Abstract
Background & objectives:
Cluster beans (Cyamopsis tetragonoloba) are rich source of soluble fibre content and are known for their cholesterol lowering effect. The beneficial anti-hypercholesterolaemic effect of whole dietary cluster beans as a source of dietary fibre was evaluated in high cholesterol diet induced hypercholesterolaemia in experimental rats.
Methods:
Male Wistar rats (90-95 g) divided in six groups of 10 rats each were used. Freeze dried tender cluster beans were included at 12.5 and 25 per cent levels in the diet of animals maintained for 8 wk either on high (0.5%) cholesterol diet or basal control diet.
Results:
Significant anti-hypercholesterolaemic effect was seen in cluster bean fed animals, the decrease in serum cholesterol being particularly in the LDL associated fraction. There was also a beneficial increase in HDL associated cholesterol fraction. Hepatic lipid profile showed a significant decrease in both cholesterol and triglycerides as a result of feeding tender cluster beans along with high cholesterol diet.
Interpretation & Conclusions:
The present experimental results showed the beneficial hypocholesterolaemic and hypolipidimic influences dietary tender cluster beans in atherogenic situation. Studies in human need to be done to confirm the results.
Keywords: Anti-hypercholestaerolemic effect, cholesterol, HDL, LDL, lipid profile, tender cluster beans
Increased blood cholesterol level is considered to be a risk factor for coronary heart disease. Dietary and pharmacologic reduction in total and LDL cholesterol decreases the risk of coronary diseases and dietary intervention is the desirable approach. Many investigators have reported that soluble dietary fibre has the ability to lower plasma cholesterol concentration in experimental animals and in humans1,2. Guar gum – a galactomannan prepared from mature cluster bean (Cyamoposis tetragonoloba) has been shown to be a potent hypocholesterolaemic agent in both normal and hypercholesterolaemic animals3. Ingestion of guar gum decreases postprandial glycaemia and insulinaemia and improves sensitivity to insulin in diabetes patients4 and several animal models of diabetes5. Guar gum, the soluble galactomannan has been shown to reduce cholesterol by multiple mechanisms, namely, interruption of enterohepatic circulation of bile acids with enhanced bile acid secretion, and inhibition of cholesterol absorption leading to increased excretion of neutral sterols; additionally guar may entrap fat micelles thereby impeding fat absorption.
Cluster beans by virtue of rich soluble fibre content may be of therapeutic value in the control of hypercholesterolaemia. In humans, guar gum has been found to be associated with a 10 per cent reduction in appetite and an increase in the feeling of satiety6. It has been claimed that hypocholesterolaemic effect of guar gum may be sustained for at least 6 to 12 months7. Guar gum has been shown to lower serum cholesterol levels in normal and hypercholesterolaemic subjects8. In the context of limited information on the hypocholesterolaemic potential of whole cluster beans as such, in the present study was carried out to evaluate the hypocholesterolaemic effect of whole cluster bean as a source of guar gum in cholesterol fed rats.
Material & Methods
Materials: Cholesterol, dipalmitoyl phosphatidylcholine, bile salts, and triolein were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Heparin and manganese chloride were obtained from SISCO Research Laboratory (Mumbai, India). Casein was purchased from Nimesh Corporation (Mumbai, India). Corn starch and cane sugar powder were locally purchased. All other chemicals and solvents were of analytical grade and solvents were distilled prior to use.
Tender cluster beans were purchased from the local market. The edible portions were freeze-dried and powdered. The powdered dry samples were analyzed in triplicate for dietary fibre as soluble dietary fibre and insoluble dietary fibre adapting the rapid enzymatic assay procedure9.
Animal diets: The animals were fed with AIN-76 semi-synthetic diet. The basal control diet consisted of: corn starch, 54 per cent; casein, 21 per cent; cane sugar powder, 10 per cent; refined groundnut oil, 9.9 per cent; AIN-76 mineral mix, 4 per cent; AIN-76 vitaminised starch, 1 per cent; and vitaminised oil, 0.1 per cent. Hypercholesterolaemic diet was made by supplementing 0.5 per cent cholesterol and 0.125 per cent bile salts (1:1 mixture of sodium cholate and sodium deoxycholate) to the above basal diet substituting the same quantity of starch. Diets were stored at 4 °C in air tight containers. The test diets were prepared by incorporating freeze-dried tender cluster bean powder at two dietary levels (12.5 and 25%) by substituting equivalent amount of starch.
Animal treatment: The experiments were performed in the Department of Biochemistry, Central Food & Technological Research Institute (CFTRI), Mysore, India. Animal were obtained from Experimental Animal Production Facility of this institute. The study protocol was approved from the Institutional Animal Ethics Committee. Groups of male Wistar rats weighing between 90-95 g were maintained for 8 wk ad libitum on: (1) Basal control diet, (2) Basal diet containing 12.5 per cent freeze dried cluster beans, (3) Basal diet containing 25 per cent freeze dried cluster beans, (4) High cholesterol diet (HCD), (5) HCD containing 12.5 per cent freeze dried cluster beans, and (6) HCD containing 25 per cent freeze dried cluster beans. All animals had free access to water throughout the experiment. Body weights were recorded at weekly intervals. At the end of the experimental regimen, animals were fasted overnight and sacrificed under ether anaesthesia. Blood was collected by cardiac puncture and serum was separated. Liver was quickly excised, washed with ice-cold saline, blotted dry, weighed and stored at -20°C until further analysis.
Lipid profile: Total lipids were extracted10 and estimated gravimetrically. Cholesterol11, triglycerides12 and phospholipids13 were determined in the lipid extracts of serum and liver using standard procedures. Serum cholesterol associated with HDL fraction was determined after precipitation of apolipoprotein-B containing lipoprotein with heparin-manganese reagent14. LDL-VLDL precipitate was extracted with chloroform: methanol (2:1 v/v) and used for cholesterol determination.
Statistical analysis: Statistical analysis was carried by Grad prism statistical software. Results were analyzed by one way ANOVA and the significance level was calculated using Tukey Kramer multiple comparison test.
Results
Tender cluster bean pods contained 2.43 g soluble dietary fibre/100 g and 5.16 g insoluble dietary fibre/ 100 g, amounting to a total dietary fibre content of 7.6 g per cent. In terms of dry cluster bean powder included in the diets, 12.5 per cent cluster bean corresponded to 1.52 per cent soluble dietary fibre and 3.23 per cent insoluble dietary fibre, while 25 per cent cluster bean corresponded to 3.04 per cent soluble dietary fibre and 6.45 per cent insoluble dietary fibre.
Feeding of a high cholesterol diet to induce hypercholesterolaemia produced more than two-fold increase (P<0.005) in serum total cholesterol concentration in rats. Both the dietary levels of freeze-dried cluster beans, i.e., 12.5 and 25 per cent supplemented with HCD significantly (P<0.05) countered this increase (Table I). The decrease in serum cholesterol brought about by dietary cluster beans was seen particularly in the LDL-VLDL fraction.
Table I.
Serum lipid profile of tender cluster bean treated animals
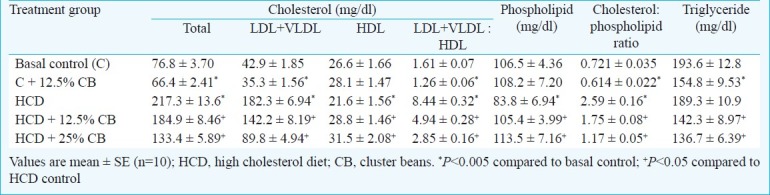
Feeding of hypercholesterolaemic diet depressed the level of HDL-cholesterol by 38 per cent (Table I). Supplementation of HCD with 12.5 per cent freeze dried cluster beans enhanced its level from 21.6 to 28.8 mg/dl, while supplementation with 25 per cent freeze dried cluster beans showed even more promising increase of raising HDL-cholesterol level to 31.5 mg/dl. Feeding of freeze-dried cluster beans along with basal control diet also produced a slight but significant decrease in total serum cholesterol and LDL-VLDL associated cholesterol, thus, showing a hypocholesterolaemic potential even in normal condition. The decrease produced by 12.5 per cent cluster beans supplementation in total cholesterol in the serum was 13.5 per cent (76.8-66.4 mg/dl), while it was 18 per cent in LDL-VLDL associated cholesterol (42.9-35.3 mg/dl). The ratio of LDL+VLDL cholesterol: HDL cholesterol was very high in HCD treatment as a result of increase in cholesterol associated with LDL fraction (Table I). Cluster bean supplementation to HCD effectively brought down this elevated ratio which signifies the atherogenicity index. The ratio of LDL+VLDL cholesterol: HDL cholesterol was also reduced by dietary cluster beans in normal animals.
In the hypercholesterolaemic situation, the level of serum phospholipid was depressed by 21 per cent, and this was significantly countered by supplementing HCD with freeze dried cluster beans (Table I). Serum phospholipid levels were completely restored to normal levels in both 12.5 and 25 per cent freeze dried cluster bean fed along with HCD. Serum phospholipid value was unaffected by dietary cluster beans in normal conditions. Cholesterol: phospholipid ratio was increased by more than three-fold under the influence of HCD, as a consequence of increased cholesterol and reduced phospholipid concentration. The increase in this ratio was effectively countered by dietary cluster beans in hypercholesterolaemic animals (Table I). Cholesterol: phospholipid ratio was also significantly reduced in normal animals under the influence dietary cluster beans. Hypercholesterolaemic situation induced by HCD did not cause much difference in the serum triglyceride value (Table I). Supplementation of HCD with 12.5 and 25 per cent freeze dried cluster beans reduced it significantly (P<0.05) to 142.3 and 136.7 mg/dl, respectively from 189.3 mg/dl. Serum triglyceride value was also reduced significantly (P<0.05) from 193.6 to 154.8 mg/dl in basal control diet supplemented with 12.5 per cent freeze dried cluster beans [Table I].
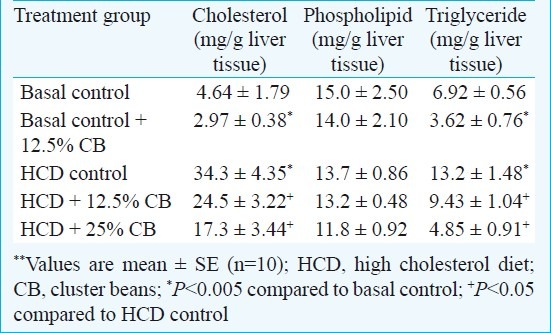
In the hypercholesterolaemic groups, HCD control had a hepatic cholesterol content of 34.3 mg/g, which amounted to 7.4-times the basal control value. Significant (P<0.05) reduction in hepatic cholesterol content was seen in rats fed with 12.5 or 25 per cent freeze-dried cluster beans along with HCD. Hepatic phospholipid content was unaffected in HCD treatment (Table II). Dietary cluster bean did not have any influence on hepatic phospholipid either in HCD fed animals or in basal diet fed animals. Hepatic triglyceride content was about double in HCD treatment. Supplementation of HCD with freeze dried cluster beans reversed it to 9.43 mg/g and 4.85 mg/g (in 12.5 and 25% groups) in the respective groups, the latter amounting to bring the triglyceride value to normal range. The triglyceride lowering effect of dietary cluster beans was also seen in basal diet fed animals where 12.5 per cent freeze dried cluster beans appreciably reduced the value to almost half (3.62 compared to 6.92 mg/g in basal control) (Table II).
Table II.
Liver lipid profile of tender cluster bean treated animals
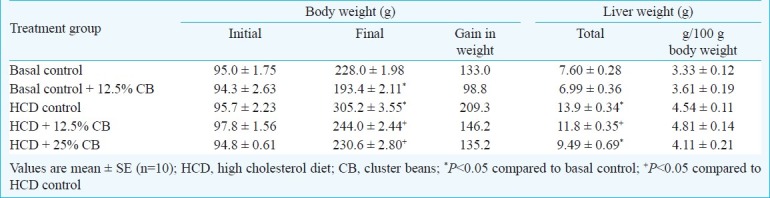
Changes in body and liver weights of rats were seen as a result of feeding tender cluster beans (Table III). Hypercholesterolaemic diet had increased the body weight of rats by 209 g; the gain in body weight was significantly (P<0.05) less in animals fed cluster beans along with cholesterol (146 and 135 g in the two groups). The gain in body weight was lowered by dietary cluster beans even in basal group (99 g as compared 133 g in basal control). The liver weights of the rats expressed as g/100 g body weight were not affected by dietary cluster beans (Table III).
Table III.
Body weight and liver weight of rats fed tender cluster beans
Discussion
Soluble dietary fibre such as guar gum has been explored for their ability to reduce plasma cholesterol and hence contributing significantly to cardio protective property. A relationship between fibre deficit diet and acceleration in the development of certain chronic and degenerative diseases has been hypothesized. Guar gum is a dietary fibre advocated for use in lowering serum total cholesterol levels in patients with hypercholesterolaemia2. Its mechanism of action is proposed to be similar to that of the bile-sequestering resins. Clinical trials indicate that guar gum may reduce serum total cholesterol by 10 to 15 per cent8, although some studies showed no significant response8. An attenuation of this effect during long term treatment has been seen but evidence of this effect is equivocal17. As an adjunct to established therapies (bezafibrate, lovastatin or gemfibrozil) guar gum has shown some promise: it may produce a further reduction in total cholesterol of about 10 per cent in patients not responding adequately to these drugs alone18.
Fernandez et al19 observed that the hypocholesterolaemic effect of guar gum is due to its ability to form viscous gel in the small intestine. Favier et al20 studied the effects of guar gum in rats adapted to 0.4 per cent cholesterol diets and reported lowered plasma cholesterol accompanied by significantly enhanced biliary bile acid excretion as well as the intestinal and cecal bile acid pool. Their study had inferred that high viscosity of guar gum may reduce the rate of gastric emptying and the diffusion of nutrients into the mucosa of small intestine. This mechanism further reduces the absorption of cholesterol, whether its origin is dietary or endogenous. The emphasis is on the capacity of fibre to entrap bile acids in small intestine and to increase their faecal excretion, which in turn accelerates cholesterol oxidation to bile acids resulting in a spillover of body's cholesterol pool, due to faecal loss of steroid20.
Moriceau et al17 investigated various aspects of enterohepatic bile acid cycling in rats adapted to fibre-free or 5 per cent guar gum diets (supplemented with 0.25% cholesterol) and concluded that the enterohepatic cycling of steroids, especially bile acids reabsorption in portal blood is reduced. Guar gum had a significant inhibitory effect on the uptake of cholesterol into micelles21. The effect of guar gum on plasma lipoproteins and cholesterol in hypercholesterolaemic adults showed that the guar gum induced a reduction in total cholesterol of 26 mg/dl and LDL cholesterol of 25 mg/dl22. A 4 wk of guar gum supplementation in diet of type I diabetic patients decreased, the serum total cholesterol level by 21 per cent23. Brown and Walter24 reported that inclusion of 3 g soluble fibre could decrease cholesterol by 0.129 mmol/l in a controlled clinical trial.
Effect of crude dietary fibre sources (supplemented at 5 or 10%) has been examined on the plasma cholesterol concentration in Sprague-Dawley rats25. The results suggested that dietary fibres exert a plasma cholesterol-lowering effect through cecal fermentation of this fibre. In fact, soluble fibre such as guar gum are almost completely fermented by gut microflora to short chain fatty acids and this process is characterized by an acidification of cecal contents. Propionate, a fermentable metabolite of soluble fibre is one of the prime components known to decrease the serum and hepatic cholesterol. Propionate concentrations at 1-1.25 mmol/l resulted in inhibition of cholesterol synthesis in vitro26.
The effects of guar gum on food intake, levels of blood serum cholesterol, triacylglycerols, glucose and LDL and HDL-cholesterol were studied in normal rats by feeding diets containing 0, 10 and 20 per cent (w/w) guar gum for 60 days27. The rats fed the guar gum diets showed significantly lower levels of blood serum cholesterol, triacylglycerols, reduced food intake and body weight gain. A concomitant increase in HDL-cholesterol with a substantial elevation of the HDL/LDL cholesterol ratio was noted. The effect of guar seed feeding for 4 wk on serum total lipids, cholesterol, triglycerides and phospholipids has been evaluated in normal and alloxan induced diabetic guinea pigs28. Blood sugar and total lipid levels were found to be decreased significantly in normal and as well as diabetic animals; free and esterified cholesterol levels were also observed to be lowered significantly in normal animals, whereas esterified fraction alone was found to be lowered in diabetics. Significant fall in the levels of other lipids – triglycerides, phospholipids and total lipids was also noticed.
It has been reported that 5 g of guar granules sprinkled over food at each main meal for a period of 4 wk decreased total plasma cholesterol29. Another study has reported that soluble dietary fibres have the ability to reduce absorption of neutral sterols, including cholesterol; and that a high faecal concentration of coprostanol, a bacteria metabolite of cholesterol, was higher in rats fed guar diet30. This increase in coprostanol suggests that soluble fibre may alter intestinal micro flora, favouring the degradation of cholesterol to coprostanol and enhancing its faecal excretion31.
Effect of incorporating tender cluster bean pod into a hypercholesterolaemic diet of rats has been studied earlier32. Cluster bean incorporated at 0.5 and 2.5 per cent levels to the high cholesterol diet and fed for 7 wk, exerted a hypocholesterolaemic effect and also lowered liver cholesterol concentration. The present study examined 5-times higher dietary level than this study, has not only confirmed the cholesterol lowering influence of tender cluster bean pods, but also shown that higher effects can be derived with this higher dietary level. Beneficial increase in the concentration of HDL associated cholesterol by dietary tender cluster beans was also shown in our study. In rats, the presence of guar gum in the diet not only was hypocholesterolaemic relative to the fibre-free diet, but also decreased food intake and body weight gain perhaps due to distension of the gastrointestinal tract33. Our study also showed reduction in body weight of rats fed with freeze-dried tender cluster beans.
In summary, the present animal study showed cholesterol lowering effect of dietary tender cluster beans in hypercholesterolaemic situation. This was also accompanied by countering of the changes in blood phospholipids, triglycerides and also hepatic lipid profile. There was a significant reduction in the weight gain of the animals by dietary cluster beans during HCD feeding. The results suggest that tender cluster beans may be effective in the treatment of hypercholesterolaemia in humans. Further studies in human volunteers need to be done to substantiate these findings. Also toxicity evaluation studies of long-term feeding of high doses of cluster beans are necessary.
Acknowledgment
The first author (SP) acknowledges the University Grants Commission, New Delhi, for the award of Junior Research Fellowship.
References
- 1.Cho IJ, Lee C, Ha TY. Hypolipidemic effect of soluble fiber isolated from seeds of cassia tora Linn.in rats fed a high-cholesterol diet. J Agric Food Chem. 2007;55:1592–6. doi: 10.1021/jf0622127. [DOI] [PubMed] [Google Scholar]
- 2.Rideout TC, Harding SV, Jones PJ, Fan MZ. Guar gum and similar soluble fibers in the regulation of cholesterol metabolism: current understandings and future research priorities. Vasc Health Risk Manag. 2008;4:1023–33. doi: 10.2147/vhrm.s3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khan AR, Khan GY, Mitchel A, Qadeer MA. Effect of guar gum on blood lipids. Am J Clin Nutr. 1981;34:2446–9. doi: 10.1093/ajcn/34.11.2446. [DOI] [PubMed] [Google Scholar]
- 4.Brennan CS. Dietary fibre, glycaemic response, and diabetes. Mol Nutr Food Res. 2005;49:560–70. doi: 10.1002/mnfr.200500025. [DOI] [PubMed] [Google Scholar]
- 5.Prieto PG, Cancelas J, Villanueva-Peñacarrillo ML, Malaisse WJ, Valverde I. Short-term and long-term effects of guar on postprandial plasma glucose, insulin and glucagon-like peptide 1 concentration in healthy rats. Horm Metab Res. 2006;38:397–404. doi: 10.1055/s-2006-944544. [DOI] [PubMed] [Google Scholar]
- 6.Evan E, Miller DS. Bulking agents in treatment of obesity. Nutr Metab. 1975;18:199–203. doi: 10.1159/000175595. [DOI] [PubMed] [Google Scholar]
- 7.Simons LA, Gayst S, Balasubramaniam S, Ruys J. Long-term treatment of hypercholesterolaemia with a new palatable formulation of guar gum. Atherosclerosis. 1982;45:101–8. doi: 10.1016/0021-9150(82)90175-7. [DOI] [PubMed] [Google Scholar]
- 8.Butt MS, Shahzadi N, Sharif MK, Nasir M. Guar gum: A miracal therapy for hypercholesterolemia, hyperglycemia and obesity. Crit Rev Food Sci Nutr. 2007;47:389–96. doi: 10.1080/10408390600846267. [DOI] [PubMed] [Google Scholar]
- 9.Asp NG, Johansson CG, Hallmer H, Siljestrom M. Rapid enzymatic assay of insoluble and soluble dietary fiber. J Agric Food Chem. 1983;31:476–82. doi: 10.1021/jf00117a003. [DOI] [PubMed] [Google Scholar]
- 10.Folch J, Lees M, Sloane-Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 11.Searcy RL, Bergquist LM. A new colour reaction for the quantification of serum cholesterol. Clin Chim Acta. 1960;5:192–7. doi: 10.1016/0009-8981(60)90035-8. [DOI] [PubMed] [Google Scholar]
- 12.Fletcher MJ. A colorimetric method for estimating serum triglycerides. Clin Chim Acta. 1968;22:393–7. doi: 10.1016/0009-8981(68)90041-7. [DOI] [PubMed] [Google Scholar]
- 13.Stewart JC. Colorimetric determination of phospholipids with ammonium ferrothiocyanate. Anal Biochem. 1980;104:10–4. doi: 10.1016/0003-2697(80)90269-9. [DOI] [PubMed] [Google Scholar]
- 14.Warnick GR, Albers JJ. A comprehensive evaluation of the heparin-manganese precipitation procedure for estimating high density lipoprotein cholesterol. J Lipid Res. 1978;19:65–76. [PubMed] [Google Scholar]
- 15.Burkitt DP, Walker AR, Painter NS. Effect of dietary fiber on stools and the transit-time, and its role in the causation of disease. Lancet. 1972;2:1408–12. doi: 10.1016/s0140-6736(72)92974-1. [DOI] [PubMed] [Google Scholar]
- 16.Burkitt DP, Trowell HC. Dietary fibre and Western diseases. Ir Med J. 1977;70:272–7. [PubMed] [Google Scholar]
- 17.Knopp RH, Superko HR, Davidson M, Insull W, Dujovne CA, Kwiterovich PO, et al. Long-term blood cholesterol-lowering effects of a dietary fiber supplement. Am J Prev Med. 1999;17:18–23. doi: 10.1016/s0749-3797(99)00039-2. [DOI] [PubMed] [Google Scholar]
- 18.Todd PA, Benfield P, Goa KL. Guar gum. A review of its pharmacological properties, and use as a dietary adjunct in hypercholesterolaemia. Drugs. 1990;39:917–28. doi: 10.2165/00003495-199039060-00007. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez ML, Sun DM, Tosca M, McNamara DJ. Guar gum effects on plasma low-density lipoprotein and hepatic cholesterol metabolism in guinea pig fed low- and high- cholesterol diets: a dose response study. Am J Clin Nutr. 1995;61:127–34. doi: 10.1093/ajcn/61.1.127. [DOI] [PubMed] [Google Scholar]
- 20.Favier ML, Bost PE, Guittard C, Demigné C, Rémésy C. The cholesterol-lowering effect of guar gum is not the result of a simple diversion of bile acids toward fecal excretion. Lipids. 1997;32:953–9. doi: 10.1007/s11745-997-0123-z. [DOI] [PubMed] [Google Scholar]
- 21.Gee JM, Blackburn NA, Johnson IT. The influence of guar gum on intestinal cholesterol transport in the rat. Br J Nutr. 1983;50:215–24. doi: 10.1079/bjn19830091. [DOI] [PubMed] [Google Scholar]
- 22.Spiller GA, Farquhar JW, Gates JE, Nichols SF. Guar gum and plasma cholesterol. Effect of guar gum and an oat fiber source on plasma lipoproteins and cholesterol in hypercholesterolemic adults. Arterioscler Thromb. 1991;11:1204–8. doi: 10.1161/01.atv.11.5.1204. [DOI] [PubMed] [Google Scholar]
- 23.Ebeling P, Yki-Jarvinen H, Aro A, Helue E, Sinisalo M, Koivisto VA. Glucose and lipid metabolism and insulin sensitivity in type I diabetes: the effect of guar gum. Am J Clin Nutr. 1988;48:98–103. doi: 10.1093/ajcn/48.1.98. [DOI] [PubMed] [Google Scholar]
- 24.Brown L, Rosner B, Willett WW, Sacks FM. Cholesterol- lowering effects of dietary fiber: a meta-analysis. Am J Clin Nutr. 1999;69:30–42. doi: 10.1093/ajcn/69.1.30. [DOI] [PubMed] [Google Scholar]
- 25.Nishimura N, Taniguchi Y, Kiriyama S. Plasma cholesterol- lowering effect on rats of dietary fiber extracted from immature plants. Biosci Biotechnol Biochem. 2000;64:2543–51. doi: 10.1271/bbb.64.2543. [DOI] [PubMed] [Google Scholar]
- 26.Nishina PM, Freedland RA. Effects of propionate on lipid biosynthesis in isolated rat hepatocytes. J Nutr. 1990;120:668–73. doi: 10.1093/jn/120.7.668. [DOI] [PubMed] [Google Scholar]
- 27.Frias AC, Sgarbieri VC. Guar gum effects on food intake, blood serum lipids and glucose levels of Wistar rats. Plant Foods Hum Nutr. 1998;53:15–28. doi: 10.1023/a:1008052216477. [DOI] [PubMed] [Google Scholar]
- 28.Srivastava A, Longia GS, Singh SP, Joshi LD. Hypoglycaemic and hypolipaemic effects of Cyamopsis tetragonoloba (guar) in normal and diabetic guinea pigs. Indian J Physiol Pharmacol. 1987;31:77–83. [PubMed] [Google Scholar]
- 29.Fuessl HS, Williams G, Adrian TE, Bloom SR. Guar sprinkled on food: effect on glycemic control, plasma lipids and gut hormones in non-insulin dependent diabetic patients. Diabet Med. 1987;4:463–8. doi: 10.1111/j.1464-5491.1987.tb00910.x. [DOI] [PubMed] [Google Scholar]
- 30.Arjmandi BH, Ahn J, Nathani S, Reeves RD. Dietary soluble fiber and cholesterol affect serum cholesterol concentration, hepatic portal venous short-chain fatty acid concentrations and fecal sterol excretion in rats. J Nutr. 1992;122:246–53. doi: 10.1093/jn/122.2.246. [DOI] [PubMed] [Google Scholar]
- 31.IIman RJ, Topping DL. Effects of dietary oat bran on fecal steroid excretion, plasma volatile fatty acids and lipid synthesis in rats. Nutr Res. 1985;5:839–46. [Google Scholar]
- 32.Sarathy R, Saraswathi G. Effect of tender cluster beans pods (Cyamopsis tetragonoloba) on cholesterol levels in rats. Am J Clin Nutr. 1983;38:295–9. doi: 10.1093/ajcn/38.2.295. [DOI] [PubMed] [Google Scholar]
- 33.Poksay KS, Schneeman BO. Pancreatic and intestinal response to dietary guar gum in rats. J Nutr. 1983;113:1544–9. doi: 10.1093/jn/113.8.1544. [DOI] [PubMed] [Google Scholar]


