Abstract
Objective
C-peptide is a main outcome measure in treatment trials of diabetes. C-peptide also has a role in the classification of diabetes, which is often difficult in adults and this is also increasingly recognised in adolescents and elders.
Aim
We aimed to describe the levels of C-peptide in relation to age and body mass index (BMI) in a large population-based cohort of adults with newly diagnosed diabetes and compare the capabilities of C-peptide, age and BMI to discriminate between autoimmune and non-autoimmune diabetes.
Subjects and methods
Blood samples from 1180 patients were analysed regarding islet cell antibody, glutamic acid decarboxylase antibody and fasting C-peptide (FCP). Receiver operating characteristics (ROC) curves were analysed to check the ability of age, BMI and C-peptide to discriminate between autoantibody-positive (Ab+) and -negative (Ab−) diabetes.
Results
Mean FCP was 0.73±0.5 (range 0.13–1.80) nmol/l in the Ab+ and 1.42±0.9 (range 0.13–8.30) nmol/l in the Ab−. FCP was 0.02 nmol/l higher per year increase in age at diagnosis of diabetes. Mean BMI was 26.0±4.8 (range 18.0–39.0) kg/m2 in the Ab+ and 28.9±5.3 (range 15.5–62.6) kg/m2 in the Ab−. FCP increased with age also within each BMI group. The highest area under the curve (AUC) in the ROC analysis was found for C-peptide, followed by age and BMI (0.78, 0.68 and 0.66 respectively).
Conclusions
At diagnosis of diabetes, C-peptide was superior to age and BMI in discriminating between autoimmune and non-autoimmune diabetes. C-peptide increased significantly with BMI and age, latter also within each BMI group. Most of the adults had normal or high levels of C-peptide at presentation of diabetes among the autoimmune patients.
Introduction
The importance of level of blood-cell function, measured as C-peptide, is well recognised in autoimmune diabetes both through its correlation with endogenous insulin secretion and in relation to complications (1, 2). Also in non-autoimmune diabetes, interest in blood-cell function has recently risen considerably (3, 4). Preservation of blood-cell function after diagnosis of diabetes is now a goal also in clinical trials of non-autoimmune diabetes (4, 5).
Classification of diabetes at presentation is often difficult in adults, especially in younger adults (6). The same difficulties are also being increasingly acknowledged in both adolescents and the elderly, where we found incidences of autoimmune diabetes as high as in the youngest age groups (7, 8). In addition to a clinical classification based on age, body mass index (BMI), ketoacidosis and other symptoms, there is also a need for better tools for classification, as type of diabetes has implications for choice of treatment and long-term prognosis, including development of complications (9). Different algorithms aimed to guide physicians in daily practice regarding classification have been published (10, 11).
Classification of diabetes with C-peptide alone was used in research settings before analysis of antibodies became prevalent (12). Classification based on treatment, a less clear definition (13, 14), was recommended by WHO until 1998, when new recommendations proposed classification on aetiological grounds into mainly autoimmune, or type 1, and non-autoimmune, or type 2, diabetes (15, 16). The main difference in the course of autoimmune and non-autoimmune diabetes is the rapid and significant decrease in endogenous insulin secretion in autoimmune diabetes compared with non-autoimmune diabetes, where insulin resistance and metabolic complications are more pronounced (14, 17).
Objective
The aim of this study was to describe the levels of C-peptide and BMI, and their relations to age and antibody status, in a large population-based study of adults with newly diagnosed diabetes and to explore the usefulness of C-peptide levels, BMI and age in classification of diabetes type at diagnosis of diabetes in adults.
Subjects and methods
Subjects
A prospective incidence study was performed during 3 years (1998–2001) in Kronoberg county, with 177 000 inhabitants. All the 25 health care centres and the two hospitals participated. Blood samples were collected at the time of diagnosis from 98% (1626/1666) of all newly diagnosed adults aged 18–100 years (8). The majority of samples, 83% (1355/1626), were collected in the morning after an overnight fast. We here report on the fasting C-peptide (FCP) levels of the 1180/1355 (87%) of the patients whose data were complete, who were aged ≥20 years, with mean age 65.5±14.3, range 20–100 years, and whose samples were collected ≤90 days from diagnosis of diabetes. Of the 1295 fasting subjects with data on sampling interval, 115 (8.5%) were excluded because the interval was ≥91 days, and for 60 subjects (4.5%), the data were incomplete. Of the 1180 samples reported here, 56.6% (668) were collected within 10 days, 23.0% (271) within 11–30 days and 20.4% (241) within 31–90 days. The population was ethnically homogeneous with 95% Caucasians. Patients with secondary or gestational diabetes were excluded from the initial incidence study. There were no significant differences in gender proportions, in the whole material with 50.7% (598/1180) men, or regarding autoantibody-positive (Ab+) vs -negative (Ab−), 45% (26/58) vs 51% (572/1120) men (not significant (NS)), or within the Ab− or Ab+ groups (P=0.55–85), or within the 10-year age groups (P=0.16–0.70). The study was approved by the Ethics Committee of Lund University and performed according to the Declaration of Helsinki.
Methods
C-peptide was centrally analysed at the Department of Clinical Chemistry, Lund University Hospital, by RIA (MD315; Euro-Diagnostica AB, Malmö, Sweden). Detection limit of the method was 0.13 nmol/l, reference range 0.25–1.0 nmol/l, and total variation (sum of intra- and inter-assay variation) was 7%. Islet cell antibody (ICA) levels were analysed with immunofluorescence with a detection limit of 9 JDF-U, sensitivity 100% and specificity 88%; glutamic acid decarboxylase antibody (GADA) levels were analysed with radioimmunoprecipitation with the lower reference limit index of 0.08, corresponding to 21 WHO-U/ml, sensitivity of 70% and specificity of 100%. Both analyses were standardised according to the Diabetes Antibody Standardization Program (18, 19).
The term autoimmune diabetes (Ab+) was used if subjects were positive to at least one of the antibodies to islet cells and/or glutamic acid decarboxylase. Those negative for both antibodies were designated non-autoimmune (Ab−). Length and weight were measured by a nurse at the health care centre or hospital department and not self-reported by the patient. Calculation of BMI was possible for 98% of the fasting subjects.
The relationship between FCP and blood glucose level at the time of sampling for FCP was investigated and the potential influence on FCP of being treated with insulin or oral hypoglycemic agents (OHAs) was also explored, along with level of blood glucose. Type of OHA was not recorded, but at least 50%, probably more, ought to have metformin only, according to the guidelines and praxis at the time, meaning that <5% of all the newly diagnosed patients have been possibly treated with sulphonylurea OHA.
Statistical analyses
Descriptive statistics are reported in 10-year age groups and five BMI groups. Differences between groups were explored by ANOVA, when appropriate with post hoc Bonferroni correction, and potential interactions between levels of FCP, BMI, age, autoimmune status and sample interval (days from diagnosis to sample collection) were explored. The non-parametric Kruskal–Wallis and Mann–Whitney U tests and simple and multiple linear and logistic regression models were also applied. The sensitivity and specificity using the parameters age, BMI and C-peptide, for identifying autoimmune subjects, were explored by receiver operating characteristic (ROC) curves. All tests were two tailed, and a significance level of 0.05 was considered significant. SPSS Software (Statistical Package for the Social Sciences, Chicago, IL, USA) version 17.0 was used.
Results
C-peptide
In all the fasting subjects with newly diagnosed diabetes, mean FCP was 1.39±0.9 (range 0.13–8.30) nmol/l. Mean FCP was significantly lower in the newly diagnosed with autoimmune diabetes than that in the non-autoimmune group (Table 1). There was no significant mean FCP difference with regard to sex in the Ab+, but in the Ab− FCP was higher in the women, 1.48±0.84, compared with the men, 1.37±0.87, P=0.03; the range was the same.
Table 1.
Fasting C-peptide levels (nmol/l), and BMI (kg/m2), per age group in adults with newly diagnosed diabetes. Values are mean±s.d.; median, min–max.
| C-peptide, fasting | BMI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | n | Autoimmune | n | Non-autoimmune | P, Ai vs non-Ai | n | Autoimmune | n | Non-autoimmune | P, Ai vs non-Ai |
| 20–29 | 7 | 0.32±0.29 | 13 | 0.91±0.93 | NS | 7 | 23.9±6.2 | 12 | 26.1±8.2 | NS |
| 0.23, 0.13–0.96 | 0.66, 0.19–3.5 | 22.8, 18.0–37.0 | 22.0, 19.0–42.0 | |||||||
| 30–39 | 3 | 0.18±0.42 | 28 | 1.38±0.67 | 0.05 | 3 | 19.0±1.0 | 27 | 32.0±7.0 | 0.04 |
| 0.19, 0.13–0.21 | 1.30, 0.13–3.10 | 19.0, 18.0–20.0 | 31.0, 16.5–45.0 | |||||||
| 40–49 | 9 | 0.51±0.31 | 102 | 1.16±0.51 | <0.0001 | 9 | 26.8±6.5 | 101 | 31.2±5.2 | <0.02 |
| 0.52, 0.13–0.96 | 1.10, 0.13–2.90 | 24.0, 20.0–39.0 | 31.0, 19.0–46.0 | |||||||
| 50–59 | 14 | 0.70±0.41 | 214 | 1.23±0.58 | <0.001 | 14 | 26.1±4.7 | 212 | 30.5±6.0 | <0.008 |
| 0.62, 0.24–1.50 | 1.10, 0.13–5.20 | 26.4, 19.5–38.0 | 29.5, 16.4–62.6 | |||||||
| 60–69 | 9 | 1.06±0.62 | 261 | 1.30±0.61 | NS | 9 | 27.3±3.7 | 260 | 29.1±4.5 | NS |
| 1.03, 0.13–1.80 | 1.10, 0.22–4.0 | 27.5, 22.0–34.0 | 28.5, 17.7–47.0 | |||||||
| 70–79 | 13 | 0.94±0.53 | 303 | 1.55±0.99 | <0.03 | 13 | 26.8±3.0 | 296 | 28.6±5.0 | NS |
| 1.10, 0.25–1.80 | 1.30, 0.13–8.10 | 27.0, 23.0–33.0 | 28.0, 15.5–45.5 | |||||||
| 80+ | 3 | 1.25±0.07 | 199 | 1.76±1.13 | NS | 3 | 28.1±1.9 | 192 | 26.2±4.3 | NS |
| 1.30, 1.20–1.30 | 1.40, 0.40–8.30 | 28.2, 26.0–30.0 | 25.0, 16.0–47.7 | |||||||
| All | 58 | 0.73±0.5 | 1120 | 1.42±0.9, | <0.0001 | 58 | 26.0±4.8 | 1100 | 28.9±5.3 | <0.0001 |
| 0.62, 0.13–1.80 | 1.20, 0.13–8.30 | 26.0, 18.0–39.0 | 28.0, 15.5–62.6 | |||||||
Ai, autoimmune; non-Ai, non-autoimmune.
The level of FCP increased significantly with age at diagnosis in both non-autoimmune and autoimmune adults with new diabetes (both P<0.0001; Table 1). For every 1-year the increase in age in the newly diagnosed, FCP was 0.02 nmol/l higher in both autoimmune and non-autoimmune diabetes (both P<0.0001); for the increase in FCP per 10-year age group, P was <0.0001 for both diabetes types. For levels of FCP and BMI per diagnosis, age and BMI group, see Tables 1 and 2.
Table 2.
Fasting C-peptide levels (nmol/l), per BMI group (kg/m2), and age (years) in adults with newly diagnosed diabetes. Values are mean±s.d., median, min–max.
| C-peptide, fasting | Age | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| BMI group | n | Autoimmune | n | Non-autoimmune | P Ai vs non-Ai | Autoimmune | Non-autoimmune | P Ai vs non-Ai | |
| A | <20 | 7 | 0.20±0.06 | 29 | 1.03±0.64 | <0.002 | 35.6±8.1 | 63.4±19.8 | <0.001 |
| 0.22, 0.13–0.31 | 1.0, 0.19–3.60 | 32.0, 26–50 | 69.0, 26–95 | ||||||
| B | 20 to <25 | 20 | 0.48±0.40 | 255 | 1.38±1.1 | <0.0001 | 51.7±17.4 | 71.7±13.9 | <0.0001 |
| 0.36, 0.13–1.80 | 1.0, 0.13–8.30 | 52.0, 22–77 | 75.0, 23–94 | ||||||
| C | 25 to <30 | 21 | 1.09±0.38 | 440 | 1.36±0.8 | NS | 65.1±11.7 | 66.7±12.3 | NS |
| 1.20, 0.52–1.80 | 1.20, 0.13–7.80 | 65.0, 40–82 | 67.0, 31–94 | ||||||
| D | 30 to <35 | 6 | 0.91±0.67 | 250 | 1.47±0.7 | <0.04 | 60.3±13.8 | 62.7±13.3, | NS |
| 1.15, 0.25–1.80 | 1.30, 0.44–5.30 | 65.5, 43–73 | 63.0, 21–96 | ||||||
| E | ≥35 | 4 | 0.61±0.32 | 126 | 1.62±0.8 | <0.02 | 42.3±14.5 | 59.1±13.9 | 0.04 |
| 0.51, 0.35–0.96 | 1.50, 0.13–6.40 | 42.0, 28–57 | 58.0, 23–93 | ||||||
| All | 58 | 0.76±0.53 | 1100 | 1.39±0.84 | <0.0001 | 55.1±16.9 | 66.1±14.0 | <0.0001 | |
| 0.62, 0.13–1.9 | 1.20, 0.13–8.3 | 55.0, 22–82 | 67.0, 21–100 | ||||||
Ai, autoimmune; non-Ai, non-autoimmune.
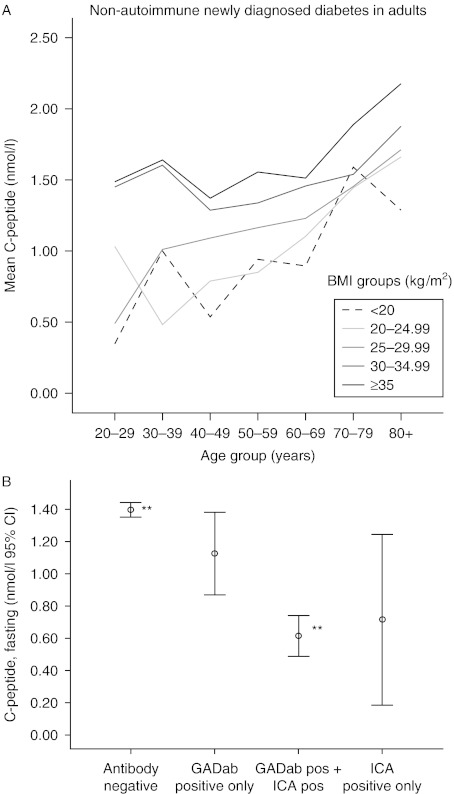
Level of FCP at onset of diabetes was independently influenced by both age and BMI. Their combined influence on the level of FCP was greatest in autoimmune diabetes: 40% (R2=0.398) compared with 8% (R2=0.083) in non-autoimmune diabetes (P<0.0001 for both). FCP was 0.04 nmol/l higher per 1 kg/m2 higher BMI in autoimmune and 0.03 nmol/l higher in non-autoimmune, diabetes (both P<0.001). Being Ab+ meant a 0.4 nmol/l lower FCP, at the same levels of age and BMI, compared with the Ab− subjects (P<0.001). Mean FCP was correlated to increasing BMI, and BMI group, and to increasing age, also within each BMI group, and this was especially apparent in non-autoimmune diabetes (Fig. 1A).
Figure 1.
(A) Mean fasting C-peptide (nmol/l), per age and BMI group in non-autoimmune diabetes. (B) Mean fasting C-peptide, including 95% confidence intervals, per antibody positivity; all in adults with newly diagnosed diabetes. **P<0.001.
In the analysis of the three interval groups sampled (≤10 days, within 11–30 days and within 31 to ≤90 days) there was no difference in levels of FCP between any of the three groups among the Ab− (1.45±0.9, 1.43±0.86 and 1.31±0.64 nmol/l; NS), while in the Ab+, FCP was significantly lower in the ≤10 days sampling, interval (0.57±0.45) compared with the 31- to 90-day interval (1.19±0.51 nmol/l), P=0.02. FCP in the 11- to 30-day group (0.79±0.47 nmol/l) did not significantly differ from any of the other two interval groups (P=0.2–0.8). Per increased interval of 1 day (from 0 to 90 days) from diabetes diagnosis to sampling, FCP was 0.003 nmol/l lower at the time of sampling in the Ab−, P=0.02; in the Ab+, it was 0.0009 higher, P=0.004. The corresponding figures for BMI were +0.02 kg/m2 among Ab−, P=0.002, and among Ab+, +0.08 kg/m2, P=0.02. In the interaction analysis with sample interval only autoimmunity significantly influenced level of FCP, and not age nor interval. Sampling interval significantly influenced level of FCP in the autoimmune subgroup but had no influence on FCP in the non-autoimmune group, confirmed by logistic regression where R2 for interval was only 0.04 (4%) in the Ab−, P=0.02, and slightly higher, 0.123 (12%), P=0.01, in the Ab+.
Of the 1180 patients, 58 (4.9%) were autoimmune. Eighty three percent (926/1120) of the Ab−, 41.4% (24/58) of the Ab+ and 80.5% of all (950/1180) were treated with diet only, no insulin or OHAs, at the time of sampling. In total, 11.7% (138/1180) were treated with OHAs: for Ab− patients, this was 11.8% (132/1120) and for Ab+33% (19/58). Nine Ab− and two Ab+ patients (11/1180, 0.9%) were treated with both OHA and insulin. Insulin treatment was already initiated in 7.6% (90/1180) of the patients, 6.3% (71/1120) Ab− and 33% (19/58) Ab+. In the Ab−, there were no significant differences in the level of mean FCP between the different treatment groups. FCP levels were as follows: for diet only, 1.43±0.8; for OHA treated, 1.36±0.96; for insulin treated, 1.35±1.1; and for OHA+insulin treated, 1.07±0.57 nmol/l; in the Ab+, 1.03±0.0.47, 0.80±0.49, 0.31±0.22 and 0.33±0.28 nmol/l respectively. In the Ab+, the insulin treated had a significantly lower mean level of C-peptide at diagnosis of diabetes than both the diet-only and the OHA-treated groups (P<0.0001 and 0.01 respectively). Complete data for multiple regression analyses were available for 88.3% (1042/1180). Mean blood glucose at sampling (in the 1042 and 1180) was 8.2±2.9 mmol/l (range 1.4–24.2) mmol/l. Only one subject had blood glucose in the hypoglycemic range, <3 mmol/l, while 79% (820/1042) had blood glucose 3.0–10.0 mmol/l, 21% (219/1042) 10.1–20.0 mmol/l and two individuals ≥20.0 mmol/l.
The relationship between FCP and blood glucose level at the time of sampling for FCP was explored in a multiple regression model including age and autoimmune status. Level of blood glucose had no influence at all on level of FCP, P=0.75. Both age and autoimmunity, however, influenced level of C-peptide significantly (P<0.0001). In another multiple regression model, the potential influence of blood glucose on FCP in relation to treatment with insulin or OHAs at the time of the sampling was calculated. There was still no correlation between FCP and blood glucose (P=0.40), and there were no correlations between FCP and treatment with OHAs (P=0.14), but there was a significant relation between being treated with insulin early and level of FCP (P=0.001).
BMI and age
Mean BMI for all was 28.8±5.4 (range 15.5–62.6) kg/m2. Mean BMI for the Ab+ was significantly lower than that for the Ab− (P<0.0001; Table 1). The women had higher mean BMI level than the men, both the autoimmune, 27.4±5.5 vs 24.5±3.4 kg/m2 (P<0.03), and the non-autoimmune, 29.4±5.6 vs 28.5±5.0 kg/m2 (P<0.01). For BMI in different age groups, see Table 1 and Fig. 1A. In the younger adult age groups, especially ages 30–50 years, among those who were newly diagnosed with non-autoimmune diabetes had the highest mean levels of BMI, which then decreased with increasing age at onset (P<0.0001). The women were older than the men at diagnosis of Ab− diabetes, 66.4±14.5, min 22, max 100 vs 64.1±14.3, min 21, max 94 years (P<0.003).
Mean BMI in the Ab− did not differ significantly from neither the only GADA+ nor the only ICA+, nor from those positive to both antibodies. There were no significant differences in BMI between the different Ab+ groups.
Autoimmunity
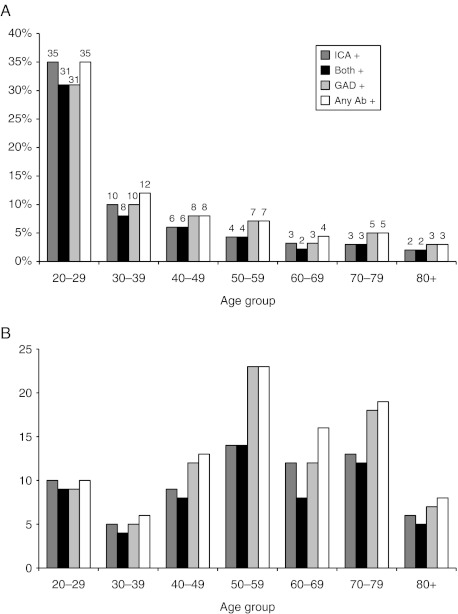
Mean FCP levels in those negative to both antibodies were significantly higher than those positive to both antibodies (P<0.001), but with correction for multiple comparisons were not significantly different from those positive to only one antibody, GADA or ICA, and there were no significant differences in the levels of C-peptide between any of the Ab+ groups (Fig. 1B). The mean FCP in only GADA+ subjects, 1.06±0.53, range 0.17–1.80, was lower than that in the Ab−, 1.42±0.85, range 0.13−8.30, but the difference did not reach significance (Fig. 1B). The majority of the Ab+ subjects were both GADA+ and ICA+ (Fig. 2A and B). The Ab+ proportion of newly diagnosed subjects was highest in the adult age groups below 60 years, peaking at age 20–29 years (Fig. 2A). The proportion of Ab+ subjects among the newly diagnosed was 3–5% in ages 60–100 years. The proportion of newly diagnosed that was Ab+ decreased with age, but the absolute numbers of Ab+ subjects were higher in the 50- to 80-year olds than in the younger age groups (Fig. 2B). Among subjects with BMI ≤20 kg/m2, 15–20% were Ab+, as were 5–7% among those with BMI 20–25 kg/m2 and <5% among BMI ≥25 kg/m2.
Figure 2.
Prevalence of antibodies per age group in percent (A) and absolute numbers (B) in adults with newly diagnosed diabetes.
ROC analysis
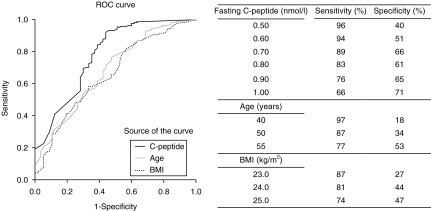
ROC curves for the fasting subjects are displayed in Fig. 3. AUC for FCP was superior to those for age and BMI for differentiating between Ab+ and Ab− subjects. AUC was 0.78±0.04 (0.71–0.85) for C-peptide, 0.68±0.04 (0.61–0.76) for age and 0.66±0.04 (0.59–0.73) for BMI (P<0.0001 for all). For sensitivity and specificity, see Fig. 3.
Figure 3.
ROC analysis of C-peptide, BMI and age to identify subjects that are autoimmune.
Discussion
C-peptide
C-peptide level at diagnosis of both autoimmune and non-autoimmune diabetes increased with age at onset, which has been described, mostly in younger patients (20). Similar to our findings, FCP increased by 2% per year from age at onset in newly diagnosed Italian type 1 patients aged 0–49 years (21). Of our newly diagnosed adults above the age of 40 years with autoimmune diabetes, 95% had detectable C-peptide compared with 60% in the 15- to 34-year olds in the Diabetes Incidence Study in Sweden (22). The same trend was found in non-diabetic subjects and was experimentally demonstrated to be due to increasing insulin resistance with age independent of BMI (23). Our elderly patients had higher FCP than those healthy elders, probably due to a dominating insulin resistance at onset of diabetes (23, 24, 25).
C-peptide at diagnosis was increased with increasing BMI level and also with age within each BMI group, emphasising the independent influence of age on C-peptide. The women had higher levels of BMI and were older at presentation of Ab− diabetes than the men, and consequently also had higher C-peptide levels compared with the men. Men are usually more prone to abdominal and visceral rather than subcutaneous fat distribution, and visceral fat is not as well reflected by BMI (26, 27).
The Botnia study found significantly lower levels of FCP than we did, 0.46 vs 0.62 nmol/l, in GADA+ vs GADA− patients (17); the levels showed the same relation but both lower than ours, probably due to the longer duration of diabetes that was 6±10 years, while in the Kronoberg study, all patients were recently diagnosed. Our levels of C-peptide may differ from those found in other studies due to differences in age, as we also included the eldest age groups, in which diabetes is most prevalent (8, 28, 29, 30). In contrast to the prevailing notion that level of blood glucose influences level of C-peptide, we found no evidence of such influence in this cohort. A minority of the patients in this study were treated with OHAs or insulin at the time of the study, and treatment was withheld on the morning of the fasting sampling, therefore it is not likely that these factors had any major influence on the results. The majority of the patients (88%) were treated with diet only at the time of sampling for FCP, and it was only in the already insulin-treated Ab+ patients that there was a significant correlation between level of blood glucose at sampling and level of FCP (P=0.003).
Body mass index
Our findings that the most obese subjects were affected by non-autoimmune diabetes earlier in life than the less obese were in accordance with a registry study from Oregon (31). The levels of BMI that we found were lower than those in the Oregon study, but higher than those in the Botnia study, probably due to differences in ethnicities and duration after onset of diabetes (17, 31). The trend of negative correlation between BMI and age in Ab− diabetes was similar in the Botnia study. We also analysed this relation in patients with Ab+ diabetes and found an opposite trend. This, in combination with that the majority of the adults with newly diagnosed diabetes, independently of presence of autoantibodies, had BMI in the overweight or obese ranges, accentuates the limitation in using BMI for distinction between diabetes types. The ROC analysis ranked BMI after both C-peptide and age regarding ability to detect autoimmune diabetes. In accordance with this, one study found that a combination of antibodies and C-peptide was found to have the highest predictive value for ketose-prone diabetes (32). The explanatory rate for C-peptide level of age and BMI was greater in autoimmune than in non-autoimmune diabetes, 39 vs 8%. This indicates that the major explanations are due to other factors and that the relations between insulin resistance and blood-cell function are more complex, which has also been demonstrated experimentally (33).
Autoimmunity
Considering that we also included the oldest age groups, with most prevalent diabetes, the prevalence of patients positive to at least one pancreatic autoantibody was in accordance with previous studies, such as UKPDS and the Botnia study (8, 17, 34). Some early studies analysed only one antibody (17, 28, 29, 34). The differences in frequency of Ab+ between studies can be explained by the number and types of antibodies analysed, duration of diabetes as well as ages in the populations studied.
The proportion of Ab+ subjects among our newly diagnosed patients decreased with age and was 3–5% in ages 60–100 years. However, the absolute numbers of subjects positive to at least one antibody was higher in the 50- to 80-year olds than in the younger adult age groups due to the larger number of individuals affected by diabetes in the older age groups (8).
Classification
Age and BMI are among the most frequently used tools for clinical classification but seldom tested in an evidence-based manner. In our large population-based cohort of newly diagnosed patients, we found that, while still not ideal, C-peptide was a better discriminator than both age and BMI for identifying those positive to at least one of GADA and/or ICA, and C-peptide AUC reached the same level in ROC analysis as several classification schemes for ketosis-prone diabetes (32).
The reasons for assigning individuals with diabetes to different subgroups are both to facilitate research regarding all aspects from pathophysiology to new treatments and prevention and for clinical use when recommending treatment to patients (35, 36). Concomitantly, there are also limitations to the use of classifications (37).
The emergence of different treatments for different types of diabetes, reactualised by the latest new group of anti-diabetic drugs, the incretin-based therapies and by treatments aiming for prevention, relief and cure of autoimmune diabetes, all put new focus on blood-cell function (4, 5, 38), which is still best estimated by C-peptide (39). C-peptide is available in standard commercial assays, is not very costly and ought to be readily available in many settings.
Previously, the difficulties of classification and thereby of recommending the optimal treatment and follow-up for patients were identified to be problems of young adults, but now it appears as a problem of all ages, not least in the elderly, where diabetes is most frequent (7, 8).
Autoantibodies predict insulin dependency within 3–6 years in the majority of adult patients diagnosed with diabetes (34, 40). In the latest recommendation by WHO, LADA is considered a variant of autoimmune, or type 1 diabetes (15, 16), and many share that view (36, 41, 42), but it is also debated (37). A useful classification of diabetes type can save time, effort and concern, and thereby costs, for health care providers and patients; it may increase patient security and possibility of empowerment and minimise risks (30, 32, 41, 43). As the number of adults with autoimmune diabetes are larger than those diagnosed with autoimmune diabetes during childhood, many patients are affected (8, 44). They have a decreasing blood-cell function and are at risk of ketoacidosis. Delay of insulin treatment and prolonged time with deteriorated blood glucose involves risk of earlier debut of complications (9, 32, 41, 45, 46).
Conclusions
C-peptide level at diagnosis of diabetes was superior to both age and BMI in discriminating between autoimmune and non-autoimmune diabetes; it increased with increasing BMI, and age, and later even within each BMI group. Analysis of C-peptide is less expensive than antibody analyses and better than both BMI and age at indicating autoimmune diabetes, information that affects both choice of treatment and follow-up; therefore, C-peptide can be a good complement in clinical practice in many settings.
Acknowledgements
The authors wish to thank the physicians and diabetes nurses of Kronoberg; the patients; PhDs Anna Lindgren, Center of Mathematical Sciences, Lund Institute of Technology, and Per Nyberg, Medical Faculty, both Lund University, for statistical advice; Ass. Prof. Anders Isaksson, Department of Clinical Chemistry, Lund University Hospital, for the analyses of C-peptide; and Lena Widén, Kronoberg County Council R&D for expert secretarial assistance.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The work was financed by the Health Care Regions of Skane and Kronoberg, Southern Sweden; Lund University funding of Clinical research (ALF) and the Swedish Council of Medical Research, grant no K97-19X12242.
References
- Faber OK, Binder C. B-cell function and blood glucose control in insulin dependent diabetics within the first month of insulin treatment. Diabetologia. 1977;13:263–268. doi: 10.1007/BF01219710. [DOI] [PubMed] [Google Scholar]
- Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care. 2003;26:832–836. doi: 10.2337/diacare.26.3.832. [DOI] [PubMed] [Google Scholar]
- Palmer AJ, Roze S, Valentine WJ, Minshall ME, Lammert M, Oglesby A, Hayes C, Spinas GA. What impact would pancreatic beta-cell preservation have on life expectancy, quality-adjusted life expectancy and costs of complications in patients with type 2 diabetes? A projection using the CORE Diabetes Model. Current Medical Research and Opinion. 2004;20(Suppl 1):S59–S66. doi: 10.1185/030079904X2024. [DOI] [PubMed] [Google Scholar]
- Marchetti P, Lupi R, Del Guerra S, Bugliani M, D'Aleo V, Occhipinti M, Boggi U, Marselli L, Masini M. Goals of treatment for type 2 diabetes: beta-cell preservation for glycemic control. Diabetes Care. 2009;32(Suppl 2):S178–S183. doi: 10.2337/dc09-S306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinman B, Gerich J, Buse JB, Lewin A, Schwartz S, Raskin P, Hale PM, Zdravkovic M, Blonde L. Efficacy and safety of the human glucagon-like peptide-1 analog liraglutide in combination with metformin and thiazolidinedione in patients with type 2 diabetes (LEAD-4 Met+TZD) Diabetes Care. 2009;32:1224–1230. doi: 10.2337/dc08-2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnqvist HJ, Littorin B, Nystrom L, Schersten B, Ostman J, Blohme G, Lithner F, Wibell L. Difficulties in classifying diabetes at presentation in the young adult. Diabetic Medicine. 1993;10:606–613. doi: 10.1111/j.1464-5491.1993.tb00132.x. [DOI] [PubMed] [Google Scholar]
- Gilliam LK, Brooks-Worrell BM, Palmer JP, Greenbaum CJ, Pihoker C. Autoimmunity and clinical course in children with type 1, type 2, and type 1.5 diabetes. Journal of Autoimmunity. 2005;25:244–250. doi: 10.1016/j.jaut.2005.09.013. [DOI] [PubMed] [Google Scholar]
- Thunander M, Petersson C, Jonzon K, Fornander J, Ossiansson B, Torn C, Edvardsson S, Landin-Olsson M. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Research and Clinical Practice. 2008;82:247–255. doi: 10.1016/j.diabres.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Brophy S, Brunt H, Davies H, Mannan S, Williams R. Interventions for latent autoimmune diabetes (LADA) in adults. Cochrane Database of Systematic Reviews. 2007;18:CD006165. doi: 10.1002/14651858.CD006165.pub2. [DOI] [PubMed] [Google Scholar]
- Fourlanos S, Perry C, Stein MS, Stankovich J, Harrison LC, Colman PG. A clinical screening tool identifies autoimmune diabetes in adults. Diabetes Care. 2006;29:970–975. doi: 10.2337/dc05-2101. [DOI] [PubMed] [Google Scholar]
- Lutgens MW, Meijer M, Peeters B, Poulsen ML, Rutten MJ, Bots ML, van der Heijden GJ, Soedamah-Muthu SS. Easily obtainable clinical features increase the diagnostic accuracy for latent autoimmune diabetes in adults: an evidence-based report. Primary Care Diabetes. 2008;2:207–211. doi: 10.1016/j.pcd.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Gjessing HJ, Matzen LE, Faber OK, Froland A. Fasting plasma C-peptide, glucagon stimulated plasma C-peptide, and urinary C-peptide in relation to clinical type of diabetes. Diabetologia. 1989;32:305–311. doi: 10.1007/BF00265547. [DOI] [PubMed] [Google Scholar]
- Report of a WHO Study Group. World Health Organisation, Tech Rep Ser no 727, 1985. [PubMed]
- Bennett P & Knowler WC. Definition, diagnosis and classification of diabetes mellitus and glucose homeostasis. In Joslins Diabetes Mellitus, pp 331–339. Ed EA Kahn. Boston: Lipincott, Williams & Wilkins, 2005.
- Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabetic Medicine. 1998;15:539–553. doi: 10.1002/(SICI)1096-9136(199807)15:7%3C539::AID-DIA668%3E3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Diagnosis and classification of diabetes mellitus. Diabetes Care 2010 33 Suppl 1 S62–S69. [DOI] [PMC free article] [PubMed]
- Tuomi T, Carlsson A, Li H, Isomaa B, Miettinen A, Nilsson A, Nissen M, Ehrnstrom BO, Forsen B, Snickars B, Lahti K, Forsblom C, Saloranta C, Taskinen MR, Groop LC. Clinical and genetic characteristics of type 2 diabetes with and without GAD antibodies. Diabetes. 1999;48:150–157. doi: 10.2337/diabetes.48.1.150. [DOI] [PubMed] [Google Scholar]
- Verge CF, Stenger D, Bonifacio E, Colman PG, Pilcher C, Bingley PJ, Eisenbarth GS. Combined use of autoantibodies (IA-2 autoantibody, GAD autoantibody, insulin autoantibody, cytoplasmic islet cell antibodies) in type 1 diabetes: Combinatorial Islet Autoantibody Workshop. Diabetes. 1998;47:1857–1866. doi: 10.2337/diabetes.47.12.1857. [DOI] [PubMed] [Google Scholar]
- Bingley PJ, Bonifacio E, Mueller PW. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes. 2003;52:1128–1136. doi: 10.2337/diabetes.52.5.1128. [DOI] [PubMed] [Google Scholar]
- Picardi A, Visalli N, Lauria A, Suraci C, Buzzetti R, Merola MK, Manfrini S, Guglielmi C, Gentilucci UV, Pitocco D, Crino A, Bizzarri C, Cappa M, Pozzilli P. Metabolic factors affecting residual beta cell function assessed by C-peptide secretion in patients with newly diagnosed type 1 diabetes. Hormone and Metabolic Research. 2006;38:668–672. doi: 10.1055/s-2006-954586. [DOI] [PubMed] [Google Scholar]
- Petrone A, Galgani A, Spoletini M, Alemanno I, Di Cola S, Bassotti G, Picardi A, Manfrini S, Osborn J, Pozzilli P, Buzzetti R. Residual insulin secretion at diagnosis of type 1 diabetes is independently associated with both, age of onset and HLA genotype. Diabetes/Metabolism Research and Reviews. 2005;21:271–275. doi: 10.1002/dmrr.549. [DOI] [PubMed] [Google Scholar]
- Jensen R, Gilliam L, Torn C, Landin-Olsson M, Palmer J, Akesson K, Kockum I, Lernmark B, Karlsson AF, Lynch KF, Breslow N, Lernmark A, Sundkvist G. Islet cell autoantibody levels after the diagnosis of young adult diabetic patients. Diabetic Medicine. 2007;24:1221–1228. doi: 10.1111/j.1464-5491.2007.02235.x. [DOI] [PubMed] [Google Scholar]
- Basu R, Dalla Man C, Campioni M, Basu A, Klee G, Toffolo G, Cobelli C, Rizza RA. Effects of age and sex on postprandial glucose metabolism: differences in glucose turnover, insulin secretion, insulin action, and hepatic insulin extraction. Diabetes. 2006;55:2001–2014. doi: 10.2337/db05-1692. [DOI] [PubMed] [Google Scholar]
- Greenbaum CJ, Anderson AM, Dolan LM, Mayer-Davis EJ, Dabelea D, Imperatore G, Marcovina S, Pihoker C. Preservation of beta-cell function in autoantibody-positive youth with diabetes. Diabetes Care. 2009;32:1839–1844. doi: 10.2337/dc08-2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai EB, Sherry NA, Palmer JP, Herold KC. The rise and fall of insulin secretion in type 1 diabetes mellitus. Diabetologia. 2006;49:261–270. doi: 10.1007/s00125-005-0100-8. [DOI] [PubMed] [Google Scholar]
- Kuk JL, Saunders TJ, Davidson LE, Ross R. Age-related changes in total and regional fat distribution. Ageing Research Reviews. 2009;8:339–348. doi: 10.1016/j.arr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gender Medicine. 2009;6(Suppl 1):60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clauson P, Linnarsson R, Gottsater A, Sundkvist G, Grill V. Relationships between diabetes duration, metabolic control and beta-cell function in a representative population of type 2 diabetic patients in Sweden. Diabetic Medicine. 1994;11:794–801. doi: 10.1111/j.1464-5491.1994.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Gottsater A, Landin-Olsson M, Fernlund P, Lernmark A, Sundkvist G. Beta-cell function in relation to islet cell antibodies during the first 3 yr after clinical diagnosis of diabetes in type II diabetic patients. Diabetes Care. 1993;16:902–910. doi: 10.2337/diacare.16.6.902. [DOI] [PubMed] [Google Scholar]
- Tfayli H, Bacha F, Gungor N, Arslanian S. Islet cell antibody-positive versus -negative phenotypic type 2 diabetes in youth: does the oral glucose tolerance test distinguish between the two? Diabetes Care. 2010;33:632–638. doi: 10.2337/dc09-0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillier TA, Pedula KL. Characteristics of an adult population with newly diagnosed type 2 diabetes: the relation of obesity and age of onset. Diabetes Care. 2001;24:1522–1527. doi: 10.2337/diacare.24.9.1522. [DOI] [PubMed] [Google Scholar]
- Balasubramanyam A, Garza G, Rodriguez L, Hampe CS, Lakshmi G, Lernmark A, Maldonado MR. Accuracy and predictive value of ketosis-prone diabetes. Diabetes Care. 2006;29:2575–2579. doi: 10.2337/dc06-0749. [DOI] [PubMed] [Google Scholar]
- Ferrannini E, Gastaldelli A, Miyazaki Y, Matsuda M, Mari A, DeFronzo RA. Beta-cell function in subjects spanning the range from normal glucose tolerance to overt diabetes: a new analysis. Journal of Clinical Endocrinology and Metabolism. 2005;90:493–500. doi: 10.1210/jc.2004-1133. [DOI] [PubMed] [Google Scholar]
- Turner R, Stratton I, Horton V. UKPDS: autoantibodies to islet-cell cytoplasm and glutamic acid decarboxylase for prediction of insulin requirement in type 2 diabetes. Lancet. 1997;350:1288–1293. doi: 10.1016/S0140-6736(97)03062-6. [DOI] [PubMed] [Google Scholar]
- Bronstein J, Lawrence RD. Two types of diabetes mellitus, with and without available plasma insulin. BMJ. 1951;1:732. doi: 10.1136/bmj.1.4709.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolandsson O, Palmer JP. Latent autoimmune diabetes in adults (LADA) is dead: long live autoimmune diabetes. Diabetologia. 2010;53:1250–1253. doi: 10.1007/s00125-010-1713-0. [DOI] [PubMed] [Google Scholar]
- Gale EAM. Declassifying diabetes. Diabetologia. 2006;49:1989–1995. doi: 10.1007/s00125-006-0348-7. [DOI] [PubMed] [Google Scholar]
- Keymeulen B, Vandemeulebroucke E, Ziegler AG, Mathieu C, Kaufman L, Hale G, Gorus F, Goldman M, Walter M, Candon S, Schandene L, Crenier L, De Block C, Seigneurin JM, De Pauw P, Pierard D, Weets I, Rebello P, Bird P, Berrie E, Frewin M, Waldmann H, Bach JF, Pipeleers D, Chatenoud L. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. New England Journal of Medicine. 2005;352:2598–2608. doi: 10.1056/NEJMoa043980. [DOI] [PubMed] [Google Scholar]
- Palmer JP, Fleming GA, Greenbaum CJ, Herold KC, Jansa LD, Kolb H, Lachin JM, Polonsky KS, Pozzilli P, Skyler JS, Steffes MW. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve beta-cell function: report of an ADA workshop, 21–22 October 2001. Diabetes. 2004;53:250–264. doi: 10.2337/diabetes.53.1.250. [DOI] [PubMed] [Google Scholar]
- Landin-Olsson M, Nilsson KO, Lernmark A, Sundkvist G. Islet cell antibodies and fasting C-peptide predict insulin requirement at diagnosis of diabetes mellitus. Diabetologia. 1990;33:561–568. doi: 10.1007/BF00404145. [DOI] [PubMed] [Google Scholar]
- Fourlanos S, Dotta F, Greenbaum CJ, Palmer JP, Rolandsson O, Colman PG, Harrison LC. Latent autoimmune diabetes in adults (LADA) should be less latent. Diabetologia. 2005;48:2206–2212. doi: 10.1007/s00125-005-1960-7. [DOI] [PubMed] [Google Scholar]
- Leslie RD, Williams R, Pozzilli P. Clinical review: type 1 diabetes and latent autoimmune diabetes in adults: one end of the rainbow. Journal of Clinical Endocrinology and Metabolism. 2006;91:1654–1659. doi: 10.1210/jc.2005-1623. [DOI] [PubMed] [Google Scholar]
- Appel SJ, Wadas TM, Rosenthal RS, Ovalle F. Latent autoimmune diabetes of adulthood (LADA): an often misdiagnosed type of diabetes mellitus. Journal of the American Academy of Nurse Practitioners. 2009;21:156–159. doi: 10.1111/j.1745-7599.2009.00399.x. [DOI] [PubMed] [Google Scholar]
- Naik RG, Brooks-Worrell BM, Palmer JP. Latent autoimmune diabetes in adults. Journal of Clinical Endocrinology and Metabolism. 2009;94:4635–4644. doi: 10.1210/jc.2009-1120. [DOI] [PubMed] [Google Scholar]
- Isomaa B, Almgren P, Henricsson M, Taskinen MR, Tuomi T, Groop L, Sarelin L. Chronic complications in patients with slowly progressing autoimmune type 1 diabetes (LADA) Diabetes Care. 1999;22:1347–1353. doi: 10.2337/diacare.22.8.1347. [DOI] [PubMed] [Google Scholar]
- Thunander M, Thorgeirsson H, Torn C, Petersson C, Landin-Olsson M. Beta-cell function and metabolic control in latent autoimmune diabetes in adults with early insulin versus conventional treatment: a 3-year follow-up. European Journal of Endocrinology. 2011;164:239–245. doi: 10.1530/EJE-10-0901. [DOI] [PMC free article] [PubMed] [Google Scholar]