Abstract
Functional MRI has been used to investigate the responsiveness of the maternal rat brain to pup-suckling under various experimental paradigms. Our research employing the lactating rat model has explored the cortical sensory processing of pup stimuli and the effect of suckling on the brain’s reward system. Suckling was observed to increase blood-oxygen-level-dependent (BOLD) signal intensity in the midbrain, striatum and prefrontal cortex, which are areas that receive prominent dopaminergic inputs. The BOLD activation of the reward system occurs in parallel with the activation of extensive cortical sensory areas. The observed regions include the olfactory cortex, auditory cortex and gustatory cortex and could correspond to cortical representations of pup odors, vocalizations and taste that are active during lactation. Activation patterns within reward regions are consistent with past research on maternal motivation and we explore the possibility that exposure to drugs of abuse might be disruptive of maternal neural responses to pups, particularly in the prefrontal cortex. Our ongoing fMRI studies support and extend past research on the maternal rat brain and its functional neurocircuitry.
Introduction
Research on the neural circuitry of lactation can provide significant insight on the mechanisms contributing to the maternal-offspring bond. Lactation is a key event during early postnatal development and its disruption in the mother can influence offspring behavior. For several years our laboratory has used MRI technology to study the maternal rat brain. Functional magnetic resonance imaging provides a unique window into brain activity during lactation. A series of experiments in awake lactating rats have focused on the neural processing of the natural suckling stimulus from pups (1–4). Brain activity in response to suckling simulation has been investigated by our group in relation to its reinforcing properties and it’s processing across cortical sensory regions. Most of the past research on the brain regions directly driven by suckling inputs has been guided by traditional neurophysiological and histological methods, as well as lesion experiments (5–11). Electrophysiological recordings taken from neurons in the somatosensory cortex of the anesthetized postpartum rat indicate that the receptive field of the ventrum skin surrounding the nipple area is doubled in size during the lactation period (9). Cortical plasticity may therefore generate greater levels of neuronal activity in the maternal brain during gestational and lactational epochs, which could potentially be studied using fMRI techniques in rodents. Studies using c-fos immunohistochemical assays, which provide cellular-level spatial resolution (12–15), are limited in the temporal window of brain activity and can miss transient stimulus-coupled neural responses. Contrary to lesion studies, functional MRI is non-invasive and allows longitudinal and developmental studies. The goals of the present mini-review are to: 1) present fMRI methods in rats as an important means for examining the neural circuitry of lactation and maternal care; 2) briefly discuss past research aimed at investigating cortical and subcortical processing of the suckling stimulus with a focus on the role of maternal brain reward regions that are also affected by drug exposure; and 3) provide potential directions on the use of fMRI and other methods to study maternal care and the processing of social stimuli in the rodent brain.
Functional MRI of Lactating Rats
Experiments using magnetic resonance phenomena as a surrogate measure of neuronal activity is increasingly used in rodents in pharmacological experiments, in studies of cortical sensory processing and in combination with optogenetic methods to corroborate the specificity of the BOLD signal (16–20). One of the major implementations of animal fMRI research has been in the study of the dynamics of the T2* weighted signal (blood oxygen level dependent or BOLD signal), thereby supporting the study of brain function with the technique. For instance, Afonso and Koretsky (2002) showed detailed layer-specific BOLD activation in the anesthetized rat somatosensory cortex at field strength as high as 11.7Tesla (21). Another study using an anesthetized preparation showed that the BOLD and cerebral blood flow weighted signals in the cat visual cortex are localized to grey matter regions, supporting the selectivity of the method to measure stimulus-coupled sensory processing (22). There have been very few awake animal imaging studies. Sachdev et al (2003) have used fMRI to study the whisker barrel cortex of the awake rat while Martin et al (23) investigated neurovascular coupling of awake animals in comparison to varying anesthetic levels. Goense et al (24) studied the dynamics of the BOLD signal in the visual cortex of awake Rhesus monkeys and correlated it with local field potentials. An important recent advance has been the use of fMRI in combination with optogenetic methods to study the relationship between sensory cortical BOLD signal responses and light-induced neuronal activity in the same region of the awake mouse (20). Stein et al. (25) and Marota et al (16) have employed the methods in anesthetized rodents to assess changes in BOLD signal following administration of drugs such as opiates and cocaine. Similar experiments using awake rat methods have provided additional information regarding stimulant induced brain activity (26). These and a growing number of other experiments have provided valuable information on brain function and strongly support and validate the judicious use of fMRI methods in studies of brain and behavior. In many of the aforementioned experimental settings rats are imaged under the effects of general anesthetics and therefore preclude some forms of neural processing that would occur only in the awake state (23). Methods developed by Ferris and colleagues have allowed fMRI studies in awake restrained rats (27–29). Experiments that employ the awake rat methods require extensive acclimation to restraint and MR sound prior to experiments and we have been able to successfully implement these procedures in our studies.
Methodological considerations using fMRI
A strength that fMRI has over other in vivo methods is its submillimeter spatial coverage combined with its ability to use a hemodynamic signal to map out neural activity across multiple brain areas with reasonable temporal resolution (22). The BOLD signal arises from changes in tissue oxy-to-deoxyhemoglobin ratio and, as such, it is primarily a hemodynamic signal restricted by the biophysical and biological properties of the local neurovasculature (30–33). Microvascular magnetic field gradients that are sensitive to changes in paramagnetic deoxy- and diamagnetic oxy-hemoglobin concentrations near brain areas of altered neuronal metabolism are a likely source of the detected fMRI signal (34). Changes in neuronal activity and the accompanying compensatory adjustments in blood flow, in blood volume and in the cerebral consumption rates for oxygen thus give rise to the BOLD signal changes. Providing a sensory stimulus to an awake, restraint-acclimatized rat inside the MRI scanner has allowed us to track sensory modality-specific changes in the BOLD signal (Fig. 1). It is noted in figure 1 that there is selective increases in BOLD bilaterally in the somatosensory cortical region representing the ventrum trunk area of the unanesthetized rat. A statistically significant increase in signal intensity in voxels localized within the somatosensory cortex can be observed at the time of stimulus onset (n = 7 used in the composite map). The results of such a study are straightforward since activity in the cortical area is anticipated in response to the somatic stimulus provided. However, one can also observe increased BOLD bilaterally in areas adjacent to the foci of activity and in thalamic regions (Fig. 1). This type of result is pervasive in awake studies. Namely, there are findings that provide a more intricate view of the brain’s endogenous response than in anesthetized preparations. There are significant lines of evidence suggesting that agents typically used for anesthetizing animals can suppress certain forms of neuronal activity and modify specific patterns of neuronal activity and metabolism. For instance, stimulation of the whisker-to-barrel cortex pathway resulted in differential cerebral blood flow changes and brain glucose utilization responses across different brain regions that were dependent on whether or not rats were anesthetized with halothane (35). The results of the latter study suggest that although the barrel cortex is active in the anesthetized state, other regions along the pathway arising from the stimulation of peripheral sensory receptors are suppressed and may thus require a conscious state (35). Firing of action potentials over localized regions of the awake rat visual cortex showed higher frequencies and bursting but lower pair-wised correlations between single units than ketamine-anesthetized rats (36). This latter study suggests that the propagation of actions potentials over localized networks is modified by the induction of an anesthetized state. Therefore, the studies discussed herein are of awake and restraint-acclimatized rats.
Fig. 1.
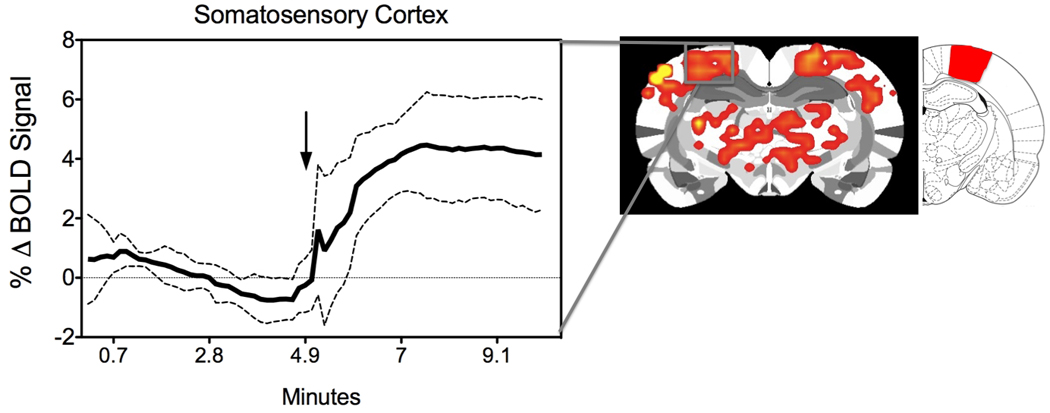
BOLD signal response in the ventrum trunk representation of the somatosensory cortex of awake rats. Seven unanesthetized virgin rats received mechanical rubbing stimulation on the ventrum skin for 5 minutes during a 10-minute fMRI scan (4). Shown is a composite brain image highlighting positive somatosensory BOLD activation. To the right of the brain map is a rat atlas map highlighting the ventrum trunk area. The timecourse to the left shows the temporal evolution of the BOLD signal (mean ± standard deviation). Arrow indicates stimulus onset.
In the case of the mammillae, primary afferent fibers terminate in the ipsilateral dorsal root ganglia between spinal segments C5 and L6 (44), with afferent relays along the lateral cervical nucleus, the dorsal column nuclei and the sensory and spinal portions of the trigeminal complex (5, 11). Fig. 2 (bottom) shows three dimensional composite maps of suckling stimulated BOLD activity in lactating rats compared to artificial suction on the mammillae, and general rubbing on the ventrum skin in lactating and virgin rats (4). We hypothesized that the sensory cortical field dedicated to the representation of the ventrum skin is expanded and this would be observed as an increased BOLD signal response to suckling in comparison to rubbing on the ventrum and suction on the mammillae. A corollary of the experimental design was the discernment of sensory cortical regions selectively representing the mammillae, which was not previously identified using electrophysiological methods (9). The physical conditions of the pup-suckling stimulus (suction strength, frequency, number of nipples stimulated) are important determinants for the milk ejection reflex in the rat (6, 7). Therefore, rather than presenting suction stimulation artificially, studies have been carried out with pups presented inside the magnet underneath the ventrum of the restrained dams in order to mimic the natural stimulus conditions (Fig. 2 top image)(1). Dams are imaged between postnatal days 4 – 8, which correspond to the epoch of high pup value for the rat dams (37). Animals are temporarily anesthetized with 2–4 % isoflurane, secured in the holder and allowed to awaken. The hind limbs of the dam are loosely tethered upward. This provides a visual inspection of her ventrum from outside the magnet and prevents the dam from kicking and injuring the pups. A padded plastic weigh boat containing four to six pups is positioned under the dam in the magnet. A thin plastic film separated the pups from the dam’s ventrum. When the shield is pulled away the pups are exposed to the six hind-limb nipples.
Fig. 2.
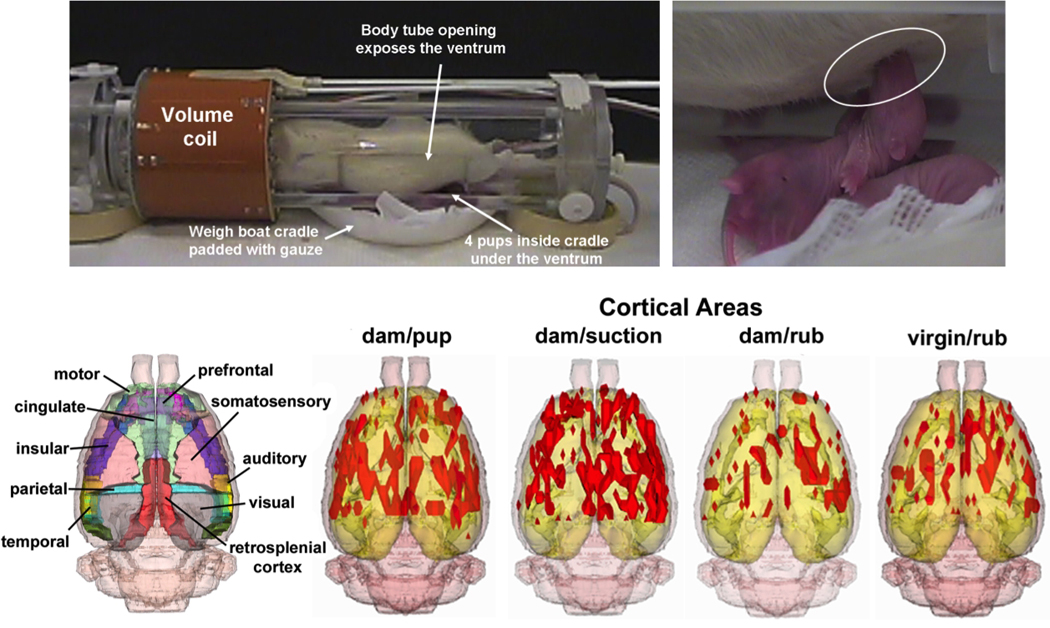
Functional MRI of lactation. Image shows the radiofrequency coil electronics and rodent restrainers adapted for lactation studies. Three-dimensional maps at the bottom show the pattern of cortical BOLD responses to suckling in comparison to general somatosensory stimulation in the trunk region or selective suction on nipples (4).
Despite its advantages, it is important to point out several concerns of fMRI in awake rats. First, the types of neural activity that can be investigated using fMRI are limited (38, 39). While in vivo extracellular recording methods permit the quantitative assessment of excitatory/inhibitory neuronal firing (single unit rates, field potential and multiunit activity), this type of detailed examination of the neural code is not achievable by fMRI studies alone (40). Second, rats are restrained for fMRI experiments thereby precluding behavioral tests during fMRI scanning. Both of these issues impose an upper limit in our ability to interpret brain function and its relation to maternal behavior using fMRI. Another important concern is that awake animals are restrained across multiple acclimation and imaging sessions. This is necessary since anesthesia influences both neuronal activity and the BOLD signal (41, 42). Prior to imaging studies, animals are acclimated to the restraint and MR noise (29). Further work is needed to resolve the permanent effects, if any, on hypothalamic-pituitary-adrenal axis function and the animal’s behavioral state under these conditions. With these concerns in mind we have designed studies to investigate the maternal rodents neural response to pups in the absence of anesthesia during scanning sessions.
Cortical Sensory Processing of the Suckling Stimulus
Female rats increase the amount of licking of the ventrum skin surface during the latter days of pregnancy. This contributes to mammillary tissue development and increases in the size of the ventrum region (43). The increased size of the cortical receptive field representing the ventrum trunk is further expanded by the high degree of sensory stimulation in this area of the body during the lactational period (9). Somatosensory information coming from the landscape of peripheral receptors (i.e. sensory receptors underlying body skin surface) is relayed to the cortex through the spinothalamic pathway and is topographically represented in the cortex. During the course of the experiment it was noted instead that dams showed a robust activation in the olfactory cortex, and other sensory cortical regions, when pups suckled. Suckling increased activity not only in the cortical map for the rat ventrum, but also in auditory, olfactory and gustatory cortices. Rather than showing selectivity in the neural responses, the data indicate that neural activity is modified over wide areas of the cerebral cortex in response to a specific stimulus delivered through one sensory modality. In order to control for the variety of sensory stimuli in addition to the suckling that are provided by pups a group of dams received selective artificially applied suction on the nipple regions and a similar widespread pattern of cortical activity was noted (4).
Somatosensory stimulation on the ventrum skin and snout is important to maintain the levels of maternal care and aggression in the rat (45–47). Therefore, the widespread activity across multiple areas of the cortex might influence maternal behavior in rats through indirect interactions with subcortical areas involved in maternal care. One area that could be indirectly influenced by somatosensory stimulation is the periaqueductal grey (PAG)(48). Lesions of the PAG interfere with kyphotic nursing postures and might be associated with altered maternal aggression (49). Infusion of the GABAA antagonist bicuculline locally in the PAG prolongs the duration of nursing behaviors while infusion of an agonist results in reduces nursing duration(48). This effect appears specific for the ventrolateral PAG. Removal of perioral sensory stimulation alters licking and retrieval behaviors but not nursing(13). Only behaviors involved in nursing but not active maternal behaviors lead to maximal expression of c-fos in regions of the PAG (13). Li et al (10) used brain c-fos immunostaining to examine the specific cellular circuitry involved in lactation in relation to responses to suckling pups as here (10). Among their findings it was noted that there was extensive c-fos activation with suckling across cortical areas responsive to sensory stimuli. These included the somatosensory, auditory and olfactory cortices, subcortical areas that are part of the sensory pathways and they also observed c-fos immunolabelling in the PAG and other regions involved in arousal and attention such as reticular formation (10). They noted that the integration of sensory modalities could occur through the RF. It is possible that our data using in vivo fMRI support their findings using ex vivo cellular imaging. An important link therefore exists between cortical sensory processing and lactation-related behavioral output through specific midbrain regions that may be of importance for our fMRI findings.
With the pattern of cortical BOLD activation in the absence of the pups one might construe that one sensory modality can activate other areas that perhaps ‘replay’ the sensory event of lactation, which includes sounds, odors and touch from pups in addition to frequent bouts of licking from the mother. In other words, ‘memory’ of the lactation event might be accessed by the suckling stimulus even in the absence of pups. Maternal memory has been studied in previous studies and has been found to be very robust (50, 51). Dams allowed a few hours of contact with newborn pups, immediately after parturition, retain their maternal behavior 10 days later, even if the pups are removed from them in the interim (50, 51). One possibility is that during the early postpartum period, the suckling stimulus has been strongly ‘conditioned’ to several sensory modalities (those neurons or groups of neurons that fire together, strongly wire together during lactation). The mechanism by which such a conditioning mechanism occurs in the somatosensory cortex and other adjacent cortical regions has been investigated to some extent in postpartum dams (9, 52).
Oxytocin is an important chemical substrate involved in the neural actions of suckling. Oxytocin’s role in suckling-stimulated BOLD signal changes was investigated in a separate fMRI study (1). During lactation, OT is released from the neurohypophysis to stimulate milk let down by exertion of pro-contractile actions in smooth muscle cells. In addition to the peripheral actions through it’s receptors, there is widespread distribution of OT receptors in the brain of male and female rats (53, 54). OT neurotransmission has been implicated in range of behavioral functions including social memory, the onset of maternal behavior and nursing (55–57). Separation of dams from continual exposure to pups reduces OT perikarya in a number of hypothalamic nuclei (58). This could have important implications for the actions of somatosensory stimulation on OT levels and maternal care in the rat. OT modulation of maternal behaviors such as retrieval and crouching are mediated through the medial POA and ventral tegmental area (VTA) (55). This latter finding suggests that stimuli eliciting the release of OT in the central nervous system of the mother could interact with the reward system through the VTA dopamine system. The mechanism of action of OT during lactation may be more global as it is released in many key limbic areas including the amygdala and septum (59, 60). Moreover, there is important evidence indicating that OT increased hippocampal long-term potentiation and can enhance reference but not working memory (61). The specific role of OT in modulating lactation-related neuronal activity was studied using fMRI following the procedures described above. It was observed that suckling-induced OT release into central neural sites increases the BOLD signal in many areas of the postpartum rat brain. This was observed in PVN, olfactory tubercle, anterior olfactory nucleus, insular cortex, piriform cortex, cortical amygdala, medial preoptic area, VTA and prefrontal cortex. In these regions, suckling and ICV oxytocin administration increased the BOLD signal. However, the magnitude of the BOLD signal changes were much more widespread with suckling than with OT administration (1). Therefore, stimulation of the ventrum and nipples in dams might evoke the release of neurotransmitters other than OT, one of these being the mesolimbic neurotransmitter dopamine (DA) (62).
Activation Of Reward Regions with Suckling Stimulation and Cocaine
Sensory stimuli from pups can be reinforcing in early postpartum dams, perhaps more so than other natural rewards. Rat dams work incessantly to care for their offspring, with continuous displays of retrieval behavior, nest building, pup grooming, arched back nursing and aggression towards nest intruders. Operant conditioning tests show that dams will bar press to obtain pups, a behavior commonly observed with other natural rewards, such as with food, water and sex, and during drug self administration (63). Suckling pups appear to produce the necessary stimuli for the reinforcement of high levels of maternal behaviors through activation of brain reward regions (2, 64, 65). The reward system is comprised of DA neurons that are located in the VTA, which send axon projections to various forebrain limbic sites, among which are the nucleus accumbens (NAC) and medial prefrontal cortex (mPFC). Of these, accumbens DA might take part in maternal motivation (62) while DA in the mPFC still needs study. The role of the VTA in maternal responsiveness and maternal motivation, which has been elegantly investigated (66), appears to be functionally intertwined with the medial preoptic area (mPOA), the bed nucleus of stria terminalis and ventral pallidum through direct synaptic connectivity (67). The core circuitry for reward and motivation in lactating rats is modulated by incoming sensory inputs that are driven by pup stimuli and by hormonal mechanisms mediated through the mPOA (67). Cortical regions that are activated with the presentation of suckling pups might also exert top-down effects through the medial PFC regions that include the anterior cingulate, prelimbic, infralimbic and orbital cortices (Fig. 4). These neocortical areas receive inputs from all areas of the cortex and also share associational inputs and their integrated outputs in layer 5 are sent to major projections sites (68) such as the dorsal striatum and ventral striatum that could play a key part in many behaviors that can impact the expression of maternal behavior. There is evidence using the retrograde tracer wheat-germ agglutinin that one of the output regions that receive a moderate input from layer 5 neurons of the prelimbic cortex is the PAG (69), which controls the arched-back nursing posture and aggression (49). On the other hand, anterograde tracer data using Phaseolus vulgaris-leucoagglutinin shows that the infralimbic region projects to preoptic area (70) that controls active components of maternal care (67). Therefore, neurons in various subareas of the medial PFC may take part in higher functions that control the levels of maternal care and this should be an important area of future work.
Fig. 4.
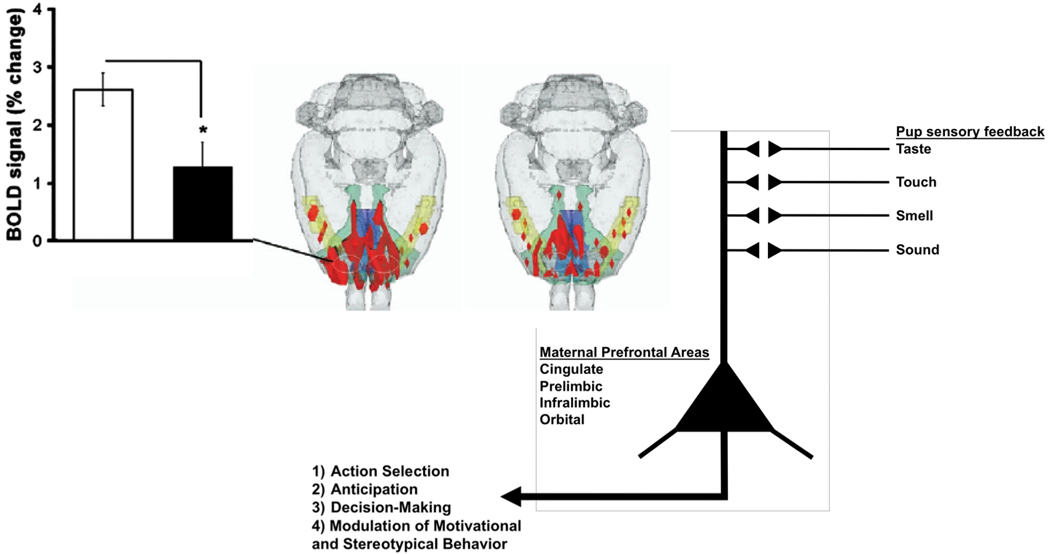
Changes in prefrontal cortical BOLD signal changes with pregestational cocaine sensitization. Lactating rats showing the lower prefrontal BOLD signal response to suckling were cocaine sensitized (black bar and 3D map on the right). A pictorial representation of the role of the medial prefrontal cortical regions in maternal responses and maternal behavior is shown to the lower right, below maps. The image is not intended to represent the synaptic physiology of the cortex, but instead to represent the integration of various sensory stimuli from pups in the cortex and the behavioral outcomes mediated through the prefrontal cortex.
Morrell and colleagues (37, 71) have shown that early postpartum rat dams prefer pups to cocaine. The preference for pups shifts over the course of the postpartum period with a gain of preference for cocaine a few days before the weaning age of pups. Using functional magnetic resonance imaging (fMRI), Ferris and co-workers provided a potential neurophysiological basis for this preference for pups over cocaine (2). They showed that suckling stimulation from pups increased the blood-oxygen-level-dependent (BOLD) signal in the striatum, prefrontal cortex and midbrain (2). The fMRI study in lactating dams provided a direct association between suckling stimuli and ‘neural activity’ in regions of the reward system, particularly forebrain regions that receive midbrain DA inputs. Suckling was found to activate these areas (increased BOLD signal intensity) while very little BOLD signal decreases were observed (Fig. 3). The balance of positive over negative BOLD signal changes was taken to represent increased neuronal activity, perhaps through the release of DA and it’s after effects on neuronal activity in the VTA and its projections sites. The notion that the observed increased in BOLD within the reward system might correspond to DA is consistent with observations of increased extracellular concentrations of DA in the maternal accumbens when dams are presented with pups (72). Correlations between the levels of maternal licking of pups and increased accumbal voltammetric DA signal have also been reported in lactating rats (62). Blocking the effects of dopamine by infusing the antagonist cis-flupenthixol was observed to reduce retrieval behavior in dams and increase the time spent lactating (65). However, this same effect of DA blockade is not observed in the dorsomedial striatum (65). We observed increases in BOLD signal in both the ventral and dorsal striatum with suckling stimulation and this seems at odds with a selective role for the ventral striatum in maternal responding (2). One possible explanation for this could be that incoming inputs from the cortex and not ascending inputs from the midbrain substantia nigra DA system underlie the increases in BOLD. Recent work from our laboratory employing more specific measurements of neuronal activity are beginning to identify cell populations within the dorsomedial striatum that increase firing rates during the retrieval of pups (Fig. 5; Febo, unpublished observations). More work is underway to examine the neuronal populations that underlie maternal behavior in the rat.
Fig. 3.
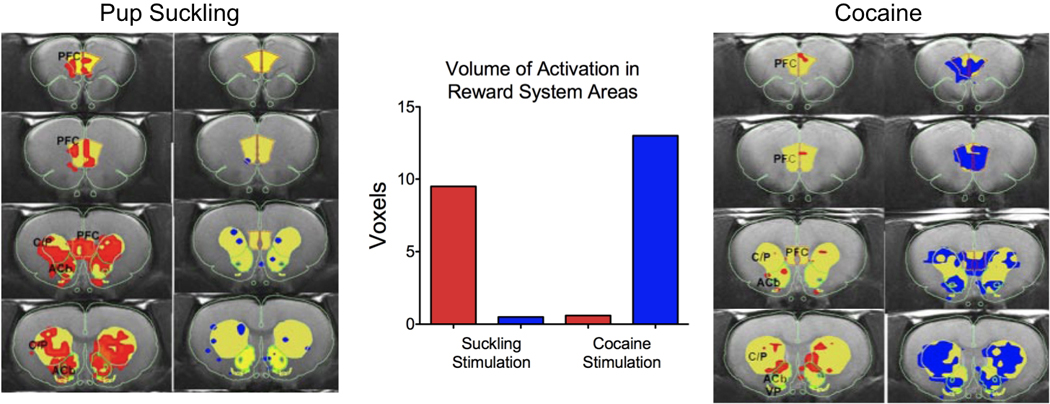
Activation of areas of the reward system of lactating rats. Comparison of the BOLD signal changes in the reward system in response to suckling pups and cocaine.
Fig. 5.
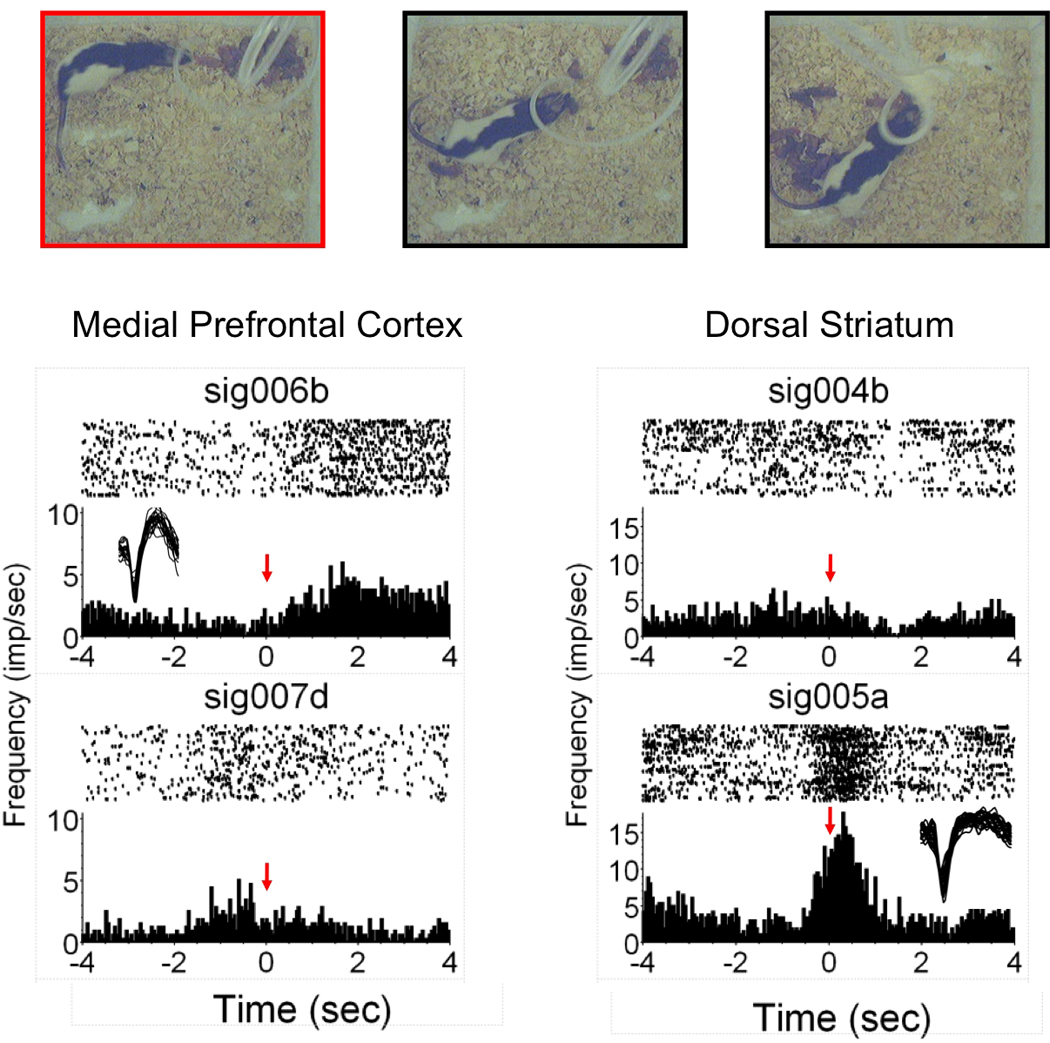
Identification of putative neurons that actively fire during maternal retrieval. Images overlying graphs show a sequence of digital video frames of retrieval behavior. The frame outlined with red indicates that it was used to generate timestamps for the analysis (as in ref. 91). Shown are data from the medial prefrontal cortex and the dorsal striatum in separate groups of animals implanted with Teflon-coated stainless steel microwire arrays. Note that unit prefrontal sig006b shows an increase in firing immediately following initiation of retrieval, whereas sig005a in the lower right shows heighted responses during the initiation of retrieval. Insets show example waveform (amplitudes 60–100 microvolts).
Dams were also given an intracerebroventricular dose of cocaine (20 µg/ 10 uL) to determine whether activation by cocaine supersedes activity observed with pup suckling. The cocaine dose had been previously demonstrated to increased BOLD signal in regions of the reward system in male and female rats (18, 73, 74). While pup suckling largely increased the BOLD signal, this was not observed with cocaine. Only large decreases in the BOLD signal were observed with cocaine (Fig. 3). The pattern of brain activation with suckling versus cocaine is consistent with behavioral data showing a preference of pups over cocaine during early lactation days (37). Suckling, a natural reinforcer in dams, produced robust increases in BOLD (possibly high rewarding effect) while cocaine, an artificial but strong reinforcer produced a robust BOLD decrease (possibly low rewarding effect). The imaging results may have importance for the expression of maternal behaviors following treatment with cocaine. Acute cocaine has disruptive effects on ongoing maternal behavior (75), however, at 24 hrs no differences are observable (76), thus supporting direct effects of cocaine on maternal behavior. Chronic gestational and postpartum cocaine has been shown to disrupt maternal retrieval and crouching (77, 78). Vernotica et al. (79) showed that cocaine dose-dependently interferes with the expression of maternal retrieval, nest building, crouching and aggressiveness towards nest intruders for up to 4 hours after treatment but not at 24 hours after the last injection. In sum, our fMRI work showing increases in BOLD in the mesolimbic and mesocortical systems is consistent with past research on the role of DA in these areas in maternal motivation. DA plays a key role in maternal care and motivation and disrupting normal DA function in mesolimbic and cortical regions may lead to alterations in maternal behavior that can impact offspring development.
Cocaine-induced changes in the physiology and neurochemistry of neurons of the reward pathway might affect the propensity for relapse to drug abuse and also affect the expression of maternal behavior. The propensity for relapse is of great concern in drug abstinent mothers who are caring for newborns (80) and further research is needed to understand the long-term effects of repeated cocaine administration on the expression of maternal behaviors. It is important to mention that maternal care is also dramatically affected by ongoing drug intake, either during the gestation and postpartum period. Gestational administration of cocaine can alter OT levels in the mPOA and amygdala and therefore affect the onset of maternal care at parturition (81). Further, our imaging studies indicate that pregestationally cocaine-sensitized female rats that are tested for maternal behaviors after an extended hiatus from drug treatment show increased retrieval behavior along with a blunted medial PFC response to pup suckling (3, 82). The results indicate that cocaine pre-exposed females displayed shorter latency to obtain pups than control mothers, in a manner suggestive of increased sensitization to pups. The putative sensitized response to pups was associated with a curtailed prefrontal cortical BOLD response to suckling, with no changes in mesolimbic or olfactory pathways (Fig. 4). The observed behavioral and physiological measures were altered even after such an extended period of withdrawal from cocaine (ca. >30 days) and with the development of sensitization. This suggests that enduring changes in prefrontal cortical DA may have occurred after repeated cocaine administration and that this brain region that is implicated in sensitization and drug seeking behavior might also play a role in maternal behavior. These latter findings also have important implications for the role of the mPFC regions in maternal behavior, particularly retrieval which could be taken to partly reflect the dams motivational state. A similar finding was reported recently by Nephew and Febo (2010) in Long Evans rats and previously in the CD-1 mouse (83). CD-1 dams given cocaine injections as virgins and then withdrawn from further treatment during gestation showed higher postpartum pup nosing and nest building. This was observed primarily in dams that were also conditioned to the test cage environment where cocaine was given (83).
Inhibition, inactivation or lesions of the medial division of the prefrontal cortex (anterior cingulate, prelimbic and infralimbic areas) can impact the quantity and quality of maternal behavior (84–86). The mPFC showed increases in BOLD signal throughout all of the neuroimaging experiments designed to study suckling in the rat. Indeed, the mPFC may play a role in maternal care that deserves further study. The role of the mPFC in maternal behavior is also supported by previous work showing changes in multiunit activity and electroencephalographic patterns in dams attending pups within the home cage environment (87, 88). The increase BOLD signal in this area of the cortex might represent an increased neuronal activity associated with the rewarding properties of pups and suckling stimulation (2), and more importantly, also associated with modulatory inputs from the cortex on maternal behavior structures (86). Neurons within the mPFC also show variations in firing rates during behavioral sequences involved in drug self-administration (89) and other goal-directed behaviors (90, 91). A role of the medial PFC subregions is still in need of further study since most of the field has focused on subcortical circuitry necessary for the expression of maternal behaviors in rats. The cortical role might be to instruct these subcortical networks and provide modulatory inputs (Fig.4). Given the complexity of the prefrontal regions in a host of higher order functions, establishing a specific role to the expression of motor and inactive components of maternal care is not simple. In primates the cortex clearly plays a role in maternal care and this has been substantiated across neuroimaging studies (92–97). Moreover, recent imaging work comparing responses in first time mothers to own vs unknown infant faces shows a significant effect in the substantia nigra and putamen nucleus (96). This effect is modulated by infant affective state. A recent neuroimaging study provides further support for the right prefrontal cortex in mothers viewing infant emotion faces (97). Importantly it was found that PFC responses were selective for maternal attachment, as increases hemodynamic signals were not observed when viewing adult emotion faces (97). Fig. 5 shows preliminary results from an ongoing experiment employing in vivo extracellular recordings to establish a link between the behavioral responses of dams and alterations of firing rates of units that were detected and classified in the medial PFC and dorsal striatum using previously published methods (91). The preliminary findings appear to support a role for these areas in maternal behavior. Maternal rats are carefully examined for maternal retrieval behavior to generate timestamps used in the neural analysis. The video frame showing the initial contact with pups preceding pick up and movement is used. Increases are observed in both mPFC units and dorsal striatal units. However, at least in these two cases there are increases with different dynamics, in the PFC there is sustained firing after contact and the dorsal striatum there is an immediate and transient increase (Fig. 5). It will be important in future work to determine the sensory cues that are provided by pups that can drive these regions of the brain to initiate maternal behaviors. We have already begun experiments using specific neurochemicals that can provide social signals (98). Rodents rely to a large extent on odors such as urine, pheromones and sounds within the ultrasonic range, and these are especially important for the maternal brain. Moving beyond the lactation studies, we have employed in vivo distal presentation of pups in our imaging work using a stimulus presentation paradigm similar to Ferris et al (99). This model has allowed the investigation of neural circuits in the maternal brain that respond to a threat to pups (an intruder male) (100). The results of this study were strikingly consistent with the activation of non-maternal, but emotionally relevant brain regions (100).
Our most recent experiments presented at the 2010 Parental Brain Conference are directed at understanding more deeply the role of drug-associated cues in relapse in the maternal rat. We know that cues can be strong motivators of drug seeking behavior, however, we also know that the early postpartum dams are more likely to prefer pups to cocaine (37, 63). Our unpublished data indicate that the preference for pups appears to hold strong even after the development of conditioned place preference for cocaine. Presentation of a drug-associated cue to dams inside the fMRI activates the prefrontal cortex strongly, however, the presence of pups significantly ameliorates activity within this area (100, 101). These and other fMRI studies will help explore maternal brain circuitry further, in both normal and disease models.
Acknowledgements
Research support received from NIH grant DA019946.
References
- 1.Febo M, Numan M, Ferris CF. Functional magnetic resonance imaging shows oxytocin activates brain regions associated with mother-pup bonding during suckling. J Neurosci. 2005;25(50):11637–11644. doi: 10.1523/JNEUROSCI.3604-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferris CF, Kulkarni P, Sullivan JM, Jr, Harder JA, Messenger TL, Febo M. Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci. 2005;25(1):149–156. doi: 10.1523/JNEUROSCI.3156-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Febo M, Ferris CF. Development Of Cocaine Sensitization Before Pregnancy Affects Subsequent Maternal Retrieval Of Pups And Prefrontal Cortical Activity During Nursing. Neuroscience. 2007 doi: 10.1016/j.neuroscience.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Febo M, Stolberg TL, Numan M, Bridges RS, Kulkarni P, Ferris CF. Nursing stimulation is more than tactile sensation: It is a multisensory experience. Horm Behav. 2008;54(2):330–339. doi: 10.1016/j.yhbeh.2008.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dubois-Dauphin M, Armstrong WE, Tribollet E, Dreifuss JJ. Somatosensory systems and the milk-ejection reflex in the rat. II. The effects of lesions in the ventroposterior thalamic complex, dorsal columns and lateral cervical nucleus-dorsolateral funiculus. Neuroscience. 1985;15(4):1131–1140. doi: 10.1016/0306-4522(85)90257-x. [DOI] [PubMed] [Google Scholar]
- 6.Sutherland RC, Juss TS, Wakerley JB. Prolonged electrical stimulation of the nipples evokes intermittent milk ejection in the anaesthetised lactating rat. Exp Brain Res. 1987;66(1):29–34. doi: 10.1007/BF00236198. [DOI] [PubMed] [Google Scholar]
- 7.Karyakin MG, Alekseev NP. The influence of tactile stimulation of nipples on the milk ejection reflex in the rat. J Comp Physiol A. 1992;170(5):645–650. doi: 10.1007/BF00199340. [DOI] [PubMed] [Google Scholar]
- 8.Lambert RC, Moos FC, Ingram CD, Wakerley JB, Kremarik P, Guerne Y, Richard P. Electrical activity of neurons in the ventrolateral septum and bed nuclei of the stria terminalis in suckled rats: statistical analysis gives evidence for sensitivity to oxytocin and for relation to the milk-ejection reflex. Neuroscience. 1993;54(2):361–376. doi: 10.1016/0306-4522(93)90258-h. [DOI] [PubMed] [Google Scholar]
- 9.Xerri C, Stern JM, Merzenich MM. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. J Neurosci. 1994:141710–141721. doi: 10.1523/JNEUROSCI.14-03-01710.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li C, Chen P, Smith MS. Neural populations in the rat forebrain and brainstem activated by the suckling stimulus as demonstrated by cFos expression. Neuroscience. 1999;94(1):117–129. doi: 10.1016/s0306-4522(99)00236-5. [DOI] [PubMed] [Google Scholar]
- 11.Stern JM, Yu YL, Crockett DP. Dorsolateral columns of the spinal cord are necessary for both suckling-induced neuroendocrine reflexes and the kyphotic nursing posture in lactating rats. Brain Res. 2002;947(1):110–121. doi: 10.1016/s0006-8993(02)02916-5. [DOI] [PubMed] [Google Scholar]
- 12.Walsh CJ, Fleming AS, Lee A, Magnusson JE. The effects of olfactory and somatosensory desensitization on Fos-like immunoreactivity in the brains of pup-exposed postpartum rats. Behav Neurosci. 1996;110(1):134–153. doi: 10.1037//0735-7044.110.1.134. [DOI] [PubMed] [Google Scholar]
- 13.Lonstein JS, Stern JM. Somatosensory contributions to c-fos activation within the caudal periaqueductal gray of lactating rats: effects of perioral, rooting, and suckling stimuli from pups. Horm Behav. 1997;32(3):155–166. doi: 10.1006/hbeh.1997.1416. [DOI] [PubMed] [Google Scholar]
- 14.Lonstein JS, Simmons DA, Swann JM, Stern JM. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience. 1998;82(1):267–281. doi: 10.1016/s0306-4522(97)00283-2. [DOI] [PubMed] [Google Scholar]
- 15.Lin SH, Miyata S, Matsunaga W, Kawarabayashi T, Nakashima T, Kiyohara T. Metabolic mapping of the brain in pregnant, parturient and lactating rats using fos immunohistochemistry. Brain Res. 1998;787(2):226–236. doi: 10.1016/s0006-8993(97)01484-4. [DOI] [PubMed] [Google Scholar]
- 16.Marota JJ, Mandeville JB, Weisskoff RM, Moskowitz MA, Rosen BR, Kosofsky BE. Cocaine activation discriminates dopaminergic projections by temporal response: an fMRI study in Rat. Neuroimage. 2000;11(1):13–23. doi: 10.1006/nimg.1999.0520. [DOI] [PubMed] [Google Scholar]
- 17.Mandeville JB, Jenkins BG, Kosofsky BE, Moskowitz MA, Rosen BR, Marota JJ. Regional sensitivity and coupling of BOLD and CBV changes during stimulation of rat brain. Magn Reson Med. 2001;45(3):443–447. doi: 10.1002/1522-2594(200103)45:3<443::aid-mrm1058>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 18.Febo M, Segarra AC, Tenney JR, Brevard ME, Duong TQ, Ferris CF. Imaging cocaine-induced changes in the mesocorticolimbic dopaminergic system of conscious rats. J Neurosci Methods. 2004;139(2):167–176. doi: 10.1016/j.jneumeth.2004.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lu H, Xi ZX, Gitajn L, Rea W, Yang Y, Stein EA. Cocaine-induced brain activation detected by dynamic manganese-enhanced magnetic resonance imaging (MEMRI) Proc Natl Acad Sci U S A. 2007;104(7):2489–2494. doi: 10.1073/pnas.0606983104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Desai M, Kahn I, Knoblich U, Bernstein J, Atallah H, Yang A, Kopell N, Buckner RL, Graybiel AM, Moore CI, Boyden ES. Mapping Brain Networks in Awake Mice Using Combined Optical Neural Control and fMRI. J Neurophysiol. doi: 10.1152/jn.00828.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Silva AC, Koretsky AP. Laminar specificity of functional MRI onset times during somatosensory stimulation in rat. Proc Natl Acad Sci U S A. 2002;99(23):15182–15187. doi: 10.1073/pnas.222561899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim DS, Duong TQ, Kim SG. High-resolution mapping of iso-orientation columns by fMRI. Nat Neurosci. 2000;3(2):164–169. doi: 10.1038/72109. [DOI] [PubMed] [Google Scholar]
- 23.Martin C, Martindale J, Berwick J, Mayhew J. Investigating neural-hemodynamic coupling and the hemodynamic response function in the awake rat. Neuroimage. 2006;32(1):33–48. doi: 10.1016/j.neuroimage.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 24.Goense JB, Logothetis NK. Neurophysiology of the BOLD fMRI signal in awake monkeys. Curr Biol. 2008;18(9):631–640. doi: 10.1016/j.cub.2008.03.054. [DOI] [PubMed] [Google Scholar]
- 25.Xi ZX, Wu G, Stein EA, Li SJ. Opiate tolerance by heroin self-administration: an fMRI study in rat. Magn Reson Med. 2004;52(1):108–114. doi: 10.1002/mrm.20119. [DOI] [PubMed] [Google Scholar]
- 26.Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF. The Neural Consequences of Repeated Cocaine Exposure Revealed by Functional MRI in Awake Rats. Neuropsychopharmacology. 2004 doi: 10.1038/sj.npp.1300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lahti KM, Ferris CF, Li F, Sotak CH, King JA. Imaging brain activity in conscious animals using functional MRI. J Neurosci Methods. 1998;82(1):75–83. doi: 10.1016/s0165-0270(98)00037-5. [DOI] [PubMed] [Google Scholar]
- 28.Ludwig R, Bodgdanov G, King J, Allard A, Ferris CF. A dual RF resonator system for high-field functional magnetic resonance imaging of small animals. J Neurosci Methods. 2004;132(2):125–135. doi: 10.1016/j.jneumeth.2003.08.017. [DOI] [PubMed] [Google Scholar]
- 29.King JA, Garelick TS, Brevard ME, Chen W, Messenger TL, Duong TQ, Ferris CF. Procedure for minimizing stress for fMRI studies in conscious rats. J Neurosci Methods. 2005;148(2):154–160. doi: 10.1016/j.jneumeth.2005.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tian P, Teng IC, May LD, Kurz R, Lu K, Scadeng M, Hillman EM, De Crespigny AJ, D'Arceuil HE, Mandeville JB, Marota JJ, Rosen BR, Liu TT, Boas DA, Buxton RB, Dale AM, Devor A. Cortical depth-specific microvascular dilation underlies laminar differences in blood oxygenation level-dependent functional MRI signal. Proc Natl Acad Sci U S A. 107(34):15246–15251. doi: 10.1073/pnas.1006735107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sheth SA, Nemoto M, Guiou M, Walker M, Pouratian N, Toga AW. Linear and nonlinear relationships between neuronal activity, oxygen metabolism, and hemodynamic responses. Neuron. 2004;42(2):347–355. doi: 10.1016/s0896-6273(04)00221-1. [DOI] [PubMed] [Google Scholar]
- 32.Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat Neurosci. 2006;9(2):260–267. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- 33.Devor A, Tian P, Nishimura N, Teng IC, Hillman EM, Narayanan SN, Ulbert I, Boas DA, Kleinfeld D, Dale AM. Suppressed neuronal activity and concurrent arteriolar vasoconstriction may explain negative blood oxygenation level-dependent signal. J Neurosci. 2007;27(16):4452–4459. doi: 10.1523/JNEUROSCI.0134-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogawa S, Lee TM, Kay AR, Tank DW. Brain magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci U S A. 1990;87(24):9868–9872. doi: 10.1073/pnas.87.24.9868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakao Y, Itoh Y, Kuang TY, Cook M, Jehle J, Sokoloff L. Effects of anesthesia on functional activation of cerebral blood flow and metabolism. Proc Natl Acad Sci U S A. 2001;98(13):7593–7598. doi: 10.1073/pnas.121179898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenberg DS, Houweling AR, Kerr JN. Population imaging of ongoing neuronal activity in the visual cortex of awake rats. Nat Neurosci. 2008;11(7):749–751. doi: 10.1038/nn.2140. [DOI] [PubMed] [Google Scholar]
- 37.Mattson BJ, Williams S, Rosenblatt JS, Morrell JI. Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci. 2001;115(3):683–694. doi: 10.1037//0735-7044.115.3.683. [DOI] [PubMed] [Google Scholar]
- 38.Buzsaki G, Kaila K, Raichle M. Inhibition and brain work. Neuron. 2007;56(5):771–783. doi: 10.1016/j.neuron.2007.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Logothetis NK. What we can do and what we cannot do with fMRI. Nature. 2008;453(7197):869–878. doi: 10.1038/nature06976. [DOI] [PubMed] [Google Scholar]
- 40.Logothetis NK, Pauls J, Augath M, Trinath T, Oeltermann A. Neurophysiological investigation of the basis of the fMRI signal. Nature. 2001;412(6843):150–157. doi: 10.1038/35084005. [DOI] [PubMed] [Google Scholar]
- 41.Smith AJ, Blumenfeld H, Behar KL, Rothman DL, Shulman RG, Hyder F. Cerebral energetics and spiking frequency: the neurophysiological basis of fMRI. Proc Natl Acad Sci U S A. 2002;99(16):10765–10770. doi: 10.1073/pnas.132272199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sicard K, Shen Q, Brevard ME, Sullivan R, Ferris CF, King JA, Duong TQ. Regional cerebral blood flow and BOLD responses in conscious and anesthetized rats under basal and hypercapnic conditions: implications for functional MRI studies. J Cereb Blood Flow Metab. 2003;23(4):472–481. doi: 10.1097/01.WCB.0000054755.93668.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roth LL, Rosenblatt JS. Mammary glands of pregnant rats: development stimulated by licking. Science. 1966;151(716):1403–1404. doi: 10.1126/science.151.3716.1403. [DOI] [PubMed] [Google Scholar]
- 44.Tasker JG, Theodosis DT, Poulain DA. Afferent projections from the mammary glands to the spinal cord in the lactating rat--I. A neuroanatomical study using the transganglionic transport of horseradish peroxidase-wheatgerm agglutinin. Neuroscience. 1986;19(2):495–509. doi: 10.1016/0306-4522(86)90276-9. [DOI] [PubMed] [Google Scholar]
- 45.Stern JM, Kolunie JM. Maternal aggression of rats is impaired by cutaneous anesthesia of the ventral trunk, but not by nipple removal. Physiol Behav. 1993;54(5):861–868. doi: 10.1016/0031-9384(93)90293-o. [DOI] [PubMed] [Google Scholar]
- 46.Kolunie JM, Stern JM, Barfield RJ. Maternal aggression in rats: effects of visual or auditory deprivation of the mother and dyadic pattern of ultrasonic vocalizations. Behav Neural Biol. 1994;62(1):41–49. doi: 10.1016/s0163-1047(05)80057-3. [DOI] [PubMed] [Google Scholar]
- 47.Lonstein JS, Gammie SC. Sensory, hormonal, and neural control of maternal aggression in laboratory rodents. Neurosci Biobehav Rev. 2002;26(8):869–888. doi: 10.1016/s0149-7634(02)00087-8. [DOI] [PubMed] [Google Scholar]
- 48.Salzberg HC, Lonstein JS, Stern JM. GABA(A) receptor regulation of kyphotic nursing and female sexual behavior in the caudal ventrolateral periaqueductal gray of postpartum rats. Neuroscience. 2002;114(3):675–687. doi: 10.1016/s0306-4522(02)00358-5. [DOI] [PubMed] [Google Scholar]
- 49.Lonstein JS, Stern JM. Role of the midbrain periaqueductal gray in maternal nurturance and aggression: c-fos and electrolytic lesion studies in lactating rats. J Neurosci. 1997;17(9):3364–3378. doi: 10.1523/JNEUROSCI.17-09-03364.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee A, Li M, Watchus J, Fleming AS. Neuroanatomical basis of maternal memory in postpartum rats: selective role for the nucleus accumbens. Behav Neurosci. 1999;113(3):523–538. doi: 10.1037//0735-7044.113.3.523. [DOI] [PubMed] [Google Scholar]
- 51.Li M, Fleming AS. Differential involvement of nucleus accumbens shell and core subregions in maternal memory in postpartum female rats. Behav Neurosci. 2003;117(3):426–445. doi: 10.1037/0735-7044.117.3.426. [DOI] [PubMed] [Google Scholar]
- 52.Guic E, Carrasco X, Rodriguez E, Robles I, Merzenich MM. Plasticity in primary somatosensory cortex resulting from environmentally enriched stimulation and sensory discrimination training. Biol Res. 2008;41(4):425–437. [PubMed] [Google Scholar]
- 53.Ostrowski NL. Oxytocin receptor mRNA expression in rat brain: implications for behavioral integration and reproductive success. Psychoneuroendocrinology. 1998;23(8):989–1004. doi: 10.1016/s0306-4530(98)00070-5. [DOI] [PubMed] [Google Scholar]
- 54.Vaccari C, Lolait SJ, Ostrowski NL. Comparative distribution of vasopressin V1b and oxytocin receptor messenger ribonucleic acids in brain. Endocrinology. 1998;139(12):5015–5033. doi: 10.1210/endo.139.12.6382. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA. Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci. 1994;108(6):1163–1171. doi: 10.1037//0735-7044.108.6.1163. [DOI] [PubMed] [Google Scholar]
- 56.Ferguson JN, Aldag JM, Insel TR, Young LJ. Oxytocin in the medial amygdala is essential for social recognition in the mouse. J Neurosci. 2001;21(20):8278–8285. doi: 10.1523/JNEUROSCI.21-20-08278.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Nishimori K, Young LJ, Guo Q, Wang Z, Insel TR, Matzuk MM. Oxytocin is required for nursing but is not essential for parturition or reproductive behavior. Proc Natl Acad Sci U S A. 1996;93(21):11699–11704. doi: 10.1073/pnas.93.21.11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Pedersen CA, Johns JM, Musiol I, Perez-Delgado M, Ayers G, Faggin B, Caldwell JD. Interfering with somatosensory stimulation from pups sensitizes experienced, postpartum rat mothers to oxytocin antagonist inhibition of maternal behavior. Behav Neurosci. 1995;109(5):980–990. doi: 10.1037//0735-7044.109.5.980. [DOI] [PubMed] [Google Scholar]
- 59.Bosch OJ, Kromer SA, Brunton PJ, Neumann ID. Release of oxytocin in the hypothalamic paraventricular nucleus, but not central amygdala or lateral septum in lactating residents and virgin intruders during maternal defence. Neuroscience. 2004;124(2):439–448. doi: 10.1016/j.neuroscience.2003.11.028. [DOI] [PubMed] [Google Scholar]
- 60.Bosch OJ, Meddle SL, Beiderbeck DI, Douglas AJ, Neumann ID. Brain oxytocin correlates with maternal aggression: link to anxiety. J Neurosci. 2005;25(29):6807–6815. doi: 10.1523/JNEUROSCI.1342-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tomizawa K, Iga N, Lu YF, Moriwaki A, Matsushita M, Li ST, Miyamoto O, Itano T, Matsui H. Oxytocin improves long-lasting spatial memory during motherhood through MAP kinase cascade. Nat Neurosci. 2003;6(4):384–390. doi: 10.1038/nn1023. [DOI] [PubMed] [Google Scholar]
- 62.Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ. Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci. 2004;24(17):4113–4123. doi: 10.1523/JNEUROSCI.5322-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee A, Clancy S, Fleming AS. Mother rats bar-press for pups: effects of lesions of the mpoa and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res. 1999;100(1–2):15–31. doi: 10.1016/s0166-4328(98)00109-0. [DOI] [PubMed] [Google Scholar]
- 64.Stern JM, Keer SE. Maternal motivation of lactating rats is disrupted by low dosages of haloperidol. Behav Brain Res. 1999;99(2):231–239. doi: 10.1016/s0166-4328(98)00108-9. [DOI] [PubMed] [Google Scholar]
- 65.Keer SE, Stern JM. Dopamine receptor blockade in the nucleus accumbens inhibits maternal retrieval and licking, but enhances nursing behavior in lactating rats. Physiol Behav. 1999;67(5):659–669. doi: 10.1016/s0031-9384(99)00116-x. [DOI] [PubMed] [Google Scholar]
- 66.Numan M, Smith HG. Maternal behavior in rats: evidence for the involvement of preoptic projections to the ventral tegmental area. Behav Neurosci. 1984;98(4):712–727. doi: 10.1037//0735-7044.98.4.712. [DOI] [PubMed] [Google Scholar]
- 67.Numan M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev Psychobiol. 2007;49(1):12–21. doi: 10.1002/dev.20198. [DOI] [PubMed] [Google Scholar]
- 68.Feldmeyer D, Sakmann B. Synaptic efficacy and reliability of excitatory connections between the principal neurones of the input (layer 4) and output layer (layer 5) of the neocortex. J Physiol. 2000;(525 Pt):131–139. doi: 10.1111/j.1469-7793.2000.00031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gabbott PL, Warner TA, Jays PR, Salway P, Busby SJ. Prefrontal cortex in the rat: projections to subcortical autonomic, motor, and limbic centers. J Comp Neurol. 2005;492(2):145–177. doi: 10.1002/cne.20738. [DOI] [PubMed] [Google Scholar]
- 70.Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse. 2004;51(1):32–58. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- 71.Mattson BJ, Williams SE, Rosenblatt JS, Morrell JI. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacology (Berl) 2003;167(1):1–8. doi: 10.1007/s00213-002-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hansen S, Bergvall AH, Nyiredi S. Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav. 1993;45(3):673–676. doi: 10.1016/0091-3057(93)90523-v. [DOI] [PubMed] [Google Scholar]
- 73.Febo M, Ferris CF, Segarra AC. Estrogen influences cocaine-induced blood oxygen level-dependent signal changes in female rats. J Neurosci. 2005;25(5):1132–1136. doi: 10.1523/JNEUROSCI.3801-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Febo M, Segarra AC, Nair G, Schmidt K, Duong TQ, Ferris CF. The neural consequences of repeated cocaine exposure revealed by functional MRI in awake rats. Neuropsychopharmacology. 2005;30(5):936–943. doi: 10.1038/sj.npp.1300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kinsley CH, Turco D, Bauer A, Beverly M, Wellman J, Graham AL. Cocaine alters the onset and maintenance of maternal behavior in lactating rats. Pharmacol Biochem Behav. 1994;47(4):857–864. doi: 10.1016/0091-3057(94)90288-7. [DOI] [PubMed] [Google Scholar]
- 76.Zimmerberg B, Gray MS. The effects of cocaine on maternal behaviors in the rat. Physiol Behav. 1992;52(2):379–384. doi: 10.1016/0031-9384(92)90287-c. [DOI] [PubMed] [Google Scholar]
- 77.Johns JM, Noonan LR, Zimmerman LI, Li L, Pedersen CA. Effects of short- and long-term withdrawal from gestational cocaine treatment on maternal behavior and aggression in Sprague-Dawley rats. Dev Neurosci. 1997;19(4):368–374. doi: 10.1159/000111234. [DOI] [PubMed] [Google Scholar]
- 78.Johns JM, Nelson CJ, Meter KE, Lubin DA, Couch CD, Ayers A, Walker CH. Dose-dependent effects of multiple acute cocaine injections on maternal behavior and aggression in Sprague-Dawley rats. Dev Neurosci. 1998;20(6):525–532. doi: 10.1159/000017353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vernotica EM, Lisciotto CA, Rosenblatt JS, Morrell JI. Cocaine transiently impairs maternal behavior in the rat. Behav Neurosci. 1996;110(2):315–323. doi: 10.1037//0735-7044.110.2.315. [DOI] [PubMed] [Google Scholar]
- 80.Harmer AL, Sanderson J, Mertin P. Influence of negative childhood experiences on psychological functioning, social support, and parenting for mothers recovering from addiction. Child Abuse Negl. 1999;23(5):421–433. doi: 10.1016/s0145-2134(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 81.Elliott JC, Lubin DA, Walker CH, Johns JM. Acute cocaine alters oxytocin levels in the medial preoptic area and amygdala in lactating rat dams: implications for cocaine-induced changes in maternal behavior and maternal aggression. Neuropeptides. 2001;35(2):127–134. doi: 10.1054/npep.2001.0854. [DOI] [PubMed] [Google Scholar]
- 82.Nephew BC, Febo M. Effect of cocaine sensitization prior to pregnancy on maternal care and aggression in the rat. Psychopharmacology (Berl) 209(1):127–135. doi: 10.1007/s00213-010-1777-z. [DOI] [PubMed] [Google Scholar]
- 83.Petruzzi S, Cirulli F, Laviola G. Prior cocaine exposure in different environments affects the behavioral responses of mouse dams. Pharmacol Biochem Behav. 1997;56(3):541–547. doi: 10.1016/s0091-3057(96)00294-8. [DOI] [PubMed] [Google Scholar]
- 84.Febo M, Felix-Ortiz AC, Johnson TR. Inactivation or inhibition of neuronal activity in the medial prefrontal cortex largely reduces pup retrieval and grouping in maternal rats. Brain Res. :132577–132588. doi: 10.1016/j.brainres.2010.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Afonso VM, Sison M, Lovic V, Fleming AS. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav Neurosci. 2007;121(3):515–526. doi: 10.1037/0735-7044.121.3.515. [DOI] [PubMed] [Google Scholar]
- 86.Pereira M, Morrell JI. Differential effects of discrete subarea-specific transient inactivation of the medial prefrontal cortex on the motivated choice of pup- versus cocaine-associated environments by early postpartum rats. Soc Neurosci Abstracts. 2010 doi: 10.1016/j.neuroscience.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hernandez-Gonzalez M, Navarro-Meza M, Prieto-Beracoechea CA, Guevara MA. Electrical activity of prefrontal cortex and ventral tegmental area during rat maternal behavior. Behav Processes. 2005;70(2):132–143. doi: 10.1016/j.beproc.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 88.Hernandez-Gonzalez M, Prieto-Beracoechea C, Navarro-Meza M, Ramos-Guevara JP, Reyes-Cortes R, Guevara MA. Prefrontal and tegmental electrical activity during olfactory stimulation in virgin and lactating rats. Physiol Behav. 2005;83(5):749–758. doi: 10.1016/j.physbeh.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 89.Chang JY, Janak PH, Woodward DJ. Comparison of mesocorticolimbic neuronal responses during cocaine and heroin self-administration in freely moving rats. J Neurosci. 1998;18(8):3098–3115. doi: 10.1523/JNEUROSCI.18-08-03098.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Peters YM, O'Donnell P, Carelli RM. Prefrontal cortical cell firing during maintenance, extinction, and reinstatement of goal-directed behavior for natural reward. Synapse. 2005;56(2):74–83. doi: 10.1002/syn.20129. [DOI] [PubMed] [Google Scholar]
- 91.Febo M. Prefrontal cell firing in male rats during approach towards sexually receptive female: interactions with cocaine. Synapse. 65(4):271–277. doi: 10.1002/syn.20843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS. A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry. 2002;51(6):431–445. doi: 10.1016/s0006-3223(01)01284-7. [DOI] [PubMed] [Google Scholar]
- 93.Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage. 2004;21(2):583–592. doi: 10.1016/j.neuroimage.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 94.Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21(1):155–166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 95.Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF. Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of child psychology and psychiatry, and allied disciplines. 2008;49(10):1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Strathearn L, Li J, Fonagy P, Montague PR. What's in a smile? Maternal brain responses to infant facial cues. Pediatrics. 2008;122(1):40–51. doi: 10.1542/peds.2007-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Nishitani S, Doi H, Koyama A, Shinohara K. Differential prefrontal response to infant facial emotions in mothers compared with non-mothers. Neurosci Res. 70(2):183–188. doi: 10.1016/j.neures.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 98.Febo M, Pira AS. Increased BOLD activation to predator stressor in subiculum and midbrain of amphetamine-sensitized maternal rats. Brain Res. 1382(1):18–27. doi: 10.1016/j.brainres.2010.11.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ferris CF, Stolberg T, Kulkarni P, Murugavel M, Blanchard R, Blanchard DC, Febo M, Brevard M, Simon NG. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008:9111. doi: 10.1186/1471-2202-9-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nephew BC, Caffrey MK, Felix-Ortiz AC, Ferris CF, Febo M. Blood oxygen level-dependent signal responses in corticolimbic 'emotions' circuitry of lactating rats facing intruder threat to pups. Eur J Neurosci. 2009;30(5):934–945. doi: 10.1111/j.1460-9568.2009.06875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Caffrey MK, Febo M. Pup presence differentially modulates maternal brain response to a cocaine-paired cue. Soc Neurosci Abstracts. 2010 [Google Scholar]


