Abstract
Background & objectives:
Chapekar established a model of ovarian tumourigenesis in mice by splenic transplantation of ovaries, resulting in sustained luteinizing hormone (LH) levels because of absence of feedback inhibition. There is increasing evidence of the differential response to LH or hCG under various experimental conditions. The effect of sustained hormonal stimulation in long term cultures is sparsely investigated. The study is aimed to determine the role of hCG and LH stress on caprine ovarian granulosa cells and their downstream signaling in short and long term cultures.
Methods:
To study the response of hCG and LH stress and downstream signaling, short term cultures were set up by exposing goat ovarian granulosa cells in primary cultures to hCG and LH stress (levels beyond their physiological doses) for 5 days (P0). Cells were sub-cultured at sixth day and subjected to prolonged LH/ hCG stress for two weeks in passage 1(P1) (long term cultures). Downstream cell signaling molecules were assessed. Intracellular cAMP was estimated by ELISA. For PKA and PKC, activity assays were performed. pERK protein expressions in short term cultures were assessed by Western blot and flowcytometry; in long term cultures, pERK expression was analyzed by flowcytometry.
Results:
Differential effects on cell proliferation were observed in long term cultures, where the untreated and hCG exposed cells showed markedly reduced cell proliferation after second week of exposure while LH treated cells continued to proliferate. Different levels of cAMP, PKA, PKC and phosphorylated ERK1/2 were observed on short term and long term LH stimulation. On sustained hormonal stimulation, cAMP levels were significantly (P<0.05) higher in hCG treated cultures as compared to controls and LH treated cultures. LH led to maximal elevation of ERK in long term cultures.
Interpretation & Conclusions:
As pERK1/2 promotes cellular proliferation, activation of ERK1/2 in LH treated cultures may be responsible for sustained growth. Prolonged LH treatment promoted growth and proliferation in caprine ovarian granulosa cells whereas prolonged exposure to hCG led to elevated levels of cAMP and decreased the rate of proliferation. Defining the signals and second messengers that act as survival or apoptotic mediators may help in elucidation of the mechanisms controlling proliferation or programmed cell death in granulosa cells.
Keywords: Caprine, cell culture, cell proliferation, granulosa cells, hCG, LH
Luteinizing hormone (LH) stimulates production of estrogen and progesterone, which play a role in ovarian function and control of reproductive cycle. There is increasing evidence linking granulosa cell proliferation and malignancy to ovarian hyperstimulation. Existing reports suggest that although LH and human chorionic gonadotropin (hCG) bind and activate the same G-protein coupled hormone receptor, these hormones can cause distinct responses in their target cells1. Earlier studies reported different binding sites for hCG and LH on the same receptor, which might result in activation of different protein kinases following signal transduction via Gs, Gi or Gq proteins2–6. The most important signaling pathway activated by hCG and LH is the protein kinase A (PKA) pathway controlling functions such as progesterone secretion by the cells in the corpus luteum2,7. Another kinase activated by hCG and LH stimulation of granulosa cells is protein kinase C (PKC)8. An increase in the concentration of cyclic AMP is demonstrated in human corpus luteum stimulated in vitro by LH and hCG. The increase by both the hormones in short time cultures (day 3) in human granulosa cells was comparable9. Crucial factors in gonadotrophin mediated signaling in the ovary include members of the mitogen activated protein kinase (MAPK) family (ERK 1/2) that are shown to be activated in response to gonadotrophins in both porcine and rat granulosa cells10–12. LH and hCG rapidly increase ERK1/2 activation in human granulosa cells, as also observed in porcine granulosa cells and a rat granulosa cell line. There are no documented studies to determine the effect of sustained hormonal stimulation in long term cultures.
To assess the effect of prolonged exposure of ovarian cells to LH stress, Chapekar established a model of ovarian tumourigenesis in mice by bilateral ovariectomy and splenic transplantation of ovaries, leading to sustained LH levels due to the absence of feedback inhibition12. Further, an in vitro model was set up by subjecting primary goat ovarian granulosa cells to LH stress. After the 8th passage, cultures became independent of LH and eventually developed into a cell line (AIMS/GRXII)13. The cell line grew for 35 passages and its growth and functional parameters were studied in detail14. After the 14th passage, one of the culture flasks revealed transformed cells, which were named AIMS/GRXVIII15. These transformed cells were characterized and these developed tumours in hamster cheek pouch.
Pilot studies conducted earlier on primary cultures of goat ovarian granulosa cells have shown that hCG treated cultures grew only up to passage 316. The differential response and signaling mediated by LH and hCG, which form the basis of LH induced proliferation in caprine ovarian granulosa cells, need to be assessed.
The present study is aimed at assessing the role of LH or hCG stress on primary cultures of goat (caprine) ovarian granulosa cells both in short term (5 days) and long term (2 wk) cultures, and their downstream signaling pathways. Granulosa cells from secondary follicles were chosen for the studies since cells at this stage are least exposed to LH in vivo.
Material & Methods
Reagents: Ovine luteinizing hormone (LH), human chorionic gonadotrophin (hCG), Hank's balanced salt solution (BSS), minimum essential medium (MEM), trypsin, trypan blue and foetal calf serum (FCS) were obtained from Sigma, USA. Amphotericin B (Amfocare, 50 mg) was bought from Medispec Pharmaceuticals Pvt. Ltd. Gentamicin (80 mg in 2 ml) was procured from Elder Pharmaceuticals (India). MOPS, β-glycerol phosphate, sodium fluoride, dithiothreitol (DTT), benzamidine were purchased from Merck, USA. Sodium vanadate, EGTA, EDTA, NP40, phenylmethanesulphonylfluoride (PMSF), leupeptin and aprotinin were purchased from Calbiochem, USA. Total ERK1/2 antibody and pERK1/2 antibody were procured from Cell Signaling Technology, USA. Goat anti-rabbit secondary antibody was purchased from Cell Signaling Technology, USA.
Granulosa cell cultures: The study was conducted in the Department of Biochemistry, All India Institute of Medical Sciences (AIIMS), New Delhi. Goat ovaries were obtained from a local abattoir. Granulosa cells from medium sized follicles were collected for all the experiments as described earlier17,18. The cells were thoroughly washed with Hank's balanced salt solution (HBSS) containing antibiotics, 8 μg/ml gentamicin and 5 μg/ml amphotericin B. The ovaries were transferred to fresh sterile flasks and thoroughly washed three times, with HBSS containing antibiotics. The medium sized follicles were punctured gently using sterile cataract knife to release granulosa cells in a petridish containing MEM with 8 μg/ ml gentamicin. Pooling cells only from medium sized follicles (minimally exposed to LH) ensured homogeneity of granulosa cells. Cells were resuspended in culture medium [MEM + 10% FCS + gentamicin (4 μg/ml)] and equal numbers of cells (2×105cells/ml) were plated in either 6-well plates or tissue culture flasks (25 cm2). The cells were incubated at 37°C under a gas phase of 5 per cent CO2 in humidified air in a CO2 incubator. Eight hours after explantation, the cultures were washed twice with HBSS containing antibiotics to remove debris and unattached cells, fed with fresh culture medium and maintained for growth in a 5 per cent CO2 incubator. The granulosa cells were treated with LH/hCG from 8 h onwards after explantation.
Hormone regimen for establishment of short term cultures of ovarian granulosa cells: The response of granulosa cells (in terms of cell proliferation-established by counting cells in Neubauer haemocytometer, and progesterone secretion) to different doses of LH and hCG was determined and dose response curves plotted (data not shown). The dosage of LH that induced maximum cell proliferation and progesterone secretion (functional response of the cells) was 3.57×103 Units/ml (3 μg/ml) and that of hCG was 25 Units /ml similar to earlier reports13,16. These doses were used subsequently for treating the short and long term cultures.
Cells were treated with LH or hCG respectively, starting from 8 h after explantation of ovarian granulosa cells in 6 well plates. With every change of medium, the cells were fed with culture medium containing LH/hCG at 48, 72 and 96 h, respectively.
Hormone regimen for establishment of long term cultures of ovarian granulosa cells: For cells grown up to 4 wk, media changes were done every fifth day and supplemented with the respective hormones at the same dosage as used for short term cultures. For estimation of levels of secreted progesterone, intracellular cAMP, and activity of PKA and PKC, cells were grown in 6- well plates and analysis was done at 5th day and two weeks. For Western blot and flowcytometric analysis, cells were augmented in 25 cm2 culture flasks.
Measurement of progesterone secretion and levels of intracellular cAMP formation: Progesterone secreted in the culture supernatant was estimated by progesterone ELISA kit (Cayman Chemicals, USA). To estimate intracellular levels of cAMP, primary culture was done and equal number of cells were plated in 6-well plates and treated with culture medium/culture medium containing hCG/culture medium containing LH, eight hours after explantation and estimation was done in both short (Passage 0, P0, 5th day) and long term cultures (Passage1, P1, subculture was done after first week of primary culture and the estimation was done at two wk). Cells were treated with or without hormone in serum free medium at different time points. Reactions were terminated by adding 0.1N HCl. ELISA for estimation of intracellular cAMP was performed as described in the kit (Cayman Chemicals, USA). The intra- and inter-assay variations in the estimation of progesterone were 5 and 11 per cent, respectively while that for the estimation of cAMP was 3 and 7 per cent, respectively.
Protein kinase A (PKA) and protein kinase C (PKC) activity assays: Primary culture of granulosa cells was done and equal number of cells were plated in 6-well plates and treated with culture medium/culture medium containing hCG/culture medium containing LH, eight hours after explantation and estimation was done in both short (Passage 0, P0, 5th day) and long term cultures (Passage 1, P1, two weeks). For PKA and PKC assay, cells were treated with serum free media for 2 h (to get rid of the effect of FCS) followed by treatment with or without hormone (LH or hCG) at different time points in serum free medium (Figs 3 a–d). Media was removed from the plates. Plates were washed with 1X ice-cold PBS (1M, pH 7.4) and then 1ml of lysis buffer [20 mM MOPS, 50 mM β-glycerol phosphate, 50 mM sodium fluoride, 1 mM sodium vanadate, 5 mM EGTA, 2 mM EDTA, 1% NP40, 1 mM dithiothreitol (DTT), 1 mM benzamidine, 1 mM PMSF and 10 μg/ml leupeptin and aprotinin] was added to the tissue culture plates and allowed to stand for 10 min on ice. After a 10 min incubation period, cells were scraped using a cell scraper and the cell lysate was centrifuged at 15,000 g for 15 min and cytosolic fraction was obtained. Protein concentration was determined using BCA method [Bicinchoninic acid (BCA) kit, Banglore Genei, India] for protein estimation and equal amount of protein was taken and PKA and PKC assays were performed using kits as per the protocol (Assay Designs, USA). For PKA assay, intra- and inter-assay variations were 5 and 8 per cent, respectively. For PKC assay, intra- and inter-assay variations were 6 and 12 per cent, respectively.
Fig. 3a.
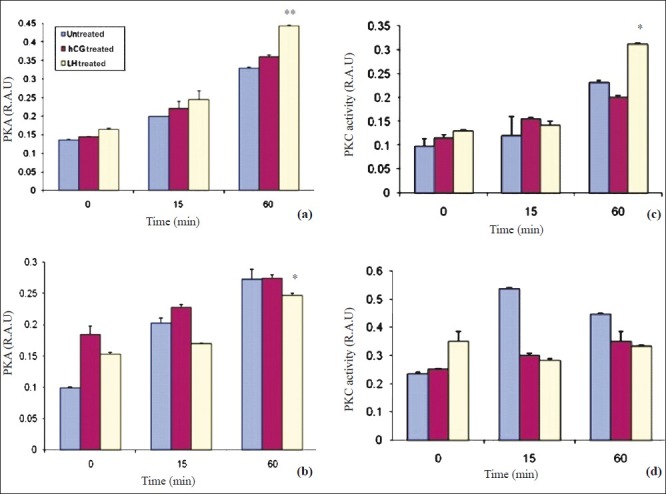
LH and hCG increased PKA activity in a time dependent manner in short term cultures of granulosa cells (day 5). Data expressed as mean ± SEM (n=3). Increase in PKA activity is seen after stimulation with LH as well as hCG, with maximum elevation seen in LH treated cultures. **P<0.001. b. LH and hCG increased PKA activity in a time dependent manner in long term cultures of granulosa cells (2 wk). Data expressed as mean ± SEM (n=3). *P<0.05 compared to LH treated cultures in a time dependent manner. c. LH and hCG stress increased PKC activity in a time dependent manner in short term cultures of granulosa cells (day 5). Data is expressed as mean±SEM. Increase in PKC activity in a time dependent manner is seen after stimulation with LH or hCG (*P<0.05). d. PKC activity is more in untreated cells as compared to hCG treated and LH treated cultures after long term stimulation (2 wk). Data expressed as mean ± SEM. Increase in PKC activity is maximum in untreated cells (P=ns).
Immunoblot analysis: Cells from short and long term cultures were treated with serum free media for two hours followed by treatment with or without hormone (LH or hCG) at different time points in serum free medium. All incubations were carried in serum free MEM. After treatment, the cells were rinsed with ice cold PBS (pH 7.4) and lysed with cell lysis buffer containing 20 mM Tris (pH 7.5), 150 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 per cent Triton X-100, 1 mM β-glycerol phosphate, 1 mM sodium vanadate (Na3VO4), 1 μg/ml leupeptin and 1mM PMSF. Proteins within aliquots of whole cell lysates (20 μg/lane) were resolved in a 10 per cent SDS-PAGE and transferred to nitrocellulose membrane using Western blotting apparatus (Bio Rad Laboratories, Inc., USA). Ponceau (Sigma, USA) stain was used to visually assess the complete transfer of protein from the gel to the membrane. The non-specific binding sites on the membrane were blocked with 1xTBS, 0.1 per cent Tween-20 with 5 per cent w/v nonfat dry milk at room temperature for 1 h. Next, the membrane was incubated with the primary antibody [Phospho p44/42 MAP Kinase (Thr202/Tyr204)] rabbit polyclonal IgG (1:1000) or MAP Kinase antibody rabbit polyclonal IgG (1:1000) or β-actin antibody overnight at 4°C, followed by two washing with TBS-Tween 20 twice. It was then incubated with secondary antibody (HRP-conjugated, 1:2000) for 1 h at room temperature. Finally, the membrane was incubated with 10 ml LumiGLO® (0.5 ml 20X LumiGLO®, 0.5 ml 20X Peroxide and 9.0 ml Millie-Q Water, Cell signaling) with gentle agitation for 1 min at room temperature and developed.
Flowcytometric analysis: Granulosa cells were pooled on the fifth day of primary culture and after subculture at passage 1 (P1, 2 wk), incubated with medium containing LH or hCG or hormone free medium for 30 min (because maximal elevation in pERK1/2 was observed at that time point in western blot), washed with ice cold PBS and analyzed by intracellular staining and flowcytometry. Cells were collected by centrifugation and the supernatant was aspirated. 0.7 million cells were resuspended in 0.5-1 ml PBS. Formaldehyde was added to a final concentration of 2 per cent and cells were fixed for 10 min at 37°C. Tubes were chilled on ice for 1 min. Cells were permeabilized by adding ice-cold 100 per cent methanol slowly to pre-chilled cells, while gently vortexing, to a final concentration of 90 per cent methanol and incubated for 30 min on ice, 2 ml incubation buffer was added to each tube and rinsed by centrifugation. Cells were resuspended in 100 μl incubation buffer per assay tube. Blocking was done in the incubation buffer for 10 min at room temperature. Phospho p44/42 MAP kinase (Thr202/Tyr204) rabbit polyclonal IgG (1:100) or MAP kinase antibody rabbit polyclonal IgG (1:25) was added to the assay tubes, incubated for 1 h at room temperature and rinsed in incubation buffer by centrifugation. Cells were then incubated with FITC-conjugated secondary goat anti rabbit antibody (1:100) (Santa Cruz, USA) diluted in incubation buffer for 30 min at room temperature and rinsed in incubation buffer by centrifugation. Finally, cells were resuspended in 0.5 ml PBS and analyzed in a flow cytometer.
Statistical analysis: Statistical analysis was carried out using unpaired t-test (Graph Pad Prism Version 5 for Windows, Graph Pad Software, San Diego, California USA). All values are given as mean ± SEM.
Results
A distinct morphology of the granulosa cells subjected to LH stress was observed, which differed from that of the hCG and untreated cell controls, respectively. Both hCG and LH treated cells in short term (5 day) cultures exhibited similar functional characteristics, assessed by their progesterone secretion (Fig. 1a). A distinct differential response of the ovarian granulosa cells was observed in both short (passage 0, P0, 5th day) and long term cultures (passage 1, P1, two wk) on subjecting these to sustained LH and hCG stimulation, respectively. Cell counts from day 0 to 4 revealed no significant difference in cell number after treatment with LH and hCG (Fig. 1b). Granulosa cells in primary cultures on sustained stimulation with LH began to proliferate and continued to grow at the same rate with subsequent subculture (P1-P3 passages) and were monitored up to 8 wk. On the other hand, granulosa cells in primary culture when subjected to hCG stress, on subculture, became slow growing after P1 as the number of days taken to become confluent increased. The untreated cells also became slow growing after subculture (P1 & P2) and began to degenerate after the P2 stage (data not shown). Since the cells subjected to hCG stress and untreated cells decreased in number in the long term cultures, further studies to assess the short term and long term effect of sustained hormonal stimulation on the downstream signaling molecules were conducted in 2 wk old (long term, P1) cultures and on day 5 of the primary culture (short term, P1) of granulosa cells.
Fig. 1a.
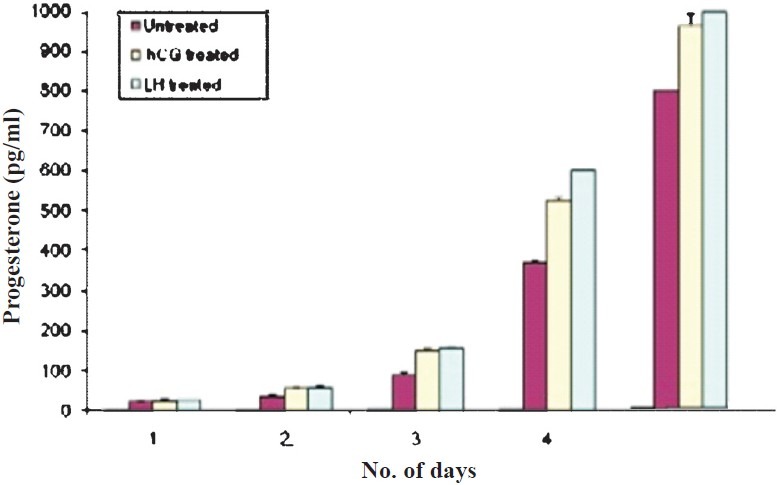
Effect of LH and hCG on progesterone secretion in granulosa cells. Values are mean ± SEM (n=3).
Fig. 1b.
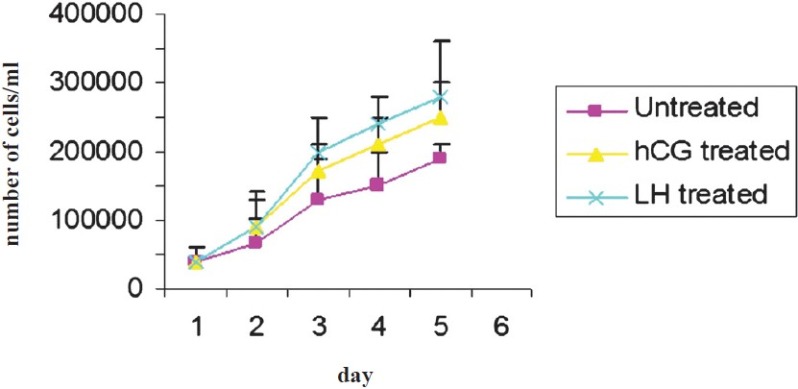
Effect of LH and hCG on cell proliferation and growth rate of granulosa cells in short term culture. Data expressed as mean ± SEM.
The effects of LH and hCG stress on the activation of cAMP, PKA and PKC and the downstream molecule ERK1/2 were assessed. The salient findings were the differences observed in the downstream signaling molecules mediated by sustained LH and hCG stress, between short term and long term granulosa cell cultures. Time kinetic studies were conducted under three different conditions (LH treated, hCG treated and untreated granulosa cells) in short term cultures at five days and in long term cultures (two wk). In short term cultures, maximally elevated cAMP levels at 15 min of hormone stimulation (LH & hCG) were observed, after which the cAMP levels declined at 4 h. cAMP levels were highest (P<0.001) in LH treated cells as compared to the other two groups (Fig. 2a). In long term cultures, cAMP levels were minimal in the LH treated cells as compared to that in hCG and untreated cells (Fig. 2b). Maximally elevated activity of PKA was observed in LH treated cells at 60 min (P<0.001) as compared to that in hCG and untreated cells at 5th day (Fig. 3a). On sustained stimulation with the gonadotrophins (in two wk old, long term cultures), the PKA activity was maximum in hCG treated cells at 60 min, as compared to LH treated and untreated cells (Fig. 3b). This is in concordance with the alterations in the levels of intracellular cAMP levels in these cells.
Fig. 2a.
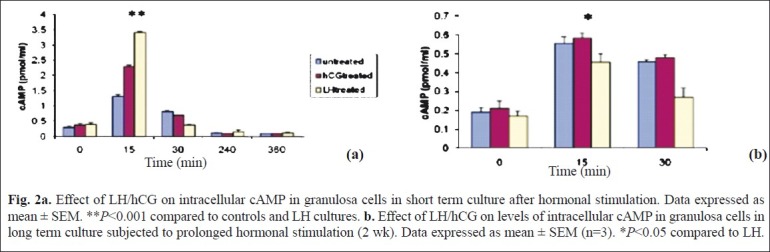
Effect of LH/hCG on intracellular cAMP in granulosa cells in short term culture after hormonal stimulation. Data expressed as mean ± SEM. **P<0.001 compared to controls and LH cultures. b. Effect of LH/hCG on levels of intracellular cAMP in granulosa cells in long term culture subjected to prolonged hormonal stimulation (2 wk). Data expressed as mean ± SEM (n=3). *P<0.05 compared to LH.
We studied the expression of total and phosphorylated ERK in the untreated hCG and LH treated cells by Western blot (Fig. 4 a, b) and flow cytometry (Fig. 4c–f. A similar increase was observed in phosphorylated ERK 1/2 (activation) in granulosa cells on hCG or LH stress in the cells in short term cultures by flow cytometry (Fig. 4 d). The expression of pERK1/2 was similar in hCG treated and LH treated cultures in short term cultures as shown by Western blot (Fig. 4 b). At two wk (long term culture), however, the activation of ERK 1/2 was maximum in LH treated cells as compared to those in hCG treated and untreated control cells on flow cytometric analysis, as shown in Fig. 4 g. Protein kinase C activity was found to be significantly (P<0.05) higher in LH treated cultures as compared to hCG treated short term cultures at 60 min of hormonal treatment. However, at 0 and 15 min, there was no difference in control or the treated groups (Fig. 3c). Both hCG and LH suppressed PKC expression in the long term cultures (Fig. 3d).
Figs. 4a and 4b.
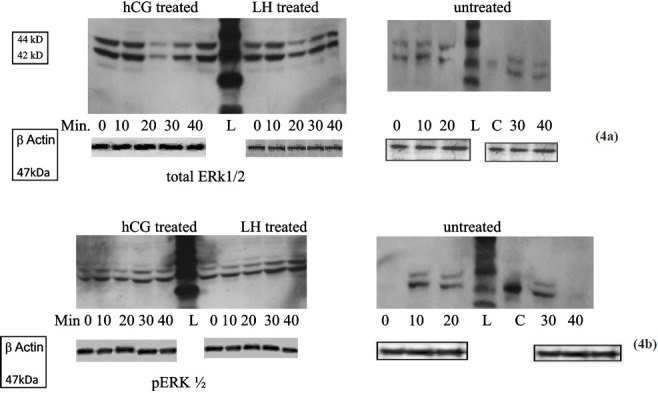
Western blot of total ERK1/2 (4a) and pERK1/2 (4b) in untreated, hCG treated and LH treated cultures at different time points. hCG and LH treated cultures show similar expression whereas there is no expression of pERK1/2 in untreated cultures at 0 and 40 min (L-ladder, C-positive control). Anti-β actin antibody was used to ensure equal loading.
Figs. 4c & 4d.
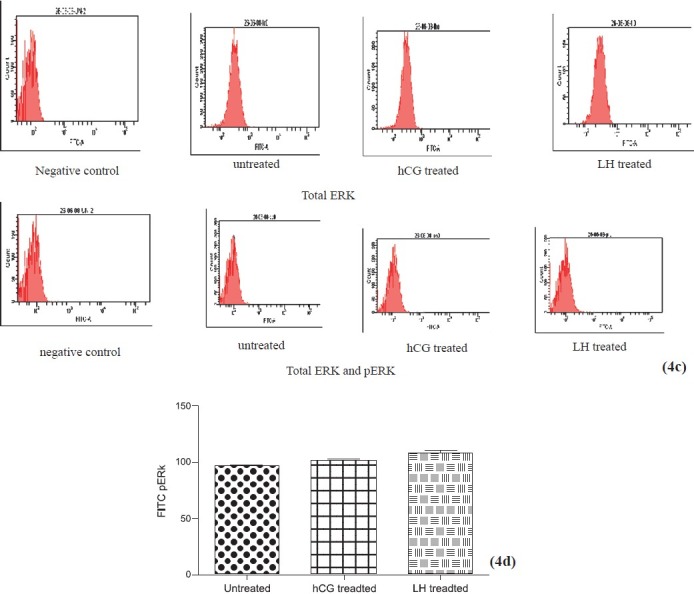
Flowcytometric analysis of ERK after short term stimulation with LH or hCG for 30 min. hCG and LH treated cultures show similar increase in total ERK (4c) and phosphorylated ERK (4d) expression. Values are mean ± SEM of 3 observations.
Figs. 4e, 4f and 4g.
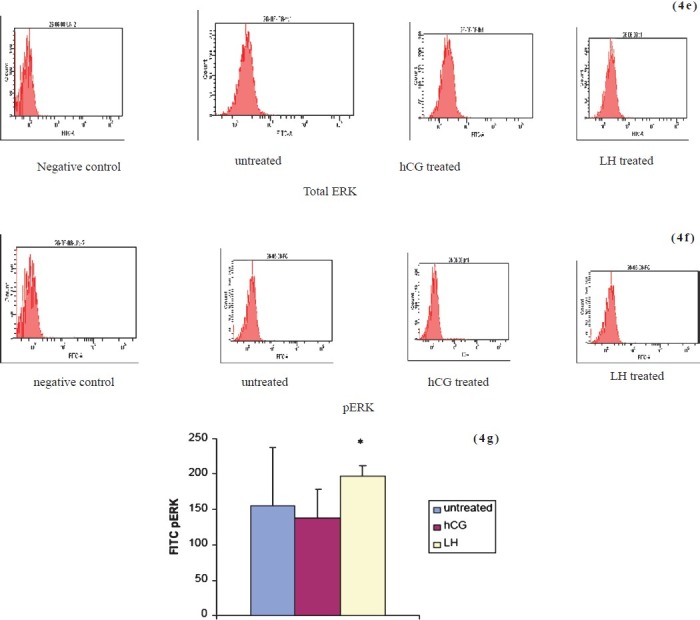
Flowcytometric analysis of total (4e) and phosphorylated ERK (4 f) after sustained stimulation with LH or hCG. Expression of phosphorylated ERK in LH treated culture is significantly higher as compared to hCG treated cultures (4 g). Values given as mean ± SEM of 3 observations. *P<0.05 compared to untreated control cells.
Discussion
The present study, conducted on short and long term primary cultures of goat ovarian granulosa cells exposed to LH and hCG stress showed differential modulation of the signaling molecules subjected to sustained stimulation with LH and hCG. In short term cultures, both LH and hCG stress supported cell growth and proliferation; in long term cultures, LH stress continued to promote sustained cell proliferation and survival, while hCG did not. There were also significant differences in molecules like cAMP and ERK which are important for signal transduction. Maximum elevation of cAMP levels was observed in granulosa cells at two wk (long term) on sustained hCG stimulation; these cells, in contrast to LH treated cells, became slow growing with decreased cell proliferation and increased doubling time. Prolonged hCG stimulation via elevation of cAMP may be leading to decreased proliferation of these cells. In case of cells subjected to prolonged LH stimulation at two weeks, cAMP levels declined in comparison to hCG and untreated cells thereby probably supporting cell proliferation and growth. Gene transcripts to phosphodiesterase 4D, cAMP specific (PDNHD) have been found to be upregulated by LH, which may contribute to LH mediated reduction in the levels of cAMP19. It is known that the gonadotrophins transmit survival signals to the follicular tissue in part through elevation of intracellular cAMP levels20,21. However, excessive cAMP levels in mature rat and human granulosa cells, obtained from preovulatory follicles, led to an opposite effect, that is accelerated apoptosis under in vitro conditions, especially upon serum deprivation22,23. Gonadotrophins activate the hormone sensitive adenylyl cyclase for a very limited period, which is immediately followed by a refractory period, where levels of cAMP decline dramatically. In contrast, forskolin or 8-Br-cAMP which produce persistently raised intracellular cAMP levels might be harmful to the cells and might lead to activation of the apoptotic process22. Apoptosis is shown to be induced in vitro, in primary granulosa cell cultures, by serum deprivation and by induction of high intracellular levels of cAMP20 which may be the cause of decreased number of cells in the hCG treated cultures in our study. The expression of pERK in both hCG and LH treated short term cultures was comparable. On sustained hormonal stimulation, expression of pERK was highest in LH treated long term cultures as compared to the hCG treated or untreated cells. Maximal elevation of pERK1/2 in LH treated long term cultures may be promoting cell proliferation and survival. ERK activation can repress BIM mRNA levels (proapoptotic proteins)24 and on the other hand is reported to increase the expression of pro-survival BCL-2 proteins25. ERK1/2 is also known to upregulate survivin which increases cell proliferation and survival26. These studies are in consonance with our finding of sustained LH stimulation supporting the granulosa cell proliferation in long term cultures. Increase in levels of pERK1/2 has been reported previously in granulosa cell tumours27. Silencing of ERK1/2 led to complete suppression of tumour cell proliferation27. It is well established that ERK1/2 pathway increases tumour survival28. Sustained hormonal stimulation in ovaries is associated with certain pathological conditions like polycystic ovarian syndrome and granulosa cell tumours, which are associated with high levels of LH. There are independent reports suggesting the role of elevated LH in granulosa cell tumourigenesis. Post-menopausal women with high levels of LH represent the largest cohort of patients with granulosa cell tumour29. Hyper secretion of LH in transgenic mice led to granulosa cell tumour30. Our results indicated that sustained hCG stimulation failed to support the proliferation of the granulosa cells and eventually led to decreased cell growth.
In conclusion, the present study demonstrated the differential response of goat ovarian granulosa cells subjected to LH or hCG stress at different stages in culture. LH stress promoted sustained proliferation of ovarian granulosa cells in long term cultures while hCG stress failed to do so. Defining the signals and second messengers that can act as survival or apoptotic signals may further elucidate the possible mechanisms controlling proliferation or programmed cell death in both the normal and transformed granulosa cells and the precise role of hormones in carcinogenesis.
Acknowledgment
Authors acknowledge the Indian Council of Medical Research (ICMR), New Delhi, for financial assistance, and thank Shri Pradeep for procuring goat ovaries for this study, Dr Neera Nath and Shrimati Meenu for helping us with the flow cytometric analysis.
References
- 1.Kammerman S, Canfield RE, Kolena J, Channing CP. The binding of iodinated hCG to porcine granulosa cells. Endocrinology. 1972;91:65–74. doi: 10.1210/endo-91-1-65. [DOI] [PubMed] [Google Scholar]
- 2.Gilchrist RL, Ryu KS, Ji I, JI TH. The luteinizing hormone/chorionic gonadotropin receptor has distinct transmembrane conductors for cAMP and inositol phosphate signals. J Biol Chem. 1996;271:19283–7. doi: 10.1074/jbc.271.32.19283. [DOI] [PubMed] [Google Scholar]
- 3.Gudermann T, Birnbaumer M, Birnbaumer L. Evidence for dual coupling of the murine luteinizing hormone receptor to adenylyl cyclase and phosphoinositide breakdown and Ca2 mobilization. Studies with the cloned murine luteinizing hormone receptor expressed in L cells. J Biol Chem. 1992;267:4479–88. [PubMed] [Google Scholar]
- 4.Herrlich A, Kuhn B, Grosse R, Schmid A, Schultz G, Gudermann T. Involvement of Gs and Gi proteins in dual coupling of the luteinizing hormone receptor to adenylyl cyclase and phospholipase C. J Biol Chem. 1996;271:16764–72. doi: 10.1074/jbc.271.28.16764. [DOI] [PubMed] [Google Scholar]
- 5.Muller T, Gromoll J, Simoni M. Absence of exon 10 of the human luteinizing hormone (LH) receptor impairs LH, but not human chorionic gonadotropin action. J Clin Endocrinol Metab. 2003;88:2242–9. doi: 10.1210/jc.2002-021946. [DOI] [PubMed] [Google Scholar]
- 6.Rajagopalan-Gupta RM, Mukherjee S, Zhu X, Ho YK, Hamm H, Birnbaumer M, et al. Roles of Gi and Gq/11 in mediating desensitization of the luteinizing hormone/choriogonadotropin receptor in porcine ovarian follicular membranes. Endocrinology. 1999;140:1612–21. doi: 10.1210/endo.140.4.6657. [DOI] [PubMed] [Google Scholar]
- 7.Ryu KS, Gilchrist RL, Koo YB, Ji I, Ji TH. Gene, interaction, signal generation, signal divergence and signal transduction of the LH/CG receptor. Int J Gynaecol Obstet. 1998;1(Suppl):S9–20. doi: 10.1016/S0020-7292(98)80001-5. [DOI] [PubMed] [Google Scholar]
- 8.Murphy BD. Models of luteinization. Biol Reprod. 2000;63:2–11. doi: 10.1095/biolreprod63.1.2. [DOI] [PubMed] [Google Scholar]
- 9.Dewi DA, Abayasekara DR, Wheeler-Jones CP. Requirement for ERK1/2 activation in the regulation of progesterone production in human granulosa-lutein cells is stimulus specific. Endocrinology. 2002;143:877–88. doi: 10.1210/endo.143.3.8677. [DOI] [PubMed] [Google Scholar]
- 10.Cameron MR, Foster JS, Bukovsky A, Wimalasena J. Activation of mitogen-activated protein kinases by gonadotrophins and cyclic adenosine 5′-monophosphates in porcine granulosacells. Biol Reprod. 1996;55:111–9. doi: 10.1095/biolreprod55.1.111. [DOI] [PubMed] [Google Scholar]
- 11.Das S, Maizels ET, DeManno D, St Clair E, Adam SA, Hunzicker-Dunn M. A stimulatory role of cyclic adenosine 3’,5’-monophosphate in follicle-stimulating hormone-activated mitogen activated protein kinase signaling pathway in rat ovarian granulosa cells. Endocrinology. 1996;137:967–74. doi: 10.1210/endo.137.3.8603610. [DOI] [PubMed] [Google Scholar]
- 12.Chapekar TN. Tumourigenesis of autologous intrasplenic ovarian transplants in swiss mice. Indian J Med Res. 1982;76:565–70. [PubMed] [Google Scholar]
- 13.Chapekar TN, Sharma N. Development of a cell-line of differentiated goat (Capra hircus) granulosa cells subjected to hormonal stress. Indian J Exp Biol. 1985;23:421–5. [PubMed] [Google Scholar]
- 14.Chapekar TN, Sharma N, Malik AK. Morphological and functional characterization of a hormonally induced AIMS/GRXII cell line. Pathobiology. 1991;59:102–8. doi: 10.1159/000163624. [DOI] [PubMed] [Google Scholar]
- 15.Chapekar TN, Malik AK. The AIMS/GRXVIII cell line: ‘spontaneous’ transformation of hormonally induced primary cells derived from goat ovarian granulosa. Pathobiology. 1991;59:345–50. doi: 10.1159/000163675. [DOI] [PubMed] [Google Scholar]
- 16.Chapekar T. Biology of cancer: some questions to answer. Neuro Endocrinol Lett. 2001;22:326–9. [PubMed] [Google Scholar]
- 17.Sen K, Chapekar TN. In vitro luteal transformation of granulosa cells of rabbit ovary in response to luteinizing hormone. Indian J Exp Biol. 1975;13:135–7. [PubMed] [Google Scholar]
- 18.Mohini P, Raj BJ, Chapekar TN. Effect of ovine luteinizing hormone and prolactin on progesterone secretion by goat granulosa cells in culture. J Endocrinol. 1980;84:311–3. doi: 10.1677/joe.0.0840311. [DOI] [PubMed] [Google Scholar]
- 19.Sasson R, Rimon E, Dantes A, Cohen T, Shinder V, Land-Bracha A, et al. Gonadotrophin-induced gene regulation in human granulosa cells obtained from IVF patients.Modulation of steroidogenic genes, cytoskeletal genes and genes coding for apoptotic signaling and protein kinases. Mol Hum Reprod. 2004;10:299–311. doi: 10.1093/molehr/gah041. [DOI] [PubMed] [Google Scholar]
- 20.Amsterdam A, Gold RS, Hosokawa K, Yoshida Y, Sasson R, Jung Y, et al. Crosstalk among multiple signaling pathways controlling ovarian cell death. Trends Endocrinol Metab. 1999;10:255–62. doi: 10.1016/s1043-2760(99)00164-2. [DOI] [PubMed] [Google Scholar]
- 21.Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15:707–24. doi: 10.1210/edrv-15-6-707. [DOI] [PubMed] [Google Scholar]
- 22.Aharoni D, Dantes A, Oren M, Amsterdam A. cAMP-mediated signals as determinants for apoptosis in primary granulosa cells. Exp Cell Res. 1995;218:271–82. doi: 10.1006/excr.1995.1156. [DOI] [PubMed] [Google Scholar]
- 23.Breckwoldt M, Several N, Aharoni D, Barash A, Segal I, Insler V, et al. Expression of Ad4-BP/cytochrome P450 side chain cleavage enzyme and induction of cell death in long-term cultures of human granulosa cells. Mol Hum Reprod. 1996;2:391–400. doi: 10.1093/molehr/2.6.391. [DOI] [PubMed] [Google Scholar]
- 24.Dazy S, Damiola F, Parisey N, Beug H, Gandrillon O. The MEK-1/ERKs signaling pathway is differentially involved in the self-renewal of early and late avian erythroid progenitor cells. Oncogene. 2003;22:9205–16. doi: 10.1038/sj.onc.1207049. [DOI] [PubMed] [Google Scholar]
- 25.Fan M, Goodwin M, Vu T, Brantley-Finley C, Gaarde WA, Chambers TC. Vinblastine induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J Biol Chem. 2000;275:29980–5. doi: 10.1074/jbc.M003776200. [DOI] [PubMed] [Google Scholar]
- 26.Siddiqa A, Long LM, Li L, Marciniak RA, Kazhdan I. Expression of HER-2 in MCF-7 breast cancer cells modulates anti-apoptotic proteins Survivin and Bcl-2 via the extracellular signal - related kinase (ERK) and phosphoinositide-3 kinase (PI3K) signaling pathways. BMC Cancer. 2008;8:129. doi: 10.1186/1471-2407-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steinmetz R, Wagoner HA, Zeng P, Hammond JR, Hannon TS, Meyers JL, et al. Mechanisms regulating the constitutive activation of the extracellular signal-regulated kinase(ERK) signaling pathway in ovarian cancer and the effect of ribonucleic acid interference for ERK1/2 on cancer cell proliferation. Mol Endocrinol. 2004;18:2570–82. doi: 10.1210/me.2004-0082. [DOI] [PubMed] [Google Scholar]
- 28.Balmanno K, Cook SJ. Tumor cell survival signaling by the ERK1/2 Pathway. Cell Death Differ. 2008;16:368–77. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- 29.Amsterdam A, Several N. Control of differentiation, transformation, and apoptosis in granulosa cells by oncogenes, oncoviruses, and tumour suppressor genes. Endocr Rev. 1997;18:435–61. doi: 10.1210/edrv.18.4.0306. [DOI] [PubMed] [Google Scholar]
- 30.Riesman KA, Clay CM, Nett TM, Wagner T, Yun J, Nilson JH. Targeted over expression of luteinizing hormone in transgenic mice leads to infertility, polycystic ovaries, and ovarian tumors. Proc Natl Acad Sci USA. 1995;92:1322–6. doi: 10.1073/pnas.92.5.1322. [DOI] [PMC free article] [PubMed] [Google Scholar]


