Abstract
Rationale: Human rhinoviruses (HRV) are the leading cause of upper respiratory infections and have been postulated to trigger asthma exacerbations. However, whether HRV are detected during crises because upper respiratory infections often accompany asthma attacks, or because they specifically elicit exacerbations, is unclear. Moreover, although several hypotheses have been advanced to explain virus-induced exacerbations, their mechanism remains unclear.
Objectives: To determine the role of HRV in pediatric asthma exacerbations and the mechanisms mediating wheezing.
Methods: We prospectively studied 409 children with asthma presenting with upper respiratory infection in the presence or absence of wheezing. Candidate viral and immune mediators of illness were compared among children with asthma with different degrees of severity of acute asthma.
Measurements and Main Results: HRV infections specifically associated with asthma exacerbations, even after adjusting for relevant demographic and clinical variables defined a priori (odds ratio, 1.90; 95% confidence interval, 1.21–2.99; P = 0.005). No difference in virus titers, HRV species, and inflammatory or allergic molecules was observed between wheezing and nonwheezing children infected with HRV. Type III IFN-λ1 levels were higher in wheezing children infected with HRV compared with nonwheezing (P < 0.001) and increased with worsening symptoms (P < 0.001). Moreover, after adjusting for IFN-λ1, children with asthma infected with HRV were no longer more likely to wheeze than those who were HRV-negative (odds ratio, 1.18; 95% confidence interval, 0.57–2.46; P = 0.66).
Conclusions: Our findings suggest that HRV infections in children with asthma are specifically associated with acute wheezing, and that type III IFN-λ1 responses mediate exacerbations caused by HRV. Modulation of IFN- λ1 should be studied as a therapeutic target for exacerbations caused by HRV.
Keywords: asthma, interferon-λ, rhinovirus, children, asthma exacerbation
At a Glance Commentary
Scientific Knowledge on the Subject
Human rhinoviruses (HRV) are the leading cause of upper respiratory infections and are thought to trigger asthma exacerbations. However, whether HRV infections play a causal role in the development of asthma exacerbations is unclear.
What This Study Adds to the Field
Our findings suggest that HRV infections in children with asthma are specifically associated with acute wheezing, and that type III IFN-λ1 responses mediate exacerbations caused by HRV. Modulation of IFN-λ1 may provide a therapeutic target for exacerbations caused by HRV.
Asthma exacerbations are the main cause of hospitalization in children, and occur in association with respiratory viral infections (1, 2). Human rhinoviruses (HRV) are frequently isolated in the upper airways of children during respiratory infections and during asthma attacks, and have been postulated to trigger these crises (3, 4). However, whether HRV are detected during asthma crises because they are the most frequent cause of upper respiratory infections (URI; which accompany asthma attacks), or because they can specifically elicit asthma exacerbations is unclear. To our knowledge, no pediatric study has compared the viral etiology of URI in patients with asthma with and without wheezing to investigate the specific association between HRV URI and asthma attacks.
Several hypotheses have been advanced to explain the mechanisms that trigger asthma crises during respiratory infections (4–7). Focused mainly on HRV-associated episodes, two nonexclusive theories attribute asthma attacks to direct viral injury and immune-mediated exacerbations (7–10). Postulated direct effects of HRV associate wheezing episodes with high viral load (9, 11) or specific HRV species (10, 12). These theories are supported in part by studies where inoculation of an attenuated HRV in adults with asthma and healthy control subjects led to higher HRV titers in the respiratory tract of the former group (13, 14), and other observations suggesting a potential association between HRV species C (HRVC) and wheezing or asthma (12, 15). Meanwhile, other leading hypotheses have been postulated to explain immune-mediated pathways to exacerbations associated with HRV. The first attributes exacerbations to respiratory tract innate immune inflammation (4) and is supported by experimental exposures and epidemiologic studies demonstrating that HRV enhance the production of inflammatory cytokines (e.g., IL-8 and IL-6) and elicit neutrophilia in the lungs of patients with asthma (16, 17). The second of these hypotheses suggests that HRV-mediated asthma crises are elicited by polarization of the adaptive immune response toward Th2 responses (8). A variety of respiratory viruses may recruit allergen-specific (not virus-specific) Th2 cells to the lungs of an atopic host, even with viruses (e.g., HRV) that do not routinely elicit virus-specific Th2 responses in healthy children (18). The third theory proposes that loss of regulatory cytokine expression (5) leads to asthma attacks. Finally, recent reports attributed asthma crises to altered modulation of type III IFN-λ after HRV infection in patients with asthma (14). A role for type III IFN-λ1 in asthma exacerbations has been postulated, but the directionality of this modulation is not clear (14).
This study investigated the specific role of HRV in the inception of asthma attacks. Then, the role of HRV load and HRV species in eliciting wheezing in children with asthma was explored. Finally, the role of immune mediators, with a special emphasis on type III IFN-λ1, in asthma crises and disease severity was analyzed.
Methods
Study Population
Children between 5 and 18 years of age with a medical diagnosis of asthma presenting with URI symptoms in the presence or absence of wheezing and visiting the Asthma Clinics of Hospital Mi Pueblo, Florencia Varela; Hospital Evita Pueblo, Berazategui; Hospital Iriarte, Quilmes; and Hospital V. Lopez y Planes, General Rodriguez in Buenos Aires, Argentina, were invited to participate. The four Asthma Clinics serve a population of low socioeconomic status in the outskirts of Buenos Aires (see Table E1 in the online supplement for socioeconomic data). Children with asthma in these neighborhoods attend the Clinics routinely, receiving free specialized medical care and are therefore unlikely to seek medical attention elsewhere. Enrollment was conducted by pediatric pulmonologists staffing the Asthma Clinics. Children with asthma with a URI and an acute asthmatic exacerbation were considered cases, whereas children with a preexisting diagnosis of asthma and with signs and symptoms of URI but no wheezing were considered control subjects. Patients with oropharyngeal malformations, neuromuscular disorders, immune suppression, or chronic cardiopulmonary diseases other than asthma were excluded from participation. Children were enrolled between March 1st (fall) and November 1st (spring) in 2007 and 2008. Children were not enrolled during the summer, because of the low incidence of asthma exacerbations. The Institutional Review Boards of the participating hospitals approved the study. Written informed consent was obtained from parents or guardians; assent was obtained from invited children.
Demographic and Clinical Data
Demographic and clinical data were ascertained from parents by questionnaire and chart review and included such variables as age, sex, breastfeeding history, number of siblings, prior asthma hospitalizations, prior intensive care unit admissions for asthma, home tobacco smoke exposure, history of allergic diseases and asthma, use of corticosteroids, birth history, allergen exposure, and other treatments at home.
Score for baseline asthma status was determined by frequency of symptoms (days per week, nights per month) and FEV1 (see Table E2) (19).
URI and asthma exacerbation severity was defined as none (URI with no wheezing), mild, moderate, or severe using a modified functionalized Global Initiative for Asthma (GINA) asthma exacerbation severity scheme (see Table E3) (20).
Viruses and Cytokines
Nasal washes were obtained by gently flushing the children's nostrils with 4 ml of sterile saline solution (AnalytiCals, Buenos Aires, Argentina). Secretions were aliquoted, immediately snap frozen using dry ice, and stored at −80°C. Aliquots of frozen samples were analyzed for viral RNA in our laboratories at Johns Hopkins University and Vanderbilt University and for cytokines (mRNA and protein) in our laboratories at Fundacion INFANT in Buenos Aires. Respiratory specimens from children were tested by MultiCode-Plx Assay (EraGen, Madison, WI) for HRV, respiratory syncytial virus, parainfluenza virus, influenza virus, human metanepneumovirus, adenovirus, and human coronaviruses. RNA was extracted from 200 μl of pooled nasal wash samples on a Roche (Indianapolis, IN) MagNApure LC automated nucleic acid extraction instrument and quantitative real-time reverse transcriptase polymerase chain reaction (RT-PCR) for HRV was performed with the Smart Cycler II (Cepheid, Sunnyvale, CA) using primers and probe sequences (CY+AGCC+TGCGTGGC, GAAACACGGACACCCAAAGTA, TCCTCCGGCCCCTGAATGYGGC) directed at a highly conserved HRV 5′-noncoding region (21). HRV viral load was quantified using an HRV RNA runoff-transcript standard curve. Conventional RT-PCR was then performed on HRV-positive samples by using primers that amplified a fragment of approximately 548 nt, encompassing the VP4/VP2 region, and the hypervariable region in the 5′-NCR (22, 23). Amplified fragments were sequenced bidirectionally with the ABI PRISM BigDye Terminator Kit (Applied Biosystems, Carlsbad, CA) on a 3730xl DNA Analyzer (Applied Biosystems). Sequences were edited and aligned with MacVector version 11.1 (MacVector, Cary, NC). Seven published strains from GenBank and 152 field strains from this study were included. Phylogenetic analysis was performed using Molecular Evolutionary Genetics Analysis (MEGA) (Tempe, AZ) version 4 with three bootstrapped replicates and the neighbor-joining algorithm with HRV87 as outgroup (24). Cytokines were measured with a cytometric bead array (Becton Dickinson, Franklin Lakes, NJ). IFN-α, IFN-λ1, and IFN-λ2/3 were measured by ELISA and real-time RT-PCR. Specific blood IgE to common foods and aeroallergens (Table E4) was tested in 89 subjects by Immunetech (Foster City, CA) using MyAllergyTest System.
Statistical Analysis
Continuous and categorical variables were compared using Wilcoxon rank-sum, chi-square, and Fisher exact tests as appropriate. Risk factors for wheezing were assessed using logistic regression with covariates selected a priori. Multivariate models with and without type III IFN-λ1 were compared using a log likelihood ratio test. A Bonferroni correction was used to account for multiple cytokine comparisons. Analyses were performed using R statistical software (www.r-project.org). Complete details are available at http://biostat.mc.vanderbilt.edu /ArchivedAnalyses.
Results
Study Population
Four hundred and nine 5- to 18-year-old children with asthma with URI symptoms (200 presenting with wheezing and 209 not wheezing) were prospectively enrolled during the fall, winter, and spring of 2007 and 2008. Fourteen (3%) families declined to participate; these children had similar characteristics as those enrolled in the study (not shown).
Wheezing children had more frequent prior hospitalizations because of asthma and admissions to intensive care unit (Table 1). Conversely, greater baseline severity of illness (National Asthma Education and Prevention Program [NAEPP] II score does not consider prior hospitalizations [19]) and more frequent use of inhaled steroids were observed in nonwheezing control subjects (Table 1).
TABLE 1.
COMPARISON OF DEMOGRAPHIC AND CLINICAL CHARACTERISTICS BY WHEEZING AND RHINOVIRUS STATUS IN THE STUDY POPULATION
| All Children |
All Children |
HRV Positive |
|||||||
| Wheezing (n = 200) | No Wheezing (n = 209) | P Value | HRV Positive (n = 180) | HRV Negative (n = 211) | P Value | Wheezing (n = 106) | No Wheezing (n = 74) | P Value | |
| Age | 8.6 (6.8, 11.2) | 8.8 (6.7, 11.2) | 0.938 | 8.6 (6.9, 11.1) | 8.8 (6.7, 11.6) | 0.589 | 8.4 (6.6, 11) | 8.9 (7.1, 11.2) | 0.297 |
| Female (%) | 91 (46) | 83 (38) | 0.279 | 80 (44.4) | 87 (41.2) | 0.591 | 48 (45.3) | 32 (43.2) | 0.906 |
| Baseline severity (%) | |||||||||
| Mild intermittent | 70 (35) | 29 (14) | <0.001 | 60 (33.3) | 36 (17.1) | 0.003 | 41 (38.7) | 19 (25.7) | <0.001 |
| Mild persistent | 79 (40) | 53 (25) | 51 (28.3) | 74 (35.1) | 38 (35.8) | 13 (17.6) | |||
| Moderate | 42 (21) | 125 (60) | 65 (36.1) | 95 (45) | 23 (21.7) | 42 (56.8) | |||
| Severe | 9 (4) | 2 (1) | 4 (2.2) | 6 (2.8) | 4 (3.8) | 0 (0) | |||
| Prior asthma hospitalization (%) | |||||||||
| 0 | 61 (31) | 95 (46) | 0.004 | 68 (37.8) | 87 (41.4) | 0.346 | 36 (34) | 32 (43.2) | 0.449 |
| 1 | 26 (13) | 27 (13) | 19 (10.6) | 29 (13.8) | 12 (11.3) | 7 (9.5) | |||
| ≥2 | 113 (57) | 86 (41) | 93 (51.7) | 94 (44.8) | 58 (54.7) | 35 (47.3) | |||
| Prior asthma ICU (%) | |||||||||
| 0 | 190 (95) | 198 (96) | 0.058 | 171 (95.5) | 200 (95.7) | 0.971 | 101 (95.3) | 70 (95.9) | 0.096 |
| 1 | 8 (4) | 2 (1) | 4 (2.2) | 5 (2.4) | 4 (3.8) | 0 (0) | |||
| ≥2 | 2 (1) | 6 (3) | 4 (2.2) | 4 (1.9) | 1 (0.9) | 3 (4.1) | |||
| Asthma in family (%) | |||||||||
| Mother | 35 (18) | 34 (16) | 0.841 | 33 (18.3) | 34 (16.1) | 0.656 | 22 (20.8) | 11 (14.9) | 0.418 |
| Father | 24 (12) | 21 (10) | 0.636 | 11 (6.1) | 27 (12.8) | 0.04 | 7 (6.6) | 4 (5.4) | 0.989 |
| Siblings | 39 (20) | 57 (27) | 0.082 | 33 (18.3) | 56 (26.5) | 0.071 | 17 (16) | 16 (21.6) | 0.449 |
| Allergies in family (%) | |||||||||
| Mother | 20 (10) | 35 (17) | 0.064 | 17 (9.4) | 36 (17.1) | 0.041 | 9 (8.5) | 8 (10.8) | 0.791 |
| Father | 7 (4) | 20 (10) | 0.023 | 4 (2.2) | 23 (10.9) | 0.002 | 2 (1.9) | 2 (2.7) | 0.882 |
| Siblings | 15 (8) | 25 (12) | 0.176 | 14 (7.8) | 25 (11.8) | 0.242 | 7 (6.6) | 7 (9.5) | 0.674 |
| Inhaled steroid use (%) | 80 (40) | 128 (61) | <0.001 | 94 (52.2) | 107 (50.7) | 0.844 | 46 (43.4) | 48 (64.9) | 0.007 |
| HRV positive (%) | 106 (56) | 74 (37) | <0.001 | 180 (100) | — | <0.001 | — | — | — |
| Positive for other viruses (%) | |||||||||
| Missing | 34 (17) | 42 (20.1) | — | 18 (10) | 40 (19) | — | 12 (11.3) | 6 (8.1) | — |
| Coinfected | 16 (8) | 13 (6.2) | 0.685 | 26 (14.4) | 3 (1.4) | <0.001 | 16 (15.1) | 10 (13.5) | 0.858 |
| Any other virus | 32 (19.3) | 24 (14.4) | 0.244 | 26 (16) | 30 (17.5) | 0.77 | 16 (17) | 10 (14.7) | 0.829 |
| RSV | 12 (7.2) | 9 (5.4) | 0.509 | 11 (6.8) | 10 (5.8) | 0.823 | 7 (7.4) | 4 (5.9) | 0.762 |
| Influenza | 8 (4.8) | 2 (1.2) | 0.061 | 3 (1.9) | 7 (4.1) | 0.338 | 3 (3.2) | 0 (0) | 0.265 |
| PIV | 6 (3.6) | 1 (0.6) | 0.067 | 4 (2.5) | 3 (1.8) | 0.717 | 4 (4.3) | 0 (0) | 0.14 |
| Adenovirus | 5 (3) | 13 (7.8) | 0.087 | 9 (5.6) | 9 (5.3) | 1 | 3 (3.2) | 6 (8.8) | 0.167 |
| HMPV | 0 (0) | 0 (0) | — | 0 (0) | 0 (0) | — | — | — | — |
| Coronaviruses | 2 (1.2) | 4 (2.4) | 0.685 | 1 (0.6) | 5 (2.9) | 0.216 | 0 (0) | 1 (1.5) | 0.42 |
Definition of abbreviations: HMPV = human metanepneumovirus; HRV = human rhinoviruses; ICU = intensive care unit; PIV = parainfluenza virus; RSV = respiratory syncytial virus.
No differences were observed when comparing breastfeeding, number of siblings, and smoking among groups.
Rhinoviruses and Asthma Exacerbations
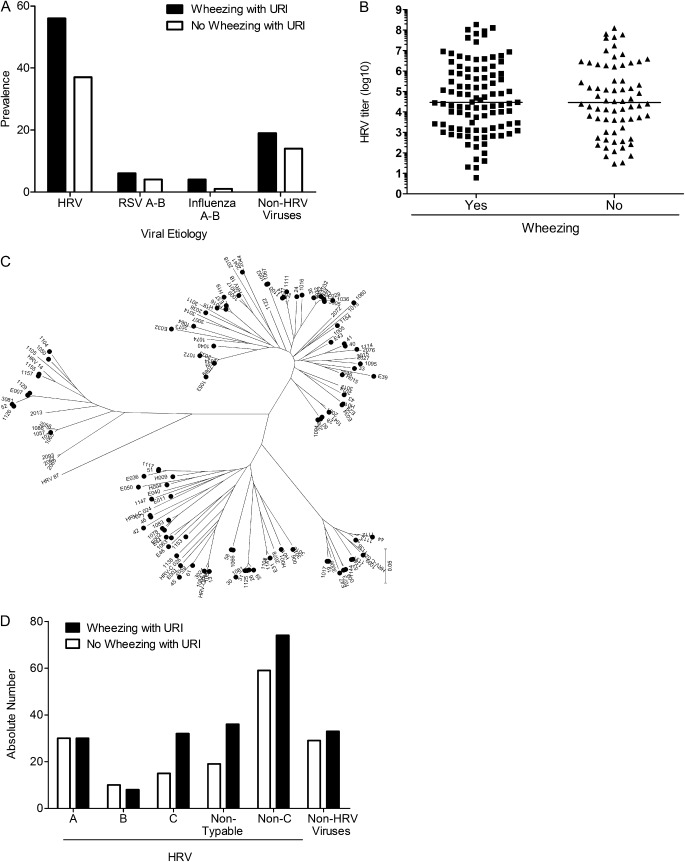
Respiratory viruses were detected in respiratory secretions of 333 (82%) children (Table 1). HRV were the agents detected most frequently, in 106 (56%) wheezing children and 74 (37%) nonwheezing patients (Figure 1A) (P < 0.001). Those who were HRV-positive were more likely to wheeze than those who were HRV-negative (odds ratio [OR], 2.17; 95% confidence interval [CI], 1.44–3.25; P < 0.001). The relationship between HRV and wheezing persisted after adjustment for age, sex, baseline asthma severity, familial history of asthma, and prior hospitalizations (OR, 1.90; 95% CI, 1.21–2.99; P = 0.005 (Table 2)]. A similar association was not observed for any other assayed viral pathogen. RNA from other respiratory viruses was detected infrequently and at similar rates between cases and control subjects (19% vs. 14%, respectively; P = 0.24) (Table 1).
Figure 1.
Rhinoviruses associate with asthma exacerbations. (A) Prevalence of respiratory viruses in respiratory secretions of wheezing (solid bars) and nonwheezing (open bars) children with asthma with upper respiratory symptoms. P = 0.005 for human rhinoviruses (HRV) wheezing versus nonwheezing; P = 0.24 for all other viruses combined. (B) Rhinovirus titer in respiratory secretions of wheezing (squares) and nonwheezing (triangles) infected children with asthma. Black lines represent median values. P = 0.68. (C) Phylogenetic tree of HRV isolated in secretions of patients with asthma. (D) Distribution of wheezing (solid bars) and nonwheezing (open bars) presentations in children with asthma with upper respiratory tract symptoms according to HRV group. P = 0.19 for C versus all non-C combined. RSV = respiratory syncytial virus; URI = upper respiratory infections.
TABLE 2.
FEATURES ASSOCIATED WITH WHEEZING, MULTIVARIATE ANALYSES
| Odds Ratio | 95% Confidence Intervals | P Value | |
| HRV positive | 1.90 | 1.21–2.99 | 0.005 |
| Age, yr | 1.05 | 0.97–1.13 | 0.243 |
| Female | 1.28 | 0.82–2 | 0.284 |
| Baseline severity | |||
| Mild intermittent | 1 | ||
| Mild persistent | 0.76 | 0.42–1.39 | 0.376 |
| Moderate-to-severe | 0.16 | 0.09–0.29 | <0.001 |
| Family history of asthma | 0.81 | 0.46–1.42 | 0.457 |
| Maternal asthma | 1.07 | 0.52–2.2 | 0.86 |
| Prior asthma hospitalizations | |||
| 0 | 1 | ||
| 1 | 1.56 | 0.76–3.23 | 0.228 |
| ≥2 | 2.6 | 1.59–4.25 | <0.001 |
Moderate-to-severe baseline asthma was associated with less frequent acute wheezing. Conversely, more than one hospitalization because of asthma increased the risk of wheezing (Table 2).
Viral Load and Asthma Exacerbations
We first compared HRV titers in respiratory secretions obtained from cases (HRV URI and wheezing) and control subjects (HRV URI and not wheezing). Comparison revealed no differences. Median viral load for cases was 42,700 (interquartile range, 2,826–942,300) and for control subjects was 52,320 (interquartile range, 6 158–1,967,000) (P = 0.68) (Figure 1B).
Subsequently, we investigated whether HRV species associated with disease exacerbations (Figures 1C and 1D). HRVC has recently been recognized to cause severe respiratory disease in children (25–27) and has been postulated to be associated with asthma exacerbations (10, 12, 15, 25). Of 180 HRV-positive samples, 60 (33%) were HRVA, 18 (10%) HRVB, 47 (26%) HRVC, and 55 (31%) could not be sequenced. Thirty-two (68%) children with HRVC wheezed, compared with 8 (44%) with HRVB, 30 (50%) with HRVA, 36 (66%) with untypable HRV, and 74 (56%) all non-C HRV combined (P = 0.19, for C vs. all non-C combined) (Figure 1D). HRVC strains were more diverse than those among species A and B (Figure 1C). The findings cannot confirm a role for HRV titers or species in asthma exacerbations.
Inflammation and HRV-mediated Asthma Exacerbation
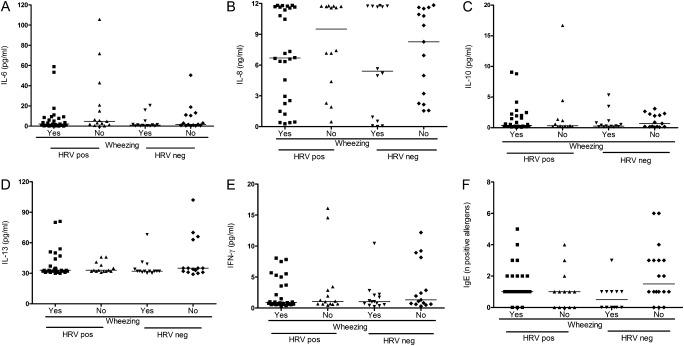
IL-6 is an inflammatory cytokine associated with production of acute-phase reactants (28), and IL-8 a potent proinflammatory cytokine with a key role in neutrophil recruitment and activation during inflammation (17, 28). Previous studies showed an association between IL-6 and IL-8 levels and production of other inflammatory molecules (17). Interestingly, no significant differences in IL-6 and IL-8 levels in respiratory secretions of cases and control subjects were observed (P = 0.59 and P = 0.11, respectively). Moreover, inflammatory cytokine production did not differ in HRV-infected and -uninfected patients (P = 0.19 for IL-6 and P = 0.90 for IL-8, respectively) (Figures 2A and 2B).
Figure 2.
Cytokine responses in the respiratory tract of children with asthma. Cytokine responses in respiratory secretions of human rhinoviruses (HRV)–infected and non–HRV-infected children with asthma with upper respiratory infections symptoms and with or without wheezing (n = 80). Cytokine determinations include (A) IL-6, (B) IL-8, (C) IL-10, (D) IL-13, and (E) IFN-γ. Comparisons of all cytokines between wheezing and nonwheezing children infected with HRV, P = NS. (F) Number of allergens with positive serum-specific IgE titers in children with asthma (n = 89) with upper respiratory infections plus wheezing (yes/no) and HRV infection (yes/no), P = NS for all comparisons. Black lines represent median values.
We then speculated that differential levels of the regulatory cytokine IL-10 could affect the inflammatory profile of HRV-mediated wheezing in the respiratory tract (5). However, IL-10 levels in respiratory secretions were similar between wheezing and nonwheezing children with HRV URI (P = 0.48) (Figure 2C), and between HRV-infected and -uninfected subjects (P = 0.57) (Figure 2C). All these observations fail to support an association between innate inflammation and HRV-associated wheezing in our patients.
Th2 Bias and HRV-mediated Asthma Exacerbation
Several studies ascribe asthma exacerbations to Th2 cytokines, IL-13 in particular (29, 30). Comparison of IL-13 levels between cases and control subjects revealed no differences (P = 0.35) (Figure 2D). No differences were observed when comparing IL-13 levels in HRV-positive and HRV-negative patients with asthma (P = 0.69) (Figure 2D).
Another mechanism of Th2 polarization is the suppression of the Th1 response, which prior studies associated with the inception of reactive airways disease in children (31, 32). Levels of IFN-γ in the respiratory tract of children infected with HRV did not differ between cases and control subjects (P = 0.36) (Figure 2E) nor between HRV-infected and -uninfected children (P = 0.93) (Figure 2E).
Finally, we investigated the role of atopy in predisposing for HRV-mediated wheezing. For this purpose, we analyzed specific IgE levels against 10 common food allergens and aeroallergens (Table E4) in a subset of 89 children, 49 with HRV and 40 without HRV (Figure 2F). Specific IgE levels were similar in wheezing and nonwheezing children infected with HRV (P = NS) regardless of HRV infection. All these findings fail to support an association between Th2 bias and HRV-mediated wheezing in our population.
Type III IFN-λ1 as a Determinant of Disease Severity
Recently, a study ascribed a role in asthma exacerbations to down-modulation of type III IFN-λ (14). Comparison of volunteers with and without asthma experimentally infected with an attenuated HRV showed lower type III IFN-λ1 and type III IFN-λ2 levels in respiratory secretions of patients with asthma (14). Conversely, other studies have shown elevated IFN-λ1 in people with asthma compared with people without asthmas (33, 34).
In our study, baseline production of type III IFN-λ1 in children with asthma without respiratory symptoms was lower than in healthy control subjects (Figure E1A). Moreover, in line with previous observations in adults (14), in vitro analysis of type III IFN-λ1 production in primary nasal epithelial cells of children with asthma post-HRV infection was suppressed compared with cytokine production in nasal epithelial cells of healthy children (Figure E1B).
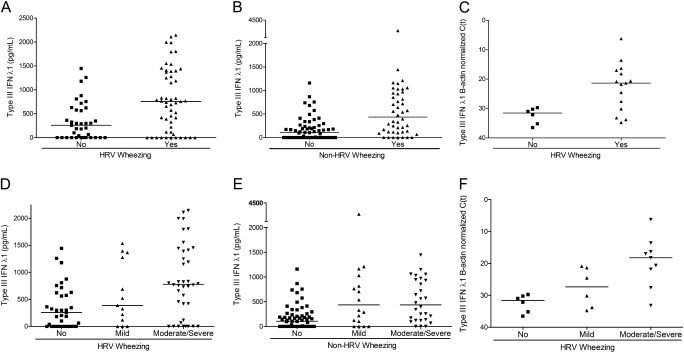
To explore whether type III IFN-λ1 levels intervene in a pathway triggering asthma exacerbations associated with HRV, we compared responses in wheezing versus nonwheezing children presenting HRV URI in a sample of 197 patients participating in our study balancing cases and control subjects (Figure 3A) (see Table E5 for a comparison of subgroup with total population). Interestingly, wheezing children infected with HRV had higher levels of type III IFN-λ1 than nonwheezing children infected with HRV with asthma (P < 0.001; because seven cytokines were tested, to control the family-wise type I error rate at 5%, “statistically significant” implies P < 0.007) (Figure 3A).
Figure 3.
Type III IFN-λ1 as a determinant of severity in asthma crises caused by human rhinoviruses (HRV). (A) Type III IFN-λ1 production in respiratory secretions of HRV-infected nonwheezing (squares) versus wheezing (triangles) children with asthma with upper respiratory infection (URI) symptoms, P < 0.001. Black lines represent median values. (B) Type III IFN-λ1 production in respiratory secretions of non–HRV-infected nonwheezing (squares) versus wheezing (triangles) children with asthma with URI symptoms, P < 0.001. Black lines represent median values. (C) Type III IFN-λ1 mRNA levels in respiratory secretions of HRV-infected nonwheezing (squares) versus wheezing (triangles) children with asthma with URI symptoms, P < 0.011. Black lines represent median values. (D) Type III IFN-λ1 production in respiratory secretions of HRV-infected children with asthma with nonwheezing (squares), mild wheezing (up-pointing triangles), and moderate-to-severe wheezing (down-pointing triangles), P < 0.001. Black lines represent median values. (E) Type III IFN-λ1 production in respiratory secretions of non–HRV-infected children with asthma with nonwheezing (squares), mild wheezing (up-pointing triangles), and moderate-to-severe wheezing (down-pointing triangles), P = 0.36. Black lines represent median values. (F) Type III IFN-λ1 mRNA levels in respiratory secretions of HRV-infected children with asthma with nonwheezing (squares), mild wheezing (up-pointing triangles), and moderate-to-severe wheezing (down-pointing triangles), P = 0.008. Black lines represent median values.
Children with asthma with URI not caused by HRV also showed a difference in type III IFN-λ1 levels between those who wheezed and those who did not wheeze (P < 0.001) (Figure 3B). Among all wheezing children, those with HRV had higher levels of type III IFN-λ1 than those without HRV (P < 0.01). Analysis of type III IFN-λ1 mRNA levels confirmed our observations (P = 0.011) (Figure 3C).
Interestingly, higher type III IFN-λ1 levels associated with worsening illness. Patients with HRV URI and no wheezing had lower median type III IFN-λ1 levels than those with mild HRV wheezing, and these had lower levels than those with moderate and severe HRV disease (P < 0.001) (Figure 3D). These observations were confirmed by assaying mRNA levels of the antiviral cytokine by real-time RT-PCR (P = 0.008) (Figure 3F). Children with wheezing resulting from triggers other than HRV also mirrored this trend (P < 0.001) (Figure 3E).
Given that type III IFN-λ1 levels correlated with wheezing and severity of acute exacerbation during HRV infection, we examined whether type III IFN-λ1 is the mechanism by which HRV elevates the risk of wheezing. Mediation was assessed by seeing if the inclusion of type III IFN-λ1 weakened the association of the virus with asthma crises (35). Among the subset of 197 children with IFN-λ1 measurements, HRV was associated with asthma exacerbations (OR, 1.90; 95% CI, 1.08–1.35; P = 0.027). After adjusting for IFN-λ1, those who were HRV-positive were no longer more likely to wheeze than those who were HRV-negative (OR, 1.31; 95% CI, 0.70–2.47; P = 0.399). IFN-λ1 remained a strong predictor of wheezing, independent of HRV status (OR, 1.22 per 100-unit increase; 95% CI, 1.13–1.32; P < 0.001). When the effect of IFN-λ1 was analyzed including age, sex, baseline severity, family history of asthma, and prior asthma hospitalizations, our observations were confirmed: IFN-λ1 remained a strong predictor of wheezing (OR, 1.2 per 100-unit increase in IFN-λ1; 95% CI, 1.11–1.31; P < 0.001) and the association between HRV and wheezing was significantly weakened by the cytokine (OR, 1.18; 95% CI, 0.57–2.46; P = 0.66). Note that in this subset of 197 patients, prior hospitalization and baseline severity of asthma also weakened the association between HRV and wheezing (P = 0.14 in absence of IFN-λ1).
Other IFNs and Asthma Exacerbations
IFN-λ2/3 has an amino acid sequence similar to IFN-λ1. Levels of type III IFN-λ2/3 were similar in wheezing and nonwheezing children infected with HRV (see Figures E2 and E3).
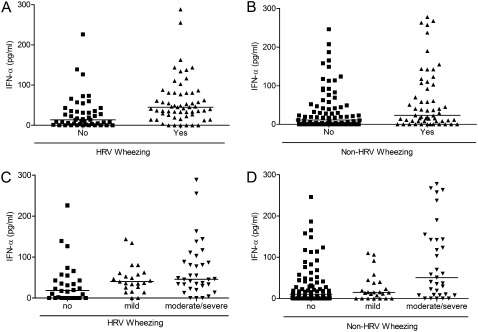
Given that type III IFN-λ1 levels correlated with wheezing and severity of acute exacerbation during HRV infection, and previous studies suggest that type I IFN and IFN-sensitive genes play an essential role in asthma exacerbations (36, 37), we examined whether type I IFN-α was also associated with asthma crises. Interestingly, type I IFN-α levels were higher in respiratory secretions of wheezing compared with nonwheezing people with asthma with URI (P < 0.001) (Figure 4) but did not explain mediation of exacerbations by type III IFN-λ1 (type III IFN-λ1 effect in the model did not change when adjusted for type I IFN-α; P < 0.001). The mediating role of type I IFN-α in HRV-associated crises was small (P = 0.027 for the relationship between HRV and exacerbations weakened to P = 0.08 when adjusted by type I IFN-α).
Figure 4.
Type I IFN-α in human rhinoviruses (HRV)–positive or -negative wheezing severity. (A) Type I IFN-α production in respiratory secretions of HRV-infected nonwheezing (squares) versus wheezing (triangles) children with asthma with upper respiratory infection symptoms, P < 0.001. Black lines represent median values. (B) Type I IFN-α production in respiratory secretions of non–HRV-infected nonwheezing (squares) versus wheezing (triangles) children with asthma with upper respiratory infection symptoms, P = 0.02. Black lines represent median values. (C) Type I IFN-α production in respiratory secretions of HRV-infected children with asthma with nonwheezing (squares), mild wheezing (up-pointing triangles), and moderate-to-severe wheezing (down-pointing triangles), P < 0.05. Black lines represent median values. (D) Type I IFN-α production in respiratory secretions of non–HRV-infected children with asthma with nonwheezing (squares), mild wheezing (up-pointing triangles), and moderate-to-severe wheezing (down-pointing triangles), P < 0.05. Black lines represent median values.
Discussion
This study shows that HRV infections specifically associate with pediatric asthma exacerbations, and that type III IFN-λ1 levels increase with worsening acute asthma symptoms during HRV infections. More importantly, our findings suggest that type III IFN-λ1 responses contribute to HRV asthma attacks, and offer a potential explanation for divergent results in earlier manuscripts (14, 30): although type III IFN-λ1 levels were deficient at baseline compared with healthy control subjects (14), they were hyperexpressed during asthma exacerbations in our population.
Type III IFN-λ1 may play different roles in the pathogenesis of asthma. Low baseline type III IFN-λ1 levels in people with asthma (14) may enhance susceptibility to HRV-infection and consequent virus-mediated asthma attacks in children. Potential markers of these low levels of defensive cytokines in the respiratory tract of people with asthma include early nasopharyngeal colonization with bacteria (38), and evidence for impaired viral clearance in these patients (13). In addition, IFN-λ1 promotes production of inflammatory chemokines and cytokines (39), and modulates proliferation of regulatory T cells and Th1/Th2 bias (40, 41). However, no evidence was found to support a role for virus titer or these innate and adaptive responses in HRV-mediated crises in our study. Importantly, IFN-λ is produced by alveolar type II cells (42), and affects receptors in epithelial cells (43). Pathways in lung epithelial cells can trigger exacerbations (44). In line with this pathogenic role for the cytokine, a study of patients with asthma described higher serum IFN-λ1 levels in dust mite–sensitized individuals and allergic people with asthma during peak pollen season compared with healthy control subjects or allergic people with asthma out of season (34). However, the mechanism by which IFN-λ1 production after HRV infection leads to wheezing is unclear and needs further investigation. It may be of interest to study whether low baseline levels of IFN-λ1 lead to higher expression levels of its receptor in respiratory epithelium and, consequently, a heightened, more exuberant cytokine effect during HRV infection. Interestingly, activation in IFN signaling pathways was significantly elevated during acute exacerbations compared with convalescence in a recent pediatric study, and subsequent baseline lung function in children with asthma varied as a function of the degree of activation of these IFN-dependent pathways (45).
Certain limitations are inherent to a study of these characteristics and stress that results should be interpreted with caution. First, in studying human populations we recognize the possibility that other environmental factors or unmeasured confounders may affect the results (35). Second, even though we examined highly representative inflammatory cytokines (28), other unmeasured inflammatory molecules could influence disease severity. Third, untypable HRV strains may obscure a species (perhaps C viruses, for which less sequence information is available) or group of viruses associated with wheezing. Fourth, our research question (determinants of wheezing in people with asthma with URI) did not include ascertainment of viral shedding in completely asymptomatic children with asthma (no URI symptoms) and because shedding in asymptomatic patients can be prevalent could overestimate the role of HRV in URI. Moreover, sampling a single time point for viral replication and the potential variability of sample dilution during lavage suggest that the lack of association between wheezing and HRV replication should be interpreted with caution. In addition, studying children during wild-type infections requires extrapolation of immune manifestations in the lungs through the analysis of upper respiratory tract secretions. The direct correlation of cytokine responses and virus titer in the upper and lower respiratory tract during viral infections has been described in other publications (46, 47).
However, this study has important strengths. First, it presents hundreds of children with asthma with URI symptoms, with and without HRV-infection or asthma exacerbations, for analysis of virus-specific triggers of acute disease. Second, its design addresses whether HRV has a specific association with asthma crises by including nonwheezing children with asthma with URI symptoms as control subjects. Third, it evaluates several leading hypotheses on immune mechanisms of asthma crises. Finally, it contextualizes the interaction between HRV and type III IFN-λ1 during asthma attacks and suggests a mechanistic role for type III IFN-λ1. Moreover, it emphasizes the potential importance of IFN, in this case λ and α, in the disease.
More than 150 HRV serotypes have been recognized (48, 49), stressing the challenges for the design of HRV-specific vaccines. Modulation of IFN-λ1 should be studied as a preventive or therapeutic target against HRV-associated asthma crises.
Supplementary Material
Footnotes
Supported by the Thrasher Research Fund (to F.P.P.) and KL2 RR24977-03 and 1K23AI091691-01 (to E.K.M.).
Author Contributions: E.K.M., J.Z.H., V.W., M.E.S., N.B., J.P.B., Y.M., A.R., A.R., M.O., N.P., M.B., D.K., S.C., F.M.F., A.C., G.K.S., J.V.W., and F.P.P. obtained data or performed experiments; E.K.M., B.E.S., M.E.S., D.H., R.L., S.J.L., J.B., and F.P.P. analyzed the data; and E.K.M., V.W., D.H., R.L., and F.P.P. wrote the manuscript.
This article has an online supplement, which is accessible from this issue's table of contents at www.atsjournals.org
Originally Published in Press as DOI: 10.1164/rccm.201108-1462OC on December 1, 2011
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ. Asthma and wheezing in the first six years of life. The Group Health Medical Associates. N Engl J Med 1995;332:133–138 [DOI] [PubMed] [Google Scholar]
- 2.Worldwide variation in prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and atopic eczema: ISAAC. The International Study of Asthma and Allergies in Childhood (ISAAC) steering committee. Lancet 1998;351:1225–1232 [PubMed] [Google Scholar]
- 3.Miller EK, Lu X, Erdman DD, Poehling KA, Zhu Y, Griffin MR, Hartert TV, Anderson LJ, Weinberg GA, Hall CB, et al. Rhinovirus-associated hospitalizations in young children. J Infect Dis 2007;195:773–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pizzichini MM, Pizzichini E, Efthimiadis A, Chauhan AJ, Johnston SL, Hussack P, Mahony J, Dolovich J, Hargreave FE. Asthma and natural colds. Inflammatory indices in induced sputum: a feasibility study. Am J Respir Crit Care Med 1998;158:1178–1184 [DOI] [PubMed] [Google Scholar]
- 5.Grissell TV, Powell H, Shafren DR, Boyle MJ, Hensley MJ, Jones PD, Whitehead BF, Gibson PG. Interleukin-10 gene expression in acute virus-induced asthma. Am J Respir Crit Care Med 2005;172:433–439 [DOI] [PubMed] [Google Scholar]
- 6.Friedlander SL, Jackson DJ, Gangnon RE, Evans MD, Li Z, Roberg KA, Anderson EL, Carlson-Dakes KT, Adler KJ, Gilbertson-White S, et al. Viral infections, cytokine dysregulation and the origins of childhood asthma and allergic diseases. Pediatr Infect Dis J 2005;24:S170–176, discussion S174–175 [DOI] [PubMed] [Google Scholar]
- 7.Busse WW, Lemanske RF, Jr, Gern JE. Role of viral respiratory infections in asthma and asthma exacerbations. Lancet 2010;376:826–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rakes GP, Arruda E, Ingram JM, Hoover GE, Zambrano JC, Hayden FG, Platts-Mills TA, Heymann PW. Rhinovirus and respiratory syncytial virus in wheezing children requiring emergency care. IgE and eosinophil analyses. Am J Respir Crit Care Med 1999;159:785–790 [DOI] [PubMed] [Google Scholar]
- 9.Message SD, Laza-Stanca V, Mallia P, Parker HL, Zhu J, Kebadze T, Contoli M, Sanderson G, Kon OM, Papi A, et al. Rhinovirus-induced lower respiratory illness is increased in asthma and related to virus load and Th1/2 cytokine and IL-10 production. Proc Natl Acad Sci U S A 2008;105:13562–13567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, Zhu Y, Szilagyi PG, Morin LL, Heil LH, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol 2009;123:98–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerna G, Piralla A, Rovida F, Rognoni V, Marchi A, Locatelli F, Meloni F. Correlation of rhinovirus load in the respiratory tract and clinical symptoms in hospitalized immunocompetent and immunocompromised patients. J Med Virol 2009;81:1498–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller EK. New human rhinovirus species and their significance in asthma exacerbation and airway remodeling. Immunol Allerg Clin North Am 2010;30:541–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wark PA, Johnston SL, Bucchieri F, Powell R, Puddicombe S, Laza-Stanca V, Holgate ST, Davies DE. Asthmatic bronchial epithelial cells have a deficient innate immune response to infection with rhinovirus. J Exp Med 2005;201:937–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Contoli M, Message SD, Laza-Stanca V, Edwards MR, Wark PA, Bartlett NW, Kebadze T, Mallia P, Stanciu LA, Parker HL, et al. Role of deficient type III interferon-lambda production in asthma exacerbations. Nat Med 2006;12:1023–1026 [DOI] [PubMed] [Google Scholar]
- 15.Bizzintino J, Lee WM, Laing IA, Vang F, Pappas T, Zhang G, Martin AC, Khoo SK, Cox DW, Geelhoed GC, et al. Association between human rhinovirus C and severity of acute asthma in children. Eur Respir J 2011;37:1037–1042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Teran LM, Johnston SL, Schroder JM, Church MK, Holgate ST. Role of nasal interleukin-8 in neutrophil recruitment and activation in children with virus-induced asthma. Am J Respir Crit Care Med 1997;155:1362–1366 [DOI] [PubMed] [Google Scholar]
- 17.Norzila MZ, Fakes K, Henry RL, Simpson J, Gibson PG. Interleukin-8 secretion and neutrophil recruitment accompanies induced sputum eosinophil activation in children with acute asthma. Am J Respir Crit Care Med 2000;161:769–774 [DOI] [PubMed] [Google Scholar]
- 18.Stephens R, Randolph DA, Huang G, Holtzman MJ, Chaplin DD. Antigen-nonspecific recruitment of Th2 cells to the lung as a mechanism for viral infection-induced allergic asthma. J Immunol 2002;169:5458–5467 [DOI] [PubMed] [Google Scholar]
- 19.National Asthma Education and Prevention Program of the National Heart Lung and Blood Institute, and the National Center for Environmental Health of the Centers for Disease Control and Prevention (NAEPP II). Key clinical activities for quality asthma care, recommendations of the National Asthma Education and Prevention Program. MMWR Recomm Rep 2003;52:3–5 [PubMed] [Google Scholar]
- 20. Global Initiative for Asthma [Internet; accessed 2006 Sept 1]. Available from: www.ginasthma.org.
- 21.Lu X, Holloway B, Dare RK, Kuypers J, Yagi S, Williams JV, Hall CB, Erdman DD. Real-time reverse transcription-PCR assay for comprehensive detection of human rhinoviruses. J Clin Microbiol 2008;46:533–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Savolainen C, Blomqvist S, Mulders MN, Hovi T. Genetic clustering of all 102 human rhinovirus prototype strains: serotype 87 is close to human enterovirus 70. J Gen Virol 2002;83:333–340 [DOI] [PubMed] [Google Scholar]
- 23.Miller EK, Edwards KM, Weinberg GA, Iwane MK, Griffin MR, Hall CB, Zhu Y, Szilagyi PG, Morin LL, Heil LH, et al. A novel group of rhinoviruses is associated with asthma hospitalizations. J Allergy Clin Immunol 2009;123:98–104, e101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamura K, Dudley J, Nei M, Kumar S. MEGA: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol 2007;24:1596–1599 [DOI] [PubMed] [Google Scholar]
- 25.Arden KE, McErlean P, Nissen MD, Sloots TP, Mackay IM. Frequent detection of human rhinoviruses, paramyxoviruses, coronaviruses, and bocavirus during acute respiratory tract infections. J Med Virol 2006;78:1232–1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McErlean P, Shackelton LA, Lambert SB, Nissen MD, Sloots TP, Mackay IM. Characterisation of a newly identified human rhinovirus, HRV-QPM, discovered in infants with bronchiolitis. J Clin Virol 2007;39:67–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lau SK, Yip CC, Lin AW, Lee RA, So LY, Lau YL, Chan KH, Woo PC, Yuen KY. Clinical and molecular epidemiology of human rhinovirus C in children and adults in Hong Kong reveals a possible distinct human rhinovirus C subgroup. J Infect Dis 2009;200:1096–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janeway CA TP, Walport M, Shlomchik M. Immunobiology. The immune system in health and disease, 6th ed New York: Garland Science Publishing; 2005. pp. 48–54 [Google Scholar]
- 29.Wills-Karp M, Luyimbazi J, Xu X, Schofield B, Neben TY, Karp CL, Donaldson DD. Interleukin-13: central mediator of allergic asthma. Science 1998;282:2258–2261 [DOI] [PubMed] [Google Scholar]
- 30.Wills-Karp M. Interleukin-13 in asthma pathogenesis. Curr Allergy Asthma Rep 2004;4:123–131 [DOI] [PubMed] [Google Scholar]
- 31.Brooks GD, Buchta KA, Swenson CA, Gern JE, Busse WW. Rhinovirus-induced interferon-gamma and airway responsiveness in asthma. Am J Respir Crit Care Med 2003;168:1091–1094 [DOI] [PubMed] [Google Scholar]
- 32.Papadopoulos NG, Stanciu LA, Papi A, Holgate ST, Johnston SL. A defective type 1 response to rhinovirus in atopic asthma. Thorax 2002;57:328–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bullens DM, Decraene A, Dilissen E, Meyts I, De Boeck K, Dupont LJ, Ceuppens JL. Type III IFN-lambda mRNA expression in sputum of adult and school-aged asthmatics. Clin Exp Allergy 2008;38:1459–1467 [DOI] [PubMed] [Google Scholar]
- 34.He S, Li T, Chen H, Ma W, Yao Q, Yang H, Wang H, Wang F, Zhao C, Yang P. CD14+ cell-derived IL-29 modulates proinflammatory cytokine production in patients with allergic airway inflammation. Allergy 2011;66:238–246 [DOI] [PubMed] [Google Scholar]
- 35.Cole SR, Hernan MA. Fallibility in estimating direct effects. Int J Epidemiol 2002;31:163–165 [DOI] [PubMed] [Google Scholar]
- 36.Bini EJ, Weinshel EH. Severe exacerbation of asthma: a new side effect of interferon-alpha in patients with asthma and chronic hepatitis C. Mayo Clin Proc 1999;74:367–370 [DOI] [PubMed] [Google Scholar]
- 37.Subrata LS, Bizzintino J, Mamessier E, Bosco A, McKenna KL, Wikstrom ME, Goldblatt J, Sly PD, Hales BJ, Thomas WR, et al. Interactions between innate antiviral and atopic immunoinflammatory pathways precipitate and sustain asthma exacerbations in children. J Immunol 2009;183:2793–2800 [DOI] [PubMed] [Google Scholar]
- 38.Bisgaard H, Hermansen MN, Buchvald F, Loland L, Halkjaer LB, Bonnelykke K, Brasholt M, Heltberg A, Vissing NH, Thorsen SV, et al. Childhood asthma after bacterial colonization of the airway in neonates. N Engl J Med 2007;357:1487–1495 [DOI] [PubMed] [Google Scholar]
- 39.Pekarek V, Srinivas S, Eskdale J, Gallagher G. Interferon lambda-1 (IFN-lambda1/IL-29) induces ELR(-) CXC chemokine mRNA in human peripheral blood mononuclear cells, in an IFN-gamma-independent manner. Genes Immun 2007;8:177–180 [DOI] [PubMed] [Google Scholar]
- 40.Mennechet FJ, Uze G. Interferon-lambda-treated dendritic cells specifically induce proliferation of foxp3-expressing suppressor T cells. Blood 2006;107:4417–4423 [DOI] [PubMed] [Google Scholar]
- 41.Jordan WJ, Eskdale J, Srinivas S, Pekarek V, Kelner D, Rodia M, Gallagher G. Human interferon lambda-1 (IFN-lambda1/IL-29) modulates the Th1/Th2 response. Genes Immun 2007;8:254–261 [DOI] [PubMed] [Google Scholar]
- 42.Wang J, Oberley-Deegan R, Wang S, Nikrad M, Funk CJ, Hartshorn KL, Mason RJ. Differentiated human alveolar type II cells secrete antiviral IL-29 (IFN-lambda 1) in response to influenza A infection. J Immunol 2009;182:1296–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Donnelly RP, Kotenko SV. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res 2010;30:555–564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hammad H, Chieppa M, Perros F, Willart MA, Germain RN, Lambrecht BN. House dust mite allergen induces asthma via toll-like receptor 4 triggering of airway structural cells. Nat Med 2009;15:410–416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bosco A, Ehteshami S, Stern DA, Martinez FD. Decreased activation of inflammatory networks during acute asthma exacerbations is associated with chronic airflow obstruction. Mucosal Immunol 2010;3:399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sheeran P, Jafri H, Carubelli C, Saavedra J, Johnson C, Krisher K, Sanchez PJ, Ramilo O. Elevated cytokine concentrations in the nasopharyngeal and tracheal secretions of children with respiratory syncytial virus disease. Pediatr Infect Dis J 1999;18:115–122 [DOI] [PubMed] [Google Scholar]
- 47.Garofalo RP, Patti J, Hintz KA, Hill V, Ogra PL, Welliver RC. Macrophage inflammatory protein-1alpha (not T helper type 2 cytokines) is associated with severe forms of respiratory syncytial virus bronchiolitis. J Infect Dis 2001;184:393–399 [DOI] [PubMed] [Google Scholar]
- 48.Palmenberg AC, Spiro D, Kuzmickas R, Wang S, Djikeng A, Rathe JA, Fraser-Liggett CM, Liggett SB. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science 2009;324:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mackay IM. Human rhinoviruses: the cold wars resume. J Clin Virol 2008;42:297–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.