Abstract
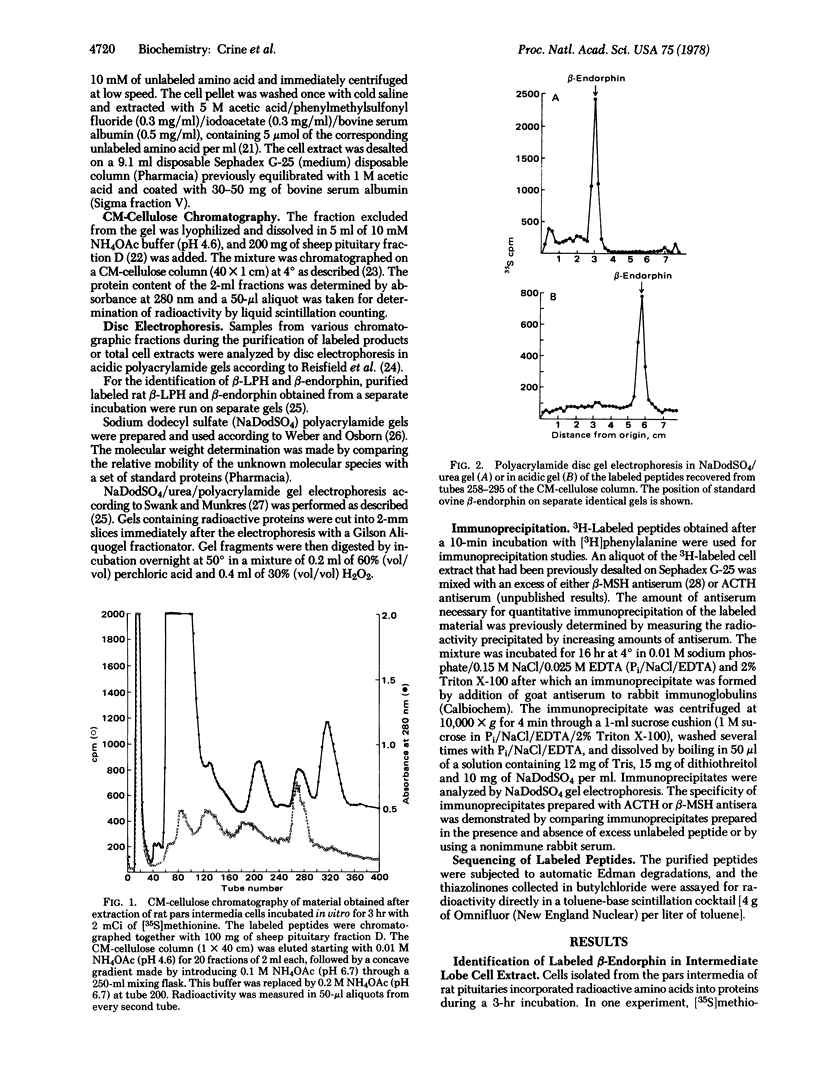
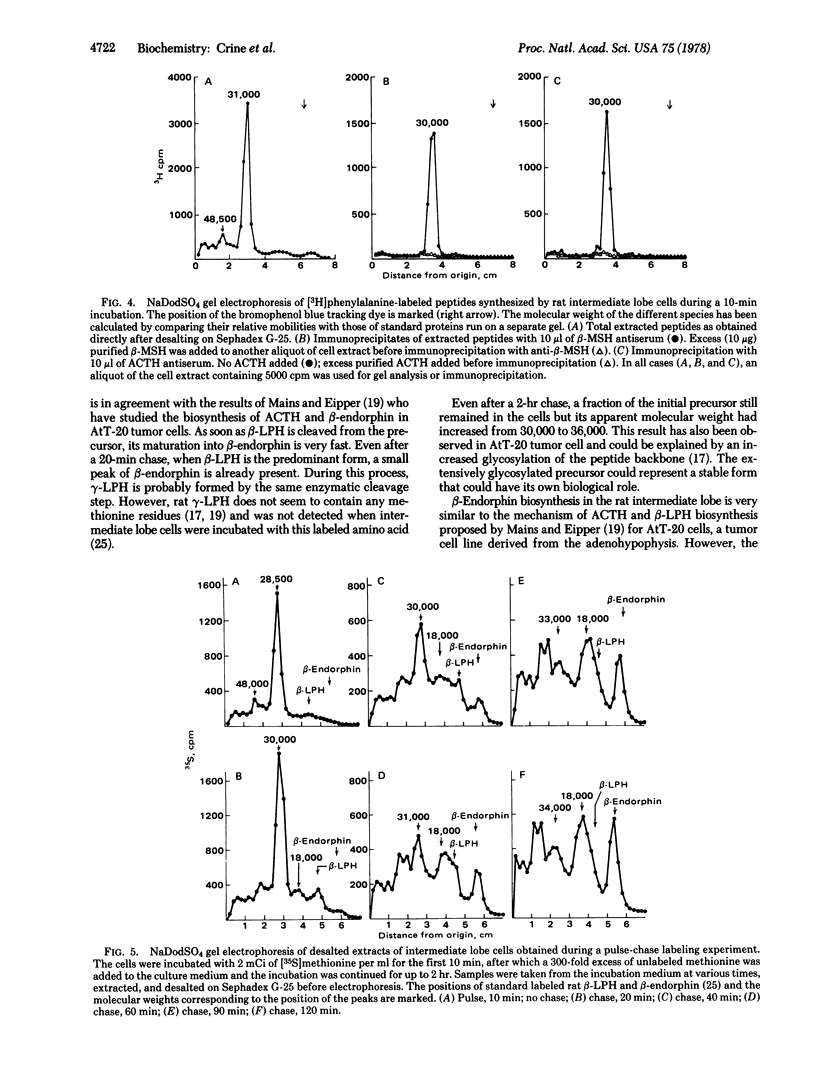
When isolated rat pars intermedia cells were incubated for 10 min with radioactive amino acids, one major labeled protein with a molecular weight of 30,000 +/- 1500 was extracted. This protein was shown to contain in its sequence the antigenic determinants for corticotropin and beta-melanotropin by immunoprecipitation. When the radioactivity incorporated into this large molecular weight protein during the first 10 min was chased by a further incubation in presence of an excess of unlabeled amino acid, the initial protein was degraded into several smaller peptides including beta-endorphin and beta-lipotropin. Another 18,000-dalton peptide was also observed and was tentatively identified as a large molecular form of corticotropin. From the kinetics of the maturation of the initial precursor, it is concluded that the initial cleavage of the 30,000-dalton peptide gives rise to beta-lipotropin and the 18,000-dalton form of corticotropin. beta-Lipotropin is subsequently cleaved to form beta-endorphin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B. I. The separation of different forms of melanocyte-stimulating hormone from the rat neurointermediate lobe by polyacrylamide gel electrophoresis, with a note on rat neurophysins. J Endocrinol. 1973 Jun;57(3):393–404. doi: 10.1677/joe.0.0570393. [DOI] [PubMed] [Google Scholar]
- Bloom F., Battenberg E., Rossier J., Ling N., Leppaluoto J., Vargo T. M., Guillemin R. Endorphins are located in the intermediate and anterior lobes of the pituitary gland, not in the neurohypophysis. Life Sci. 1977 Jan 1;20(1):43–47. doi: 10.1016/0024-3205(77)90126-6. [DOI] [PubMed] [Google Scholar]
- Chrétien M., Seidah N. G., Benjannet S., Dragon N., Routhier R., Motomatsu T., Crine P., Lis M. A betaLPH precursor model: recent developments concerning morphine-like substances. Ann N Y Acad Sci. 1977 Oct 28;297:84–107. doi: 10.1111/j.1749-6632.1977.tb41847.x. [DOI] [PubMed] [Google Scholar]
- Crine P., Benjannet S., Seidah N. G., Lis M., Chrétien M. In vitro biosynthesis of beta-endorphin in pituitary glands. Proc Natl Acad Sci U S A. 1977 Apr;74(4):1403–1406. doi: 10.1073/pnas.74.4.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crine P., Benjannet S., Seidah N. G., Lis M., Chrétien M. In vitro biosynthesis of beta-endorphin, gamma-lipoprotein, and beta-lipotropin by the pars intermedia of beef pituitary glands. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4276–4280. doi: 10.1073/pnas.74.10.4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraicer J., Gosbee J. L., Bencosme S. A. Pars intermedia and pars distalis: two sites of ACTH production in the rat hypophysis. Neuroendocrinology. 1973;11(3):156–176. doi: 10.1159/000122129. [DOI] [PubMed] [Google Scholar]
- Kraicer J., Morris A. R. In vitro release of ACTH from dispersed rat pars intermedia cells. I. Effect of secretagogues. Neuroendocrinology. 1976;20(1):79–96. doi: 10.1159/000122471. [DOI] [PubMed] [Google Scholar]
- LaBella F., Downey G., Queen G., Pinsky C. Endorphin activity in anterior, intermediate, and posterior pituitary. Can J Physiol Pharmacol. 1976 Dec;54(6):946–948. doi: 10.1139/y76-132. [DOI] [PubMed] [Google Scholar]
- LaBella F., Queen G., Senyshyn J., Lis M., Chretien M. Lipotropin: localization by radioimmunoassay of endorphin precursors in pituitary and brain. Biochem Biophys Res Commun. 1977 Mar 21;75(2):350–357. doi: 10.1016/0006-291x(77)91049-x. [DOI] [PubMed] [Google Scholar]
- Li C. H., Barnafi L., Chrétien M., Chung D. Isolation and amino-acid sequence of beta-LPH from sheep pituitary glands. Nature. 1965 Dec 11;208(5015):1093–1094. doi: 10.1038/2081093b0. [DOI] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Biosynthesis of adrenocorticotropic hormone in mouse pituitary tumor cells. J Biol Chem. 1976 Jul 10;251(13):4115–4120. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Coordinate synthesis of corticotropins and endorphins by mouse pituitary tumor cells. J Biol Chem. 1978 Feb 10;253(3):651–655. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon H. D., Li C. H., Jennings B. M. Immunohistochemical and histochemical studies of pituitary beta-lipotrophs. Anat Rec. 1973 Mar;175(3):529–537. doi: 10.1002/ar.1091750303. [DOI] [PubMed] [Google Scholar]
- Moriarty C. M., Moriarty G. C. Bioactive and immunoactive ACTH in the rat pituitary: influence of stress and adrenalectomy. Endocrinology. 1975 Jun;96(6):1419–1425. doi: 10.1210/endo-96-6-1419. [DOI] [PubMed] [Google Scholar]
- Moriarty G. C. Adenohypophysis: ultrastructural cytochemistry. A review. J Histochem Cytochem. 1973 Oct;21(10):855–894. doi: 10.1177/21.10.855. [DOI] [PubMed] [Google Scholar]
- Pelletier G., Leclerc R., Labrie F., Cote J., Chretien M., Lis M. Immunohistochemical localization of beta-lipotropic hormone in the pituitary gland. Endocrinology. 1977 Mar;100(3):770–776. doi: 10.1210/endo-100-3-770. [DOI] [PubMed] [Google Scholar]
- Pezalla P. D., Clarke W. C., Lis M., Seidah N. G., Chrétien M. Immunological characterization of beta-lipotropin fragments (endorphin, beta-MSH, and N-fragment) from fish pituitaries. Gen Comp Endocrinol. 1978 Feb;34(2):163–168. doi: 10.1016/0016-6480(78)90207-1. [DOI] [PubMed] [Google Scholar]
- REISFELD R. A., LEWIS U. J., WILLIAMS D. E. Disk electrophoresis of basic proteins and peptides on polyacrylamide gels. Nature. 1962 Jul 21;195:281–283. doi: 10.1038/195281a0. [DOI] [PubMed] [Google Scholar]
- Richelson E., Thompson E. J. Transport of neurotransmitter precursors into cultured cells. Nat New Biol. 1973 Feb 14;241(111):201–204. doi: 10.1038/newbio241201a0. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: cell-free synthesis of the precursor and identification of corticotropin peptides in the molecule. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4826–4830. doi: 10.1073/pnas.74.11.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: identification of beta-lipotropin peptides and their arrangement relative to corticotropin in the precursor synthesized in a cell-free system. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5300–5304. doi: 10.1073/pnas.74.12.5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott A. P., Lowry P. J., Ratcliffe J. G., Rees L. H., Landon J. Corticotrophin-like peptides in the rat pituitary. J Endocrinol. 1974 Jun;61(3):355–367. doi: 10.1677/joe.0.0610355. [DOI] [PubMed] [Google Scholar]
- Scott A. P., Ratcliffe J. G., Rees L. H., Landon J., Bennett H. P., Lowry P. J., McMartin C. Pituitary peptide. Nat New Biol. 1973 Jul 18;244(133):65–67. doi: 10.1038/newbio244065a0. [DOI] [PubMed] [Google Scholar]
- Seidah N. G., Gianoulakis C., Crine P., Lis M., Benjannet S., Routhier R., Chrétien M. In vitro biosynthesis and chemical characterization of beta-lipotropin, gamma-lipotropin, and beta-endorphin in rat pars intermedia. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3153–3157. doi: 10.1073/pnas.75.7.3153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M., Nicholson W. E., Orth D. N., Mitchell W. M., Island D. P., Liddle G. W. Preliminary characterization of the pituitary melanocyte stimulating hormones of several vertebrate species. Endocrinology. 1972 Jan;90(1):249–256. doi: 10.1210/endo-90-1-249. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Thody A. J. Different forms of melanocyte-stimulating hromone in the pituitary gland of the rat. Gen Comp Endocrinol. 1969 Dec;13(3):477–481. doi: 10.1016/0016-6480(69)90272-x. [DOI] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]



