Abstract
Platelet transfusion has long been practiced with rudimentary knowledge about optimal storage conditions and their implications for efficacy and, particularly, safety. Recent concerns about complications such as inflammation, thrombosis and altered recipient immunity have been raised about platelet transfusion. This review will discuss recent important findings that have raised these issues about platelet transfusion associated morbidity, mortality and the possible role of platelet storage in these associations.
Introduction
Platelets are responsive cells essential for maintenance of vascular integrity, participating in hemostasis, thrombosis and host immune responses. With a lifespan of about 8–10 days, platelets are continuously generated from bone marrow megakaryocytes which release platelets into the bloodstream to maintain levels of 150,000–400,000 platelets per microliter of blood. Thrombocytopenia, or low platelet count, can arise from multiple factors including bone marrow disorders, anti-neoplastic chemotherapy or hematopoietic stem cell transplantation, and is often prophylactically treated with platelet transfusion in the absence of actual bleeding. Platelets are collected and stored by multiple means, including platelet separation from individually donated whole blood units, or through apheresis procedures (Table 1). Over 9 million platelet concentrate equivalents were transfused in the United States in 1999 (about 2 million doses) [1]. Transfusion adverse reactions such as fever, chills, rigors and more rarely, life-threatening acute lung injury, occur in up to 30% of platelet transfusion recipients, which is significantly higher than red cell transfusions [2]. Some of the reasons for these differences have only recently become clear, and while removal of leukocytes from stored platelets minimizes these reactions, they remain quite common. In contrast, leukocyte reduction of red cell transfusions has made febrile reactions to this therapy rare.
Table 1.
Suggested improvements for various types of donation and storage.
| Type of PlateletAcquisition | When used | Adverse Events | Possible Areas for Improvement |
|---|---|---|---|
| Single Donor Pheresis Platelets | Thrombocytopenia |
|
|
| Whole Blood Pooled Platelets |
|
Platelet Storage Issues
Platelet concentrates currently can be transfused up to five days after preparation, but there is concern that platelet efficacy and safety may decline during storage, perhaps due to platelet activation, which increases over time. Amongst the issues that may be involved in this “storage lesion” are increases in platelet surface P-selectin [3] and platelet-derived soluble mediators histamine [4], CD40 ligand (CD40L) [5], CCL5 (RANTES), CXCR4 (platelet factor 4), transforming growth factor-β and CXCL8 (IL-8) [3]. Besides enhancing platelet activation, increased soluble CD40L levels have been associated with an increased risk of allergic and febrile reactions in platelet transfusion recipients, as well as lung injury [6,7]. Once platelets become activated to release these mediators, they may well be less effective in hemostasis upon transfusion. In addition to changes in soluble mediators, the entire platelet proteome has also been shown to change over time in storage, likely contributing to their functional decline [8,9].
Platelet microparticles generated during storage may be an important contributor to adverse reactions to transfusions. Microparticles are submicron vesicles formed during a membrane ruffling process, and contain RNA, cytoplasmic and membrane proteins derived from the parent cell. Roughly 80% of circulating microparticles in human blood are platelet-derived, while the remaining percentages are mainly produced by endothelial cells, leukocytes or erythrocytes. Platelet microparticles provide an anionic binding surface for coagulation factors, such as tissue factor, with their characteristically exposed phosphatidyl serine, thus assisting in the hemostatic process [10]. Microparticles can signal target cells via surface receptor interaction or translocation of internal RNA, lipids and protein [11,12]. Besides hemostasis and thrombosis, the communicative role of platelet microparticles has been proposed in diabetes, inflammation, malignancy, infection, angiogenesis and immunity [13–15]. Platelets have been shown to release microparticles during storage of whole blood, accumulating through day 5 and remaining elevated through an additional 30 days of storage [16]. Similar findings were reported for platelet concentrates [3,17]. Due to platelet microparticle involvement in hemostasis and thrombosis, removal of these active small vesicles from transfused platelets speculatively could decrease prothrombotic complications of recipients. Therefore, studies addressing changes in levels and composition of platelet microparticles in stored platelet concentrates, and their effects after transfusion would be of great interest.
The increase of activated platelets during storage could be due to conditions that differ from their activation-regulated in vivo environment, along with influences of in vitro platelet apoptosis [18,19] or other forms of cell death and senescence. Senescent platelets normally are cleared from circulation by the spleen and liver [20], but senescent platelets in a stored concentrate remain present and may alter activation states of residual platelets through the release of soluble mediators and cell to cell interactions. Platelet aggregates of great size are most likely filtered from the unit before transfusion, but soluble prothrombotic or proinflammatory mediators are able to pass through filters and be transfused along with the platelets.
ABO Compatibility and Platelet Transfusions
It has been demonstrated that ABO blood group antigens are present on the platelet surface, possibly on glycoproteins IIa, IIIa and Ib, and IIb [21, 22]. Refractoriness, or the inability to increase platelet counts post-transfusion, is a common complication that may be largely prevented by removal of leukocytes prior to transfusion and transfusing only ABO identical platelets to the recipient [23]. Recently, a large and elaborate observational study compared the absolute and corrected (normalized with body mass and number of transfused platelets) platelet count increments of platelet transfusion recipients from ABO compatible (mostly identical) or incompatible donors. The study demonstrated that ABO compatible transfusions resulted in a significantly higher corrected platelet count within 24 hours post transfusion [24]. The increased platelet count of the ABO compatible group was modest, but the difference may have been underestimated, as only one time point was measured and the pooled transfused platelet concentrates were not necessarily uniform. Previous smaller randomized trials and observational studies have confirmed that ABO identical platelets, particularly when given repeatedly, yield much improved count increments, reduced transfusion refractoriness and conceivably reduced bleeding compared with use of ABO unselected platelet transfusions [25]. Unfortunately, this practice has not been considered in many transfusion centers due to logistic reasons such as cost and inventory. Improving clinical practice of platelet transfusion would greatly benefit patients and resource utilization.
Human Leukocyte Antigen (HLA)-A,B Compatibility and Platelet Transfusions
HLA-A,B (Class I Major Histocompatability Complex) proteins are exposed on the platelet surface as in many other cells. These proteins are highly polymorphic among humans, making exact matches difficult. The major cause of refractoriness to platelet transfusions is antibodies to HLA induced by previous pregnancies or non-leukocytereduced transfusions. In general, the white cells in transfused blood are substantially more immunogenic than the platelets, thus it is known that leukoreduction filtrated transfusions yield refractoriness rates of <10% as compared with 50% in repetitively transfused patients receiving non-leukoreduced transfusions. In our center, transfusing only ABO identical, leukoreduced transfusions, refractoriness rates are <2% (unpublished data).
HLA’s influence on platelet transfusions has been under investigation for over half a century. Finding HLA matches is an elaborate process which increases time and cost of transfusion. However, matching, selective mismatching or serologic crossmatching are beneficial for patients who develop refractoriness despite other precautionary measures. Due to the great variation of HLA determinants, new and more efficient methods of screening compatible donor units are under investigation, and will be helpful to decrease time and cost of providing platelets to HLA alloimmunized refractory patients. Antibody Specificity Prediction or selective mismatching is one method which determines the recipient’s HLA-specific antibodies followed by selection of a donor whose platelets lack corresponding reactive antigens [26]. Another method involves an algorithm called HLAMatcher, which analyzes recipient DNA to identify HLA surface epitopes that may be recognized by antibodies, therefore ruling out a repertoire of antibodies present in that patient. Similar screening of platelet donors or concentrates would then be performed, thus eliminating donors with antigens to recipient crossreactive antibodies. This method more quickly assists in pairing of acceptable mismatches and expands the compatible transfusion options from a limited storage pool [27].
Transfused Platelets Contribute to Inflammation and Organ Injury
In addition to their major roles in hemostasis, platelets can function as innate immune cells that respond to, and release inflammatory mediators. Platelet α-granules contain numerous potent immunologic mediators including prostaglandin E2, RANTES, platelet factor-4, transforming growth factor-β and IL-8, which can be released upon platelet activation. Cytokines such as IL-1β are translated from platelet mRNA and released during activation. Additionally, activated platelets abundantly express P-selectin and CD40L on their surface, and upon cleavage, soluble CD40L can be released from the platelet surface. Platelet mediators can attract white blood cells and activate endothelial cells to enhance differentiation, humoral immunity and inflammation, or form plateletleukocyte complexes to assist in transendothelial migration [28,29]. Platelet microparticles released from activated platelets have high levels of surface P-selectin and contain other inflammatory mediators which function in transcellular communication, both at the site of platelet activation and at sites distal to their release. For example, platelet microparticles have been shown to deposit RANTES on activated endothelium, thus attracting monocytes and contributing to atherosclerotic lesions [30]. Excessive platelet activation can also contribute to inflammatory conditions such as migraines, rheumatoid arthritis, psoriasis, inflammatory pulmonary disease, inflammatory bowel disease and even malignancies [31].
Platelets are known to progressively activate over time in storage for transfusion and proinflammatory mediators are released into the supernatant of the stored unit [5]. These mediators may also interact with other cells within the stored product, and can cause an inflammatory reaction upon transfusion. For example, membrane-bound and soluble forms of CD40L significantly increase in platelet concentrates over storage time, and concentrate supernatant has been found to stimulate CD40 expressing cells in vitro. Specifically, platelet CD40L signaling stimulated fibroblast production of prostaglandin E2 and IL-6 and activated CD40 expressing polymorphonuclear leukocytes and induced damage to endothelial cells [5,6]. These cytokines likely contribute to the development of inflammatory reactions such as transfusion-related acute lung injury (TRALI), which is the leading reported cause of transfusion-related death [32]. This lung injury occurs within 6 hours of transfusion, and is initiated when transfused plasma mediators such as antibodies or bioactive lipids influence recipient vascular endothelium and leukocytes causing sequestration of neutrophils in lung capillary beds. Activation of neutrophils causes pulmonary inflammation and edema in animal models that lead to characteristic TRALI findings including hypoxemia, tachypnea, cyanosis, dyspnea and fever [32].
The most common reaction to platelet transfusion is a febrile nonhemolytic transfusion reaction, occurring between 5–30% of platelet transfusion patients. More often, transfusion of the supernatant plasma portion of platelet concentrates rather than the cellular portion, causes adverse reactions such as rigors or fever. Plasma concentration of IL-1β and IL-6 levels increase with storage time and correlate with these reactions [33].
Platelet Transfusions are associated with Thrombosis
Platelets are essential for hemostasis, preventing serious or even fatal blood loss after blood vessel injury. However, pathologic platelet activation can lead to thrombosis, contributing to common conditions such as acute coronary syndrome, ischemic stroke, transient ischemic attack and deep vein thrombosis. During platelet activation, many prothrombotic soluble mediators, such as adenosine diphosphate, thromboxane A2, serotonin and epinephrine are released in order to recruit and activate more platelets [34]. In the context of platelet transfusions, activation status of both donor and recipient platelets likely influence each other and may lead to thrombotic complications for platelet recipients. While the evidence that platelet transfusion is pro-thrombotic is epidemiologic, it is a truism in hematology that any therapy that is pro-hemostatic has thrombosis as one of its major risks. Thus it would be surprising if platelet transfusion did not contribute to thrombosis in some recipients, but this concept is a relatively new one in the half century history of platelet transfusion research.
A recent retrospective study assessed platelet transfusions given to 15,237 hospitalized cancer patients across 60 medical centers between 1995 and 2003. Risks of death, as well as venous and arterial thromboembolism were significantly increased in platelet transfusion recipients compared to patients who did not receive transfusions [35]. Similarly, survival of liver transplant patients decreased with increasing numbers of transfused platelet units in a dose-dependent manner [36]. While these are observational epidemiologic associations, the effects are large, mechanistically reasonable, and thus unlikely to be entirely explained by confounding factors. Additional adverse events such as infection, increased vasopressor and respiratory medication use, stroke and death were also associated with platelet transfusion in coronary artery bypass graft surgery patients [37]. These studies are important to generate further hypotheses about previously unappreciated risks associated with platelet transfusions, but much more investigation will be needed to determine causality and underlying mechanisms mediating these associations with adverse clinical outcomes after platelet transfusions.
Decreasing the Risks and Morbidity of Platelet Transfusions
Decreasing storage time before transfusion correlates to somewhat better outcomes, in terms of platelet count increments but the clinical significance of this benefit is unknown. Reducing platelet storage time limits would be a simple improvement in theory, however, it would likely dramatically reduce available transfusions and is not yet practical nor indicated by clinical outcome studies.
Leukocyte reduction and ultraviolet-B radiation are both known to reduce HLA antibody formation and recipient refractoriness [23]. Photochemical treatment can be used to abrogate the infectivity and immunogenicity of viruses, bacteria, and contaminating white blood cells from a platelet transfusion. While the advantage of eliminating pathogens is obvious, these treatments also raise levels of some soluble inflammatory cytokines above untreated units over time [3]. Cytokine levels of untreated platelet storage units also increase over time and contribute to the previously discussed adverse recipient reactions [3,5]. Leukocyte reduction at the time of collection, rather than immediately pre-transfusion, was significantly better for prevention of mediator accumulation [33]. These observations highlight the impact of contaminating white blood cells, which is another area in need of further investigation.
Our group is currently investigating the addition of anti-platelet small molecules to platelet units to reversibly attenuate platelet activation during storage, with the potential for reducing the number of activated platelets, microparticles and soluble proinflammatory mediators in stored concentrates. Stored supernatant plasma in platelet concentrates, and not the cells, is the major contributor to adverse reactions of platelet transfusion recipients, which indicates involvement of soluble factors [33,38]. Saline washing of platelet concentrates before transfusion is one attractive and simple way to significantly reduce soluble mediators and adverse reactions after transfusion, possibly including cardiac failure and acute lung injury [39]. In a small pilot randomized trial, patients with acute leukemia receiving washed platelet transfusions experienced a trend toward improved survival compared to patients receiving unwashed platelets, and significantly so for patients under 50 years old [40].
Conclusion
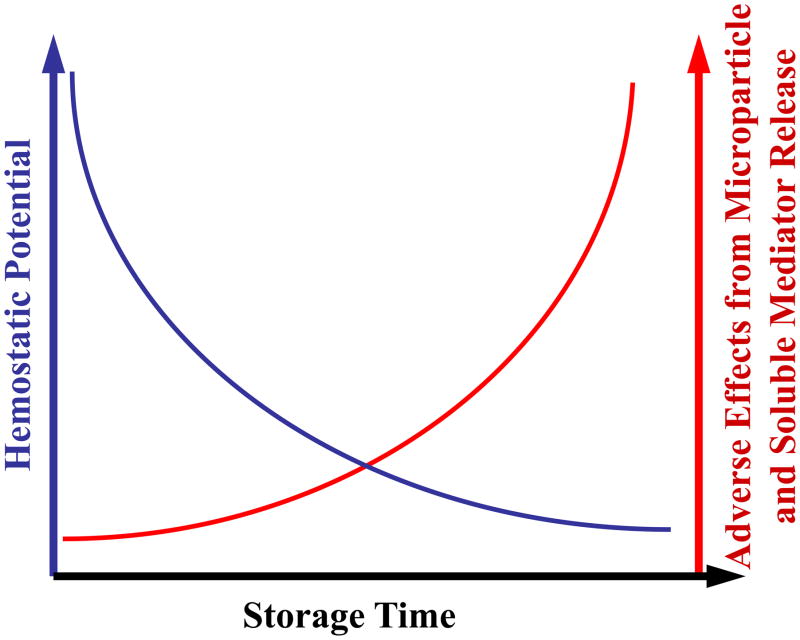
During storage for transfusion, platelets experience decreased efficacy/viability, while levels of prothrombotic and proinflammatory soluble mediators and microparticles increase. This trend over time could be due to activation and platelet death within the unit. Figure 1 shows schematically and speculatively how the balance of hemostatic potential of platelets versus inflammatory mediators worsens over storage time, again suggesting improved storage conditions or modifications of storage media need to be investigated. Washing stored platelets before transfusion has been proposed to reduce harmful side effects but remains controversial. Anti-platelet and other function-preserving small molecule additions to stored platelets are currently under investigation as possible means to prevent platelet deterioration during storage. Main themes that we consider particularly fruitful for future platelet transfusion research include: 1) the use of therapeutic platelet transfusion only in bleeding patients rather than prophylactic administration, 2) a better understanding of the platelet storage lesion, including loss of hemostatic function and increases in undesirable soluble mediators or platelet microparticles over storage time, and 3) optimized treatment of concentrates during storage, including small molecule platelet activation inhibitors and/or washing protocols that preserve platelet function while maximizing removal of harmful mediators.
Figure 1.
A schematic of platelet storage. Hemostatic potential decreases while soluble mediators and microparticles increase during storage. Upon transfusion, donor platelets may need to become activated for sufficient hemostatic effect. However as a stored platelet concentrate ages, the hemostatic potential of the platelets has been shown to decrease in number and function (blue line). Adverse effects occurring in the recipient after transfusion are probably more likely to occur after prolonged storage of platelet concentrates. Recent investigations suggest that adverse events after platelet transfusion may well be associated with increased accumulation of microparticles and soluble inflammatory and thrombotic mediators during platelet storage (red line).
Acknowledgments
This work was supported in part by T32-HL066988, HL100051, HL095467 and ES01247.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sullivan MT, Wallace EL. Blood collection and transfusion in the United States in 1999. Transfusion. 2005;45:141–148. doi: 10.1111/j.1537-2995.2004.03288.x. [DOI] [PubMed] [Google Scholar]
- 2.Heddle NM, et al. A prospective study to identify the risk factors associated with acute reactions to platelet and red cell transfusions. Transfusion. 1993;33:794–797. doi: 10.1046/j.1537-2995.1993.331094054613.x. [DOI] [PubMed] [Google Scholar]
- 3.Apelseth TO, et al. Cytokine accumulation in photochemically treated and gamma-irradiated platelet concentrates during storage. Transfusion. 2006;46:800–810. doi: 10.1111/j.1537-2995.2006.00800.x. [DOI] [PubMed] [Google Scholar]
- 4.Konca K, et al. The effect of cromoglycate on time-dependent histamine and serotonin concentrations in stored blood products. Transfus Apher Sci. 2006;34:193–198. doi: 10.1016/j.transci.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman J, et al. Release of biologically active CD154 during collection and storage of platelet concentrates prepared for transfusion. J Thromb Haemost. 2007;5:788–796. doi: 10.1111/j.1538-7836.2007.02412.x. [DOI] [PubMed] [Google Scholar]
- 6.Khan SY, et al. Soluble CD40 ligand accumulates in stored blood components, primes neutrophils through CD40, and is a potential cofactor in the development of transfusion-related acute lung injury. Blood. 2006;108:2455–2462. doi: 10.1182/blood-2006-04-017251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Blumberg N, et al. An association of soluble CD40 ligand (CD154) with adverse reactions to platelet transfusions. Transfusion. 2006;46:1813–1821. doi: 10.1111/j.1537-2995.2006.00979.x. [DOI] [PubMed] [Google Scholar]
- 8.Thon JN, et al. Comprehensive proteomic analysis of protein changes during platelet storage requires complementary proteomic approaches. Transfusion. 2008;48:425–435. doi: 10.1111/j.1537-2995.2007.01546.x. [DOI] [PubMed] [Google Scholar]
- 9.Springer DL, et al. Platelet proteome changes associated with diabetes and during platelet storage for transfusion. J Proteome Res. 2009;8:2261–2272. doi: 10.1021/pr800885j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horstman LL, Ahn YS. Platelet microparticles: a wide-angle perspective. Crit Rev Oncol Hematol. 1999;30:111–142. doi: 10.1016/s1040-8428(98)00044-4. [DOI] [PubMed] [Google Scholar]
- 11.Ratajczak J, et al. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 12.Barry OP, et al. Transcellular activation of platelets and endothelial cells by bioactive lipids in platelet microparticles. J Clin Invest. 1997;99:2118–2127. doi: 10.1172/JCI119385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng B, et al. Circulating level of microparticles and their correlation with arterial elasticity and endothelium-dependent dilation in patients with type 2 diabetes mellitus. Atherosclerosis. 2009;208:264–269. doi: 10.1016/j.atherosclerosis.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 14.Nomura S. Dynamic role of microparticles in type 2 diabetes mellitus. Curr Diabetes Rev. 2009;5:245–251. doi: 10.2174/157339909789804404. [DOI] [PubMed] [Google Scholar]
- 15.Italiano JE, Jr, et al. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol. 2010 doi: 10.1097/MOH.0b013e32833e77ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sugawara A, et al. Preventing platelet-derived microparticle formation-- and possible side effects-with prestorage leukofiltration of whole blood. Arch Pathol Lab Med. 2010;134:771–775. doi: 10.5858/134.5.771. [DOI] [PubMed] [Google Scholar]
- 17.Nomura S, et al. Platelets expressing P-selectin and platelet-derived microparticles in stored platelet concentrates bind to PSGL-1 on filtrated leukocytes. Clin Appl Thromb Hemost. 2000;6:213–221. doi: 10.1177/107602960000600406. [DOI] [PubMed] [Google Scholar]
- 18.Leytin V, Freedman J. Platelet apoptosis in stored platelet concentrates and other models. Transfus Apher Sci. 2003;28:285–295. doi: 10.1016/S1473-0502(03)00048-X. [DOI] [PubMed] [Google Scholar]
- 19.Mason KD, et al. Programmed anuclear cell death delimits platelet life span. Cell. 2007;128:1173–1186. doi: 10.1016/j.cell.2007.01.037. [DOI] [PubMed] [Google Scholar]
- 20.Kaplan JE, Saba TM. Platelet removal from the circulation by the liver and spleen. Am J Physiol. 1978;235:H314–320. doi: 10.1152/ajpheart.1978.235.3.H314. [DOI] [PubMed] [Google Scholar]
- 21.Santoso S, et al. Blood group A and B determinants are expressed on platelet glycoproteins IIa, IIIa, and Ib. Thromb Haemost. 1991;65:196–201. [PubMed] [Google Scholar]
- 22.Ogasawara K, et al. Study on the expression of ABH antigens on platelets. Blood. 1993;82:993–999. [PubMed] [Google Scholar]
- 23.Slichter SJ, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105:4106–4114. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pavenski K, et al. Posttransfusion platelet count increments after ABOcompatible versus ABO-incompatible platelet transfusions in noncancer patients: an observational study. Transfusion. 2010;50:1552–1560. doi: 10.1111/j.1537-2995.2010.02602.x. [DOI] [PubMed] [Google Scholar]
- 25.Heal JM, et al. The role of ABO matching in platelet transfusion. Eur J Haematol. 1993;50:110–117. doi: 10.1111/j.1600-0609.1993.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 26.Petz LD, et al. Selecting donors of platelets for refractory patients on the basis of HLA antibody specificity. Transfusion. 2000;40:1446–1456. doi: 10.1046/j.1537-2995.2000.40121446.x. [DOI] [PubMed] [Google Scholar]
- 27.Duquesnoy RJ. Structural epitope matching for HLA-alloimmunized thrombocytopenic patients: a new strategy to provide more effective platelet transfusion support? Transfusion. 2008;48:221–227. doi: 10.1111/j.1537-2995.2007.01516.x. [DOI] [PubMed] [Google Scholar]
- 28.Elzey BD, et al. The emerging role of platelets in adaptive immunity. Cell Immunol. 2005;238:1–9. doi: 10.1016/j.cellimm.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 29.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 30.Mause SF, et al. Platelet microparticles: a transcellular delivery system for RANTES promoting monocyte recruitment on endothelium. Arterioscler Thromb Vasc Biol. 2005;25:1512–1518. doi: 10.1161/01.ATV.0000170133.43608.37. [DOI] [PubMed] [Google Scholar]
- 31.McNicol A, Israels SJ. Beyond hemostasis: the role of platelets in inflammation, malignancy and infection. Cardiovasc Hematol Disord Drug Targets. 2008;8:99–117. doi: 10.2174/187152908784533739. [DOI] [PubMed] [Google Scholar]
- 32.Silliman CC, McLaughlin NJ. Transfusion-related acute lung injury. Blood Rev. 2006;20:139–159. doi: 10.1016/j.blre.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 33.Heddle NM, et al. The role of the plasma from platelet concentrates in transfusion reactions. N Engl J Med. 1994;331:625–628. doi: 10.1056/NEJM199409083311001. [DOI] [PubMed] [Google Scholar]
- 34.Angiolillo DJ, et al. Basic principles of platelet biology and clinical implications. Circ J. 2010;74:597–607. doi: 10.1253/circj.cj-09-0982. [DOI] [PubMed] [Google Scholar]
- 35.Khorana AA, et al. Blood transfusions, thrombosis, and mortality in hospitalized patients with cancer. Arch Intern Med. 2008;168:2377–2381. doi: 10.1001/archinte.168.21.2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.de Boer MT, et al. The impact of intraoperative transfusion of platelets and red blood cells on survival after liver transplantation. Anesth Analg. 2008;106:32–44. doi: 10.1213/01.ane.0000289638.26666.ed. table of contents. [DOI] [PubMed] [Google Scholar]
- 37.Spiess BD, et al. Platelet transfusions during coronary artery bypass graft surgery are associated with serious adverse outcomes. Transfusion. 2004;44:1143–1148. doi: 10.1111/j.1537-2995.2004.03322.x. [DOI] [PubMed] [Google Scholar]
- 38.Vo TD, et al. Platelet washing to prevent recurrent febrile reactions to leucocyte-reduced transfusions. Transfus Med. 2001;11:45–47. doi: 10.1046/j.1365-3148.2001.00280.x. [DOI] [PubMed] [Google Scholar]
- 39.Blumberg N, et al. An association between decreased cardiopulmonary complications (transfusion-related acute lung injury and transfusion-associated circulatory overload) and implementation of universal leukoreduction of blood transfusions. Transfusion. 2010;50:2738–2744. doi: 10.1111/j.1537-2995.2010.02748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blumberg N, et al. A randomized trial of washed red blood cell and platelet transfusions in adult acute leukemia [ISRCTN76536440] BMC Blood Disord. 2004;4:6. doi: 10.1186/1471-2326-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]