Abstract
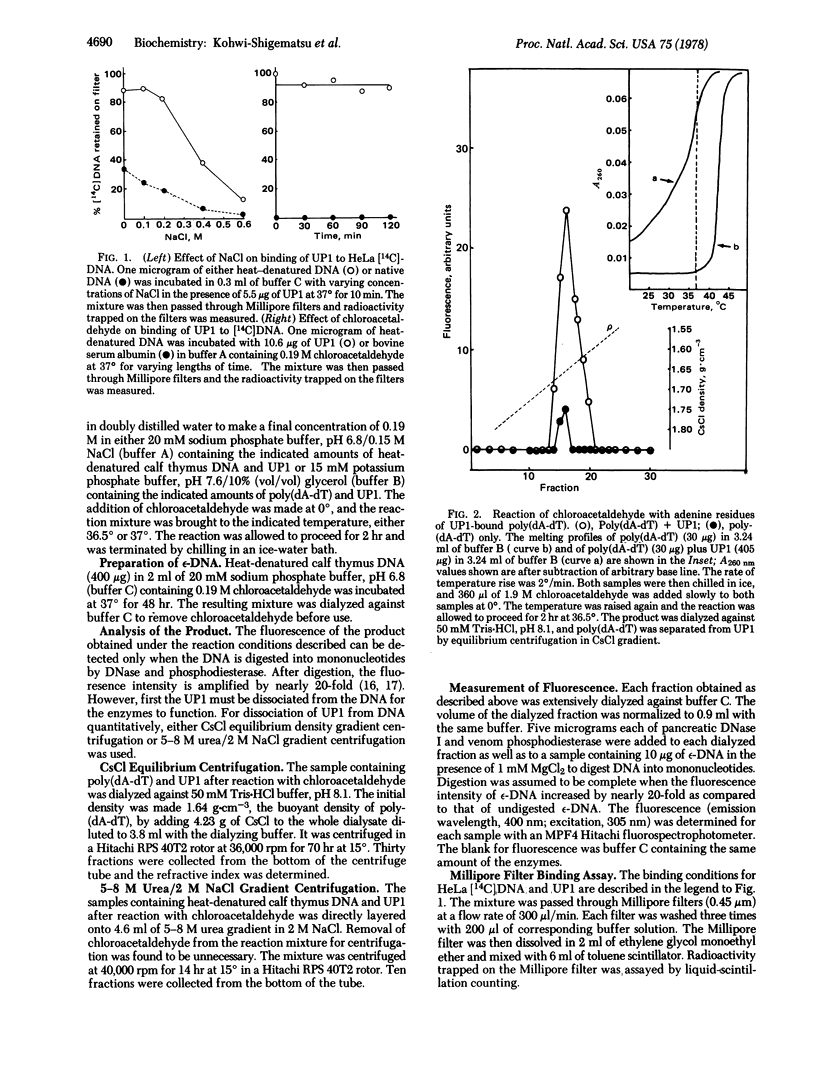
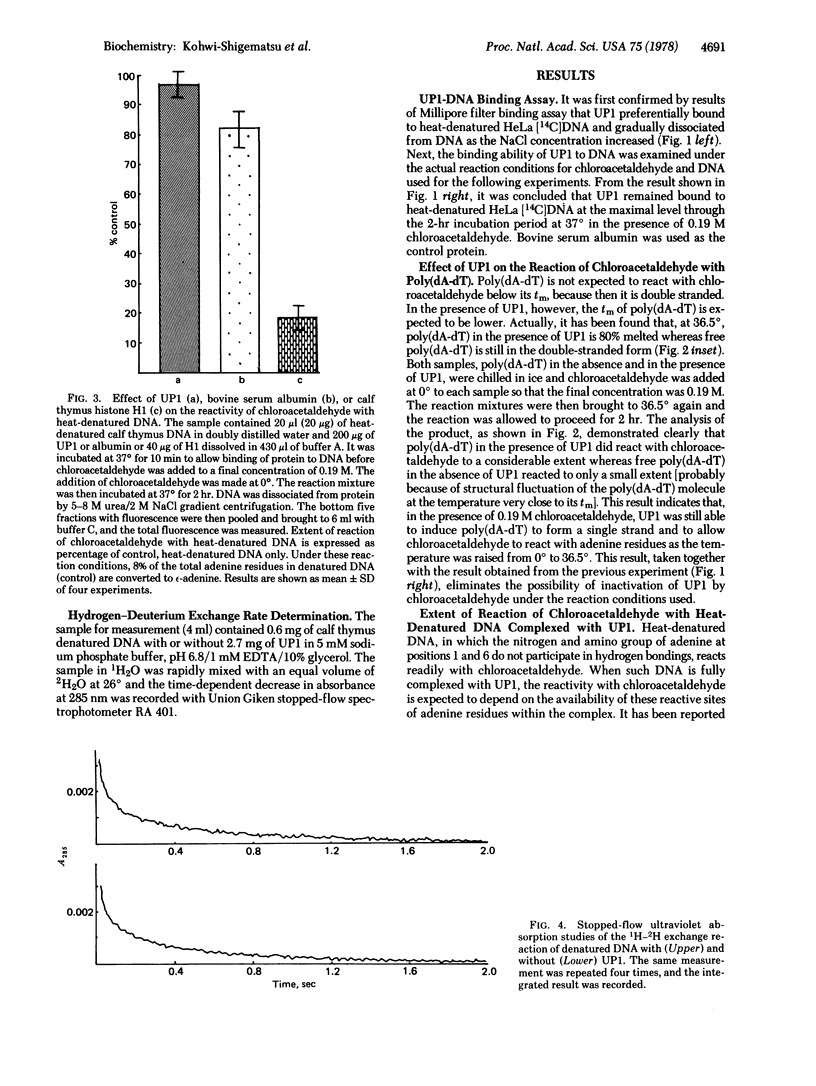
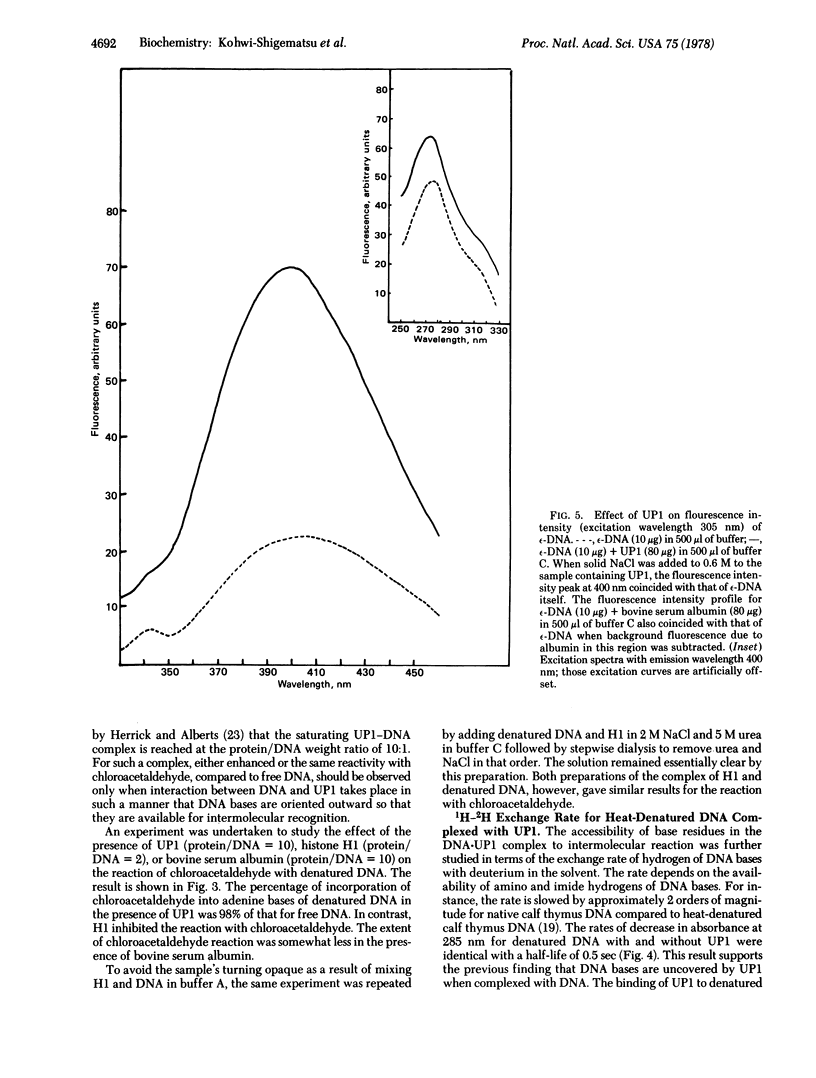
The reaction of chloroacetaldehyde with adenine bases in DNA to give a fluorescent product was used to study the availability to intermolecular reaction of positions 1 and 6 of adenine in DNA complexes with calf thymus DNA helix-destabilizing protein. No inhibition of this reaction was observed when heat-denatured DNA was complexed with the protein at a protein/DNA weight ratio of 10:1, compared to free DNA. On the contrary, the same reaction was inhibited markedly for denatured DNA in the presence of calf thymus histone HI at protein/DNA weight ratio of 2:1. Furthermore, the exchange rate for hydrogens of amino and imide groups of DNA bases in DNA strands with deuterium in the solvent was totally unaffected upon complexing of DNA with the DNA helix-destabilizing protein as examined by stopped-flow ultraviolet spectroscopy. These results indicate that the DNA helix-destabilizing protein forms a complex with single-stranded DNA, leaving DNA bases uncovered by the protein. The fluorescence intensity of DNA pretreated with chloroacetaldehyde was amplified by nearly 3-fold upon addition of the DNA helix-destabilizing protein. The possibility of "unstacking" of DNA bases induced by the protein is discussed.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberts B., Sternglanz R. Recent excitement in the DNA replication problem. Nature. 1977 Oct 20;269(5630):655–661. doi: 10.1038/269655a0. [DOI] [PubMed] [Google Scholar]
- Banks G. R., Spanos A. The isolation and properties of a DNA-unwinding protein from Ustilago maydis. J Mol Biol. 1975 Mar 25;93(1):63–77. doi: 10.1016/0022-2836(75)90360-5. [DOI] [PubMed] [Google Scholar]
- Barrio J. R., Secrist J. A., 3rd, Leonard N. J. Fluorescent adenosine and cytidine derivatives. Biochem Biophys Res Commun. 1972 Jan 31;46(2):597–604. doi: 10.1016/s0006-291x(72)80181-5. [DOI] [PubMed] [Google Scholar]
- Carroll R. B., Neet K. E., Goldthwait D. A. Self-association of gene-32 protein of bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2741–2744. doi: 10.1073/pnas.69.9.2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner D., Furth J. J. De novo synthesis of a polymer of deoxyadenylate and deoxythymidylate by calf thymus DNA polymerase alpha. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3944–3946. doi: 10.1073/pnas.72.10.3944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henner D., Further J. J. Primed and unprimed synthesis of poly (dA-dT) by calf thymus DNA polymerase alpha. J Biol Chem. 1977 Mar 25;252(6):1932–1937. [PubMed] [Google Scholar]
- Herrick G., Alberts B. Nucleic acid helix-coil transitions mediated by helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2133–2141. [PubMed] [Google Scholar]
- Herrick G., Alberts B. Purification and physical characterization of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2124–2132. [PubMed] [Google Scholar]
- Herrick G., Delius H., Alberts B. Single-stranded DNA structure and DNA polymerase activity in the presence of nucleic acid helix-unwinding proteins from calf thymus. J Biol Chem. 1976 Apr 10;251(7):2142–2146. [PubMed] [Google Scholar]
- Huberman J. A., Kornberg A., Alberts B. M. Stimulation of T4 bacteriophage DNA polymerase by the protein product of T4 gene 32. J Mol Biol. 1971 Nov 28;62(1):39–52. doi: 10.1016/0022-2836(71)90129-x. [DOI] [PubMed] [Google Scholar]
- Kimura K., Nakanishi M., Yamamoto T., Tsuboi M. A correlation between the secondary structure of DNA and the reactivity of adenine residues with chloroacetaldehyde. J Biochem. 1977 Jun;81(6):1699–1703. doi: 10.1093/oxfordjournals.jbchem.a131629. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee C. H., Wetmur J. G. Physical studies of chloroacetaldehyde labelled fluorescent DNA. Biochem Biophys Res Commun. 1973 Feb 5;50(3):879–885. doi: 10.1016/0006-291x(73)91327-2. [DOI] [PubMed] [Google Scholar]
- Moise H., Hosoda J. T4 gene 32 protein model for control of activity at replication fork. Nature. 1976 Feb 12;259(5543):455–458. doi: 10.1038/259455a0. [DOI] [PubMed] [Google Scholar]
- Molineux I. J., Friedman S., Gefter M. L. Purification and properties of the Escherichia coli deoxyribonucleic acid-unwinding protein. Effects on deoxyribonucleic acid synthesis in vitro. J Biol Chem. 1974 Oct 10;249(19):6090–6098. [PubMed] [Google Scholar]
- Nakanishi M., Tsuboi M., Saijo Y., Nagamura T. Stopped-flow ultraviolet spectroscopy for hydrogen-exchange studies of nucleic acids. FEBS Lett. 1977 Sep 1;81(1):61–64. doi: 10.1016/0014-5793(77)80928-9. [DOI] [PubMed] [Google Scholar]
- Nossal N. G. DNA synthesis on a double-stranded DNA template by the T4 bacteriophage DNA polymerase and the T4 gene 32 DNA unwinding protein. J Biol Chem. 1974 Sep 10;249(17):5668–5676. [PubMed] [Google Scholar]
- Otto B., Baynes M., Knippers R. A single-strand-specific DNA-binding protein from mouse cells that stimulates DNA polymerase. Eur J Biochem. 1977 Feb 15;73(1):17–24. doi: 10.1111/j.1432-1033.1977.tb11287.x. [DOI] [PubMed] [Google Scholar]
- Reuben R. C., Gefter M. L. A DNA-binding protein induced by bacteriophage T7. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1846–1850. doi: 10.1073/pnas.70.6.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal N., Delius H., Kornberg T., Gefter M. L., Alberts B. A DNA-unwinding protein isolated from Escherichia coli: its interaction with DNA and with DNA polymerases. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3537–3541. doi: 10.1073/pnas.69.12.3537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolman G. L., Barrio J. R., Leonard N. J. Chloroacetaldehyde-modified dinucleoside phosphates. Dynamic fluorescence quenching and quenching due to intramolecular complexation. Biochemistry. 1974 Nov 19;13(24):4869–4878. doi: 10.1021/bi00721a001. [DOI] [PubMed] [Google Scholar]



