Abstract
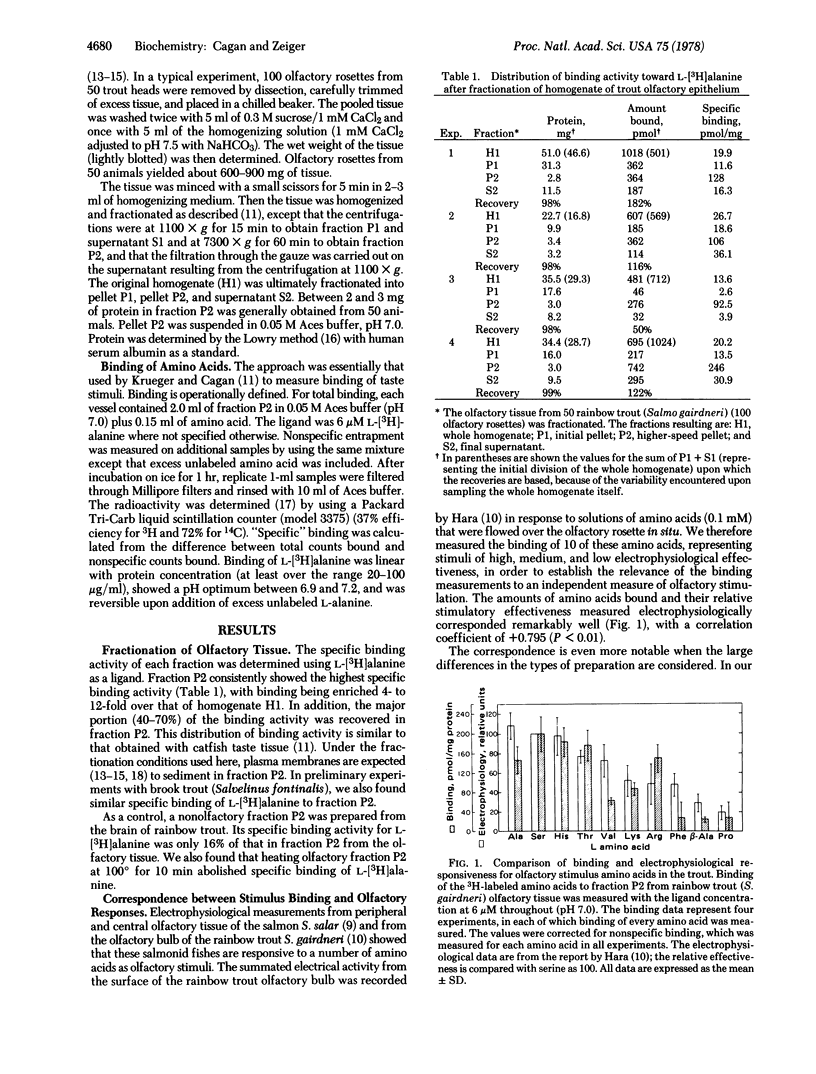
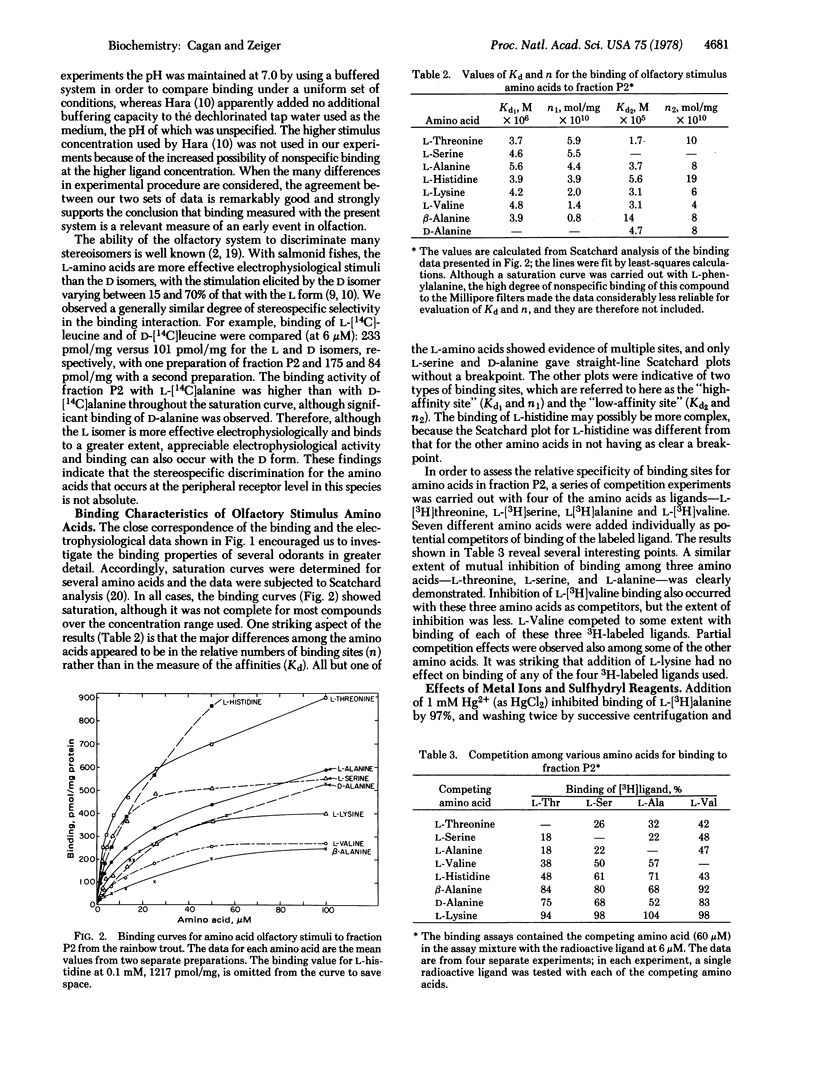
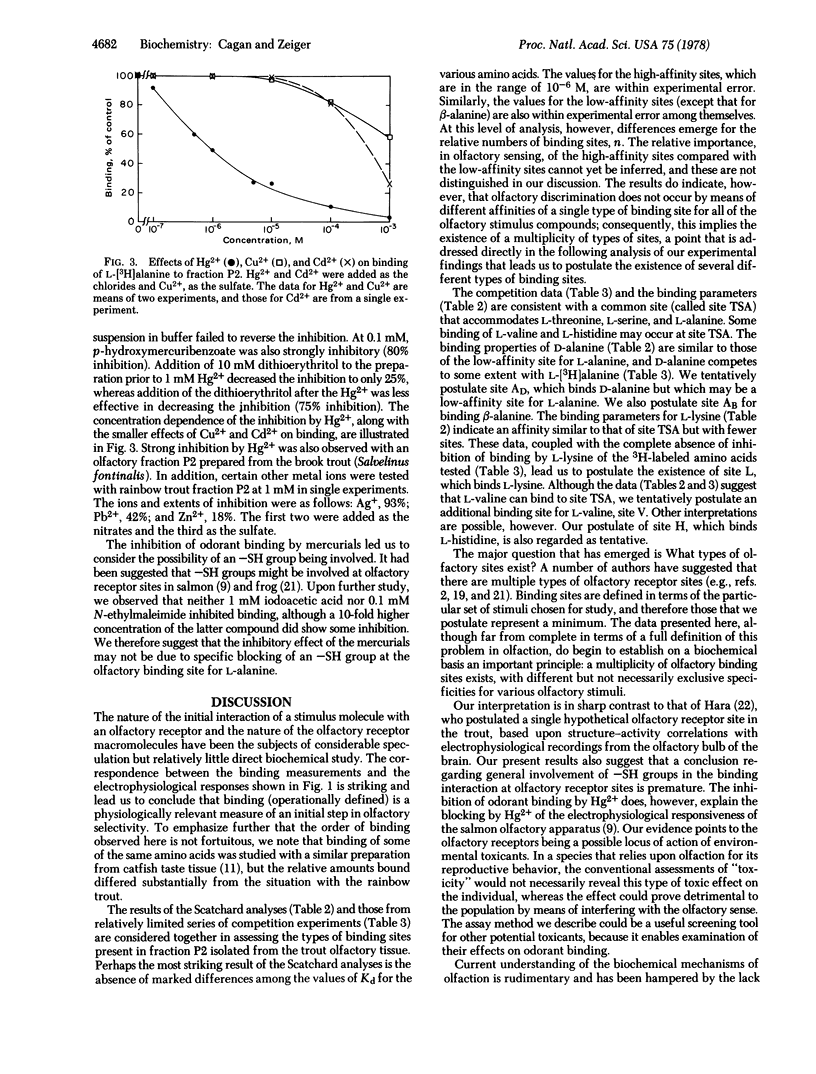
The extent of binding of 10 radioactively labeled odorant amino acids to a sedimentable fraction (fraction P2) derived from the olfactory rosettes of the rainbow trout Salmo gairdneri corresponded closely with their reported relative stimulatory effectiveness measured electrophysiologically. L isomers were bound to a greater extent than their respective D isomers. Binding of L-alanine was strongly and irreversibly inhibited by mercurials but was not affected by sulfhydryl-blocking reagents. Binding was saturable and reversible. Scatchard analyses gave evidence of two types of binding sites for most of the amino acids studied. The Kd values of the higher-affinity binding sites were similar among the amino acids, being in the range of 10(-6) M; differences occurred in the relative numbers of sites, n. These results, coupled with those from competition experiments, lead to the postulate that a multiplicity of types of olfactory binding sites exist in the trout: site TSA, which binds L-threonine, L-serine, and L-alanine; site L, which binds L-lysine; and site AB which binds beta-alanine. Tentative assignments are: site V, which binds L-valine; site H, which binds L-histidine; and site AD, which binds D-alanine. Site AD may be a lower affinity site for L-alanine. Binding of olfactory stimulus molecules appears to be an initial discrimination step in olfaction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ash K. O. Ascorbic acid: cofactor in rabbit olfactory preparations. Science. 1969 Aug 29;165(3896):901–902. doi: 10.1126/science.165.3896.901. [DOI] [PubMed] [Google Scholar]
- Ash K. O. Chemical sensing: an approach to biological molecular mechanisms unsig difference spectroscopy. Science. 1968 Oct 25;162(3852):452–454. doi: 10.1126/science.162.3852.452. [DOI] [PubMed] [Google Scholar]
- Ash K. O., Skogen J. D. Chemosensing: selectivity, sensitivity and additive effects on a stimulant-induced activity of olfactory preparations. J Neurochem. 1970 Aug;17(8):1143–1153. doi: 10.1111/j.1471-4159.1970.tb03362.x. [DOI] [PubMed] [Google Scholar]
- Gennings J. N., Gower D. B., Bannister L. H. Studies on the receptors to 5alpha-androst-16-en-3-one and 5alpha-androst-16-en-3alpha-ol in sow nasal mucosa. Biochim Biophys Acta. 1977 Feb 28;496(2):547–556. doi: 10.1016/0304-4165(77)90335-x. [DOI] [PubMed] [Google Scholar]
- Getchell M. L., Gesteland R. C. The chemistry of olfactory reception: stimulus-specific protection from sulfhydryl reagent inhibition. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1494–1498. doi: 10.1073/pnas.69.6.1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski J., Gannon F. Current models of steroid hormone action: a critique. Annu Rev Physiol. 1976;38:425–450. doi: 10.1146/annurev.ph.38.030176.002233. [DOI] [PubMed] [Google Scholar]
- Hara T. J. Olfactory responses to amino acids in rainbow trout, Salmo gairdneri. Comp Biochem Physiol A Comp Physiol. 1973 Feb 1;44(2):407–416. doi: 10.1016/0300-9629(73)90493-3. [DOI] [PubMed] [Google Scholar]
- Hara T. J. Structure-activity relationships of amino acids in fish olfaction. Comp Biochem Physiol A Comp Physiol. 1976;54(1):31–36. doi: 10.1016/s0300-9629(76)80067-9. [DOI] [PubMed] [Google Scholar]
- Hasler A. D. Guideposts of Migrating Fishes. Science. 1960 Sep 23;132(3430):785–792. doi: 10.1126/science.132.3430.785. [DOI] [PubMed] [Google Scholar]
- Krueger J. M., Cagan R. H. Biochemical studies of tast sensation. Binding of L-[3H]alanine to a sedimentable fraction from catfish barbel epithelium. J Biol Chem. 1976 Jan 10;251(1):88–97. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- NEVILLE D. M., Jr The isolation of a cell membrane fraction from rat liver. J Biophys Biochem Cytol. 1960 Oct;8:413–422. doi: 10.1083/jcb.8.2.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray T. K. A modified method for the isolation of the plasma membrane from rat liver. Biochim Biophys Acta. 1970 Jan 6;196(1):1–9. doi: 10.1016/0005-2736(70)90159-8. [DOI] [PubMed] [Google Scholar]



