Abstract
There is an emergent literature suggesting that East Asians and Westerners differ in cognitive processes because of cultural biases to process information holistically (East Asians) or analytically (Westerners). To evaluate the possibility that such differences are accompanied by differences in brain structure, we conducted a large comparative study on cognitively matched young and old adults from two cultural/ethnic groups—Chinese Singaporeans and non-Asian Americans—that involved a total of 140 persons. Young predominantly White American adults were found to have higher cortical thickness in frontal, parietal, and medial-temporal polymodal association areas in both hemispheres. These findings were replicated using voxel-based morphometry applied to the same data set. Differences in cortical thickness observed between young volunteers were not significant in older subjects as a whole. However, group differences were evident when high-performing old were compared. Although the observed differences in gray matter may be rooted in strategic differences in cognition arising from ethnic/cultural differences, alternative explanations involving genetic heritage and environmental factors are also considered.
INTRODUCTION
Structural imaging studies have revealed that experience plays a role in sculpting the structure of the brain. Relative enlargement of functionally relevant brain regions has been shown to correlate with superior performance in domains as diverse as second language acquisition (Golestani, Molko, Dehaene, LeBihan, & Pallier, 2007; Green, Crinion, & Price, 2007), musical ability (Gaser & Schlaug, 2003), spatial navigational ability (Maguire et al., 2000), and specific motor skills (Draganski et al., 2004). Moreover, in the case of spatial navigation and juggling, regional brain volume appears to be modulated by experience in a behaviorally meaningful manner, as individuals with the greater volume show better performance (Draganski & May, 2008). Overall, the data suggest that experience can modify brain structure and that morphometry is a valuable tool for studying human brain plasticity in vivo (May & Gaser, 2006).
It would follow that sustained biases in behavior such as those mediated by culture could influence brain structure or function. There is an emergent literature suggesting that East Asians and Westerners differ in cognitive processes because of collectivist versus individualistic biases endemic in East Asian and Western cultures, respectively (Nisbett & Masuda, 2003). As a result, East Asians tend to process information holistically and Westerners analytically (Nisbett & Miyamoto, 2005; Park & Gutchess, 2002). These behavioral observations are supported by eyetracking data (Chua, Boland, & Nisbett, 2005) and fMRI studies (Han & Northoff, 2008; Goh et al., 2007). However, the extent to which these factors relate to differences in brain structure has not been adequately evaluated and thus is the focus of the present research.
Although the impetus for comparisons of brain structures in East Asians and Westerners in the present article stems from the growing focus on the role of cultural values in neural and behavioral function, it is also likely that differences in diet, environmental exposure and/or genetics affect neural structure. With this caveat in place, a careful examination of ethnic/cultural differences in brain structures could prove useful at methodological and neurocognitive levels.
At the methodological level, the existence of significant morphological differences between persons of different ethnicity might motivate the creation and use of ethnic/culture-specific brain templates for the purpose of evaluating functional imaging data.
At a neurocognitive level, the discovery of colocalized functional and structural brain changes might influence data interpretation, for example, how we might evaluate claims concerning the unique demands of Chinese character processing on functional engagement of the left pFC (Kochunov et al., 2003; Tan et al., 2001).
At a conceptual level, the study of ethnic/culture differences is an important methodology for understanding neuroplasticity, particularly when individuals of different ages are sampled. How different are the groups at younger ages relative to older ages? Differences that magnify with age are more likely due to experience and environmental factors, whereas differences that decrease with age are more likely due to the increasing influence of biological control over the aging process (Park, Nisbett, & Hedden, 1999).
To date, three structural imaging studies have examined differences in brain structure in East Asians and Westerners, although none have included comparisons of young and old adults. The first of these was a study that examined differences in the locus of intersubject variation in brain shape across Japanese and European volunteers, using MRI and three-dimensional reconstruction (Zilles, Kawashima, Dabringhaus, Fukuda, & Schormann, 2001). They reported shorter and wider hemispheres in Japanese compared to Europeans. In the other two studies, the focus was on the role of life-long use of significantly different languages (Green et al., 2007; Kochunov et al., 2003), with a general finding of increased tissue in left inferior frontal cortex and left middle temporal gyrus as well as right superior temporal gyrus. The differences observed were hypothesized to be a result of the neural requirements for fluency in spoken and written Chinese, as distinct from those required to support English.
At present there are no studies of which we are aware that examined differences in brain structure as a function of age across cultures/ethnicity. This is an important comparison because it affords a life span perspective on cultural/ethnic contributions to brain structure, providing a window as to whether differences observed in young adults persist in a cohort of old adults who have undergone significantly different experiences as well as environmental exposures relative to young adults.
The study has four main methodological and design features. First, prior studies used relatively rudimentary measures to ensure the equivalence of imaging data collection across study sites. Here, we harnessed many advances in imaging and image analysis methodologies accumulated as a result of multicenter imaging research collaboration (Jack et al., 2008; Keator et al., 2008), for example, phantom quality assurance data (Mallozzi, Blezek, Gunter, Jack, & Levy, 2006), imaging sequence uniformity, verification of imaging data quality at the individual subject level (Jack et al., 2008), and gradient unwarping (Jovicich et al., 2006). In addition, we obtained data from the same model of MR scanner using the same type of imaging coils. Second, to obviate concerns that our findings might be restricted by analysis methodology (May & Gaser, 2006), we evaluated brain structure using both of the most widely used semiautomated brain morphometry tools—Freesurfer (Martinos Imaging Centre, Charlestown, MA) and voxel-based morphometry (VBM; FMRIB, Oxford, United Kingdom). Third, to maximize within-group homogeneity in terms of environment, genetics, and cultural values, we selected only Singaporeans of Chinese ethnicity for participation in the East Asian sample. In the U.S. sample, individuals of Asian heritage were excluded. Finally and perhaps most importantly, young and old participants were carefully matched for cognitive function across cultures, using nonverbal tests of speed and working memory. The young Singaporeans and Americans we tested were equivalent in cognitive function as were the old Singaporeans and Americans. As is typical for cognitive aging samples, the older adults showed decreased speed and working memory function relative to young adults (Park et al., 2002).
On the basis of the extant literature, we hypothesized that the more analytic, focused cognitive bias amongst Americans relative to the Singaporean sample (Nisbett & Miyamoto, 2005) would give rise to greater cortical thickness or gray matter density in the frontal lobes bilaterally because of greater focus on engagement of these areas. We also hypothesized that the holistic bias to processing visual information would result in larger hippocampi among the East Asian subjects given the importance of the latter in contextual processing (Cohen et al., 1999). In view of functional imaging evidence that shows an interaction between age and culture in visual processing (Goh et al., 2007), we postulated that age by culture effects would be found in the ventral visual cortex.
METHODS
Participants
Two hundred eleven (211) right-handed healthy volunteers were recruited from two separate sites, young and old adults in Singapore (SG) and young and old adults in Champaign, Illinois. The young adults in Singapore were students from the local universities and in the United States were students at the University of Illinois at Urbana-Champaign, Illinois. The older adults at both sites were healthy community-dwelling older adults who were recruited via community organizations and advertisements. The 211 volunteers comprised 104 young adults (age range=18–30 years, with 50 from Singapore) and 107 older adults (age range = 60–85 years, with 50 from Singapore).
Volunteers were excluded if there was (1) evidence of psychiatric illness within the past 2 years, including substance abuse, (2) a history of recreational drug use in the previous 6 months, (3) less than 10 years of education, (4) less than 20/30 vision after correction, and (5) a history of CNS disease or brain injury, if they were (6) left-handed, (7) pregnant, or (8) had a Mini-Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975) score lower than 26, and (9) if there were conditions that would contraindicate MRI. Because all instructions were presented in English, individuals who were not fluent in English were excluded (English is one of the four official languages in Singapore).
Of the 211 volunteers recruited, 2 were not analyzed on account missing data. In addition, 40 were not sufficiently well positioned in the center of the coil (the imaging “sweet spot” for this scanner). This occurred for a variety of reasons, but most commonly because participants’ necks were too short or their shoulders were too large for the head to be properly placed. Six volunteers’ MR images did not have sufficient gray/white matter contrast to permit high-quality image segmentation. This resulted in 163 remaining participants. Data associated with a further 23 Singaporean volunteers were discarded to ensure that the two groups were balanced in terms of age, sex, education, and number. This resulted in the analysis of data from140 participants (Table 1). The analyzed subjects comprised 39 young Singaporean volunteers (mean age = 22.8 years, SD = 1.5 years; 20 women; mean education = 14.1 years, SD = 0.5 years; mean MMSE = 29.5, SD = 0.8), 31 elderly Singaporean volunteers (mean age = 64.2 years, SD = 2.9 years; 18 women; mean education = 13.2 years, SD = 1.5 years; mean MMSE = 28.2, SD = 1.1), 39 young U.S. volunteers (mean age = 21.1 years, SD = 1.8 years; 20 women; mean education = 14.2 years, SD = 0.7 years; mean MMSE = 29.1, SD = 1.0), and 31 elderly U.S. volunteers (mean age = 64.6 years, SD = 3.2 years; 18 women; mean education = 15.9 years, SD = 1.9 years; mean MMSE = 28.1, SD = 1.2). Although Singaporean elderly participants had fewer years of formal education than U.S. participants, they can still be considered to be enriched on account of their having received an average of 3 years more education than a large cohort of elders recently studied in a mixed targeted recruitment/community-based study on healthy cognitive aging (Chee et al., 2009). Moreover, extensive psychometric data were collected suggesting general equivalency in the samples (described below).
Table 1.
Key Volunteer Characteristics (N = 140)
| YU | OU | YS | OS | |
|---|---|---|---|---|
| n | 39 | 31 | 39 | 31 |
| Mean age | 21.05 (1.76) | 64.58 (3.21) | 22.82 (1.50) | 64.23 (2.85) |
| Age range | 19–28 | 61–71 | 20–28 | 61–71 |
| Female/male | 20/19 | 18/13 | 20/19 | 18/13 |
| Years of education | 14.15 (0.67) | 15.90 (1.85) | 14.10 (0.45) | 13.23 (1.52) |
| MMSE | 29.10 (0.97) | 28.10 (1.19) | 29.54 (0.76) | 28.19 (1.08) |
YU = young U.S.; OU = older U.S.; YS = young SG; OS = older SG.
Values in parentheses are standard deviations.
Only individuals of Chinese ethnicity were included in the Singapore sample (e.g., Indians, Malays, and Whites were excluded). Individuals of East Asian descent were excluded form the U.S. sample. The U.S. group’s race/ethnicity was as follows: young U.S.: 34 Whites, 4 Blacks, 1 Latino/Hispanic; old U.S.: 29 White, 2 Blacks.
Neuropsychological Assessment and Cultural Values Questionnaire
All volunteers received a battery of cognitive tests and also completed the Schwartz Value Scale (Schwartz, 1992), a questionnaire designed to assess how strongly participants subscribed to traditional Eastern versus Western values. The cognitive tests included three measures of processing speed: pattern matching (Salthouse, 1996), the Wechsler Adult Intelligence Scale—Revised (WAIS-R) Digit-Symbol (Wechsler, 1981), and a dot-matching task requiring comparison of 3 × 3 dot matrices developed by Park et al. (2005) for cross-cultural research. There were two measures of working memory: the Wechsler Memory Scale, Third Edition (WMS-III) Corsi Blocks Forward and Backward, and Letter–Number Sequencing (Wechsler, 1997). In addition, the WMS-III Mental Control Task was included (Wechsler, 1997) as well as the MMSE (Folstein et al., 1975). All of the tasks except the MMSE relied on visuospatial, numbers, or letters rather than words.
The neuropsychological assessments took place onsite at the respective hosting laboratory in each country. The personnel involved in test administration underwent common training and conferred frequently across the course of the data acquisition.
Imaging Protocol
Image acquisition at each site took place on 3T Siemens Allegra (Siemens, Erlangen, Germany) systems. Measures to document the similarity of functional imaging data obtained fromthese two systems have been published (Sutton et al., 2008). Identical imaging protocols were used at both sites. Care was taken to ensure optimal placement of each volunteer’s head given the nonlinear spatial distortion characteristics of the imaging systems involved. The principal sequence relevant to this study was a T1-weighted MP-RAGE optimized for gray–white matter contrast; repetition time = 2300 msec, inversion time = 900 msec, flip angle = 9°, bandwidth = 240 Hz/pixel, field of view = 256 × 240 mm, matrix = 256 × 256, resulting voxel dimensions = 1.0 × 1.0 × 1.1 mm, and acquisition time = 9 min 14 sec. Images were screened by a neurologist for pathological findings.
Image Analyses
Quality Assurance
Because data were collected at two different sites, a great deal of care was taken to ensure intersite comparibility of data between scanners. A 165-sphere ADNI Magphan phantom (The Phantom Laboratory, Salem, NY) was scanned daily at both sites before the data acquisition to evaluate geometric distortion and signal-to-noise ratio. To evaluate geometric distortion, we segmented the phantom images obtained using the MPRAGE sequence, determined the locations of the centroids of the segmented spheres, and noted the deviations from ideal. To compare data across sites, we evaluated the spatial deviations from ideal across sites.
Image Processing
The primary tool used for analyzing brain structure was Freesurfer 3.0.5 (Martinos Imaging Centre). Processing each MR image involved removal of nonbrain tissue using a hybrid watershed algorithm (Segonne et al., 2004), bias field correction (this was performed with a custom Allegra-specific tool), automated Talairach transformation, segmentation of subcortical white matter and gray matter (Fischl et al., 2002, 2004), intensity normalization, tessellation of the gray/white matter boundary, automated correction of topology defects, surface deformation to form the gray/white matter boundary and gray/cerebrospinal fluid boundary, and parcellation of cerebral cortex (Desikan et al., 2006; Fischl et al., 2004) on the basis of gyral and sulcal information derived from manually traced brains. Morphometric evaluation of each hemisphere was conducted independently. Each pial surface and gray–white junction mesh was carefully reviewed by a single researcher (ZH), and the meshes were edited manually as necessary to conform to visually determined anatomical boundaries. All measures were corrected for head size before statistical analysis.
Estimated total intracranial volumes
The estimated total intracranial volume (eTIV) was calculated using a validated method described elsewhere (Buckner et al., 2004; Mathalon, Sullivan, Rawles, & Pfefferbaum, 1993). Briefly, an Atlas Scaling Factor was determined on the basis of the transformation matrix of atlas normalization for each individual volunteer. The Atlas Scaling Factor was then used to scale the total intracranial volume of the standardized atlas brain to compute a given volunteer’s eTIV.
Volumes of interest
The VOI reported in the present study involved select subcortical regions—thalamus, caudate, putamen, and hippocampus as well as the ventricles. Total ventricular volume was defined as the total volume of lateral ventricles, third ventricle, fourth ventricle, and fifth ventricle. Total cerebral volume was the sum of total cerebral gray matter volume, total cerebral white matter volume, and total subcortical structure volume. These VOI were adjusted for eTIV before analyses to account for group and gender differences in head size. The adjustment was performed using an ANCOVA approach (Buckner et al., 2004; Mathalon et al., 1993), such that
b being the slope of the linear regression between the VOI volume and the eTIV. It has been reported that the relationship between total intracranial volume and VOI volume changes with age (O’Brien et al., 2006). Therefore, VOI volumes were adjusted independently for young and old groups to the same average intracranial volume for the whole group. As ventricular measurements are positively skewed, we log-transformed raw measurements before making adjustments for head size differences.
Cortical thickness
FreeSurfer creates cortical models by forming the optimal boundaries separating gray/white and gray/cerebrospinal fluid. The thickness of the cortical gray matter was defined as the closest distance from the gray/white to the gray/cerebrospinal fluid boundary at each vertex on the tessellated surface. Procedures for the measurement of cortical thickness have been previously validated against histological analysis (Rosas et al., 2002) and manual measurements (Fischl et al., 2004; Kuperberg et al., 2003). The surface data were smoothed using a Gaussian kernel with an FWHM of 25 mm. Statistical maps of differences in cortical thickness were thresholded at a false discovery rate level of 0.05.
Secondary analysis with FSL-VBM
A long-standing issue with automated brain morphometry studies is the concern that results obtained from different methods may be difficult to compare (Klauschen, Goldman, Barra, Meyer-Lindenberg, & Lundervold, 2008). To address this concern, we also processed image data with FMRIB Software Library VBM (FSL-VBM) (Smith et al., 2004), using VBM (Good et al., 2001; Ashburner & Friston, 2000). First, skull, dura, and other nonbrain tissues were removed with BET (Jenkinson, Bannister, Brady, & Smith, 2002). Next, tissue-type segmentation was preformed using FAST4 (Zhang, Brady, & Smith, 2001). The resulting gray matter partial volume images were then aligned to MNI152 standard space using the affine registration tool FLIRT (Jenkinson et al., 2002; Jenkinson & Smith, 2001), followed optionally by nonlinear registration using FNIRT (Andersson, Jenkinson, & Smith, 2007), which uses a b-spline representation of the registration warp field (Rueckert et al., 1999). The resulting images were averaged to create a study-specific template, to which the native gray matter images were then nonlinearly reregistered. To correct for local expansion or contraction, we modulated the registered partial-volume images by division with the Jacobian of the warp field. The modulated segmented images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm. Finally, a voxelwise statistical map was computed using permutation-based nonparametric testing, using a false-positive cutoff of 5% corrected for multiple comparisons. These points were mapped on their corresponding locations on gray-white junction remapped into spherical coordinate space and compared with the cortical thickness maps created using Freesurfer.
Anatomical labeling convention
The labels applied to neuroanatomical sites showing cortical thickness or gray matter density changes were determined by referring to the group-specific automated surface parcellation generated by Freesurfer. This method has been previously validated using manually segmented images and represents an unbiased labeling strategy (Desikan et al., 2006). In addition, as many readers might be interested in correlating these structural findings with functional data, we provided the corresponding Brodmann’s area (BA) labels.
Support vector machine classification
To cross validate the discriminability between U.S. young and SG young in an unbiased fashion, we carried out classifications with cortical thickness as the feature of interest. LIBSVM 2.87 (Chih-Chung Chang, 2001) with linear kernel and default cost parameter was used for the classification. Threefold cross validation was performed on each vertex and the resulting accuracies were mapped onto the cortical surface.
RESULTS
Neuropsychological and Schwartz Value Scale Data
The results from the neuropsychological testing are presented in Table 2. For every cognitive task, there was an age main effect. In addition, there was a group main effect on the Corsi Blocks Forward, F(1, 136) = 4.63, p < .05, η2 = .03, because of somewhat higher performance in the U.S. participants. In addition, there was an Age × Group interaction on the WAIS-R Digit-Symbol Task, F(1, 136) = 6.23, p < .05, η2 = .04, that occurred because the difference between young and old was greater in Singaporeans than Americans. No other effects involving group were significant, and the two that we reported were only significant at the .05 level. Thus, the neuropsychological data suggest that the groups were well matched for cognitive function and that differences in brain structures cannot be attributed to differences in basic cognitive function.
Table 2.
Neuropsychological Test Performance Scores
| Mean (SD) | Significance of F Values | |||||||
|---|---|---|---|---|---|---|---|---|
| Test | Subscale | YU | OU | YS | OS | Age | Group | Age × Group |
| Schwartz Value Scale | Power | 8.7 (4.4) | 8.5 (4.5) | 12.8 (3.7) | 12.7 (3.9) | * | ||
| Achievement | 17.8 (3.1) | 16.5 (3.9) | 18.3 (3.1) | 17.4 (3.9) | ||||
| Hedonism | 9 (1.9) | 8.1 (2.2) | 8.1 (2.5) | 7.2 (1.7) | ** | ** | ||
| Stimulation | 11.4 (3.7) | 10 (3.3) | 12 (3.1) | 10.7 (3.3) | ** | |||
| Self-direction | 23.6 (3.7) | 24 (3.3) | 23.6 (3.1) | 23.8 (3.3) | ||||
| Universalism | 35.5 (6.9) | 34.9 (6.1) | 33.9 (5.0) | 38.7 (6.7) | ** | ** | ||
| Benevolence | 23.8 (3.7) | 25.1 (3.9) | 25.4 (3.1) | 26.7 (3.3) | * | * | ||
| Tradition | 12.8 (5.0) | 16.5 (5.6) | 18.3 (3.1) | 23.8 (3.3) | * | * | ||
| Conformity | 15.3 (3.7) | 18.4 (3.3) | 17.8 (3.7) | 21.4 (2.2) | * | * | ||
| Security | 18.8 (3.7) | 21.9 (4.5) | 22 (3.7) | 26 (3.3) | * | * | ||
| Pattern matching | 41.9 (5.6) | 28 (6.7) | 43.2 (6.2) | 26.4 (5.6) | * | |||
| WAIS-R Digit Symbol | 73.3 (9.4) | 55.9 (7.7) | 78.9 (9.4) | 53.7 (8.9) | * | ** | ||
| Dot matching | 16.2 (2.5) | 10.1 (2.8) | 17.4 (3.1) | 9.9 (3.3) | * | |||
| WMS-III Corsi Blocks | Forward | 10.3 (1.9) | 8.2 (1.7) | 9.6 (1.2) | 7.7 (1.7) | * | ** | |
| Backward | 9.5 (1.9) | 7.5 (1.7) | 9.2 (1.2) | 8 (1.7) | * | |||
| WMS-III Letter Number Sequencing | 14.3 (3.1) | 10.2 (2.8) | 13.2 (3.1) | 9.4 (2.2) | * | |||
| WMS-III Mental Control | 28.6 (5.6) | 24.3 (4.5) | 28.6 (3.1) | 22.2 (4.5) | * | |||
| MMSE | 29.1 (1.2) | 28.1 (1.1) | 29.5 (0.6) | 28.2 (1.1) | * | |||
Values in parentheses are standard deviations; YU = young U.S.; OU = older U.S.; YS = young SG; OS = older SG.
p < .05.
p < .01.
An analysis of the subscales from the Schwartz Value Scale (Schwartz, 1992) indicated that the groups held to values commonly associated with their respective cultures. Singaporeans rated higher than U.S. participants in collectivistic values that included benevolence, F(1, 136) = 7.24, p < .01, η2 = .05, tradition, F(1, 136) = 71.10, p < .01, η2 = .34, conformity, F(1, 136) = 22.24, p < .01, η2 = .14, and security, F(1, 136) = 31.68, p < .01, η2 = .19. There was an Age × Group interaction on the universalism subscale, F(1, 136) = 6.06, p < .05, η2 = .04, which resulted from the particularly high score recorded by older Singaporeans. Singaporeans also scored higher than Americans on the power subtest, F(1, 136) = 34.08, p < .01, η2 = .20, perhaps reflecting the greater emphasis East Asian cultures place on authority. Americans scored higher than East Asians on the hedonism subscale, F(1, 136) = 6.04, p < .05, η2 = .04, reflecting the high value placed on personal pleasure and satisfaction in this culture.
Age modulated values, there being main effects of age on almost every variable. Young adults rated higher on hedonism, F(1, 136) = 6.57, p < .05, η2 = .05, and stimulation, F(1, 136) = 5.20, p < .05, η2 = .04, whereas older adults scored higher in benevolence, F(1, 136) = 5.30, p < .05, η2 = .04, tradition, F(1, 136) = 36.43, p < .01, η2 = .21, conformity, F(1, 136) = 31.17, p < .01, η2 = .19, and security, F(1, 136) = 30.14, p < .01, η2 = .18. This indicates an overall trend toward collectivism with increased age.
Quality Assurance
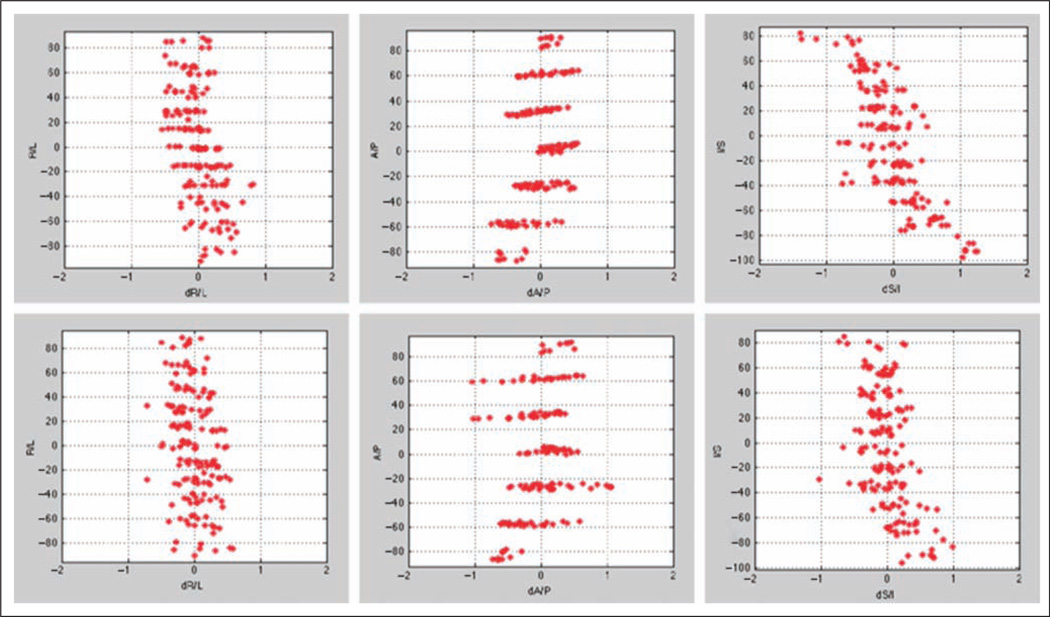
To ensure that signal quality was equivalent across scanners, we measured absolute displacement of each sphere within a phantom. The absolute displacement of each sphere from the phantom was mainly within 1 mm (Figure 1). Notably, given that most of the brain regions reported here are on the lateral surface, distortions along the right-left direction were the lowest, being within 0.5 mm of ideal. As cortical thickness modeling results in inferences that concern submillimeter differences between the compared groups, it is important to point out that these differences are relative, and the critical metric is how well the scanners at the two sites corresponded in terms of geometric deviations from ideal at each point in imaging space. We found that although some spheres differed from ideal in a significant manner, the differences between displacements from ideal were within 0.5 mm across sites.
Figure 1.
Spatial deviations of individual reference spheres within a geometry calibration (ADNI) phantom for the scanner used on Singaporeans (upper row) and Americans (lower row). Data have undergone gradient unwarping.
Head Size and Volume of Selected Subcortical Structures
Measurements of head size and selected subcortical structures are displayed in Table 3. As shown, young U.S. volunteers had larger intracranial volumes than their SG counterparts, but these differences were nonsignificant in the comparison between older Singaporean and U.S. volunteers. Intracranial volumes are uncorrected values. In contrast, the volumes of other subcortical structures (thalamus, caudate, putamen, and hippocampal formation) were adjusted for head size. Nearly all measures in Table 3 showed a reduction in volume with age, except for ventricular volume, which increased. Table 4 presents correlation values for the age effect for each structure. These are reported to allow for a direct comparison with an aging study of a different sample of Singaporeans conducted by Chee et al. (2009). The age effects generalized across both cultures. In summary, the volumetric measures show little evidence of systematic differences related to group/ethnicity, a finding similar to the neuropsychological data.
Table 3.
Structural Volumes
| Main Structural Volumes (cm3) |
Mean (SD) | Significance of F Values | |||||
|---|---|---|---|---|---|---|---|
| YU | OU | YS | OS | Age | Group | Age × Group | |
| Total Intracranial Vol. | 1594.84 (181.12) | 1431.11 (141.60) | 1466.01 (136.42) | 1391.36 (154.49) | ** | ** | |
| Thalamus | 15.21 (1.22) | 12.65 (0.83) | 14.97 (1.10) | 13.02 (0.73) | ** | ||
| Caudate | 7.70 (0.72) | 7.11 (0.84) | 7.58 (0.81) | 6.98 (0.52) | ** | ||
| Putamen | 11.38 (0.97) | 9.67 (0.89) | 11.48 (1.22) | 9.94 (0.72) | ** | ||
| Hippocampus | 8.56 (0.54) | 7.86 (0.63) | 8.56 (0.56) | 7.75 (0.46) | ** | ||
| Ventriclesa | 1.17 (0.15) | 1.42 (0.16) | 1.22 (0.13) | 1.42 (0.13) | ** | ||
| Cerebral White Matter | 455.03 (21.25) | 473.42 (21.41) | 467.40 (23.64) | 475.37 (21.71) | ** | ||
| Cerebral Gray Matter | 476.32 (20.23) | 424.72 (21.25) | 472.89 (19.93) | 420.13 (19.27) | ** | ||
| Total Cerebral Vol. | 1010.82 (38.97) | 927.90 (40.92) | 1010.02 (42.72) | 920.36 (31.65) | ** | ||
Values in parentheses are standard deviations; YU = young U.S.; OU = older U.S.; YS = young SG; OS = older SG.
Ventricle volumes were log-transformed.
p < .01.
Table 4.
Age-Related Correlations for VOI Volumes
| VOI | SG (rage) | U.S. (rage) |
|---|---|---|
| Intracranial | −.24* | −.45** |
| Thalamus | −.71** | −.78** |
| Caudate | −.38** | −.35** |
| Putamen | −.60** | −.68** |
| Hippocampus | −.62** | −.53** |
| Ventriclesa | .65** | .67** |
| Cerebral GM | −.81** | −.80** |
| Cerebral WM | .17 | .38** |
| Total cerebrum | −.76** | −.75** |
Ventricle volumes were log-transformed.
p < .05.
p < .01.
Significant Differences in Cortical Thickness and Gray Matter Density between Young Participants
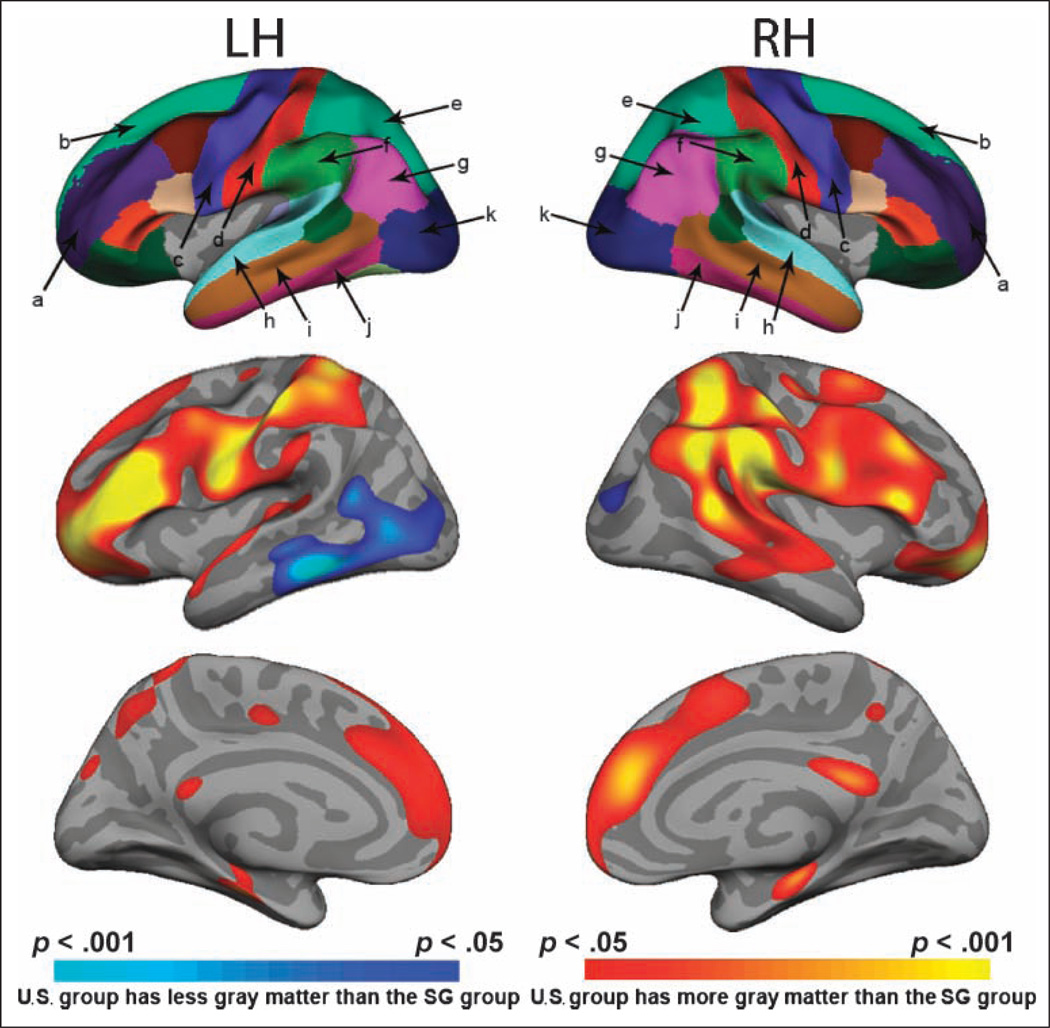
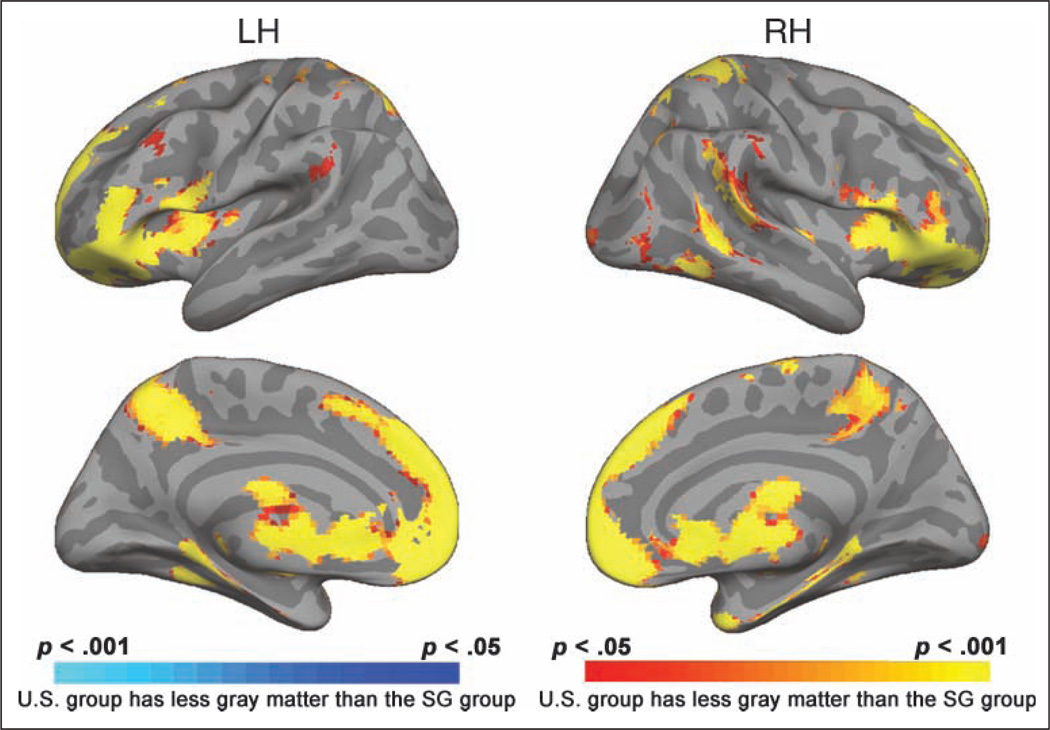
We found significantly higher cortical thickness in U.S. young relative to SG young in several cortical regions that correspond to polymodal association cortex. On the lateral surface of the cerebral cortex (Figure 2), these included bilateral inferior frontal gyrus (corresponding to lateral and anterior pFC BA 45, 47 and BA 10, 11), the left precentral gyrus (premotor cortex, BA 8), the right supramarginal gyrus with part of the angular gyrus (BA 39, 40), and the right superior parietal lobule (BA 7). On the medial cerebral cortical surface, U.S. young had thicker cortex in the superior frontal gyrus bilaterally (BA 9, 10), the right medial-temporal region (BA 35), and around the precuneus bilaterally (BA 7). Only one region was thicker in SG young, and this was in the left inferior temporal gyrus (BA 37).
Figure 2.
Upper panel shows the result of the automated cortical parcellation process in which specific areas on the lateral cortical surface are labeled. Cortical labeling: (a) middle frontal, (b) superior frontal, (c) precentral, (d) postcentral, (e) superior parietal, (f) supramarginal, (g) inferior parietal, (h) superior temporal, (i) middle temporal, (j) inferior temporal, (k) lateral occipital. The lower panels show cortical surface thickness revealing regions where U.S. young (yellow-red) and SG young subjects (blue) show statistically greater cortical thickness. Map thresholds are indicated by the color bars.
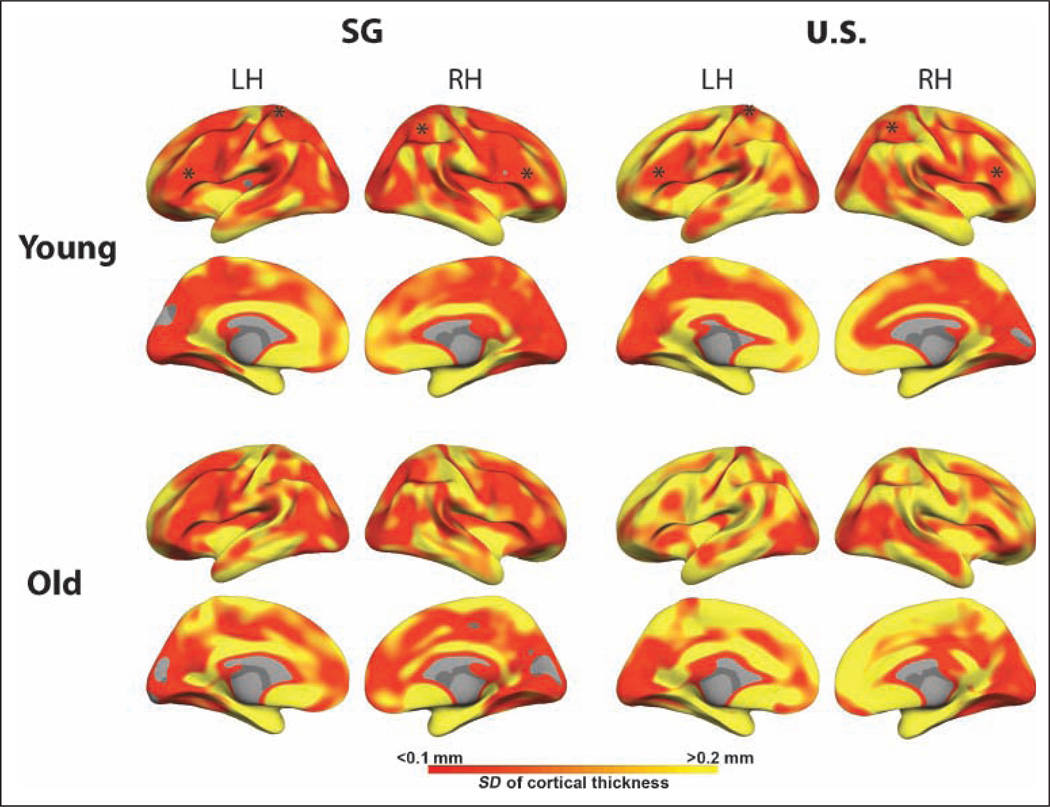
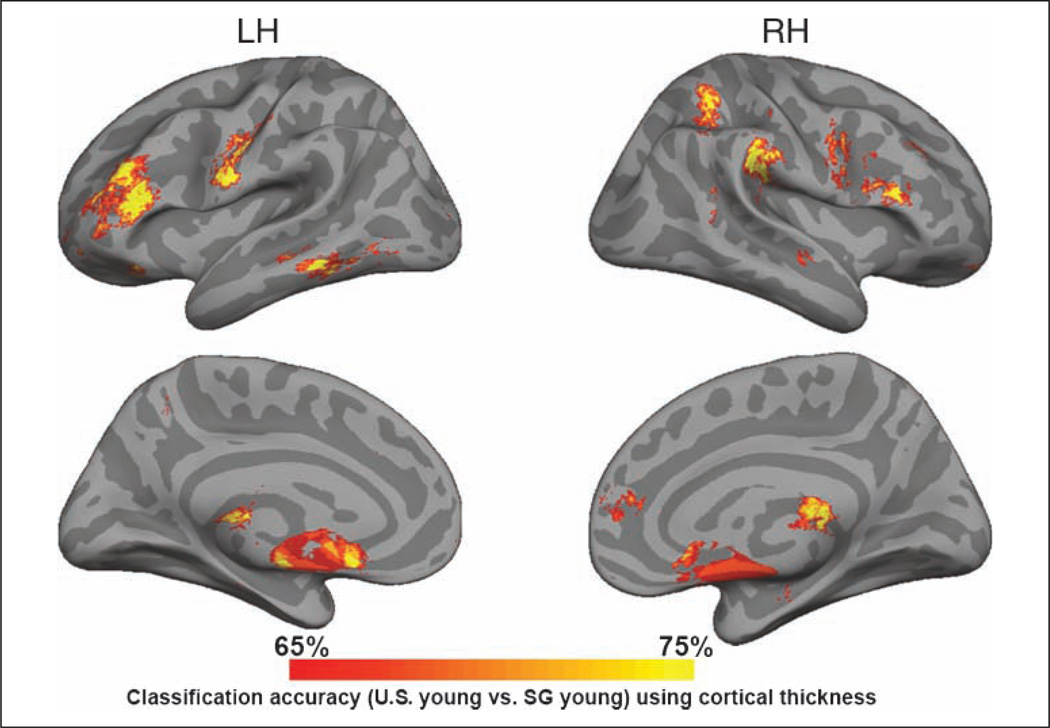
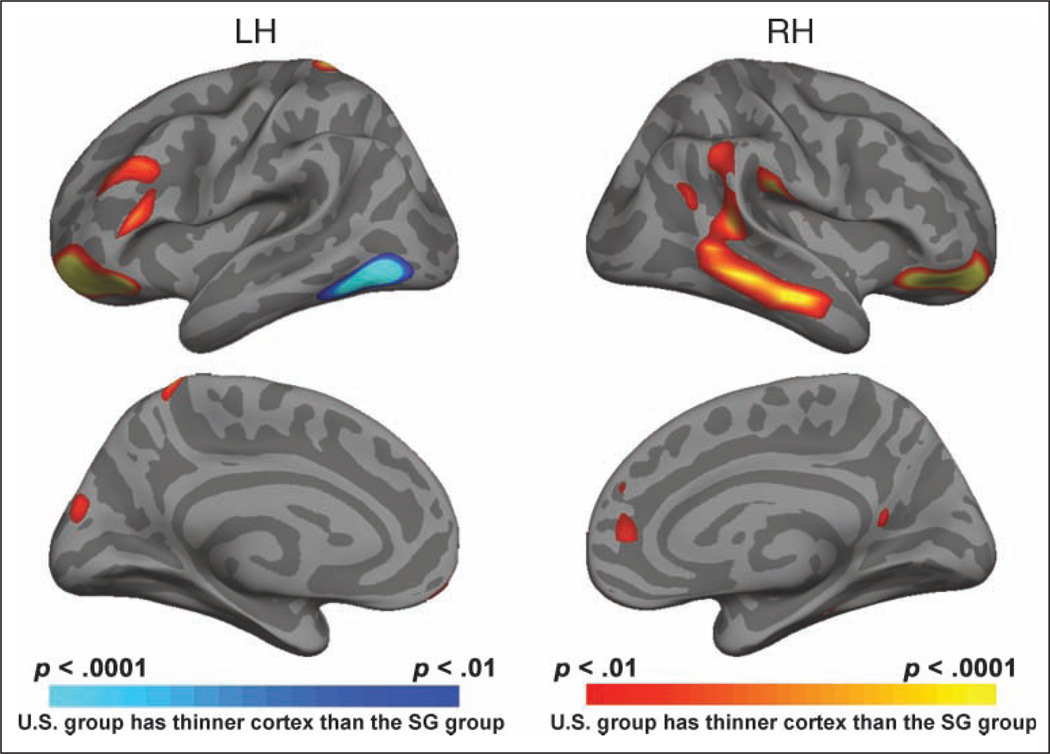
Notably, the variations we observed in thickness as a function of group occurred in regions that showed less local variability in cortical thickness, adding confidence that the findings are reliable (Figure 3). In addition, to further assess the reliability of our data, the data from all 78 young volunteers were subject to a support vector machine classifier that identified vertices on the surface mesh that discriminated between the U.S. young and the SG young on the basis of cortical thickness. Surface regions showing a classification accuracy of 65% or more were found in bilateral inferior prefrontal regions, left precentral region, right supramarginal gyrus, superior parietal lobule, and medial-temporal region (Figure 5). Analysis using VBM yielded findings that broadly concurred with the cortical thickness–based analysis, adding further confidence to the findings. Since thresholding and smoothing are different for cortical thickness analysis and VBM, it is not appropriate to use “similarity indices” such as the Jaccard similarity index or Dice coefficient to compare the two methods, as ground truth is not available. Importantly, we report that using two very different methodologies for measurement of gray matter, we find reasonably convergent results.
Figure 3.
Cortical surface maps denoting the extent of variation of cortical thickness by region and in each of the four groups. Asterisks in the frontal and dorsal parietal regions identify regions where group differences in cortical thickness were reliably identified in young subjects. Note that these lie in regions where there is relatively low within-group variation of thickness.
Figure 5.
Cortical surface maps showing the locations where support vector machine-based classification (accuracy > 65%) was able to differentiate U.S. and SG young on the basis of cortical thickness. The concurrence between this map and the preceding one provides convergent information regarding the reliability of detection of between group differences in cortical structure.
With this last consideration in mind, we note that regions showing higher gray matter density that concurred with the cortical thickness findings were bilateral inferior prefrontal regions, right supramarginal gyrus, superior parietal lobule, and medial-temporal region on the lateral cortical faces and the precuneus and anterior medial prefrontal region bilaterally on the medial faces of the cerebral cortex (Figure 6). The only clear differences between Freesurfer and VBM were in the left premotor and inferior temporal gyri.
Figure 6.
VBM-identified differences in gray matter density projected on FreeSurfer-derived cortical surface. These maps show significant gray matter density differences between U.S. young and SG young (compare these with the FreeSurfer analyses in Figure 5).
Differences in Cortical Thickness in Old Participants
We did not observe any significant differences between U.S. old and SG old except that the latter had a thicker left inferior temporal gyrus. As with the analysis for young volunteers, this finding was not present in the VBM analysis.
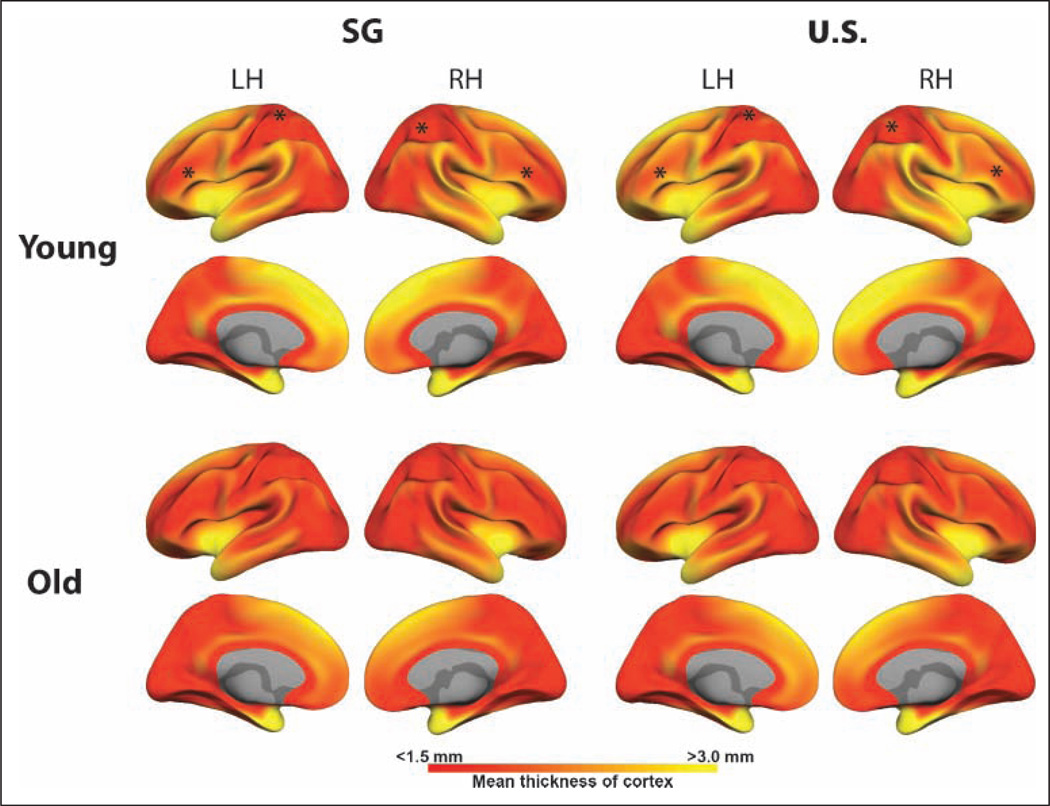
Careful inspection of the cortical thickness and variability of thickness measures in this group, however, suggests that the absence of a difference does not equate to there being no difference between U.S. and SG old persons. In particular, the high variability of cortical thickness in elders particularly in the frontal lobe lowers the likelihood that a significant between-group difference would be detected (Figures 3 and 4).
Figure 4.
Cortical surface maps displaying the mean cortical thickness by region in each of the four groups. Asterisks in the frontal and dorsal parietal regions mark regions where significant group differences in cortical thickness were identified (see Figure 2).
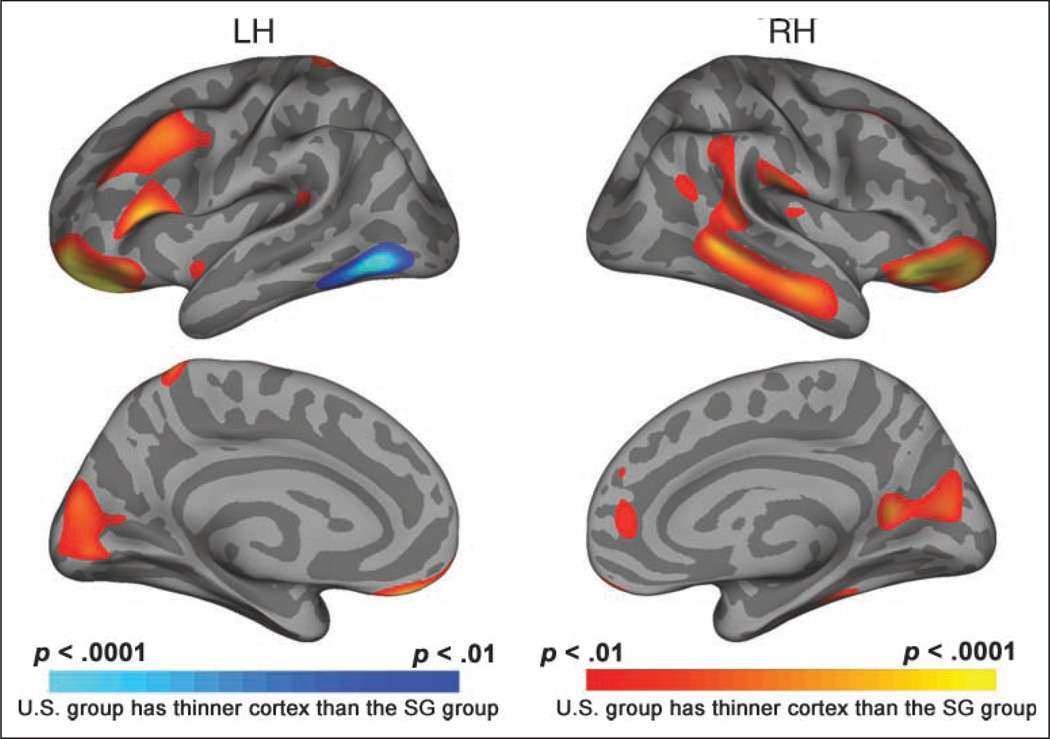
In view of evidence that processing speed accounts for the majority of age-related variance on cognitive tasks (Salthouse, 1996), we performed a median split of the old participants in each elderly group on the basis of their WAIS-R Digit-Symbol scores. When we compared U.S. and SG high-performing elderly in this manner, we found group differences in cortical thickness similar to the group differences observed between the young U.S. and the SG volunteers (Figure 7). A conjunction analysis (Figure 8) showed large area of overlap between Figures 2 and 7. The comparison between low-performing elderly did not show significant group differences.
Figure 7.
Cortical thickness maps identifying regions that have greater cortical thickness in a subgroup of U.S. and SG elderly who scored comparably higher on a test of processing speed. The similarity between this comparison and the group comparison between U.S. and SG young suggests that the group differences identified are stable across age in higher functioning elderly adults.
Figure 8.
Conjunction map showing cortical areas that show common between-group differences in young (Figure 2) and high-performing old (Figure 7) subjects.
Correlation between Measures of Brain Structure and the Schwartz Value Scale
After controlling for the effect of group, there was no significant correlation between subscale scores within the Schwartz Value Scale and cortical thickness in either elderly or young persons.
Gender Effects
We also examined our data for gender differences and found minimal evidence for differences—young women showed greater cortical thickness in the postcentral gyrus bilaterally and the anterior-most portion of the right superior parietal lobule. Critically, the loci of these effects did not overlap with areas showing group differences in cortical thickness (with the exception of the anterior most portion of the right superior parietal lobule), suggesting the group differences reported above cannot be attributed to gender. This finding allowed us to collapse morphometric data across gender for greater power (Hulshoff Pol et al., 2006; Salat et al., 2004).
DISCUSSION
In the largest and most thoroughly controlled comparison of brain structure between two ethnic/cultural groups to date, we found that young predominantly White American adults had thicker cortex in several parts of frontal, parietal, and medial-temporal polymodal association cortices in both hemispheres. We did not find group differences in hippocampal or ventral visual cortex volume that were predicted on the basis of expected cultural effects on brain structure. There were pronounced effects of age common across both groups.
Although the differences in gray matter observed between U.S. and SG volunteers were not significant across the entire sample of older subjects, when the high-performing elderly were analyzed separately, U.S. old showed greater thickness in frontal and right inferior temporal areas and SG old showed a small patch of thicker gray matter in the left inferior temporal lobe. These findings were similar to that observed in the comparison between young persons in both groups. While seeking to comment on cultural effects on brain structure, we first review our findings in light of genetic/environmental alternative explanations and also relate our findings to the small body of data on structural differences in Asian and European brains.
Genetic and Environmental Factors Underlying Differences in Cortical Thickness
Both genetic and environmental factors can affect gray matter volume. Genetic influences have been observed at the whole brain (Baare et al., 2001), lobar (Geschwind, Miller, DeCarli, & Carmelli, 2002; Thompson et al., 2001), and region-specific levels (Hulshoff Pol et al., 2006). Genetically influenced covariation in cortical thickness in spatially disparate areas may be related to architectonics (Shaw et al., 2008) and may have functional consequences. Interestingly, cortex with the most complex laminar architecture—association cortex—appears to be under the strongest influence of genetic factors. This is congruent with the related observation that intelligence has a high degree of heritability (Hulshoff Pol et al., 2006; Posthuma et al., 2002), which may be in turn captured in the form of variation in cortical thickness within the frontal and parietal lobes (Narr et al., 2007) of persons belonging to a particular population. The last detail is critical to highlight because if it were not for the broadly matched cognitive scores across the two populations, the cortical thickness differences in the young observed here might be attributed to ethnic differences in intelligence or at least cognitive potential (Rushton & Jensen, 2008).
At the present time, genetic influences on cortical thickness and cognitive ability have been deduced solely from twin studies. The specific genes thought to be responsible for these differences are unknown. Moreover, because we carefully matched samples, both old and young, for critical components of intellectual ability, the differences in cortical thickness observed are not easily attributed to differences in basic processing speed, working memory, or inhibitory capabilities.
There are numerous ways in which environmental factors could influence brain structure and function. In addition to the experience related variables described earlier, education (Fotenos, Mintun, Snyder, Morris, & Buckner, 2008; Staff, Murray, Deary, & Whalley, 2004) as well as a variety of cardiovascular risk/fitness factors (Murray et al., 2005; Colcombe et al., 2004; Raz, Rodrigue, & Acker, 2003) and diet (Kalmijn et al., 2004; Mattson, 2003) could affect the aging process (Bamshad, 2005; Kirkwood, 2005). Further, the effect of environmental factors can be expected to cumulate with age. Interestingly, we found no evidence for greater differences in cortical thickness in our older participants. This suggests that either environmental factors that influence cortical thickness exerted similar effects in both groups or age-related changes in brain volume were large and consistent across groups, dominating the weaker ethnic/culture effects.
Age Effects and Brain Structure
Age had a profound effect on brain size in this sample. The effect of age was broadly equivalent between the U.S. and SG groups. Previous work has shown that despite differences in diet, lifestyle, and culture, Chinese elderly display a comparable rate and pattern of brain atrophy to their White counterparts (Chee et al., 2009). Nevertheless, it is noteworthy that this is the first direct comparison of two culturally and ethnically different samples within a single research study. Finally, the finding of similar age-related declines in structure across groups illustrates the robustness of these changes and their relatively large effect size relative to those pertaining to culture/ethnicity.
The thinness of cerebral cortex in elderly subjects (around 1.5–2.0 mm in most VOIs) and the greater variability (Figures 3 and 4) of these measures could easily obscure a relatively subtle between-group difference. Although the magnitude and sample-indifferent nature of the age effects was notable, it was striking that ethnic/cultural differences in frontal thickness observed in the young U.S. sample were maintained in the old adults when only high performers were examined. Park and Gutchess (2002) suggested that culture effects observed in young adults might be obliterated by the effects of aging on the brain. The present data suggest this to be the case for lower performers.
Comparison with Prior Cross-ethnic Studies on Brain Structure
There are two published studies in the literature that address differences between primarily White and East Asian samples of young adults. Zilles et al. (2001) compared Japanese and European brains using MRI and reported differences in variability in hemispheric shape in occipital and temporal lobe. The present study does not characterize shape data. Genetic studies show relatively little variation of gray matter in the occipital lobe (Geschwind et al., 2002; Thompson et al., 2001), and we found it to be consistently thin across all four groups.
The other study used deformation field morphometry and reported small areas of increased cortex in left frontal, temporal, and parietal lobes in educationally matched Chinese bilinguals (Kochunov et al., 2003). The frontal regions that showed structural differences were also those that were more highly activated in fMRI experiments evaluating language, supporting the authors’ claim that the Chinese language makes higher demands on processing within left hemisphere (Tan et al., 2001).
Crinion et al. (2009) found that Chinese speakers of both European and Asian ancestry showed higher gray matter density in left insula and right anterior temporal regions. This finding suggests that there may be language-driven effects associated with speaking Chinese and being multilingual that are independent of culture/ethnicity.
Critically, in the current study, all SG volunteers were at least bilingual in Chinese and English, and most of their American counterparts were monolingual, yet we did not find the proposed plasticity-driven increase in thickness in relevant language areas for the SG subjects. The present findings argue for caution when drawing inferences from cross-group studies seeking to relate differences in structure to some behavioral variable of interest.
Cultural Influences on Brain Structure
Given the above caveats, it is reasonable to posit that some of the differences in cortical thickness in association cortices observed here may relate to differences in culture-based cognitive processes documented in behavioral studies (Nisbett & Miyamoto, 2005). There is a wealth of findings demonstrating the bias of individuals from East Asian cultures to process information “holistically,” integrating context with target, relative to a more focused, object-centric approach used by “Westerners.” The latter bias in perceptual processing is hypothesized to lend itself to analytical thinking. The finding of thicker polymodal association cortex in U.S. young would be consistent with such a framework although as we have discussed, other unknown genetic or environmental variables may be contributory.
An important point not generally made in more culturally driven explanations of neurocognition is that “cultural effects” are driven by biases that have genetic or environmental underpinnings. For example, it is possible that there is a genetic basis in individuals of European decent that favors the formulation of associations in sensory information. This in turn could bias neural circuitry toward an analytic form of reasoning as opposed to holistic processing.
Alternatively, a plausible environmental explanation for the bias could stem from the urban crowding characteristic of Asian cities—the 24 most densely populated cities in the world are in Asia (City-Mayors-Statistics, 2009). This could impose selection pressures that favor paying close attention to context and less to central figures. In support of this argument, both Japanese and Americans rated photographs of Japanese cities as more complex than photos of New York City (Miyamoto, Nisbett, & Masuda, 2006). Moreover, priming participants with Japanese pictures resulted in more attention to context, suggesting that the perceptual environment may drive some of the differences observed in the present study.
Conclusions
We found robust differences in cortical thickness in polymodal frontal and parietal association cortex between cognitively well-matched young adults who differed in ethnicity and culture using two semiautomated brain morphometric techniques. We have adopted a conservative interpretation of these data and suggest that the findings could have arisen from cultural (external) as well as genetic (inherent) influences that ultimately could be interrelated, meriting further investigation.
Acknowledgments
This work was supported by the BMRC (04/1/36/19/372) and the STaR award awarded to Michael Chee as well as by the National Institute on Aging, grant nos. R01 AGO15047 and R01AGO60625-15 awarded to Denise Park. Sam Sim and Karren Chen contributed to neuropsychological evaluation at the Singapore site.
REFERENCES
- Andersson JLR, Jenkinson M, Smith S. Non-linear optimisation (FMRIB Tech. Rep. No. TR07JA1) 2007 Retrieved from http://www.fmrib.ox.ac.uk/analysis/techrep/
- Ashburner J, Friston KJ. Voxel-based morphometry—The methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Baare WF, Hulshoff Pol HE, Boomsma DI, Posthuma D, de Geus EJ, Schnack HG, et al. Quantitative genetic modeling of variation in human brain morphology. Cerebral Cortex. 2001;11:816–824. doi: 10.1093/cercor/11.9.816. [DOI] [PubMed] [Google Scholar]
- Bamshad M. Genetic influences on health: Does race matter? Journal of the American Medical Association. 2005;294:937–946. doi: 10.1001/jama.294.8.937. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, et al. A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. Neuroimage. 2004;23:724–738. doi: 10.1016/j.neuroimage.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Chee MW, Chen K, Zheng H, Chan PL, Isaac V, Sim KY, et al. Cognitive function and brain structure correlations in healthy elderly East Asians. Neuroimage. 2009;46:257–269. doi: 10.1016/j.neuroimage.2009.01.036. [DOI] [PubMed] [Google Scholar]
- Chih-Chung Chang C-JL. LIBSVM: A library for support vector machines. 2001 Retrieved from http://www.csie.ntu.edu.tw/~cjlin/libsvm. [Google Scholar]
- Chua HF, Boland JE, Nisbett RE. Cultural variation in eye movements during scene perception. Proceedings of the National Academy of Sciences, U.S.A. 2005;102:12629–12633. doi: 10.1073/pnas.0506162102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- City-Mayors-Statistics. The largest cities in the world by land area, population and density. [Retrieved March 5, 2009];2009 from http://www.citymayors.com/statistics/largest-cities-density-125.html. [Google Scholar]
- Cohen NJ, Ryan J, Hunt C, Romine L, Wszalek T, Nash C. Hippocampal system and declarative (relational) memory: Summarizing the data from functional neuroimaging studies. Hippocampus. 1999;9:83–98. doi: 10.1002/(SICI)1098-1063(1999)9:1<83::AID-HIPO9>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, et al. Cardiovascular fitness, cortical plasticity. and aging. Proceedings of the National Academy of Sciences, U.S.A. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crinion JT, Green DW, Chung R, Ali N, Grogan A, Price GR, et al. Neuroanatomical markers of speaking Chinese. Human Brain Mapping. 2009;30:4108–4115. doi: 10.1002/hbm.20832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Busch V, Schuierer G, Bogdahn U, May A. Neuroplasticity: Changes in grey matter induced by training. Nature. 2004;427:311–312. doi: 10.1038/427311a. [DOI] [PubMed] [Google Scholar]
- Draganski B, May A. Training-induced structural changes in the adult human brain. Behavioural Brain Research. 2008;192:137–142. doi: 10.1016/j.bbr.2008.02.015. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, et al. Sequence-independent segmentation of magnetic resonance images. Neuroimage. 2004;23(Suppl. 1):S69–S84. doi: 10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: Evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Archives of Neurology. 2008;65:113–120. doi: 10.1001/archneurol.2007.27. [DOI] [PubMed] [Google Scholar]
- Gaser C, Schlaug G. Brain structures differ between musicians and non-musicians. Journal of Neuroscience. 2003;23:9240–9245. doi: 10.1523/JNEUROSCI.23-27-09240.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind DH, Miller BL, DeCarli C, Carmelli D. Heritability of lobar brain volumes in twins supports genetic models of cerebral laterality and handedness. Proceedings of the National Academy of Sciences, U.S.A. 2002;99:3176–3181. doi: 10.1073/pnas.052494999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh JO, Chee MW, Tan JC, Venkatraman V, Hebrank A, Leshikar ED, et al. Age and culture modulate object processing and object-scene binding in the ventral visual area. Cognitive, Affective & Behavioral Neuroscience. 2007;7:44–52. doi: 10.3758/cabn.7.1.44. [DOI] [PubMed] [Google Scholar]
- Golestani N, Molko N, Dehaene S, LeBihan D, Pallier C. Brain structure predicts the learning of foreign speech sounds. Cerebral Cortex. 2007;17:575–582. doi: 10.1093/cercor/bhk001. [DOI] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Green DW, Crinion J, Price CJ. Exploring cross-linguistic vocabulary effects on brain structures using voxel-based morphometry. Bilingualism (Cambridge, England) 2007;10:189–199. doi: 10.1017/s1366728907002933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Northoff G. Culture-sensitive neural substrates of human cognition: A transcultural neuroimaging approach. Nature Reviews Neuroscience. 2008;9:646–654. doi: 10.1038/nrn2456. [DOI] [PubMed] [Google Scholar]
- Hulshoff Pol HE, Schnack HG, Posthuma D, Mandl RC, Baare WF, van Oel C, et al. Genetic contributions to human brain morphology and intelligence. Journal of Neuroscience. 2006;26:10235–10242. doi: 10.1523/JNEUROSCI.1312-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Jr, Bernstein MA, Fox NC, Thompson P, Alexander G, Harvey D, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. Journal of Magnetic Resonance Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, et al. Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kalmijn S, van Boxtel MP, Ocke M, Verschuren WM, Kromhout D, Launer LJ. Dietary intake of fatty acids and fish in relation to cognitive performance at middle age. Neurology. 2004;62:275–280. doi: 10.1212/01.wnl.0000103860.75218.a5. [DOI] [PubMed] [Google Scholar]
- Keator DB, Grethe JS, Marcus D, Ozyurt B, Gadde S, Murphy S, et al. A national human neuroimaging collaboratory enabled by the Biomedical Informatics Research Network (BIRN) IEEE Transactions on Information Technology in Biomedicine. 2008;12:162–172. doi: 10.1109/TITB.2008.917893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TB. Understanding the odd science of aging. Cell. 2005;120:437–447. doi: 10.1016/j.cell.2005.01.027. [DOI] [PubMed] [Google Scholar]
- Klauschen F, Goldman A, Barra V, Meyer-Lindenberg A, Lundervold A. Evaluation of automated brain MR image segmentation and volumetry methods. Human Brain Mapping. 2008;30:1310–1327. doi: 10.1002/hbm.20599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochunov P, Fox P, Lancaster J, Tan LH, Amunts K, Zilles K, et al. Localized morphological brain differences between English-speaking Caucasians and Chinese-speaking Asians: New evidence of anatomical plasticity. NeuroReport. 2003;14:961–964. doi: 10.1097/01.wnr.0000075417.59944.00. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Maguire EA, Gadian DG, Johnsrude IS, Good CD, Ashburner J, Frackowiak RS, et al. Navigation-related structural change in the hippocampi of taxi drivers. Proceedings of the National Academy of Sciences, U.S.A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallozzi RP, Blezek DJ, Gunter JL, Jack CR, Levy JR. Phantom-based evaluation of gradient non-linearity for quantitative neurological MRI studies. Paper presented at the ISMRM 14th Scientific Meeting and Exhibition; Seattle, WA. 2006. [Google Scholar]
- Mathalon DH, Sullivan EV, Rawles JM, Pfefferbaum A. Correction for head size in brain-imaging measurements. Psychiatry Research. 1993;50:121–139. doi: 10.1016/0925-4927(93)90016-b. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Gene-diet interactions in brain aging and neurodegenerative disorders. Annals of Internal Medicine. 2003;139:441–444. doi: 10.7326/0003-4819-139-5_part_2-200309021-00012. [DOI] [PubMed] [Google Scholar]
- May A, Gaser C. Magnetic resonance-based morphometry: A window into structural plasticity of the brain. Current Opinion in Neurology. 2006;19:407–411. doi: 10.1097/01.wco.0000236622.91495.21. [DOI] [PubMed] [Google Scholar]
- Miyamoto Y, Nisbett RE, Masuda T. Culture and the physical environment. Holistic versus analytic perceptual affordances. Psychological Science. 2006;17:113–119. doi: 10.1111/j.1467-9280.2006.01673.x. [DOI] [PubMed] [Google Scholar]
- Murray AD, Staff RT, Shenkin SD, Deary IJ, Starr JM, Whalley LJ. Brain white matter hyperintensities: Relative importance of vascular risk factors in nondemented elderly people. Radiology. 2005;237:251–257. doi: 10.1148/radiol.2371041496. [DOI] [PubMed] [Google Scholar]
- Narr KL, Woods RP, Thompson PM, Szeszko P, Robinson D, Dimtcheva T, et al. Relationships between IQ and regional cortical gray matter thickness in healthy adults. Cerebral Cortex. 2007;17:2163–2171. doi: 10.1093/cercor/bhl125. [DOI] [PubMed] [Google Scholar]
- Nisbett RE, Masuda T. Culture and point of view. Proceedings of the National Academy of Sciences, U.S.A. 2003;100:11163–11170. doi: 10.1073/pnas.1934527100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbett RE, Miyamoto Y. The influence of culture: Holistic versus analytic perception. Trends in Cognitive Sciences. 2005;9:467–473. doi: 10.1016/j.tics.2005.08.004. [DOI] [PubMed] [Google Scholar]
- O’Brien LM, Ziegler DA, Deutsch CK, Kennedy DN, Goldstein JM, Seidman LJ, et al. Adjustment for whole brain and cranial size in volumetric brain studies: A review of common adjustment factors and statistical methods. Harvard Review of Psychiatry. 2006;14:141–151. doi: 10.1080/10673220600784119. [DOI] [PubMed] [Google Scholar]
- Park DC, Gutchess AH. Aging, cognition, and culture: A neuroscientific perspective. Neuroscience and Biobehavioral Reviews. 2002;26:859–867. doi: 10.1016/s0149-7634(02)00072-6. [DOI] [PubMed] [Google Scholar]
- Park DC, Hedden T, Jing Q, Jiao S, Lautenschlager G, Nisbett RE. Culture and the aging mind. University of Illinois; 2005. [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychology and Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Park DC, Nisbett R, Hedden T. Aging, culture, and cognition. Journals of Gerontology, Series B, Psychological Sciences and Social Sciences. 1999;54:P75–P84. doi: 10.1093/geronb/54b.2.p75. [DOI] [PubMed] [Google Scholar]
- Posthuma D, De Geus EJ, Baare WF, Hulshoff Pol HE, Kahn RS, Boomsma DI. The association between brain volume and intelligence is of genetic origin. Nature Neuroscience. 2002;5:83–84. doi: 10.1038/nn0202-83. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM, Acker JD. Hypertension and the brain: Vulnerability of the prefrontal regions and executive functions. Behavioral Neuroscience. 2003;117:1169–1180. doi: 10.1037/0735-7044.117.6.1169. [DOI] [PubMed] [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, et al. Regional and progressive thinning of the cortical ribbon in Huntington’s disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. [DOI] [PubMed] [Google Scholar]
- Rueckert D, Sonoda LI, Hayes C, Hill DL, Leach MO, Hawkes DJ. Nonrigid registration using free-form deformations: Application to breast MR images. IEEE Transactions on Medical Imaging. 1999;18:712–721. doi: 10.1109/42.796284. [DOI] [PubMed] [Google Scholar]
- Rushton JP, Jensen AR. James Watson's most inconvenient truth: Race realism and the moralistic fallacy. Medical Hypotheses. 2008;71:629–640. doi: 10.1016/j.mehy.2008.05.031. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, et al. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Salthouse TA. General and specific speed mediation of adult age differences in memory. Journals of Gerontology, Series B, Psychological Sciences and Social Sciences. 1996;51:P30–P42. doi: 10.1093/geronb/51b.1.p30. [DOI] [PubMed] [Google Scholar]
- Schwartz SH. Universals in the content and structure of values: Theoretical advances and empirical tests in 20 countries. Vol. 25. San Diego, CA: Academic Press; 1992. [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, et al. A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, et al. Neurodevelopmental trajectories of the human cerebral cortex. Journal of Neuroscience. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl. 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Staff RT, Murray AD, Deary IJ, Whalley LJ. What provides cerebral reserve? Brain. 2004;127:1191–1199. doi: 10.1093/brain/awh144. [DOI] [PubMed] [Google Scholar]
- Sutton BP, Goh J, Hebrank A, Welsh RC, Chee MW, Park DC. Investigation and validation of intersite fMRI studies using the same imaging hardware. Journal of Magnetic Resonance Imaging. 2008;28:21–28. doi: 10.1002/jmri.21419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan LH, Liu HL, Perfetti CA, Spinks JA, Fox PT, Gao JH. The neural system underlying Chinese logograph reading. Neuroimage. 2001;13:836–846. doi: 10.1006/nimg.2001.0749. [DOI] [PubMed] [Google Scholar]
- Thompson PM, Cannon TD, Narr KL, van Erp T, Poutanen VP, Huttunen M, et al. Genetic influences on brain structure. Nature Neuroscience. 2001;4:1253–1258. doi: 10.1038/nn758. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale—Revised. San Antonio, CA: The Psychological Corporation; 1981. [Google Scholar]
- Wechsler D. Wechsler Memory Scale—Third Edition (WMS-III) San Antonio, TX: The Psychological Corporation; 1997. [Google Scholar]
- Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation-maximization algorithm. IEEE Transactions on Medical Imaging. 2001;20:45–57. doi: 10.1109/42.906424. [DOI] [PubMed] [Google Scholar]
- Zilles K, Kawashima R, Dabringhaus A, Fukuda H, Schormann T. Hemispheric shape of European and Japanese brains: 3-D MRI analysis of intersubject variability, ethnical, and gender differences. Neuroimage. 2001;13:262–271. doi: 10.1006/nimg.2000.0688. [DOI] [PubMed] [Google Scholar]