Abstract
Introduction
Straightforward studies compared adeno-associated virus (AAV) serotypes to determine the most appropriate one for robust expression in the CNS. AAV9 was efficient when directly injected into the brain, but more surprisingly, AAV9 produced global expression in the brain and spinal cord after a peripheral, systemic route of administration to neonatal mice.
Areas covered
Topics include AAV9 gene delivery from intraparenchymal, intravenous, intrathecal and intrauterine routes of administration, and related preclinical studies and disease models. Systemic AAV9 gene transfer yields remarkably consistent neuronal expression, though only in early development. AAV9 is versatile to study neuropathological proteins: microtubule-associated protein tau and transactive response DNA-binding protein 43 kDa (TDP-43).
Expert opinion
AAV9 will be more widely used based on current data, although other natural serotypes and recombineered vectors may also support or improve upon wide-scale expression. A peripheral-to-central gene delivery that can affect the entire CNS without having to inject the CNS is promising for basic functional experiments, and potentially for gene therapy. Systemic or intra-cerebrospinal fluid routes of AAV9 administration should be considered for spinal muscular atrophy, lysosomal storage diseases and amyotrophic lateral sclerosis, if more neuronal expression can be achieved in adults, or if glial expression can be exploited.
Keywords: adeno-associated virus, amyotrophic lateral sclerosis, frontotemporal lobar degeneration, gene therapy, gene transfer, lysosomal storage disease, microtubule-associated protein tau, spinal cord, spinal muscular atrophy, TDP-43
1. Introduction
Somatic cell gene transfer via viral vectors is an indispensable tool in the neurosciences, particularly for whole animal studies. Adeno-associated virus (AAV) vectors are efficient for gene delivery to neurons and have, therefore, been used extensively in both basic and clinical studies, due to the efficient expression, and the relatively minor immune response [1–3]. Natural AAV is a single-stranded parvovirus with a genome of approximately 4.7 kb [4]. It is a dependovirus because it requires helper functions from other viruses to complete its life cycle. AAV infection is not associated with any known disease [4]. Most people have been exposed to wild-type AAV and are seropositive for AAV antibodies [4]. Recombinant AAV vectors only retain 4% of the viral genome, the inverted terminal repeats, express no viral genes and are thought to persist as nonintegrated episomes [1–3]. All of these properties contribute to why gene transfer using the recombinant vector is relatively well-tolerated in various target tissues in various species. The first characterizations of AAV gene transfer in the brain of rodents were by Kaplitt et al. [5] and McCown et al. [6], which demonstrated the potential for widespread, long-term and well-tolerated transgene expression. The efficiency improved due to a number of refinements in production/purification, promoter, AAV capsid serotype (subtype) and delivery methods [1]. Gene transfer in the CNS with AAV vectors has been extensively reviewed [1–3], while here we focus on recent results with the AAV9 serotype, which has become the vector of choice for many investigators.
2. Discovery of AAV9 and intraparenchymal brain injections
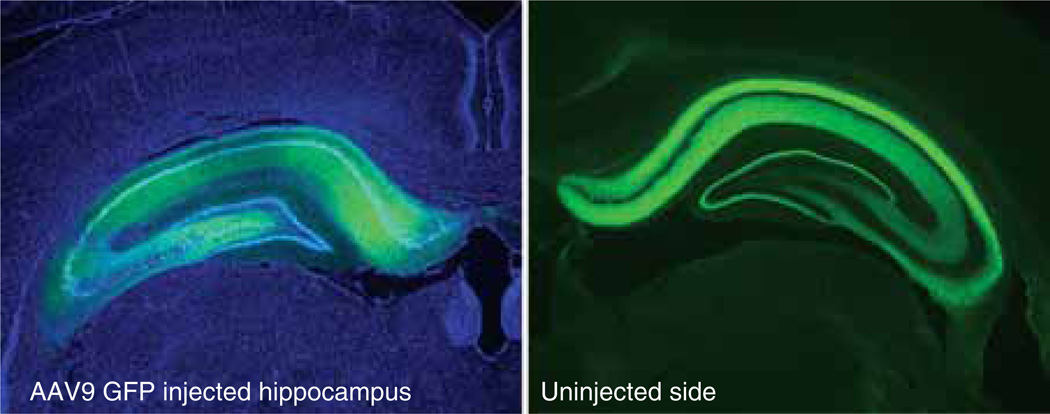
Families, or clades, of natural AAV subtypes were found in humans and nonhuman primates, and characterized, and harnessed into recombinant expression vectors by Gao et al. [7]. Most of the AAV capsid variants are about 85 – 90% homologous to each other [7], but different serotype expression patterns are observed in the rat brain [8,9]. Most notably, the early characterized AAV2 vector has a uniquely limited spread and expression pattern in brain. Cearley and Wolfe [10] compared several serotypes in the mouse brain for levels of transgene expression (beta-glucuronidase) and concluded that AAV9 was particularly efficient, producing greater expression than AAV7 or AAV8. These conclusions were corroborated in the rat brain, both with green fluorescent protein (GFP) reporter gene expression [11] and with the neurodegenerative disease-related protein, microtubule-associated protein tau [12]. Several tau AAV vectors were injected into the substantia nigra of rats, and tau levels were analyzed by western blots at 1, 2 and 4 weeks. AAV9 expression kinetics clearly peaked earlier than AAV2 or AAV8. The work with AAV9 in the brain up until 2008 described a highly efficient vector for neurons after intraparenchymal injections to the CNS. Intrahippocampal injection of AAV9 GFP is shown in Figure 1.
Figure 1. Intraparenchymal (intrahippocampal) injection of an adeno-associated virus 9 (AAV9) vector for green fluorescent protein (GFP) in the rat.
Left) Injected side with nuclear counterstain in blue. Right) Contralateral, uninjected side showing anterograde projections from the injected side. There are three points worth noting about the panel on the left: i) efficient gene transfer throughout the dorsal–ventral and medial–lateral extents of the hippocampus; ii) targeting, lack of GFP expression in the cortex above or thalamus below; and iii) lack of needle track damage in the overlying (injected) cortex. Investigators must consider all of these points in evaluating their injections. For example, the memory-related behavioral assays ran in Dayton et al. [67] after hippocampal injections of AAV9 would be difficult to interpret if there were expression in, or damage to, the cortex. Three-week expression interval. Images from Klein et al. [11].
3. Expansive CNS gene transfer via intravenous administration to neonatal mice and rats
The advent of AAV9 was more fully realized, however, by Foust et al. [13] and Duque et al. [14], injecting AAV9 vectors intravenously to neonatal and adult mice in [13], and neonatal and adult mice and cats in [14]. Based on earlier work by Foust et al. [15] with AAV8, it was surprising that AAV9 produced such robust expression throughout the CNS after a peripheral, intravenous administration [13]. The authors demonstrated greater neuronal transduction when neonatal subjects were injected intravenously compared with that in adult subjects, which showed predominantly astroglial versus neuronal transduction, using GFP expression in mice [13]. However, Duque et al. [14] did report neuronal expression in the CNS after intravenous AAV9 injections to adult mice, with an efficiency of up to 28% of spinal motor neurons transduced. These studies are a breakthrough because a large fraction of the entire brain and spinal cord can be affected without having to inject the CNS; global CNS gene transfer from a peripheral delivery is now possible. It is not fully understood why neuronal transduction is not as efficient in adult subjects, although astroglial differentiation and developmental changes in the blood–brain barrier have been hypothesized [13,16]. However, aberrant glial function has been described in a number of neurodegenerative diseases, so targeting expression to glia in adults could be relevant [17].
Wang et al. [18] adapted the intravenous method to neonatal rats with AAV9 GFP and delineated the transduced regions in the brain and spinal cord. The use of rats has potential advantages to mice for anatomical manipulations and specific behavioral tasks (e.g., food reward paradigms). There was efficient and consistent transduction of motor neurons throughout the entire spinal cord, with an estimated 78% transduction rate. In addition to motor neurons, outstanding expression occurs in dorsal root ganglion neurons, and cerebellar Purkinje neurons (Figure 2), similar to results in mice [13,14]. In contrast to the mice studies, Wang et al. used a single-stranded DNA AAV9 vector, which is far less efficient for gene transfer than self-complementary double-stranded AAV vectors first developed by McCarty et al. [19] and used in [13,14]. Nevertheless, the spinal motor neuron transduction rate of 78% is the highest ever reported to our knowledge. Self-complementary vectors are thus not required for efficient CNS expression from intravenous injections, although their greater efficiency is important for lower effective vector doses, and easing the amounts of vector needed in production. The disadvantage of self-complementary vectors is their smaller payload, and the single-stranded vectors in Wang et al. [18] could thus utilize a large cytomegalovirus/chicken beta-actin hybrid promoter (1.7 kb) to express a 43 kDa functional gene product, transactive response DNA-binding protein (TDP-43) on a wide-scale basis in the spinal cord and brain.
Figure 2. Intravenous injection of an adeno-associated virus 9 (AAV9) vector for green fluorescent protein (GFP) to a neonatal rat.
A) Biophotonic imaging of GFP throughout the entire spinal cord in a GFP rat, while an untreated rat is blank. Red indicates higher levels of GFP epifluorescence relative to blue. B) GFP expression in large neurons in the ventral horn of the lumbar spinal cord. C) Dorsal root ganglia. D) Cerebellum (with nuclear counterstain in blue). The most evident GFP-expressing cells in the cerebellum are Purkinje neurons. In contrast to focal, intraparenchymal brain injections, which transduce a circumscribed population of neurons, the intravenous AAV9 injections transduce neurons throughout the entire brain and spinal cord. 4 (B–D) or 12 (A) week expression intervals. Epifluorescence in A, C and immunofluorescence in B, D. A, B from Wang et al. [18].
Several studies in neonatal mice have confirmed the reproducibility of the intravenous method with either single- or double-stranded AAV9 [20–22]. Miyake et al. [20] demonstrated stable expression at an interval of 18 months, so the intravenous technique produces essentially permanent expression as with intraparenchymal brain injections [1]. Of note, the AAV10 serotype has also been shown to support widespread CNS gene transfer after systemic delivery to neonatal mice [20,22]. While intravenous AAV9 injections to neonatal subjects produce robust neuronal expression in the CNS, the injections also produce considerable expression in heart, liver and skeletal muscle [18,20,23,24], as expected based on earlier work administering AAV9 systemically to mice [25,26].
Hypothetically, gene therapy of a neurodegenerative disease would occur in an adult, in which widespread neuronal transduction would not be predicted from a systemic injection, though the glial transduction could have clinical relevance as mentioned [17]. Of greater currency, the neuronal transduction in neonates is a useful tool for functional studies and enhances the overall array of mammalian modeling systems.
4. Intravenous AAV9 injections to nonhuman primates
Consistent with mice and rats, efficient neuronal transduction was achieved after intravenous delivery of AAV9 to a neonatal rhesus macaque [23]. Several studies have also reported gene transfer to the CNS of monkeys at various ages after intravascular AAV9 administration, with differing degrees of neuronal versus glial transduction [24,27,28]. Consistent with mice [13], the CNS transduction was mainly in glial cells after intravascular administration to adult monkeys in [24,28]. As shown in AAV immunization studies in rats [29,30], the presence of anti-AAV neutralizing antibodies in monkeys greatly reduced transgene expression [27,28]. Neutralizing antibodies may be prevalent in humans [4] and thus be a barrier to human gene therapy. On the other hand, similar to intravenous gene transfer to neonatal rodents, and more readily applicable than gene therapy, modeling strategies will be enhanced by relevant nonhuman primate models, in which intravenous gene transfer to neonates supports efficient transduction of motor neurons [23,24]. Of practical concern, overall doses of greater than 1014 vector genomes of AAV9 were used in macaques in [23,24]. The large vector doses per kilogram that are needed for intravenous delivery to primates underscore the importance of strategies that would lower the effective dose, such as using self-complementary AAV9 and strong promoters.
5. Intrathecal/intra-cisterna magna routes of AAV9 administration (mice, pigs, monkeys)
Though more invasive than an intravenous injection, a route of administration into the central canal of the spinal cord could better target the cord and avoid expression in peripheral organs and could require lower vector doses that those used for the intravenous delivery. Snyder et al. [31] demonstrated motor neuron expression in mice after intrathecal administration of AAV9, which produced more widespread spinal expression than intraparenchymal injections as expected. Intrathecal AAV9 gene delivery to pigs was described in Bevan et al. [24] and Federici et al. [32]. Both studies described efficient transduction of spinal motor neurons. Interestingly, Federici et al. [32] determined injection parameters and doses to achieve expression throughout the spinal cord, and in both intrathecal pig studies, expression was successfully restricted to the CNS [24,32]. In monkeys, the data demonstrated more robust expression in the CNS after intra-cisterna magna versus intravenous administration [28]. The intra-cerebrospinal fluid strategies result in more targeted CNS expression, although as with intravenous injections to monkeys [27], the presence of antibodies against AAV nearly completely blocked the expression from intra-cisterna magna injections [28].
6. Intrauterine route of administration (mice, monkeys)
Gene delivery in utero could potentially be considered for gene therapy of diseases with a gene defect and perinatal morbidity/mortality. Similar to intravenous injections to neonates, intravenous AAV9 delivery to fetal mice and fetal macaques has also produced extensive neuronal expression throughout the CNS [21,33], which could have potential clinical relevance. The intrauterine route of administration is even more efficient to approach complete neuronal transduction in the CNS, because a shift toward more astroglial expression was observed with postnatal injections [21]. Again, while there could be clinical relevance, advantages of intrauterine gene transfer may be of greatest importance for basic functional and developmental studies, due to the potential for even greater efficiency of neuronal transduction relative to newborns.
7. Strategies to improve expression in CNS neurons and in clinically relevant adults
Choice of promoter is critical for efficient and sustained AAV gene transfer to neurons [1–3,34,35]. Historically, most studies of intraparenchymal AAV injections to the brain have reported selective neurotropism [1–3], in contrast to the intravenous studies with AAV9 using cytomegalovirus or cytomegalovirus/chicken beta-actin promoters [13,14]. Tissue-specific promoters could help restrict expression to neurons and to specific neuronal populations. For example, a promoter region for human neurofilament heavy chain was used in transgenic rats to drive selective expression in motor neurons [36]. This sequence is too large to package into an AAV vector, but further work could potentially minimize the sequence that confers motor neuron specificity. Alternatively, incorporation of tissue-specific endogenous microRNAs could repress expression in unwanted organs [37].
The requirement of fetuses or neonates to achieve widespread neuronal expression could be considered a caveat for gene therapy, because very young subjects may not be relevant for ageing-related diseases such as Alzheimer’s disease. Some of the intravenous AAV9 studies have shown some degree of neuronal expression in adult subjects [14,24,27]. One method that has been reported to enhance neuronal expression in adults is the use of mannitol to relax the blood–brain barrier to allow vector entry into the CNS [38], although overall, results with mannitol and systemic AAV9 are inconclusive [27,39]. Laboratory-generated vectors with empirically determined novel tropisms (directed evolution) may be necessary to improve neuronal transduction in adults, while novel recombineered vectors could also potentially evade immunosurveillance of natural AAV serotypes [17,30,40].
8. Preclinical gene therapy strategies for the brain and spinal cord using AAV9
Widespread brain and spinal gene transfer resulting from a peripheral injection is particularly important for therapeutics for two reasons: one, because a minimally invasive intervention avoids injecting the CNS; and two, because expression is achieved throughout the entire CNS, which may be necessary for a disease such as Alzheimer’s disease, which affects large portions of the brain, or diseases that affect large portions of the spinal cord. There are a number of spinal cord diseases that meet the criteria for considering gene therapy, that is, life-threatening disease without cure or ameliorative, and disease forms with inherited mutations. For example, there are genetic forms of spinal muscular atrophy (SMA) and amyotrophic lateral sclerosis (ALS), and there are no efficacious medications for these diseases involving the spinal cord [41–43]. Another reason that gene transfer is important is that a corrective protein may need to be administered on a long-term basis for a chronic disease, and a one-time gene transfer treatment can produce sustained expression. For example, repeated or chronic protein delivery to the CNS is difficult. A secretable protein factor, such as a neurotrophic factor, is particularly advantageous, as the spread of the recombinant growth factor or corrective enzyme (e.g., beta-glucuronidase for the lysosomal storage disease mucopolysaccharidosis VII [10]) could be potent over a region larger than the area of the transduced cells, thus lowering the number of gene copies needed to exert a widespread effect. Therapeutic gene delivery of a secretable factor would also bypass the need for neuronal transduction. Gene therapy paradigms in rodent models of spinal cord diseases using various AAV gene transfer modalities have been previously reviewed [44–46].
Owing to the efficient cardiac gene transfer of AAV9 [25], a number of studies have demonstrated positive effects of AAV9 vectors in models of heart disease, for example, Fechner et al. [47]. Likewise, the efficient expression in skeletal muscle from intravenous AAV9 has been exploited for preclinical gene therapy in a canine muscular dystrophy model [48]. Efforts with AAV9 in preclinical gene therapy paradigms for the CNS are outlined in Table 1. Intraparenchymal injections of specific AAV9 vectors have been used for protective effects in a Parkinson’s disease model [49]. Intraparenchymal and combined intracerebroventricular and intravenous routes of administrations of AAV9 vectors have been successful in mouse models of lysosomal storage diseases [39,50,51], which provide the rational basis for clinical development.
Table 1.
Preclinical gene transfer strategies in the central nervous system using the adeno-associated virus serotype 9 (AAV9) vector.
| Disease model | Subjects | Route | AAV9 vector | Therapeutic outcomes | Ref. |
|---|---|---|---|---|---|
| Parkinson’s disease | Adult | Intraparenchymal Striatum | EPO | Protection of dopamine neurons and motor function | [49] |
| Mucopolysaccharidosis VII | Adult | Intraparenchymal VTA | GUSB | Reduced lysosomal storage pathology | [50] |
| Mucopolysaccharidosis IIIB | Juvenile | IV | NAGLU | Reduced lysosomal storage pathology and neurodegeneration | [39] |
| Multiple sulfatase deficiency | Neonatal | IV and ICV | SUMF1 | Reduced inflammation and lysosomal storage pathology, behavioral correction, improved lifespan | [51] |
| Spinal muscular atrophy | Neonatal | IV | SMN | Improved motor and neuromuscular function, motor neuron survival, improved body weight and lifespan | [23,52–55] |
| Spinal muscular atrophy | Neonatal | ICV | SMN | Improved motor function and muscle size, improved body weight and lifespan | [55] |
| Visceral | Adult | IV | GLT-1 | Increased glutamate uptake, anti-nociception | [74] |
All studies were conducted in mice except [49], which used rats.
EPO: Erythropoietin; GLT-1: Glutamate transporter; GUSB: Beta-glucuronidase; ICV: Intracerebroventricular; IV: Intravenous; NAGLU: alpha-N-acetylglucosaminidase; SMN: Survival motor neuron; SUMF1: Sulfatase-modifying factor 1; VTA: Ventral tegmental area.
SMA is attributed to mutations in the gene called survival motor neuron, and survival motor neuron knockout mouse models are used to mimic the disease [41]. Because SMA is due to a single gene defect and may be a fatal disease (depending on disease subtype) without cure, it could be a good candidate disease for experimental strategies such as gene therapy. Interestingly, survival motor neuron gene delivery with intravenous AAV9 to the mouse model of SMA restores function and either improves or fully rescues their lifespan [23,52–55], which is promising for humans. Of note, an improved therapeutic outcome resulted from an intracerebroventricular versus intravenous route of AAV9 survival motor neuron gene delivery [55], suggesting that it is more beneficial to contain expression within the CNS in SMA models. However, expression of survival motor neuron from intravenous AAV9 administration has been shown to correct cardiac abnormalities of SMA mice [52]. More clinical development of AAV9-mediated gene therapy to replace survival motor neuron function in SMA is warranted.
ALS is another spinal cord disease without cure and with a number of specific genetic etiologies [43–46]. The transgenic rodent models of ALS based on mutations in the copper zinc superoxide dismutase one (SOD1), which occur in familial ALS, are very well characterized, faithful to the spinal neurodegeneration and paralysis in human ALS and thus widely used [56]. Gene transfer was effective in improving grip strength, motor function and the lifespan when peptidergic growth factors such as insulin-like growth factor one or vascular endothelial growth factor were expressed in mSOD1 mice with AAV, by injecting the vector into brain regions that innervate the spinal cord [57,58]. Gene knockdown strategies to reduce the expression of the pathogenic mutant SOD1 in mSOD1 mice with AAV have also improved their function by preserving their grip strength, for example, Miller et al. [59]. Successful studies will achieve widespread spinal expression from intravenous or intrathecal AAV9 in mSOD1 models, which will underscore the utility of AAV9, and explore therapeutic opportunities for ALS that were not possible before AAV9.
9. Expressing a neuropathological protein (AAV9 Tau and AAV9 TDP-43)
Viral vector expression of neuropathological proteins in the CNS is an alternative modeling system of specific neurodegenerative diseases compared with more standard methods of neurochemical lesioning or germ-line transgenic mice [1,60]. Vector-based methods are particularly useful to target expression of the most relevant brain regions and to avoid embryonic lethality. Modeling neurodegenerative diseases in rats with AAV9 is outlined in Table 2. As mentioned, Klein et al. [12] utilized the high gene transfer efficiency of AAV9 to express the microtubule-associated protein tau in the substantia nigra, which is relevant to diseases with tau neurofibrillary tangles in the brain, such as frontotemporal lobar degeneration (FTLD) with tau pathology [61–63]. AAV9 Tau led to more pronounced and near-complete striatal dopamine loss compared with AAV2 or AAV8 Tau vectors, thus graded, early- and late-stage disease models are possible via different gene transfer efficiencies [12]. Of note, an advantage of the somatic cell gene transfer approach is the ability to induce expression at any age to produce age-related models relevant to age-related neurodegenerative diseases [64]. In terms of tau neurofibrillary tangle pathology, earlier work confirmed the presence of straight tau filaments by electron microscopy, using an AAV2 Tau vector in the substantia nigra with the P301L disease-related tau mutation [1].
Table 2.
Neurodegenerative disease modeling using the adeno-associated virus serotype 9 (AAV9) vector.
| Disease model | Transgene | Subjects | Injection method | Relevant outcomes | Ref. |
|---|---|---|---|---|---|
| FTLD-Tau | Tau | Adult | Intraparenchymal substantia nigra | Neuronal loss, motor deficit | [12] |
| FTLD-Tau | Tau | Aged adult | Intraparenchymal substantia nigra | Neuronal loss, motor deficit Neuronal loss, motor deficit, microgliosis, age-related effects | [64] |
| FTLD-TDP | TDP-43 | Adult | Intraparenchymal substantia nigra | Neuronal loss, apoptosis, astrogliosis, microgliosis, motor deficit | [65] |
| FTLD-TDP | TDP-43 | Adult | Intraparenchymal hippocampus | Neuronal loss, microgliosis, memory-related deficit | [67] |
| ALS | TDP-43 | Adult | IV | Lower motor neuron loss, paralysis, muscle wasting, microgliosis | [18] |
Human wild-type forms of tau or TDP-43 were expressed in rats, except [12], which used the P301L form of tau related to a subtype of FTLD-Tau.
ALS: Amyotrophic lateral sclerosis; FTLD: Frontotemporal lobar degeneration; IV: Intravenous; TDP: Transactive response DNA-binding protein.
To mimic subtypes of the neurodegenerative disease FTLD-TDP with pathology in the nigrostriatal system [62,65], Tatom et al. [65] expressed TDP-43 in the substantia nigra of rats with AAV9 to develop an assay of relevant TDP-43 neuropathology and neurodegeneration. The human wild-type TDP-43 vector produced consistent, dose-dependent gliosis, dopaminergic neuron loss and motor deficits. There were only few examples of cells with disease-relevant cytoplasmic TDP-43 deposition using the wild-type TDP-43, which expressed mainly in the nucleus as expected [65]. TDP-43 is a major pathological protein in both FTLD-TDP and in ALS, with pathology found in the spinal cord and brain [62,63]. Both lower motor neurons in the spinal cord and upper motor neurons in the motor cortex degenerate during ALS progression. For these reasons, the AAV9 transduction patterns in Foust et al. [13] were intriguing because it appeared that the neuronal populations affected in ALS were efficiently transduced.
One concern about attempting to model a spinal cord disease state that would be consistent with germ-line transgenic rodent models was variability of the transduction produced by an intravenous gene delivery. However, extensive experience with the technique has confirmed consistent intravenous delivery, circulation to the brain and spinal cord, and gene transfer in the CNS. Relevant aspects of ALS rapidly manifested when wild-type TDP-43 was expressed, with severe muscle atrophy and limb paralysis, and moderate loss of spinal motor neurons. The intravenous AAV9 method was thus successful for modeling a spinal cord disease state comparable with germ-line TDP-43 transgenic mice and rats [44]. There was great reproducibility of the TDP-43 phenotype, which is due in part to the potent neurotoxicity of wild-type TDP-43 hyperexpression, but also to the highly consistent intravenous delivery technique. The relevant and consistent models are relatively cost-effective compared with developing and maintaining transgenic lines, which is important in light of new discoveries of genes associated to ALS [43], which could be assayed individually and in combination more rapidly by a somatic cell gene transfer approach than with transgenics. The approach will also be useful for gene structure–function studies in rodents, because designed gene variants can be assayed rapidly relative to making transgenic lines for each hypothesis.
In modeling neurodegenerative diseases, there are two potential philosophies: one, to express the neuropathological protein only in the neuronal populations affected in disease; and two, to ubiquitously express the protein and study selective vulnerability. The intravenous TDP-43 vector model from Wang et al. [18] expresses in peripheral tissues as well as in many brain regions, so changes in vector design or delivery would be needed to approach selective expression in motor neurons. However, unpublished studies by Klein et al. have shown that expression of TDP-43 in the spinal cord is necessary to induce ALS-relevant paralysis because when a different promoter was used to express TDP-43 (tet-off promoter, Haberman et al., [66]), which expresses in liver and heart but not the spinal cord, there was no observable disease state induced. Results with AAV9-expressing neuropathological proteins either with intraparenchymal [12,64,65,67] or intravenous [18] vector delivery have been impressively consistent, which support the use of AAV9 for versatile assays of neurodegenerative diseases. The intrauterine route of administration of AAV9 [21,33] could have utility for modeling, by further increasing neuronal transduction efficiency. AAV TDP-43 vectors can be used to generate nonhuman primate models of neurodegenerative diseases. For example, focal injections of an AAV1 TDP-43 vector to the cervical spinal cord of macaques has recently been reported, which produced ALS-relevant TDP-43 neuropathology in the cervical spinal cord and forelimb paresis [68]. It will be interesting to study intrathecal or intravenous delivery of AAV1 or AAV9 TDP-43 vectors to see whether ALS-like neuropathology and paralysis will be more widespread throughout the spinal cord and brain, which should be highly relevant to human and which we are attempting.
10. Expert opinion
There is a consensus that AAV9 is an efficient serotype for gene transfer in the brain, so more studies using AAV9 are expected. The widespread gene transfer derived from intravenous or intrathecal AAV9 has advanced potential applications of global CNS gene therapy. Further refinements will be necessary to achieve neuronal-specific expression in clinically relevant, symptomatic adult paradigms. It will be interesting to determine whether AAV9 has the unique attribute for wide-scale transduction after systemic or intra-cerebrospinal fluid routes of administration. When we injected AAV1 or AAV10 directly into the hippocampus or substantia nigra, they appeared to work as well as AAV9 [9,11,12], which is consistent with other studies [69–71]. In addition to AAV9, AAV10 yields efficient CNS gene transfer from intravenous injections to neonates [20,22].
Due to the widespread spinal and brain expression that is now possible, more preclinical work with AAV9 in animal models of ALS is expected, while clinical development of AAV9 gene therapy for fatal forms of SMA and lysosomal storage diseases should be pursued. Functional translation of preclinical gene therapy in rodents and dogs to primates and humans has been historically difficult [72], but we are hopeful that the widespread AAV9 expression can be harnessed for clinical utility of specific conditions. Recent successes in human gene therapy for hemophilia B and a form of blindness called Leber’s congenital amaurosis with AAV suggest there will be a growing number of specific indications that work in humans [73].
Nonetheless in consideration of potential caveats, if therapy was pursued in a young individual with a wide-scale approach, the efficient expression in the developing CNS could be dangerous in ways that we do fully not understand. The concept of gene doping is unnerving by any route of administration, and even more with a wide-scale approach involving the CNS during development. More practically speaking, developmental side effects in basic studies involving young subjects must be considered.
While human AAV9 gene therapy might be worth considering someday, AAV9 is an important vector for modeling neurodegenerative diseases in animals today. Disease models are facilitated with AAV9 due to its efficiency and versatility for either focal [12,64,65,67] or widespread [18] expression, as well as expression in different species such as nonhuman primates. The rapid and cost-effective models are consistent and should expedite gene variant structure–function comparisons and gene combinations that would take longer and cost more in transgenicmice, which is an important point considering the growing list of genes mutated in neurodegenerative diseases, and the specific pathological isoforms and comorbidities that are found. The ultimate goal in this regard is an improved model with a mechanism that is more relevant to human disease because a more faithful mechanism could enable discovery and development of an efficacious small molecule that would otherwise be missed in models with irrelevant mechanisms.
Article highlights.
AAV9 is an efficient natural adeno-associated virus (AAV) serotype for transgene expression in neurons.
Surprisingly, the AAV9 serotype produced wide-scale neuronal transduction in the CNS from a peripheral, systemic injection in neonatal subjects.
AAV9 has been used in a number of gene therapy and neurodegenerative disease modeling paradigms, by several routes of administration.
There are remaining caveats to overcome in approaching human gene therapy with AAV9. On the other hand, the remarkably consistent and efficient gene transfer with AAV9 has enhanced basic research on neurodegenerative diseases.
This box summarizes key points contained in the article.
Acknowledgements
We are grateful to G Gao, JM Wilson and colleagues at the University of Pennsylvania Gene Therapy Center for providing the materials to make recombinant AAV9. J-C Paterna, D Knight, R Zweig, M Kiaei, Y Keflemariam and I Elnagar critiqued the manuscript.
Declaration of interest
The authors have received funding from the NIH (Grant NS048450) and the Michael J. Fox Foundation.
Bibliography
Papers of special note have been highlighted as either of interest (•) or of considerable interest (••) to readers.
- 1.Klein RL, Wang DB, King MA. Versatile somatic gene transfer for modeling neurodegenerative diseases. Neurotox Res. 2009;16:329–342. doi: 10.1007/s12640-009-9080-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McCown TJ. Adeno-associated virus (AAV) vectors in the CNS. Curr Gene Ther. 2011;11:181–188. doi: 10.2174/156652311795684759. [DOI] [PubMed] [Google Scholar]
- 3.Lentz TB, Gray SJ, Samulski RJ. Viral vectors for gene delivery to the central nervous system. Neurobiol Dis. 2011 doi: 10.1016/j.nbd.2011.09.014. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berns KI, Linden RM. The cryptic life style of adeno-associated virus. Bioessays. 1995;17:237–245. doi: 10.1002/bies.950170310. [DOI] [PubMed] [Google Scholar]
- 5.Kaplitt MG, Leone P, Samulski RJ, et al. Long-term gene expression and phenotypic correction using adeno-associated virus vectors in the mammalian brain. Nat Genet. 1994;8:148–154. doi: 10.1038/ng1094-148. [DOI] [PubMed] [Google Scholar]
- 6.McCown TJ, Xiao X, Li J, et al. Differential and persistent expression patterns of CNS gene transfer by an adeno-associated virus (AAV) vector. Brain Res. 1996;713:99–107. doi: 10.1016/0006-8993(95)01488-8. [DOI] [PubMed] [Google Scholar]
- 7. Gao G, Vandenberghe LH, Alvira MR, et al. Clades of Adeno-associated viruses are widely disseminated in human tissues. J Virol. 2004;78:6381–6388. doi: 10.1128/JVI.78.12.6381-6388.2004. •• Cloning of AAV9 and other natural serotypes.
- 8.Burger C, Gorbatyuk OS, Velardo MJ, et al. Recombinant AAV viral vectors pseudotyped with viral capsids from serotypes 1, 2, and 5 display differential efficiency and cell tropism after delivery to different regions of the central nervous system. Mol Ther. 2004;10:302–317. doi: 10.1016/j.ymthe.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 9.Klein RL, Dayton RD, Leidenheimer NL, et al. Efficient neuronal gene transfer with AAV8 leads to neurotoxic levels of tau or green fluorescent protein. Mol Ther. 2006;13:517–527. doi: 10.1016/j.ymthe.2005.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cearley CN, Wolfe JH. Transduction characteristics of adeno-associated virus vectors expressing cap serotypes 7, 8, 9, and Rh10 in the mouse brain. Mol Ther. 2006;13:528–537. doi: 10.1016/j.ymthe.2005.11.015. • Efficient AAV9 gene transfer in the brain.
- 11.Klein RL, Dayton RD, Tatom JB, et al. AAV8,9, Rh10, Rh43 vector gene transfer in the rat brain: effects of serotype, promoter and purification method. Mol Ther. 2008;16:89–96. doi: 10.1038/sj.mt.6300331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Klein RL, Dayton RD, Tatom JB, et al. Tau expression levels from various adeno-associated virus vector serotypes produce graded neurodegenerative disease states. Eur J Neurosci. 2008;27:1615–1625. doi: 10.1111/j.1460-9568.2008.06161.x. • Enhanced neuronal transduction of AAV9 demonstrated functionally, in an in vivo assay of dopaminergic neurodegeneration.
- 13. Foust KD, Nurre E, Montgomery CL, et al. Intravascular AAV9 preferentially targets neonatal neurons and adult astrocytes. Nat Biotechnol. 2009;27:59–65. doi: 10.1038/nbt.1515. • • Peripheral administration of AAV9 produced widespread transgene expression in the CNS.
- 14. Duque S, Joussemet B, Riviere C, et al. Intravenous administration of self-complementary AAV9 enables transgene delivery to adult motor neurons. Mol Ther. 2009;17:1187–1196. doi: 10.1038/mt.2009.71. • • Neuronal gene transfer in the CNS of neonatal and adult mice and cats after peripheral administration.
- 15.Foust KD, Poirier A, Pacak CA, et al. Neonatal intraperitoneal or intravenous injections of recombinant adeno-associated virus type 8 transduce dorsal root ganglia and lower motor neurons. Hum Genet Ther. 2008;19:61–70. doi: 10.1089/hum.2007.093. [DOI] [PubMed] [Google Scholar]
- 16.Saunders NR, Joakim Ek C, Dziegielewska KM. The neonatal blood-brain barrier is functionally effective, and immaturity does not explain differential targeting of AAV9. Nat Biotechnol. 2009;27:804–805. doi: 10.1038/nbt0909-804. [DOI] [PubMed] [Google Scholar]
- 17.Koerber JT, Klimczak R, Jang JH, et al. Molecular evolution of adeno-associated virus for enhanced glial gene delivery. Mol Ther. 2009;17(12):2088–2095. doi: 10.1038/mt.2009.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang DB, Dayton RD, Henning PP, et al. Expansive gene transfer in the rat CNS rapidly produces amyotrophic lateral sclerosis relevant sequelae when TDP-43 is overexpressed. Mol Ther. 2010;18:2064–2074. doi: 10.1038/mt.2010.191. • Intravenous AAV9 delivery yielded a highly consistent rat model of ALS.
- 19.McCarty DM, Monahan PE, Samulski RJ. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001;8:1248–1254. doi: 10.1038/sj.gt.3301514. [DOI] [PubMed] [Google Scholar]
- 20.Miyake N, Miyake K, Yamamoto M, et al. Global gene transfer into the CNS across the BBB after neonatal systemic delivery of single-stranded AAV vectors. Brain Res. 2011;1389:19–26. doi: 10.1016/j.brainres.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 21.Rahim AA, Wong AMS, Hoefer K, et al. Intravenous administration of AAV2/9 to the fetal and neonatal mouse leads to differential targeting of CNS cell types and extensive transduction of the nervous system. FASEB J. 2011;25:3505–3518. doi: 10.1096/fj.11-182311. [DOI] [PubMed] [Google Scholar]
- 22.Zhang H, Yang B, Mu X, et al. Several rAAV vectors efficiently cross the blood-brain barrier and transduce neurons and astrocytes in the neonatal mouse central nervous system. Mol Ther. 2011;19:1440–1448. doi: 10.1038/mt.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Foust KD, Wang X, McGovern VL, et al. Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol. 2010;28:271–274. doi: 10.1038/nbt.1610. • The SMN gene transfer results in this paper and ones like it support gene therapy clinical trials for spinal muscular atrophy.
- 24.Bevan AK, Duque S, Foust KD, et al. Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther. 2011;19:1971–1980. doi: 10.1038/mt.2011.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inagaki K, Fuess S, Storm TA, et al. Robust systemic transduction with AAV9 vectors in mice: efficient global cardiac gene transfer superior to that of AAV8. Mol Ther. 2006;14:45–53. doi: 10.1016/j.ymthe.2006.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zincarelli C, Soltys S, Rengo G, Rabinowitz JE. Analysis of AAV serotypes 1–9 mediated gene expression and tropism in mice after systemic injection. Mol Ther. 2008;16:1073–1080. doi: 10.1038/mt.2008.76. [DOI] [PubMed] [Google Scholar]
- 27.Gray SJ, Matagne V, Bachaboina L, et al. Preclinical differences of intravascular AAV9 delivery to neurons and glia: a comparative study of adult mice and nonhuman primates. Mol Ther. 2011;19:1058–1069. doi: 10.1038/mt.2011.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samaranch L, Salegio EA, San Sebastian W, et al. AAV9 transduction in the central nervous system of non-human primates. Hum Gene Ther. 2011 doi: 10.1089/hum.2011.200. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanftner LM, Suzuki BM, Doroudchi MM, et al. Striatal delivery of rAAV-hAADC to rats with preexisting immunity to AAV. Mol Ther. 2004;9:403–409. doi: 10.1016/j.ymthe.2003.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Peden CS, Burger C, Muzyczka N, Mandel RJ. Circulating anti-wild-type adeno-associated virus type 2 (AAV2) antibodies inhibit recombinant AAV2 (rAAV2)-mediated, but not rAAV5-mediated, gene transfer in the brain. J Virol. 2004;78:6344–6359. doi: 10.1128/JVI.78.12.6344-6359.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Snyder BR, Gray SJ, Quach ET, et al. Comparison of adeno-associated viral vector serotypes for spinal cord and motor neuron gene delivery. Hum Genet Ther. 2011;22:1129–1135. doi: 10.1089/hum.2011.008. [DOI] [PubMed] [Google Scholar]
- 32.Federici T, Taub JS, Baum GR, et al. Robust spinal motor neuron transduction following intrathecal delivery of AAV9 in pigs. Gene Ther. 2011 doi: 10.1038/gt.2011.130. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 33.Mattar CN, Waddington SN, Biswas A, et al. Systemic delivery of scAAV9 in fetal macaques facilitates neuronal transduction of the central and peripheral nervous systems. Gene Ther. 2012 doi: 10.1038/gt.2011.216. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Xu R, Janson CG, Mastakov M, et al. Quantitative comparison of expression with adeno-associated virus (AAV-2) brain-specific gene cassettes. Gene Ther. 2001;8:1323–1332. doi: 10.1038/sj.gt.3301529. [DOI] [PubMed] [Google Scholar]
- 35.Gray SJ, Foti SB, Schwartz JW, et al. Optimizing promoters for recombinant adeno-associated virus-mediated gene expression in the peripheral and central nervous system using self-complementary vectors. Hum Genet Ther. 2011;22:1143–1153. doi: 10.1089/hum.2010.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang C, Tong J, Bi F, et al. Mutant TDP-43 in motor neurons promotes the onset and progression of ALS in rats. J Clin Invest. 2012;122:107–118. doi: 10.1172/JCI59130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xie J, Xie Q, Zhang H, et al. MicroRNA-regulated, systemically delivered rAAV9: a step closer to CNS-restricted transgene expression. Mol Ther. 2011;19:526–535. doi: 10.1038/mt.2010.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCarty DM, DiRosario J, Gulaid K, et al. Mannitol-facilitated CNS entry of rAAV2 vector significantly delayed the neurological disease progression in MPS IIIB mice. Gene Ther. 2009;16:1340–1352. doi: 10.1038/gt.2009.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fu H, Dirosario J, Killedar S, et al. Correction of neurological disease of mucopolysaccharidosis IIIB in adult mice by rAAV9 trans-blood-brain barrier gene delivery. Mol Ther. 2011;19:1025–1033. doi: 10.1038/mt.2011.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gray SJ, Blake BL, Criswell HE, et al. Directed evolution of a novel adeno-associated virus (AAV) vector that crosses the seizure-compromised blood-brain barrier (BBB) Mol Ther. 2010;18:570–578. doi: 10.1038/mt.2009.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Passini MA, Bu J, Roskelley EM, et al. CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J Clin Invest. 2010;120:1253–1264. doi: 10.1172/JCI41615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boillee S, Vande Velde C, Cleveland DW. ALS: a disease of motor neurons and their nonneuronal neighbors. Neuron. 2006;52(1):39–59. doi: 10.1016/j.neuron.2006.09.018. Review. [DOI] [PubMed] [Google Scholar]
- 43.Fecto F, Siddique T. Making connections: pathology and genetics link amyotrophic lateral sclerosis with frontotemporal lobe dementia. J Mol Neurosci. 2011;45:663–675. doi: 10.1007/s12031-011-9637-9. [DOI] [PubMed] [Google Scholar]
- 44.Wang DB, Gitcho MA, Kraemer BC, et al. Genetic strategies to study TDP-43 in rodents and to develop preclinical therapeutics for amyotrophic lateral sclerosis. Eur J Neurosci. 2011;34:1179–1188. doi: 10.1111/j.1460-9568.2011.07803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nizzardo M, Simone C, Falcone M, et al. Research advances in gene therapy approaches for the treatment of amyotrophic lateral sclerosis. Cell Mol Life Sci. 2011 doi: 10.1007/s00018-011-0881-5. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Federici T, Boulis NM. Gene therapy for amyotrophic lateral sclerosis. Neurobiol Dis. 2011 Aug 25; doi: 10.1016/j.nbd.2011.08.018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 47.Fechner H, Sipo I, Westermann D, et al. Cardiac-targeted RNA interference mediated by an AAV9 vector improves cardiac function in coxsackievirus B3 cardiomyopathy. J Mol Med (Berl) 2008;86:987–997. doi: 10.1007/s00109-008-0363-x. [DOI] [PubMed] [Google Scholar]
- 48.Kornegay JN, Li J, Bogan JR, et al. Widespread muscle expression of an AAV9 human mini-dystrophin vector after intravenous injection in neonatal dystrophin-deficient dogs. Mol Ther. 2010;18:1501–1508. doi: 10.1038/mt.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xue YQ, Ma BF, Zhao LR, et al. AAV9-mediated erythropoietin gene delivery into the brain protects nigral dopaminergic neurons in a rat model of Parkinson’s disease. Gene Ther. 2010;17:83–94. doi: 10.1038/gt.2009.113. [DOI] [PubMed] [Google Scholar]
- 50.Cearley CN, Wolfe JH. A single injection of an adeno-associated virus vector into nuclei with divergent connections results in widespread vector distribution in the brain and global correction of a neurogenetic disease. J Neurosci. 2007;27:9928–9940. doi: 10.1523/JNEUROSCI.2185-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Spampanato C, De Leonibus E, Dama P, et al. Efficacy of a combined intracerebral and systemic gene delivery approach for the treatment of a severe lysosomal storage disorder. Mol Ther. 2011;19:860–869. doi: 10.1038/mt.2010.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bevan AK, Hutchinson KR, Foust KD, et al. Early heart failure in the SMNDelta7 model of spinal muscular atrophy and correction by postnatal scAAV9-SMN delivery. Hum Mol Genet. 2010;19:3895–3905. doi: 10.1093/hmg/ddq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Valori CF, Ning K, Wyles M, et al. Systemic delivery of scAAV9 expressing SMN prolongs survival in a model of spinal muscular atrophy. Sci Transl Med. 2010;2:35ra42. doi: 10.1126/scitranslmed.3000830. [DOI] [PubMed] [Google Scholar]
- 54.Dominguez E, Marais T, Chatauret N, et al. Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum Mol Genet. 2011;20:681–693. doi: 10.1093/hmg/ddq514. [DOI] [PubMed] [Google Scholar]
- 55.Glascock JJ, Shababi M, Wetz MJ, et al. Direct central nervous system delivery provides enhanced protection following vector mediated gene replacement in a severe model of spinal muscular atrophy. Biochem Biophys Res Commun. 2012;417:376–381. doi: 10.1016/j.bbrc.2011.11.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harvey BK, Richie CT, Hoffer BJ, et al. Transgenic animal models of neurodegeneration based on human genetic studies. J Neural Transm. 2011;118:27–45. doi: 10.1007/s00702-010-0476-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dodge JC, Haidet AM, Yang W, et al. Delivery of AAV-IGF-1 to the CNS extends survival in ALS mice through modification of aberrant glial cell activity. Mol Ther. 2008;16:1056–1064. doi: 10.1038/mt.2008.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dodge JC, Treleaven CM, Fidler JA, et al. AAV4-mediated expression of IGF-1 and VEGF within cellular components of the ventricular system improves survival outcome in familial ALS mice. Mol Ther. 2010;18:2075–2084. doi: 10.1038/mt.2010.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miller TM, Kaspar BK, Kops GJ, et al. Virus-delivered small RNA silencing sustains strength in amyotrophic lateral sclerosis. Ann Neurol. 2005;57:773–776. doi: 10.1002/ana.20453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Decressac M, Mattsson B, Bjorklund A. Comparison of the behavioural and histological characteristics of the 6-OHDA and alpha-synuclein rat models of Parkinson’s disease. Exp Neurol. 2012 doi: 10.1016/j.expneurol.2012.02.012. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 61.Kumar-Singh S, Van Broeckhoven C. Frontotemporal lobar degeneration: current concepts in the light of recent advances. Brain Pathol. 2007;17:104–114. doi: 10.1111/j.1750-3639.2007.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geser F, Lee VM, Trojanowski JQ. Amyotrophic lateral sclerosis and frontotemporal lobar degeneration: a spectrum of TDP-43 proteinopathies. Neuropathology. 2010;30:103–112. doi: 10.1111/j.1440-1789.2009.01091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Neumann M, Sampathu DM, Kwong LK, et al. Ubiquitinated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Science. 2006;314:130–133. doi: 10.1126/science.1134108. •• Cytoplasmic TDP-43 lesions are prevalent in neurodegenerative diseases.
- 64.Klein RL, Dayton RD, Diaczynsky CG, Wang DB. Pronounced microgliosis and neurodegeneration in aged rats after tau gene transfer. Neurobiol Aging. 2010;31:2091–2102. doi: 10.1016/j.neurobiolaging.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tatom JB, Wang DB, Dayton RD, et al. Mimicking aspects of frontotemporal lobar degeneration and Lou Gehrig’s disease in rats via TDP-43 overexpression. Mol Ther. 2009;17:607–613. doi: 10.1038/mt.2009.3. • Intra-substantia nigra injections of AAV9 yielded an early rodent model of TDP-43 diseases.
- 66.Haberman RP, McCown TJ, Samulski RJ. Inducible long-term gene expression in brain with adeno-associated virus gene transfer. Gene Ther. 1998;5:1604–1611. doi: 10.1038/sj.gt.3300782. [DOI] [PubMed] [Google Scholar]
- 67.Dayton RD, Wang DB, Cain CD, et al. Frontotemporal lobar degeneration-related proteins induce only subtle memory-related deficits when bilaterally overexpressed in the dorsal hippocampus. Exp Neurol. 2012;233:807–814. doi: 10.1016/j.expneurol.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Uchida A, Sasaguri H, Kimura N, et al. Non-human primate model of amyotrophic lateral sclerosis with cytoplasmic mislocalization of TDP-4. Brain. 2012 doi: 10.1093/brain/awr348. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang C, Wang CM, Clark KR, Sferra TJ. Recombinant AAV serotype 1 transduction efficiency and tropism in the murine brain. Gene Ther. 2003;10:1528–1534. doi: 10.1038/sj.gt.3302011. [DOI] [PubMed] [Google Scholar]
- 70.Sondhi D, Hackett NR, Peterson DA, et al. Enhanced survival of the LINCL mouse following CLN2 gene transfer using the rh. 10 rhesus macaque-derived adeno-associated virus vector. Mol Ther. 2007;15:481–491. doi: 10.1038/sj.mt.6300049. [DOI] [PubMed] [Google Scholar]
- 71.White E, Bienemann A, Sena-Esteves M, et al. Evaluation and optimization of the administration of recombinant adeno-associated viral vectors (serotypes 2/1, 2/2, 2/rh8, 2/9, and 2/rh10) by convection-enhanced delivery to the striatum. Hum Gene Ther. 2011;22:237–251. doi: 10.1089/hum.2010.129. [DOI] [PubMed] [Google Scholar]
- 72.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 73.Wilson JM. It’s time for gene therapy to get disruptive! Hum Gene Ther. 2012;23:1–3. doi: 10.1089/hum.2011.2530. [DOI] [PubMed] [Google Scholar]
- 74.Lin Y, Roman K, Foust KD, et al. Glutamate transporter GLT-1 upregulation attenuates visceral nociception and hyperalgesia via spinal mechanisms not related to anti-inflammatory or probiotic effects. Pain Res Treat. 2011 doi: 10.1155/2011/507029. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]