Abstract
The Notch pathway powerfully influences stem cell maintenance, development and cell fate and is increasingly recognized for the key roles it plays in cancer. Notch promotes cell survival, angiogenesis and treatment resistance in numerous cancers, making it a promising target for cancer therapy. It also crosstalks with other critical oncogenes, providing a means to affect numerous signaling pathways with one intervention. While the gamma-secretaase inhibitors are the only form of Notch inhibitors in clinical trials, other forms of Notch inhibition have been developed or are theoretically feasible. In this chapter we review the rationales for Notch inhibition in cancer and then discuss in detail the various modalities for Notch inhibition, both current and speculative.
INTRODUCTION: BACKGROUND OF THE NOTCH PATHWAY IN CANCER
In the current era in oncology, much of the hope for powerful new therapies lies with targeted inhibition of pathways dysregulated in cancer. An initial wave of targeted pathway inhibitors has yielded some successes but more disappointments and major efforts are underway to refine our application of some of these approaches. However, there is no slowdown in attempting to find newer and perhaps more effective targets in cancer cells and the Notch pathway is generating growing enthusiasm in this regard. As is described in detail elsewhere in this volume, Notch is a key player in development, stem cell maintenance and cell survival and its specific roles in individual cancers are covered in other chapters here. In this chapter, the rationale for Notch inhibition as a cancer therapy and its potential drawbacks will be discussed, with extended description of established and experimental methods for Notch inhibition.
RATIONALE FOR NOTCH INHIBITION
Numerous functions have been ascribed to Notch, with some of these helping to explain its cancer-promoting effects in many tissues. Notch helps maintain certain stem cell populations,1–5 but interestingly it is also a master regulator of cell fate at critical differentiation branch points in various organ systems.5–8 Notch seems more likely to play an oncogenic role in cell types that it favors in development and differentiation, such as glial cells or T-cells.9–12 Notch activity promotes cell survival and has anti-apoptotic function13–15 and numerous mechanisms have been proposed for this. It can also drive cell division in some settings and in some settings may be required for the cell cycle.16,17
Notch is one of the most powerful of the stem cell-promoting pathways, in conjunction with the Hedgehog and Wnt pathways, making it highly relevant for cancer given the undifferentiated/de-differentiated state of most cancer cells. Stem cell pathways such as Notch may be especially attractive targets given the growing evidence for the cancer stem cell hypothesis. This hypothesis states that cancers contain a usually small subpopulation that retains stem cell character and gives rise to the other cells making up tumors [reviewed in refs. 18,19]. Various terms exist for this subpopulation, including “cancer-initiating cells,” “cancer stem cells,” or, given the uncertainty about their nature"cancer stem-like cells.” Despite variability in nomenclature, there is general agreement on the criteria that define these cells in the laboratory. Their isolation and culture has allowed detailed study of cancer stem cells and a number of features have emerged. They are capable of unlimited self-renewal, generation of more differentiated progeny and formation of cancers in animal models.20,21 These cells are more resistant than bulk cancer cells or established older cancer cell lines to standard treatments such as chemotherapy and radiation.22,23 However, cancer stem cells seem equally sensitive—or even more so—to potential therapies blocking prominent stem cell pathways like Notch.24–26 Inhibition of these pathways may cause differentiating effects in cancer stem cells, as well as more commonly seen cytotoxic effects. In keeping with this, a few reports have shown differentiating effects in cancer stem cells secondary to Notch inhibition.24,26
Some of the impact of Notch inhibition in cancer cells results from its extensive crosstalk with critical cancer proteins and pathways. Numerous studies have shown that Notch activity sustains the PI3kinase/Akt pathway27–30 and Notch has also been demonstrated to operate in an interdependent fashion with the Ras pathway.31,32 Notch regulates expression of important receptor tyrosine kinases such as the epidermal growth factor receptor (EGFR) and the vascular endothelial growth factor receptor-1 (VEGFR-1)33–35 and also interacts with fibroblast growth factor receptor (FGFR) signaling.36 Notch and the NF-kB pathway are intimately intertwined, with multiple points of interaction described37–41 The myc oncogene is a direct target of Notch, mediating much of the oncogenic effects of Notch in T-cell malignancies.42 In some instances, other oncogenic pathways have been shown to boost Notch or its downstream activity, as is the case for the hypoxia/HIF-1alpha pathway.43 Most of the best-known oncogenic pathways have been shown to cross-talk with the Notch pathway at some level; however, it is important to note that some of these interactions are context-dependent and do not occur in all cellular backgrounds.
The direct effects of Notch inhibition on cancer cells may vary. Given the interaction of Notch with important anti-apoptotic pathways such as Akt, it is perhaps not surprising that Notch inhibition has most frequently been shown to trigger apoptosis in cancer cells.14,15,24,29,33,44 Notch inhibition has also been shown to slow cancer cell proliferation, though Notch activity has generally not been considered essential for the cell cycle. However, some evidence indicates that there may be important roles for Notch in the cell cycle in some settings.45 Senescence has also been linked to the Notch pathway. The downstream mediator of Notch HES1 has been shown to play a critical role in blocking senescence46 and this is supported by recent results presented at a meeting that the combination of a Notch-inhibiting agent and a chemotherapy drug triggers senescence in glioblastoma cells.47 While Notch inhibition has not yet been associated with autophagy in cancer cells, this may just be a matter of time given the connections of Notch to Akt/mTOR signaling.
While Notch blockade can have direct inhibitory effects on cancer cells, it also may influence cancer indirectly through impacting cancer-supporting processes such as angiogenesis. A number of reports have shown direct antiangiogenic effects from Notch inhibition.48–51 A major role for Notch in blood vessels is supported by the vascular nature of the defects in the human disease CADASIL (cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy), caused by NOTCH3 mutations.52 Furthermore, in mice the knockouts of NOTCH1 or its ligands Delta-like-1 or Jagged-1 are embryonic lethal due principally to vascular defects.53,54 Recently, signaling via the Notch ligand Delta-like-4 was found by multiple groups to regulate endothelial sprouting and its inhibition led to disordered and unproductive endothelial growth and decreased tumor size—even in cancers resistant to VEGF inhibition.49,51,55 In addition, small-molecule Notch-inhibiting drugs have been shown to have potent antiangiogenic effects in animal models of cancer.56–58 The precise mechanisms by which Notch regulates the vasculature seem to be diverse. Delta-like-4/Notch signaling directly regulates angiogenic endothelial cells and Notch also seems to regulate aspects of vascular development such as arterial versus venous fate.59–61 More specifically for cancer, endothelial cells and cancer cells have been shown to communicate via Notch signaling to promote angiogenesis and cancer growth.62 Notch also regulates expression of vascular endothelial growth factor recptor-1 (VEGFR-1), a key receptor for vascular formation.35 The diverse roles of Notch in angiogenesis may have implications for cancer; by blocking processes such as angiogenesis, Notch inhibition may be valuable even in cancers whose cells are not directly sensitive.
As is becoming clear for many targeted inhibitors in cancer, Notch inhibition may be best not as solitary therapy but in combination with other agents. A growing body of evidence demonstrates that Notch inhibitors sensitize to more standard treatments such as radiation therapy and chemotherapy.47,63,64 Notch inhibitors are also being assessed in combination with other targeted inhibitors65 and such an approach may be important to maximize effects given the likelihood that most cancers will have lesions in multiple oncogenic pathways. That single cancers are driven by genetic lesions in multiple pathways has been extensively demonstrated in glioblastoma, the most common and aggressive primary brain tumor.66 This has raised concerns that inhibition of individual signaling pathways will almost never be sufficient for cancer therapy, despite the phenomenon of “oncogene addiction” established in recent years. Notch inhibition may be particularly effective when combined with inhibitors of other key stem cell pathways. For example, recently-presented but still unpublished results show potent anti-cancer effects from combining a Notch-inhibiting drug and a Hedgehog pathway inhibitor in glioblastoma stem cell lines.67
POTENTIAL RISKS OF NOTCH INHIBITION
Notch inhibition as cancer therapy may also pose significant risks and the potential risks and benefits are summarized in Table 1. One major side effect that emerged from the pioneering trials of a first-line Notch-inhibiting drug is gastrointestinal toxicity and diarrhea.68 This is likely an on-target toxicity, given reports that Notch drives gastrointestinal precursor cells toward an epithelial fate and away from a secretory cell fate; Notch inhibition thus causes an imbalance with too many secretory goblet cells.5,69 This showed its potential to be a dose-limiting toxicity in these earliest trials and it is likely one that will be problematic for any systemically-delivered Notch inhibitor. However, potential answers to this have already arisen. It has been found that intermittent dosing schedules of a Notch inhibitor can largely spare the gut while maintaining anti-tumor efficacy. In addition, it has been found that corticosteroid administration, already a component of some cancer regimens, may help ameliorate the gut toxicity of Notch inhibition.70
Table 1.
Potential advantages and disadvantages of Notch inhibition for cancer therapy
| Advantages | Disadvantages |
|---|---|
| May be especially active against resistant tumor stem cell subpopulation |
Gastrointestinal toxicity |
| Inhibiting other key cancer pathways and proteins through crosstalk—Ras, Akt, NF-κB, EGF, VEGF |
Increased risk of cancers for which Notch acts as a tumor suppressor |
| Apoptotic effects | May diminish normal stem cell populations such as in the brain |
| Cell cycle inhibition | Effect on endothelial cells may stimulate vascular tumor formation over the long term |
| Senescence? | |
| Antiangiogenic effects Sensitizes to other treatments, such as radiation and chemotherapy |
Two other theoretical risks of long-term Notch inhibition have been posited. One is the potential for damage to normal stem cells in the body, which may rely on Notch signaling to varying degrees. Possible results of this are difficult to determine, but could include anything from hematopoietic collapse to subtle cognitive decline. No signs of such toxicities have been uncovered in the earliest clinical trials, but the dosing was relatively short in those trials. Even if such toxicities emerge, it is possible that they too might be addressed by intermittent dosing of a Notch inhibitor. The other theoretical risk may be even more concerning, as it involves an increased incidence of certain cancers. While Notch plays an oncogenic role in most tissues, it acts as a tumor suppressor in some, such as certain skin cells, neuroendocrine lung cells and B-cells.71–73 Thus long-term Notch inhibition may increase the risk of cancers in these cellular compartments, though this has not yet been demonstrated. On the other hand, Notch-activating agents may have therapeutic potential for these cancers—though with the corresponding risk of increasing risk of other cancers. Despite the potential risks of Notch inhibition, it generally seems well-tolerated and these risks have not appreciably dampened the growing enthusiasm for Notch inhibitors as cancer therapies.
STRATEGIES FOR NOTCH INHIBITION
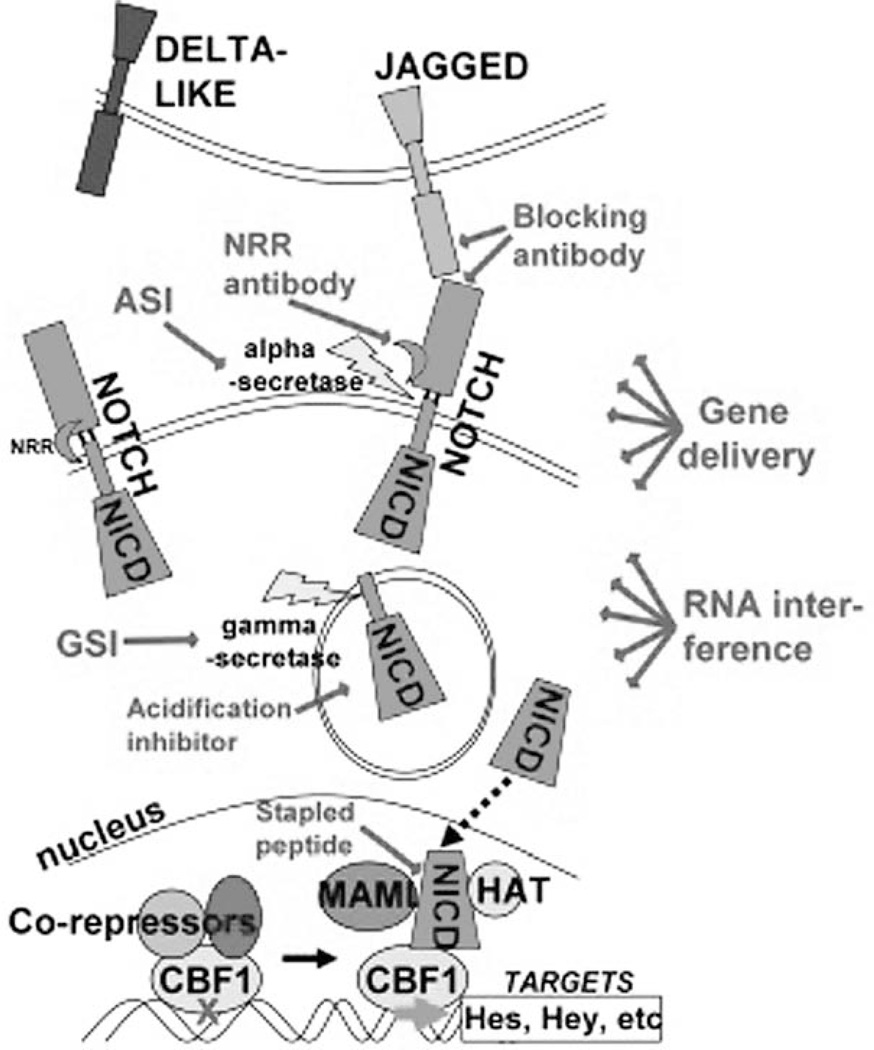
All current and experimental approaches for inhibiting Notch are discussed below, as well as some theoretical means. These are depicted in Figure 1. Potential benefits and drawbacks of each method are summarized in Table 2.
Figure 1.
Depiction of Notch pathway and loci at which current and experiemental inhibitors block. Abbreviations: NPR- negative regulatory region; NICD- Notch intracellular domain; ASI- alpha-secretase inhibitor; GSI- gamma-secretase inhibitor; MAML- Mastermind-like; HAT- Histone acetyl-transferase
Table 2.
Benefits and drawbacks of different strategies for Notch inhibition in cancer
| Benefits | Drawbacks | |
|---|---|---|
| Gamma-secretase inhibitors |
Effective Notch inhibitors in most settings. Oral agents Already in clinical trials Numerous GSIs already developed or in the pipeline |
Nonspecific. GI toxicity. |
| Alpha-secretase inhibitors |
May be active outside the cell, so not vulnerable to efflux pumps. Oral agents. |
Nonspecific. Likely GI toxicity. |
| Small-molecule blockers |
Potential for oral bioavailability and for specificity for individual Notch family members or ligands. |
Purely theoretical at this stage. |
| Endosomal acidification inhibitors |
Includes known agents, which may include some already tested in humans. May be oral. |
May be highly nonspecific and be similar to GSIs. |
| Blocking or NRR (negative regulatory region) antibodies |
Can be specific for individual Notch family members or Notch ligands. Targeting of individual Notch family members may minimize side effects such as GI toxicity. |
Difficult access—may be most useful intravascularly or with local delivery. Large molecule. |
| Stapled peptide | Highly specific for the Notch pathway. Relatively small molecule. |
Difficult access to cells—may be most useful intravascularly or with local delivery. |
| Delivery of notch-inhibiting genes |
May be quite specific. | Difficult access, likely requiring viral or liposomal delivery. Probably inefficient. |
| Delivery of siRNAs, shRNAs, or microRNAs |
siRNAs/shRNAs can be very potent Notch inhibitors. MicroRNAs are found endogenously and are likely tolerated well by normal cells. |
Difficult access, likely requiring viral or liposomal delivery. Likely inefficient but efficiency may be boosted by transduced cells shedding microvesicles taken up by nearby cells. |
Small Molecule Inhibitors
Gamma-Secretase Inhibitors
Developing inhibitors of the Notch pathway is complicated by the fact that pathway members themselves do not have enzymatic activity, as it is typically easiest to develop small-molecule inhibitors of enzymes. However, canonical signaling by the Notch pathway does require two enzymatic cleavages that occur following ligand binding to Notch, the first by the alpha-secretase complex and the second by the gamma-secretase complex. These enzymes are amenable to blockade by small-molecule agents and gamma-secretase inhibitors (GSIs) represent the pioneering class of Notch inhibitors both in the laboratory and in the clinic. It is important to note that gamma-secretase also has other targets besides the four Notch proteins, including the Notch ligands Delta-like and Jagged, APP (amyloid precursor protein), CD44, ErbB4, LRP, syndecan-3, p75 NTR, Apo ER2, DCC, Nectin-1alpha, E-cadherin and possibly N-cadherin.74–84 GSIs were first developed as potential therapies for Alzheimer’s disease and only later were adapted for cancer therapy. This lack of specificity may be problematic for their use in humans, but on the other hand it may be helpful as some of the other GSI targets have themselves been identified as potential targets for cancer therapy.85–87 A few reports in the literature indicate that at least in some cancer settings the inhibition of Notch is responsible for most of the cytotoxicity of GSIs, evident because restoring expression of the constitutively-active Notch intracellular domain can rescue the cells.25,88
Most of the experimental work with Notch inhibitors in the laboratory has been done with GSIs and early clinical trials have already taken place with the Merck GSI MK-0752. The first Phase I clinical trials of MK-0752 were in patients with T-cell leukemia/lymphoma and other Phase I trials are ongoing in patients with solid tumors and in patients with breast cancer. Stable disease and one response have been observed in patients with high-grade glioma and stable disease was also observed in patients with two other cancer types.89 A host of other clinical trials are being initiated for patient populations with a variety of cancers, with GSIs either alone or in combination with other agents.
Chemically, a number of structures have been used as the basis for GSIs. The most commonly used is a modified di- or tri-peptide with one to two aromatic hydrocarbon rings included. This has yielded hydrophobic compounds which are cell-permeable and that act as reversible inhibitors of gamma-secretase. In the laboratory, the most widely employed is DAPT (N-[N-(3,5-Difluorophenylacetyl-L-alanyl)]-S-phenylglycine t-Butyl ester) and another frequently-used compound is the structurally similar Lilly GSI L685,458. A structurally different compound which is also available preclinically is compound E ((s,s)-2-(3,5-Difluorophenyl)-acetylamino]-N-(1-methyl-2-oxo-5-phenyl-2,3-dihydro-1H-benzo[e][1,4]diazepin-3-yl)-propionamide). Another class of GSIs includes diazepine-type structures, with DBZ (dibenzazepine) as an example. Other GSIs are based on an isocoumarin foundation, such as JLK6 (7-amino-4-chloro-3-methoxyisocoumarin) and these can bind and inhibit gamma-secretase irreversibly. One sulfonamide-based GSI, Compound 18 ([11-endo]-N-(5,6,7,8,9,10-hexahydro- 6,9-methano benzo[9][8]annulen-11-yl)-thiophene-2-sulfonamide), is reported to be potent.25,88 Other structures have also been demonstrated to inhibit gamma-secretase activity.90
Other drugs in wide usage have been found to have some level of GSI activity, including many nonsteroidal anti-inflammatory drugs (NSAIDs).91,92 Some of these drugs have anti-cancer and antiangiogenic effects and Notch inhibition via GSI activity might be one mechanism to explain this. NSAIDs have been explored as GSIs particularly in the field of Alzheimer’s disease and there have been attempts to derive selective NSAID GSIs which can block amyloid precursor processing but spare Notch processing, to avoid potential side effects from Notch inhibition. One early report described flurbiprofen as such an NSAID.92 The study of selective GSIs also raises the possibility that agents could be derived that more specifically inhibit Notch processing, sparing other gamma-secretase functions. While GSIs are highly nonspecific and imperfect Notch inhibitors, they still may demonstrate clinical utility and more refined later-generation GSIs may yet emerge.
Other Potential Approaches to Small-Molecule Inhibitors
While attention has focused only on gamma-secretase as a vulnerable point in Notch processing, it may also be feasible to utilize alpha-secretase inhibitors (ASIs) that have been developed for purposes other than Notch inhibition. The alpha-secretase enzymes that cleave Notch are thought to be ADAM-10 and -17 (A Disintegrin And Metalloprotease)93,94 and inhibitors that block both these ADAMs have been developed.95 There may be theoretical advantages of an ASI over a GSI; for example, an ASI would not have to enter the cell to act. We are in the process of testing ASIs as Notch inhibitors in cancer.
At the theoretical level, it could be possible to develop small-molecule inhibitors of Notch that act in very different fashions. While inhibition of an enzymatic activity is typically the most straightforward strategy to block a protein or pathway, examples are beginning to emerge of the potential druggability of protein-protein interactions. This was powerfully shown in a recent report in which a small-molecule blocker was derived to interrupt the interaction of the fusion protein EWS-Fli1 with the RNA helicase RHA.96 This work demonstrated that small molecules could be discovered to disrupt the binding of even highly disordered proteins, lacking alpha helices or beta pleated sheets at the binding domains. Another example of a small-molecule agent blocking a protein-protein interaction is the molecule nutlin, which interferes with p53/MDM2 interaction.97 A number of protein-protein interactions in the Notch pathway would be logical targets for disruption, including Notch—Notch ligand, Notch intracellular domain (NICD)—CBF1 transcription factor, or NICD—mastermind-like (MAML).
There are likely other points in Notch processing and the Notch pathway that are amenable to blockade. One promising approach was described in a recent poster presentation, but has yet to be published.98 It relies on the discovery that the gamma-secretase cleavage of Notch occurs not at the cell membrane but in acidic endosomes.99 Numerous drug compounds with the potential to interfere with endosomal acidification were screened for their ability to reduce Notch activity and this yielded promising hits. The Na+/H+ antiporter Monensin emerged as a potent Notch inhibitor. How acidification inhibitors such as this compare to the GSIs in terms of efficiency and specificity remains to be determined.
Antibody Inhibitors of Notch Activity
Antibody inhibitors remain a prominent means for blocking protein-protein interactions or part of a protein structure and thus represent one modality for disrupting Notch activity. One potential advantage of antibody inhibitors is their specificity, allowing for targeting of individual Notch family members or ligands. Antibodies are large molecules, though and delivery/access to cancer cells could be highly problematic. For certain cancers such as brain tumors, local delivery may be an option, but for most metastatic cancers it is necessary to have efficient systemic distribution. Thus, antibody inhibitors of Notch may be most easily applied toward hematopoietic malignancies or for antiangiogenic uses.
Along these lines, antibodies to the Notch ligand Delta-like-4 represent a highly promising antiangiogenic strategy. As mentioned earlier, Notch signaling via the ligand Delta-like-4 was reported by multiple groups to suppress angiogenic sprouting by endothelial cells. Counter-intuitively, blocking Delta-like-4 with specific antibodies did not promote tumor angiogenesis, but instead led to chaotic, dysfunctional vasculature and subsequent tumor regression.49,51,55 Importantly, this occurred even in cancer models that were resistant to VEGF antibodies, an established and powerful antiangiogenic approach already in the clinic. This has prompted an aggressive effort to develop Delta-like-4 antibodies for clinical usage. While this may ultimately prove fruitful, one recent study suggests a potentially significant hazard. Prolonged treatment with a Delta-like-4 antibody in mice led to the development of vascular/endothelial cell-based tumors resembling hemangioblastoma.100 In hindsight this makes some sense, given the suppressive effect of Delta-like-4/Notch signaling on endothelial cell outgrowth. If this adverse effect is borne out by others as well, it may present a major obstacle to the usage of Delta-like-4 antibodies in the clinic.
Blocking antibodies to Notch or its ligands may serve not only antiangiogenic functions but also directly inhibit cancer cells. A growing number of reports describe the development of antibodies to specific Notch family members, sometimes with different functions. Antibodies to Notch-3 were reported that can either block or stimulate receptor signaling.101 Some of these antibodies seem to work by binding a region of Notch that obscures the target site for alpha-secretase (the negative regulatory region, or NRR), either by exposing the target site or reinforcing its blockade. This raises the interesting prospect that antibodies could fine-tune Notch activity, increasing or attenuating signaling by individual Notch family members by disparate mechanisms. One exciting report has just emerged in which anti-NRR antibodies were developed that specifically block activity of either Notch-1 or Notch-2.102 The Notch-1 anti-NRR showed good antitumor effects, but without the gut toxicity associated with combined Notch-1 and Notch-2 inhibition. These Notch isoform-specific antibodies may represent a powerful new modality for cancer therapy, with good effectiveness but decreased toxicity.
Novel Methods of Notch Inhibition
Alternatives for Notch inhibition other than the more standard approaches described above are also becoming possible, in some cases taking advantage of new technologies. One recently-described approach uses a stapled peptide to block interaction of Mastermind-like with the Notch intracellular domain. While protein-protein interactions have generally been considered “undruggable,” as discussed earlier, the stapled peptide approach represents a recent development for inhibiting some of these interactions. This strategy was introduced by the Verdine and Korsmeyer labs at Harvard, initially for the development of a Bcl-2 inhibitor.103 Some protein-protein interactions include an alpha-helix at the interface point, which raised the prospect of using the isolated alpha-helical peptide as a blocker. However, by themselves these peptides are not structurally stable and are also too large and charged to pass the plasma membrane. The Verdine laboratory noted that with the incorporation of two modified residues into the alpha-helical peptide, a hydrocarbon chain could be linked in parallel to the peptide to lock its structure. Some of these peptides retained the ability to block the relevant protein–protein interaction. Surprisingly, the stapled peptides were also found to pass through the plasma membrane, allowing blockade of intracellular protein interactions. A recent report describes a stapled peptide, derived from an alpha-helix in the Mastermind-like (MAML) protein, that blocks canonical Notch signaling.104 This peptide, termed SAHM1, could have therapeutic potential for Notch-dependent hematopoietic cancers such as T-cell acute lymphoblastic leukemia, or possibly with local delivery to solid tumors.
Genetic strategies for Notch inhibition may also find limited application in cancer therapy, particularly for hematopoietic malignancies or localized tumors, such as in brain or lung. One form of this could consist of delivery of a gene or pseudogene encoding a Notch-inhibiting protein or peptide. A dominant-negative form of Mastermind-like has been used in the laboratory to inhibit canonical Notch signaling via CBF1105 and if this could be delivered in vivo it would serve as a highly specific Notch inhibitor. Other genes known to down-regulate Notch could also serve this function, such as the Numb/Numb-like or FBXW-7 genes.106,107 Agents that up-regulate expression of these endogenous Notch-inhibiting genes could be another means of blocking Notch activity.
Delivery of RNA interference represents a similar strategy for Notch-inhibiting cancer therapy, but possibly one with more potential for clinical success. As with Notch-inhibiting genes, delivery remains the principle hurdle in developing such approaches, but it is relatively less challenging to deliver small oligonucleotides than it is whole genes. Either small interfering RNAs or endogenous or artificial microRNAs could be delivered. Small interfering RNAs (siRNAs) are synthetic 19–27 base pair double-stranded oligonucleotides designed to perfectly match a sequence in a target mRNA and they are incorporated into the cell’s RISC complex (RNA-induced silencing complex) with subsequent destruction of the target mRNA. MicroRNAs utilize the same cellular machinery but represent an endogenous form of RNA interference that was discovered more recently than the siRNAs. It is estimated that approximately 1,000 microRNAs exist in the human genome, in both intronic and intergenic regions. The microRNAs originate as small oligonucleotide hairpins that are then processed into mature double-stranded microRNAs similar to siRNAs.108 However, unlike siRNAs the microRNAs usually target the 3′ untranslated region of target genes and complementarity for the target 3′-UTRs is imperfect. They also more frequently cause translational suppression of targets, but sometimes may cause mRNA cleavage.109 MicroRNAs each target numerous genes and in general each gene is targeted by more than one microRNA. MicroRNAs thus offer the potential to simultaneously target more than one gene of interest, though the target genes may not be suppressed as efficiently as by siRNAs. For example, the microRNA miR-326 has been shown to target both Notch-1 and Notch-2 and to decrease Notch activity.110 The tumor-suppressive microRNA miR-34a has also been shown to target Notch-1 and Notch-2111 and microRNA-206 has been shown to target Notch-3.112 miR-124 inhibits the important Notch mediator Hes-1.113 In some cases, transfecting these microRNAs has been shown to not only diminish Notch activity but also to kill cancer cells and in the case of miR-326 and glioblastoma cells it was suggested that the principle mechanism for cell kill is Notch inhibition.110 With respect to whether siRNAs or microRNAs would be more successful agents for Notch inhibition and cancer therapy, this remains an open question.
At present the potential delivery modalities for genetic strategies such as RNA interference include viral or liposomal vectors. For these approaches to be successful there will have to be an advance in the technology for delivery, given the requirement that all or nearly all of the cancer cells would have to receive the payload. However, recent studies suggest that this requirement may not be as stringent as once thought, because cancer cells have been shown to shed large amounts of microvesicles that can transmit cytoplasmic contents to nearby cells.114,115 Results are beginning to emerge that enough siRNAs or microRNAs can be transferred in this fashion to suppress gene expression in neighboring cells. Thus, even if a limited percentage of cancer cells is transfected with a therapeutic vector, the transfected cancer cells may “share” with nearby untransfected cancer cells to produce good results.
CONCLUSION
The Notch pathway has tremendous potential as a new target in cancer therapy. Notch inhibition in cancer cells has the potential to slow cell proliferation, cause apoptosis, induce differentiation and possibly trigger other terminal cell fates such as senescence. These effects are unsurprising given the extensive crosstalk of Notch with major cancer pathways such as Ras, Akt and NF-κB. Importantly, Notch may be a particularly powerful target for the tumor stem cell subset, which is resistant to standard treatments such as chemotherapy and radiation but seems especially sensitive to inhibition of stem cell pathways such as Notch. Even if Notch inhibitors alone do not yield major responses and cures, there is growing evidence that synergy can result from combining Notch inhibition with already-existing treatment modalities such as chemotherapy, radiation and other pathway inhibitors. Optimism for Notch should be tempered somewhat by adverse effects such as gastrointestinal toxicity that are beginning to be observed in clinical trials and no doubt other problems from long-term Notch inhibition remain to be discovered. The field is also hampered by limited existing options for Notch inhibitors; new agents are desperately needed. While gamma-secretase inhibitors are already in clinical trials as Notch-inhibiting agents and are clinically promising, they are highly nonspecific. Other experimental means of Notch inhibition include alpha-secretase inhibitors, peptide or antibody blockers, stapled peptides and genetic strategies such as RNA interference. At present the difficulties in successfully bringing Notch inhibition to the clinic all appear surmountable and there is growing optimism that Notch inhibition will become an exciting new approach to cancer.
REFERENCES
- 1.Gaiano N, Nye JS, Fishell G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron. 2000;26:395–404. doi: 10.1016/s0896-6273(00)81172-1. [DOI] [PubMed] [Google Scholar]
- 2.Henrique D, Hirsinger E, Adam J, et al. Maintenance of neuroepithelial progenitor cells by Delta-Notch signalling in the embryonic chick retina. Curr Biol. 1997;7:661–670. doi: 10.1016/s0960-9822(06)00293-4. [DOI] [PubMed] [Google Scholar]
- 3.Kageyama R, Ohtsuka T. The Notch-Hes pathway in mammalian neural development. Cell Res. 1999;9:179–188. doi: 10.1038/sj.cr.7290016. [DOI] [PubMed] [Google Scholar]
- 4.Kumano K, Chiba S, Kunisato A, et al. Notch1 but not Notch2 is essential for generating hematopoietic stem cells from endothelial cells. Immunity. 2003;18:699–711. doi: 10.1016/s1074-7613(03)00117-1. [DOI] [PubMed] [Google Scholar]
- 5.van Es JH, van Gijn ME, Riccio O, et al. Notch/gamma-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature. 2005;435:959–963. doi: 10.1038/nature03659. [DOI] [PubMed] [Google Scholar]
- 6.Amsen D, Blander JM, Lee GR, et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell. 2004;117:515–526. doi: 10.1016/s0092-8674(04)00451-9. [DOI] [PubMed] [Google Scholar]
- 7.Fre S, Huyghe M, Mourikis P, et al. Notch signals control the fate of immature progenitor cells in the intestine. Nature. 2005;435:964–968. doi: 10.1038/nature03589. [DOI] [PubMed] [Google Scholar]
- 8.Morrison SJ, Perez SE, Qiao Z, et al. Transient Notch activation initiates an irreversible switch from neurogenesis to gliogenesis by neural crest stem cells. Cell. 2000;101:499–510. doi: 10.1016/s0092-8674(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 9.Ellisen LW, Bird J, West DC, et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell. 1991;66:649–661. doi: 10.1016/0092-8674(91)90111-b. [DOI] [PubMed] [Google Scholar]
- 10.Purow BW, Haque RM, Noel MW, et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 2005;65:2353–2363. doi: 10.1158/0008-5472.CAN-04-1890. [DOI] [PubMed] [Google Scholar]
- 11.Shih AH, Holland EC. Notch signaling enhances nestin expression in gliomas. Neoplasia. 2006;8:1072–1082. doi: 10.1593/neo.06526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weng AP, Ferrando AA, Lee W, et al. Activating mutations of NOTCH1 in human T-cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 13.Jundt F, Anagnostopoulos I, Forster R, et al. Activated Notch1 signaling promotes tumor cell proliferation and survival in Hodgkin and anaplastic large cell lymphoma. Blood. 2002;99:3398–3403. doi: 10.1182/blood.v99.9.3398. [DOI] [PubMed] [Google Scholar]
- 14.Miele L, Osborne B. Arbiter of differentiation and death: Notch signaling meets apoptosis. J Cell Physiol. 1999;181:393–409. doi: 10.1002/(SICI)1097-4652(199912)181:3<393::AID-JCP3>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Shelly LL, Fuchs C, Miele L. Notch-1 inhibits apoptosis in murine erythroleukemia cells and is necessary for differentiation induced by hybrid polar compounds. J Cell Biochem. 1999;73:164–175. doi: 10.1002/(sici)1097-4644(19990501)73:2<164::aid-jcb3>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 16.Guo D, Ye J, Dai J, et al. Notch-1 regulates Akt signaling pathway and the expression of cell cycle regulatory proteins cyclin D1, CDK2 and p21 in T-ALL cell lines. Leuk Res. 2009;33:678–685. doi: 10.1016/j.leukres.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 17.Joshi I, Minter LM, Telfer J, et al. Notch signaling mediates G1/S cell-cycle progression in T-cells via cyclin D3 and its dependent kinases. Blood. 2009;113:1689–1698. doi: 10.1182/blood-2008-03-147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dontu G, Al-Hajj M, Abdallah WM, et al. Stem cells in normal breast development and breast cancer. Cell Prolif. 2003;36(Suppl 1):59–72. doi: 10.1046/j.1365-2184.36.s.1.6.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eyler CE, Rich JN. Survival of the fittest: cancer stem cells in therapeutic resistance and angiogenesis. J Clin Oncol. 2008;26:2839–2845. doi: 10.1200/JCO.2007.15.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Kotliarova S, Kotliarov Y, et al. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 2006;9:391–403. doi: 10.1016/j.ccr.2006.03.030. [DOI] [PubMed] [Google Scholar]
- 21.Singh SK, Clarke ID, Terasaki M, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- 22.Bao S, Wu Q, McLendon RE, et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 23.Liu G, Yuan X, Zeng Z, et al. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol Cancer. 2006;5:67. doi: 10.1186/1476-4598-5-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fan X, Khaki L, Zhu TS, et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 28:5–16. doi: 10.1002/stem.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan X, Matsui W, Khaki L, et al. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 2006;66:7445–7452. doi: 10.1158/0008-5472.CAN-06-0858. [DOI] [PubMed] [Google Scholar]
- 26.Sikandar SS, Pate KT, Anderson S, et al. NOTCH signaling is required for formation and self-renewal of tumor-initiating cells and for repression of secretory cell differentiation in colon cancer. Cancer Res. 70:1469–1478. doi: 10.1158/0008-5472.CAN-09-2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perumalsamy LR, Nagala M, Sarin A. Notch-activated signaling cascade interacts with mitochondrial remodeling proteins to regulate cell survival. Proc Natl Acad Sci USA. 107:6882–6887. doi: 10.1073/pnas.0910060107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Efferson CL, Winkelmann CT, Ware C, et al. Downregulation of Notch pathway by a gamma-secretase inhibitor attenuates AKT/mammalian target of rapamycin signaling and glucose uptake in an ERBB2 transgenic breast cancer model. Cancer Res. 70:2476–2484. doi: 10.1158/0008-5472.CAN-09-3114. [DOI] [PubMed] [Google Scholar]
- 29.Meurette O, Stylianou S, Rock R, et al. Notch activation induces Akt signaling via an autocrine loop to prevent apoptosis in breast epithelial cells. Cancer Res. 2009;69:5015–5022. doi: 10.1158/0008-5472.CAN-08-3478. [DOI] [PubMed] [Google Scholar]
- 30.Palomero T, Sulis ML, Cortina M, et al. Mutational loss of PTEN induces resistance to NOTCH1 inhibition in T-cell leukemia. Nat Med. 2007;13:1203–1210. doi: 10.1038/nm1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fitzgerald K, Harrington A, Leder P. Ras pathway signals are required for notch-mediated oncogenesis. Oncogene. 2000;19:4191–4198. doi: 10.1038/sj.onc.1203766. [DOI] [PubMed] [Google Scholar]
- 32.Weijzen S, Velders MP, Elmishad AG, et al. The Notch ligand Jagged-1 is able to induce maturation of monocyte-derived human dendritic cells. J Immunol. 2002;169:4273–4278. doi: 10.4049/jimmunol.169.8.4273. [DOI] [PubMed] [Google Scholar]
- 33.Konishi J, Yi F, Chen X, et al. Notch3 cooperates with the EGFR pathway to modulate apoptosis through the induction of bim. Oncogene. 29:589–596. doi: 10.1038/onc.2009.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Purow BW, Sundaresan TK, Burdick MJ, et al. Notch-1 regulates transcription of the epidermal growth factor receptor through p53. Carcinogenesis. 2008;29:918–925. doi: 10.1093/carcin/bgn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Funahashi Y, Shawber CJ, Vorontchikhina M, et al. Notch regulates the angiogenic response via induction of VEGFR-1. J Angiogenes Res. 2:3. doi: 10.1186/2040-2384-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon K, Nery S, Rutlin ML, et al. Fibroblast growth factor receptor signaling promotes radial glial identity and interacts with Notch1 signaling in telencephalic progenitors. J Neurosci. 2004;24:9497–9506. doi: 10.1523/JNEUROSCI.0993-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang J, Shelly L, Miele L, et al. Human Notch-1 inhibits NF-kappa B activity in the nucleus through a direct interaction involving a novel domain. J Immunol. 2001;167:289–295. doi: 10.4049/jimmunol.167.1.289. [DOI] [PubMed] [Google Scholar]
- 38.Cheng P, Zlobin A, Volgina V, et al. Notch-1 regulates NF-kappaB activity in hemopoietic progenitor cells. J Immunol. 2001;167:458–467. doi: 10.4049/jimmunol.167.8.4458. [DOI] [PubMed] [Google Scholar]
- 39.Oswald F, Liptay S, Adler G, et al. NF-kappaB2 is a putative target gene of activated Notch-1 via RBP-Jkappa. Mol Cell Biol. 1998;18:2077–2088. doi: 10.1128/mcb.18.4.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Espinosa L, Santos S, Ingles-Esteve J, et al. p65-NFkappaB synergizes with Notch to activate transcription by triggering cytoplasmic translocation of the nuclear receptor corepressor N-CoR. J Cell Sci. 2002;115:1295–1303. doi: 10.1242/jcs.115.6.1295. [DOI] [PubMed] [Google Scholar]
- 41.Nickoloff BJ, Qin JZ, Chaturvedi V, et al. Jagged-1 mediated activation of notch signaling induces complete maturation of human keratinocytes through NF-kappaB and PPARgamma. Cell Death Differ. 2002;9:842–855. doi: 10.1038/sj.cdd.4401036. [DOI] [PubMed] [Google Scholar]
- 42.Weng AP, Millholland JM, Yashiro-Ohtani Y, et al. c-Myc is an important direct target of Notch1 in T-cell acute lymphoblastic leukemia/lymphoma. Genes Dev. 2006;20:2096–2109. doi: 10.1101/gad.1450406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gustafsson MV, Zheng X, Pereira T, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 44.Nefedova Y, Sullivan DM, Bolick SC, et al. Inhibition of Notch signaling induces apoptosis of myeloma cells and enhances sensitivity to chemotherapy. Blood. 2008;111:2220–2229. doi: 10.1182/blood-2007-07-102632. [DOI] [PubMed] [Google Scholar]
- 45.Ishikawa Y, Onoyama I, Nakayama KI, et al. Notch-dependent cell cycle arrest and apoptosis in mouse embryonic fibroblasts lacking Fbxw7. Oncogene. 2008;27:6164–6174. doi: 10.1038/onc.2008.216. [DOI] [PubMed] [Google Scholar]
- 46.Sang L, Coller HA, Roberts JM. Control of the reversibility of cellular quiescence by the transcriptional repressor HES1. Science. 2008;321:1095–1100. doi: 10.1126/science.1155998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gilbert CA, Hermance N, Daou M-C, et al. Glioma treatment with temozolomide and Notch inhibition suppresses neurosphere formation and xenograft formation. AACR Meeting Abstracts. 2010;2010 [Google Scholar]
- 48.Caiado F, Real C, Carvalho T, et al. Notch pathway modulation on bone marrow-derived vascular precursor cells regulates their angiogenic and wound healing potential. PLoS ONE. 2008;3:e3752. doi: 10.1371/journal.pone.0003752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hellstrom M, Phng LK, Hofmann JJ, et al. Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature. 2007;445:776–780. doi: 10.1038/nature05571. [DOI] [PubMed] [Google Scholar]
- 50.Li JL, Sainson RC, Shi W, et al. Delta-like 4 Notch ligand regulates tumor angiogenesis, improves tumor vascular function and promotes tumor growth in vivo. Cancer Res. 2007;67:1244–1253. doi: 10.1158/0008-5472.CAN-07-0969. [DOI] [PubMed] [Google Scholar]
- 51.Noguera-Troise I, Daly C, Papadopoulos NJ, et al. Blockade of Dll4 inhibits tumour growth by promoting nonproductive angiogenesis. Nature. 2006;444:1032–1037. doi: 10.1038/nature05355. [DOI] [PubMed] [Google Scholar]
- 52.Joutel A, Corpechot C, Ducros A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 53.Hrabe de Angelis M, McIntyre J, 2nd, Gossler A. Maintenance of somite borders in mice requires the Delta homologue DII1. Nature. 1997;386:717–721. doi: 10.1038/386717a0. [DOI] [PubMed] [Google Scholar]
- 54.Xue Y, Gao X, Lindsell CE, et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- 55.Ridgway J, Zhang G, Wu Y, et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature. 2006;444:1083–1087. doi: 10.1038/nature05313. [DOI] [PubMed] [Google Scholar]
- 56.Masuda S, Kumano K, Suzuki T, et al. Dual antitumor mechanisms of Notch signaling inhibitor in a T-cell acute lymphoblastic leukemia xenograft model. Cancer Sci. 2009;100:2444–2450. doi: 10.1111/j.1349-7006.2009.01328.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Luistro L, He W, Smith M, et al. Preclinical profile of a potent gamma-secretase inhibitor targeting notch signaling with in vivo efficacy and pharmacodynamic properties. Cancer Res. 2009;69:7672–7680. doi: 10.1158/0008-5472.CAN-09-1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paris D, Quadros A, Patel N, et al. Inhibition of angiogenesis and tumor growth by beta and gamma-secretase inhibitors. Eur J Pharmacol. 2005;514:1–15. doi: 10.1016/j.ejphar.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 59.Hirashima M, Suda T. Differentiation of arterial and venous endothelial cells and vascular morphogenesis. Endothelium. 2006;13:137–145. doi: 10.1080/10623320600698078. [DOI] [PubMed] [Google Scholar]
- 60.Liu ZJ, Shirakawa T, Li Y, et al. Regulation of Notch1 and Dll4 by vascular endothelial growth factor in arterial endothelial cells: implications for modulating arteriogenesis and angiogenesis. Mol Cell Biol. 2003;23:14–25. doi: 10.1128/MCB.23.1.14-25.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lawson ND, Scheer N, Pham VN, et al. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:675–683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- 62.Zeng Q, Li S, Chepeha DB, et al. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell. 2005;8:13–23. doi: 10.1016/j.ccr.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 63.Meng RD, Shelton CC, Li YM, et al. gamma-Secretase inhibitors abrogate oxaliplatin-induced activation of the Notch-1 signaling pathway in colon cancer cells resulting in enhanced chemosensitivity. Cancer Res. 2009;69:73–82. doi: 10.1158/0008-5472.CAN-08-2088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang J, Wakeman TP, Lathia JD, et al. Notch promotes radioresistance of glioma stem cells. Stem Cells. 2010;28:17–28. doi: 10.1002/stem.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Osipo C, Patel P, Rizzo P, et al. ErbB-2 inhibition activates Notch-1 and sensitizes breast cancer cells to a gamma-secretase inhibitor. Oncogene. 2008;27:5019–5032. doi: 10.1038/onc.2008.149. [DOI] [PubMed] [Google Scholar]
- 66.Stommel JM, Kimmelman AC, Ying H, et al. Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science. 2007;318:287–290. doi: 10.1126/science.1142946. [DOI] [PubMed] [Google Scholar]
- 67.Schreck CA, Taylor P, Bar EE, et al. Targeting Notch in malignant brain tumors: Crosstalk with Hedgehog as a potential mechanism of treatment resistance. AACR Meeting Abstracts. 2010;2010 [Google Scholar]
- 68.Garber K. Notch emerges as new cancer drug target. J Natl Cancer Inst. 2007;99:1284–1285. doi: 10.1093/jnci/djm148. [DOI] [PubMed] [Google Scholar]
- 69.Zecchini V, Domaschenz R, Winton D, et al. Notch signaling regulates the differentiation of postmitotic intestinal epithelial cells. Genes Dev. 2005;19:1686–1691. doi: 10.1101/gad.341705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Real PJ, Tosello V, Palomero T, et al. Gamma-secretase inhibitors reverse glucocorticoid resistance in T-cell acute lymphoblastic leukemia. Nat Med. 2009;15:50–58. doi: 10.1038/nm.1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nicolas M, Wolfer A, Raj K, et al. Notch1 functions as a tumor suppressor in mouse skin. Nat Genet. 2003;33:416–421. doi: 10.1038/ng1099. [DOI] [PubMed] [Google Scholar]
- 72.Sriuranpong V, Borges MW, Ravi RK, et al. Notch signaling induces cell cycle arrest in small cell lung cancer cells. Cancer Res. 2001;61:3200–3205. [PubMed] [Google Scholar]
- 73.Zweidler-McKay PA, He Y, Xu L, et al. Notch signaling is a potent inducer of growth arrest and apoptosis in a wide range of B-cell malignancies. Blood. 2005;106:3898–3906. doi: 10.1182/blood-2005-01-0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hoe HS, Rebeck GW. Regulation of ApoE receptor proteolysis by ligand binding. Brain Res Mol Brain Res. 2005;137:31–39. doi: 10.1016/j.molbrainres.2005.02.013. [DOI] [PubMed] [Google Scholar]
- 75.Ikeuchi T, Sisodia SS. The Notch ligands, Delta1 and Jagged2, are substrates for presenilin-dependent “gamma-secretase” cleavage. J Biol Chem. 2003;278:7751–7754. doi: 10.1074/jbc.C200711200. [DOI] [PubMed] [Google Scholar]
- 76.Kanning KC, Hudson M, Amieux PS, et al. Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J Neurosci. 2003;23:425–436. doi: 10.1523/JNEUROSCI.23-13-05425.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim DY, Ingano LA, Kovacs DM. Nectin-1alpha, an immunoglobulin-like receptor involved in the formation of synapses, is a substrate for presenilin/gamma-secretase-like cleavage. J Biol Chem. 2002;277:49976–49981. doi: 10.1074/jbc.M210179200. [DOI] [PubMed] [Google Scholar]
- 78.Lammich S, Okochi M, Takeda M, et al. Presenilin-dependent intramembrane proteolysis of CD44 leads to the liberation of its intracellular domain and the secretion of an Abeta-like peptide. J Biol Chem. 2002;277:44754–44759. doi: 10.1074/jbc.M206872200. [DOI] [PubMed] [Google Scholar]
- 79.Marambaud P, Shioi J, Serban G, et al. A presenilin-1/gamma-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. Embo J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Parisiadou L, Fassa A, Fotinopoulou A, et al. Presenilin 1 and cadherins: stabilization of cell-cell adhesion and proteolysis-dependent regulation of transcription. Neurodegener Dis. 2004;1:184–191. doi: 10.1159/000080984. [DOI] [PubMed] [Google Scholar]
- 81.Scacheri PC, Rozenblatt-Rosen O, Caplen NJ, et al. Short interfering RNAs can induce unexpected and divergent changes in the levels of untargeted proteins in mammalian cells. Proc Natl Acad Sci USA. 2004;101:892–897. doi: 10.1073/pnas.0308698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Schulz JG, Annaert W, Vandekerckhove J, et al. Syndecan 3 intramembrane proteolysis is presenilin/gamma-secretase-dependent and modulates cytosolic signaling. J Biol Chem. 2003;278:48651–48657. doi: 10.1074/jbc.M308424200. [DOI] [PubMed] [Google Scholar]
- 83.Tanigaki K, Nogaki F, Takahashi J, et al. Notch1 and Notch3 instructively restrict bFGF-responsive multipotent neural progenitor cells to an astroglial fate. Neuron. 2001;29:45–55. doi: 10.1016/s0896-6273(01)00179-9. [DOI] [PubMed] [Google Scholar]
- 84.Taniguchi Y, Kim SH, Sisodia SS. Presenilin-dependent “gamma-secretase” processing of deleted in colorectal cancer (DCC) J Biol Chem. 2003;278:30425–30428. doi: 10.1074/jbc.C300239200. [DOI] [PubMed] [Google Scholar]
- 85.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 46:1271–1277. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 86.Xu Y, Stamenkovic I, Yu Q. CD44 attenuates activation of the hippo signaling pathway and is a prime therapeutic target for glioblastoma. Cancer Res. 70:2455–2464. doi: 10.1158/0008-5472.CAN-09-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang L, Rahn JJ, Lun X, et al. Gamma-secretase represents a therapeutic target for the treatment of invasive glioma mediated by the p75 neurotrophin receptor. PLoS Biol. 2008;6:e289. doi: 10.1371/journal.pbio.0060289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fan X, Mikolaenko I, Elhassan I, et al. Notch1 and notch2 have opposite effects on embryonal brain tumor growth. Cancer Res. 2004;64:787–793. doi: 10.1158/0008-5472.CAN-04-1446. [DOI] [PubMed] [Google Scholar]
- 89.LoRusso PM, DeMuth T, Heath E, et al. Phase I study of the gamma secretase inhibitor MK-0752 in patients withmetastatic breast and other advanced solid tumors. AACR Meeting Abstracts. 2009;2009 [Google Scholar]
- 90.Lewis SJ, Smith AL, Neduvelil JG, et al. A novel series of potent gamma-secretase inhibitors based on a benzobicyclo[4.2.1]nonane core. Bioorg Med Chem Lett. 2005;15:373–378. doi: 10.1016/j.bmcl.2004.10.062. [DOI] [PubMed] [Google Scholar]
- 91.Eriksen JL, Sagi SA, Smith TE, et al. NSAIDs and enantiomers of flurbiprofen target gamma-secretase and lower Abeta 42 in vivo. J Clin Invest. 2003;112:440–449. doi: 10.1172/JCI18162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Weggen S, Eriksen JL, Das P, et al. A subset of NSAIDs lower amyloidogenic Abeta42 independently of cyclooxygenase activity. Nature. 2001;414:212–216. doi: 10.1038/35102591. [DOI] [PubMed] [Google Scholar]
- 93.Brou C, Logeat F, Gupta N, et al. A novel proteolytic cleavage involved in Notch signaling: the role of the disintegrin-metalloprotease TACE. Mol Cell. 2000;5:207–216. doi: 10.1016/s1097-2765(00)80417-7. [DOI] [PubMed] [Google Scholar]
- 94.Hartmann D, de Strooper B, Serneels L, et al. The disintegrin/metalloprotease ADAM 10 is essential for Notch signalling but not for alpha-secretase activity in fibroblasts. Hum Mol Genet. 2002;11:2615–2624. doi: 10.1093/hmg/11.21.2615. [DOI] [PubMed] [Google Scholar]
- 95.Zhou BB, Peyton M, He B, et al. Targeting ADAM-mediated ligand cleavage to inhibit HER3 and EGFR pathways in nonsmall cell lung cancer. Cancer Cell. 2006;10:39–50. doi: 10.1016/j.ccr.2006.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Erkizan HV, Kong Y, Merchant M, et al. A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing’s sarcoma. Nat Med. 2009;15:750–756. doi: 10.1038/nm.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Vassilev LT, Vu BT, Graves B, et al. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 98.Moellering R, Ramirez R, Verdine G, et al. Rational targeting of Notch receptor trafficking. AACR Meeting Abstracts. 2009;2009 [Google Scholar]
- 99.Vaccari T, Lu H, Kanwar R, et al. Endosomal entry regulates Notch receptor activation in Drosophila melanogaster. J Cell Biol. 2008;180:755–762. doi: 10.1083/jcb.200708127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Yan M, Callahan CA, Beyer JC, et al. Chronic DLL4 blockade induces vascular neoplasms. Nature. 463:E6–E7. doi: 10.1038/nature08751. [DOI] [PubMed] [Google Scholar]
- 101.Li K, Li Y, Wu W, et al. Modulation of notch signaling by antibodies specific for the extracellular negative regulatory region of Notch3. J Biol Chem. 2008 doi: 10.1074/jbc.M800170200. [DOI] [PubMed] [Google Scholar]
- 102.Wu Y, Cain-Hom C, Choy L, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 103.Walensky LD, Kung AL, Escher I, et al. Activation of apoptosis in vivo by a hydrocarbon-stapled BH3 helix. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Moellering RE, Cornejo M, Davis TN, et al. Direct inhibition of the NOTCH transcription factor complex. Nature. 2009;462:182–188. doi: 10.1038/nature08543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Maillard I, Weng AP, Carpenter AC, et al. Mastermind critically regulates Notch-mediated lymphoid cell fate decisions. Blood. 2004;104:1696–1702. doi: 10.1182/blood-2004-02-0514. [DOI] [PubMed] [Google Scholar]
- 106.Guo M, Jan LY, Jan YN. Control of daughter cell fates during asymmetric division: interaction of Numb and Notch. Neuron. 1996;17:27–41. doi: 10.1016/s0896-6273(00)80278-0. [DOI] [PubMed] [Google Scholar]
- 107.Tsunematsu R, Nakayama K, Oike Y, et al. Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during vascular development. J Biol Chem. 2004;279:9417–9423. doi: 10.1074/jbc.M312337200. [DOI] [PubMed] [Google Scholar]
- 108.Murchison EP, Hannon GJ. miRNAs on the move: miRNA biogenesis and the RNAi machinery. Curr Opin Cell Biol. 2004;16:223–229. doi: 10.1016/j.ceb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 109.Yekta S, Shih IH, Bartel DP. MicroRNA-directed cleavage of HOXB8 mRNA. Science. 2004;304:594–596. doi: 10.1126/science.1097434. [DOI] [PubMed] [Google Scholar]
- 110.Kefas B, Comeau L, Floyd DH, et al. The neuronal microRNA miR-326 acts in a feedback loop with notch and has therapeutic potential against brain tumors. J Neurosci. 2009;29:15161–15168. doi: 10.1523/JNEUROSCI.4966-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Y, Guessous F, Zhang Y, et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69:7569–7576. doi: 10.1158/0008-5472.CAN-09-0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Song G, Zhang Y, Wang L. MicroRNA-206 targets notch3, activates apoptosis and inhibits tumor cell migration and focus formation. J Biol Chem. 2009;284:31921–31927. doi: 10.1074/jbc.M109.046862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang C, Yao N, Lu CL, et al. Mouse microRNA-124 regulates the expression of Hes1 in P19 cells. Front Biosci (Elite Ed) 2:127–132. doi: 10.2741/e74. [DOI] [PubMed] [Google Scholar]
- 114.Skog J, Wurdinger T, van Rijn S, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nat Cell Biol. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yuan A, Farber EL, Rapoport AL, et al. Transfer of microRNAs by embryonic stem cell microvesicles. PLoS One. 2009;4:e4722. doi: 10.1371/journal.pone.0004722. [DOI] [PMC free article] [PubMed] [Google Scholar]