Abstract
Background & objectives:
Reflux oesophagitis (RE), is one of the most prevalent chronic gastrointestinal disorders commonly referred to as gastroesophageal reflux disease (GERD) and requires long term therapy. The present study was designed to investigate the protective effects of Panax quinquefolium (PQ), administered with variable doses, on experimentally induced reflux oesophagitis (RE) in rats.
Methods:
Forty two female Sprague-Dawley (180-220 g) rats were randomly divided to receive standardized root powder of PQ (50-200mg/kg, po), standard anti-reflux (omeprazole, 5 mg/kg, ip) and anti-oxidant (α-tocopherol, 16 mg/kg, po). After 45 min drug pretreatment, RE was produced in rats by simultaneous ligation of the pyloric end and forestomach. Several parameters, including macroscopic lesion index, glutathione system, lipid peroxidation (LPO) and tissue myeloperoxidase (MPO) activity were measured. Alterations in ICAM-1, CINC-2 and MCP-1 gene expression were examined through reverse transcriptase polymerase chain reaction (RT-PCR).
Results:
PQ significantly attenuated the severity of the macroscopic signs of RE-induced tissue damage, replenished the depleted GSH level and reduced the RE-associated LPO levels dose dependently. In contrast, omeprazole though effectively improved the mucosal damage, it failed to bring significant attenuation of RE-associated changes in LPO, GSH level and MPO activity. α-Tocopherol significantly ameliorated RE-induced tissue injury and improved LPO level and GSH/GSSG ratio but failed to counteract RE-induced MPO activity. PQ at dose of 100 mg/kg significantly downregulated ICAM-1 and CINC-2 expression whereas it showed no effect over MCP-1 expression.
Interpretation & conclusions:
The present data indicate that PQ protects against RE-induced oesophageal damage via a mechanism that inhibits the influx of inflammatory cell to oesophagus and a consequence excessive oxidative load, opening the avenue to its promising protective role in patients with gastroesophageal reflux disease (GERD).
Keywords: Acid reflux, anti-inflammatory, oesophagitis, oxidative stress, Panax quinquefolium
Reflux oesophagitis (RE) commonly referred as gastroesophageal reflux disease (GERD), is a chronic and relapsing oesophageal pathology, having significant impact on the quality of life. Population based studies showed that up to 15 per cent of individual have heartburn and/or regurgitation at least once a week and 7 per cent have symptoms daily1. Gastroesophageal reflux (GER) has also been implicated in the development of Barrett oesophagus (BE) and posed greater risk to the development of oesophageal cancer2. Conventional anti-reflux treatments were aimed primarily at reducing gastric acidity via using proton pump inhibitors or H2 receptor antagonists. Though these antisecretory treatments can effectively reduce periods of active disease and help to maintain remission, but these often bring marginal results, patients become refractory and there are tolerance and various side effects3.
The aetiology of RE is complex and multifactorial rather than a single cause. Conceptually, the contributing factors include abnormal reflux of the gastric contents from the stomach to the oesophagus due to the incompetent barriers present at the gastro-esophageal junction leading to oesophageal inflammation. It has been suggested that beside acid other contributing mechanisms were also associated with the pathophysiology of RE as no good correlation was found to exist between the amount of refluxed materials and the degree of oesophageal damage4. Various studies in experimental RE and human subjects have reported that some of the substances that are considered to be critical in the aetiology of RE are classic inflammatory product5 and reactive oxygen species6. It has also been demonstrated that inflammatory cytokines especially chemokines, play an important role in inducing early inflammatory changes in patients with GERD7. Thus, therapeutic agents which possess the properties to scavenge toxic free radicals and modulate inflammatory response might have a significant effect in ameliorating tissue destruction induced by RE.
The natural herbal American ginseng (Panax quinquefolium; PQ) belongs to the family Araliaceae and is well-known for its antioxidant and free radical scavenging activities8. The root of Panax species is known to contain various saponins, including ginsenosides Rb1, Rb2, Rc, Rd, Re and Rg1 which are claimed to possess remarkable immunomodulating, tumour therapeutic9 and antiulcer activity10. PQ is believed to improve mental performance and detrimental end points associated with diseases such as cardiovascular disease, diabetes and influenza11. Since RE posed considerable amount of oxidative stress and inflammatory loads to the oesophagus, we hypothesized that American ginseng can be beneficial. We undertook this study to investigate the effect of crude root powder of PQ in experimentally induced RE in rats.
Material & Methods
Animals: Experimental protocols were approved by the Institutional Ethical and Usage Committee of Central Drug Research Institute (CDRI), Lucknow. The guidelines of the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA) were followed. Female Sprague Dawley rats, weighing 180-220 g procured from National Laboratory Animal Centre, CDRI, were used in the study. Rats were housed three to four per cage, in a room at 22 ± 2°C, with a 12 h/12 h light/dark cycle. Standard chow pellets and water were given ad libitum, except during the period when food deprivation was applied.
Chemicals: Reduced glutathione (GSH), glutathione reductase, thiobarbituric acid, 1, 33 -tetraethoxypropane and 5, 5′-dithiobis (2-nitrobenzoic acid) (DTNB), P. quinquefolium (PQ) crude root powder, omeprazole (Omz) and α-tocopherol (Toco) were purchased from Sigma Aldrich (St. Louis, MO, USA). All the other chemicals were of analytical or HPLC grade, which were purchased locally.
Induction of reflux oesophagitis (RE): Animals were deprived of food for 18 h before induction of oesophagitis but water was provided ad libitum. RE was induced according to the method described earlier12 with slight modifications. Briefly, under pentobarbital anaesthesia (30 mg/kg, i.p.) the abdomen was incised along the midline and then both the pyloric end of the stomach and limiting ridge (transitional region between the fore stomach and corpus) were simultaneously ligated tightly, resulting in the reflux of the gastric juice into the oesophagus. After 5 h of ligation the animals were killed by cervical dislocation, oesophagus was removed and incised lengthwise. Haemorrhagic lesions were observed under Trinocular zoom microscope (SZ-CTV Olympus, Japan) and area of lesions (sq. mm) developed were measured using Biovis image analyzer software (Expert Vision Lab Private Ltd., Mumbai, India). The lesion were scored adopting the criteria proposed earlier12 for the mesuresment of severity: 0=no visible lesions; 1= a few erosions; 2= total area of lesions <30 mm2; 3= total area of lesions >30 mm2; 4= perforation.
Drug preparation and treatment schedule: For pilot investigation, a dose dependent study was conducted with standardized PQ root powder (50-200 mg/kg) on oesophageal parameters of oxidative and inflammatory load succeeding RE infliction. Omz was used at a dose of 5 mg/kg body weight and Toco at 16 mg/kg as standard anti-reflux and antioxidant drugs, respectively. The doses of Omz and Toco were selected on the basis of our pilot studies and available literature13. Suspensions of standardized root powder of PQ, Omz and Toco were prepared afresh before administration, in 1 per cent sodium carboxy methyl cellulose (CMC). PQ and Toco were administered orally using a ball ended feeding needle and Omz was given intraperitoneally (i.p.). After 1 wk of acclimatization, the rats (n=42) were randomly divided into 7 groups of 6 rats each.
The respective groups comprised: (a) Control group- rats received vehicle (1% CMC) at a dose of 1 ml/kg, p.o. before sham operation; (b) RE group: rats were operated to induce RE and was left untreated; (c) Drug treated RE group: comprising of PQ-50+RE, PQ-100+RE and PQ-200+RE groups receiving PQ at the subsequent doses of 50, 100 and 200 mg/kg ,p.o. respectively, Omz+RE group- received reference anti-reflux drug, omeprazole (5 mg/kg, i.p.) and Toco+RE group- received reference antioxidant drug, α- tocopherol (16 mg/kg, p.o.) 45 min prior to the induction of RE.
Sample preparation: For the estimations of GSH/ GSSG levels and lipid peroxidation (LPO) the excised oesophageal tissue was homogenized in ice cold 50 mM phosphate buffer (pH 7.0) containing 0.1 mM EDTA by Ultra-Turrax homogenizer (Model T25, IKA-Laborthechnik, Germany) and centrifuged at 10000 g for 15 min at 4°C, supernatant thus obtained was used for the analysis of oesophageal malondialdehyde (MDA) and glutathione levels.
Lipid peroxidation (LPO) assay: MDA levels (a thiobarbituric acid reactive species: TBARS) were measured using 1, 1, 3, 3-tetraethoxypropane as a standard14. The absorbance of reaction mixture was measured at 532 nm using spectrophotometer (Shimadzu, model 1201, USA) and expressed as nM/mg protein.
Total glutathione (GSH) and oxidized glutathione (GSSG) levels: GSH was determined by its reaction with DTNB (Ellman's reagent) to yield a yellow chromophore which was measured spectrophotometrically15. The mixture was vortexed and the absorbance was taken at 412 nm within 15 min and GSH levels were determined from a standard curve. For GSSG assay the samples were incubated at room temperature with 2-vinylpyridine and triethanolamine to derivatize the reduced GSH present in the tissue. Breifly, 100 μl of homogenized tissue samples were incubated with 2 μl of 2-vinylpyridine and 20 μl of 10 per cent triethanolamine solution for 1 h. The results were expressed as GSH/GSSG ratio.
Myeloperoxidase (MPO) activity assay: Tissue-associated MPO activity was assessed as a marker of inflammation according to the method described by Grisham et al16 with slight modification. Briefly, the oesophageal tissue was homogenized in 0.1 M phosphate-buffer and centrifuged at 20000 g (Sigma centrifuge, model 3 K30, USA) at 4 °C. Pellet thus obtained was rehomogenized in 100mM phosphate buffer containing 0.5 per cent hexadecyltrimethylammonium bromide (HETAB, pH 5.4) and 5 mM EDTA and subjected to a freeze-thaw cycle thrice before centrifugation to yield clear supernatant. The reaction mixture consisted of 200 μl of 100 mM phosphate buffer, pH 5.4, 20 μl of 20 mM 3, 3’, 5, 5’-tetramethylbenzidine dihydrochloride (TMB) and 30 μl of the supernatant. The reaction was started by adding 50 μl of 0.01 per cent H2O2 and the conversion of the substrate was noted at 655 nm spectrophotometrically and expressed as mU/mg protein.
Protein assay: The protein content of the samples was determined by Lowry's method17 using bovine serum albumin as standard.
Semi-quantitative RT-PCR (reverse transcriptase polymerase chain reaction) analysis of CINC-2, ICAM-1 and MCP-1: Total RNA was extracted from oesophageal samples using TRIzol Reagent (Invitrogen™, Carlsbad CA, USA). cDNA was generated from 5 μg of total RNA using ReverAid™ H Minus First strand cDNA synthesis kit (Fermentas life sciences, Burlington, Canada) following manufacturer's instructions. Genes for CINC-2, MCP-1, ICAM-1 and β-actin were amplified with specific primer sets as previously described18,19. cDNA samples were annealed at 94°C (5 min) and amplified for 28 cycles with the following cycling conditions: 94°C for 1 min, respective annealing temperature for CINC-2 -65°C, ICAM-1-58°C, MCP-1 -65°C and β-actin -55°C for 1 min and extension at 72°C for 1 min followed by a final extension at 72°C for 10 min and was run on Bioer XP Cycler. PCR products were electrophoresed on a 1.0 per cent agarose gel using O′RangeRuler™ 100-bp DNA Ladder (Fermentas life sciences, Burlington, Canada).The intensities of the respective genes were measured using Biovis gel documentation software (Expert Vision Lab Private Ltd., India) and expressed as relative intensity of PCR-product/β-actin ratio.
Statistical analysis: The statistical analysis of data was done using analysis of variance (ANOVA) with post-hoc analysis. The Newman Kuels post-hoc test was applied to serve as significant among groups.
Results
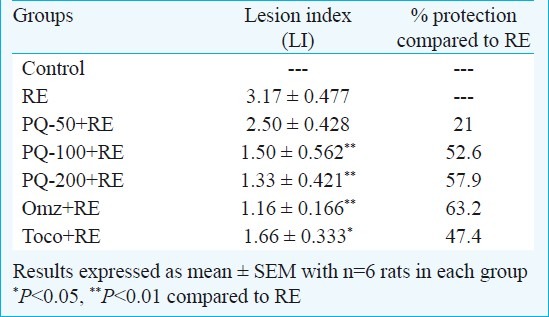
Effect of PQ on oesophagitis lesion index (LI): Five h after induction of oesophagitis, RE group displayed macroscopically evident sign of extensive oesophageal injury assessed in term of oesophageal lesion index (Table I). Administration of PQ (50, 100 and 200 mg/kg, po) reduced the severity of oesophagitis in a dose-dependent fashion, showing 21, 52 and 58 per cent protection, respectively. Significant protection was offered in PQ-100+RE and PQ-200+RE groups (P<0.01) compared to the RE group. Also, Omz+RE and Toco+RE group exhibited significant protection (P<0.01 and P<0.05, respectively) compared to RE group.
Table I.
Effect of graded doses of P. quinquefolium (PQ) on oesophageal lesion index in reflux oesophagitis (RE) rats in comparision to standard drugs omeprazole (5 mg/kg, ip) and tocopherol (16 mg/kg, po)
Effect of PQ on oesophageal LPO: LPO represents a sensitive indicator of free radical mediated membrane damage. A significant increase in the MDA levels was observed in RE group (P<0.001) compared to control rats. PQ tested at all the three doses attenuated the RE-augmented level of MDA. PQ-100+RE and PQ-200+RE groups achieved significant (P<0.001) reduction in MDA level as also PQ-50+RE group (P<0.05) when compared to RE rats. The Omz+RE and groups Toco+RE also demonstrated significant (P<0.05, P<0.001, respectively) attenuation of MDA level compared to RE (Table II).
Table II.
Effect of graded doses of P. quinquefolium (PQ) on MDA level, GSH/GSSG ratio and MPO activiity in reflux oesophagitis (RE) rats in comparision to standard drugs omeprazole (5 mg/kg, ip) and tocopherol (16 mg/kg, po)
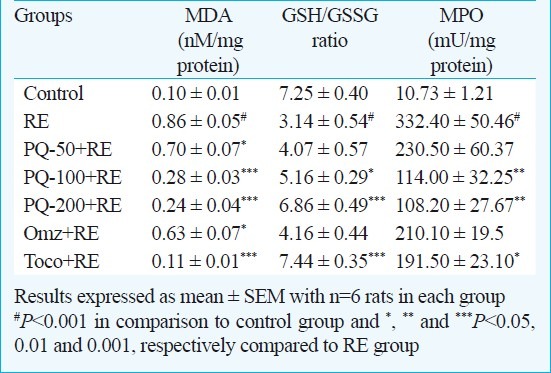
Effect of PQ on oesophageal GSH/GSSG ratio: Induction of reflux oesophagitis (RE group) significantly decreased GSH/GSSG ratio (P<0.001) compared to control animals. In PQ-100+RE and PQ-200+RE groups the ratio of GSH/GSSG increased significantly (P<0.05 and P<0.001 respectively) compared to RE-group. Omz+RE group rats did not show significant change in the ratio of GSH/GSSG but Toco+RE group exhibited significant increase (P<0.001) in the GSH/GSSG ratio over RE-rats (Table II).
Effect of PQ on oesophageal MPO activity: Onset of RE caused significant elevation (P<0.001) of MPO activity in oesophageal tissue with respect to control group. PQ-100+RE and PQ-200+RE significantly (P<0.01) reduced MPO activity. Toco+RE group also showed significant reduction (P<0.05) of MPO activity compared to RE group.
PQ at 100 and 200 mg/kg brought about significant attenuation of RE-related changes in LI, LPO, GSH and MPO activity. PQ (100 and 200 mg/kg doses) reverted the RE-aggravated MPO activity more effectively than reference anti-reflux and antioxidant drug used in this study. Therefore, 100 mg/kg PQ was used as penultimate dose for further experimental study on expression of proinflammatory genes, CINC-2, ICAM-1 and MCP-1.
Effect of PQ on mRNA level of CINC-2, ICAM-1 and MCP-1: In order to verify whether PQ is imparting any effect in controlling the inflammatory events initiated by reflux oesophagitis, the effect of PQ-100 on the mRNA levels of CINC-2, ICAM-1 and MCP-1 was studied. The gene expression levels of CINC-2, ICAM-1 and MCP-1 were significantly increased (P<0.001, P<0.01 and P<0.001, respectively) in the oesophageal lesions compared to control rats. PQ-100+RE group revealed a significant attenuation of gene expression of CINC-2 and ICAM-1 compared to RE group (P<0.05 and P<0.01, respectively), whereas no alteration was observed in the RE-upregulated expression of MCP-1 gene (Fig.).
Fig.
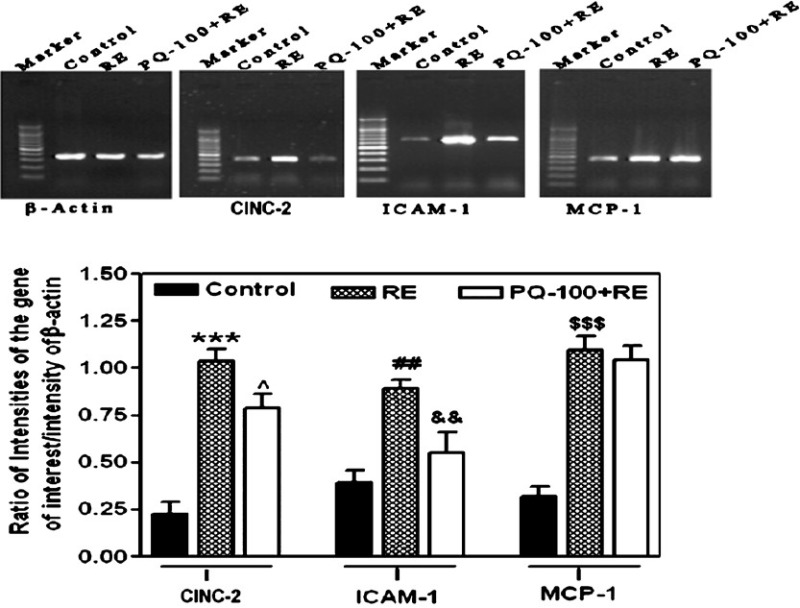
Effect of P. quinquefolium (PQ) at the dose of 100 mg/kg, p.o. on gene expression level of CINC-2, ICAM-1 and MCP-1 in reflux oesophagitis (RE) rats in comparison to control rats. Results were expressed as mean ± SEM with 6 rats in each group. For CINC-2, ***P<0.001 in comparison to control rats and ^P<0.05 compared to RE group. For ICAM-1, ##P<0.01 in comparison to control rats and &&P<0.01 compared to RE group. For MCP-1, $$$P<0.001 in comparison to control rats. The gel images are representative of three different experiments.
Discussion
The results of the present study clearly demonstrated that supplementation of PQ significantly ameliorated RE-induced oesophageal damage. Several herbal therapies have been proposed for the treatment of GERD20, however, the role of PQ against RE is not documented. The oesophageal lesion index has been correlated earlier with the production of excessive free radicals and electrophilic intermediates formation21. This oxidative load leads to membrane lipid peroxidation, which not only affects normal cellular functions, but also aggravates the oxidative stress through production of lipid derived radicals22. Our result showed that RE induction significantly increased the oesophageal level of lipid peroxidation as depicted by increased MDA levels. PQ pre-treatment potently reduced RE-accumulated level of MDA in a dose dependent fashion. There is evidence that the medicinal efficacy of PQ is closely linked to its protective properties against free radical attack23. This stabilizing effect of PQ extract can be attributed to its ability to scavenge hydroxyl radicals that troubled the cellular integrity via potentiating the oxidation process of unsaturated fatty acids present within the lipid membrane24.
A significant decline was seen in the ratio of GSH/GSSG with RE induction reflecting the compromised status of GSH dependent system. PQ treatment significantly increased this RE-declined ratio of GSH/GSSG. The ability of PQ to attenuate the RE-related alterations in the levels of MDA and GSH was observed to be as effective as reference antioxidant drug. The present data reflected that beside free radical scavenging property, PQ also reinforced the cellular endogenous anti-oxidant machinery to combat against the free radical attack.
Previous studies on oesophagitis showed that acid constituent were the most noxious agent of gastric juice that potentiates the process of free radical generation21. PQ is known for its anti-ulcer functions attributable to its reducing effect on the luminal acid output10, thus we compared its attenuating effect on oxidative stress parameter with acid suppressant drug omeprazole. PQ ameliorated the RE-related oxidative load more effectively than omeprazole, reflecting that the mechanism of PQ in RE was independent of its acid suppressing ability.
Potential sources of ROS in the oesophagus during oesophagitis include activated inflammatory cells (e.g. neutrophils), the hypoxanthine-xanthine oxidase reaction, disrupted mitochondrial electron transport, metabolism of arachidonate via the lipoxygenase pathway, and vascular endothelial cells25. A study demonstrated that 30 to 45 per cent of free radicals in inflamed oesophageal mucosa can be attributed to neutrophils26. Activated neutrophils are also a potential source of oxygen metabolites and MPO, an activating cytotoxic enzyme released from them. Our data revealed that onset of RE increased the inflammatory cell infilteration as manifested by significant increase in the oesophageal MPO activity, and PQ supplementation significantly reduced MPO activity in RE rats. Further, α-tocopherol and omeprazole were not as efficacious as PQ in reverting the RE-aggravated MPO activity indicating that protective mechanism of PQ was more dependent on its anti-inflammatory functions rather than anti-oxidant or anti-secretory actions. Since antioxidant treatment did not bring about effective reversal of MPO activity, one can exclude the possibility of observed reduction in MPO activity as a consequence of reduction in oxidative load of oesophagus.
Studies on rat chronic oesophagitis showed that recruitment of inflammatory cell to inflamed mucosa is dependent on the expression of adhesion molecules on the endothelial cells27 which helped in process of leukocyte extravasation. Besides, the mucosa of patients with oesophagitis also displayed marked elevation of chemokines molecules viz., IL-8, MCP-1, and RANTES (regulated on activation normal T-cell expressed and presumably secreted) that facilitate leukocyte emigration toward sites of inflammation7. A significant upregulation of ICAM-1 molecule was observed in our study after RE induction. Over-expression of chemokines, CINC-2 (homologue of human IL-8) and MCP-1 was also achieved on RE infliction. Accumulating evidence implicates IL-8 and MCP-1 as major mediators of acute neutrophil and chronic macrophage mediated inflammation respectively27. PQ pretreatment potently dowregulated the expression of ICAM-1 and CINC-2, however, it failed to significantly suppress MCP-1 expression in RE. Thus it is reasonable to believe that PQ inhibits influx of inflammatory cells probably by suppressing the expression of adhesion molecule (ICAM-1) and release of chemotactic substance (CINC-2) that promotes acute inflammation, while its role on the marker of chronic inflammation i.e. MCP-1 remained ambiguous. The ambiguity of PQ on MCP-1 might be due to the limitation of our RE-model which being acute in nature would be able to mimic only early inflammatory responses associated with human oesophagitis28.
In conclusion, the findings of present study suggested that PQ imparted protection against experimental RE probably through subsiding the RE-associated inflammatory responses and a consequence oxidative stress. The plausible mechanism of PQ anti-inflammatory functions against RE appeared to be suppression of RE-induced ICAM-1 and CINC-2 expression. Further studies are required to test its potential to be used as an alternative therapy against GERD.
Acknowledgment
The authors acknowledge Indian Council of Medical Research (ICMR), New Delhi, India, for financial support. CDRI communication No. 8074.
References
- 1.Goyal RK. Diseases of the esophagus. In: Kasper DL, Braunwald E, Fauci AS, Hauser SL, Longo DL, Jameson JL, editors. Harrison's principles of internal medicine. New York: McGraw-Hill; 2005. pp. 1739–46. [Google Scholar]
- 2.Kato M. Gastroesophageal reflux disease (GERD).Barrett esophagus. Nippon Rinsho. 2009;67:2357–65. [PubMed] [Google Scholar]
- 3.Katz PO. Optimizing medical therapy for gastroesophageal reflux disease: state of the art. Rev Gastroenterol Disord. 2003;3:59–69. [PubMed] [Google Scholar]
- 4.Lanas A, Royo Y, Ortego J, Molina M, Sainz R. Experimental esophagitis induced by acid and pepsin in rabbits mimicking human reflux esophagitis. Gastroenterology. 1999;116:97–107. doi: 10.1016/s0016-5085(99)70233-7. [DOI] [PubMed] [Google Scholar]
- 5.Yoshida N. Inflammation and oxidative stress in gastroesophageal reflux disease. J Clin Biochem Nutr. 2007;40:13–23. doi: 10.3164/jcbn.40.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oh TY, Lee JS, Ahn BO, Cho H, Kim W, Kim YB, et al. Oxidative stress is more important than acid in the pathogenesis of reflux oesophagitis in rats. Gut. 2001;49:364–71. doi: 10.1136/gut.49.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Isomoto H, Wang A, Mizuta Y, Akazawa Y, Ohba K, Omagari K, et al. Elevated levels of chemokines in esophageal mucosa of patients with reflux esophagitis. Am J Gastroenterol. 2003;98:551–6. doi: 10.1111/j.1572-0241.2003.07303.x. [DOI] [PubMed] [Google Scholar]
- 8.Li J, Huang M, Teoh H, Man RY. Panax quinquefolium saponins protects low density lipoproteins from oxidation. Life Sci. 1999;64:53–62. doi: 10.1016/s0024-3205(98)00533-5. [DOI] [PubMed] [Google Scholar]
- 9.Xiaoguang C, Hongyen L, Xiaohong L, Zhaodi F, Yan L, Lihua T, et al. Cancer chemopreventive and therapeutic activities of red ginseng. J Ethnopharmacol. 1998;60:71–8. doi: 10.1016/s0378-8741(97)00133-5. [DOI] [PubMed] [Google Scholar]
- 10.Jeong CS, Hyun JE, Kim YS. Ginsenoside Rb1: the anti-ulcer constituent from the head of Panax ginseng. Arch Pharm Res. 2003;26:906–11. doi: 10.1007/BF02980198. [DOI] [PubMed] [Google Scholar]
- 11.Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. J Nutr. 2007;137(1 Suppl):183S–5S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 12.Nakamura K, Ozawa Y, Furuta Y, Miyazaki H. Effects of sodium polyacrylate (PANa) on acute esophagitis by gastric juice in rats. Jpn J Pharmacol. 1982;32:445–56. doi: 10.1254/jjp.32.445. [DOI] [PubMed] [Google Scholar]
- 13.Rao CV, Vijayakumar M. Effect of quercetin, flavonoids and alpha-tocopherol, an antioxidant vitamin, on experimental reflux oesophagitis in rats. Eur J Pharmacol. 2008;589:233–8. doi: 10.1016/j.ejphar.2008.04.062. [DOI] [PubMed] [Google Scholar]
- 14.Colado MI, O’Shea E, Granados R, Misra A, Murray TK, Green AR. A study of the neurotoxic effect of MDMA (‘ecstasy’) on 5-HT neurones in the brains of mothers and neonates following administration of the drug during pregnancy. Br J Pharmacol. 1997;121:827–33. doi: 10.1038/sj.bjp.0701201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson ME. Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 1985;113:548–55. doi: 10.1016/s0076-6879(85)13073-9. [DOI] [PubMed] [Google Scholar]
- 16.Grisham MB, Benoit JN, Granger DN. Assessment of leukocyte involvement during ischemia and reperfusion of intestine. Methods Enzymol. 1990;186:729–42. doi: 10.1016/0076-6879(90)86172-r. [DOI] [PubMed] [Google Scholar]
- 17.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75. [PubMed] [Google Scholar]
- 18.Hsieh CS, Huang CC, Wu JJ, Chaung HC, Wu CL, Chang NK, et al. Ascending cholangitis provokes IL-8 and MCP-1 expression and promotes inflammatory cell infiltration in the cholestatic rat liver. J Pediatr Surg. 2001;36:1623–8. doi: 10.1053/jpsu.2001.27933. [DOI] [PubMed] [Google Scholar]
- 19.Bonello N, Jasper MJ, Norman RJ. Periovulatory expression of intercellular adhesion molecule-1 in the rat ovary. Biol Reprod. 2004;71:1384–90. doi: 10.1095/biolreprod.104.030650. [DOI] [PubMed] [Google Scholar]
- 20.Chris D. Meletis, Zabriskie N.Natural approaches for gastroesophageal reflux disease and related disorders. Altern Complemen Therap. 2007;13:64–70. [Google Scholar]
- 21.Wetscher GJ, Perdikis G, Kretchmar DH, Stinson RG, Bagchi D, Redmond EJ, et al. Esophagitis in Sprague-Dawley rats is mediated by free radicals. Dig Dis Sci. 1995;40:1297–305. doi: 10.1007/BF02065542. [DOI] [PubMed] [Google Scholar]
- 22.Davies KJ. Oxidative stress, antioxidant defenses, and damage removal, repair, and replacement systems. IUBMB Life. 2000;50:279–89. doi: 10.1080/713803728. [DOI] [PubMed] [Google Scholar]
- 23.Kitts DD, Wijewickreme AN, Hu C. Antioxidant properties of a North American ginseng extract. Mol Cell Biochem. 2000;203:1–10. doi: 10.1023/a:1007078414639. [DOI] [PubMed] [Google Scholar]
- 24.Zhang D, Yasuda T, Yu Y, Zheng P, Kawabata T, Ma Y, et al. Ginseng extract scavenges hydroxyl radical and protects unsaturated fatty acids from decomposition caused by iron-mediated lipid peroxidation. Free Radic Biol Med. 1996;20:145–50. doi: 10.1016/0891-5849(95)02020-9. [DOI] [PubMed] [Google Scholar]
- 25.Grisham MB, Hernandez LA, Granger DN. Xanthine oxidase and neutrophil infiltration in intestinal ischemia. Am J Physiol. 1986;251:G567–74. doi: 10.1152/ajpgi.1986.251.4.G567. [DOI] [PubMed] [Google Scholar]
- 26.Wetscher GJ, Hinder RA, Klingler P, Gadenstatter M, Perdikis G, Hinder PR. Reflux esophagitis in humans is mediated by oxygen-derived free radicals. Am J Surg. 1995;170:552–6. doi: 10.1016/s0002-9610(99)80014-2. [DOI] [PubMed] [Google Scholar]
- 27.Hamaguchi M, Fujiwara Y, Takashima T, Hayakawa T, Sasaki E, Shiba M, et al. Increased expression of cytokines and adhesion molecules in rat chronic esophagitis. Digestion. 2003;68:189–97. doi: 10.1159/000075698. [DOI] [PubMed] [Google Scholar]
- 28.Yamaguchi T, Yoshida N, Tomatsuri N, Takayama R, Katada K, Takagi T, et al. Cytokine-induced neutrophil accumulation in the pathogenesis of acute reflux esophagitis in rats. Int J Mol Med. 2005;16:71–7. [PubMed] [Google Scholar]


