Abstract
Background: Several clinical trials have investigated the effects of flaxseed and flaxseed-derived products (flaxseed oil or lignans) on blood lipids; however, the findings have been inconsistent.
Objective: We aimed to identify and quantify the effectiveness of flaxseed and its derivatives on blood lipid profiles.
Design: A comprehensive literature search was performed on the basis of English reports of randomized controlled trials of flaxseed or its derivatives on lipid profiles in adults, which were published from January 1990 to October 2008. Attempts also were made to access unpublished data. Study quality was assessed by using the Jadad score, and a meta-analysis was conducted.
Results: Twenty-eight studies were included. Flaxseed interventions reduced total and LDL cholesterol by 0.10 mmol/L (95% CI: −0.20, 0.00 mmol/L) and 0.08 mmol/L (95% CI: −0.16, 0.00 mmol/L), respectively; significant reductions were observed with whole flaxseed (−0.21 and −0.16 mmol/L, respectively) and lignan (−0.28 and −0.16 mmol/L, respectively) supplements but not with flaxseed oil. The cholesterol-lowering effects were more apparent in females (particularly postmenopausal women), individuals with high initial cholesterol concentrations, and studies with higher Jadad scores. No significant changes were found in the concentrations of HDL cholesterol and triglycerides.
Conclusions: Flaxseed significantly reduced circulating total and LDL-cholesterol concentrations, but the changes were dependent on the type of intervention, sex, and initial lipid profiles of the subjects. Further studies are needed to determine the efficiency of flaxseed on lipid profiles in men and premenopausal women and to explore its potential benefits on other cardiometabolic risk factors and prevention of cardiovascular disease.
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of mortality worldwide (1). Healthy dietary practices presumably play critical roles in the disease prevention. In recent years, a growing body of evidence from epidemiologic studies and randomized controlled trials (RCTs) has shown the cardiovascular protective effects of various foods and dietary factors, such as nuts, fish, plant sterols, soy protein, and isoflavones (2, 3). Flaxseed (linseed, Linum usitatissimum), an edible oil seed/grain and one of the oldest arable crops, was recently acknowledged as a functional food (4) and gained much attention because of its unique nutrient components and potential effect on the prevention of CVD (5). In addition to being the richest plant source of α-linolenic acid (ALA; 50–62% of flaxseed oil, or ≈22% of whole flaxseed) and lignans (range: 0.2–13.3 mg/g flaxseed), flaxseed is an essential source of dietary fiber (28% by weight), of which 25% is in the soluble form (5–7).
Previous animal studies suggest that flaxseed reduces both total and LDL cholesterol (8, 9). Flaxseed lignans also are shown to have cholesterol-lowering effect and could regress the atherosclerotic process (10, 11). Owing to the promising results in preclinical models, many clinical trials have been performed to determine the outcomes of flaxseed intervention (whole flaxseed, flaxseed oil, or lignans) on various cardiometabolic risk factors, particularly blood lipids (5, 7, 12, 13). However, the findings from most of the previous clinical trials were inconsistent, and the discrepancies could be attributed to small sample size, insufficient study duration, variation in study designs, and diversity of the test product. Therefore, given the increased statistical power afforded by meta-analysis and the enhanced precision of estimating effect sizes across several modest-sized trials, we conducted such an analysis to evaluate whether administration of flaxseed or its derivatives could improve blood lipids (total, LDL, and HDL cholesterol and triglycerides).
METHODS
Study identification and selection
Two researchers (AP and DY) independently searched PubMed (www.nlm.nih.gov/pubs/factsheets/pubmed.html), the Cochrane Library (www3.interscience.wiley.com/cgi-bin/mrwhome/106568753/HOME), clinicaltrials.gov (http://clinicaltrials.gov/), the World Health Organization ICTRP (International Clinical Trial Registry Platform; www.who.int/trialsearch), and the ProQuest Digital Dissertations Database (www.proquest.co.uk/en-UK/catalogs/databases/detail/pqdt.shtml) for English-language reports of clinical trials published from January 1990 to October 2008 and studies describing the effects of flaxseed or its derivatives (in the form of whole or ground flaxseed, flaxseed oil, or lignan supplement) in adult humans were selected. The key words “flax* OR linseed* OR lignan* OR Linum usitatissimum,” with the constraints noted above, were used in the literature search. Bibliographies of selected studies and relevant reviews (5, 7, 12, 13) were checked to ensure a complete collection. Attempts were also made to contact investigators for unpublished data. Studies were chosen for analysis if they met the following criteria: 1) the subjects consumed flaxseed or its derivatives for >2 wk; 2) the study was an RCT with either a parallel or crossover design; 3) the study reported the dose of flaxseed, lignans, or ALA when whole or ground flaxseed, lignan supplement, or flaxseed oil were administered, respectively; 4) the effects of flaxseed or its derivatives on lipid profiles (total, LDL, and HDL cholesterol and triglycerides) could be extracted from the report; and 5) the control regimen did not contain phytoestrogens, ALA, fish oil, or hormone replacements. According to these criteria, a total of 30 citations were identified (14–43).
When a decision of inclusion or rejection could not be made based on the title and abstract of a specific publication, the full text of the article was obtained and the eligibility for inclusion was assessed independently by 2 assessors (AP and DY) using an inclusion-exclusion form. Differences about inclusion of studies and interpretation of data between the researchers were resolved by discussion until a consensus was reached.
Data extraction and quality assessment
Study characteristics [including authors, publication year, sample size and attrition, dose and type of the intervention, control treatment, study duration, study design (crossover or parallel), and participant information (sex, initial health status, and menopausal status for women)] and blood lipid data were extracted independently by 2 assessors (AP and DY) and tabulated for analysis. Likewise, study quality was independently assessed by using the Jadad score level-of-evidence rating for RCTs (44). The score included randomization, blinding, description of withdrawals and dropouts, methods of randomization, and double-blinding status. The total score was the sum of the 5 points, which generated a scale from 0 to 5; higher numbers represented better quality. Other aspects, such as funding source and geographic locations of the studies, were also indicated.
Measurements from the full study period were used for the analysis (16–18, 23, 25, 35, 42, 43). In studies with multiple arms and multiple comparisons (15, 25, 41, 43), the “shared” group (eg, the intervention arm if there were multiple control arms or the control arm if the intervention was administered with different doses) was equally split into ≥2 groups with smaller sample sizes, and ≥2 comparisons were included in the meta-analysis to avoid double counting and correlated comparisons (46). Fish oil as one of the control regimens was included in 4 studies (15, 25, 27, 42), and participants in the fish-oil groups were excluded from analysis according to the aforementioned exclusion criteria. One study used whole flaxseed plus fiber as one of the interventions (37), and participants in this arm were not included in the analysis because the specific effects of flaxseed could not be ascertained. In a recent study (15), flaxseed oil intervention was reported to have a nonsignificant influence on blood lipids; however, the relevant data were not included in the publication. Therefore, the net changes in outcomes in this study were replaced by “zero,” and the SDs were replaced by the average SDs of the other studies.
For continuous outcomes in parallel studies, the means and SDs of changes from baseline to endpoint (both intervention and control groups) were extracted. In crossover studies, the means and SDs were used separately on interventions and controls. This step provided a conservative estimate of the effects and reduced the power of the crossover studies to show real influences of the interventions. For all data, SDs were calculated from SEs or CIs, whenever it was necessary, and the data were also estimated from the figures if no numerical forms were presented. For those with missing SDs for the changes, the average of the SDs of the initial and endpoint of relevant biomarkers was adapted in the analysis (because the SD of the change was approximately similar to the average of the SDs of the baseline and endpoint variables) (45). In addition, the change-from-baseline SDs were also imputed by using correlation coefficient methods referenced in the Cochrane Handbook (46).
Meta-analysis and statistical analysis
The estimate of principal effect was defined as the mean difference (net change in mmol/L) in lipid concentrations between the subjects assigned to consume flaxseed or its derivatives and those assigned to the control regimens. For the computation of pooled effects, each study was assigned a weight consisting of the reciprocal of its variance (46). The meta-analysis was performed by using RevMan for WINDOWS software (version 5.0.16; updated on 18 September 2008; http://www.cc-ims.net/RevMan/RevMan5; Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008).The fixed-effect models and random-effects models were used to calculate the weighted mean difference and 95% CI for each lipid variable according to the level of heterogeneity. If significant heterogeneity was found in the test, the results were consequently presented by using the random-effects models. Otherwise, the results were presented based on fixed-effect models. Moreover, potential publication bias was examined by using funnel plots in which the SEMs of the studies were plotted against their corresponding effect sizes (46).
Meta-regression was implemented to examine characteristics of the studies that were hypothesized to influence the observed treatment effects (47). The assumption of heterogeneity implied by the use of random-effects models is plausible because of differences in characteristics of the study populations (eg, sex), types of interventions (eg, whole or ground flaxseed, lignan supplement, or flaxseed oil), study duration, and study quality. Some studies were conducted in so-called healthy individuals; however, the definition of “healthy” varied substantially in different studies (18, 20, 22, 25, 26, 29, 40), and, in many cases, these individuals had elevated lipid concentrations. Therefore, the studies were categorized according to the baseline lipid concentrations. To explore the possible influence of the aforementioned covariates on net lipid changes, a series of prespecified subgroup analyses were further conducted based on biological plausibility and the literature. Of note, in one study (15), only 5% (3 of 62) of the subjects were women; therefore, this study was categorized as a study of “men” in the subgroup analysis.
RESULTS
Characteristics of the studies
A total of 160 citations were yielded from the literature search (137 from PubMed, 17 from references of the selected papers, 2 from clinicaltrials.gov, and 4 from dissertations); 82 citations were retrieved for a complete evaluation after the titles and abstracts were screened. Finally, 30 citations derived from 28 RCTs were included. The review flow diagram is depicted in Figure 1. Of the 30 citations reporting data on blood lipids, 4 citations (27, 28, 38, 39) were found to be duplicative. Thus, 2 of the citations (27, 38) were selected for data extraction, which left a total of 28 studies for the analysis. The primary characteristics of these 28 studies are outlined in Table 1.
FIGURE 1.
Review flow diagram according to the Quality of Reporting of Meta-analyses (QUOROM) statement.
TABLE 1.
Characteristics of the 28 included studies, with 36 comparisons1
| Author, publication year, and reference | Enrollment | Completer | Male | Menopausal status | Intervention | WF | ALA | LIG | Duration | Patient features | Jadad score | Design | Type of diet |
| n | n | % | g/d | g/d | mg/d | wk | |||||||
| Arjmandi et al, 1998 (14) | 38 | 34 | 0 | Yes | WF/SFS | 38 | 8.5 | NR | 6 | HC | 4 | RC | Usual |
| Barcelo-Coblijn et al, 2008 (15) | 62 | 61 | 95 | NR | FXO/SUO/FO | NA | 1.2 | NA | 12 | 8 HC, 54 NC | 4 | RP | Usual |
| Barcelo-Coblijn et al, 2008 (15) | 62 | 61 | 95 | NR | FXO/SUO/FO | NA | 2.4 | NA | 12 | 8 HC, 54 NC | 4 | RP | Usual |
| Barcelo-Coblijn et al, 2008 (15) | 62 | 61 | 95 | NR | FXO/SUO/FO | NA | 3.6 | NA | 12 | 8 HC, 54 NC | 4 | RP | Usual |
| Bloedon et al, 2008 (16) | 62 | 60 | 50 | Yes | WF/wheat bran | 40 | 3.8 | 640 | 5, 10 | HC | 4 | RP | LF, LC diet |
| Cornish et al, 2008 (17) | 39 | 39 | 100 | NA | LIG/PB | NA | NA | 543 | 8, 16, 26 | NR | 4 | RP | Usual+exercise |
| Cornish et al, 2008 (17) | 53 | 42 | 0 | NR | LIG/PB | NA | NA | 543 | 8, 16, 26 | NR | 4 | RP | Usual+exercise |
| Cunnane et al, 1995 (18) | 10 | 10 | 50 | No | GF/wheat flour | 50 | 9.0 | NR | 2, 4 | Healthy | 3 | RC | Usual |
| Demark-Wahnefried et al, 2008 (19) | 80 | 71 | 100 | NA | WF/control | 30 | NR | NR | 4.5 | PC | 4 | RP | LF diet |
| Demark-Wahnefried et al, 2008 (19) | 81 | 78 | 100 | NA | WF/control | 30 | NR | NR | 4.5 | PC | 4 | RP | Usual |
| Dodin et al, 2005 (20) | 199 | 179 | 0 | Yes | WF/wheat germ | 40 | 9.1 | 21 | 52 | Healthy | 5 | RP | Usual |
| Faintuch et al, 2007 (21) | 24 | 24 | 17 | NR | WF/MF | 30 | 5.0 | NR | 2 | Obese | 4 | RC | Usual |
| Hallund et al, 2006 (22) | 23 | 22 | 0 | Yes | LIG/PB | NA | NA | 500 | 6 | Healthy | 4 | RC | Usual |
| Harper et al, 2006 (23) | 56 | 49 | 12 | NR | FXO/OO | NA | 3.0 | NA | 12, 26 | NR | 5 | RP | AHA 1 diet |
| Jenkins et al, 1999 (24) | 37 | 29 | 76 | Yes | DF/wheat bran | 50 | NR | NR | 3 | HC | 3 | RC | NCEP 2 diet |
| Kaul et al, 2008 (25) | 88 | 88 | 39 | No | FXO/SUO/FO | NA | 1.0 | NA | 6, 12 | Healthy | 4 | RP | Usual |
| Kaul et al, 2008 (25) | 88 | 88 | 39 | No | FXO/HO/FO | NA | 1.0 | NA | 6, 12 | Healthy | 4 | RP | Usual |
| Kelley et al, 1993 (26) | 16 | 10 | 100 | NA | FXO/SUO | NA | 18.7 | NA | 8 | Healthy | 3 | RC | SF |
| Kestin et al, 1990 (27) | 39 | 33 | 100 | NA | FXO/SAO/FO | NA | 9.2 | NA | 6 | HC | 4 | RP | Usual |
| Li et al, 1999 (29) | 22 | 17 | 100 | NA | FXO/CO | NA | 15.4 | NA | 4 | Healthy | 2 | RP | Usual |
| Lucas et al, 2002 (30) | 58 | 36 | 0 | Yes | WF/wheat | 40 | 8.0 | NR | 12 | NR | 4 | RP | Usual |
| Mandasescu et al, 2005 (31) | 40 | 40 | NR | NR | GF/control | 20 | 4.5 | NR | 8.5 | HC | 2 | RP | HL diet |
| Mantzioris et al, 1994 (32) | 30 | 30 | 100 | NA | FXO/n−6 oil | NA | 12.6 | NA | 4 | NC | 3 | RP | Usual |
| Mantzioris et al, 1994 (32) | 30 | 28 | 100 | NA | FXO + FO/n−6 oil + FO | NA | 12.6 | NA | 4 | NC | 3 | RP | Usual |
| Marblestone, 2008 (33) | 12 | 11 | 0 | No | LIG/psyllium | NA | NA | 200 | 14 | HC | 5 | RP | Usual |
| Pan et al, 2007 (34) | 73 | 68 | 37 | Yes | LIG/PB | NA | NA | 360 | 12 | T2DM | 5 | RC | Usual |
| Pang et al, 1998 (35) | 29 | 29 | 100 | NA | FXO/SAO | NA | 12.2 | NA | 3, 6 | NC | 3 | RP | SF |
| Paschos et al, 2007 (36) | 40 | 35 | 100 | NA | FXO/SAO | NA | 8.1 | NA | 12 | HC | 3 | RP | Usual |
| Patade et al, 2008 (37) | 55 | 42 | 0 | Yes | WF/control/WF + fiber | 30 | 6.0 | NR | 12 | HC | 2 | RP | Usual |
| Rallidis et al, 2003 (38) | 90 | 76 | 100 | NA | FXO/SAO | NA | 8.1 | NA | 12 | HC | 3 | RP | Usual |
| Schwab et al, 2006 (40) | 16 | 14 | 57 | NR | FXO/HO | NA | 15.9 | NA | 4 | Healthy | 4 | RC | Usual |
| Singer et al, 1990 (41) | 44 | 44 | 100 | NA | FXO/OO | NA | 38.0 | NA | 2 | NR | 2 | RP | SF |
| Singer et al, 1990 (41) | 44 | 44 | 100 | NA | FXO/SUO | NA | 38.0 | NA | 2 | NR | 2 | RP | SF |
| Wilkinson et al, 2005 (42) | 57 | 57 | 100 | NA | FXO/SUO/SUO + FO | NA | 15.0 | NA | 6, 12 | HC | 3 | RP | Usual |
| Zhang et al, 2008 (43) | 66 | 55 | 54 | NR | LIG/PB | NA | NA | 300 | 2, 4, 6, 8 | HC | 4 | RP | Usual |
| Zhang et al, 2008 (43) | 66 | 55 | 54 | NR | LIG/PB | NA | NA | 600 | 2, 4, 6, 8 | HC | 4 | RP | Usual |
AHA, American Heart Association; ALA, α-linolenic acid; CO, canola oil; DF, defatted flaxseed; FO, fish oil; FXO, flaxseed oil; GF, ground flaxseed; HC, hypercholesterolemia; HL, hypolipidic; HO, hempseed oil; LC, low cholesterol; LF, low fat; LIG, lignans; MF, manioc flour; NA, not applicable; NC, normal lipid concentrations; NCEP, National Cholesterol Education Program; NR, not reported; OO, olive oil; PB, placebo; PC: prostate cancer; RC, randomized crossover design; RP, randomized parallel design; SAO, safflower oil; SF, supplied food; SFS, sunflower seed; SUO, sunflower oil; T2DM, type 2 diabetes mellitus; WF, whole flaxseed.
Overall, 1539 subjects were randomly assigned in these trials, and 1381 (89.7%) participants completed the studies. Of the 28 trials used in the meta-analysis, 6 trials were conducted exclusively in women [5 in postmenopausal women (14, 20, 22, 30, 37) and 1 in premenopausal women (33)], 10 trials were conducted in men (19, 26, 27, 29, 32, 35, 36, 38, 41, 42), 10 trials were conducted in both sexes (15–18, 21, 23–25, 34, 40, 43), 1 trial did not indicate the sex composition of the sample (31), and 1 trial reported results separately by sex (17).
Flaxseed in whole (14, 16, 19–21, 30, 37), ground (18, 31), or defatted (24) form (generically called whole flaxseed) was tested in 10 of 28 trials with doses from 20.0 to 50.0 g (median: 38.0 g; 10 g ≈ 1 tablespoon). Wheat (18, 30), wheat bran/germ (16, 20, 24), manioc flour (21), or sunflower seed (14) were chosen as the control regimen in these studies. However, in 2 of the studies (19, 31), the participants in the control arms adhered to low-fat diets or maintained their regular diets, whereas the participants in the intervention arms received the same diet plus additional flaxseed. In another study (37), regular muffins and bread were compared with flaxseed-containing muffins and bread plus additional flaxseed powder in the control and the intervention groups. Flaxseed oil has been tested in nearly half (13/28) of the trials (15, 23, 25–27, 29, 32, 35, 36, 38, 40–42), with doses of 1.0 to 38.0 g for ALA (median: 12.4 g; 7 g ≈ 1 tablespoon). The control regimens included oils enriched in the monounsaturated fatty acids (MUFAs) olive or canola oil (23, 29, 41) or in the n−6 polyunsaturated fatty acids (PUFAs) hempseed, safflower, or sunflower oil (15, 25–27, 32, 35, 36, 38, 40–42). In the remaining 5 trials (17, 22, 33, 34, 43), flaxseed lignan supplement was used with doses from 200 to 600 mg for lignans (median: 430 mg), and the controls were assigned to placebo (17, 22, 34, 43) or psyllium (33).
The trials varied in length from 2 to 52 wk, with a median duration of 8.5 wk. Most of the trials (20 trials) adopted parallel study designs (15–17, 19, 20, 23, 25, 27, 29–33, 35–38, 41–43), whereas 8 trials used crossover designs (14, 18, 21, 22, 24, 26, 34, 40). The study qualities of the selected trials were diverse; 16 studies were classified as high quality (Jadad score of 4 or 5) (14–17, 19–23, 25, 27, 30, 33, 34, 40, 43), and 12 studies were classified as low quality (Jadad score of 2 or 3) (18, 24, 26, 29, 31, 32, 35–38, 41, 42). Most of the study reports did not indicate the method for sequence generation, and 2 studies (29, 31) did not mention whether the trials were randomized. Indeed, no difference was found when these 2 studies were included in the meta-analysis or not. For practical reasons, double-blinding was not applied in some studies, especially when whole or ground flaxseed was used as the intervention.
In most studies, the investigators attempted to maintain the participants' dietary habits and provided similar amounts of total fat and saturated fat in both the intervention and control arms. In 8 studies, the diets of both groups were exclusively offered by the investigators (26, 31, 35, 41), or the participants were instructed to follow the National Cholesterol Education Program Step II (24), American Heart Association Step I (23), or low-fat, low-cholesterol dietary patterns (16, 19). In one study, both groups received the same exercise interventions (17). Most of the trials were conducted in the United States (14–16, 19, 23, 26, 30, 33, 37), Canada (17, 18, 20, 24, 25), and European countries (22, 31, 36, 39–42), which are major production and consumption areas of flaxseed and its products; the other studies were conducted in Australia (27, 29, 32, 35), China (34, 43), and Brazil (21).
In addition, no differences in body weight or lipid concentrations were reported between the comparison groups at baseline. Most of the trials were designed to maintain body weight, and no significant weight changes were documented, except in one study in which flaxseed prevented the increase in body weight compared with the control regimen (20).
Changes in blood lipid concentrations
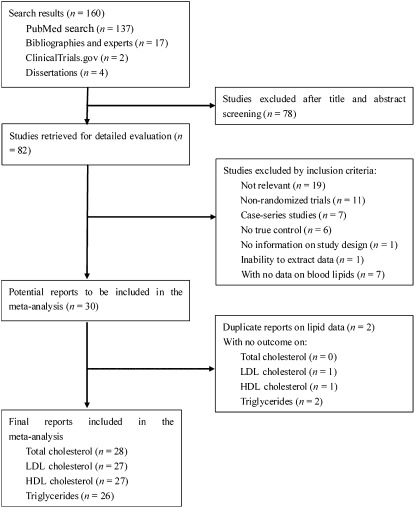
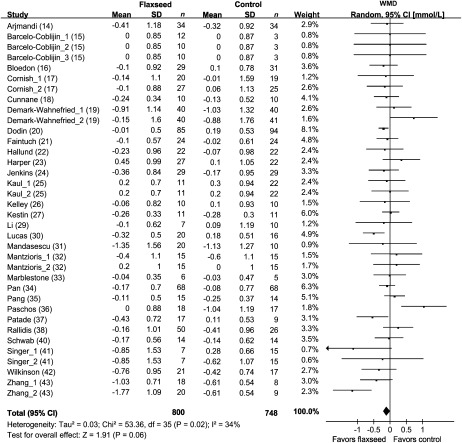
The results for total cholesterol were reported in 36 comparisons from 28 studies representing 1548 participants (Figure 2), whereas blood LDL-cholesterol concentrations were shown in 35 comparisons from 27 studies including 1471 subjects (Figure 3). Because the test for heterogeneity was significant for total cholesterol (P = 0.02) and marginally significant for LDL cholesterol (P = 0.10), we reported the results from random-effects models. For the overall population, total cholesterol decreased by 0.10 mmol/L (95% CI: −0.20, 0.00 mmol/L; P = 0.06), and LDL cholesterol decreased by 0.08 mmol/L (95% CI: −0.16, 0.00 mmol/L; P = 0.04) in the intervention groups compared with the control arms.
FIGURE 2.
Net changes (95% CI) in total cholesterol associated with flaxseed intervention expressed as the change during the intervention with flaxseed or its derivatives minus the change during the control regimens. WMD, weighted mean difference; Random, random-effects model. The horizontal lines denote the 95% CIs, some of which extend beyond the limits of the scales. The square represents the point estimate of each study. The diamond represents the overall pooled estimate of the treatment effect. Overall, total cholesterol decreased by 0.10 mmol/L (95% CI: −0.20, 0.00 mmol/L; P = 0.06) in the intervention groups compared with the control arms.
FIGURE 3.
Net changes (95% CI) in LDL cholesterol associated with flaxseed intervention expressed as the change during the intervention with flaxseed or its derivatives minus the change during the control regimens. WMD, weighted mean difference; Random, random-effects model. The horizontal lines denote the 95% CIs, some of which extend beyond the limits of the scales. The square represents the point estimate of each study. The diamond represents the overall pooled estimate of the treatment effect. Overall, LDL cholesterol decreased by 0.08 mmol/L (95% CI: −0.16, 0.00 mmol/L; P = 0.04) in the intervention groups compared with the control arms.
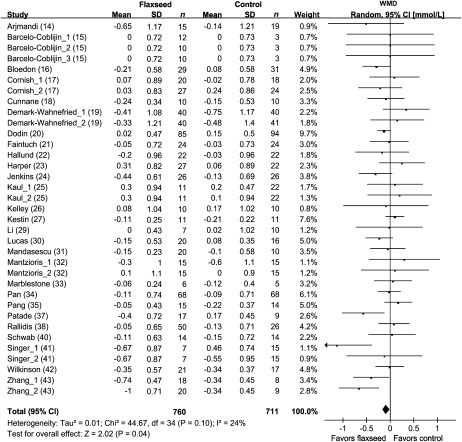
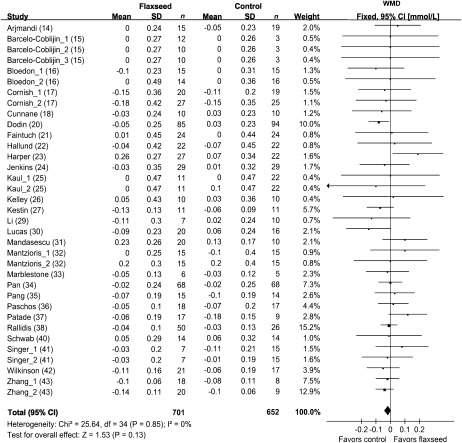
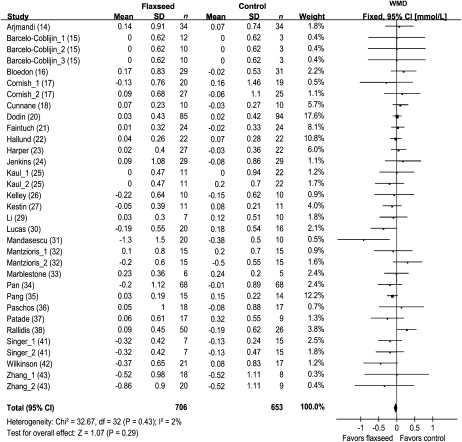
Concentrations of blood HDL cholesterol were reported in 35 comparisons from 27 studies representing 1353 participants (Figure 4). The results of blood triglycerides were shown in 33 comparisons from 26 studies including 1359 subjects (Figure 5). Because no significant heterogeneity was found for HDL cholesterol (P = 0.85) and triglycerides (P = 0.43), the results were reported based on fixed-effect models. Overall, intervention of flaxseed or its derivatives did not significantly affect HDL-cholesterol or triglycerides concentrations.
FIGURE 4.
Net changes (95% CI) in HDL cholesterol associated with flaxseed intervention expressed as the change during the intervention with flaxseed or its derivatives minus the change during the control regimens. WMD, weighted mean difference; Fixed, fixed-effect model. The horizontal lines denote the 95% CIs, some of which extend beyond the limits of the scales. The square represents the point estimate of each study. The diamond represents the overall pooled estimate of the treatment effect. Overall, no significant effect of flaxseed intervention on HDL cholesterol was found (−0.02 mmol/L; 95% CI: −0.04, 0.00 mmol/L; P = 0.13).
FIGURE 5.
Net changes (95% CI) in triglycerides associated with flaxseed intervention expressed as the change during the intervention with flaxseed or its derivatives minus the change during the control regimens. WMD, weighted mean difference; Fixed, fixed-effect model. The horizontal lines denote the 95% CIs. The square represents the point estimate of each study. The diamond represents the overall pooled estimate of the treatment effect. Overall, no significant effect of flaxseed intervention on triglycerides was found (−0.03 mmol/L; 95% CI: −0.08, 0.02 mmol/L; P = 0.29).
Subgroup analysis
Meta-regression analysis showed that sex, type of intervention (whole flaxseed, flaxseed oil, or lignan supplement), study quality (measured by the Jadad score), and initial lipid concentrations (high or low) influenced the net changes in total and LDL cholesterol (data not shown). Therefore, subgroup analyses were conducted based on these variables, and the results are summarized in Table 2.
TABLE 2.
Pooled estimates of effects on total and LDL cholesterol within various subgroups1
| Variable | No. of comparisons | Net change (95% CI) | P value | P value for heterogeneity | Analysis model2 |
| mmol/L | |||||
| Total cholesterol | |||||
| Intervention | |||||
| Whole flaxseed | 11 | −0.19 (−0.29, −0.09) | 0.0003 | 0.20 | Fixed-effect |
| Lignan supplement | 7 | −0.28 (−0.55, −0.01) | 0.04 | 0.06 | Random-effects |
| Flaxseed oil | 18 | 0.06 (−0.07, 0.19) | 0.36 | 0.44 | Fixed-effect |
| Initial total cholesterol concentration | |||||
| <5.7 mmol/L | 18 | 0.03 (−0.11, 0.17) | 0.68 | 0.84 | Fixed-effect |
| ≥5.7 mmol/L | 18 | −0.17 (−0.32, −0.03) | 0.02 | 0.004 | Random-effects |
| Sex | |||||
| Female | 7 | −0.24 (−0.36, −0.12) | <0.0001 | 0.49 | Fixed-effect |
| Male | 16 | 0.09 (−0.05, 0.23) | 0.21 | 0.27 | Fixed-effect |
| Both | 13 | −0.15 (−0.27, −0.02) | 0.02 | 0.13 | Fixed-effect |
| Jadad score | |||||
| Low, 2 or 3 | 14 | −0.04 (−0.26, 0.19) | 0.75 | 0.04 | Random-effects |
| High, 4 or 5 | 22 | −0.13 (−0.24, −0.02) | 0.02 | 0.10 | Random-effects |
| LDL cholesterol | |||||
| Intervention | |||||
| Whole flaxseed | 11 | −0.16 (−0.25, −0.06) | 0.001 | 0.30 | Fixed-effect |
| Lignan supplement | 7 | −0.16 (−0.31, −0.01) | 0.03 | 0.12 | Fixed-effect |
| Flaxseed oil | 17 | 0.06 (−0.04, 0.17) | 0.24 | 0.72 | Fixed-effect |
| Initial LDL-cholesterol concentration | |||||
| <3.4 mmol/L | 17 | 0.00 (−0.12, 0.12) | 1.00 | 0.42 | Fixed-effect |
| ≥3.4 mmol/L | 18 | −0.13 (−0.23, −0.02) | 0.02 | 0.08 | Random-effects |
| Sex | |||||
| Female | 7 | −0.17 (−0.28, −0.06) | 0.003 | 0.48 | Fixed-effect |
| Male | 15 | 0.07 (−0.04, 0.18) | 0.21 | 0.62 | Fixed-effect |
| Both | 13 | −0.14 (−0.25, −0.03) | 0.01 | 0.16 | Fixed-effect |
| Jadad score | |||||
| Low, 2 or 3 | 13 | −0.10 (−0.26, 0.06) | 0.22 | 0.09 | Random-effects |
| High, 4 or 5 | 22 | −0.08 (−0.16, −0.01) | 0.03 | 0.22 | Fixed-effect |
The meta-analysis was conducted by using RevMan 5.0 software (Cochrane Information Management System; http://www.cc-ims.net/RevMan/RevMan5). Net change is expressed as the change during intervention with flaxseed or its derivatives minus the change during the control regimens.
The random-effects models were used if significant heterogeneity was found in the test. Otherwise, the results were based on fixed-effect models.
A significant reduction in total cholesterol was found in studies using whole flaxseed, with a net change of −0.19 mmol/L (95% CI: −0.29, −0.09 mmol/L). Total cholesterol concentrations also decreased significantly in the interventions using lignan supplement (−0.28 mmol/L; 95% CI: −0.55, −0.01 mmol/L). Similarly, concentrations of LDL cholesterol declined significantly in studies using whole flaxseed (−0.16 mmol/L; 95% CI: −0.25, −0.06 mmol/L) and lignan (−0.16 mmol/L, 95% CI: −0.31, −0.01 mmol/L) supplements. No significant changes in total and LDL cholesterol were detected with the intervention of flaxseed oil.
In general, the reduction in total cholesterol was greater in women than in men, with mean changes of −0.24 mmol/L (95% CI: −0.36, −0.12 mmol/L) and 0.09 mmol/L (95% CI: −0.05, 0.23 mmol/L), respectively. Only moderate reductions (−0.15 mmol/L; 95% CI: −0.27, −0.02 mmol/L) were observed in the studies including both sexes. Similarly, the reductions in LDL cholesterol were −0.17 mmol/L (95% CI: −0.28, −0.06 mmol/L) in females, −0.14 mmol/L (95% CI: −0.25, −0.03 mmol/L) in studies with both sexes, and 0.07 mmol/L (95% CI: −0.04, 0.18 mmol/L) in males. The tests of heterogeneity were not significant in any of the analyses; thus, the results were reported from fixed-effect models.
Subgroup analysis suggested that total and LDL cholesterol were reduced to a greater degree in the high-quality studies, with net changes of −0.13 mmol/L (95% CI: −0.24, −0.02 mmol/L) and −0.08 mmol/L (95% CI: −0.16, −0.01 mmol/L), respectively. However, no significant changes were detected in the low-quality studies.
We also stratified the studies according to the initial lipid status using cutoffs of 5.7 and 3.4 mmol/L for total and LDL cholesterol, respectively. Significant reductions in total cholesterol were found in the studies including subjects with high initial concentrations (−0.17 mmol/L; 95% CI: −0.32, −0.03 mmol/L) but not in the studies enrolling subjects with low initial concentrations (0.03 mmol/L; 95% CI: −0.11, 0.17 mmol/L). The same pattern was observed for LDL cholesterol, ie, a change of −0.13 mmol/L (95% CI: −0.23, −0.02 mmol/L) and 0.00 mmol/L (95% CI: −0.12, 0.12 mmol/L) for the studies with high or low initial concentrations, respectively. No significant changes in HDL cholesterol or triglycerides were found across any subgroups (data not shown).
Publication bias
The funnel plot of the effects on blood lipids indicated no significant publication bias (see Supplemental Figure 1 under “Supplemental data” in the online issue).
DISCUSSION
Overall, flaxseed supplementation was associated with a decrease in blood total and LDL-cholesterol concentrations but did not substantially affect HDL cholesterol and triglycerides. These changes varied substantially depending on the treatment form of flaxseed, quality of the study, sex, and initial lipid profile of the subjects.
Our results showed that whole flaxseed interventions were associated with significant reductions in total and LDL cholesterol, whereas flaxseed oil interventions were not. Flaxseed contains a large amount of fiber (28% by weight), and 25% of the fiber is in the soluble form (4, 5, 7). Dietary soluble fiber has been proven to have cholesterol-lowering effects, causing significant decreases in total and LDL cholesterol (−0.045 and −0.057 mmol/L per gram, respectively) (48). The daily doses used in the flaxseed interventions included in this meta-analysis ranged from 20.0 to 50.0 g/d (median dose: 38.0 g). Therefore, estimates of the effect of the soluble fiber on total and LDL cholesterol were calculated as −0.12 and −0.15 mmol/L, respectively. Additionally, flaxseed is one of the richest sources of dietary lignans (range: 0.2–13.3 mg/g) (5–7), and purified lignans also have been shown to reduce total and LDL cholesterol in animal studies (10, 11). Human data, however, are still limited; only 5 studies have used lignan supplements (17, 22, 33, 34, 43). Moreover, the results were largely determined by one of the studies (43). Hence, further studies are still needed to confirm whether lignans alone have a cholesterol-lowering effect.
In contrast, this meta-analysis found that flaxseed oil treatment did not significantly reduce total and LDL-cholesterol concentrations compared with the control regimens. Such results are consistent with a recent meta-analysis that reported neutral effects of ALA on total and LDL cholesterol compared with the control arms (49). One of the plausible explanations for the null findings is that the effects of flaxseed oil may have been masked by the use of MUFA- or n−6 PUFA–enriched oils as the control regimen in these studies. Previous studies have indeed shown that dietary replacement of MUFAs or n−6 PUFAs for saturated fatty acids also has a cholesterol-lowering effect (50, 51). It was noticed that flaxseed oil treatment induced a modest but nonsignificant decrease in total and LDL cholesterol compared with baseline values (data not shown). Prospective epidemiologic studies also reported a reduced relative risk of coronary heart disease when saturated or trans fatty acids were replaced with ALA (52). Taken together, our results and those of others (49) indicate that the effect of ALA on blood lipids is similar to that of MUFAs or n−6 PUFAs, and whether replacement of ALA for saturated or trans fatty acids could lower blood lipids remains to be elucidated. Nevertheless, flaxseed oil has consistently been found to induce a significant increase in the proportion of n–3 fatty acids in the plasma (16, 18, 25–27) or erythrocyte membranes (15, 42) and a decrease in the ratio of n–6 to n–3, which may have other benefits for CVD (53). However, the long-term consequences of such an effect remain unclear.
According to the results of this meta-analysis, it appears that initial cholesterol profiles exert a powerful moderating effect on changes in lipid concentrations: the beneficial effects of flaxseed and its derivatives were only observed among those with relatively high initial cholesterol concentrations. Therefore, we speculate that these high initial concentrations made the subjects more likely to be influenced by the intervention. An alternative explanation, however, is regression toward the mean. It was also observed that the high-quality studies seemed to have a greater cholesterol-lowering effect than did the poor-quality studies (measured by the Jadad score). This may be attributed to the fact that generally adequate sample sizes in the high-quality studies permitted a greater statistical power to detect any beneficial effects of the treatments.
The cholesterol-lowering effect of flaxseed and its products was more remarkable in women than in men. This difference was more striking in postmenopausal women, who tended to experience increased total and LDL-cholesterol concentrations as a result of estrogen decline (54). The actual causes for the sex difference are unknown. However, we noticed the following: 1) most of the comparisons (14 of 17) in men used flaxseed oil as the interventions, whereas all of the comparisons in women used flaxseed (5 of 8) or lignans (3 of 8) as the interventions; 2) most of the comparisons (12 of 17) in men had low initial cholesterol concentrations, whereas all of the comparisons in women (8 of 8) had a high initial cholesterol concentrations; 3) more than half of the comparisons (10 of 17) in men were of low study quality, whereas most of the comparisons (6 of 8) in women were of high study quality. Therefore, we are uncertain whether the observed differential sex effects were the consequence of the study design (type of intervention, initial cholesterol profiles of the subjects, and study quality) or inherent biological variation. Therefore, the effectiveness of flaxseed or lignan interventions on blood lipids in hypercholesterolemic men or premenopausal women still remains unclear and needs to be evaluated in the future.
Finally, we did not observe any significant effects of flaxseed or its derivatives on HDL cholesterol and triglycerides. These findings are generally consistent with individual reports, in which 70–90% of trials showed neutral effects on these 2 lipid variables.
The intervention using whole flaxseed, in females or in individuals with high initial lipid concentrations, reduce total or LDL cholesterol by ≈ 0.2 mmol/L, which is estimated to result in a reduction of ≈3% in all-cause mortality and of 6% in both coronary heart disease–related mortality and total events (55). Therefore, the effects of flaxseed on dyslipidemia appear to be clinically significant. Although we believe that this meta-analysis provides useful information for clinicians and researchers alike, the findings must be interpreted with caution because of the following weaknesses. For some studies, the SDs of the net changes were not available and were estimated as the average of the SDs of the baseline and endpoint biomarkers. For the crossover studies, we used means and SDs separately on intervention and control phases. Furthermore, the effects of flaxseed on lipid profiles are not uniform because of the substantial heterogeneity among individual studies, ie, the studies were conducted in a variety of populations and had different study designs, methods, and criteria. Indeed, we found that the cholesterol-lowering effects were related to the type of intervention, study quality, sex, and initial lipid profiles. However, influences of the other covariates, such as the quality of products and the amount of specific bioactive components in flaxseed and their bioavailability could not be fully determined because of the lack of information in most of the existing studies. Efforts should be made to include detailed nutrient contents in future studies.
In this meta-analysis, we assessed the effectiveness of flaxseed and its derivatives on circulating lipid concentrations by reviewing available published and unpublished RCTs. The results suggest that flaxseed consumption reduces blood total and LDL-cholesterol concentrations, and this effect was more evident in studies that used whole flaxseed, that enrolled women (particularly postmenopausal women), and that were of high quality and in subjects with high initial cholesterol concentrations. Therefore, flaxseed consumption may be a worthwhile dietary approach for preventing hypercholesterolemia, particularly in specific patient subgroups. This systematic review not only provides a thorough synthesis of recent studies that evaluated the lipid-modulating effects of flaxseed but also facilitates the identification of future research priorities. Further studies are needed with large sample sizes, adequate durations, and solid study designs to investigate the effectiveness of flaxseed supplementation on cardiometabolic risk factors other than blood lipids, on various chronic diseases (eg, the metabolic syndrome and diabetes), and ultimately on CVD-related morbidity and mortality. Such studies are particularly important in individuals at high risk of CVD and need to be carried out in a wide variety of populations (eg, men and pre- and postmenopausal women) given the evidence of differential effects.
Supplementary Material
Acknowledgments
We thank Philip D Chilibeck and Bonnie Marblestone for providing their unpublished data. We appreciate Yiping Qiu and Zhijie Yu for their valuable comments.
The authors' responsibilities were as follows—AP and DY: study design, data collection, meta-analysis, and manuscript production; and WD-W, OHF, and XL: study design and editing of the manuscript. All authors were responsible for critical revisions and final approval of the manuscript. One author (OHF) was employed by Unilever when the work was done, but he does not own stock or any other financial interest in Unilever. He has since left Unilever and moved to the University of Warwick. Unilever does not currently sell flaxseed products and does not plan to. None of the other authors had any financial or personal conflicts of interest.
REFERENCES
- 1.World Health Organization The global burden of disease: 2004 update. Available from: http://www.who.int/healthinfo/global_burden_disease/2004_report_update/en/index.html (cited 11 November 2008)
- 2.Castro IA, Barroso LP, Sinnecker P. Functional foods for coronary heart disease risk reduction: a meta-analysis using a multivariate approach. Am J Clin Nutr 2005;82:32–40 [DOI] [PubMed] [Google Scholar]
- 3.Rudkowska I, Jones PJ. Functional foods for the prevention and treatment of cardiovascular diseases: cholesterol and beyond. Expert Rev Cardiovasc Ther 2007;5:477–90 [DOI] [PubMed] [Google Scholar]
- 4.Thompson LU, Cunnane SC. Flaxseed in human nutrition. 2nd ed. Champaign, IL: AOCS Publishing, 2003 [Google Scholar]
- 5.Bloedon LT, Szapary PO. Flaxseed and cardiovascular risk. Nutr Rev 2004;62:18–27 [DOI] [PubMed] [Google Scholar]
- 6.Muir AD. Flax lignans—analytical methods and how they influence our understanding of biological activity. J AOAC Int 2006;89:1147–57 [PubMed] [Google Scholar]
- 7.Hall C, III, Tulbek MC, Xu Y. Flaxseed. Adv Food Nutr Res 2006;51:1–97 [DOI] [PubMed] [Google Scholar]
- 8.Prasad K. Dietary flax seed in prevention of hypercholesterolemic atherosclerosis. Atherosclerosis 1997;132:69–76 [DOI] [PubMed] [Google Scholar]
- 9.Lucas EA, Lightfoot SA, Hammond LJ, et al. Flaxseed reduces plasma cholesterol and atherosclerotic lesion formation in ovariectomized Golden Syrian hamsters. Atherosclerosis 2004;173:223–9 [DOI] [PubMed] [Google Scholar]
- 10.Prasad K. Regression of hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Atherosclerosis 2008;197:34–42 [DOI] [PubMed] [Google Scholar]
- 11.Prasad K. Reduction of serum cholesterol and hypercholesterolemic atherosclerosis in rabbits by secoisolariciresinol diglucoside isolated from flaxseed. Circulation 1999;99:1355–62 [DOI] [PubMed] [Google Scholar]
- 12.Basch E, Bent S, Collins J, et al. Flax and flaxseed oil (Linum usitatissimum): a review by the Natural Standard Research Collaboration. J Soc Integr Oncol 2007;5:92–105 [DOI] [PubMed] [Google Scholar]
- 13.Oomah BD. Flaxseed as a functional food source. J Sci Food Agric 2001;81:889–94 [Google Scholar]
- 14.Arjmandi BH, Khan DA, Juma S, et al. Whole flaxseed consumption lowers serum LDL-cholesterol and lipoprotein (a) concentrations in postmenopausal women. Nutr Res 1998;18:1203–14 [Google Scholar]
- 15.Barcelo-Coblijn G, Murphy EJ, Othman R, Moghadasian MH, Kashour T, Friel JK. Flaxseed oil and fish-oil capsule consumption alters human red blood cell n–3 fatty acid composition: a multiple-dosing trial comparing 2 sources of n–3 fatty acid. Am J Clin Nutr 2008;88:801–9 [DOI] [PubMed] [Google Scholar]
- 16.Bloedon LT, Balikai S, Chittams J, et al. Flaxseed and cardiovascular risk factors: results from a double blind, randomized, controlled clinical trial. J Am Coll Nutr 2008;27:65–74 [DOI] [PubMed] [Google Scholar]
- 17.Cornish SM, Chilibeck PD, Paus-Jennsen L, et al. A randomized controlled trial of the effects of flaxseed lignan complex on metabolic syndrome composite score and bone mineral in older adults. Appl Physiol Nutr Metab 2009;34:89–98 [DOI] [PubMed] [Google Scholar]
- 18.Cunnane SC, Hamadeh MJ, Liede AC, Thompson LU, Wolever TM, Jenkins DJ. Nutritional attributes of traditional flaxseed in healthy young adults. Am J Clin Nutr 1995;61:62–8 [DOI] [PubMed] [Google Scholar]
- 19.Demark-Wahnefried W, Polascik TJ, George SL, et al. Flaxseed supplementation (not dietary fat restriction) reduces prostate cancer proliferation rates in men presurgery. Cancer Epidemiol Biomarkers Prev 2008;17:3577–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dodin S, Lemay A, Jacques H, Legare F, Forest JC, Masse B. The effects of flaxseed dietary supplement on lipid profile, bone mineral density, and symptoms in menopausal women: a randomized, double-blind, wheat germ placebo-controlled clinical trial. J Clin Endocrinol Metab 2005;90:1390–7 [DOI] [PubMed] [Google Scholar]
- 21.Faintuch J, Horie LM, Barbeiro HV, et al. Systemic inflammation in morbidly obese subjects: response to oral supplementation with alpha-linolenic acid. Obes Surg 2007;17:341–7 [DOI] [PubMed] [Google Scholar]
- 22.Hallund J, Ravn-Haren G, Bugel S, Tholstrup T, Tetens I. A lignan complex isolated from flaxseed does not affect plasma lipid concentrations or antioxidant capacity in healthy postmenopausal women. J Nutr 2006;136:112–6 [DOI] [PubMed] [Google Scholar]
- 23.Harper CR, Edwards MC, Jacobson TA. Flaxseed oil supplementation does not affect plasma lipoprotein concentration or particle size in human subjects. J Nutr 2006;136:2844–8 [DOI] [PubMed] [Google Scholar]
- 24.Jenkins DJ, Kendall CW, Vidgen E, et al. Health aspects of partially defatted flaxseed, including effects on serum lipids, oxidative measures, and ex vivo androgen and progestin activity: a controlled crossover trial. Am J Clin Nutr 1999;69:395–402 [DOI] [PubMed] [Google Scholar]
- 25.Kaul N, Kreml R, Austria JA, et al. A comparison of fish oil, flaxseed oil and hempseed oil supplementation on selected parameters of cardiovascular health in healthy volunteers. J Am Coll Nutr 2008;27:51–8 [DOI] [PubMed] [Google Scholar]
- 26.Kelley DS, Nelson GJ, Love JE, et al. Dietary alpha-linolenic acid alters tissue fatty acid composition, but not blood lipids, lipoproteins or coagulation status in humans. Lipids 1993;28:533–7 [DOI] [PubMed] [Google Scholar]
- 27.Kestin M, Clifton P, Belling GB, Nestel PJ. n−3 fatty acids of marine origin lower systolic blood pressure and triglycerides but raise LDL cholesterol compared with n−3 and n−6 fatty acids from plants. Am J Clin Nutr 1990;51:1028–34 [DOI] [PubMed] [Google Scholar]
- 28.Abbey M, Clifton P, Kestin M, Belling B, Nestel P. Effect of fish oil on lipoproteins, lecithin:cholesterol acyltransferase, and lipid transfer protein activity in humans. Arteriosclerosis 1990;10:85–94 [DOI] [PubMed] [Google Scholar]
- 29.Li D, Sinclair A, Wilson A, et al. Effect of dietary alpha-linolenic acid on thrombotic risk factors in vegetarian men. Am J Clin Nutr 1999;69:872–82 [DOI] [PubMed] [Google Scholar]
- 30.Lucas EA, Wild RD, Hammond LJ, et al. Flaxseed improves lipid profile without altering biomarkers of bone metabolism in postmenopausal women. J Clin Endocrinol Metab 2002;87:1527–32 [DOI] [PubMed] [Google Scholar]
- 31.Mandasescu S, Mocanu V, Dascalita AM, et al. Flaxseed supplementation in hyperlipidemic patients. Rev Med Chir Soc Med Nat Iasi 2005;109:502–6 [PubMed] [Google Scholar]
- 32.Mantzioris E, James MJ, Gibson RA, Cleland LG. Dietary substitution with an alpha-linolenic acid-rich vegetable oil increases eicosapentaenoic acid concentrations in tissues. Am J Clin Nutr 1994;59:1304–9 [DOI] [PubMed] [Google Scholar]
- 33.Marblestone B. The effects of flaxseed SDG on perimenopausal women with mild hyperlipidemia. San Diego, CA: University of San Diego, 2008 [Google Scholar]
- 34.Pan A, Sun J, Chen Y, et al. Effects of a flaxseed-derived lignan supplement in type 2 diabetic patients: a randomized, double-blind, cross-over trial. PLoS One 2007;2:e1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pang D, Allman-Farinelli MA, Wong T, Barnes R, Kingham KM. Replacement of linoleic acid with alpha-linolenic acid does not alter blood lipids in normolipidaemic men. Br J Nutr 1998;80:163–7 [PubMed] [Google Scholar]
- 36.Paschos GK, Zampelas A, Panagiotakos DB, et al. Effects of flaxseed oil supplementation on plasma adiponectin levels in dyslipidemic men. Eur J Nutr 2007;46:315–20 [DOI] [PubMed] [Google Scholar]
- 37.Patade A, Devareddy L, Lucas EA, Korlagunta K, Daggy BP, Arjmandi BH. Flaxseed reduces total and LDL cholesterol concentrations in Native American postmenopausal women. J Womens Health 2008;17:355–66 [DOI] [PubMed] [Google Scholar]
- 38.Rallidis LS, Paschos G, Liakos GK, Velissaridou AH, Anastasiadis G, Zampelas A. Dietary alpha-linolenic acid decreases C-reactive protein, serum amyloid A and interleukin−6 in dyslipidaemic patients. Atherosclerosis 2003;167:237–42 [DOI] [PubMed] [Google Scholar]
- 39.Rallidis LS, Paschos G, Papaioannou ML, et al. The effect of diet enriched with alpha-linolenic acid on soluble cellular adhesion molecules in dyslipidaemic patients. Atherosclerosis 2004;174:127–32 [DOI] [PubMed] [Google Scholar]
- 40.Schwab US, Callaway JC, Erkkila AT, Gynther J, Uusitupa MI, Jarvinen T. Effects of hempseed and flaxseed oils on the profile of serum lipids, serum total and lipoprotein lipid concentrations and haemostatic factors. Eur J Nutr 2006;45:470–7 [DOI] [PubMed] [Google Scholar]
- 41.Singer P, Jaeger W, Berger I, et al. Effects of dietary oleic, linoleic and alpha-linolenic acids on blood pressure, serum lipids, lipoproteins and the formation of eicosanoid precursors in patients with mild essential hypertension. J Hum Hypertens 1990;4:227–33 [PubMed] [Google Scholar]
- 42.Wilkinson P, Leach C, Ah-Sing EE, et al. Influence of alpha-linolenic acid and fish-oil on markers of cardiovascular risk in subjects with an atherogenic lipoprotein phenotype. Atherosclerosis 2005;181:115–24 [DOI] [PubMed] [Google Scholar]
- 43.Zhang W, Wang X, Liu Y, et al. Dietary flaxseed lignan extract lowers plasma cholesterol and glucose concentrations in hypercholesterolaemic subjects. Br J Nutr 2008;99:1301–9 [DOI] [PubMed] [Google Scholar]
- 44.Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996;17:1–12 [DOI] [PubMed] [Google Scholar]
- 45.Cappuccio FP, Kerry SM, Forbes L, Donald A. Blood pressure control by home monitoring: meta-analysis of randomized trials. BMJ 2004;329:145–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Higgins JPT, Green S, eds. Cochrane handbook for systematic reviews of interventions. Version 5.0.1. New York, NY: Wiley, 2008 [Google Scholar]
- 47.van Houwelingen HC, Arends LR, Stijnen T. Advanced methods in meta-analysis: multivariate approach and meta-regression. Stat Med 2002;21:589–624 [DOI] [PubMed] [Google Scholar]
- 48.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol-lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr 1999;69:30–42 [DOI] [PubMed] [Google Scholar]
- 49.Wendland E, Farmer A, Glasziou P, Neil A. Effect of α linolenic acid on cardiovascular risk markers: a systematic review. Heart 2006;92:166–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fernandez ML, West KL. Mechanisms by which dietary fatty acids modulate plasma lipids. J Nutr 2005;135:2075–8 [DOI] [PubMed] [Google Scholar]
- 51.Harris WS. n−3 fatty acids and serum lipoproteins: human studies. Am J Clin Nutr 1997;65:1645S–54S [DOI] [PubMed] [Google Scholar]
- 52.Hu FB, Stampfer MJ, Manson JE, et al. Dietary fat intake and the risk of coronary heart disease in women. N Engl J Med 1997;337:1491–9 [DOI] [PubMed] [Google Scholar]
- 53.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233:674–88 [DOI] [PubMed] [Google Scholar]
- 54.Fukami K, Koike K, Hirota K, Yoshikawa H, Miyake A. Perimenopausal changes in serum lipids and lipoproteins: a 7-year longitudinal study. Maturitas 1995;22:193–7 [DOI] [PubMed] [Google Scholar]
- 55.Gould AL, Davies GM, Alemao E, Yin DD, Cook JR. Cholesterol reduction yields clinical benefits: meta-analysis including recent trials. Clin Ther 2007;29:778–94 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.