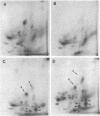
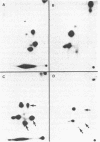
Abstract
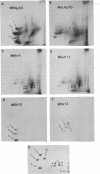
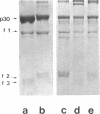
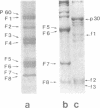
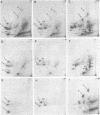
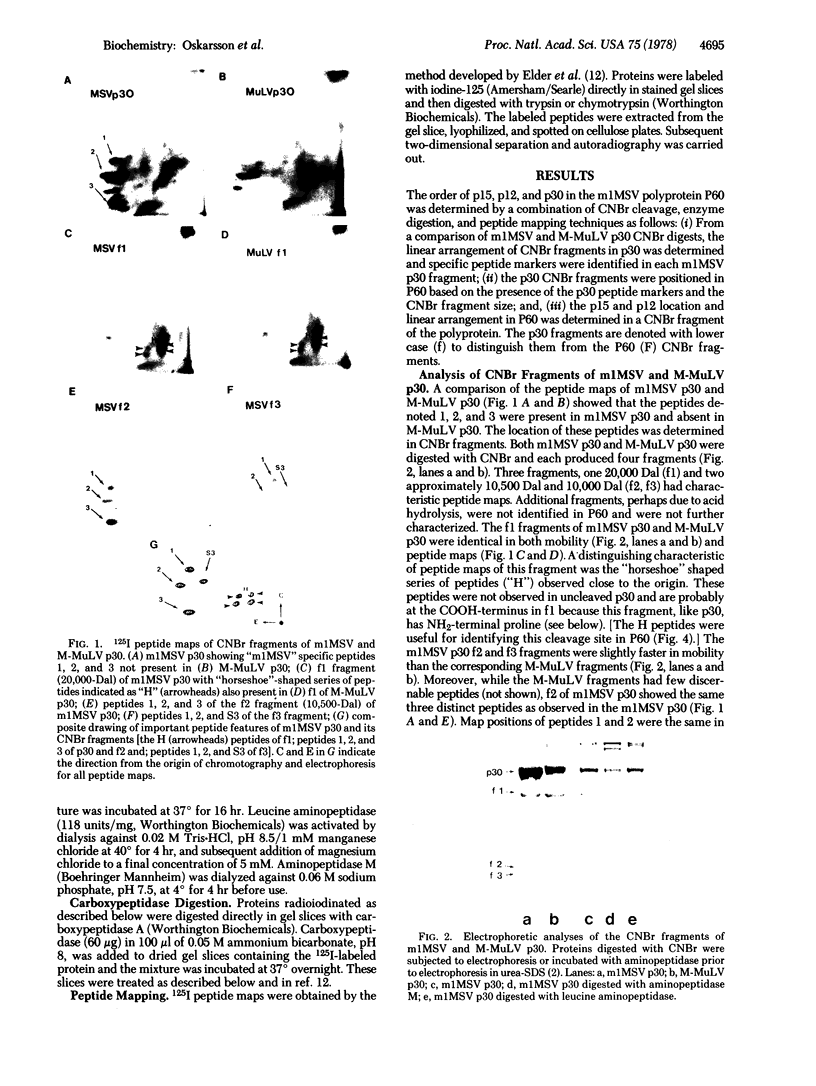
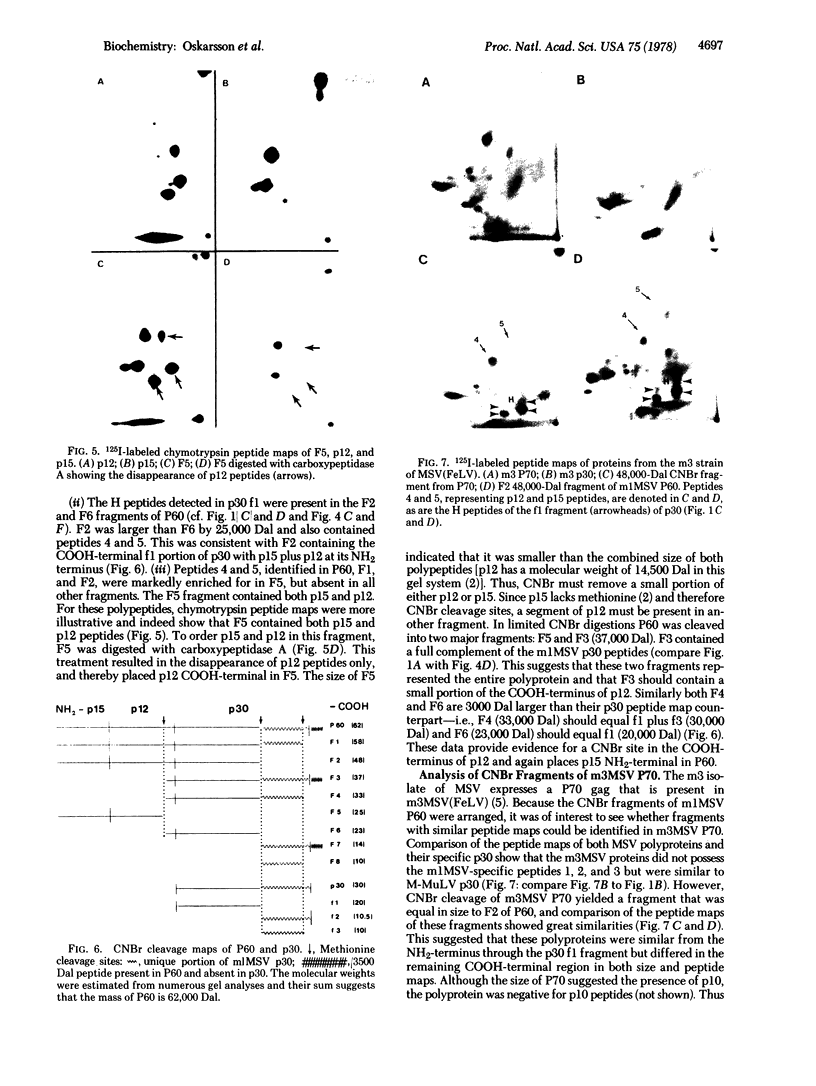
The gene order of the ml Moloney sarcoma virus (mlMSV) specific pP60gag (P60) was determined by direct chemical analysis of the polyprotein. P60 was cleaved with cyanogen bromide (CNBr) into eight partial and complete fragments ranging in mass from 10,000 daltons to 58,000 daltons. Peptide maps of these fragments were compared to maps of p15, p12, and three CNBr fragments of p30. The polarity of p15 and p12 in a CNBr fragment of P60 was determined by carboxypeptidase A digestion; likewise the CNBr fragments of p30 were ordered by aminopeptidase digestion. The linear arrangement of P60 CNBr fragments gave the gene order of NH2-p15-p12-p30-COOH. The m3 isolate of MSV expresses a P70 gag polyprotein. Peptide maps of 48,000-dalton CNBr fragments of m3 P70 and ml P60 were similar and suggested that both polyproteins were similar through the NH2-terminal two-thirds of p30. However, the presence of peptides unique to the 10,500-dalton COOH-terminal fragment of m1MSV p30 and not present in the p30 of either m3MSV or Moloney leukemia virus suggested that the gag gene deletion in the m1 isolate begins in the p30 reading frame.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbacid M., Stephenson J. R., Aaronson S. A. gag Gene of mammalian type-C RNA tumour viruses. Nature. 1976 Aug 12;262(5569):554–559. doi: 10.1038/262554a0. [DOI] [PubMed] [Google Scholar]
- Bernstein A., Mak T. W., Stephenson J. R. The Friend virus genome: evidence for the stable association of MuLV sequences and sequences involved in erythroleukemic transformation. Cell. 1977 Sep;12(1):287–294. doi: 10.1016/0092-8674(77)90206-9. [DOI] [PubMed] [Google Scholar]
- Bister K., Hayman M. J., Vogt P. K. Defectiveness of avian myelocytomatosis virus MC29: isolation of long-term nonproducer cultures and analysis of virus-specific polypeptide synthesis. Virology. 1977 Oct 15;82(2):431–448. doi: 10.1016/0042-6822(77)90017-4. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Pickett R. A., 2nd, Hampton J., Lerner R. A. Radioiodination of proteins in single polyacrylamide gel slices. Tryptic peptide analysis of all the major members of complex multicomponent systems using microgram quantities of total protein. J Biol Chem. 1977 Sep 25;252(18):6510–6515. [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Peebles P. T., Haapala D. K., Bassin R. H. Reversion of murine sarcoma virus transformed mouse cells: variants without a rescuable sarcoma virus. Science. 1972 Jun 2;176(4038):1033–1035. doi: 10.1126/science.176.4038.1033. [DOI] [PubMed] [Google Scholar]
- Fischinger P. J., Nomura S., Tuttle-Fuller N., Dunn K. J. Revertants of mouse cells transformed by murine sarcoma virus. 3. Metastable expression of virus functions in revertants retransformed by murine sarcoma virus. Virology. 1974 May;59(1):217–229. [PubMed] [Google Scholar]
- Gautsch J. W., Elder J. H., Schindler J., Jensen F. C., Lerner R. A. Structural markers on core protein p30 of murine leukemia virus: functional correlation with Fv-1 tropism. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4170–4174. doi: 10.1073/pnas.75.9.4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Davidson N. A heteroduplex study of the sequence relationships between the RNAs of M-MSV and M-MLV. Cell. 1977 Mar;10(3):469–477. doi: 10.1016/0092-8674(77)90034-4. [DOI] [PubMed] [Google Scholar]
- Kato T., Sasaki M., Kimura S. Application of the dansylation reaction to the characterization of low molecular weight peptides by dodecyl sulfate-polyacrylamide-gel electrophoresis. Anal Biochem. 1975 Jun;66(2):515–522. doi: 10.1016/0003-2697(75)90618-1. [DOI] [PubMed] [Google Scholar]
- Oroszlan S., Copeland T., Summers M. R., Smythers G., Gilden R. V. Amino acid sequence homology of mammalian type C RNA virus major internal proteins. J Biol Chem. 1975 Aug 25;250(16):6232–6239. [PubMed] [Google Scholar]
- Oskarsson M. K., Long C. W., Robey W. G., Scherer M. A., Vande Woude G. F. Phosphorylation and nucleic acid binding properties of m1 Moloney murine sarcoma virus-specific pP60gag. J Virol. 1977 Jul;23(1):196–204. doi: 10.1128/jvi.23.1.196-204.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oskarsson M. K., Robey W. G., Harris C. L., Fischinger P. J., Haapala D. K., Vande Woude G. F. A p60 polypeptide in the feline leukemia virus pseudotype of Moloney sarcoma virus with murine leukemia virus p30 antigenic determinants. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2380–2384. doi: 10.1073/pnas.72.6.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson L., Andersson P., Olshevsky U., Weinberg R., Baltimore D., Gesteland R. Translation of MuLV and MSV RNAs in nuclease-treated reticulocyte extracts: enhancement of the gag-pol polypeptide with yeast suppressor tRNA. Cell. 1978 Jan;13(1):189–199. doi: 10.1016/0092-8674(78)90149-6. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Oskarsson M. K., Vande Woude G. F., Naso R. B., Arlinghaus R. B., Haapala D. K., Fischinger P. J. Cells transformed by certain strains of Moloney sarcoma virus contain murine p60. Cell. 1977 Jan;10(1):79–89. doi: 10.1016/0092-8674(77)90142-8. [DOI] [PubMed] [Google Scholar]
- Sherr C. J., Sen A., Todaro G. J., Sliski A., Essex M. Pseudotypes of feline sarcoma virus contain an 85,000-dalton protein with feline oncornavirus-associated cell membrane antigen (FOCMA) activity. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1505–1509. doi: 10.1073/pnas.75.3.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J. R., Khan A. S., Sliski A. H., Essex M. Feline oncornavirus-associated cell membrane antigen: evidence for an immunologically crossreactive feline sarcoma virus-coded protein. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5608–5612. doi: 10.1073/pnas.74.12.5608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]