SUMMARY
Nonalcoholic fatty liver disease (NAFLD) is associated with increased cardiovascular and liver-related mortality. NAFLD is characterized by both triglyceride and free cholesterol (FC) accumulation without a corresponding increment in cholesterol esters. The aim of this study was to evaluate the expression of cholesterol metabolic genes in NAFLD and relate these to disease phenotype. NAFLD was associated with increased SREBP-2 maturation, HMG CoA reductase (HMGCR) expression and decreased phosphorylation of HMGCR. Cholesterol synthesis was increased as measured by the circulating desmosterol:cholesterol ratio. miR-34a, a microRNA increased in NAFLD, inhibited sirtuin-1 with downstream dephosphorylation of AMP kinase and HMGCR. Cholesterol ester hydrolase was increased while ACAT-2 remained unchanged. LDL receptor expression was significantly decreased and similar in NAFLD subjects on or off statins. HMGCR expression was correlated with FC, histologic severity of NAFLD and LDL-cholesterol. These data demonstrate dysregulated cholesterol metabolism in NAFLD which may contribute to disease severity and cardiovascular risks.
Keywords: Nonalcoholic fatty liver disease, Nonalcoholic steatohepatitis, fatty liver, atherosclerosis, cholesterol, HMG CoA reductase, lipogenesis, hypercholesterolemia
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is a common cause of chronic liver disease (Browning et al., 2004; Wanless and Lentz, 1990). The clinical-histologic phenotype of NAFLD extends from a nonalcoholic fatty liver (NAFL) to nonalcoholic steatohepatitis (NASH) (Ludwig et al., 1980). NASH can progress to cirrhosis in up to 15% of affected subjects (Adams et al., 2005; Ekstedt et al., 2006). Several studies have also linked NAFLD to an increased risk of cardiovascular disease, a leading cause of death in this population (Brea et al., 2005; Hanley et al., 2005; Targher et al., 2007; Villanova et al., 2005). The potential mechanisms underlying these findings remain to be fully defined.
NAFLD has classically been associated with increased hepatic triglycerides (Browning and Horton, 2004). Lipidomic analyses of NAFLD have however demonstrated that there is also an accumulation of free cholesterol (FC) without a similar increment in cholesterol esters (CE) in both NAFL and NASH (Puri et al., 2007). We evaluated the expression of genes involved in hepatic cholesterol metabolism to better understand the molecular basis for FC accumulation. Given the known cellular toxicity of FC and the linkage of NAFLD with cardiac disease (Devries-Seimon et al., 2005; Geng et al., 2003; Ginsberg, 2006), we further evaluated the relationship of changes in the molecular pathways of cholesterol metabolism to liver disease severity and known risk factors for cardiovascular disease.
A previous study found increased HMG CoA reductase (HMGCR) expression in NASH but did not clarify if this was due to underlying obesity or the liver disease (Caballero et al., 2009). It also did not provide detailed information on the status of all cholesterol metabolic pathways in the various phenotypes of NAFLD. We evaluated the mRNA and protein expression of genes involved in cholesterol metabolism in subjects with biopsy-proven NAFL or NASH and compared them to lean normal individuals and age, gender and weight matched controls without liver disease. We demonstrate numerous changes in the expression status of genes involved in cholesterol metabolism and clarify those that are related to underlying obesity versus those related to NAFLD. We further evaluated the functional significance of the observed changes by measurement of surrogate markers of cholesterol synthesis.
A key finding is that the expression of HMGCR is increased in both NAFL and NASH and that it is related to FC levels and the severity of the liver disease. Moreover, the HMGCR is relatively dephosphorylated in both phenotypes of NAFLD and thus in its active form (Beg et al., 1985, 1987). MiR-34a is a microRNA that is overexpressed in NASH (Cheung et al., 2008). It inhibits sirtuin-1 which is known to dephosphorylate AMP kinase a known regulator of HMGCR phosphorylation (Beg et al., 1984; Chaudhary and Pfluger, 2009; Clarke and Hardie, 1990). To evaluate the potential role of miR-34a as a regulator of HMGCR phosphorylation, the effects of overexpression and silencing of miR-34a were evaluated in Huh-7 cells. We demonstrate that miR-34a enhances HMGCR dephosphorylation which represents a novel function of miR-34a of potential clinical relevance in NASH.
LDL-cholesterol (LDL-C) is a well-known risk factor for cardiac disease. We also demonstrate that HMGCR and LDL receptor (LDLR) are directly and inversely related to LDL-C respectively in subjects with NAFLD. Although statin therapy lowers LDL-C in subjects with NAFLD, the LDLR and HMGCR expression remain at the levels seen in those not on statins. Together, these data suggest widespread abnormalities in hepatic cholesterol metabolism in NAFLD which are tightly associated with disease activity and cardiovascular risk factors specifically LDL-C.
RESULTS
1. The patient population studied
A total of 20 subjects with NAFL and NASH each were enrolled and compared to 20 obese controls and 6 lean normal controls. The summary of demographic, clinical and laboratory data are shown in Table 1. Subjects with NAFL or NASH were similar with respect to gender, ethnicity, body mass index, alkaline phosphate, total cholesterol (TC), and LDL-C to the obese control group. As expected, lean normal subjects had lower insulin concentrations, LDL-C and aspartate and alanine aminotransferases compared to subjects with NAFL or NASH. NASH subjects had significantly higher triglyceride levels compared with the lean and obese controls (P<0.01 and 0.006 respectively). Lean normal control subjects had lower LDL-C compared to those with NAFL or NASH (p<0.0001) while only those with NASH had a significantly higher LDL-C compared to obese controls (p< 0.005). A small minority of subjects in the obese controls, NAFL and NASH had type 2 diabetes mellitus that was controlled with diet alone. These subsets did not differ from the rest of the subjects in their groups with respect to other parameters.
Table 1.
Baseline demographic, clinical and laboratory data
| Parameter | Lean normal N= 6 Mean ± S.D. |
Obese normal N= 20 Mean ± S.D. |
NAFL N= 20 Mean ± S.D. |
NASH N=20 Mean ± S.D. |
P value |
|---|---|---|---|---|---|
| Age (yrs) | 42.5 ± 7.5 | 44 ± 6.5 | 46.4 ± 7.5 | 52.2 ± 7.7 | n.s. |
| Males:females (n) | 3:5 | 6:14 | 7:13 | 8:12 | n.s. |
| Caucasian (n) | 6 | 16 | 15 | 18 | n.s. |
| BMI (kg/m2) | 21.4 ± 2.2 | 33.2 ± 2.2 | 34.1 ± 3 | 34.2 ± 3 | < 0.0001* |
| Type 2 diabetes mellitus (n) | 0 | 3 | 5 | 5 | n.s. |
| Hypertension (n) | 0 | 8 | 12 | 12 | 0.02** |
| AST (IU/l) | 18 ± 5 | 19 ± 7 | 47 ± 21 | 56 ± 23 | <0.002** |
| ALT (IU/l) | 22 ± 6 | 24 ± 5 | 69 ± 32 | 72 ± 37 | <0.003** |
| Alk phos (IU/l) | 86 ± 21 | 90 ± 9 | 97 ± 18 | 101 ± 21 | n.s. |
| Bilirubin (mg/dl) | 0.2 ± 0.08 | 0.3 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | n.s. |
| Albumin (gm/dl) | 4.2 ± 0.2 | 4 ± 0.4 | 3.9 ± 0.3 | 4 ± 0.3 | n.s. |
| Fasting blood sugar (mg/dl) | 78 ± 8 | 85 ± 9 | 92 ± 12 | 93 ± 14 | n.s. |
| Fasting insulin (uIU/dl) | 6.5 ± 3 | 17.2 ± 8 | 18 ± 12 | 22 ± 9 | <0.004* |
| Hemoglobin A1C (%) | 5.9 ± 0.3 | 6.3 ± 1 | 6.5 ± 1.5 | 6.3 ± 1.2 | n.s. |
| Total cholesterol (mg/dl) | 135 ± 21 | 142 ± 34 | 155 ± 32 | 146 ± 34 | n.s. |
| LDL-cholesterol (mg/dl) | 82 ± 27 | 118 ± 16 | 128 ± 17 | 132 ± 14 | <0.01*** |
| HDL-cholesterol (mg/dl) | 62 ± 8 | 46 ± 10 | 42 ± 12 | 38 ± 13 | <0.03** |
| Triglycerides (mg/dl) | 145 ± 35 | 152 ± 41 | 175 ± 45 | 186 ± 33 | <0.01* |
| Steatosis grade | 0 | 0 | 2.5 | 2.8 | N/A |
| Inflammation grade | 0 | 0 | 0 | 2.4 | N/A |
| Cytologic ballooning grade | 0 | 0 | 0 | 1.9 | N/A |
| Fibrosis stage | 0 | 0 | 0 | 1.6 | N/A |
lean vs other groups
NAFL or NASH vs either control group
NAFL and NASH higher than lean control, but only NASH significantly higher than obese controls
2. Status of Cholesterol metabolic pathways
A: Cholesterol synthetic pathways are up regulated in NAFLD
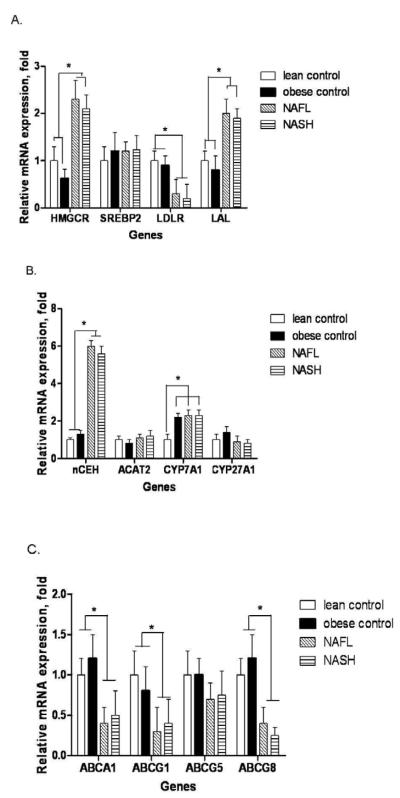
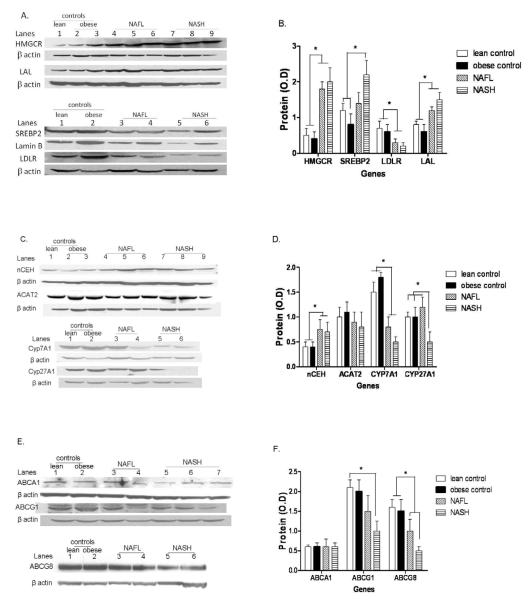
The expression of HMGCR, the rate-limiting enzyme for cholesterol synthesis, was similar in lean vs. obese normal controls. Both NAFL and NASH were associated with a 2-3 fold increase in HMGCR mRNA expression (p< 0.001 vs. lean or obese controls for both) and 3-4 fold increase in HMGCR protein levels (Figs. 1a and 2a-b). The increase in HMGCR expression was not accompanied by a concomitant increase in the mRNA levels of SREBP-2 the principal transcriptional regulator of HMGCR (Fig.1a). However, the nuclear protein content of SREBP-2 was increased significantly in NASH (Fig.2a and b). There were no significant differences between diabetic versus nondiabetic subjects within individual groups.
FIGURE 1.
mRNA expression of key genes associated with hepatic cholesterol metabolism. (A): obese normal controls had the same level of expression of HMGCR, SREBP-2, LDLR and LAL as lean normal controls. However, both NAFL and NASH were associated with a significantly increased HMGCR (* p< 0.001 for both vs. either control) and LAL (* p< 0.002 for both vs. either control) expression and decreased LDLR expression (* p< 0.01 for both NAFL or NASH vs. either control), (B): NAFL and NASH were associated with significantly elevated nCEH expression (* p< 0.0001 for both vs. lean or obese controls). Obese controls, NAFL and NASH had a significantly increased CYP7A expression compared to lean controls (* p< 0.02), (C): Compared to lean and obese normal controls which were similar, NAFL and NASH were associated with a significant decrease in expression of ABCA1, ABCG1 and ABCG8 (* p< 0.05 for all). Mean ± S.D. shown for all data.
FIGURE 2.
Western blot analysis and graphic summary of protein expression (mean ± S.D.) of HMGCR, LAL, SREBP-2 and LDLR in lean controls, obese controls, NAFL and NASH (panels A and B respectively), nCEH, ACAT2, CYP7A and CYP27A (panels C and D), and ABCA1, ABCG1 and ABCG8 (panels E and F). β-actin was used as a loading control for all except SREBP-2 which was performed on nuclear extracts and where lamin B was used as the loading control. NAFL and NASH were associated with a highly significant increase in HMGCR (* p< 0.0001 for both). SREBP-2 levels were significantly higher in NASH compared to controls (* p< 0.02). LDLR was significantly decreased in both NAFL and NASH (* p< 0.03 for both compared to controls) while LAL was increased (p< 0.02 for both compared to controls). NAFL and NASH were also associated with significantly increased nCEH (* p< 0.01 for both vs. controls) along with decreased expression of CYP7A (* p< 0.05 for both). There was a stepwise decrease in ABCG8 expression from normal to NAFL to NASH (p< 0.05 for NASH vs. lean and obese controls). CYP27A was decreased in NASH alone compared to controls and NAFL (p< 0.02 vs. both controls and NAFL).
B: Cholesterol uptake pathways are down regulated in NAFLD
LDL-C is taken up via LDLR and then de-esterified in late endosomes and the FC returned to the plasma membrane or used for cellular needs (Ikonen, 2008; Tabas, 2000). The mRNA and protein levels of LDLR and Lysosomal acid lipase (LAL) were similar in lean and obese controls. In contrast to the increase in HMGCR expression, both mRNA and protein levels of LDLR were significantly decreased in subjects with NAFL or NASH (Figures 1a and 2a-b). The expression of PCSK9, a known regulator of LDLR (Wang et al., 2011), was similar across the study groups (supplemental Fig. 2). LAL expression was increased in NAFL and NASH. Once again, there were no significant differences between diabetic versus nondiabetic subjects within individual groups.
C: Increased expression of Cholesterol de-esterification pathways in NAFLD
Neutral cholesterol ester hydrolase (nCEH) de-esterifies CE and returns cholesterol to the intrahepatic FC pool. There was a highly significant increase in the mRNA and protein levels of nCEH in both subjects with NAFL or NASH compared to controls (Figure 1b and 2c-d).
D: Decreased expression of cholesterol metabolizing pathways in NAFLD
In contrast to the observed increase in nCEH expression, the expression of ACAT-2 was not significantly altered in subjects with either NAFL or NASH (Fig. 1b and 2c-d). The mRNA levels of cholesterol 7α hydroxylase (CYP7A) increased significantly in obese controls compared to lean controls; this was accompanied by a smaller but not statistically significant increase in CYP7A protein levels in obese controls. On the other hand, there was a highly significant step wise decrease in CYP7A protein in NAFL and NASH compared to both groups of controls (Fig. 2c-d). CYP27A mRNA levels did not change in NAFLD. However, CYP27A protein levels were significantly decreased in NASH alone (Fig. 2c-d).
E: Cholesterol exporter expression is decreased in NAFLD
The mRNA levels of ABCA1 and ABCG1 were both significantly decreased in NAFL as well as NASH compared to either control group (Fig. 1c). However, while ABCA1 protein levels were similar across the four groups, ABCG1 protein levels were significantly decreased in NASH only compared to the two control groups (Fig. 2e-f). The protein levels of ABCG1 in NAFL were intermediate between the controls and NASH and were not significantly different from either (Fig. 2c-d). There were no significant changes in the expression of the biliary canalicular cholesterol transporter ABCG5; however, there was a stepwise decrease in ABCG8 mRNA and protein levels from normal to NAFL to NASH which was significant for NASH (Figs 1c and 2c-d).
3. NAFLD phenotype is related to HMGCR expression and FC accumulation
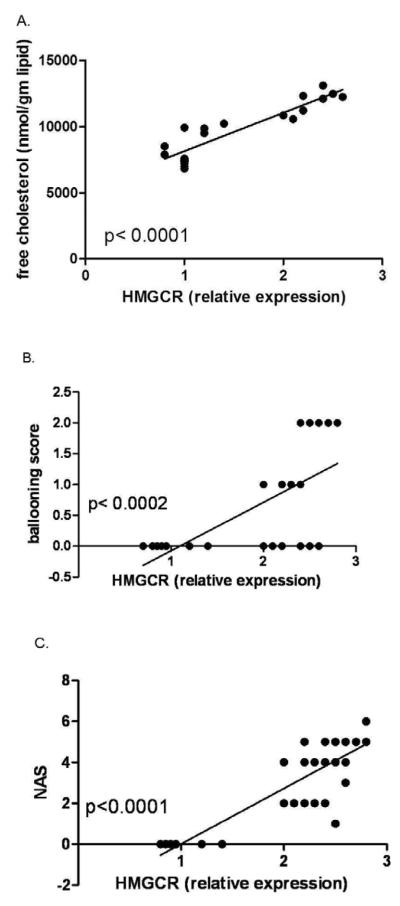
HMGCR expression alone was strongly related to hepatic FC (Fig. 3a). There was no significant correlation between HMGCR expression and the severity of hepatic steatosis or inflammation. There was however a significant direct relationship between the presence and severity of cytologic ballooning with hepatic FC as well as HMGCR expression (Fig. 3b). The NAFLD activity score was also significantly related to HMGCR expression (Fig.3c). NASH was also associated with significantly lower levels of CYP27A and a trend for lower biliary cholesterol transporter ABCG8 expression compared to those with NAFL.
FIGURE 3.
Relationship of HMGCR expression versus FC (A) in 8 subjects with NAFLD and 8 controls where enough liver tissue for simultaneous FC measurement, mRNA and protein analysis was available. There was a strong and direct relationship between HMGCR and hepatic FC (p< 0.0001). The relationship of HMGCR and cytologic ballooning scores (B) and the NAFLD activity score (C) in subjects with NAFL or NASH are also shown. There was also a strong and direct relationship to cytologic ballooning and the overall NAFLD activity score (NAS). There was no relationship to other features of NASH (data not shown).
4. NAFLD is associated with dephosphorylation of HMGCR and increased FC synthesis
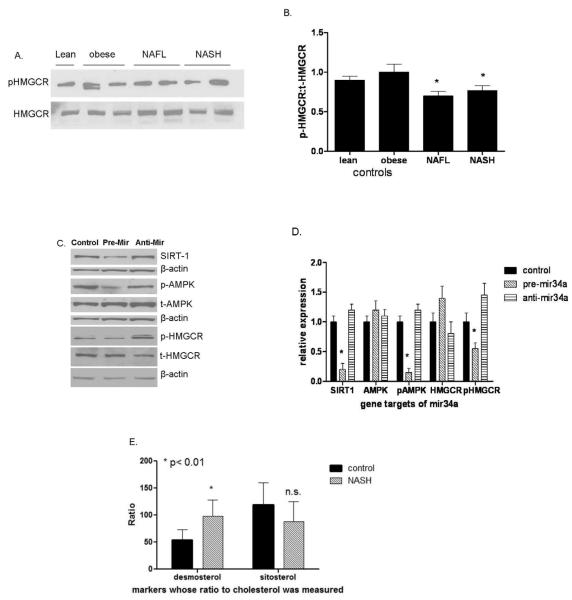
Compared to both lean and obese controls, both NAFL and NASH were associated with a decrease in HMGCR phosphorylation indicating that not only is HMGCR expression increased but that it is more biologically active (Fig. 4a-b) (Burg and Espenshade, 2011). Mir-34a, a miRNA that is increased in NASH, suppresses sirtuin-1 which regulates the activity of AMP kinase (AMPK) a known regulator of HMGCR phosphorylation (Chaudhary and Pfluger, 2009; Cheung et al., 2008; Clarke and Hardie, 1990). To further evaluate the effect of miR-34a on HMGCR phosphorylation, the impact of miR-34a over-expression or silencing on its sequential downstream targets sirtuin-1, AMPK and HMGCR were studied (Figure 4 c-d). MiR-34a over-expression decreased sirtuin-1 levels and the levels of phosphorylated AMPK and HMGCR. Conversely, silencing miR-34a had the opposite effect. The functional impact of the relatively dephosphorylated state of HMGCR in NASH was confirmed by a significantly increased desmosterol:total cholesterol ratio in such subjects compared to controls (Fig. 4e) (Matthan and Lichtenstein, 2004; Matthan et al., 2010). The sitosterol:total cholesterol ratio, a marker of cholesterol absorptive activity (Matthan et al., 2010), was not significantly changed in subjects with NASH.
FIGURE 4.
NAFL and NASH were associated with less phosphorylation of the HMGCR compared to lean or obese controls (Western blot of representative subjects (A) and graphic summary of data (n=6 for each group), p< 0.05 for both (B). In another set of experiments, miR-34a, a microRNA that is overexpressed in NASH, was over-expressed or silenced in Huh-7 cells (panels C and D). Over-expression of miR-34a suppressed Sirt1 and the phosphorylation of downstream AMP kinase and HMGCR. Silencing miR-34a had the opposite effect. These data indicate that miR-34a can modulate the phosphorylation of HMGCR. To further determine if these changes were reflected in increased cholesterol synthetic activity, the circulating desmosterol to cholesterol ratio was measured in subjects with NAFLD (n=10) versus lean controls (n=10) (E). The desmosterol:cholesterol ratio in subjects with NAFLD was almost double that in controls confirming that there was increased cholesterol synthetic activity. The sitosterol:cholesterol ratios were not significantly different indicating that cholesterol absorption was not significantly different. Mean ± S.D. shown for all graphical data.
5. LDL-C is related to hepatic cholesterol metabolic pathways
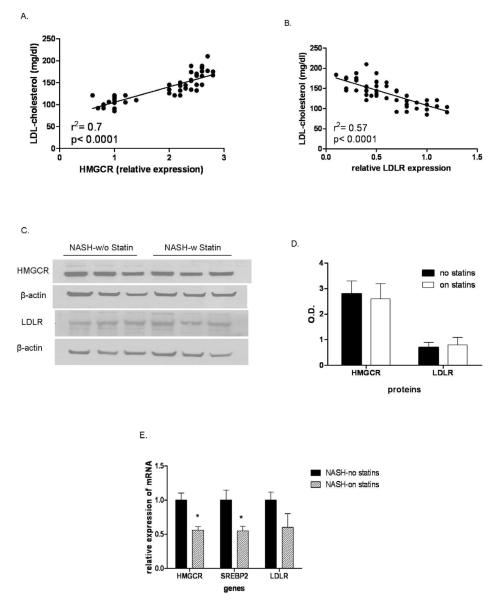
The levels of LDL-C were closely related to HMGCR expression and inversely related to LDLR expression (Fig. 5a-b). Many subjects with NAFL or NASH receive statins to decrease their LDL-C, a key pro-atherogenic risk factor. To further investigate if statin therapy could reverse or modify the changes noted in hepatic cholesterol metabolic pathways, a second group of subjects with NASH receiving statins (n=6) at the time of diagnosis of NASH were compared to those not on statins or any other hypolipidemic agents. These two groups were comparable with respect to demographic, clinical and laboratory parameters with the exception of lower LDL-C in those on statins (117 ± 24 vs. 132 ± 14 mg/dl, p< 0.02). Subjects with NASH on statins however had lower HMGCR and LDLR mRNA compared to those not on statins and the protein expression were similar in the two groups (Fig. 5c-e). The ballooning and NAFLD activity scores were somewhat lower in those taking statins but this was not statistically significant.
FIGURE 5.
Circulating LDL-C was directly related to HMGCR expression levels (A) and inversely related to LDLR expression (B) (data for all subjects from all groups shown). Subjects with NASH who were on statins for more than six months were compared to those who were not on statins. HMGCR and LDLR protein levels, shown as a Western blot in three representative subjects each (C), were similar in both groups (shown graphically for six subjects each in panel D). mRNA levels of HMGCR and SREBP-2 were paradoxically lower in subjects on statins (p< 0.05 for both) compared to those not on statins (E). Data for 6 subjects on statins were compared to 20 subjects not on statins. Mean ± S.D. shown for all graphical data.
DISCUSSION
The liver plays a central role in cholesterol homeostasis. The current study provides evidence for multiple and complex alterations in the pathways of cholesterol homeostasis in both NAFL and in NASH, the two major phenotypes of NAFLD. While these studies in human tissues did not permit interventions to define the precise role of specific changes in the accumulation of FC in NAFLD, they do provide a detailed snap-shot of changes in cholesterol metabolic pathways in this condition and clues to the basis for FC accumulation in afflicted subjects.
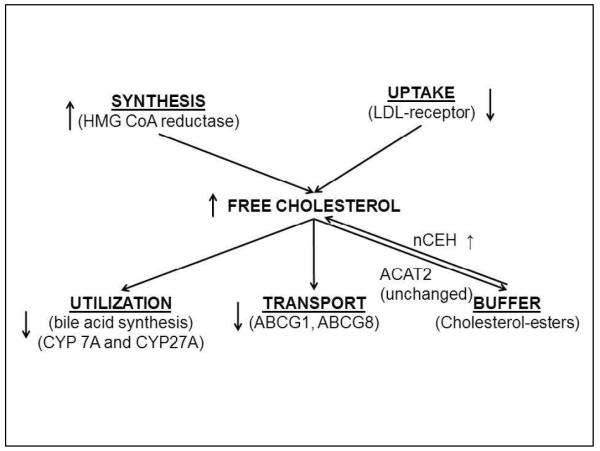
The present studies indicate that a combination of increased production, decreased utilization and transport (Fig. 6) could contribute to the accumulation of FC in NAFLD. These include activation of HMGCR and nCEH on one hand and inhibition of CYP7A and ABCG8 on the other. Of these, the increase in HMGCR was most dominant and its expression was tightly linked to both hepatic FC and the NAFLD activity score. It was also associated with the serum LDL-C levels. These data support the concept that HMGCR over-expression is a key factor driving both the LDL-C and accumulation of hepatic FC in NAFLD.
FIGURE 6.
A schematic showing the key molecular changes in cholesterol metabolic pathways in NASH compared to lean or obese controls. Increased HMGCR and nCEH expression would be expected to increase the FC pool. Utilization of FC for bile acid synthesis is decreased due to decreased CYP7A and CYP27A expression. ABCG1 and ABCG8 expression are also decreased which would be expected to decrease export of cholesterol from hepatocytes.
The increase in both HMGCR mRNA and protein levels in NAFLD suggests that it is transcriptionally activated. Increased activation of SREBP-2, the principal transcriptional activator of HMGCR (Horton et al., 1998), appears to be the mechanism for increased HMGCR expression. These findings appear to be specific for NAFLD because they were not seen in obese, weight-matched controls. We have also not seen increased HMGCR mRNA expression in hepatitis C (provided in supplemental Fig. 1). There are several potential mechanisms that could contribute to SREBP-2 activation in NAFLD including increased FC content, unfolded protein response (Puri et al., 2007; Puri et al., 2008). The precise mechanisms now await experimental elucidation.
HMGCR is not only increased in NAFLD but is also relatively dephosphorylated and in its active form in NAFLD (Clarke and Hardie, 1990). The functional consequence of increased HMGCR activity was corroborated by the nearly doubled levels of the desmosterol:cholesterol ratio, a marker of cholesterol synthetic activity (Matthan et al., 2010), in subjects with NAFLD. Our data indicate that miR-34a can modulate HMGCR phosphorylation and support the concept that increased miR-34a may play a role in maintaining HMGCR in its active form in NAFLD. It is however recognized that the only way to be certain is to suppress miR-34a and evaluate its impact on HMGCR and hepatic cholesterol accumulation in NAFLD. Unfortunately there are no reagents currently available to selectively suppress miR-34a in humans. Also, diet-induced animal models of NAFLD have a proportional increase in FC and CE (personal observation) and are thus different from humans with NAFLD (Puri et al., 2007).
It is well known that uptake of LDL-C via LDLR provides feedback inhibition of HMGCR by inhibition SREBP-2 activation (Goldstein and Brown, 2009). One could therefore hypothesize that decreased hepatic uptake of LDL-derived cholesterol due to decreased LDLR in NAFLD causes disinhibition of SREBP-2 maturation and consequent transcriptional activation of HMGCR. However, this would also be expected to increase LDLR expression unless it was unable to overcome the effects of the primary cause for LDLR suppression. PCSK9 is a known inhibitor of LDLR expression (Horton et al., 2007). However, PCSK9 mRNA was similar across our study groups suggesting that other mechanisms are responsible for the low LDLR expression in NAFLD.
NASH is also associated with a decrease in CYP7A and CYP27A levels; CYP27A is a mitochondrial protein and its decrease may reflect mitochondrial injury that is present in NASH but not in NAFL (Caldwell et al., 2009; Perez-Carreras et al., 2003). Also, biliary cholesterol would be expected to be decreased by the decrease in ABCG8. Cholesterol gallstones are however prevalent in this obese population of subjects (Chen et al., 2006). This suggests that ABCG5/ABCG8-independent biliary cholesterol secretion is present in NASH. These possibilities and their implications need further clarification.
Prior studies have focused on the role of free fatty acids as a key driver of lipotoxicity in NAFLD and support the concept that triglyceride synthesis diminishes fatty acid toxicity by acting as a sump to limit fatty acid accumulation (Feldstein et al., 2004; Yamaguchi et al., 2008). The current study extends the concept of lipotoxicity to FC accumulation by providing evidence for a relationship between HMGCR activity and FC accumulation to the severity of histologic injury and stage of disease. From a cardiovascular point of view the direct relation between LDL-C and HMGCR expression suggests that the liver disease contributes to LDL-mediated cardiovascular risk. Also, the lower than expected LDLR expression on statin therapy may negatively impact the benefits of statins in this population. This however requires prospective validation because it is at least theoretically possible that subjects on statins had a lower LDLR prior to starting treatment compared to those not on statins.
The inclusion of subjects with type 2 diabetes could be considered as a potential confounder. However, given the high prevalence of diabetes in subjects with NASH, the failure to include such subjects would limit the generalizability of the data. These patients were otherwise similar to the rest of their cohorts and no differences were noted between these subjects and the rest of the subjects within each group. It is however possible that with more severe diabetes additional differential changes in cholesterol metabolic pathways could be uncovered.
In summary, the current study provides evidence for widespread abnormalities in cholesterol homeostatic pathways in NAFLD. The key findings are an increase in HMGCR expression and decreased expression of LDL receptors and bile acid synthetic enzymes. The mechanisms for these abnormalities and the impact of the changes in specific pathways on disease activity and progression as well as accelerated atherosclerosis in NASH await elucidation.
EXPERIMENTAL PROCEDURES
Human Subjects and Liver Biopsies
Four groups of subjects were studied: (1) biopsy-proven NASH, (2) biopsy proven nonalcoholic fatty liver (NAFL), (3) weight- and gender-matched obese subjects with normal liver histology, and (4) lean subjects with normal liver histology. Normal liver status was defined as normal liver enzymes and imaging studies along with an asymptomatic status. Fatty liver and steatohepatitis were diagnosed using standard criteria used before (Kleiner et al., 2005). The nonalcoholic nature of the disease was established clinically using a daily intake cutoff of < 20 gm of alcohol for females and < 30 gm/day for males within the past five years, as used in other studies of this disease (Chalasani et al., 2009). Subjects with diet-controlled type 2 diabetes mellitus were allowed to be included because many subjects with NAFLD have type 2 diabetes their exclusion could limit the generalizability of the data.
The exclusion criteria for the study included failure to obtain informed consent, concurrent pregnancy, presence of advanced liver fibrosis (stage 3 or 4 disease), liver failure (elevated bilirubin or INR > 1.5), greater than 5% weight gain or loss within prior 3 months, HIV infection, concomitant presence of other liver diseases and use of drugs known to affect lipid metabolism e.g. statins, fibrates, polyunsaturated fatty acids etc. or impact NASH e.g. vitamin E, thiazolidinediones and pentoxyfylline. For studies on the effect of statins, subjects who met inclusion criteria for NASH or NAFL but were on a stable dose of statins for over 6 months were studied.
Liver tissue was obtained at the time of clinically indicated liver biopsies or at the beginning of a non-hepatic elective operation (bariatric surgery or cholecystectomy) for obese normal subjects. Lean normal liver tissue was obtained from a tissue repository in the department of surgical pathology at the author’s institution. Tissue was snap frozen in liquid nitrogen for future use. All subjects provided informed consent. The study was approved by the institutional IRB (VCU IRB # 1960).
Quantification of gene expression using real time PCR
Primers were designed by using Beacon Designer™ software (Bio-Rad Laboratories, Inc.) and verified by using BLAST search (Table S1). These included ABCA1, ABCG1, ABCG5, ABCG8, ACAT2, CYP7A1, CYP27A1, HMGCR, LAL, LDLR, nCEH, PCSK9 and SREBP-2. Total RNA from liver biopsies was extracted with a commercial RNA isolation kit (Trizol, Invitrogen), in each case according to the manufacturer’s instructions. Following purification with RNase-free DNase to remove genomic DNA from the preparation, cDNA was synthesized from 4 μg of total RNA by using Maloney-murine leukemia virus reverse transcriptase and oligo(dT) primers and subjected to PCR by the manufacturer’s instructions. The quantitative RT-PCR was performed using SYBR Green PCR master mix (BioRad, Hercules, CA) on an ABI Prism 7300 Sequence Detection System as described before (Puri et al., 2008): 50°C for 2 minutes and then at 95°C for 10 minutes followed by 40 cycles of amplification (95°C for 15 seconds; 60°C for 30 seconds; 80°C for 30 seconds). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as the endogenous normalizer. The specificity of qPCR was established by incorporating no template and no reverse transcript controls. Total RNA extracted from a histologically normal liver of a lean normal individual obtained at the time of living donor liver transplantation was used as an internal calibrator across all experiments. Cycle threshold (Ct) values were normalized to GAPDH and comparative quantification of target mRNA done by the ΔΔCt method using integrated software with Stratagene Mx3000P® QPCR system.
Quantitative RT-PCR measurement of miRNA
This was performed as previously described. Total RNA and small micro RNAs were isolated and extracted from frozen liver tissues or cell cultures as previously described (Puri et al., 2008). cDNA was synthesized using TaqMan MicroRNA Reverse Transcriptase (Applied Biosystems, Foster City, CA) according to manufacturer’s instructions. RT-PCR was performed in triplicates using an Applied Biosystems 7300 Sequence Detection system, as described by Bloomston (Bloomston et al., 2007). The relative abundance of miRNA was calculated after normalization to small nuclear RNA U6 (RNU6B, Applied Biosystems) to the manufacturer’s protocol.
Protein extraction and Western blot analysis
This was performed as previously described by us (Puri et al., 2008). Human liver tissues were homogenized using lysis buffer (Sigma, St.Louis, MO) and then sonicated on ice with a Sonicator cell disrupter, Model 100 Sonic Dismembrator (Thermo Fisher Scientific Inc., Waltham, MA; power 2, 6 pulses ×2). Cell lysates were centrifuged at 12000 g x 30 minutes and proteins in the supernatants quantified and separated using 4–12% NuPAGE® Novex Bis-Tris Mini Gels (Invitrogen) and were transferred to a nitrocellulose membrane for 1 h at 40 V using a Western blot apparatus (Invitrogen). After overnight incubation with primary antibody, membranes were washed and incubated with HRP-conjugated secondary antibodies (Pierce Biotechnology, Inc., Rockford, IL) and were detected using the SuperSignal chemiluminescence kit (Pierce). The signal capture and protein levels analyses were performed as described before (Cheung et al., 2008). SREBP-2 and Lamin B were measured in nuclear extracts by Western blots as described before (Cheung et al., 2008).
Cell culture and transfection protocols
Huh-7 cell line was grown in DMEM containing 10% fetal bovine serum (FBS), 100 IU/ml penicillin and 100 g/ml Streptomycin. Total RNA from Huh-7 cell was extracted with a commercial RNA isolation kit (Trizol, Invitrogen), in each case according to the manufacturer’s instructions. miR-34a and miR-34a-inhibitor were purchased from Dharmacon RNA Technologies(Lafayette, CO, USA). Cells were plated to 50% confluency and were transfected with 40 nmol/l miR-34a, miR-34a inhibitor or negative control by Lipofectamine 2000 (Invitrogen) in Opti-MEM (Invitrogen, Carlsbad, CA), according to the manufacturer’s protocol. Transfection efficiency was monitored with FAM dye-labeled Pre-miR™ Negative Control #1 (Ambion. Co.). After 48 h, cells were harvested for analysis.
Measurement of desmosterol, sitosterols
Briefly, 200 microliters of serum was spiked with deuterated internal standard and the sterols in the sample were extracted from the specimen using hexane. The organic supernatant was dried down using a turbovap at 40°C and the samples were reconstituted in 80:20 methanol:isopropanol and analyzed via liquid chromatography tandem mass spectrometry (LC-MSMS), monitoring specific m/z transitions for each sterol. The analysis is performed on an AB Sciex 5500 MSMS with Shimadzu Prominence 20 series pumps.
Statistical analysis and sample size estimation
Sample size estimations were performed using N Query Advisor 7.0. To test the hypothesis that size (mean levels controls vs. disease) of 1.25 for NAFL and 1.5 for NASH, and assuming a standard deviation of 25% for each of the groups and a power of 80% to detect changes at a p value of 0.05, a total of 6 subjects would be required in each arm. If the effect size was a 2-fold change in NASH, and 1.5 fold change in NAFL, the sample size dropped to 3 subjects in each arm. Not knowing the exact standard deviation for each of the biologic parameters to be measured, a minimum sample size of 20 subjects in each group was planned with the exception of lean normals where 6 subjects were studied (mainly due to limited availability of tissue). The RNA and protein levels for a given gene were compared across groups using Kruskal Wallis analysis of variance (ANOVA), a distribution-free test. A Bonnferroni post-test was used for multiple comparisons. Significance was set at a p value of 0.05.
Supplementary Material
SUPPLEMENTAL FIGURE 1: HMGCR mRNA expression in lean controls, obese controls and subjects with hepatitis C (n=20) were compared. HMGCR mRNA expression was significantly lower than controls in subjects with hepatitis C virus infection.
SUPPLEMENTAL FIGURE 2: PCSK9 mRNA expression was measured in lean and obese controls (n=6 each) and in subjects with NAFL or NASH (n=8 each). There were no significant across group differences by ANOVA.
HIGHLIGHTS.
There is increased HMGCR and decreased LDL receptor expression in NAFLD.
HMGCR expression correlates with FC accumulation and histologic severity of NAFLD.
LDL-C varies directly with HMGCR and inversely with LDLR expression in NAFLD.
miR-34a dephosphorylates HMGCR and may increase cholesterol synthesis in NAFLD.
ABBREVIATIONS
- NAFLD
Nonalcoholic fatty liver disease
- NASH
Nonalcoholic steatohepatitis NAFL: Nonalcoholic fatty liver
- FC
Free Cholesterol
- TC
Total cholesterol
- CE
Cholesterol ester
- LDL
Low density lipoprotein
- LDLR
Low density lipoprotein receptor
- nCEH
neutral cholesterol ester hydrolase
- ACAT
Acyl CoA cholesterol acyl transferase
- CYP7A1
Cholesterol 7α hydroxylase
- CYP27A1
Cholesterol 27α hydroxylase
- LAL
Lysosomal acid lipase
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work has only been presented in part at the annual meeting of the American Association for Study of Liver Diseases in San Francisco in 2008. This work was supported by two grants from the National Institutes of Health to Dr. Sanyal K24 DK 02755 and T32 DK-007150-33. The authors are indebted to Dr. Michael Pandak, Shunlin Ren and Phillip Hylemon for their advice.
REFERENCES
- Adams LA, Sanderson S, Lindor KD, Angulo P. The histological course of nonalcoholic fatty liver disease: a longitudinal study of 103 patients with sequential liver biopsies. J Hepatol. 2005;42:132–138. doi: 10.1016/j.jhep.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Beg ZH, Stonik JA, Brewer HB., Jr. In vivo modulation of rat liver 3-hydroxy-3-methylglutaryl-coenzyme A reductase, reductase kinase, and reductase kinase kinase by mevalonolactone. Proceedings of the National Academy of Sciences. 1984;81:7293–7297. doi: 10.1073/pnas.81.23.7293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beg ZH, Stonik JA, Brewer HB., Jr. Phosphorylation of hepatic 3-hydroxy-3-methylglutaryl coenzyme A reductase and modulation of its enzymic activity by calcium-activated and phospholipid-dependent protein kinase. The Journal of Biological Chemistry. 1985;260:1682–1687. [PubMed] [Google Scholar]
- Beg ZH, Stonik JA, Brewer HB., Jr. Modulation of the enzymic activity of 3-hydroxy-3-methylglutaryl coenzyme A reductase by multiple kinase systems involving reversible phosphorylation: a review. Metabolism: clinical and experimental. 1987;36:900–917. doi: 10.1016/0026-0495(87)90101-6. [DOI] [PubMed] [Google Scholar]
- Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C, Croce CM. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- Brea A, Mosquera D, Martin E, Arizti A, Cordero JL, Ros E. Nonalcoholic fatty liver disease is associated with carotid atherosclerosis: a case-control study. Arteriosclerosis,Thrombosis, and Vascular Biology. 2005;25:1045–1050. doi: 10.1161/01.ATV.0000160613.57985.18. [DOI] [PubMed] [Google Scholar]
- Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- Burg JS, Espenshade PJ. Regulation of HMG-CoA reductase in mammals and yeast. Progress in lipid research. 2011;50:403–410. doi: 10.1016/j.plipres.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caballero F, Fernandez A, De Lacy AM, Fernandez-Checa JC, Caballeria J, Garcia-Ruiz C. Enhanced free cholesterol, SREBP-2 and StAR expression in human NASH. J Hepatol. 2009;50:789–796. doi: 10.1016/j.jhep.2008.12.016. [DOI] [PubMed] [Google Scholar]
- Caldwell SH, de Freitas LA, Park SH, Moreno ML, Redick JA, Davis CA, Sisson BJ, Patrie JT, Cotrim H, Argo CK, Al-Osaimi A. Intramitochondrial crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2009;49:1888–1895. doi: 10.1002/hep.22851. [DOI] [PubMed] [Google Scholar]
- Chalasani NP, Sanyal AJ, Kowdley KV, Robuck PR, Hoofnagle J, Kleiner DE, Unalp A, Tonascia J. Pioglitazone versus vitamin E versus placebo for the treatment of non-diabetic patients with non-alcoholic steatohepatitis: PIVENS trial design. Contemporary Clinical Trials. 2009;30:88–96. doi: 10.1016/j.cct.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary N, Pfluger PT. Metabolic benefits from Sirt1 and Sirt1 activators. Current Opinion in Clinical Nutrition and Metabolic Care. 2009;12:431–437. doi: 10.1097/MCO.0b013e32832cdaae. [DOI] [PubMed] [Google Scholar]
- Chen CH, Huang MH, Yang JC, Nien CK, Etheredge GD, Yang CC, Yeh YH, Wu HS, Chou DA, Yueh SK. Prevalence and risk factors of gallstone disease in an adult population of Taiwan: an epidemiological survey. J Gastroenterol Hepatol. 2006;21:1737–1743. doi: 10.1111/j.1440-1746.2006.04381.x. [DOI] [PubMed] [Google Scholar]
- Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA, Sanyal AJ. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;48:1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke PR, Hardie DG. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. The EMBO J. 1990;9:2439–2446. doi: 10.1002/j.1460-2075.1990.tb07420.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devries-Seimon T, Li Y, Yao PM, Stone E, Wang Y, Davis RJ, Flavell R, Tabas I. Cholesterol-induced macrophage apoptosis requires ER stress pathways and engagement of the type A scavenger receptor. J Cell Biol. 2005;171:61–73. doi: 10.1083/jcb.200502078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, Kechagias S. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- Feldstein AE, Werneburg NW, Canbay A, Guicciardi ME, Bronk SF, Rydzewski R, Burgart LJ, Gores GJ. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology. 2004;40:185–194. doi: 10.1002/hep.20283. [DOI] [PubMed] [Google Scholar]
- Geng YJ, Phillips JE, Mason RP, Casscells SW. Cholesterol crystallization and macrophage apoptosis: implication for atherosclerotic plaque instability and rupture. Biochem Pharmacol. 2003;66:1485–1492. doi: 10.1016/s0006-2952(03)00502-1. [DOI] [PubMed] [Google Scholar]
- Ginsberg HN. Is the slippery slope from steatosis to steatohepatitis paved with triglyceride or cholesterol? Cell Metabolism. 2006;4:179–181. doi: 10.1016/j.cmet.2006.08.010. [DOI] [PubMed] [Google Scholar]
- Goldstein JL, Brown MS. The LDL receptor. Arteriosclerosis, Thrombosis, and Vascular Biology. 2009;29:431–438. doi: 10.1161/ATVBAHA.108.179564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Jr., Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005;54:3140–3147. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends in Biochemical Sciences. 2007;32:71–77. doi: 10.1016/j.tibs.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton JD, Shimomura I, Brown MS, Hammer RE, Goldstein JL, Shimano H. Activation of cholesterol synthesis in preference to fatty acid synthesis in liver and adipose tissue of transgenic mice overproducing sterol regulatory element-binding protein-2. The J Clin Invest. 1998;101:2331–2339. doi: 10.1172/JCI2961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonen E. Cellular cholesterol trafficking and compartmentalization. Nat Rev Mol Cell Biol. 2008;9:125–138. doi: 10.1038/nrm2336. [DOI] [PubMed] [Google Scholar]
- Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- Ludwig J, Viggiano TR, McGill DB, Oh BJ. Nonalcoholic steatohepatitis: Mayo Clinic experiences with a hitherto unnamed disease. Mayo Clin Proc. 1980;55:434–438. [PubMed] [Google Scholar]
- Matthan NR, Lichtenstein AH. Approaches to measuring cholesterol absorption in humans. Atherosclerosis. 2004;174:197–205. doi: 10.1016/S0021-9150(03)00248-X. [DOI] [PubMed] [Google Scholar]
- Matthan NR, Resteghini N, Robertson M, Ford I, Shepherd J, Packard C, Buckley BM, Jukema JW, Lichtenstein AH, Schaefer EJ. Cholesterol absorption and synthesis markers in individuals with and without a CHD event during pravastatin therapy: insights from the PROSPER trial. J Lipid Res. 2010;51:202–209. doi: 10.1194/jlr.M900032-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Carreras M, Del Hoyo P, Martin MA, Rubio JC, Martin A, Castellano G, Colina F, Arenas J, Solis-Herruzo JA. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38:999–1007. doi: 10.1053/jhep.2003.50398. [DOI] [PubMed] [Google Scholar]
- Puri P, Baillie RA, Wiest MM, Mirshahi F, Choudhury J, Cheung O, Sargeant C, Contos MJ, Sanyal AJ. A lipidomic analysis of nonalcoholic fatty liver disease. Hepatology. 2007;46:1081–1090. doi: 10.1002/hep.21763. [DOI] [PubMed] [Google Scholar]
- Puri P, Mirshahi F, Cheung O, Natarajan R, Maher JW, Kellum JM, Sanyal AJ. Activation and dysregulation of the unfolded protein response in nonalcoholic fatty liver disease. Gastroenterology. 2008;134:568–576. doi: 10.1053/j.gastro.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Tabas I. Cholesterol and phospholipid metabolism in macrophages. Biochim Biophys Acta. 2000;1529:164–174. doi: 10.1016/s1388-1981(00)00146-3. [DOI] [PubMed] [Google Scholar]
- Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, Day C, Arcaro G. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- Villanova N, Moscatiello S, Ramilli S, Bugianesi E, Magalotti D, Vanni E, Zoli M, Marchesini G. Endothelial dysfunction and cardiovascular risk profile in nonalcoholic fatty liver disease. Hepatology. 2005;42:473–480. doi: 10.1002/hep.20781. [DOI] [PubMed] [Google Scholar]
- Wang Z, Yao T, Song Z. Chronic alcohol consumption disrupted cholesterol homeostasis in rats: down-regulation of low-density lipoprotein receptor and enhancement of cholesterol biosynthesis pathway in the liver. Alcoholism, Clin Exp Res. 2011;34:471–478. doi: 10.1111/j.1530-0277.2009.01111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanless IR, Lentz JS. Fatty liver hepatitis (steatohepatitis) and obesity: an autopsy study with analysis of risk factors. Hepatology. 1990;12:1106–1110. doi: 10.1002/hep.1840120505. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K, Yang L, McCall S, Huang J, Yu XX, Pandey SK, Bhanot S, Monia BP, Li YX, Diehl AM. Diacylglycerol acyltranferase 1 anti-sense oligonucleotides reduce hepatic fibrosis in mice with nonalcoholic steatohepatitis. Hepatology. 2008;47:625–635. doi: 10.1002/hep.21988. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTAL FIGURE 1: HMGCR mRNA expression in lean controls, obese controls and subjects with hepatitis C (n=20) were compared. HMGCR mRNA expression was significantly lower than controls in subjects with hepatitis C virus infection.
SUPPLEMENTAL FIGURE 2: PCSK9 mRNA expression was measured in lean and obese controls (n=6 each) and in subjects with NAFL or NASH (n=8 each). There were no significant across group differences by ANOVA.