHF animals, as well as repeated measurements within groups (*, P<0.05; **, P<0.01; ***, P<0.001; ns, not significant).
Pair-feeding obese C57Bl/6J mice a HF diet aggravates insulin sensitivity, compared to mice switched to a LF diet with the same caloric intake
In order to compare the effects of our dietary interventions on insulin sensitivity we measured fasting plasma values for glucose and insulin and calculated surrogate markers for insulin sensitivity. Values after 9 weeks on a HF diet were compared to their corresponding values at the end of the study. Mice that remained on a HF diet for another 9 weeks experienced decreased insulin sensitivity, as calculated by QUICKI (0.24±0.00 to 0.23±0.00, P=0.0185), HOMA (32.5±7.2 to 57.2±11.2, P=0.0359) and log(HOMA) (1.48±0.08 to 1.72±0.09, P=0.0187). Switching mice from a HF diet to a LF diet decreased fasting glucose levels from 316±28 mg/dL to 204±8 mg/dL (P=0.0134) and decreased fasting insulin levels from 40.8±5.0 μU/mL to 16.9±2.2 μU/mL (P=0.0061). QUICK, HOMA, and log(HOMA) all improved from 0.24±0.00 to 0.28±0.01 (P=0.0026), 30.6±2.9 to 8.6±1.3 (P=0.0026) and 1.48±0.04 to 0.91±0.07 (P=0.0020), respectively. More interestingly, restricting mice in the RHF-P group to the lower amount of calories of the RLF group but maintaining their HF diet did not improve their fasting glucose levels (289±12 mg/dL to 250±15 mg/dL, P=0.2021), and increased fasting insulin levels from 43.7±8.2 μU/mL to 72.6±7.1 μU/mL (P=0.0202). In contrast to the RLF mice, these mice did not improve their insulin sensitivity as calculated by QUICKI (0.25±0.00 to 0.24±0.00, P=0.0876), HOMA (30.4±5.2 to 44.5±15.6, P=0.0880) and log(HOMA) (1.46±0.07 to 1.63±0.06, P=0.0903).
Plasma liver function tests improved in C57Bl/6J mice switched to a LF diet, but not in mice that had been fed a HF diet restricted to the caloric intake of mice on a LF diet
To detect the presence of hepatic steatosis, liver enzymes were measured on all experimental groups (Figure 4). When compared to their corresponding base-line parameters, RHF mice exhibited an increase in mean ALT values (P=0.0054), suggesting progression of hepatic steatosis. RLF animals exhibited a decrease in mean ALT values (P=0.0476), whereas RHF-P mice showed no improvement (P=0.1562). Cholesterol levels decreased in the RLF group (P=0.0470), whereas both RHF-P and RHF animals showed elevations (P=0.0018 and P=0.0017, respectively). Triglyceride levels significantly increased in all three groups. This indicates that even though both RLF and RHF-P groups consumed an equal amount of calories, the RLF mice showed resolution of both their liver injury and elevated cholesterol, whereas the higher fat content in the diet of the RHF-P mice resulted in maintenance of the elevated ALT levels and progression of cholesterol elevation.
Switching to a LF diet resolved hepatic steatosis, but obese C57Bl/6J mice pair-fed a HF diet maintained their hepatic steatosis as determined by histology
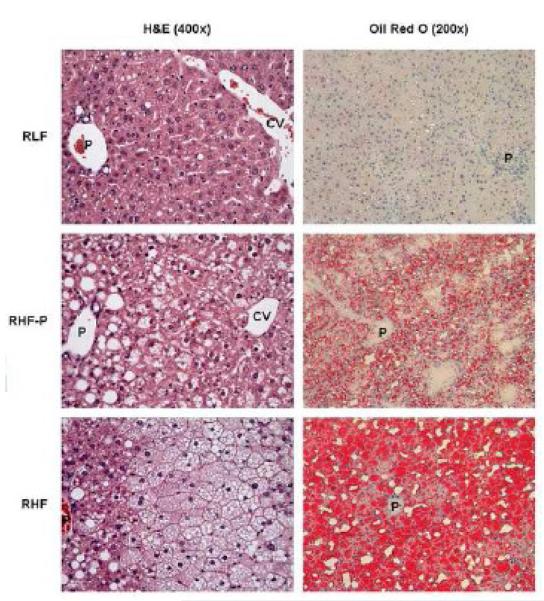
Figure 5 shows liver histopathological features of all groups studied. Liver sections from RHF mice showed extensive macrovesicular steatosis diffusely affecting the liver parenchyma, with ballooning of hepatocytes present mostly in the central vein area. In contrast, liver sections from RLF mice exhibited hepatic architecture with only rare microvesicular steatosis in midzone hepatocytes with an occasional hepatocyte showing macrovesicular steatosis. Liver sections from the RHF-P group showed diffuse macro- and microvesicular steatosis. Macrovesicular changes were most marked in the periportal and midzone hepatocytes, whereas ballooned hepatocytes were noted in the central vein area. Inflammatory changes suggestive for steatohepatitis were not observed in any of the groups.
Figure 5.
Representative liver sections stained with hematoxylin and eosin (H&E; left panels), and Oil Red O (right panels). RLF livers exhibited normal hepatic architecture, with occasional fat droplets. RHF-P livers revealed moderate macrovesicular steatosis around the portal tract, and moderate microvesicular steatosis around the central vein. RHF livers revealed extensive microvesicular steatosis around the central vein, and extensive macrovesicular steatosis around the portal tract. Original magnification 20x. P indicates portal tract; CV, central vein.
Switching to a LF diet resolved hepatic steatosis, but obese C57Bl/6J mice pair-fed a HF diet maintained their hepatic steatosis as determined by MR spectroscopy
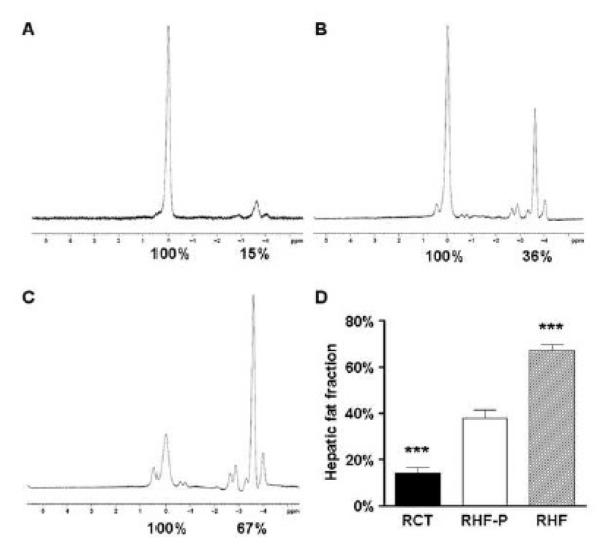
In order to objectively quantify the effects of the dietary interventions, MR spectroscopy was performed on livers. Representative spectra of the different groups are shown in Figure 6. RHF mice that had been fed a 60% HF diet ad libitum for 19 weeks increased their liver fat content to a value of 67.1±2.5%, compared to the hepatic fat fraction of HF mice at 9 weeks (34.5±8.2%; P=0.0079). RHF-P mice, in contrast, exhibited a similar hepatic fat fraction of 37.7±3.7%, when compared to HF mice (P=0.7389). This indicates that a profound reduction of caloric intake, but not dietary fat content, halted progression of hepatic steatosis. RLF animals that had a similar caloric intake to RHF-P mice but were switched to a 10% LF diet were found to have their liver fat content reduced to 14.2±2.3%, when compared to HF mice at 9 weeks (P=0.0317). These data corroborate our finding on histology that switching to a LF diet was efficacious in reducing hepatic steatosis, whereas mice that had been pair-fed a diet with a 60% fat content showed no resolution.
Figure 6.
Magnetic resonance spectra for RLF (A), RHF-P (B) and RHF (C) livers. Percent fat content was determined relative to water (100%) by numerical integration of the areas under the lipid and water peaks. Mean hepatic fat fraction as measured by magnetic resonance spectroscopy (D). Statistical significance is calculated between the RHF-P animals and the difference between RLF animals and RHF animals (*, P<0.05; **, P<0.01). Variance statistic is SEM.
HF feeding affected hepatic gene expression levels in both the 9 weeks and 19 weeks experiments
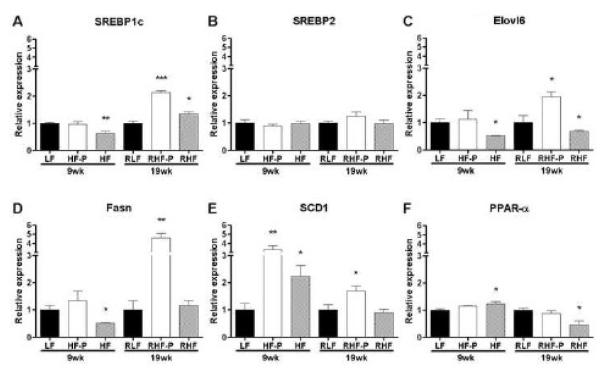
To explore the mechanism why mice on a 60% HF diet that were pair-fed to mice on a 10% LF diet developed hepatic steatosis (HF-P) and did not reverse their fatty liver in the reversal experiment (RHF-P), we examined hepatic transcript levels of genes involved in lipid metabolism (Figure 7). In the 9 weeks experiment, feeding mice a HF diet ad libitum resulted in significantly reduced Elovl6, SREBP1c and Fasn levels, whereas SREBP2 levels remained unchanged, suggesting a decrease in de novo lipogenesis compared to LF controls. SCD1 gene expression levels, however, were significantly increased. Being a downstream target of SREBP1c activation, increased SCD1 gene expression suggests that the increased dietary free fatty acid influx was mainly responsible for the net build-up of fat in the liver, followed by de novo lipogenesis. PPAR-α levels were somewhat increased illustrating the liver’s response to oxidize the accumulated fat. In HF-P mice, however, none of the genes related to de novo lipogenesis were upregulated in the 9 weeks experiment. In contrast, SCD1 transcript levels were upregulated threefold compared to LF mice, suggesting that in this 9 weeks experiment the development of hepatic steatosis in the HF pair-fed group was mainly attributed to increased delivery and uptake of long chain fatty acids into the hepatocytes, followed by early de novo lipogenesis.
Figure 7.
Upregulation of genes related to lipogenesis in livers of mice pair-fed a HF diet and in mice that were fed a HF diet ad libitum, compared to LF-fed controls. Hepatic expression of (A) SREBP1c, (B) SREBP2, (C) Elovl6, (D) Fasn, (E) SCD1 and (F) PPAR-α. Relative transcript levels were quantified by TaqMan quantitative RTPCR, normalized by β2MG and expressed in arbitrary units versus LF (9 weeks experiment) or RLF (19 weeks experiment) controls (*, P<0.05; **, P<0.01; ***, P<0.001). Variance statistic is SEM.
In the 19 weeks reversal experiment, the gene transcript levels of SREBP1c, Elovl6, Fasn as well as SCD1 in RHF-P mice were all markedly upregulated, which is compatible with increased de novo lipogenesis combined with increased uptake of free fatty acids. In contrast, SREBP2 and PPAR-α levels remained unchanged. This suggests that in the 19 weeks reversal experiment, both de novo lipogenesis and increased uptake of free fatty acids were responsible for maintaining the hepatic steatosis. This is in sharp contrast with the 10% LF pair-fed RLF animals that reversed their steatosis markedly.
Discussion
Hepatic steatosis in patients with NAFLD is a consequence of increased free fatty acid delivery to the liver combined with increased de novo lipogenesis (25). Although the pathogenesis of this common disorder remains poorly understood, it is generally accepted that over-consumption and insulin resistance may play an important role in the development of NAFLD. Unlike the other components of the metabolic syndrome, the association between dietary macronutrient intake and NALFD has not been established (26). In this study, we sought to determine the effects of dietary fat content on the development of NAFLD and other components of the metabolic syndrome, corrected for caloric intake using a pair-feeding design. Our results indicate that the development of hepatic steatosis in a murine DIO-model can be prevented and reversed when switching to a standard diet low in fat. Moreover, we demonstrate that a diet composed of 60% fat calories, when corrected for caloric intake, contributed to the development of hepatic steatosis by activating genes related to both de novo lipogenesis and increased uptake of free fatty acids.
Different dietary manipulations have been shown to induce obesity, insulin resistance and NAFLD in different strains and species of rodents, thereby reproducing the human condition. This suggests that over-consumption with either carbohydrates, fats, or both may play a role in the development of hepatic steatosis associated with obesity and insulin resistance (27). Our experimental HF diet contains predominantly saturated fat and has small amount of polyunsaturated fat, quite similar to the diets consumed by obese and NAFLD patients (28). Likewise, saturated fat intake positively correlates with insulin sensitivity and transaminase levels in NALFD patients (29). In accordance with these reports, our results show that mice fed a 60% fat diet consistently developed hyperglycemia, hyperinsulinemia, insulin resistance, visceral adiposity and hepatic steatosis. Since HF feeding is associated with hyperphagia, one of our aims was to elucidate whether the effects observed in the HF groups resulted from increased caloric or fat intake. A previous report showed that reducing the number of calories consumed from a HF diet attenuated but did not prevent the development of insulin resistance and obesity in mice, thus indicating a role of HF diet independent of caloric intake (13). In addition to corroborating these findings, we now demonstrate that the development of hepatic steatosis directly results from an increased fat intake that is independent from the amount of calories consumed.
SCD1 is induced by dietary carbohydrates, saturated fat, and cholesterol through activation of SREBP-1c and liver X receptor (30). Increased delivery and uptake of the long chain saturated fatty acids palmitate (C16:0) and stearate (C18:0) by hepatocytes is associated with an increase in SCD1 expression and activity, resulting in a net formation of the monounsaturated fatty acids palmitoleate (C16:1) and oleate (C18:1), triglyceride storage and development of hepatic steatosis (31, 32). In our 9 weeks study, the development of hepatic steatosis was mainly a result from an overflow of free long chain fatty acids, as demonstrated by increased transcript levels of SCD1, but absence of SREBP1c, Elovl6 or Fasn upregulation. Even when mice were pair-fed to LF animals to correct for HF-diet associated hyperphagia the effects remained, dissecting the pathway of overfeeding from diet composition.
In our reversal experiment, hepatic transcript levels of SREBP1c, Elovl6 and Fasn were increased. SREBP1c is independently activated by insulin, glucose and fructose, and is a key transcriptional activator of hepatic lipogenic genes such as Fasn and SCD1 (33). SREBP1c inhibits insulin receptor substrate-2 signaling (34), and overexpression of SREBP1c in livers of transgenic mice leads to marked increases in de novo lipogenesis and development of hepatic steatosis (35). Elov6 is an enzyme catalyzing the conversion of the long chain saturated fatty acid palmitate (C16:0) to stearate (C18:0). Mice deficient in the gene for Elevl6 that were fed a HF diet had markedly reduced SREBP1c expression levels and were able to maintain insulin receptor substrate-2 signaling, despite the development of hepatic steatosis and obesity (36). Elevated transcript levels of Elov6 as seen in our experiments may have further activated SREBP1c expression. Fasn catalyzes the synthesis of long chain fatty acids from acetyl-CoA and malonyl-CoA and is one of the rate limiting steps in de novo lipogenesis (37). The increased transcript levels of Fasn in the RHF-P mice may have contributed to the increased synthesis of fatty acids, ultimately resulting in hepatic steatosis.
Weight loss and increased physical activity are generally recommended as treatments for patients with NAFLD, despite the lack of scientific evidence regarding diet composition and its effect on liver histology and long-term outcomes of NAFLD (38, 39). This paucity of data makes it difficult to make evidence-based recommendations about dietary modification to treat NAFLD. In mice receiving a HF diet for 16 weeks, complete reversal of both insulin resistance and obesity has been demonstrated after switching to a diet low in fat (15). Our findings show that reducing the amount of fat in the diet of obese mice with established insulin resistance and NAFLD not only resulted in weight loss and improved insulin sensitivity but also in complete reversal of hepatic steatosis, independent from caloric intake. Although these results can not immediately be extrapolated to humans, our findings justify further investigation regarding the role of diets low in total saturated, and trans fat in the treatment of patients with NAFLD.
Three study limitations may warrant consideration. First, it can be hypothesized that a HF diet results in a reduction in the metabolic rate, shifting the energy equation toward energy storage. It may be possible that despite pair-feeding, in our study the HF fed animals were in greater positive energy balance. This may be a consequence of some decrease in activity and/or energy expenditure. Although this was not assessed in our study, previous reports show that the development of obesity was unrelated to motor activity in mice, and that rats fed a 59% fat diet demonstrated a transient decrease in energy expenditure at 30 days, but no difference at 70 days compared to LF fed controls (14, 40). Likewise, humans fed a 40% fat diet demonstrated weight gain without decreased energy expenditure (41). Data that HF diet induced obesity is affected by metabolic rate is lacking, however, it remains possible that the metabolic rate in the animals used in our study under our particular experimental conditions was decreased.
Second, the observed results may be a consequence of fat source, rather than fat content. Indeed, our HF diet is mainly derived from saturated fat (45% saturated, 24% trans, 24% monounsaturated, and 7% polyunsaturated fatty acids), whereas the LF purified rodent diet is comprised of polyunsaturated fatty acids (100% soybean oil). Saturated fatty acids may promote endoplasmatic reticulum stress and hepatocyte injury resulting in hepatic dysfunction in rodents (42). Also, a saturated fat intake exceeding 10% of total energy may promote insulin resistance in humans and therefore may not be suitable for NAFLD patients (28). Polyunsaturated fatty acids, in contrast, decrease cardiovascular disease when consumed as an alternative to saturated fats (43). A recent study showed that mice fed a saturated LF diet gained significantly more weight compared to mice fed an unsaturated LF diet, whereas HF diets resulted in significant weight gain regardless of fat composition (44). In this study, however, liver fatty acid content was not examined. It may therefore be hypothesized that weight gain and the development of hepatic steatosis may at least in part be a result of dietary saturated fat. This hypothesis is beyond the scope of the current study, but remains under investigation in our ongoing research.
Finally, the degree to which these findings apply to humans remains speculative. Humans are biologically different from rodents as they tend to have lower rates of de novo lipogenesis, which might attenuate the effects of macronutrient dietary content on hepatic lipid accumulation. The DIO model in male C57Bl/6J mice fed a HF diet, however, is the most widely referenced model in diabetes and obesity research, and closely resembles the development of the metabolic syndrome in response to the increasing sedentary lifestyle in humans. In contrast, many other experimental models utilized to investigate the pathophysiology of NAFLD are either based on genetic leptin defects or the methionine and choline deficiency diets. Although scientific evidence is lacking, a low glycemic diet with decreased saturated and trans-fat intake, but increased mono and polyunsaturated fatty acid consumption, is currently recommended to treat patients with NAFLD (39). The results from our study showing reversal of hepatic steatosis when obese mice had been switched to a diet low in saturated and trans-fats, but high in polyunsaturated fats, in part, support these recommendations in terms of dietary fat composition but suggest that total fat provided is a prominent factor as well.
In conclusion, this study recapitulates the effects of high dietary fat content on the development and reversal of visceral obesity and insulin resistance independent of caloric intake. Onset of hepatic steatosis in this model resulted from an increased dietary intake of fat, in addition to excess caloric intake. Moreover, we demonstrated that hepatic steatosis was successfully reversed after switching obese mice to a diet with an appropriate fat composition and content. These results may serve to further investigate the role of diets low in total saturated, and trans fat in the treatment of patients with NAFLD.
Table 3.
Body weights, tissue weights, caloric intake and surrogate markers for insulin resistance in mice that had been fed an ad libitum high fat diet for 9 weeks, and after 1 week recovery from phlebotomy had been switched to the intervention diets for another 9 weeks.
| RLF (n=5) | RHF-P (n=5) | RHF (n=5) | |
|---|---|---|---|
| 19 weeks | 19 weeks | 19 weeks | |
| Body weight gain (g) | −6.8 ± 0.9 ** | 0.3 ± 1.1 | 11.3 ± 0.8 *** |
| Liver weight (g) | 0.98 ± 0.06 *** | 1.63 ± 0.07 | 3.52 ± 0.40 ** |
| Liver/body weight ratio | 0.034 ± 0.00 *** | 0.044 ± 0.00 | 0.072 ± 0.01 ** |
| Total caloric intake / mouse (kcal) | 468 | 492 | 754 |
| Caloric efficiency (g/kcal) | −0.0145 | 0.0006 | 0.0150 |
| Relative Inguinal fat mass (%) | 3.73 ± 0.72 ** | 7.37 ± 0.58 | 9.36 ± 0.48 * |
| Mesenteric fat pad (%) | 1.91 ± 0.29 ** | 3.50 ± 0.23 | 4.38 ± 0.14 * |
| Retroperitoneal fat pad (%) | 1.67 ± 0.35 * | 2.74 ± 0.26 | 4.32 ± 0.24 ** |
| Epidydimal fat pad (%) | 4.56 ± 0.61 *** | 8.44 ± 0.45 | 12.28 ± 0.70 ** |
| White adipose tissue fat index (%) | 11.87 ± 1.92 ** | 22.05 ± 1.40 | 29.33 ± 1.36 ** |
| Fasting glucose (mg/dL) | 204 ± 8 | 250 ± 15 | 252 ± 11 |
| Fasting insulin (μU/mL) | 16.9 ± 2.2 ** | 72.6 ± 7.1 | 90.4 ± 13.8 |
| QUICKI | 0.28 ± 0.01 *** | 0.24 ± 0.00 | 0.23 ± 0.00 |
| HOMA | 8.6 ± 1.3 ** | 44.5 ± 5.6 | 57.2 ± 11.2 |
| Log(HOMA) | 0.91 ± 0.07 *** | 1.63 ± 0.06 | 1.72 ± 0.09 |
Values given are means ± SEM; RHF-P group was used as reference;
P<0.05;
P<0.01;
P<0.001.
RLF indicates reversal low fat; RHF-P, reversal high fat pair-fed; RHF, reversal high fat; QUICKI, quantitative insulin-sensitivity check index; HOMA, homeostasis model assessment.
Acknowledgements
The authors are grateful to Dr. Bruce R. Bistrian (Beth Israel Deaconess Medical Center, Boston, MA) for insightful discussion on the data and thoughtful comments on the manuscript, to Anisha K. Sharma and Dr. Yury Popov (both Beth Israel Deaconess Medical Center, Boston, MA) for excellent technical assistance and to Helen Wang (Beth Israel Deaconess Medical Center, Boston, MA) for help with designing some of the TaqMan probes and primers used in our study.
Dr. De Meijer was recipient of fellowships from the foundations Stichting Prof. Michaël-van Vloten Fonds (Venray, The Netherlands), VSBfonds (Utrecht, The Netherlands), Gerrit Jan Mulder Stichting (Rotterdam, The Netherlands), Prins Bernhard Cultuurfonds (Amsterdam, The Netherlands), and Dr. Saal van Zwanenberg Stichting (Oss, The Netherlands). Dr. Le was the recipient of the Joshua Ryan Rappaport Fellowship(Boston, MA). Dr. Puder was supported by the National Institutes of Health (grant DK069621-05) and the Children’s Hospital Surgical Foundation (Boston, MA). The funders did not participate in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
3. Conflicts of interest: The authors have no conflicts of interest to disclose.
4. Institutional approval: Animal protocols complied with the National Institutes of Health Animal Research Advisory Committee guidelines and were approved by the Children’s Hospital Boston Animal Care and Use Committee (protocol no. A06-08-065R).
References
- 1.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 2.Powell EE, Cooksley WG, Hanson R, et al. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 3.Teli MR, James OF, Burt AD, et al. The natural history of nonalcoholic fatty liver: a follow-up study. Hepatology. 1995;22:1714–1719. [PubMed] [Google Scholar]
- 4.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 5.Bellentani S, Saccoccio G, Masutti F, et al. Prevalence of and risk factors for hepatic steatosis in Northern Italy. Ann Intern Med. 2000;132:112–117. doi: 10.7326/0003-4819-132-2-200001180-00004. [DOI] [PubMed] [Google Scholar]
- 6.Fan CY, Pan J, Usuda N, et al. Steatohepatitis, spontaneous peroxisome proliferation and liver tumors in mice lacking peroxisomal fatty acyl-CoA oxidase. Implications for peroxisome proliferator-activated receptor alpha natural ligand metabolism. J Biol Chem. 1998;273:15639–15645. doi: 10.1074/jbc.273.25.15639. [DOI] [PubMed] [Google Scholar]
- 7.Nomura H, Kashiwagi S, Hayashi J, et al. Prevalence of fatty liver in a general population of Okinawa, Japan. Jpn J Med. 1988;27:142–149. doi: 10.2169/internalmedicine1962.27.142. [DOI] [PubMed] [Google Scholar]
- 8.Ratziu V, Giral P, Charlotte F, et al. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 9.Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- 10.Clark JM, Diehl AM. Hepatic steatosis and type 2 diabetes mellitus. Curr Diab Rep. 2002;2:210–215. doi: 10.1007/s11892-002-0085-3. [DOI] [PubMed] [Google Scholar]
- 11.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 12.Collins S, Martin TL, Surwit RS, et al. Genetic vulnerability to diet-induced obesity in the C57BL/6J mouse: physiological and molecular characteristics. Physiol Behav. 2004;81:243–248. doi: 10.1016/j.physbeh.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Petro AE, Cotter J, Cooper DA, et al. Fat, carbohydrate, and calories in the development of diabetes and obesity in the C57BL/6J mouse. Metabolism. 2004;53:454–457. doi: 10.1016/j.metabol.2003.11.018. [DOI] [PubMed] [Google Scholar]
- 14.Brownlow BS, Petro A, Feinglos MN, et al. The role of motor activity in diet-induced obesity in C57BL/6J mice. Physiol Behav. 1996;60:37–41. doi: 10.1016/0031-9384(95)02210-4. [DOI] [PubMed] [Google Scholar]
- 15.Parekh PI, Petro AE, Tiller JM, et al. Reversal of diet-induced obesity and diabetes in C57BL/ 6J mice. Metabolism. 1998;47:1089–1096. doi: 10.1016/s0026-0495(98)90283-9. [DOI] [PubMed] [Google Scholar]
- 16.Howard BV, Manson JE, Stefanick ML, et al. Low-fat dietary pattern and weight change over 7 years: the Women’s Health Initiative Dietary Modification Trial. Jama. 2006;295:39–49. doi: 10.1001/jama.295.1.39. [DOI] [PubMed] [Google Scholar]
- 17.Astrup A. Dietary management of obesity. JPEN J Parenter Enteral Nutr. 2008;32:575–577. doi: 10.1177/0148607108321707. [DOI] [PubMed] [Google Scholar]
- 18.Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360:859–873. doi: 10.1056/NEJMoa0804748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 20.Reeves PG, Nielsen FH, Fahey GC., Jr AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–1951. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 21.Hermanowski-Vosatka A, Balkovec JM, Cheng K, et al. 11beta-HSD1 inhibition ameliorates metabolic syndrome and prevents progression of atherosclerosis in mice. J Exp Med. 2005;202:517–527. doi: 10.1084/jem.20050119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muniyappa R, Lee S, Chen H, et al. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 23.Alwayn IP, Andersson C, Lee S, et al. Inhibition of matrix metalloproteinases increases PPAR-alpha and IL-6 and prevents dietary-induced hepatic steatosis and injury in a murine model. Am J Physiol Gastrointest Liver Physiol. 2006;291:G1011–1019. doi: 10.1152/ajpgi.00047.2006. [DOI] [PubMed] [Google Scholar]
- 24.Popov Y, Patsenker E, Fickert P, et al. Mdr2 (Abcb4)-/- mice spontaneously develop severe biliary fibrosis via massive dysregulation of pro- and antifibrogenic genes. J Hepatol. 2005;43:1045–1054. doi: 10.1016/j.jhep.2005.06.025. [DOI] [PubMed] [Google Scholar]
- 25.Donnelly KL, Smith CI, Schwarzenberg SJ, et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest. 2005;115:1343–1351. doi: 10.1172/JCI23621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harrison SA, Day CP. Benefits of lifestyle modification in NAFLD. Gut. 2007;56:1760–1769. doi: 10.1136/gut.2006.112094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis. 2001;21:89–104. doi: 10.1055/s-2001-12932. [DOI] [PubMed] [Google Scholar]
- 28.Musso G, Gambino R, De Michieli F, et al. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology. 2003;37:909–916. doi: 10.1053/jhep.2003.50132. [DOI] [PubMed] [Google Scholar]
- 29.Tiikkainen M, Bergholm R, Vehkavaara S, et al. Effects of identical weight loss on body composition and features of insulin resistance in obese women with high and low liver fat content. Diabetes. 2003;52:701–707. doi: 10.2337/diabetes.52.3.701. [DOI] [PubMed] [Google Scholar]
- 30.Flowers MT, Ntambi JM. Role of stearoyl-coenzyme A desaturase in regulating lipid metabolism. Curr Opin Lipidol. 2008;19:248–256. doi: 10.1097/MOL.0b013e3282f9b54d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sampath H, Miyazaki M, Dobrzyn A, et al. Stearoyl-CoA desaturase-1 mediates the prolipogenic effects of dietary saturated fat. J Biol Chem. 2007;282:2483–2493. doi: 10.1074/jbc.M610158200. [DOI] [PubMed] [Google Scholar]
- 32.Li ZZ, Berk M, McIntyre TM, et al. Hepatic lipid partitioning and liver damage in nonalcoholic fatty liver disease: role of stearoyl-CoA desaturase. J Biol Chem. 2009;284:5637–5644. doi: 10.1074/jbc.M807616200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Horton JD, Goldstein JL, Brown MS. SREBPs: activators of the complete program of cholesterol and fatty acid synthesis in the liver. J Clin Invest. 2002;109:1125–1131. doi: 10.1172/JCI15593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 35.Shimomura I, Bashmakov Y, Horton JD. Increased levels of nuclear SREBP-1c associated with fatty livers in two mouse models of diabetes mellitus. J Biol Chem. 1999;274:30028–30032. doi: 10.1074/jbc.274.42.30028. [DOI] [PubMed] [Google Scholar]
- 36.Matsuzaka T, Shimano H, Yahagi N, et al. Crucial role of a long-chain fatty acid elongase, Elovl6, in obesity-induced insulin resistance. Nat Med. 2007;13:1193–1202. doi: 10.1038/nm1662. [DOI] [PubMed] [Google Scholar]
- 37.Hillgartner FB, Salati LM, Goodridge AG. Physiological and molecular mechanisms involved in nutritional regulation of fatty acid synthesis. Physiol Rev. 1995;75:47–76. doi: 10.1152/physrev.1995.75.1.47. [DOI] [PubMed] [Google Scholar]
- 38.Bellentani S, Grave R Dalle, Suppini A, et al. Behavior therapy for nonalcoholic fatty liver disease: The need for a multidisciplinary approach. Hepatology. 2008;47:746–754. doi: 10.1002/hep.22009. [DOI] [PubMed] [Google Scholar]
- 39.Zivkovic AM, German JB, Sanyal AJ. Comparative review of diets for the metabolic syndrome: implications for nonalcoholic fatty liver disease. Am J Clin Nutr. 2007;86:285–300. doi: 10.1093/ajcn/86.2.285. [DOI] [PubMed] [Google Scholar]
- 40.Wilsey J, Zolotukhin S, Prima V, et al. Central leptin gene therapy fails to overcome leptin resistance associated with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1011–1020. doi: 10.1152/ajpregu.00193.2003. [DOI] [PubMed] [Google Scholar]
- 41.Piers LS, Walker KZ, Stoney RM, et al. The influence of the type of dietary fat on postprandial fat oxidation rates: monounsaturated (olive oil) vs saturated fat (cream) Int J Obes Relat Metab Disord. 2002;26:814–821. doi: 10.1038/sj.ijo.0801993. [DOI] [PubMed] [Google Scholar]
- 42.Wang D, Wei Y, Pagliassotti MJ. Saturated fatty acids promote endoplasmic reticulum stress and liver injury in rats with hepatic steatosis. Endocrinology. 2006;147:943–951. doi: 10.1210/en.2005-0570. [DOI] [PubMed] [Google Scholar]
- 43.Laaksonen DE, Nyyssonen K, Niskanen L, et al. Prediction of cardiovascular mortality in middle-aged men by dietary and serum linoleic and polyunsaturated fatty acids. Arch Intern Med. 2005;165:193–199. doi: 10.1001/archinte.165.2.193. [DOI] [PubMed] [Google Scholar]
- 44.Townsend KL, Lorenzi MM, Widmaier EP. High-fat diet-induced changes in body mass and hypothalamic gene expression in wild-type and leptin-deficient mice. Endocrine. 2008;33:176–188. doi: 10.1007/s12020-008-9070-1. [DOI] [PubMed] [Google Scholar]