Abstract
We report on the significant improvement of central macular thickness in a case of clinically significant macular edema after dexamethasone 0.7 mg sustained-release intravitreal implant (Ozurdex®; Allergan, Inc, Irvine, CA, USA). Patient presented to us with persistent clinically significant macular edema (CSME) in both eyes. Right eye received dexamethasone implant and left eye received two intravitreal bevacizumab injections 1.25 mg/0.05 mL (Avastin®; Genentech Inc., South San Francisco, CA, USA) with an interval of four weeks. After six weeks of follow-up, dexamethasone implant in the right eye showed normal macular thickness whereas persistent macular edema (ME) was found even after second intravitreal bevacizumab injection in the left eye.
Keywords: Clinically significant macular edema, dexamethasone, implant, ozurdex
Focal/grid laser photocoagulation is still the gold standard treatment for diabetic macular edema (DME).[1] However, for patients with refractory DME, treatment/s beyond laser therapy is to be looked at. In a large case series, bevacizumab has been used in the treatment of DME successfully.[2,3] Glucocorticoid such as intravitreal triamcinolone (IVTA) has been shown to be useful for the treatment of refractory DME resistant to grid or focal laser, however, the recurrence of DME and safety of triamcinolone on ocular cells was questioned by many researchers.[4–6] Dexamethasone is a known potent anti-inflammatory agent with a favorable side-effect profile. Previous studies have shown that dexamethasone biodegradable drug delivery system (Ozurdex®; Allergan Inc, Irvine, California, USA) can improve visual acuity (BCVA) and macular thickness in cases of vein occlusion and non-infectious uveitis.[7,8] Ozurdex is in Phase III of FDA approval for DME. Here, we describe our experience with off-label use of 0.7 mg dexamethasone intravitreal implant in the treatment of refractory clinically significant macular edema (CSME) with intravitreal bevacizumab use in the other eye as a comparative arm. To the best of our knowledge this is the first case comparing efficacy of dexamethasone implant and bevacizumab in a single patient.
Case Report
A 43-year-old male presented with refractory CSME in both eyes. Grid laser treatment was performed in both eyes seven months ago. In spite of seven months of follow-up after grid laser with good systemic control, persistent CSME was found in both the eyes with central macular thickness (CMT) of 311 μm and 452 μm in the right and left eye respectively [Figs. 1a and 1b]. Intravitreal bevacizumab was given in the left eye and the right eye was kept under observation. Four weeks after bevacizumab injection, CMT was reduced to 355 μm in the left eye with persistent CSME observed with maximum macular thickness (MMT) of 461 μm [Fig. 1d]. CMT increased in the right eye to 350 μm [Fig. 1c]. Intravitreal dexamethasone implant was injected in the right eye whereas second bevacizumab injection was given in the left eye. At six weeks of follow-up, CMT returned to 261 μm in the right eye [Fig. 1e] whereas CMT and MMT were 306 μm and 420 μm respectively in the left eye [Fig. 1f].
Figure 1.
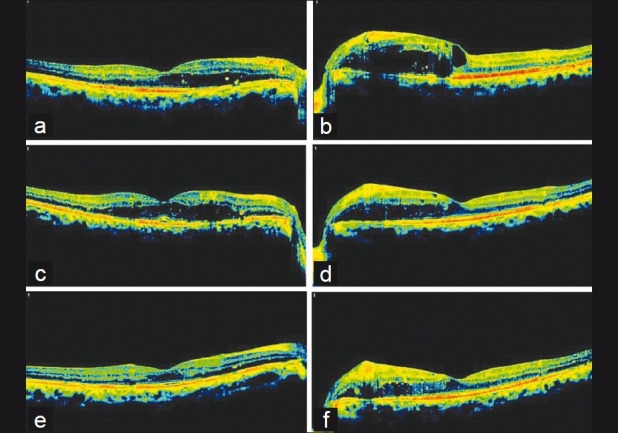
Persistent macular edema (ME) in the right eye after 7 months follow-up of grid laser with central macular thickness (CMT) 311μm. Intervention-None. (b) Persistent ME in the left eye after 7 months of grid laser. CMT is 452 μm and maximum macular thickness (MMT) is 553 μm. Intervention- 1st Intravitreal Bevacizumab. (c) Right eye macular SD-OCT after 4 weeks of follow-up (ME worsened and CMT increased to 350 μm). Intervention- Dexamethasone sustained-release implant. (d) Left eye macular SD-OCT after 4 weeks of 1st Intravitreal Avastin (ME improved and CMT and MMT reduced to 355 μm and 461 μm respectively). Intervention- 2nd Intravitreal Bevacizumab. (e) Right eye macular SD-OCT after 6 weeks of implant (ME subsided and CMT became 261μm). (f) Left eye macular SD-OCT after 6 weeks of 2nd Intravitreal Bevacizumab. Persistent ME with CMT 306 μm and MMT 420 μm (SD-OCT-Spectral Domain Optical Coherence Tomography)
High intraocular pressure was recorded in the right eye (26 mmHg) and patient was kept on antiglaucoma medications. Patient also reported snake-like floater in the right eye. There was no other significant adverse effect noted during this short follow-up.
Discussion
In the present case, dexamethasone sustained-release implant was able to successfully bring down the CMT to the normal level in refractory CSME within six weeks whereas persistent CSME was observed even after using two bevacizumab injections in the other eye. As this is a single case with a very short follow-up, it is very difficult to comment on the efficacy of dexamethasone implant per se but comparison with fellow eye (bevacizumab) in similar systemic metabolic conditions makes it an interesting observation even at short term. It is important to evaluate the efficacy of dexamethasone sustained-release implant for the long term because that will give a clue towards its cost-effectiveness compared to the existing therapies. It should not be concluded with this report that dexamethasone sustained-release implant is a better therapeutic option than bevacizumab because there was difference in the nature of macular edema in both the eyes i.e. right eye had early edema in comparison to left eye and both the therapies caused reduction in the macular thickness.
Recent results of RESOLVE and READ 2 study have shown successful use of ranibizumab in cases of DME. As far as the frequency of ranibizumab is concerned, the READ 2 study has shown reduction in the frequency of ranibizumab injection to 2.9 when it is combined with laser, compared to 5.3 and 4.4 in ranibizumab and laser alone patients respectively during 18 months of follow-up.[9,10]
This case report provides an insight for future long-term studies. Future comparative clinical studies with a large sample size and long-term follow-up after FDA approval of dexamethasone sustained-release implant for DME will be able to provide better results and guidelines.
References
- 1.Diabetic Retinopathy Clinical Research Network. A randomized trial comparing intravitreal triamcinolone acetonide and focal/grid photocoagulation for diabetic macular edema. Ophthalmology. 2008;115:1447–9. doi: 10.1016/j.ophtha.2008.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mehta S, Blinder KJ, Shah GK, Kymes SM, Schlief SL, Grand MG. Intravitreal bevacizumab for the treatment of refractory diabetic macular edema. Ophthalmic Surg Lasers Imaging. 2010;41:323–9. doi: 10.3928/15428877-20100430-05. [DOI] [PubMed] [Google Scholar]
- 3.Arevalo JF, Fromow-Guerra J, Quiroz-Mercado H, Sanchez JG, Wu L, Maia M, et al. Primary intravitreal bevacizumab (Avastin) for diabetic macular edema: Results from the Pan-American Collaborative Retina Study Group at 6-month follow-up. Ophthalmology. 2007;114:743–50. doi: 10.1016/j.ophtha.2006.12.028. [DOI] [PubMed] [Google Scholar]
- 4.Yilmaz T, Weaver CD, Gallagher MJ, Cordero-Coma M, Cervantes-Castaneda RA, Klisovic D, et al. Intravitreal triamcinolone acetonide injection for treatment of refractory diabetic macular edema: A systematic review. Ophthalmology. 2009;116:902–11. doi: 10.1016/j.ophtha.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 5.Sharma A, Pirouzmanesh A, Andley UP, Kenney MC, Pirouzmanesh A, Kuppermann BD. Paper presented at: The Association for Research in Vision and Ophthalmology annual meeting. Fort Lauderdale, FL: 2008. Evaluation of in-vitro effects of triamcinolone acetonide and dexamethasone on human lens epithelial cells. April 27-May 1. [Google Scholar]
- 6.Kuppermann BD, Patil AJ, Sharma A, Estrago Franco MF, Mansoor S, Raymond V, et al. Paper presented at: the Association for Research in Vision and Ophthalmology annual meeting. Fort Lauderdale, FL: 2008. Effects of triamcinolone acetonide on human trabecular meshwork cells invitro. April 27-May 1. [Google Scholar]
- 7.Myung JS, Aaker GD, Kiss S. Treatment of noninfectious posterior uveitis with dexamethasone intravitreal implant. Clin Ophthalmol. 2010;4:1423–6. doi: 10.2147/OPTH.S15696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haller JA, Bandello F, Belfort R, Jr, Blumenkranz MS, Gillies M, Heier J, et al. Randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with macular edema due to retinal vein occlusion. Ophthalmology. 2010;117:1134–46. doi: 10.1016/j.ophtha.2010.03.032. [DOI] [PubMed] [Google Scholar]
- 9.Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, et al. Two-year outcomes of the ranibizumab for edema of the macula in diabetes (READ-2) study. Ophthalmology. 2010;117:2146–51. doi: 10.1016/j.ophtha.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): A 12-month, randomized, controlled, double-masked, multicenter phase II study. Diabetes Care. 2010;33:2399–405. doi: 10.2337/dc10-0493. [DOI] [PMC free article] [PubMed] [Google Scholar]


