Abstract
The last 350 years since the publication of the first medical monograph on rickets (old English term wrickken) (Glisson et al., 1651) have seen spectacular advances in our understanding of mineral-homeostasis. Seminal and exciting discoveries have revealed the roles of PTH, vitamin D, and calcitonin in regulating calcium and phosphate, and maintaining healthy teeth and skeleton. However, it is clear that the PTH/Vitamin D axis does not account for the entire picture, and a new bone-renal metabolic milieu has emerged, implicating a novel set of matrix proteins, hormones, and Zn-metallopeptidases. The primary defects in X-linked hypophosphatemic rickets (HYP) and autosomal-dominant hypophosphatemic rickets (ADHR) are now identified as inactivating mutations in a Zn-metalloendopeptidase (PHEX) and activating mutations in fibroblast-growth-factor-23 (FGF23), respectively. In oncogenic hypophosphatemic osteomalacia (OHO), several tumor-expressed proteins (MEPE, FGF23, and FRP-4) have emerged as candidate mediators of the bone-renal pathophysiology. This has stimulated the proposal of a global model that takes into account the remarkable similarities between the inherited diseases (HYP and ADHR) and the tumor-acquired disease OHO. In HYP, loss of PHEX function is proposed to result in an increase in uncleaved full-length FGF23 and/or inappropriate processing of MEPE. In ADHR, a mutation in FGF23 results in resistance to proteolysis by PHEX or other proteases and an increase in half-life of full-length phosphaturic FGF23. In OHO, over-expression of FGF23 and/or MEPE is proposed to result in abnormal renal-phosphate handling and mineralization. Although this model is attractive, many questions remain unanswered, suggesting a more complex picture. The following review will present a global hypothesis that attempts to explain the experimental and clinical observations in HYP, ADHR, and OHO, plus diverse mouse models that include the MEPE null mutant, HYP-PHEX transgenic mouse, and MEPE-PHEX double-null-mutant.
Keywords: PHEX, MEPE, FGF23, mineralization, hypophosphatemia, phosphaturic, osteomalacia, rickets
(1) Introduction
X-linked hypophosphatemic rickets (HYP) is characterized by defective renal phosphate handling (renal-leak), aberrant vitamin D metabolism, and defective calcification of bone (rickets in children and osteomalacia in adults) (Rowe, 1994, 1997, 1998; Quarles and Drezner, 2001). Tumor-induced osteomalacia (OHO) has many features in common with HYP and is proposed to have an overlapping pathophysiology (Rowe, 1997, 1998; Drezner, 2001; Quarles and Drezner, 2001). The primary defect in HYP is a defective Zn-metalloendopeptidase called PHEX, formerly PEX (HYP Consortium et al., 1995; Rowe et al., 1996a, 1997; Francis et al., 1997). The acronym PHEX stands for Phosphate-regulating gene with Homologies to Endopeptidases on the X-chromosome. PHEX is predominantly expressed in osteoblasts and odontoblasts, and there is overwhelming evidence confirming that a loss of PHEX function indirectly results in the secretion of osteoblast-specific factors that inhibit renal phosphate handling and mineralization in vitro and in vivo (Meyer et al., 1989a,b; Ecarot et al., 1992a,b, 1994a,b, 1995; Nesbitt et al., 1992, 1995, 1999; Yamamoto et al., 1992; Rifas et al., 1995, 1997; Halstead et al., 1996; Lajeunesse et al., 1996; Carpenter et al., 1998; Xiao et al., 1998; Miao et al., 2001; Shih et al., 2002). These uncharacterized HYP-osteoblast secreted phosphate-inhibitors and mineralization-inhibitors have been described as phosphatonins (PTNs) and minhibins (MNHs), respectively (Econs and Drezner, 1994; Rowe et al., 2004).
Recently, the ‘autosomal-dominant’ form of hypophosphatemic-rickets (ADHR) has been shown to be caused by activating mutations in FGF23 (White et al., 2000). The FGF23 missense-mutations increase resistance to proteolysis and, consequently, half-life of the full-length mutated FGF23 molecule. Thus, the full-length mutant-form is proposed to have an adverse impact on renal phosphate handling and mineralization in ADHR. FGF23 bone-tissue expression is very low. Indeed, several groups find no evidence for expression of FGF23 in bone (Yamashita et al., 2000; Guo et al., 2001, 2002; Liu et al., 2001; White et al., 2001; Bai et al., 2002). However, when more sensitive techniques were used, FGF23 mRNA expression was detected in bone-tissue and in SV-40 transformed cell-lines derived from HYP osteoblasts and osteogenic cells from normal human bone (Liu et al., 2003b; Riminucci et al., 2003). Unlike MEPE or PHEX, low-level FGF23 mRNA in bone is not developmentally coordinated with maturation of the normal osteoblast in vitro (Gowen et al., 2003; Liu et al., 2003a). Low-level bone-expression of FGF23 does not preclude bone-expressed FGF23 from having a major role in bone-renal homeostasis. However, this suggests that extra-osseous expressed FGF23 may also play a key role, particularly since FGF23 expression was originally localized to the ventrolateral thalamic nucleus of the brain, and PHEX is also expressed in brain, parathyroid glands, and bone (Blydt-Hansen et al., 1999; Meyer and Meyer, 2000; Meyer et al., 2000; Yamashita et al., 2000).
The evidence strongly suggests that several factors adversely affect renal phosphate-handling independently in HYP and or OHO (FGF23, FRP-4, MEPE, etc.). Frizzled related protein 4 (FRP-4) is the most recent candidate phosphatonin suggested to play a role in OHO and HYP (De Beur et al., 2002; Berndt et al., 2003). New research is now beginning to unravel the relationship between these candidate phosphatonins and minhibins. For example, the key questions are: (1) Are these factors components of contiguous, overlapping, or separate pathways? (2) Do they exert their biological effects directly or indirectly? (3) Are the proposed phosphatonins/minhibins osteoblastic and/or extra-osseous in provenance, or both?
There are clearly complex molecular and physicochemical interactions between and among matrix-proteins required for mineralization, matrix-proteins inhibiting mineralization, bone remodeling, and bone-renal phosphate-homeostasis. Perturbations in proteolytic processing, protein-protein interactions, and signal-transduction, not surprisingly, affect the dynamic equilibrium of these complex multifaceted processes in unexpected ways. For example, a small acidic, protease-resistant MEPE-peptide (ASARM peptide) could potentially be the first biological bisphosphonate described (Rowe et al., 2000, 2004). This peptide occurs in MEPE and some related family proteins (SIBLINGs), and the acronym ASARM stands for Acidic-Serine-Aspartate-Rich-MEPE-associated motif (Rowe et al., 2000, 2004). The biological properties of the ASARM peptide are remarkably similar, in some aspects, to those of etidronate. The ASARM peptide (like etidronate) inhibits mineralization in vivo and in vitro and affects renal phosphate handling. Many uncertainties remain, and determining the physiological importance of these new molecules will require a careful disentangling of their pathophysiological roles. The rewards will be high, since there is clear potential for the therapeutic treatment of hypophosphatemic and hyperphosphatemic conditions (renal osteodystrophy, end-stage renal failure, renal toxicity, renal-transplantation), bone-mineral and phosphate loss disorders (HYP, OHO, ADHR), ectopic calcification, periodontal disease, and osteoporosis.
(2) PHEX: a Protease and Ligand?
PHEX belongs to the M13 family and MA clan of Zn-metalloendopeptidases. The prototypic member of this group of type II integral membrane glycoproteins is neutral endopeptidase (NEP) (Letarte et al., 1988; Shipp et al., 1988). These proteins have a short cytoplasmic N-terminal region, a single transmembrane domain, and a large extracellular C-terminal domain with a zinc binding motif. Other members of this group include: endothelin-converting enzyme-1 (ECE-1α, ECE-Iβ, and ECE-II), ECE-like enzyme/distress-induced neuronal endopeptidase (ECEL1/DINE), soluble endopeptidase/NEP-like enzyme-1/neprilysin 2 (SEP/NL1/NEP2), membrane metalloendopeptidase-like 2 (MMEL2), and Kell blood group protein antigen (KELL) (Letarte et al., 1988; Shipp et al., 1988; Rawlings and Barrett, 1995, 1999; Valdenaire et al., 2000; Turner et al., 2001; Bianchetti et al., 2002). Neprilysin is also called common acute lymphoblastic leukemia antigen (CALLA), CD10, NEP, or enkephilinase (Letarte et al., 1988; Shipp et al., 1988). The M13 zinc metalloendopeptidases are integrally involved in several diseases, including renal function defects, bone mineral loss disorders, cardiovascular disease, arthritis and inflammatory disorders, and cancer. They are also involved in many essential elements of cellular regulation and physiology (Turner et al., 2001).
PHEX does contain key residues required for catalytic activity of small peptides (Campos et al., 2003), but a defined physiological substrate remains to be identified. The natural substrates for NEP are small (< 3 kDa), and this is also likely for PHEX (Oefner et al., 2000; Campos et al., 2003). The PHEX gene is similar to the NEP family of zinc metallopeptidases in several important aspects: (1) large number of small exons (22 characterized to date for PHEX with 749 amino acids); (2) short cytoplasmic N-terminal region encompassing a small hydrophobic transmembrane domain; (3) large extracellular C-terminal domain that includes a zinc-binding motif; (4) highly conserved amino acid zinc-binding motif (HEFTH in PHEX, and HEITH in NEP), conforming to the neprilysin family HEXXH and located in the same region of the protein (Chingwen and Hersh, 1995; Turner et al., 2001); (5) the number and spacing of the cysteine residues ‘10’ are almost identical to those of neprilysin, and are highly conserved; and (6) conservation of the amino acids asparagine, alanine, histidine, glutamic acid, histidine, glutamic acid, aspartic acid, histidine, and arginine at positions 538, 539, 580, 581, 584, 642, 646, 710, and 717, respectively (these residues are variously important for sequestering the zinc atom, substrate specificity, stabilization of the transition state, and catalysis) (Le Moual et al., 1991, 1992a,b, 1993, 1994; Beaumont et al., 1991, 1992; Dion et al., 1993, 1995; Turner and Tanzawa, 1997; Marie-Claire et al., 2000; Oefner et al., 2000; Turner et al., 2001).
Initial information concerning the structure and nature of the PHEX gene product catalytic site was acquired by the mutation-analysis of 99 HYP families and the use of computer-informatics to compare known physicochemical data and site-directed mutagenesis-studies published for M13 and M3 metallopeptidases (Rowe et al., 1997). Several other studies have reported mutations in the PHEX gene in families with X-linked rickets, and also in the mouse homologue, and there is an excellent curated Web site detailing all known and published PHEX mutations (Sabbagh et al., 2000). Overall amino acid sequence similarity between PHEX and NEP is 60%, with 34% identity. Of interest is the change in amino acid 583 (valine in NEP, isoleucine in PHEX). This amino acid is 3 residues 5′ to the zinc-binding motif (VGIHEITH) in neprilysin, and site-directed mutagenesis studies confirm its role in NEP substrate specificity (Chingwen and Hersh, 1995; Rawlings and Barrett, 1995; Turner et al., 2001).
Full-length FGF23 and MEPE do not appear to be PHEX substrates (Guo et al., 2002; Campos et al., 2003; Liu et al., 2003a,b). (See Section 3 for a fuller discussion of FGF23 and PHEX.) Remarkably, PHEX protects full-length MEPE from proteolysis, notably cathepsin-B cleavage (in vitro) (Guo et al., 2002; Rowe et al., 2004). This protection may also prevent release of the acidic COOH-terminal MEPE ASARM peptide in vivo (see Fig. 1). In addition, osteocalcin is not degraded by PHEX and inhibits PHEX cleavage of PTHrP (107-139) (Boileau et al., 2001). PTHrp (107-139) is one of the very few naturally occurring, small-peptide substrates cleaved by PHEX (Boileau et al., 2001), and Danio rerio is the simplest organism containing a PHEX gene. However, PTHrp (107-139) is absent in bony fish (Bianchetti et al., 2002), suggesting that PTHrp (107-139) either is an unlikely PHEX substrate or has emerged as an alternative substrate in higher organisms. For further PHEX phylogenetic details, the reader is directed to an excellent and very informative Web site at http://www-igbmc.u-strasbg.fr/BioInfo/ENDOPEPTIDASES_M13/M13_homepage.html (Bianchetti et al., 2002). Detailed M13 Zn-metallopeptidase-alignments have also been published (Barrett, 1995; Rawlings and Barrett, 1995, 1999; Valdenaire et al., 2000; Valdenaire and Schweizer 2000; Bianchetti et al., 2002), and the MEROPS database provides direct online access at http://www.merops.co.uk.
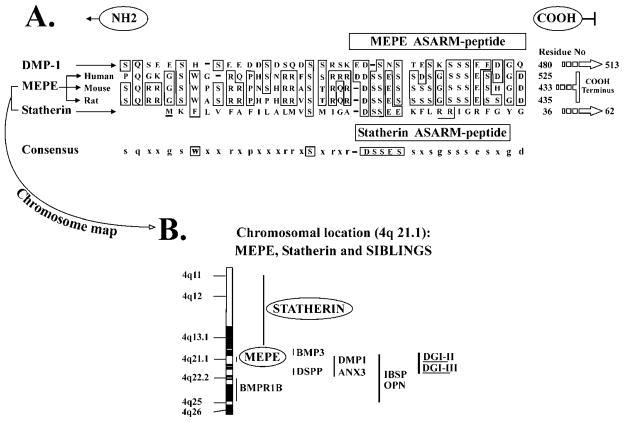
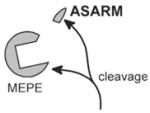
Figure 1.
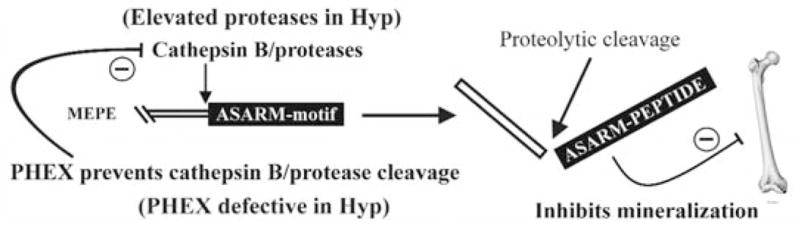
Proposed role for PHEX, MEPE, and the ASARM peptide in mineralization. MEPE and osteoblastic proteases (NEP, ECEL-1/DINE, and cathepsin-D) are markedly up-regulated in murine hyposteoblasts that have defective phex (Jo et al., 2000, 2001; Argiro et al., 2001; Bai et al., 2002; Dubois et al., 2002; Guo et al., 2002; Liu et al., 2003). PHEX/phex protects MEPE from cathepsin-B and general protease degradation and prevents release of the ASARM peptide (Guo et al., 2002). Cathepsin-B is also expressed in the osteoblast (Aisa et al., 1996, 2003). Thus, in HYP/hyp, elevated MEPE (with elevated osteoblastic proteases and loss of PHEX protease protection of MEPE) results in elevated levels of protease-resistant MEPE-ASARM peptide. The MEPE-ASARM peptide inhibits mineralization in vivo, and the osteopontin ASARM peptide potently inhibits calcium oxalate crystallization and crystal growth (Rowe et al., 2000, 2004; Hoyer et al., 2001). Also, the salivary statherin ASARM peptide (see Fig. 2) plays a direct biological role in inhibiting spontaneous precipitation of supersaturated salivary calcium and phosphate and maintaining the mineralization dynamics of tooth enamel (Schlesinger and Hay, 1977; Bennick et al., 1981; Raj et al., 1992; Long et al., 1998). Of related interest, the MEPE knockout, as expected, has accelerated mineralization and increased bone density and bone formation (Gowen et al., 2003). Mineral maturity (mineral crystal size and perfection) throughout all anatomic regions of the osteopontin knock-out mouse bone are also significantly increased (Boskey et al., 2002).
Although osteocalcin is not cleaved by PHEX, the negatively charged Gla residues in osteocalcin are thought to interact with the highly charged and atypical S1′ pocket (Boileau et al., 2001). A similar interaction may also occur with the highly charged ASARM peptide region of MEPE (Boileau et al., 2001; Campos et al., 2003). Interestingly, a single yeast two-hybrid experiment with MEPE and PHEX did not indicate an interaction between the two proteins (Guo et al., 2002). However, the folding and/or post-translational processing of PHEX and/or MEPE in yeast compared with insect or mammalian PHEX may have confounded the demonstration of a direct interaction. Further experiments are needed to confirm the nature (direct/indirect) of PHEX and its non-proteolytic cell-surface interactions with MEPE, osteocalcin, and perhaps other matrix proteins. Moreover, since KELL interacts with the XK protein (non-proteolytically), and other M13 members form homo-and/or heterodimers, it is reasonable to speculate that PHEX may well function as a small peptide protease and also as a matrix-protein ligand.
In this regard, PHEX does cleave at acidic amino acid residues (Asp or Glu) when locked in the S1 subsite and has a strong preference for Asp (Boileau et al., 2001; Campos et al., 2003). This was confirmed elegantly by PHEX cleavage analysis of small internally quenched fluorogenic peptides (IQFP) (Campos et al., 2003). More specifically, PHEX cleavage was found to occur at the amino terminus of acidic amino acid residues (Asp or Glu) adjacent to a small IQFP, MEPE-ASARM peptide (Campos et al., 2003). Thus, it is possible to speculate that, in the intact full-length MEPE-protein, the MEPE-PHEX association may allow for interaction with the MEPE COOH-terminal ASARM motif (Guo et al., 2002; Liu et al., 2003a). However, this interaction may not necessarily lead to proteolysis, due to the conformational dynamics of the MEPE-PHEX interaction (Campos et al., 2003). The MEPE-PHEX interaction in vivo may therefore prevent proteolytic release of the protease-resistant MEPE-ASARM peptide by protecting MEPE from localized matrix proteases. Moreover, PHEX is localized to the cell-surface plasma membrane of osteoblasts, and its long carboxy terminal region extends into the extracellular milieu, ideally situated for matrix protein interactions (Sabbagh et al., 2001). Also, several PHEX missense-mutations (C85R, G579R, and S711R) in HYP patients result in sequestration of the disease-causing mutant PHEX-proteins in the endoplasmic reticulum (ER) and failure in targeting to the plasma-membrane (Sabbagh et al., 2001, 2003). HYP mutation G579R lies one amino acid residue upstream from the PHEX catalytic domain of the Zn-binding motif HEXXH, and HYP mutation S711R lies in the conserved HSP motif shown to be important in the stabilization of the transition state (Dion et al., 1993). Thus, both mutations were expected to abrogate PHEX small-peptide catalytic activity. Remarkably, both mutations prevent PHEX targeting to the plasma-membrane and cause protein sequestration in the Golgi (Sabbagh et al., 2001, 2003).
PHEX plays a major role in mineralization and is expressed predominantly in bones and teeth. The bone expression is localized to osteoblasts, osteocytes, and odontoblasts but not pre-osteoblasts (Du et al., 1996; Beck et al., 1997; Guo and Quarles, 1997; Ruchon et al., 1998, 2000b; Thompson et al., 2002). There is temporal and coordinated expression of PHEX in vitro and in vivo during osteoblast differentiation, and loss of PHEX function results in defective mineralization (Ecarot-Charrier et al., 1988; Ecarot et al., 1992a; Ruchon et al., 1998, 2000b; Xiao et al., 1998; Ecarot and Desbarats, 1999; Miao et al., 2001; Shih et al., 2002; Thompson et al., 2002). PHEX also plays a major role in renal phosphate handling but is not expressed in the kidney, suggesting the secondary involvement of a circulating systemic factor. Recent studies confirm that PHEX expression in brain and PTH glands may also play a key role in phosphate homeostasis (Blydt-Hansen et al., 1999; Meyer and Meyer, 2000; Brewer et al., 2003).
In summary, although there is no direct in vivo evidence, it is tempting to speculate that PHEX and possibly KELL have evolved functionally to fulfill dual roles as oligopeptidases and ‘non-proteolytic’ protein-ligands. A supplementary protein-protein ‘non-protease’ role for PHEX and KELL is not unprecedented, since all M13 Zn-metalloendopeptidase family members form homodimer and/or heterodimer complexes, and some have no known physiological substrate (Lee et al., 1999; Turner et al., 2001). PHEX, for example, interacts non-proteolytically with osteocalcin as well as with MEPE (Boileau et al., 2001; Guo et al., 2002). Moreover, KELL-antigen (M13 family member) binds to XK protein and does not seem to have a confirmed physiological substrate, although it can act as an endothelin-converting enzyme (Lee et al., 1999, 2000, 2003). No substrates have been identified for XCE, a recently identified M13 family member (Schweizer et al., 1999; Valdenaire and Schweizer, 2000). The physiological substrate for PHEX also remains elusive (Quarles and Drezner, 2001). The compound complexity of the pathways and molecular interactions involved has been illustrated dramatically by recent HYP-mouse PHEX transgenic experiments. Specifically, two groups re-introduced normal wild-type PHEX into a HYP mouse (PHEX is the primary defect). Remarkably, this failed to cure the phosphate and mineralization phenotypes (Liu et al., 2002; Erben et al., 2003). The possible reasons for this will be discussed in the following sections.
(3) FGF23—an Extra-osseous and/or Osteoblastic Phosphatonin: ADHR and OHO
There are several excellent reviews that describe the evidence for the central involvement of FGF23 in several phosphate-wasting orders, and these include ADHR, HYP, OHO, and McCune-Albright syndrome (MAS), fibrous dysplasia of bone (FD), and humoral hypercalcemia of malignancy (Strewler, 2001; Fukumoto and Yamashita, 2002; Jan de Beur and Levine, 2002; Kronenberg, 2002; Kumar, 2002; Shimada et al., 2002; Drezner, 2003; Quarles, 2003; Tenenhouse and Murer, 2003). Using FGF-15 homology database searches, investigators originally identified FGF23 by in silico cloning (Yamashita et al., 2000). This was followed by the discovery of focused COOH-terminal FGF23 mutations in patients with ADHR (White et al., 2000). These activating mutations occur in a 176-RXXR-179 motif and confer resistance to furin-type convertases. The mutated protein is proposed to have an increased half-life, and the resulting full-length uncleaved form of FGF23 is phosphaturic in vivo and, in contrast to PTH-induced phosphaturia, does not lead to up-regulation of 1,25-vitamin D3 (Shimada et al., 2001, 2002; Saito et al., 2003; Yamashita et al., 2002). Also, CHO cells expressing FGF23 heterotransplanted into athymic nude mice elicit osteomalacia, depressed 1,25-vitamin D3 levels, and hypophosphatemia, closely duplicating the features of OHO (Shimada et al., 2001). Thus, full-length (uncleaved) FGF23 is an ideal candidate for a phosphaturic hormone or phosphatonin.
Original studies, although demonstrating in vivo hypophosphatemic effects with FGF23, were unable to confirm direct in vitro inhibition with the use of OK cells (Shimada et al., 2001). Subsequent studies, using identical cell-lines, have indicated either a heparin requirement for FGF23 phosphate-uptake inhibition activity (Yamashita et al., 2002) or heparin-mediated inhibition of FGF23 phosphate-uptake inhibition activity in vitro (Bowe et al., 2001). Moreover, FGF23 is reported to mediate phosphate-uptake inhibition via activation of a mitogen-activated protein kinase pathway via FGF receptor 3c in OK cells in vitro (Yamashita et al., 2002). Levels of renal Npt2 type IIa and type IIc Na-dependent phosphate co-transporter (Npt2a,b) mRNA and proteins are reduced in rat renal brush-border membranes (ex vivo), following in vivo over-expression with full-length mutant FGF23 (Segawa et al., 2003). The over-expression was achieved by direct injection of naked human FGF23 DNA from wild-type or mutant constructs. Since the defect in phosphate re-absorption in HYP mice is secondary to a decrease in renal abundance of type IIa and type IIc Na/Pi co-transporter (Npt2) mRNA and protein, this is compelling evidence for a link with aberrant processing of FGF23 in HYP (Tenenhouse et al., 2003; Tenenhouse and Murer, 2003).
Without doubt, inappropriately reduced serum 1,25-vitamin D3 plays a key secondary role in HYP. Recent elegant in vivo studies indicate that FGF23 acts on renal phosphate transport indirectly by influencing vitamin D metabolism and suppressing serum levels of 1,25-vitamin D3 (Shimada et al., 2004). Specifically, in mice given a single bolus tail-vein injection of recombinant FGF23, a dramatic reduction in 1,25-vitamin D3 was observed after 3 hrs, whereas a small reduction in serum PO4 occurred only after 9 hrs. Moreover, FGF23-mediated changes in serum 1,25-vitamin D3 were observed at doses as low as 0.18 μg, in dramatic contrast to the much higher doses (from 4.5 μg to 9 μg) required to elicit small changes in serum PO4. All the changes described were independent of PTH. The authors concluded that FGF23 may indirectly regulate serum phosphate by controlling extra-renal factors, and they excluded intestinal phosphate absorption as an alternative site of control. The FGF23-mediated changes in vitamin D metabolism were shown to be due to a suppression of the anabolic 25-hydroxyvitamin D (3)-1(alpha)-hydroxylase (1α-hydroxylase) and an increase in expression of the catabolic 24-hydroxylase. The mitochondrial renal 1α-hydroxylase is responsible for synthesis of the metabolically active 1,25-vitamin D3, and the 24-hydroxylase clears 1,25-vitamin D3 via the C24 oxidation pathway.
In HYP, catabolic 24-hydroxylase activity is markedly increased (activity, protein, and mRNA) (Roy et al., 1994). However, with regard to the 1α-hydroxylase and HYP, a more complex pattern has emerged. Recent experiments with HYP and normal kidney-homogenates have demonstrated a paradoxical increased expression of 1α-hydroxylase (mRNA) relative to normals, but an inappropriately reduced enzyme activity (Fujiwara et al., 2003). Moreover, phosphate-depleted normal animals exhibited a three-fold increase in 1α-hydroxylase activity that was accompanied by increased mRNA expression. In contrast, phosphate-depleted HYP mice did not exhibit an increase in 1α-hydroxylase enzyme activity but, remarkably, did exhibit increased mRNA expression that was identical in magnitude to that of phosphate-depleted normals. However, similar studies by other investigators, who used purified renal-mitochondria as opposed to whole-kidney homogenates, indicate an additional order of complexity (Azam et al., 2003). Specifically, HYP animals subjected to a normal diet also had increased 1α-hydroxylase mRNA, but, in contrast to the study where whole-kidney homogenates were used (Fujiwara et al., 2003), a commensurate increase in 1α-hydroxylase enzyme activity was recorded. Moreover, HYP animals subjected to a high-phosphorus diet continued to show a marked increase in 1α-hydroxylase mRNA and 1α-hydroxylase activity (mitochondrial) relative to normals. Remarkably, only in HYP animals fed a low-phosphorus diet was a suppression of 1α-hydroxylase mRNA and 1α-hydroxylase enzyme activity (renal mitochondrial) measured. Both studies (whole-kidney homogenate and renal mitochondrial) agree that 1α-hydroxylase mRNA is paradoxically elevated in HYP animals relative to normals when both are fed normal-phosphorus diets. Thus, there may well be a factor present in HYP whole-kidney homogenates that poisons or inhibits 1α-hydroxylase activity.
Clearly the dysfunctional changes in vitamin D metabolism in HYP, ADHR, and OHO are complex. However, the recent in vivo FGF23 experiments by Shimada et al. (2004) (discussed above) have shifted the paradigm and provided compelling evidence that the primary action of FGF23 is on vitamin D metabolism, and that the effects on phosphate could be secondary (independent of PTH) (Shimada et al., 2004). Also, analysis of recent data by Larsson et al. (2003) indicates that FGF23 levels do not change in response to variations in phosphate uptake in healthy volunteers, although serum FGF23 levels do increase in patients with impaired renal function (Larsson et al., 2003). Indeed, FGF23 could be a central conduit through which several hypophosphatemic disorders (notably HYP, ADHR, and OHO) are linked. In this regard, several investigators have reported an association between serum levels of FGF23 and phosphate levels in HYP, OHO, McCune-Albright syndrome (MAS), fibrous dysplasia of bone (FD), and humoral hypercalcemia of malignancy (Fukumoto and Yamashita, 2002; Shimada et al., 2002; Liu et al., 2003a; Riminucci et al., 2003; Singh and Kumar, 2003; Weber et al., 2003). FGF23 appears to be elevated in some but not all cases of OHO, and although there is no statistical correlation with circulating FGF23 in HYP patients, there is a small but significant correlation reported for the degree of hypophosphatemia (Yamazaki et al., 2002; Jonsson et al., 2003; Larsson et al., 2003; Weber et al., 2003). The diffuse correlation between FGF23 and HYP could well be due to the FGF23 ‘primary’ effect on vitamin D metabolism and a secondary bone-renal dynamic effect on serum phosphate.
Key to the putative HYP, FGF23 association is the primary defect in HYP, a Zn metalloendopeptidase (PHEX) described earlier (HYP Consortium et al., 1995; Rowe et al., 1996a, 1997; Francis et al., 1997). This led to the proposal that full-length FGF23 (phosphaturic) is normally inactivated by PHEX, and that the mutated ADHR FGF23 protein is resistant to PHEX proteolysis (Bowe et al., 2001; Kumar, 2002). An initial study, by an indirect approach, confirmed that mutated ‘ADHR’ FGF23 was resistant to PHEX cleavage, and that wild-type FGF23 is sensitive to PHEX cleavage (Bowe et al., 2001). However, other studies, with direct techniques, have failed to demonstrate cleavage of full-length FGF23 or peptides by PHEX (Liu et al., 2003a). Although it now seems unlikely that PHEX cleaves FGF23 (mutant or wild-type), it is possible that PHEX has a secondary impact on the processing of FGF23, and/or affects the levels of FGF23 expression (Liu et al., 2003a,b). This could occur at extra-osseous sites, particularly since FGF23 expression was primarily localized to the ventrolateral thalamic nucleus of the brain, and PHEX is also expressed in the brain and parathyroid glands (Blydt-Hansen et al., 1999; Meyer and Meyer, 2000; Meyer et al., 2000; Yamashita et al., 2000).
(4) Matrix Extracellular PhosphoglycoprotEin (MEPE): Osteoblastic Phosphatonin & Minhibin
Matrix Extracellular PhosphoglycoprotEin (MEPE) was first cloned from a tumor resected from a patient with tumor-induced osteomalacia (OHO) (Rowe et al., 2000). MEPE belongs to a distinct family of proteins that have recently been named Short Integrin Binding Ligand Interacting Glycoproteins (SIBLINGs) (Rowe et al., 2000; Fisher et al., 2001). These proteins (MEPE, osteopontin [OPN], dentin-matrix-protein-1 [DMP-1], bone-sialoprotein [BSP], dentin-sialophosphoprotein [DSPP], and enamelin [ENM]) all map to 4q21 and have many shared features. These include RGD motifs, protein glycosylation, phosphorylated residues (serines), similar genomic structures, and ASARM motif in diverse structural contexts (OPN, MEPE, DMP-1, and DSPP), and all have links to bone-dentin mineralization and/or phosphate calcium homeostasis (Fig. 2) (Rowe et al., 2000; Fisher et al., 2001). MEPE was originally cloned by expression-screening of an OHO-tumor cDNA library with polyclonal antibodies that neutralized OHO tumor-secreted renal-phosphate-uptake-inhibiting factor(s) (Rowe et al., 1996b, 2000). This was followed by the cloning of rat MEPE (OF45) and mouse MEPE (Argiro et al., 2001; Petersen et al., 2000). The MEPE null-mutant mouse has increased bone mass, resistance to aging-associated trabecular bone loss, increased mineralization-apposition rate (MAR), and a dramatically accelerated mineralization rate in ex vivo osteoblast cultures (Gowen et al., 2003). MEPE is markedly up-regulated in X-linked hypophosphatemic rickets HYP-osteoblasts and OHO-tumors and is exclusively expressed in osteoblasts, osteocytes, and odontoblasts (Rowe et al., 1996b, 2000, 2004; Petersen et al., 2000; Argiro et al., 2001; Bai et al., 2002; De Beur et al., 2002; Guo et al., 2002; MacDougall et al., 2002; Gowen et al., 2003). We have recently confirmed that MEPE inhibits phosphate-uptake and mineralization in vivo and in vitro (Dobbie et al., 2003; Rowe et al., 2004). MEPE-mediated in vivo phosphaturia in rodents can be induced via bolus administration or infusion (Dobbie et al., 2003; Rowe et al., 2004). The in vitro mineralization-inhibition observed with MEPE is mediated by a short cathepsin-B-released carboxy-terminal MEPE-peptide (ASARM peptide), and this peptide likely also inhibits phosphate uptake (Rowe et al., 2000, 2004).
Figure 2.
Salivary-statherin & MEPE consensus ASARM motif: mineralization-inhibition and ancestral genes on chromosome 4. MEPE, DMP-1, and the SIBLINGs are related to an ancestral mineralization-gene (salivary statherin) that is also thought to function in the transport of calcium and phosphate in salivary glands. Statherin maps to chromosome 4 in the SIBLING/MEPE region and also contains an ASARM motif. (A) A clustal alignment of the COOH terminal region of human-DMP-1, human-MEPE, mouse-MEPE, and rat-MEPE with human-statherin (62-residue protein). In MEPE and DMP-1, the ASARM peptide occupies the most distal COOH-region of the molecule and is highlighted with a boxed cartouche labeled ‘MEPE ASARM peptide’. The boxed cartouche labeled as ‘statherin-ASARM peptide’ contains the sequence shown to play a biological role in inhibiting spontaneous precipitation of supersaturated salivary calcium and phosphate and maintaining the mineralization dynamics of tooth enamel (Schlesinger and Hay, 1977; Bennick et al., 1981; Raj et al., 1992; Long et al., 1998). Statherin is also thought to function in the transport of calcium and phosphate in salivary glands (Raj et al., 1992). In both MEPE and statherin, cathepsin-B and/or general protease cleavage results in the release of MEPE and statherin protease-resistant ASARM peptides. Both MEPE and statherin ASARM peptides are phosphorylated, protease-resistant, acidic and highly charged molecules with low pI’s. They also share biological properties. For example, a feature of the MEPE ASARM region is the repeat (D) SSES/E sequence. This short sequence has been shown to be a key inhibitor of hydroxyapatite crystal formation and mineralization in salivary statherin (Raj et al., 1992; Long et al., 1998). (B) Schematic presentation of the remarkable clustering of MEPE, DMP-1, statherin, and other SIBLING genes on chromosome 4.
Dramatic changes in serum MEPE (41% drop) have also been reported in healthy subjects given high-phosphate diets (Allen et al., 2003). The antibodies used to screen circulating MEPE were not specific for the full-length molecule and likely not specific for the carboxy-terminal MEPE ASARM peptide. Thus, the dramatic reduction reported in circulating MEPE may reflect an increase in MEPE expression, accompanied by increased proteolytic turnover and release of protease-resistant ASARM peptide, and is consistent with the ASARM model (see HYP below for fuller discussion). This is supported by the finding that 1,25-vitamin D3 suppresses MEPE expression in rodents, and that levels of 1,25-vitamin D3 are reduced in individuals on high-phosphate diets (Argiro et al., 2001). Also, the vitamin D receptor null-mutant mouse has markedly increased levels of mRNA MEPE expression (Okano et al., 2003). In summary, there is strong evidence that the MEPE ASARM peptide plays a key role in modulating mineralization and likely affects renal-phosphate handling via a unique PHEX-MEPE protein-protein interaction and coordinately inhibits mineralization (minhibin) (Guo et al., 2002; Rowe et al., 2004). This is discussed further below.
(5) MEPE, PHEX, STATHERIN, & ASARM peptide: Mineralization in Bones, Teeth, and Saliva
(5a) PHEX protects MEPE from protease cleavage
As discussed earlier, the physiological substrate for PHEX remains elusive, and although small synthetic peptides of FGF23 and MEPE are substrates, several studies have failed to confirm cleavage of full-length FGF23 and/or MEPE (Guo et al., 2002; Campos et al., 2003; Liu et al., 2003a). Remarkably, PHEX and a mutated carboxy-terminal PHEX-fragment protect MEPE from cathepsin-B cleavage in vitro. Also, cathepsin-B is expressed in the osteoblast (Guo et al., 2002; Aisa et al., 2003), and cathepsin-B cleavage of MEPE results in the specific release of ASARM peptide (Figs. 1, 2A) (Rowe et al., 2004). The cleavage sites are highly conserved in all cloned species (mouse, rat, monkey, and human), and the ASARM peptide is extraordinarily resistant to a vast array of proteases, including carboxypeptidases (B/T), cathepsins-(BDGK), trypsin, granzyme-A, papain, pepsin, kallikrein, plasmin, nardilysin, NEP, and ECEL1/DINE. Interestingly, the renal-phosphate inhibitory activity of conditioned media from OHO tumors cannot be inactivated by proteolytic digestion with trypsin, proteinase K, and/or papain (Miyauchi et al., 1988; Wilkins et al., 1995; Jonsson et al., 2001). However, these enzymes efficiently abolish the capacity of PTH and parathyroid extracts to inhibit renal phosphate uptake in vitro (Jonsson et al., 2001). Size ultrafiltration experiments also indicate that tumor phosphatonin-minhibin activity is due to small (< 5 kDa) protease-resistant molecules (Miyauchi et al., 1988; Cai et al., 1994; Wilkins et al., 1995; Lajeunesse et al., 1996; Nelson et al., 1996, 2001; Jonsson et al., 2001). These findings are consistent with the notion that the small proteolytically resistant ASARM peptide is a phosphatonin and/or minhibin (Rowe et al., 2004). Of direct relevance is the finding that cathepsin D, neprilysin (NEP), and ECEL1/DINE proteases are markedly up-regulated in HYP-mice osteoblasts and bone marrow stromal cells (BMSC) (Jo et al., 2000, 2001; Dubois et al., 2002). Indeed, these authors reported a strong correlation between the inhibition of P(i) uptake by conditioned media (CM) from HYP cells and elevated NEP-like activities. Other investigators also confirm NEP mRNA and protein expression in mouse bone tissue, including bone-forming cells, osteoblast precursors, pre-osteoblasts, osteoblasts, and osteocytes (Ruchon et al., 2000a). Moreover, cathepsin D has been reported to have an inhibitory effect on osteoblast cell mineralization, and an improvement of the HYP-mouse mineralization defect occurs on the addition of pepstatin (a cathepsin-D inhibitor) (Jo et al., 2000, 2001). Also, incubating HYP osteoblasts with phosphoramidon (protease inhibitor) prevents the production of an osteoblast-secreted inhibitor of renal phosphate (P[i]) uptake (Jo et al., 2000, 2001; Dubois et al., 2002). This is consistent with a protease-inhibitor (phosphoramidon, pepstatin)-mediated reduction in free ASARM peptide in the HYP osteoblast.
Thus, the reported massive up-regulation of MEPE, the excess protease expression, and the lack of functional PHEX would collectively increase the levels of MEPE ASARM peptide dramatically in HYP (see Fig. 1). This in turn would cause the observed periosteocytic defects in mineralization, leading to rickets, dental abnormalities, and/or osteomalacia. Of additional interest is the finding that normal human-osteoblast cathepsin-B production, secretion, and activity are markedly stimulated by interleukin-β-1, PTH, and dexamethasone bone-resorbing agents (Oursler et al., 1993; Aisa et al., 1996).
(5b) Osteopontin, statherin, supersaturated calcium phosphate, saliva & ASARM peptide
A remarkable association to an ancestral mineralization gene is provided by our observation that statherin also contains an ASARM motif (Rowe et al., 2004) and maps to the same region of chromosome 4q21 (Figs. 2A, 2B). Statherin, a small 63-residue salivary protein, maintains mineral solution dynamics of enamel by virtue of its ability to inhibit spontaneous precipitation and crystal growth from supersaturated solutions of calcium phosphate minerals (Raj et al., 1992; Schwartz et al., 1992; Long et al., 1998). Statherin’s role in preserving the calcium-phosphate supersaturated state of saliva is crucial for re-calcification and stabilization of tooth enamel and for the inhibition of formation of mineral accretions on tooth surfaces. In addition, statherin has been proposed to function in the transport of calcium and phosphate during secretion in the salivary glands (Raj et al., 1992). As with the MEPE ASARM peptide, a single cathepsin-B site is present in statherin that would potentially release the highly charged and phosphorylated aspartate-serine-rich statherin ASARM peptide. Recently, an osteopontin-derived ASARM peptide was found to be a potent inhibitor of calcium-oxalate formation and mineralization (Hoyer et al., 2001; Wesson et al., 2003), and, for the first time, we demonstrated, in a biological system, that the MEPE ASARM peptide is a powerful inhibitor of BMP2-mediated mineralization of murine-osteoblast (2T3) cells in vitro (Rowe et al., 2004).
(5c) MEPE null-mutant has increased bone and mineralization apposition rate
If the proposed ASARM peptide model is valid, then a MEPE knockout should result in increased mineralization. Recently, an elegant study by Gowen et al. (2003) described a MEPE (OF45) knockout. The animal has increased bone mass, and increased numbers and thickness of trabeculae and cortical bone mass. In vitro, osteoblasts exhibit an accelerated mineralization rate, and in vivo, the mineralization apposition rate (MAR) is dramatically increased compared with that in normals. This is consistent with MEPE’s proposed role as a controller of the rates of development and sizes of mineral structures (ASARM peptide), as well as the bone formation rate. The null change in serum phosphate is consistent with the ASARM model and is described in more detail below. (See Section 6e, ‘MEPE null-mutant model’.) Interestingly, a more detailed FTIRM analysis of the osteopontin (Opn) knockout (osteopontin ‘ASARM peptide’ inhibits calcification of oxalate; Hoyer et al., 2001) revealed that the relative amounts of mineral in the more mature areas of bone (central cortical bone) are significantly increased in these animals (Boskey et al., 2002). Moreover, mineral maturity (mineral crystal size and perfection) throughout all anatomic regions of the Opn-deficient bone is significantly increased, together with hyperoxaluria-induced renal-stone formation (Boskey et al., 2002; Wesson et al., 2003). Further experiments are needed to determine whether this phenotype is also exhibited to a greater or lesser extent in the MEPE knock-out, confirming the ASARM peptide model.
(6) Global ASARM Hypothesis
Fig. 1 presents a summarized scheme of the proposed interaction among PHEX, MEPE, and proteases in HYP. PHEX may regulate mineralization by modulating proteolytic activation/inactivation of MEPE and perhaps other matrix-proteins (Fig. 2) and may coordinately regulate the release of ASARM peptide(s) (mineralization-inhibitor). Specifically, PHEX may sequester and thus transiently protect MEPE from extracellular-matrix proteases. The PHEX-mediated sequestration and protection of MEPE are then proposed to result in a decrease in the release of protease-resistant MEPE-ASARM peptide. Although the physiological substrate for PHEX remains elusive, the possibility of an indirect modulation of MEPE processing and/or release of ASARM peptide by PHEX cannot be excluded. PHEX may also have a dual role as a highly specific oligopeptidase and matrix-protein-ligand (discussed in Section 2). The following global scheme takes into account the diverse factors required for mineralization, inhibiting mineralization, and bone remodeling. Moreover, the dichotomy of osteoblastic factors and extra-osseous factors involved in regulating renal phosphate handling and mineralization is also considered.
Figs. 3 to 7 collectively depict a global hypothesis that attempts to explain the observed bone-renal physiology, bone-mineral loss disorders, defective renal phosphate handling, and diverse mouse models (null-mutants, transgenics, and double-null/transgenic mice) available and predicted. The Table presents an index with key iconic features used for specific molecules depicted in Figs. 3 to 7. In all Figs. (3 to 7), the lower left part of the scheme represents the osteoblast (wavy line), the upper left the ventrolateral-thalamic nucleus (VLTN), or, less specifically, the brain. Letters encircled at different points in each diagram are specific reference points for descriptions in the text. For example, reference point A in Fig. 3 will be referred to as Fig. 3A when discussed in the text. The upper right portion of the schemes represents the kidney, and the central cartoon depicts the upper femur. The VLTN, or brain, is also representative of extra-osteoblastic sites of FGF23 (VLTN) and PHEX (brain) expression. FGF23 expression occurs in the VLTN region of the brain, and expression in bone and other tissues is very low, as indicated earlier (White et al., 2000; Yamashita et al., 2000; Guo et al., 2001, 2002; Bai et al., 2002; Liu et al., 2003a; Quarles, 2003). Within the cartoon representing bone (specifically the growth plate of the femur encompassing the epiphyseal, metaphyseal, and diaphyseal regions) is a white region representative of osteoid or unmineralized bone-matrix. The size of the osteoid in the different models is schematically representative of the degree of mineralization. The condition described in each Fig. is indicated in a box above the representation of the femur. Finally, an animated scheme of the ASARM model (flash-media) can be accessed at the following url: http://periodontics.uthscsa.edu/rowe/asarm-modelandfgf23.html.
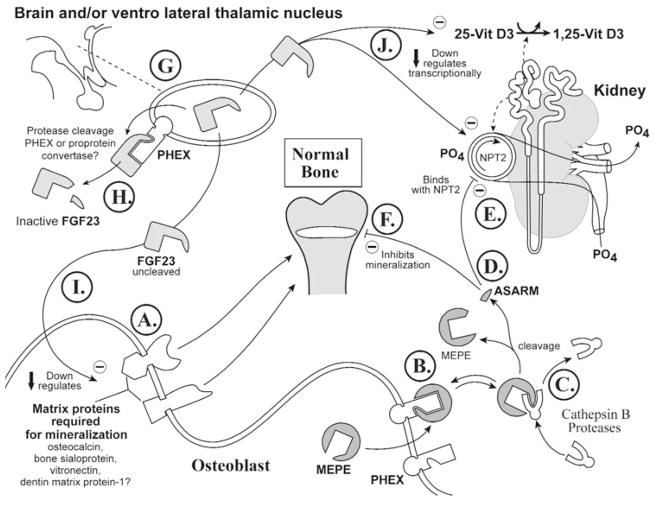
Figure 3.
Normal physiology. A scheme representing the proposed dynamic and interactive roles of PHEX, FGF23, MEPE, and matrix proteins in maintaining normal tooth-bone-renal-mineralization and phosphate homeostasis. See text for detailed description (specifically, the sub-paragraph entitled ‘Normal Physiology [Global Hypothesis]’). Also refer to the Table for more details concerning the icons used to represent the diverse molecules, pathways, and tissues.
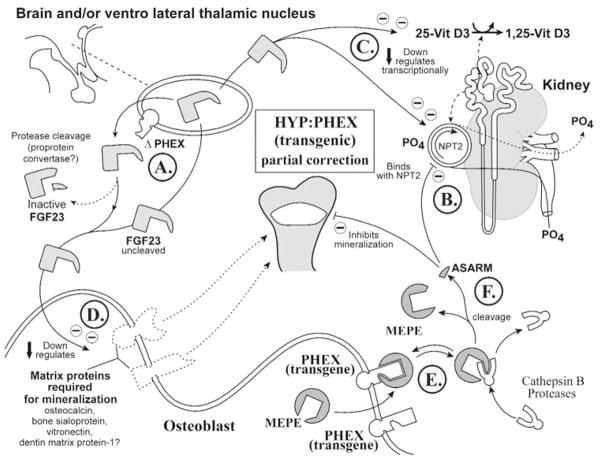
Figure 7.
The phex transgenic hyp-mouse: hyp-mice with re-introduced wild-type phex genes have partially corrected mineralization phenotypes and remain hypophosphatemic. See text for a full explanation of the proposed model for the experimental observations (specifically, the subparagraph entitled ‘HYP-PHEX transgenic [partial correction of mineralization phenotype] [Global Hypothesis]’). Also refer to the Table for more details concerning the icons used to represent the diverse molecules, pathways, and tissues.
TABLE.
Summarized Index Showing Representative Icons and Detailed Descriptions for Specific Molecules and Features Depicted in Figs. 3–7
| Molecule, Hormone, Matrix-protein, Enzyme, Protein, and Description | Normal | Cleaved | Mutated | Reduced Expression |
|---|---|---|---|---|
| FGF23: Full-length FGF23 is the active form (inhibits phosphate uptake and mineralization in vivo). Cleaved FGF23 is inactive, and a COOH terminal mutation(s) in ADHR is proposed to increase resistance to proteolysis and thus full-length protein stability (activating mutation). FGF23 may also play a role in OHO and HYP. FGF23 cleavage by PHEX is equivocal, although processing may be influenced indirectly by PHEX. |  |
 |
 |
NA |
| MEPE: Cleavage of MEPE results in release of ASARM peptide. The ASARM peptide is resistant to proteolysis (most known proteases) and is very stable. MEPE expression is elevated in HYP and is secreted by OHO tumors. Moreover, ASARM peptide inhibits mineralization. In contrast, MEPE ASARM peptide, when part of a matrix-protein (uncleaved from MEPE-protein) and bound to extracellular matrix, may well be required as a nucleator/enhancer of mineralization. Elevated MEPE expression and protease activity in HYP are predicted to result in increased levels of free ASARM peptide. Moreover, free ASARM peptide may also contribute to the inhibition of renal phosphate-uptake (see below). |  |
 |
NA | NA |
| Matrix-proteins required for mineralization (osteocalcin, bone sialoprotein, vitronectin, DMP-1): These proteins are down-regulated in HYP, and the DMP-1 null-mutant has a rickets-like bone phenotype. Of relevance to the functional role of the ASARM peptide present in DMP-1, MEPE, BSP, and OPN is the finding that mineralization enhancement occurs when matrix proteins such as DMP-1 and DSP are irreversibly bound to matrix (collagen). In contrast, unbound matrix phosphoproteins inhibit mineralization. This may well be analogous to the presence of free, protease-resistant, acidic phosphorylated ASARM peptide in Hyp (minhibin osteoblastic-phosphatonin). | 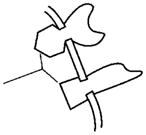 |
NA | NA | 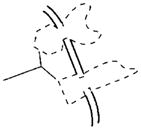 |
| PHEX: This gene is mutated in Hyp, and evidence suggests that one of its functions is to protect MEPE from proteolytic cleavage and thus release of ASARM peptide. PHEX may also indirectly influence FGF23 processing at an extra-osseous site. Although low-level expression of FGF23 does occur in bone, there are no reports of FGF23 expression in primary osteoblasts. However, low-level FGF23 expression has been reported in an SV40 transgenic (stably transformed) normal mouse osteoblastic cell-line (NORM-SV40). Moreover, FGF23 expression in an Hyp SV40 transgenic Hyp-mouse osteoblastic cell-line (Hyp-SV40) was relatively increased. Also, unlike MEPE, there is no correlation with osteoblast developmental expression and FGF23 expression. Direct PHEX cleavage of FGF23 is equivocal (see text for further discussion). | 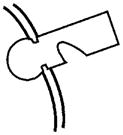 |
NA | 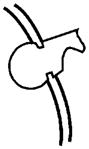 |
NA |
| ASARM peptide (MEPE): In free form, this peptide inhibits mineralization. As part of a matrix protein (MEPE, DMP-1) bound to extracellular matrix, it may well be required as a nucleator/enhancer of mineralization. The ASARM peptide is resistant to most known proteases and is very stable. Elevated MEPE expression and protease activity in HYP are predicted to result in increased levels of ASARM peptide. Moreover, evidence that PHEX protects MEPE from proteolysis suggests that this may also contribute to the increased levels of ASARM peptide in HYP (defective PHEX). Free ASARM peptide may also contribute to the inhibition of renal-phosphate uptake. The renal-phosphate inhibition is likely steric and would exacerbate transcriptional effects on NPT2 expression mediated directly or indirectly by other factors (FGF23). |  |
NA | NA | NA |
| Proteases/Cathepsin B: Protease ECEL-1/DINE, NEP, and cathepsin D are markedly elevated in HYP osteoblasts and bone marrow stem cells. In combination with the documented increased in MEPE expression, this may contribute to a marked elevation of free MEPE ASARM peptide in Hyp. The ASARM peptide is resistant to proteolysis. |  |
NA | NA | NA |
(6a) Normal physiology (see Fig. 3)
In this scheme (see Fig. 3A; osteoblast; lower left), the proteins required for mineralization (osteocalcin, bone sialoprotein, vitronectin, and DMP-1) are in dynamic balance with the ASARM peptide, an inhibitor of mineralization (Rowe et al., 2004). These proteins (required for mineralization) are down-regulated in rickets, and the DMP-1 null-mutant is reported to have a bone phenotype reminiscent of rickets (defective, hypomineralization) (Miao et al., 2001, 2004; Huang et al., 2003). In contrast, osteoblast-expressed MEPE protein is up-regulated in rickets, and there is in vitro, ‘indirect’, evidence suggesting that PHEX may sequester MEPE and/or other matrix proteins (osteocalcin) on the plasma-membrane (see Fig. 3B; cell-surface of the osteoblast) (Argiro et al., 2001; Boileau et al., 2001; Sabbagh et al., 2001; Bai et al., 2002; Guo et al., 2002; Liu et al., 2003a). Analysis of the same in vitro data indicates that PHEX-sequestered MEPE is protected from matrix proteases. The PHEX sequestration is likely reversible, and free unbound-MEPE is vulnerable to cleavage by extracellular matrix proteases/cathepsin B (see Fig. 3C). In HYP, these proteases (ECEL1/DINE, NEP, cathepsin D) are markedly elevated, as is MEPE (Jo et al., 2000, 2001; Argiro et al., 2001; Bai et al., 2002; Dubois et al., 2002; Guo et al., 2002; Liu et al., 2002, 2003a). Cleavage of MEPE would result in the release of the protease-resistant stable ASARM peptide (Figs. 3C, 3D). Free ASARM peptide may also sterically inhibit the NPT2 co-transporter (see Fig. 3E; kidney, upper right) by direct binding. Whether this NPT2 interaction occurs during normal physiology or is one of the pathophysiological factors that contributes to the inhibition of phosphate uptake in HYP and/or OHO remains to be resolved experimentally.
FGF23 expression occurs in the brain and, more specifically, in the ventrolateral-thalamic nucleus (Yamashita et al., 2000) (see Fig. 3G, upper left), and PHEX may well indirectly influence the cleavage and inactivation of FGF23 by pro-protein convertases (Fig. 3H). There are no specific reports of PHEX expression in the VLTN, but PHEX is ubiquitously expressed, and there is high-level expression in the parathyroid glands and brain (Blydt-Hansen et al., 1999; Meyer and Meyer, 2000; Brewer et al., 2003). Indeed, in one study, the levels of brain PHEX mRNA expression in growing animals was markedly higher than in calvariae and femurs (Meyer and Meyer, 2000). Moreover, this same study reported a marked and significant increase in PHEX mRNA expression in the brains of animals fed a low-phosphate diet. PHEX expression in the parathyroid glands is also altered in some unique cases of patients with tertiary hyperparathyroidism (Blydt-Hansen et al., 1999). These authors reported that PHEX mRNA levels were several-fold greater in parathyroid glands from two patients and suggested that PHEX may also have a role in the normal regulation of PTH (Blydt-Hansen et al., 1999). Thus, indirect regulation of FGF23 by PHEX may also occur in the brain or parathyroid glands, although studies are needed to confirm this.
Full-length uncleaved FGF23 molecule may indirectly/directly down-regulate the proteins required for mineralization (Figs. 3I, 3A; osteoblast, lower left). The proteins required for mineralization depicted in the scheme are all down-regulated in HYP, and the DMP-1 null-mutant mouse is reported to have a defective bone-tooth mineralization phenotype (Miao et al., 2001, 2004; Huang et al., 2003). In contrast, MEPE (mineralization inhibitor via ASARM peptide) expression is elevated in HYP (Argiro et al., 2001; Bai et al., 2002; Guo et al., 2002; Liu et al., 2003a). Thus, FGF23 and/or other cytokines are likely directly/indirectly responsible for the changes in expression of these proteins. Specifically, several experiments have shown that full-length FGF23 administered in vivo causes rickets and defective mineralization (Shimada et al., 2001, 2002, 2004; Bai et al., 2003; Segawa et al., 2003). FGF23-mediated mineralization-inhibition has not been reported in vitro, suggesting an indirect FGF23 signal-mediated mechanism that could be modulated indirectly by PHEX at extra-osseous sites (possibly brain or parathyroid glands; Fig. 3H). Also, full-length FGF23 indirectly or directly suppresses expression of the NPT2 phosphate co-transporter (NPT2), suppresses expression of 1α-hydroxylase, and increases expression of 24-hydroxylase (decrease in serum 1,25-vitamin D3) (Fig. 3J) (Shimada et al., 2001, 2004; Bai et al., 2003; Segawa et al., 2003).
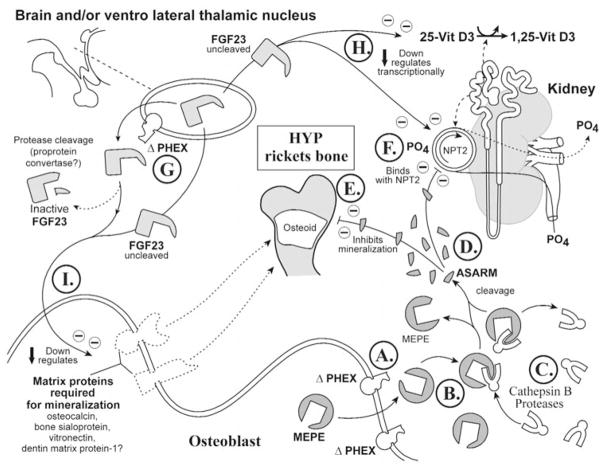
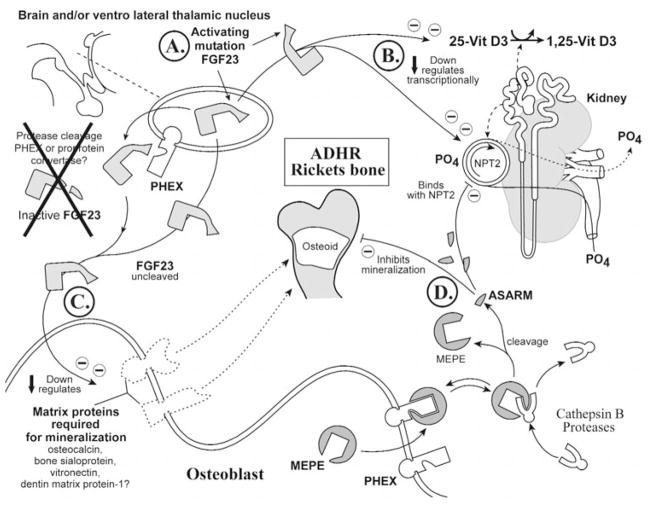
(6b) X-linked hypophosphatemic rickets (Hyp) (Fig. 4)
Figure 4.
X-linked rickets (Hyp). A cartoon illustrating the proposed changes in X-linked hypophosphatemic rickets. The primary defect in this disease is the PHEX gene, and this has an impact on renal phosphate handling, vitamin D metabolism, and mineralization. For comparison with normal states, see Fig. 3. See text for detailed description (specifically, the subparagraph entitled ‘X-linked hypophosphatemic rickets [Hyp] [Global Hypothesis]’). Also refer to the Table for more details concerning the icons used to represent the diverse molecules, pathways, and tissues.
In Hyp/HYP, PHEX is defective, as depicted in the lower right panel (osteoblast) and upper-left panel, depicting the brain, parathyroid glands, and/or ventrolateral-thalamic nucleus (VLTN) (Figs. 4A, 4G; see Table for iconography). Also, the levels of MEPE expression are dramatically increased in Hyp osteoblasts relative to normal osteoblasts (Fig. 4B) (Argiro et al., 2001; Bai et al., 2002; Guo et al., 2002; Liu et al., 2003a). The ‘ASARM model’ would predict that PHEX sequestration of MEPE does not occur due to mutated PHEX, and this results in increased MEPE vulnerability to proteolysis (Fig. 4A). Also, protease (ECEL1/DINE, NEP, cathepsin D) expression is markedly increased in Hyp (Fig. 4C) (Jo et al., 2000, 2001; Dubois et al., 2002).
The increased MEPE and protease expression and loss of PHEX sequestration (protection) of MEPE result in the generation of large quantities of protease-resistant ASARM peptide (Fig. 4D; pathological). The free ‘rogue’ ASARM peptide (highly stable and resistant to proteases) locally inhibits mineralization of osteoid (Fig. 4E; large osteoid seam depicted). Given the physiocochemical properties shared with the bisphosphonates and PFA (acidic, phosphorylated, low pI, highly charged, hydrophilic, and small size), the ASARM peptide may alter phosphate-uptake and mineralization via a similar mechanism. Indeed, inhibition of mineralization is likely somewhat analogous to the inhibition elicited by bisphosphonates (Bonjour et al., 1975; Trechsel et al., 1977; McCloskey et al., 1988) and has been reported for osteopontin, statherin, and MEPE ASARM peptide (Raj et al., 1992; Gururaja and Levine, 1996; Hoyer et al., 2001; Tamaki et al., 2002; Rowe et al., 2004). Moreover, circulating ASARM peptide likely inhibits renal phosphate uptake by directly binding (reversibly) to NPT2, again in a manner also proposed to be analogous to bisphosphonates and phosphonoformic-acid (Fig. 4F) (Loghman-Adham et al., 1987, 1992; VanScoy et al., 1988; Loghman-Adham and Dousa, 1992). Remarkably, in vivo or in vitro, PFA and bisphosphonates can elicit dual actions on phosphate-uptake (inhibition or enhancement) (Walton et al., 1975; Muhlbauer et al., 1981; Loghman-Adham and Dousa, 1992). This dual biological response is dependent on mode of administration.
FGF23 may also play a key role in the pathophysiology of HYP. Specifically, inappropriate processing of FGF23 at an extra-osseous site will directly or indirectly suppress NPT2 gene expression (Fig. 4G). Thus, the direct phosphate-inhibitory effect of free ASARM peptide is compounded by FGF23 (Figs. 4D, 4F). Also, as previously mentioned, matrix proteins (required for mineralization) are suppressed and MEPE expression is increased in Hyp (Argiro et al., 2001; Miao et al., 2001; Bai et al., 2002; Guo et al., 2002; Liu et al., 2003a). This is also likely mediated directly or indirectly by FGF23 (Figs. 4I, 4B). Finally, full-length unprocessed FGF23 directly alters vitamin D metabolism, as depicted in the scheme (Fig. 4H).
(6c) Autosomal-dominant hypophosphatemic rickets (ADHR) (Fig. 5)
Figure 5.
Autosomal-dominant hypophosphatemic rickets (ADHR). Global scheme proposing the changes in autosomal-dominant hypophosphatemic rickets (ADHR). See text for detailed description (specifically, the subparagraph entitled ‘Autosomal-dominant hypophosphatemic rickets [ADHR] [Global Hypothesis]’). Also refer to the Table for more details concerning the icons used to represent the diverse molecules, pathways, and tissues.
As discussed earlier, in ADHR, mutated FGF23 results in increased stability and half-life of the full-length, phosphaturic, FGF23 molecule (activating mutation; Fig. 5A, upper left VLTN/brain). This is proposed to suppress expression of proteins required for mineralization (Fig. 5C), increase expression of proteins inhibiting mineralization (MEPE, ASARM peptide; Fig. 5D), inhibit renal phosphate uptake and 1α-hydroxylase expression, and/or increase 24-hydroxylase activities (Fig. 5B). There is no direct evidence for PHEX-mediated proteolytic inactivation of FGF23, although processing of FGF23 by PHEX may occur indirectly (see Section on FGF23 for fuller discussion) (Liu et al., 2003a; Quarles, 2003). Moreover, as discussed earlier, there is no direct evidence that FGF23 directly effects expression of matrix proteins. However, expression of these proteins is altered in Hyp (no published data for ADHR), and some studies correlate FGF23 levels with the degree of hypophosphatemia in Hyp.
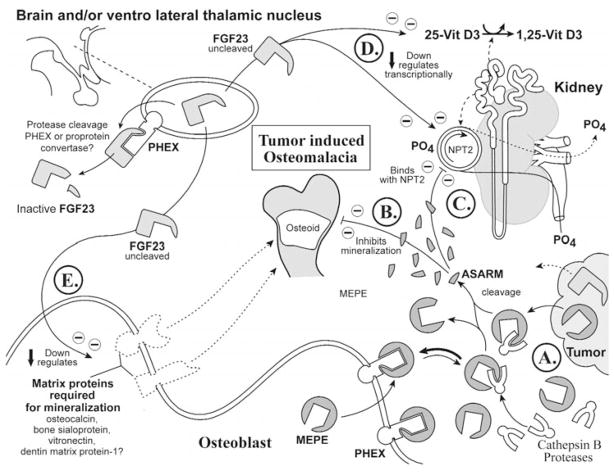
(6d) Tumor-induced osteomalacia (OHO) (Fig. 6)
Figure 6.
Oncogenic hypophosphatemic hypophosphatemia (OHO). Global scheme proposing the changes in oncogenic hypophosphatemic osteomalacia, also known as tumor-induced osteomalacia (OHO). See text for detailed description (specifically, the subparagraph entitled ‘Tumor-induced osteomalacia [OHO] [Global Hypothesis]’). Also refer to the Table for more details concerning the icons used to represent the diverse molecules, pathways, and tissues.
Over-expression of MEPE and thus ASARM peptide and/or FGF23 has an adverse impact on renal phosphate handling, vitamin D metabolism, and mineralization, as depicted in the scheme (Fig. 6A; see lower right for tumor representation). Of interest is the finding that MEPE is expressed in all OHO tumors screened to date and is notably absent from non-phosphaturic tumors (Rowe et al., 2000; Seufert et al., 2001; Shimada et al., 2001; De Beur et al., 2002). Moreover, as discussed earlier, DMP-1 is also over-expressed by many of these tumors (Shimada et al., 2001; De Beur et al., 2002) and also has a carboxy-terminal ASARM motif (Rowe et al., 2000) (Fig. 1). Thus, extensive proteolysis and release of the protease-resistant ASARM peptide may well mediate the pathophysiological changes in specific MEPE- and DMP-1-expressing tumors (Figs. 6B, 6C). FGF23-expressing tumors are predicted to result in a similar inhibition of mineralization and hypophosphatemia via a different mechanism, as discussed earlier (see HYP, ADHR, and also Figs. 6D, 6E). The observation that not all phosphaturic OHO tumors express FGF23 but all screened tumors (to date) do express MEPE (Rowe et al., 2000; Seufert et al., 2001; Shimada et al., 2001; De Beur et al., 2002) suggests that MEPE could be downstream of FGF23 in the phosphate/mineralization signaling cascade and supports the ASARM model. However, this is speculative and remains to be confirmed experimentally.
Proteolysis of FGF23 leads to inactivation of phosphaturic activity, and, as previously discussed, some but not all OHO tumors are predicted to secrete large quantities of wild-type FGF23 that overwhelm the cellular proteolytic machinery (see section on FGF23). Moreover, there is strong evidence, from several groups, of the involvement of tumor-secreted phosphaturic factors of diverse sizes (Aschinberg et al., 1977; Popovtzer, 1981; Miyauchi et al., 1988; Cai et al., 1994; Wilkins et al., 1995; Lajeunesse et al., 1996; Nelson et al., 1996, 1997, 1998, 2001; Rowe et al., 1996b, 2000; Jonsson et al., 2001). In this regard, the majority of the reports directly implicate low-molecular-weight protease-resistant peptides and confirm the seminal findings by Miyauchi et al. (1988) concerning the phosphaturic effects of heterotransplanting tumors into nude mice. For example, Jonsson et al. (2001) partially purified a low-molecular-weight phosphaturic factor (high-pressure liquid chromatography, etc.) from four OHO tumors. The phosphate-uptake inhibiting activity (OK renal cells, in vitro) of the low-molecular-weight tumor-factor was resistant to the following proteases: trypsin, proteinase K, and papain. In contrast, the ability of PTH and parathyroid extracts to inhibit renal phosphate was abolished by treatment with the same enzymes (Jonsson et al., 2001). The HPLC characteristics they described for this molecule are remarkably similar to those of the synthetic MEPE ASARM peptide (unpublished observations). Full-length FGF23 is phosphaturic in vivo, and cleavage of this molecule inactivates its activity; thus, it is unlikely that these small phosphaturic molecule(s) are derived from FGF23. However, it is possible that, in some tumors, over-expression of FGF23 may indirectly/directly alter expression of smaller phosphaturic and mineralization inhibiting peptides derived from the bone-matrix (MEPE?). This remains to be tested experimentally.
(6e) MEPE null-mutant
Disruption of MEPE/OF45 expression in the mouse results in increased bone-mass, resistance to age-associated trabecular bone loss, increased mineralization apposition rate, accelerated mineralization in ex vivo osteoblast cultures, and normo-phosphatemia (Gowen et al., 2003). This phenotype is consistent with the ASARM model for the following reasons. Although there are no published reports concerning the relative expression levels of matrix proteins in the MEPE null-mutant, given the null-phenotype, matrix-proteins required for mineralization (DMP-1, osteocalcin, BSP, and vitronectin) are likely normal or slightly elevated. Thus, an absence of the MEPE-ASARM peptide (mineralization inhibitor) required to counterbalance the mineralization/bone-formation process is wholly compatible with the observed MEPE null-mutant bone-phenotype. Moreover, the normal phosphate homeostasis (as observed) is also expected, due to the absence of ASARM peptide and the presence of normal FGF23/PHEX expression.
(6f) HYP-PHEX transgenic mouse (Fig. 7)
Surprisingly, osteoblast-targeted re-introduction of normal PHEX into Hyp mice (PHEX-defective mice) only partially corrects the mineralization phenotype, and the mice retain hypophosphatemia (Bai et al., 2002; Liu et al., 2002; Erben et al., 2003). However, this is consistent with the ASARM model. In two of the three reported transgenic studies, normal PHEX expression was targeted to the osteoblast (Fig. 7E, lower right osteoblast). Thus, extra-osteoblastic (brain) mutated-PHEX protein could still, potentially, indirectly result in abnormal processing of FGF23, PTH, and/or other cytokines (Fig. 7A; upper left VLTN). This in turn is proposed to result in abnormal suppression (via uncleaved/unprocessed FGF23 and/or other cytokines) of NPT2 expression and vitamin-D metabolism, resulting in hypophosphatemia (Figs. 7B, 7C). Also, proteins required for mineralization will still be suppressed due to the direct/indirect PHEX extra-osseous (brain) abnormal FGF23 processing (Fig. 7D; see lower left osteoblast representation). However, normal osteoblast-expressed PHEX is proposed to contribute to a partial correction of the mineralization phenotype (Fig. 7E). This is because MEPE sequestration by normal PHEX will prevent or reduce release of free ASARM peptide (Fig. 7F). Moreover, normal PHEX has also been reported to suppress MEPE expression (Argiro et al., 2001; Bai et al., 2002; Gowen et al., 2003). Thus, although there is still low-level expression of proteins required for mineralization (extra-osseous full-length FGF23-mediated), there is a reduction in the levels of osteoblast-derived mineralization inhibitor (ASARM peptide). This results in only a partial amelioration of the mineralization defect, with commensurate full hypophosphatemia.
Further support for the role of the brain in PHEX-mediated bone-renal homeostasis has come from the study of transgenic Hyp-mice expressing the PHEX-gene via a ‘partially’ ubiquitous promoter (Erben et al., 2003). Remarkably, the hypophosphatemic phenotype was not cured (as in the osteoblast promoter-targeted transgenics). Improvement of the bone-phenotype, however, was markedly better than the targeted osteoblastic PHEX-Hyp transgenic-mice, but not completely ameliorated. On closer inspection, however, the only tissue not expressing normal PHEX in these animals (partially ubiquitous promoter) was brain. This strongly suggests that PHEX may play a key role in processing bone-renal factors in the brain as well as in bone (extra-osseous and osteoblastic pathways). The partial correction of the mineralization defect in all transgenics described (partial-global and osteoblast-targeted) is not likely due to persistent hypophosphatemia, since NPT2 null-mutant mice with severe hypophosphatemia do not display a rickets or defective bone-mineralization phenotype (Beck et al., 1998; Tenenhouse et al., 2003).
In summary, in Hyp-transgenic mice that express normal PHEX in all tissues except brain, full-length-FGF23 (brain-derived) may partially suppress expression of the osteoblast matrix-proteins required for mineralization. However, this negative impact on mineralization is likely to be counterbalanced by a reduction in free MEPE-ASARM peptide (mineralization inhibitor) due to normal osteoblast transgenic-PHEX sequestration of full-length MEPE. The net result is a partial but not complete correction of the mineralization defect and a continued hypophosphatemia.
(6h) (Hyp-MEPE) double-null-mutant
A key component in this experimental animal model is the use of the Hyp mouse as the Phex-deficient phenotype. Thus, the Phex mutation will be truly global, and defective Phex protein will occur in all tissues. Remarkably, like the ‘Hyp-Phex’ transgenic mice (see earlier discussion; Section 6f), a combined Mepe and Hyp null-mutant mouse (MEPE−/−: Phex −/−) retains hypophosphatemia but has a partial correction of the mineralization defect (Liu et al., 2003c). This phenotype can be explained when one considers that defective Phex likely continues to affect extra-osteoblastic (brain) processing of FGF23 indirectly in the double-null-mutant mouse. This will in turn result in increased levels of uncleaved full-length FGF23 and thus commensurate suppression of the matrix proteins required for mineralization. In addition, the uncleaved FGF23 will indirectly suppress NPT2 expression and also directly affect vitamin D metabolism (hypophosphatemia and low serum 1,25-vitamin D3). The absence of MEPE will remove the mineralization inhibitor component (ASARM peptide) of the mineralization axis (proteins required for mineralization and mineralization inhibitors). However, the FGF23-mediated suppression of proteins required for mineralization will quench a full improvement in mineralization phenotype, and only a partial improvement in mineralization results. In addition, although MEPE-ASARM peptide is absent, a continued hypophosphatemia persists due to FGF23 suppression (direct or indirect) of NPT2 nuclear expression. Finally, the ASARM motif is a feature of the extracellular matrix (SIBLING proteins, etc.). Thus, a continued elevated presence of ASARM peptide(s) may still be a contributory disease factor, due to the elevated osteoblastic-protease expression in Hyp. Moreover, as in the Hyp-Phex transgenic mice, the partial correction of the mineralization defect is unlikely, due to the persistent hypophosphatemia, since NPT2 null-mutant mice with severe hypophosphatemia do not display a rickets or defective bone-mineralization phenotype (Beck et al., 1998; Tenenhouse et al., 2003).
(7) Haploinsufficiency & the ASARM Model: When Too Little is Too Much and Too Much is Too Little
Haploinsufficiency refers to the inability of a single wild-type allele to confer a normal phenotype (i.e., the null allele is dominant). This implies that two wild-type alleles are barely sufficient to maintain normal functioning, or that one defective allele has an adverse impact on the normal allele. Haploinsufficiency is very rare, since most individuals are heterozygous for multiple loss-of-function mutations in essential genes. Moreover, multiple new mutations arise each generation such that haploinsufficiency at most loci would, in fact, be incompatible with life itself. Therefore, the finding that the Mepe null-mutant (Mepe (−/−) and the Hyp mouse (Phex −/−) both exhibit haploinsufficiency is truly remarkable and in itself compelling evidence of a MEPE-PHEX association, regardless of the other phenotypic links described earlier (Qiu et al., 1993, 2003; Wang et al., 1999; Gowen et al., 2003). Moreover, the fact that X-linked hypophosphatemic rickets is a dominant disease is perplexing, and the discovery that PHEX function (a putative protease) is independent of gene dose is fascinating. This is because males have one X chromosome (XY) and females two (XX). The ASARM model, however, is consistent with these findings. In heterozygous Hyp females, both the normal and abnormal Phex alleles are expressed (Wang et al., 1999). Thus, in Hyp female heterozygotes, there is a chimeric population of normal and abnormal osteoblasts (lyonization or X-chromosome inactivation; Lyon, 1988), with commensurate excess of localized bone production of ASARM peptide. In Hyp hemizygous males (XY), all osteoblasts will be Phex-defective and will therefore secrete ASARM peptide, as will female homozygous Hyposteoblasts. Thus, only one defective Phex allele is required to produce sufficient quantities of bone-population-derived mineralization-inhibitor (ASARM peptides). This is supported by the finding of haploinsufficiency in the MEPE null-mutant and the opposite bone phenotype. Specifically, a single autosomal (chromosome 4)-defective MEPE allele (heterozygote) is also sufficient to produce disease. Thus, in MEPE-null heterozygotes, the overall production of MEPE in bone will be reduced (potentially by half), and this will be compounded by sequestration by phex (normal in all mepe null-mutant osteoblasts). In addition, Phex suppresses Mepe expression (Argiro et al., 2001; Bai et al., 2003; Gowen et al., 2003), and this will also add to the reduction in free ASARM peptide.
As a caveat, it should be noted that although there is unequivocal absence of gene-dose effect in Hyp mice with respect to hypophosphatemia, mineralization defects, skeletal mass, renal mitochondrial 24-hydroxylase, tail-length, Phex, and alkaline phosphatase, etc. (Kay et al., 1991; Shetty and Meyer, 1991; Scriver and Tenenhouse, 1992; Qiu et al., 1993, 2003; Wang et al., 1999), the data are less clear in humans. Some studies support haploinsufficiency in man (Petersen et al., 1992; Scriver and Tenenhouse, 1992); some do not (Whyte et al., 1996). Of additional interest is the finding that vertebral length is influenced by gene dose in the Hyp mouse (Qiu et al., 2003). This discrepancy between vertebral growth and all the other gene-dose independent parameters in the Hyp mouse was suggested to be due to the involvement of Phex in distinct pathways (Qiu et al., 2003). Moreover, parental genomic imprinting does not appear to play a role (Qiu et al., 2003).
(8) Summary
An intricate tapestry of hormones, cytokines, matrix proteases, and extracellular matrix-proteins has evolved to maintain bone-tooth balance and skeletal integrity. Phosphate and calcium are the fundamental building blocks of this exquisitely balanced order. They are assembled to form the superbly sculpted structures of the skeleton and the teeth. This living work of art requires finely tuned molecular mechanisms to maintain the dynamic among bone formation, bone resorption, mineralization, and inhibition of mineralization. Some new features of this complex biology have emerged, and new tools are now available. These new tools will help unravel the tangled and wrickkened complexity of interactions in disease and health.
Acknowledgments
The author thanks Mr. David Baker (UTHSCSA media services) for expert and helpful advice in the graphical design of the Figures and illustrations. The author also acknowledges with gratitude the generous financial support and awards from the Children’s Cancer Research Center (CCRC) of the University of Texas Health Science Center at San Antonio (UTHSCSA), National Institutes of Health (NIH) grants 1 RO3 DE015900-01 (National Institute of Dental & Craniofacial Research; NIDCR) and 1 RO1 AR51598-01 (National Institute of Arthritis and Musculoskeletal and Skin Diseases; NIAMS).
Addendum
As this journal goes to press, two new publications have provided additional support for the model presented in this review. Studies by Jain et al. (2004) have confirmed that circulating serum MEPE in normal subjects (476 ± 247 ng/mL) is strongly correlated with serum PO4, PTH, and bone mineral density. Serum levels of matrix extracellular phosphoglycoprotein (MEPE) in normal humans correlate with serum phosphorus, parathyroid hormone, and bone mineral density (Jain et al., 2004). Moreover, the levels of serum MEPE are also correlated with age in normal individuals. Finally, we have confirmed that PHEX and MEPE interact specifically via the ASARM motif, with surface plasmon resonance (Rowe et al., 2004). Surface plasmon resonance (SPR) confirms that MEPE binds to PHEX via the MEPE-ASARM motif: a model for impaired mineralization in X-linked rickets (HYP).
References
- Aisa MC, Rahman S, Senin U, Maggio D, Russell RG. Cathepsin B activity in normal human osteoblast-like cells and human osteoblastic osteosarcoma cells (MG-63): regulation by interleukin-1 beta and parathyroid hormone. Biochim Biophys Acta. 1996;1290:29–36. doi: 10.1016/0304-4165(95)00182-4. [DOI] [PubMed] [Google Scholar]
- Aisa MC, Beccari T, Costanzi E, Maggio D. Cathepsin B in osteoblasts. Biochim Biophys Acta. 2003;1621:149–159. doi: 10.1016/s0304-4165(03)00054-0. [DOI] [PubMed] [Google Scholar]
- Allen HC, Fedarko NS, Jain A, Whybro A, Barker ME, Eastell R, et al. The response of MEPE to long-term and short-term phosphate supplementation in healthy volunteers (abstract) J Bone Miner Res. 2003;18:S116. [Google Scholar]
- Argiro L, Desbarats M, Glorieux FH, Ecarot B. Mepe, the gene encoding a tumor-secreted protein in oncogenic hypophosphatemic osteomalacia, is expressed in bone. Genomics. 2001;74:342–351. doi: 10.1006/geno.2001.6553. [DOI] [PubMed] [Google Scholar]
- Aschinberg LC, Solomon LM, Zeis PM, Justice P, Rosenthal IM. Vitamin D-resistant rickets associated with epidermal nevus syndrome: demonstration of a phosphaturic substance in the dermal lesions. J Pediatr. 1977;91:56–60. doi: 10.1016/s0022-3476(77)80444-7. [DOI] [PubMed] [Google Scholar]
- Azam N, Zhang MY, Wang X, Tenenhouse HS, Portale AA. Disordered regulation of renal 25-hydroxyvitamin D-1alpha-hydroxylase gene expression by phosphorus in X-linked hypophosphatemic (hyp) mice. Endocrinology. 2003;144:3463–3468. doi: 10.1210/en.2003-0255. [DOI] [PubMed] [Google Scholar]
- Bai X, Miao D, Panda D, Grady S, McKee MD, Goltzman D, et al. Partial rescue of the Hyp phenotype by osteoblast-targeted PHEX (Phosphate-Regulating Gene with Homologies to Endopeptidases on the X Chromosome) expression. Mol Endocrinol. 2002;16:2913–2925. doi: 10.1210/me.2002-0113. [DOI] [PubMed] [Google Scholar]
- Bai XY, Miao D, Goltzman D, Karaplis AC. The autosomal dominant hypophosphatemic rickets R176Q mutation in FGF23 resists proteolytic cleavage and enhances in vivo biological potency. J Biol Chem. 2003;278:9843–9849. doi: 10.1074/jbc.M210490200. [DOI] [PubMed] [Google Scholar]
- Barrett AJ. Proteolytic enzymes: aspartic and metallo peptidases. In: Barrett AJ, editor. Methods in enzymology. 1. Vol. 248. New York: Academic Press; 1995. pp. 1–873. [Google Scholar]
- Beaumont A, Le Moual H, Boileau G, Crine P, Roques BP. Evidence that both arginine 102 and arginine 747 are involved in substrate binding to neutral endopeptidase (EC 3.4.24.11) J Biol Chem. 1991;266:214–220. [PubMed] [Google Scholar]
- Beaumont A, Barbe B, Le Moual H, Boileau G, Crine P, Fournie-Zaluski MC, et al. Charge polarity reversal inverses the specificity of neutral endopeptidase-24.11. J Biol Chem. 1992;267:2138–2141. [PubMed] [Google Scholar]
- Beck L, Soumounou Y, Martel J, Krishnamurthy G, Gauthier C, Goodyer CG, et al. Pex/PEX tissue distribution and evidence for a deletion in the 3′ region of the Pex gene in X-linked hypophosphatemic mice. J Clin Invest. 1997;99:1200–1209. doi: 10.1172/JCI119276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck L, Karaplis AC, Amizuka N, Hewson AS, Ozawa H, Tenenhouse HS. Targeted inactivation of Npt2 in mice leads to severe renal phosphate wasting, hypercalciuria, and skeletal abnormalities. Proc Natl Acad Sci USA. 1998;95:5372–5377. doi: 10.1073/pnas.95.9.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennick A, McLaughlin AC, Grey AA, Madapallimattam G. The location and nature of calcium-binding sites in salivary acidic proline-rich phosphoproteins. J Biol Chem. 1981;256:4741–4746. [PubMed] [Google Scholar]
- Berndt T, Craig TA, Bowe AE, Vassiliadis J, Reczek D, Finnegan R, et al. Secreted frizzled-related protein 4 is a potent tumor-derived phosphaturic agent. J Clin Invest. 2003;112:785–794. doi: 10.1172/JCI18563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchetti L, Oudet C, Poch O. M13 endopeptidases: new conserved motifs correlated with structure, and simultaneous phylogenetic occurrence of PHEX and the bony fish. Proteins. 2002;47:481–488. doi: 10.1002/prot.10075. [DOI] [PubMed] [Google Scholar]
- Blydt-Hansen TD, Tenenhouse HS, Goodyer P. PHEX expression in parathyroid gland and parathyroid hormone dys-regulation in X-linked hypophosphatemia. Pediatric Nephrol. 1999;13:607–611. doi: 10.1007/s004670050669. [DOI] [PubMed] [Google Scholar]
- Boileau G, Tenenhouse HS, DesGroseillers L, Crine P. Characterization of PHEX endopeptidase catalytic activity: identification of parathyroid-hormone-related peptide 107-139 as a substrate and osteocalcin, PPi and phosphate as inhibitors. Biochem J. 2001;355:707–713. doi: 10.1042/bj3550707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonjour JP, Trechsel U, Fleisch H, Schenk R, DeLuca HF, Baxter LA. Action of 1,25-dihydroxyvitamin D3 and a diphosphonate on calcium metabolism in rats. Am J Physiol. 1975;229:402–408. doi: 10.1152/ajplegacy.1975.229.2.402. [DOI] [PubMed] [Google Scholar]
- Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD. Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int. 2002;71:145–154. doi: 10.1007/s00223-001-1121-z. [DOI] [PubMed] [Google Scholar]
- Bowe AE, Finnegan R, Jan de Beur SM, Cho J, Levine MA, Kumar R, et al. FGF-23 inhibits renal tubular phosphate transport and is a PHEX substrate. Biochem Biophys Res Commun. 2001;284:977–981. doi: 10.1006/bbrc.2001.5084. [DOI] [PubMed] [Google Scholar]
- Brewer AJ, Canaff L, Hendy GN, Tenenhouse HS, Gauthier C, Chau H, et al. Differential regulation of PHEX expression in bone and parathyroid gland by chronic renal insufficiency and 1,25-dihydroxyvitamin D3. Am J Physiol Renal Physiol. 2003;286:F739–F748. doi: 10.1152/ajprenal.00321.2003. [DOI] [PubMed] [Google Scholar]
- Cai Q, Hodgson SF, Kao PC, Lennon VA, Klee GG, Zinsmiester AR, et al. Brief report: inhibition of renal phosphate transport by a tumor product in a patient with oncogenic osteomalacia. N Engl J Med. 1994;330:1645–1649. doi: 10.1056/NEJM199406093302304. [DOI] [PubMed] [Google Scholar]
- Campos M, Couture C, Hirata IY, Juliano MA, Loisel TP, Crine P, et al. Human recombinant endopeptidase PHEX has a strict S1′ specificity for acidic residues and cleaves peptides derived from fibroblast growth factor-23 and matrix extracellular phosphoglycoprotein. Biochem J. 2003;373:271–279. doi: 10.1042/BJ20030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter TO, Moltz KC, Ellis B, Andreoli M, McCarthy TL, Centrella M, et al. Osteocalcin production in primary osteoblast cultures derived from normal and Hyp mice. Endocrinology. 1998;139:35–43. doi: 10.1210/endo.139.1.5677. [DOI] [PubMed] [Google Scholar]
- Chingwen L, Hersh LB. Neprilysin: assay methods, purification, and characterisation. In: Barrett AJ, editor. Methods in enzymology. 1. Vol. 248. New York: Academic Press; 1995. pp. 253–263. Proteolytic enzymes: aspartic and metallo peptidases. [DOI] [PubMed] [Google Scholar]
- De Beur SM, Finnegan RB, Vassiliadis J, Cook B, Barberio D, Estes S, et al. Tumors associated with oncogenic osteomalacia express genes important in bone and mineral metabolism. J Bone Miner Res. 2002;17:1102–1110. doi: 10.1359/jbmr.2002.17.6.1102. [DOI] [PubMed] [Google Scholar]
- Dion N, Le Moual H, Crine P, Boileau G. Kinetic evidence that His-711 of neutral endopeptidase 24.11 is involved in stabilization of the transition state. FEBS Lett. 1993;318:301–304. doi: 10.1016/0014-5793(93)80533-z. [DOI] [PubMed] [Google Scholar]
- Dion N, Le Moual H, Fournie-Zaluski MC, Roques BP, Crine P, Boileau G, et al. Evidence that Asn542 of neprilysin (EC 3.4.24.11) is involved in binding of the P2′ residue of substrates and inhibitors. Biochem J. 1995;311:623–627. doi: 10.1042/bj3110623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbie H, Shirley DG, Faria NJ, Rowe PSN, Slater JM, Unwin RJ. Infusion of the bone-derived protein MEPE causes phosphaturia in rats (abstract) J Am Soc Nephrol. 2003;14:468A. [Google Scholar]
- Drezner MK. Tumor-induced osteomalacia. Rev Endocr Metab Disord. 2001;2:175–186. doi: 10.1023/a:1010006811394. [DOI] [PubMed] [Google Scholar]
- Drezner MK. Hypophosphatemic rickets. Endocr Dev. 2003;6:126–155. doi: 10.1159/000072774. [DOI] [PubMed] [Google Scholar]
- Du L, Desbarats M, Viel J, Glorieux FH, Cawthorn C, Ecarot B. cDNA cloning of the murine Pex gene implicated in X-linked hypophosphatemia and evidence for expression in bone. Genomics. 1996;36:22–28. doi: 10.1006/geno.1996.0421. [DOI] [PubMed] [Google Scholar]
- Dubois SG, Ruchon AF, Delalandre A, Boileau G, Lajeunesse D. Role of abnormal neutral endopeptidase-like activities in Hyp mouse bone cells in renal phosphate transport. Am J Physiol Cell Physiol. 2002;283:C1414–C1421. doi: 10.1152/ajpcell.00135.2002. [DOI] [PubMed] [Google Scholar]
- Ecarot B, Desbarats M. 1,25-(OH)(2)D-3 down-regulates expression of Phex, a marker of the mature osteoblast. Endocrinology. 1999;140:1192–1199. doi: 10.1210/endo.140.3.6593. [DOI] [PubMed] [Google Scholar]
- Ecarot B, Glorieux FH, Desbarats M, Travers R, Labelle L. Defective bone formation by Hyp mouse bone cells transplanted into normal mice: evidence in favor of an intrinsic osteoblast defect. J Bone Miner Res. 1992a;7:215–220. doi: 10.1002/jbmr.5650070213. [DOI] [PubMed] [Google Scholar]
- Ecarot FH, Glorieux FH, Desbarats M, Travers R, Labelle L. Effect of dietary phosphate deprivation and supplementation of recipient mice on bone formation by transplanted cells from normal and X-linked hypophosphataemic mice. J Bone Miner Res. 1992b;7:523–530. doi: 10.1002/jbmr.5650070508. [DOI] [PubMed] [Google Scholar]
- Ecarot B, Caverzasio J, Desbarats M, Bonjour JP, Glorieux FH. Phosphate transport by osteoblasts from X-linked hypophosphatemic mice. Am J Physiol. 1994;266:E33–E38. doi: 10.1152/ajpendo.1994.266.1.E33. [DOI] [PubMed] [Google Scholar]
- Ecarot B, Glorieux FH, Desbarats M, Travers R, Labelle L. Effect of 1,25-dihydroxyvitamin D3 treatment on bone formation by transplanted cells from normal and X-linked hypophosphatemic mice. J Bone Miner Res. 1995;10:424–431. doi: 10.1002/jbmr.5650100313. [DOI] [PubMed] [Google Scholar]
- Ecarot-Charrier B, Glorieux FH, Travers R, Desbarats M, Bouchard F, Hinek A. Defective bone formation by transplanted Hyp mouse bone cells into normal mice. Endocrinology. 1988;123:768–773. doi: 10.1210/endo-123-2-768. [DOI] [PubMed] [Google Scholar]
- Econs MJ, Drezner MK. Tumour induced osteomalacia—unveiling a new hormone. N Engl J Med. 1994;330:1679–1681. doi: 10.1056/NEJM199406093302310. [DOI] [PubMed] [Google Scholar]
- Erben RG, Maye D, Weber K, Johnson T, Jonsson K, Jüppner H, et al. Ubiquitous overexpression of Phex does not fully rescue the Hyp mouse phenotype (abstract) J Bone Miner Res. 2003;18:S22. doi: 10.1359/JBMR.050212. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Torchia DA, Fohr B, Young MF, Fedarko NS. Flexible structures of SIBLING proteins, bone sialoprotein, and osteopontin. Biochem Biophys Res Commun. 2001;280:460–465. doi: 10.1006/bbrc.2000.4146. [DOI] [PubMed] [Google Scholar]
- Francis F, Strom TM, Hennig S, Boeddrich A, Lorenz B, Brandau O, et al. Genomic organisation of the human PEX gene mutated in X-linked dominant hypophosphataemic rickets. Gen Res. 1997;7:573–585. doi: 10.1101/gr.7.6.573. [DOI] [PubMed] [Google Scholar]
- Fujiwara I, Aravindan R, Horst RL, Drezner MK. Abnormal regulation of renal 25-hydroxyvitamin D-1alpha-hydroxylase activity in X-linked hypophosphatemia: a translational or post-translational defect. J Bone Miner Res. 2003;18:434–442. doi: 10.1359/jbmr.2003.18.3.434. [DOI] [PubMed] [Google Scholar]
- Fukumoto S, Yamashita T. Fibroblast growth factor-23 is the phosphaturic factor in tumor-induced osteomalacia and may be phosphatonin. Curr Opin Nephrol Hypertens. 2002;11:385–389. doi: 10.1097/00041552-200207000-00003. [DOI] [PubMed] [Google Scholar]
- Glisson F, Bate G, Regemorter A. Peter Cole, Cornhill Royal Exchange. London: Bedford Royal Infirmary Medical Library; 1651. A treatise of the rickets: being a disease common to children. [Google Scholar]
- Gowen LC, Petersen DN, Mansolf AL, Qi H, Stock JL, Tkalcevic GT, et al. Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem. 2003;278:1998–2007. doi: 10.1074/jbc.M203250200. [DOI] [PubMed] [Google Scholar]
- Guo R, Quarles LD. Cloning and sequencing of human PEX from a bone cDNA library: evidence for its developmental stage-specific regulation in osteoblasts. J Bone Miner Res. 1997;12:1009–1017. doi: 10.1359/jbmr.1997.12.7.1009. [DOI] [PubMed] [Google Scholar]
- Guo R, Liu S, Spurney RF, Quarles LD. Analysis of recombinant Phex: an endopeptidase in search of a substrate. Am J Physiol Endocrinol Metab. 2001;281:E837–E847. doi: 10.1152/ajpendo.2001.281.4.E837. [DOI] [PubMed] [Google Scholar]
- Guo R, Rowe PS, Liu S, Simpson LG, Xiao ZS, Quarles LD. Inhibition of MEPE cleavage by Phex. Biochem Biophys Res Commun. 2002;297:38–45. doi: 10.1016/s0006-291x(02)02125-3. [DOI] [PubMed] [Google Scholar]
- Gururaja TL, Levine MJ. Solid-phase synthesis and characterization of human salivary statherin: a tyrosine-rich phosphoprotein inhibitor of calcium phosphate precipitation. Pept Res. 1996;9:283–289. [PubMed] [Google Scholar]
- Halstead LR, Weinstein RS, Cheng SL, Rifas L, Avioli LV. Comparison of 22-oxacalcitriol and 1,25(OH)2D3 on bone metabolism in young X-linked hypophosphatemic male mice. Am J Physiol. 1996;270:E141–E147. doi: 10.1152/ajpendo.1996.270.1.E141. [DOI] [PubMed] [Google Scholar]
- Hoyer JR, Asplin JR, Otvos L. Phosphorylated osteopontin peptides suppress crystallization by inhibiting the growth of calcium oxalate crystals. Kidney Int. 2001;60:77–82. doi: 10.1046/j.1523-1755.2001.00772.x. [DOI] [PubMed] [Google Scholar]
- Huang H, Ye L, Xie Y, Ko S, Harris SE, Bonewald L, et al. Bone repair is dramatically delayed in dentin matrix protein-1 (Dmp1) deficient mice (abstract) J Bone Miner Res. 2003;18:S197. [Google Scholar]
- Francis F, Hennig S, Korn B, Reinhardt R, de Jong D, et al. HYP Consortium. A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. The HYP Consortium. Nat Genet. 1995;11:130–136. doi: 10.1038/ng1095-130. [DOI] [PubMed] [Google Scholar]
- Jain A, Fedarko NS, Collins MT, Gelman R, Ankrom MA, Tayback M, et al. Serum levels of matrix extracellular phosphoglycoprotein (MEPE) in normal humans correlate with serum phosphorus, parathyroid hormone and bone mineral density. J Clin Endocrinol Metab. 2004;89:4158–4161. doi: 10.1210/jc.2003-032031. [DOI] [PubMed] [Google Scholar]
- Jan de Beur SM, Levine MA. Molecular pathogenesis of hypophosphatemic rickets. J Clin Endocrinol Metab. 2002;87:2467–2473. doi: 10.1210/jcem.87.6.8688. [DOI] [PubMed] [Google Scholar]
- Jo OD, Shih R, Sun A, Pham P, Yanagawa J, Yanagawa N. Cathepsin D (Cat D) and bone defect in hypophosphatemic (Hyp) mice (abstract) J Am Soc Nephrol. 2000;11:408A. [Google Scholar]
- Jo OD, Yang F, Kuizon B, Shih HM, Yanagawa N. Increased apoptosis in Hyp mouse bone. Potential role of cathepsin D (abstract) J Am Soc Nephrol. 2001;12:743A. [Google Scholar]
- Jonsson KB, Mannstadt M, Miyauchi A, Yang IM, Stein G, Ljunggren O, et al. Extracts from tumors causing oncogenic osteomalacia inhibit phosphate uptake in opossum kidney cells. J Endocrinol. 2001;169:613–620. doi: 10.1677/joe.0.1690613. [DOI] [PubMed] [Google Scholar]
- Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, et al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med. 2003;348:1656–1663. doi: 10.1056/NEJMoa020881. [DOI] [PubMed] [Google Scholar]
- Kay GF, Thakker RV, Rastan S. Generation of a Mus spretus/Mus domesticus backcross segregating for Ta and Hyp by in vitro fertilisation (abstract) Cytogenet Cell Genet. 1991;58:2137. [Google Scholar]
- Kronenberg HM. NPT2a—the key to phosphate homeostasis. N Engl J Med. 2002;347:1022–1024. doi: 10.1056/NEJMe020098. [DOI] [PubMed] [Google Scholar]
- Kumar R. New insights into phosphate homeostasis: fibroblast growth factor 23 and frizzled-related protein-4 are phosphaturic factors derived from tumors associated with osteomalacia. Curr Opin Nephrol Hypertens. 2002;11:547–553. doi: 10.1097/00041552-200209000-00011. [DOI] [PubMed] [Google Scholar]
- Lajeunesse D, Meyer RA, Jr, Hamel L. Direct demonstration of a humorally-mediated inhibition of renal phosphate transport in the Hyp mouse. Kidney Int. 1996;50:1531–1538. doi: 10.1038/ki.1996.468. [DOI] [PubMed] [Google Scholar]
- Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB. Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int. 2003;64:2272–2279. doi: 10.1046/j.1523-1755.2003.00328.x. [DOI] [PubMed] [Google Scholar]
- Le Moual H, Devault A, Roques BP, Crine P, Boileau G. Identification of glutamic acid 646 as a zinc-coordinating residue in endopeptidase-24.11. J Biol Chem. 1991;266:15670–15674. [PubMed] [Google Scholar]
- Le Moual H, Beaumont A, Roques BP, Crine P, Boileau G. Identification of two arginine residues involved in the binding of substrate to neutral endopeptidase 24-11. Matrix Suppl. 1992a;1:99. [PubMed] [Google Scholar]
- Le Moual H, Crine P, Boileau G. Identification of Glu 646 of neutral endopeptidase 24-11 as a zinc binding residue. Matrix Suppl. 1992b;1:100. [PubMed] [Google Scholar]
- Le Moual H, Roques BP, Crine P, Boileau G. Substitution of potential metal-coordinating amino acid residues in the zinc-binding site of endopeptidase-24.11. FEBS Lett. 1993;324:196–200. doi: 10.1016/0014-5793(93)81392-d. [DOI] [PubMed] [Google Scholar]
- Le Moual H, Dion N, Roques BP, Crine P, Boileau G. Asp650 is crucial for catalytic activity of neutral endopeptidase 24-11. Eur J Biochem. 1994;221:475–480. doi: 10.1111/j.1432-1033.1994.tb18760.x. [DOI] [PubMed] [Google Scholar]
- Lee S, Lin M, Mele A, Cao Y, Farmar J, Russo D, et al. Proteolytic processing of big endothelin-3 by the Kell blood group protein. Blood. 1999;94:1440–1450. [PubMed] [Google Scholar]
- Lee S, Russo D, Redman CM. The Kell blood group system: Kell and XK membrane proteins. Sem Hematol. 2000;37:113–121. doi: 10.1016/s0037-1963(00)90036-2. [DOI] [PubMed] [Google Scholar]
- Lee S, Debnath AK, Redman CM. Active amino acids of the Kell blood group protein and model of the ectodomain based on the structure of neutral endopeptidase 24.11. Blood. 2003;102:3028–3034. doi: 10.1182/blood-2003-05-1564. [DOI] [PubMed] [Google Scholar]
- Letarte M, Vera S, Tran R, Addis JB, Onizuka RJ, Quackenbush EJ, et al. Common acute lymphocytic leukemia antigen is identical to neutral endopeptidase. J Exp Med. 1988;168:1247–1253. doi: 10.1084/jem.168.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Guo R, Quarles LD. Cloning and characterization of the proximal murine phex promoter. Endocrinology. 2001;142:3987–3995. doi: 10.1210/endo.142.9.8403. [DOI] [PubMed] [Google Scholar]
- Liu S, Guo R, Tu Q, Quarles LD. Overexpression of Phex in osteoblasts fails to rescue the Hyp mouse phenotype. J Biol Chem. 2002;277:3686–3697. doi: 10.1074/jbc.M107707200. [DOI] [PubMed] [Google Scholar]
- Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD. Regulation of FGF23 expression but not degradation by phex. J Biol Chem. 2003a;278:37419–37426. doi: 10.1074/jbc.M304544200. [DOI] [PubMed] [Google Scholar]
- Liu S, Guo R, Xiao G, Quarles LD. Regulation of FGF23 expression but not metabolism by Phex (abstract) J Bone Miner Res. 2003b;18:S50. [Google Scholar]
- Liu S, Brown TA, Xiao Z, Guo R, Quarles LD. Deletion of Mepe in Hyp mice fails to correct hypophosphatemia but partially rescues abnormal mineralization ex vivo (abstract) J Bone Miner Res. 2003c;18:S24. [Google Scholar]
- Loghman-Adham M, Dousa TP. Dual action of phosphono-formic acid on Na(+)-phosphate cotransport in opossum kidney cells. Am J Physiol. 1992;263:F301–F310. doi: 10.1152/ajprenal.1992.263.2.F301. [DOI] [PubMed] [Google Scholar]
- Loghman-Adham M, Szczepanska-Konkel M, Yusufi AN, Van Scoy M, Dousa TP. Inhibition of Na+-Pi cotransporter in small gut brush border by phosphonocarboxylic acids. Am J Physiol. 1987;252:G244–G249. doi: 10.1152/ajpgi.1987.252.2.G244. [DOI] [PubMed] [Google Scholar]
- Loghman-Adham M, Szczepanska-Konkel M, Dousa TP. Phosphate transport in brush border membranes from uremic rats. Response to phosphonoformic acid. J Am Soc Nephrol. 1992;3:1253–1259. doi: 10.1681/ASN.V361253. [DOI] [PubMed] [Google Scholar]
- Long JR, Dindot JL, Zebroski H, Kiihne S, Clark RH, Campbell AA, et al. A peptide that inhibits hydroxyapatite growth is in an extended conformation on the crystal surface. Proc Natl Acad Sci USA. 1998;95:12083–12087. doi: 10.1073/pnas.95.21.12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyon MF. X-chromosome inactivation and the location and expression of X-linked genes. Am J Hum Genet. 1988;42:8–16. [PMC free article] [PubMed] [Google Scholar]
- MacDougall M, Simmons D, Gu TT, Dong J. MEPE/OF45, a new dentin/bone matrix protein and candidate gene for dentin diseases mapping to chromosome 4q21. Connect Tissue Res. 2002;43:320–330. doi: 10.1080/03008200290000556. [DOI] [PubMed] [Google Scholar]
- Marie-Claire C, Tiraboschi G, Ruffet E, Inguimbert N, Fournie-Zaluski MC, Roques BP. Exploration of the S(′ )(1) subsite of neprilysin: a joined molecular modeling and site-directed mutagenesis study. Proteins. 2000;39:365–371. doi: 10.1002/(sici)1097-0134(20000601)39:4<365::aid-prot90>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- McCloskey EV, Yates AJ, Gray RE, Hamdy NA, Galloway J, Kanis JA. Diphosphonates and phosphate homeostasis in man. Clin Sci (Lond) 1988;74:607–612. doi: 10.1042/cs0740607. [DOI] [PubMed] [Google Scholar]
- Meyer MH, Meyer RA., Jr MRNA expression of Phex in mice and rats: the effect of low phosphate diet. Endocrine. 2000;13:81–87. doi: 10.1385/ENDO:13:1:81. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Jr, Meyer MA, Gray RW. Parabiosis suggests a humoral factor is involved in X-linked hypophosphataemia in mice. J Bone Miner Res. 1989a;4:493–500. doi: 10.1002/jbmr.5650040407. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Jr, Tenenhouse HS, Meyer MA, Klugerman AH. The renal phosphate transport defect in normal mice parabiosed to X-linked hypophosphataemic mice persists after parathyroidectomy. J Bone Miner Res. 1989b;4:523–532. doi: 10.1002/jbmr.5650040411. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Young CG, Meyer MH, Garges PL, Price DK. Effect of age on the expression of Pex (Phex) in the mouse. Calcif Tissue Int. 2000;66:282–287. doi: 10.1007/s002230010057. [DOI] [PubMed] [Google Scholar]
- Miao D, Bai X, Panda D, McKee M, Karaplis A, Goltzman D. Osteomalacia in hyp mice is associated with abnormal phex expression and with altered bone matrix protein expression and deposition. Endocrinology. 2001;142:926–939. doi: 10.1210/endo.142.2.7976. [DOI] [PubMed] [Google Scholar]
- Miao D, Bai X, Panda D, Karaplis AC, Goltzman D, McKee MD. Cartilage abnormalities are associated with abnormal Phex expression and with altered matrix protein and MMP-9 localization in Hyp mice. Bone. 2004;34:638–647. doi: 10.1016/j.bone.2003.12.015. [DOI] [PubMed] [Google Scholar]
- Miyauchi A, Fukase M, Tsutsumi M, Fujita T. Hemangio-pericytoma induced osteomalacia: tumour transplantation in nude mice causes hypophosphataemia and tumour extracts inhibit renal 25-hydroxyvitamin D α1-hydroxylase activity. J Clin Endocrinol Metab. 1988;67:46–53. doi: 10.1210/jcem-67-1-46. [DOI] [PubMed] [Google Scholar]
- Muhlbauer RC, Bonjour JP, Fleisch H. Tubular handling of phosphate along the nephron of thyroparathyroidectomized rats injected with ethane-1-hydroxy-1,1-diphosphonate. Clin Sci (Lond) 1981;60:171–177. doi: 10.1042/cs0600171. [DOI] [PubMed] [Google Scholar]
- Nelson AE, Namkung HJ, Patava J, Wilkinson MR, Chang AM, Reddel RR, et al. Characteristics of tumor cell bioactivity in oncogenic osteomalacia. Molec Cell Endocrinol. 1996;124:17–23. doi: 10.1016/s0303-7207(96)03928-7. [DOI] [PubMed] [Google Scholar]
- Nelson AE, Robinson BG, Hogan JJ, Dwight T, Mason RS. Mechanism of inhibition of renal phosphate uptake by a tumor-derived factor in oncogenic osteomalacia (abstract) J Bone Miner Res. 1997;12:F679. [Google Scholar]
- Nelson AE, Mason RS, Hogan JJ, Diamond T, Robinson BG. Tumor expression studies indicate that HEM-1 is unlikely to be the active factor in oncogenic osteomalacia. Bone. 1998;23:549–553. doi: 10.1016/s8756-3282(98)00136-7. [DOI] [PubMed] [Google Scholar]
- Nelson AE, Hogan JJ, Holm IA, Robinson BG, Mason RS. Phosphate wasting in oncogenic osteomalacia: PHEX is normal and the tumor-derived factor has unique properties. Bone. 2001;28:430–439. doi: 10.1016/s8756-3282(01)00417-3. [DOI] [PubMed] [Google Scholar]
- Nesbitt T, Coffman TM, Griffiths R, Drezner MK. Crosstransplantation of kidneys in normal and Hyp mice. Evidence that the Hyp mouse phenotype is unrelated to an intrinsic renal defect. J Clin Invest. 1992;89:1453–1459. doi: 10.1172/JCI115735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesbitt T, Econs MJ, Byun JK, Martel J, Tenenhouse HS, Drezner MK. Phosphate transport in immortalized cell cultures from the renal proximal tubule of normal and Hyp mice: evidence that the HYP gene locus product is an extrarenal factor. J Bone Miner Res. 1995;10:1327–1333. doi: 10.1002/jbmr.5650100909. [DOI] [PubMed] [Google Scholar]
- Nesbitt T, Fujiwara I, Thomas R, Xiao ZS, Quarles LD, Drezner MK. Coordinated maturational regulation of PHEX and renal phosphate transport inhibitory activity: evidence for the patho-physiological role of PHEX in X-linked hypophosphatemia. J Bone Miner Res. 1999;14:2027–2035. doi: 10.1359/jbmr.1999.14.12.2027. [DOI] [PubMed] [Google Scholar]
- Oefner C, D’Arcy A, Hennig M, Winkler FK, Dale GE. Structure of human neutral endopeptidase (neprilysin) complexed with phosphoramidon. J Mol Biol. 2000;296:341–349. doi: 10.1006/jmbi.1999.3492. [DOI] [PubMed] [Google Scholar]
- Okano T, Tsugawa N, Hirami C, Kato S. Functional properties of cultured normal and vitamin D receptor knockout mice calvarial osteoblasts (abstract) J Bone Miner Res. 2003;18:S141. [Google Scholar]
- Oursler MJ, Riggs BL, Spelsberg TC. Glucocorticoid-induced activation of latent transforming growth factor-beta by normal human osteoblast-like cells. Endocrinology. 1993;133:2187–2196. doi: 10.1210/endo.133.5.8404670. [DOI] [PubMed] [Google Scholar]
- Petersen DJ, Boniface AM, Schranck FW, Rupich RC, Whyte MP. X-linked hypophosphataemic rickets: a study with (literature review) of linear growth response to calcitriol and phosphate therapy. J Bone Miner Res. 1992;7:583–597. doi: 10.1002/jbmr.5650070602. [DOI] [PubMed] [Google Scholar]
- Petersen DN, Tkalcevic GT, Mansolf AL, Rivera-Gonzalez R, Brown TA. Identification of osteoblast/osteocyte factor 45 (OF45), a bone-specific cDNA encoding an RGD-containing protein that is highly expressed in osteoblasts and osteocytes. J Biol Chem. 2000;275:36172–36180. doi: 10.1074/jbc.M003622200. [DOI] [PubMed] [Google Scholar]
- Popovtzer MM. Tumour induced hypophosphataemic osteomalacia: evidence for a phosphaturic cyclic AMP-independent action of tumour extract (abstract) Clin Res. 1981;29:418A. [Google Scholar]
- Qiu ZQ, Tenenhouse HS, Scriver CR. Parental origin of mutant allele does not explain absence of gene dose effect in X-linked Hyp mice. Genet Res Camb. 1993;62:39–43. doi: 10.1017/s0016672300031542. [DOI] [PubMed] [Google Scholar]
- Qiu ZQ, Travers R, Rauch F, Glorieux FH, Scriver CR, Tenenhouse HS. Effect of gene dose and parental origin on bone histomorphometry in X-linked Hyp mice. Bone. 2003;34:134–139. doi: 10.1016/j.bone.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Quarles LD. FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab. 2003;285:E1–E9. doi: 10.1152/ajpendo.00016.2003. [DOI] [PubMed] [Google Scholar]
- Quarles LD, Drezner MK. Pathophysiology of X-linked hypophosphatemia, tumor-induced osteomalacia, and autosomal dominant hypophosphatemia: a perPHEXing problem. J Clin Endocrinol Metab. 2001;86:494–496. doi: 10.1210/jcem.86.2.7302. [DOI] [PubMed] [Google Scholar]
- Raj PA, Johnsson M, Levine MJ, Nancollas GH. Salivary statherin. Dependence on sequence, charge, hydrogen bonding potency, and helical conformation for adsorption to hydroxyapatite and inhibition of mineralization. J Biol Chem. 1992;267:5968–5976. [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ. Evolutionary families of metallopeptidases. Methods Enzymol. 1995;248:183–228. doi: 10.1016/0076-6879(95)48015-3. [DOI] [PubMed] [Google Scholar]
- Rawlings ND, Barrett AJ. MEROPS: the peptidase database. Nucleic Acids Res. 1999;27:325–331. doi: 10.1093/nar/27.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rifas L, Gupta A, Hruska KA, Avioli LV. Altered osteoblast gluconeogenesis in X-linked hypophosphatemic mice is associated with a depressed intracellular pH. Calcif Tissue Int. 1995;57:60–63. doi: 10.1007/BF00298998. [DOI] [PubMed] [Google Scholar]
- Rifas L, Cheng S, Halstead LR, Gupta A, Hruska KA, Avioli LV. Skeletal casein kinase activity defect in the HYP mouse. Calcif Tissue Int. 1997;61:256–259. doi: 10.1007/s002239900331. [DOI] [PubMed] [Google Scholar]
- Riminucci M, Collins MT, Fedarko NS, Cherman N, Corsi A, White KE, et al. FGF-23 in fibrous dysplasia of bone and its relationship to renal phosphate wasting. J Clin Invest. 2003;112:683–692. doi: 10.1172/JCI18399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe PS. Molecular biology of hypophosphataemic rickets and oncogenic osteomalacia. Hum Genet. 1994;94:457–467. doi: 10.1007/BF00211008. [DOI] [PubMed] [Google Scholar]
- Rowe PS. The PEX gene: its role in X-linked rickets, osteomalacia, and bone mineral metabolism. Exp Nephrol. 1997;5:355–363. [PubMed] [Google Scholar]
- Rowe PS. The role of the PHEX gene (PEX), in families with X-linked hypophosphataemic rickets. Curr Opin Nephrol Hypertens. 1998;7:367–376. doi: 10.1097/00041552-199807000-00004. [DOI] [PubMed] [Google Scholar]
- Rowe PS, Goulding JN, Francis F, Oudet C, Econs MJ, Hanauer A, et al. The gene for X-linked hypophosphataemic rickets maps to a 200–300 kb region in Xp22.1, and is located on a single YAC containing a putative vitamin D response element (VDRE) Hum Genet. 1996a;97:345–352. doi: 10.1007/BF02185769. [DOI] [PubMed] [Google Scholar]
- Rowe PS, Ong A, Cockerill F, Goulding J, Hewison M. Candidate 56 and 58 kDa protein(s) responsible for mediating the renal defects in oncogenic hypophosphataemic osteomalacia. Bone. 1996b;18:159–169. doi: 10.1016/8756-3282(95)00458-0. [DOI] [PubMed] [Google Scholar]
- Rowe PS, Oudet C, Francis F, Sinding C, Pannetier S, Econs MJ, et al. Distribution of mutations in the PEX gene in families with X-linked hypophosphataemic rickets (HYP) Hum Mol Genet. 1997;6:539–549. doi: 10.1093/hmg/6.4.539. [DOI] [PubMed] [Google Scholar]
- Rowe PS, de Zoysa P, Dong R, Wang H, White K, Econs MJ, et al. MEPE, a new gene expressed in bone-marrow and tumours causing osteomalacia. Genomics. 2000;67:54–68. doi: 10.1006/geno.2000.6235. [DOI] [PubMed] [Google Scholar]
- Rowe PS, Kumagai Y, Gutierrez G, Garrett IR, Blacher R, Rosen D, et al. MEPE has the properties of an osteoblastic phosphatonin and minhibin. Bone. 2004;34:303–319. doi: 10.1016/j.bone.2003.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S, Martel J, Ma S, Tenenhouse HS. Increased renal 25-hydroxyvitamin D3-24-hydroxylase messenger ribonucleic acid and immunoreactive protein in phosphate deprived Hyp mice: a mechanism for accelerated 1,25-dihydroxyvitamin D3 catabolism in X-linked hypophosphataemic rickets. Endocrinology. 1994;134:1761–1767. doi: 10.1210/endo.134.4.8137741. [DOI] [PubMed] [Google Scholar]
- Ruchon AF, Marcinkiewicz M, Siegfried G, Tenenhouse HS, DesGroseillers L, Crine P, et al. Pex mRNA is localized in developing mouse osteoblasts and odontoblasts. J Histochem Cytochem. 1998;46:459–468. doi: 10.1177/002215549804600405. [DOI] [PubMed] [Google Scholar]
- Ruchon AF, Marcinkiewicz M, Ellefsen K, Basak A, Aubin J, Crine P, et al. Cellular localization of neprilysin in mouse bone tissue and putative role in hydrolysis of osteogenic peptides. J Bone Miner Res. 2000a;15:1266–1274. doi: 10.1359/jbmr.2000.15.7.1266. [DOI] [PubMed] [Google Scholar]
- Ruchon AF, Tenenhouse HS, Marcinkiewicz M, Siegfried G, Aubin JE, Desgroseillers L, et al. Developmental expression and tissue distribution of Phex protein: effect of the Hyp mutation and relationship to bone markers. J Bone Miner Res. 2000b;15:1440–1450. doi: 10.1359/jbmr.2000.15.8.1440. [DOI] [PubMed] [Google Scholar]
- Sabbagh Y, Jones AO, Tenenhouse HS. PHEXdb, a locus-specific database for mutations causing X-linked hypophosphatemia. Hum Mutat. 2000;16:1–6. doi: 10.1002/1098-1004(200007)16:1<1::AID-HUMU1>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Sabbagh Y, Boileau G, DesGroseillers L, Tenenhouse HS. Disease-causing missense mutations in the PHEX gene interfere with membrane targeting of the recombinant protein. Hum Mol Genet. 2001;10:1539–1546. doi: 10.1093/hmg/10.15.1539. [DOI] [PubMed] [Google Scholar]
- Sabbagh Y, Boileau G, Campos M, Carmona AK, Tenenhouse HS. Structure and function of disease-causing missense mutations in the PHEX gene. J Clin Endocrinol Metab. 2003;88:2213–2222. doi: 10.1210/jc.2002-021809. [DOI] [PubMed] [Google Scholar]
- Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, et al. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278:2206–2211. doi: 10.1074/jbc.M207872200. [DOI] [PubMed] [Google Scholar]
- Schlesinger DH, Hay DI. Complete covalent structure of statherin, a tyrosine-rich acidic peptide which inhibits calcium phosphate precipitation from human parotid saliva. J Biol Chem. 1977;252:1689–1695. [PubMed] [Google Scholar]
- Schwartz SS, Hay DI, Schluckebier SK. Inhibition of calcium phosphate precipitation by human salivary statherin: structure-activity relationships. Calcif Tissue Int. 1992;50:511–517. doi: 10.1007/BF00582164. [DOI] [PubMed] [Google Scholar]
- Schweizer A, Valdenaire O, Koster A, Lang Y, Schmitt G, Lenz B, et al. Neonatal lethality in mice deficient in XCE, a novel member of the endothelin-converting enzyme and neutral endopeptidase family [In Process Citation] J Biol Chem. 1999;274:20450–20456. doi: 10.1074/jbc.274.29.20450. [DOI] [PubMed] [Google Scholar]
- Scriver CR, Tenenhouse HS. X-linked hypophosphataemia: a homologous phenotype in humans and mice with unusual organ-specific gene dosage. J Inherit Metab Dis. 1992;15:610–624. doi: 10.1007/BF01799618. [DOI] [PubMed] [Google Scholar]
- Segawa H, Kawakami E, Kaneko I, Kuwahata M, Ito M, Kusano K, et al. Effect of hydrolysis-resistant FGF23-R179Q on dietary phosphate regulation of the renal type-II Na/Pi transporter. Pflügers Arch. 2003;446:585–592. doi: 10.1007/s00424-003-1084-1. [DOI] [PubMed] [Google Scholar]
- Seufert J, Ebert K, Muller J, Eulert J, Hendrich C, Werner E, et al. Octreotide therapy for tumor-induced osteomalacia. N Engl J Med. 2001;345:1883–1888. doi: 10.1056/NEJMoa010839. [DOI] [PubMed] [Google Scholar]
- Shetty NS, Meyer RA., Jr Craniofacial abnormalities in mice with X-linked hypophosphatemic genes (Hyp or Gy) Teratology. 1991;44:463–472. doi: 10.1002/tera.1420440412. [DOI] [PubMed] [Google Scholar]
- Shih NR, Jo OD, Yanagawa N. Effects of PHEX antisense in human osteoblast cells. J Am Soc Nephrol. 2002;13:394–399. doi: 10.1681/ASN.V132394. [DOI] [PubMed] [Google Scholar]
- Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, et al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA. 2001;98:6500–6505. doi: 10.1073/pnas.101545198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada T, Muto T, Urakawa I, Yoneya T, Yamazaki Y, Okawa K, et al. Mutant FGF-23 responsible for autosomal dominant hypophosphatemic rickets is resistant to proteolytic cleavage and causes hypophosphatemia in vivo. Endocrinology. 2002;143:3179–3182. doi: 10.1210/endo.143.8.8795. [DOI] [PubMed] [Google Scholar]
- Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19:429–435. doi: 10.1359/JBMR.0301264. [DOI] [PubMed] [Google Scholar]
- Shipp MA, Richardson NE, Sayre PH, Brown NR, Masteller EL, Clayton LK, et al. Molecular cloning of the common acute lymphoblastic leukemia antigen (CALLA) identifies a type II integral membrane protein. Proc Natl Acad Sci USA. 1988;85:4819–4823. doi: 10.1073/pnas.85.13.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh RJ, Kumar R. Fibroblast growth factor 23 concentrations in humoral hypercalcemia of malignancy and hyper-parathyroidism. Mayo Clin Proc. 2003;78:826–829. doi: 10.4065/78.7.826. [DOI] [PubMed] [Google Scholar]
- Strewler GJ. FGF23, hypophosphatemia, and rickets: has phosphatonin been found? Proc Natl Acad Sci USA. 2001;98:5945–5946. doi: 10.1073/pnas.11154898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki N, Tada T, Morita M, Watanabe T. Comparison of inhibitory activity on calcium phosphate precipitation by acidic proline-rich proteins, statherin, and histatin-1. Calcif Tissue Int. 2002;71:59–62. doi: 10.1007/s00223-001-1084-0. [DOI] [PubMed] [Google Scholar]
- Tenenhouse HS. Recent advances in epithelial sodium-coupled phosphate transport. Curr Opin Nephrol Hypertens. 1999;8:407–414. doi: 10.1097/00041552-199907000-00003. [DOI] [PubMed] [Google Scholar]
- Tenenhouse HS, Murer H. Disorders of renal tubular phosphate transport. J Am Soc Nephrol. 2003;14:240–248. doi: 10.1097/01.asn.0000045045.47494.71. [DOI] [PubMed] [Google Scholar]
- Tenenhouse HS, Martel J, Gauthier C, Segawa H, Miyamoto KI. Differential effects of Npt2a gene ablation and the X-linked Hyp mutation on renal expression of type IIc Na/Pi cotransporter. Am J Physiol Renal Physiol. 2003;285:F1271–F1278. doi: 10.1152/ajprenal.00252.2003. [DOI] [PubMed] [Google Scholar]
- Thompson DL, Sabbagh Y, Tenenhouse HS, Roche PC, Drezner MK, Salisbury JL, et al. Ontogeny of Phex/PHEX protein expression in mouse embryo and subcellular localization in osteoblasts. J Bone Miner Res. 2002;17:311–320. doi: 10.1359/jbmr.2002.17.2.311. [DOI] [PubMed] [Google Scholar]
- Trechsel U, Schenk R, Bonjour JP, Russell RG, Fleisch H. Relation between bone mineralization, Ca absorption, and plasma Ca in phosphonate-treated rats. Am J Physiol. 1977;232:E298–E305. doi: 10.1152/ajpendo.1977.232.3.E298. [DOI] [PubMed] [Google Scholar]
- Turner AJ, Tanzawa K. Mammalian membrane metallopeptidases: NEP, ECE, KELL, and PEX. FASEB J. 1997;11:355–364. doi: 10.1096/fasebj.11.5.9141502. [DOI] [PubMed] [Google Scholar]
- Turner AJ, Isaac RE, Coates D. The neprilysin (NEP) family of zinc metalloendopeptidases: genomics and function. Bioessays. 2001;23:261–269. doi: 10.1002/1521-1878(200103)23:3<261::AID-BIES1036>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Valdenaire O, Schweizer A. Endothelin-converting enzyme-like I (ECELI; ‘XCE’): a putative metallopeptidase crucially involved in the nervous control of respiration. Biochem Soc Trans. 2000;28:426–430. [PubMed] [Google Scholar]
- Valdenaire O, Rohrbacher E, Langeveld A, Schweizer A, Meijers C. Organization and chromosomal localization of the human ECEL1 (XCE) gene encoding a zinc metallopeptidase involved in the nervous control of respiration. Biochem J. 2000;346:611–616. [PMC free article] [PubMed] [Google Scholar]
- VanScoy M, Loghman-Adham M, Onsgard M, Szczepanska-Konkel M, Homma S, Knox FG, et al. Mechanism of phosphaturia elicited by administration of phosphonoformate in vivo. Am J Physiol. 1988;255:F984–F994. doi: 10.1152/ajprenal.1988.255.5.F984. [DOI] [PubMed] [Google Scholar]
- Walton RJ, Russell RG, Smith R. Changes in the renal and extrarenal handling of phosphate induced by disodium etidronate (EHDP) in man. Clin Sci Mol Med. 1975;49:45–56. doi: 10.1042/cs0490045. [DOI] [PubMed] [Google Scholar]
- Wang L, Du L, Ecarot B. Evidence for Phex haploinsufficiency in murine X-linked hypophosphatemia. Mamm Genome. 1999;10:385–389. doi: 10.1007/s003359901007. [DOI] [PubMed] [Google Scholar]
- Weber TJ, Liu S, Indridason OS, Quarles LD. Serum FGF23 levels in normal and disordered phosphorus homeostasis. J Bone Miner Res. 2003;18:1227–1234. doi: 10.1359/jbmr.2003.18.7.1227. [DOI] [PubMed] [Google Scholar]
- Wesson JA, Johnson RJ, Mazzali M, Beshensky AM, Stietz S, Giachelli C, et al. Osteopontin is a critical inhibitor of calcium oxalate crystal formation and retention in renal tubules. J Am Soc Nephrol. 2003;14:139–147. doi: 10.1097/01.asn.0000040593.93815.9d. [DOI] [PubMed] [Google Scholar]
- White KE, Evans WE, O’Riordan JLH, Speer MC, Econs MJ, Lorenz-Depiereux B, et al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet. 2000;26:345–348. doi: 10.1038/81664. [DOI] [PubMed] [Google Scholar]
- White KE, Jonsson KB, Carn G, Hampson G, Spector TD, Mannstadt M, et al. The autosomal dominant hypophosphatemic rickets (ADHR) gene is a secreted polypeptide over-expressed by tumors that cause phosphate wasting. J Clin Endocrinol Metab. 2001;86:497–500. doi: 10.1210/jcem.86.2.7408. [DOI] [PubMed] [Google Scholar]
- Whyte MP, Schranck FW, Armamento-Villareal R. X-linked hypophosphatemia: a search for gender, race, anticipation, or parent of origin effects on disease expression in children. J Clin Endocrinol Metab. 1996;81:4075–4080. doi: 10.1210/jcem.81.11.8923863. [DOI] [PubMed] [Google Scholar]
- Wilkins GE, Granleese S, Hegele RG, Holden J, Anderson DW, Bondy GP. Oncogenic osteomalacia: evidence for a humoral phosphaturic factor. J Clin Endocrinol Metab. 1995;80:1628–1634. doi: 10.1210/jcem.80.5.7745010. [DOI] [PubMed] [Google Scholar]
- Xiao ZS, Crenshaw M, Guo R, Nesbitt T, Drezner MK, Quarles LD. Intrinsic mineralization defect in Hyp mouse osteoblasts. Am J Physiol. 1998;275:E700–E708. doi: 10.1152/ajpendo.1998.275.4.E700. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Ecarot B, Glorieux FH. Abnormal response of osteoblasts from Hyp mice to 1,25-dihydroxyvitamin D3. Bone. 1992;13:209–215. doi: 10.1016/8756-3282(92)90199-7. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Yoshioka M, Itoh N. Identification of a novel fibroblast growth factor, FGF-23, preferentially expressed in the ventrolateral thalamic nucleus of the brain. Biochem Biophys Res Commun. 2000;277:494–498. doi: 10.1006/bbrc.2000.3696. [DOI] [PubMed] [Google Scholar]
- Yamashita T, Konishi M, Miyake A, Inui KI, Itoh N. Fibroblast growth factor (FGF)-23 inhibits renal phosphate reabsorption by activation of the mitogen-activated protein kinase pathway. J Biol Chem. 2002;277:28265–28270. doi: 10.1074/jbc.M202527200. [DOI] [PubMed] [Google Scholar]
- Yamazaki Y, Okazaki R, Shibata M, Hasegawa Y, Satoh K, Tajima T, et al. Increased circulatory level of biologically active full-length FGF-23 in patients with hypophosphatemic rickets/osteomalacia. J Clin Endocrinol Metab. 2002;87:4957–4960. doi: 10.1210/jc.2002-021105. [DOI] [PubMed] [Google Scholar]