Abstract
Proton-coupled folate transporter (PCFT) mediates folate intestinal absorption and transport across the choroid plexus, processes defective in subjects with hereditary folate malabsorption (HFM). PCFT is also widely expressed in human solid tumors where it contributes to the transport of pemetrexed and other antifolates. This study defines the basis for the functional changes due to a P425R mutation detected in a subject with HFM. Among various substitutions, only positively charged mutants (P425R and P425K) lost function but in a highly selective manner. Transport of reduced folates mediated by P425R-PCFT was virtually abolished; the methotrexate influx Kt was increased fivefold (from 2 to 10 μM). In contrast, the pemetrexed influx Kt mediated by P425R-PCFT was decreased 30% compared with wild-type (WT)-PCFT. Methotrexate inhibition of pemetrexed influx was competitive with a Ki for WT-PCFT comparable to its influx Kt. However, the methotrexate influx Ki for P425R-PCFT was ∼15-fold higher than the WT-PCFT influx Kt and threefold higher than the methotrexate influx Kt for the P425R-PCFT mutant. The confirmed secondary structure and homology modeling place the P425 residue at the junction of the 6th external loop and 12th transmembrane domain, remote from the aqueous translocation pathway, a prediction confirmed by the failure to label P425C-PCFT with N-biotinylaminoethyl methanethiosulfonate-biotin and the absence of inhibition of P425C-PCFT function by water-soluble sulfhydryl reagents. Hence, despite its location, the P425R-PCFT mutation produces a conformational change that fully preserves pemetrexed binding but markedly impairs binding of methotrexate and other folates to the carrier.
Keywords: heme carrier protein 1, intestinal folate transport
tetrahydrofolate cofactors, the vitamin B9 family, are one-carbon donors that play an essential role in nucleic acid biosynthesis and methylation reactions (32). The first step in meeting mammalian requirements for this vitamin is absorption across the apical brush-border membrane of the proximal small intestine, a process mediated by the proton-coupled folate transporter (PCFT-SLC46A1; Refs. 13, 30, 32). PCFT is also required for the transport of folates from blood to cerebrospinal fluid across the choroid plexus barrier (28, 30, 34). These physiological roles of PCFT were established by the defects that occur when there are loss-of-function mutations in this gene in subjects with the autosomal recessive disorder hereditary folate malabsorption (HFM; Refs. 2a, 13, 33). PCFT has a narrow range of activities for a variety of folate and antifolate substrates at its optimal low pH. However, transport becomes much more selective at physiological pH where, unlike for most folates and antifolates, the carrier retains a particularly high affinity for the new-generation antifolate pemetrexed currently in clinical use for the treatment of mesothelioma and the nonsquamous subtypes of nonsmall cell carcinoma of the lung (5, 18, 26).
Insights into PCFT residues and domains that play a critical role in its function have derived from several different approaches. From site-directed mutagenesis, the E185 residue [5th transmembrane domain (TMD)] was identified as required for proton coupling (24) and H281 (7th TMD) as critical to proton binding that allosterically modulates folate binding. H247 (large intracellular loop) interacts with S172 (2nd intracellular loop) to modulate folate access to the aqueous translocation pathway (23). D109 (1st intracellular loop) is absolutely required for PCFT function (19). A variety of residues have been studied based on their role in HFM. 1) R113 was mutated in two subjects with HFM; this residue is near irreplaceable; charge preservation (R113H and R113K) permitted only slight preservation of function (7, 33). 2) The D156 residue is critical for protein stability; the D156Y mutant detected in a subject with HFM, along with other substitutions at this residue, save for glutamate, results in an unstable protein (19). 3) The R376 residue was mutated in two subjects with HFM. R376Y resulted in a complete loss of function. R376Q preserved some selective function (11). PCR-based random mutagenesis identified other residues required for PCFT function and, in several cases, recapitulated mutations found in subjects with HFM and identified a mutational hotspot located in a narrow region of the 5′-end of the pcft gene (35).
The current study exploited the selective preservation of transport activity of a mutated PCFT associated with HFM, P425R, to undertake a detailed characterization of the functional impact of this mutation. The studies indicate that despite the location of the P425 residue at the boundary of the 6th external loop and 12th TMD, an area remote from the aqueous translocation pathway and folate binding pocket, the mutation at this site produced a highly selective alteration in binding of its transport substrates.
MATERIALS AND METHODS
Chemicals.
[3H]methotrexate {[3′,5′,7-3H(N)]-MTX}, [3H]folic acid, [3H](6S)5-formyltetrahydrofolate (5-formylTHF), [3H](6S)5-methyltetrahydrofolate (5-methylTHF), and [3H]pemetrexed were obtained from Moravek Biochemicals (Brea, CA). Purity was confirmed initially and periodically by liquid chromatography (29). EZ-Link sulfo-NHS-LC-biotin [sulfosuccin-imidyl-6-(biotinamido) hexanoate] was purchased from Pierce Biotechnology (Rockford, IL), streptavidin-agarose beads from Fischer Scientific (Pittsburgh, PA), and protease inhibitor cocktail from Roche Applied Science (Mannheim, Germany). The sulfhydryl reactive reagent N-biotinylaminoethyl methanethiosulfonate (MTSEA-biotin) was purchased from Biotium (Hayward, CA). Methanethiosulfonate-ethyltrimethylammonium (MTSET+) and methanethiosulfonate-ethylsulfonate (MTSES−) were obtained from Affymetrix (Santa Clara, CA).
Construction of mutant plasmids by site-directed mutagenesis.
Mutations in pcft cDNA were generated with the Quick Change II XL site-directed mutagenesis kit (Stratagene, La Jolla, CA) using the following primers: ATGAAGGGGTTCCGTTTCCTCCTGGGAG, ATGAAGGGGTTCAAATTCCTCCTGGGAG, ATGAAGGGGTTCGATTTCCTCCTGGGAG, ATGAAGGGGTTCTCTTTCCTCCTGGGAG, ATGAAGGGGTTCTTTTTCCTCCTGGGAG, ATGAAGGGGTTCGCTTTCCTCCTGGGAG, and ATGAAGGGGTTCTGTTTCCTCCTGGGAG (P425R, P425K, P425D, P425S, P425F, P425F, P425A, and P425C, respectively, 5′ to 3′ direction). Mutant constructs were verified by DNA sequencing in the Albert Einstein Cancer Center Genomics Shared Resource. A PCFT pcDNA3.1(+) expression vector was used as template that encodes hemagglutinin (HA)-tagged PCFT at the COOH terminus.
Cell lines, cell culture conditions, and transient transfections.
HeLa-R1–11 cells, the recipient for transient transfections in this study, have a genomic deletion of the reduced folate carrier along with silencing of PCFT expression due to methylation of the promoter and a loss of gene copies (2, 31). R1–11 cells were maintained in RPMI-1640 medium supplemented with 5% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. R-1–11 cells (3.5 × 105/vial) were seeded into 17-mm liquid scintillation vials in preparation for transport studies. In separate experiments, 6 × 105 cells/well were seeded in sixwell plates in preparation for analyses of PCFT protein in the crude membrane preparation and accessibility to biotinylation at the cell surface. Forty-eight hours later, these cells were transfected with PCFT constructs (0.8 μg/vial or 2 μg/well of a 6-well plate) with lipofectamine 2000 (3 μl/vial for transport studies and 5 μl/well for Western blot analysis; Invitrogen, Carlsbad, CA). Two days later, the cells were processed for transport or protein analyses. For transient transfections, the same amount of plasmid was used for each construct, with cells seeded at the same density. In each experiment mutant, wild-type and vector-only transfections were performed, each in duplicate. Transport studies were repeated at least three times. With this experimental approach, PCFT transport activities achieved with each transfection were highly reproducible (SE < ±20%).
Membrane transport analyses (functional characterization).
In preparation for influx determinations, R1–11 transfectants, grown as a monolayer at the bottom of glass vials as described above, were washed twice with 2 ml of HBS buffer (20 mM HEPES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, and 5 mM dextrose at pH 7.4) and incubated with the same HBS buffer (2 ml per vial) in a water bath (37°C) for 20 min. The buffer was then aspirated, and 500 μl of transport buffer, either HBS or MBS (20 mM MES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, and 5 mM dextrose), were added containing tritiated folates or antifolates. The HBS transport buffer was adjusted with 1 N NaOH for studies at pH ≥7.0, while MES was adjusted with 1 N HCl for studies at pH <7.0. Transport was halted after 1 min by the addition of 10 vol (5 ml) of ice-cold HBS buffer (pH 7.4), an interval over which uptake mediated by PCFT is unidirectional (27). Cells were then washed three times with 5 ml of ice-cold HBS, followed by the addition of 500 μl of 0.2 M NaOH, and the cells were digested by incubation for 1 h at 65°C. A portion of the hydrolysate (400 μl) was then analyzed on a liquid scintillation spectrometer; the protein content of another portion of hydrolysate (10 μl) was determined using the Pierce kit (Thermo Scientific, Rockford, IL). Influx is expressed as picomoles of tritiated substrate per milligrams of protein per minute or percentage of wild-type activity. Influx kinetics were determined based on the relationship between influx and extracellular substrate concentration by a nonlinear regression best fit to the Michaelis-Menten equation. Nonspecific uptake and adsorption to the cell surface were assessed in vector-only-transfected cells and subtracted from total uptake.
A variety of screening procedures were developed to rapidly assess the mechanistic basis for the loss of function of mutated PCFTs. A PCFT mutation can result in decreased proton binding to the carrier, which, in turn, allosterically results in decreased binding of folate substrate. In this case, decreasing the pH increases proton and folate binding, tending to correct the transport defect. This is assessed by comparing MTX influx at pH 4.5 and 5.5 (19, 23). To assess for a defect in proton coupling, transport is assessed at pH 7.4 in the absence of a transmembrane proton gradient as well as pH 5.5. A mutation that disrupts a residue required for proton coupling results in impaired transport at low pH but transport is unchanged at pH 7.4 in the absence of a proton gradient (24). Finally, to screen for changes in influx Kt and/or Vmax, the ratio of influx mediated by the mutated PCFT to influx mediated by wild-type PCFT was assessed at substrate concentrations of 1 and 100 μM. If the ratio is low at the low concentration but increases at the higher concentrations, the mutation results in an increase in the influx Kt. If the ratio is high at the low concentration but decreases as the concentration is increased, the influx Kt for the mutated PCFT is decreased. If the ratio remains low at the highest concentration, the influx Vmax is decreased.
Sulfhydryl modification by MTS reagents.
As described above, R1–11 cells were seeded and transfected with PCFT expression vectors, P425C, or wild-type PCFT. Two days later, cells were washed with HBS buffer twice and treated with fresh MTSET (positive charge) or MTSES (negative charge) solution in HBS buffer (pH 7.4, final concentration ≈3 mM) at room temperature. After 30 min, cells were washed twice with HBS buffer and influx of [3H]MTX was measured as described above.
Cell surface biotinylation and Western blot analyses.
This assay was described previously (19, 24). Briefly, 2 days after transfection, cells were washed twice with PBS (2 ml) at pH 8.0 and treated with 1 mg/ml EZ-Link Sulfo-NHS-LC-biotin in PBS (pH 8.0) at room temperature for 30 min. Cells were then washed twice and treated with hypotonic buffer (0.5 mM Na2HPO4 and 0.1 mM EDTA at pH 7.0) containing protease inhibitors and kept on ice for 30 min. Cells were then scraped from the plates and centrifuged at 14,000 rpm for 15 min at 4°C. The membrane fraction was pelleted and resuspended in 400 μl of lysis buffer (0.1% SDS, 1% Triton X-100, 1 mM EDTA, 150 mM NaCl, and 20 mM Tris pH 7.4), containing protease inhibitors and rotated in a cold room (4°C) for 30 to 120 min. Supernatant (25 μl) was taken from each sample for Western blot analysis (designated as the crude membrane protein fraction). After centrifugation at 14,000 rpm at 4°C for 15 min, supernatant was mixed with streptavidin-agarose beads (50 μl that had been prewashed three times with lysis buffer) overnight at 4°C. The beads were then washed four times with lysis buffer (500 μl) containing protease inhibitors. After the final wash, bead-bound protein was stripped by heating for 5 min at 95° in 2× SDS-PAGE loading buffer containing dithiothreitol and loaded directly onto polyacrylamide gels. The crude membrane fraction was mixed with 2× SDS-PAGE loading buffer (1:1) containing dithiothreitol at room temperature before Western blot analysis. For the crude membrane samples, blots were first probed with a rabbit β-actin antibody (Cell Signaling Technology, Denvers, MA) then stripped with stripping buffer (100 mM 2-β-mercaptoethanol, 2% SDS, and 62.5 mM Tris·HCl pH 6.7) and reprobed with anti-HA antibody (Sigma, St. Louis, MO). For biotinylated samples, blots were probed directly with anti-HA antibody. Blots were developed with the Amersham ECL Plus reagent (GE Healthcare, Piscataway, NJ). The level of expression for wild-type PCFT and P425R was determined by using ImageJ software (http://rsbweb.nih.gov/ij/download.html).
Biotinylation and immunoprecipitation by MTSEA-biotin.
In this assay, described previously (36), cells were prepared in the same way as in the cell surface biotinylation assay but were treated with MTSEA-biotin. MTSEA-biotin was prepared by dissolving in DMSO at a concentration of 2 mg/100 μl followed by dilution with HBS buffer at a ratio of 1 to 100. After cells were washed with HBS twice, they were treated with 1 ml of HBS containing MTSEA-biotin (500 μM). After 30 min at room temperature, the solution was aspirated and cells were washed with 2 ml of HBS at least twice. The steps that followed were identical to the cell surface biotinylation and Western blot analyses.
Molecular model.
A homology model for PCFT has been employed by this laboratory as an adjunct to the interpretation of experimental observations within a three-dimensional context. In the current study, this model was further refined using the HHpred (21), a Hidden Markov model-based fold-recognition and alignment method. The best scoring template remained the crystal structure of the glycerol-3-phosphate transporter from Escherichia coli (PDB code 1pw54) as in other studies (8, 19, 23). The optimal alignment between PCFT and 1pw54 was obtained in the current study directly from HHpred and served as input to comparative protein structure modeling with Modeller (4, 17). The quality of the model, and the justification for its use, were verified through energetic analysis using statistical pair potentials implemented in Prosa (20). Additional verification was conducted by predicting the location of transmembrane segments using HMMTOP (22) and comparing it with the location of transmembrane helices in the three-dimensional model. The previous workflow described the most successful model built, but other alignment techniques [such as Muscle (3), Clustalw (1), MMM (14, 15), and Align2D (9, 10)] and their corresponding comparative models were also explored.
Statistical analysis.
Statistical comparisons were performed by the two-tailed Student's paired t-test, the differences were considered significant at P ≤ 0.05. (GraphPad Prism, version 3.0 for windows).
RESULTS
Evaluation of the expression and function of P425R and other PCFT mutants at this residue.
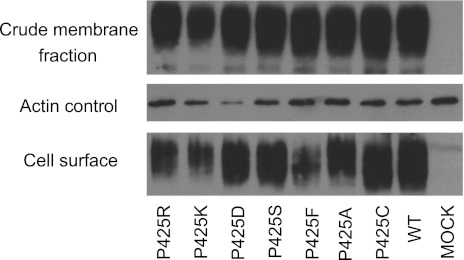
In a previous study (33), a homozygous P425R PCFT mutation was identified in a subject with HFM. To define the impact of this mutation and the role of this residue in PCFT function, seven P425 mutants, including P425R, were generated by site-directed mutagenesis. These included positive (-K), negative (-D), polar (-C, -S), and neutral (-F, -A) substitutions to assess structural/size and charge/polarity requirements at this residue. As indicated in Fig. 1, all mutant PCFT proteins were present in the crude membrane preparation at levels comparable to wild-type PCFT. All were expressed at the cell surface; the mutant PCFT identified in the patient with HFM, P425R, was accessible to biotinylation at the plasma membrane but at a somewhat reduced level and comparable with what was observed for the like-charged P425K and the neurtal P425F PCFT mutants. Based on densitometric measurements, the P425R mutant detected at the cell surface was 0.58 the level of wild-type PCFT (Table 1).
Fig. 1.
Expression and accessibility of P425 proton-coupled folate transporter (PCFT) mutants. Western blot analysis of the crude membrane fraction (top) and protein biotinylated at the cell surface (bottom) for wild-type (WT) and P425 mutant PCFTs. Actin was the loading control (middle). Western blot is representative of 2 independent experiments.
Table 1.
Summary of MTX and PMX influx kinetic values for the wild-type and P425R mutant PCFT
|
Vmax |
|||||||
|---|---|---|---|---|---|---|---|
| Kt, μM | Mutant/WT | Ki, μM | Rate: pmol·mg protein−1·min−1 | Relative expression at the cell surface (P425R/WT) | Corrected for expression | Mutant/WT–corrected for expression | |
| WT | |||||||
| MTX | 2.03 ± 0.54 | 3.1 ± 0.4 | 277.4 ± 22.9 | 1.0 | |||
| PMX | 0.60 ± 0.08 | 102.9 ± 3.9 | |||||
| P425R | |||||||
| MTX | 10.1 ± 1.53 | 4.98 | 29 ± 2 | 120.4 ± 8.6 | 0.58 | 208 | 0.73 |
| PMX | 0.41 ± 0.07 | 0.68 | 25.3 ± 1.2 | 44 | 0.44 | ||
Values are means ± SE. A comparison of methotrexate (MTX) and pemetrexed (PMX) influx kinetics (Kt and Kt) based on experiments represented in the data of Figs. 4 and 5. Relative expression was measured by ImagejJ densitometry from 2 separate experiments and used to normalize the influx Vmax values. PCFT, proton-coupled folate transporter; WT, wild type.
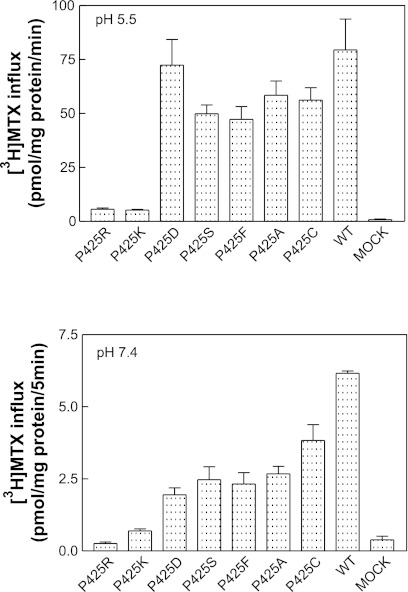
Figure 2 represents the functional analyses of these mutant proteins. The mutations that resulted in a prominent loss of MTX transport function at pH 5.5 were the patient, P425R, and the like-charged P425K PCFT constructs (Fig. 2, top); this could not be explained on the basis of the very modest decrease in protein expression. All the other mutants preserved the majority of transport function at pH 5.5 irrespective of polarity (-S, -C), neutrality or size (-F, -A), or opposite charge (-D). Transport function was also markedly decreased at pH 7.4 (Fig. 2, bottom) for the P425R and P425K mutants, and there was also a loss of function for the other mutants at this pH, greater than observed at pH 5.5. These observations exclude the possibility that the loss of function was due to a defect in proton coupling. If that were the case, function would have been preserved at pH 7.4 in the absence of a transmembrane pH gradient. There was no increase in MTX influx relative to wild-type PCFT when the pH was decreased to 4.5 (data not shown) excluding a role for this residue as a determinant of proton binding, which, in turn, allosterically enhances folate binding to its site on the carrier.
Fig. 2.
Functional assessment of P425 PCFT mutants. Top: [3H]methotrexate (MTX) influx was assessed at a concentration of 0.5 μM and pH 5.5 over 1 min. Bottom: [3H]MTX influx was assessed at a concentration of 1 μM at pH 7.4. Data are the means ± SE from 3 experiments performed on different days.
Substrate-dependent changes in transport mediated by the P425 PCFT mutants.
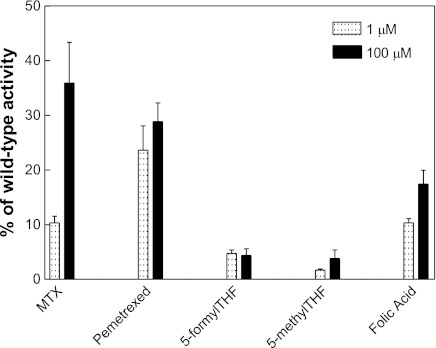
The functional impact of the P425R mutation was substrate and concentration dependent as indicated in Fig. 3. At an extracellular concentration of 1 μM, MTX influx mediated by the mutant was 10% that of wild-type PCFT. This increased to nearly 40% of wild-type activity when the MTX concentration was increased to 100 μM. A comparable level of activity was observed when folic acid was the substrate but with a much lesser increase when the concentration was increased to 100 μM. The greatest loss of activity was observed for 5-methylTHF and 5-formylTHF, and this was independent of substrate concentration. What was most striking was the preservation of [3H]pemetrexed activity (∼2.5-fold greater than MTX at a concentration of 1 μM), which was unaffected by concentration, suggesting that unlike MTX and folic acid, the Kt for this substrate was not increased due to the P425R mutation. These observations suggested a marked increase in the influx Kt for MTX, to a lesser extent for folic acid, and a marked decrease in influx Vmax for 5-formylTHF and 5-methylTHF mediated by P425R PCFT. The marked loss of activity for the reduced folates precluded an accurate determination of influx kinetics. In the following section, influx parameters were evaluated, in detail, for MTX and pemetrexed.
Fig. 3.
Substrate and concentration dependence of transport. Influx of [3H]MTX, [3H]pemetrexed, [3H]5-formyltetrahydrofolate (5-formylTHF), [3H]5-methyltetrahydrofolate (5-methylTHF) and [3H]folic acid was assessed at substrate concentrations of 1 and 100 μM at pH 5.5 over 1 min at 37°C. Data are the means ± SE from 3 experiments performed on different days.
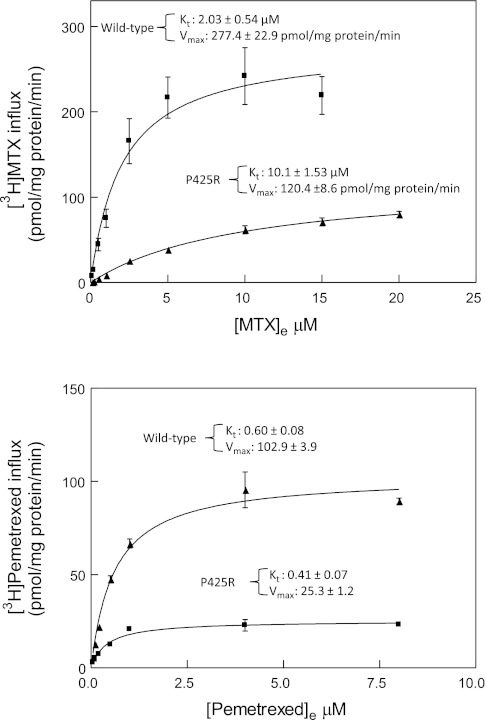
Kinetic basis for changes in transport activities mediated by the mutant P425R PCFT.
An analysis of influx kinetics revealed a fivefold increase (P = 0.002) in the MTX influx Kt mediated by the P425R mutant PCFT (Fig. 4, top). In contrast, there was a ∼30% decrease in the pemetrexed influx Kt (P = 0.03) mediated the P425R mutant relative to wild-type PCFT (Fig. 4, bottom). There was a 2.3-fold and 4-fold decrease (P = 0.005) in influx Vmax (MTX and pemetrexed, respectively) for the P425R mutant relative to wild-type PCFT. This decrease is related in part to the decreased level of P425R protein accessible at the cell surface (Fig. 1 and Table 1). After normalization based on protein expression, the magnitude of difference in Vmax between the wild-type and PCFT mutant was decreased to 1.3-fold and 2.3-fold for MTX and pemetrexed, respectively. This difference in decrease in influx Vmax for MTX and pemetrexed between the wild-type and P425R mutant PCFT was not significant (P = 0.1). Most importantly, while the P425R mutation markedly suppressed the apparent affinity of the carrier for MTX, the apparent affinity for pemetrexed was fully preserved; indeed, there was a small but statistically significant increase. Influx kinetic parameters are summarized in Table 1.
Fig. 4.
Comparison of MTX and pemetrexed influx kinetics mediated by the wild-type and P425R mutant PCFTs. Top: [3H]MTX influx kinetics for P425R and wild-type PCFT. Bottom: [3H]pemetrexed influx kinetics. Transport was measured at pH 5.5 over 1 min at 37°C. Nonspecific uptake was assessed in vector-only-transfected cells and subtracted from total uptake. Kinetic parameters were determined by nonlinear regression best fit to Michaelis-Menten equation, v = Vmax * S/[Kt + S]. Data are the mean ± SE from 3 experiments performed on different days.
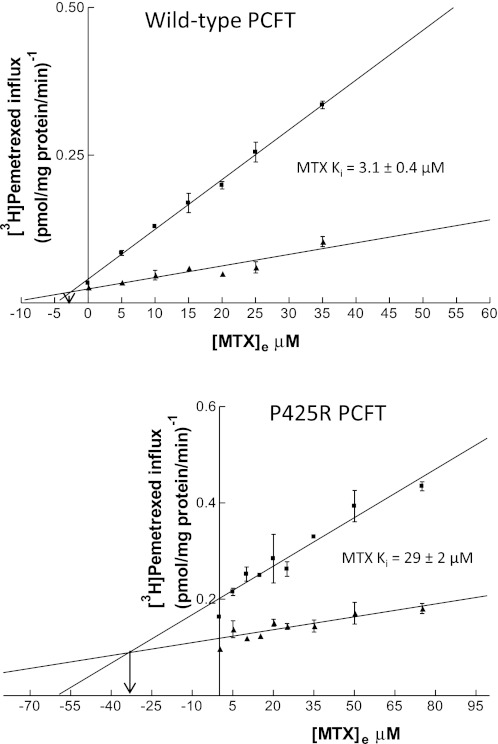
To more specifically assess and quantitate MTX binding to the wild-type and mutated carriers, inhibition constants were obtained based on inhibition of [3H]pemetrexed influx by MTX. As indicated in the Dixon plots in Fig. 5, MTX inhibition of both the wild-type (top) and mutated PCFT (bottom) was clearly competitive. Based on the average of four experiments, the influx Ki for MTX of 3.1 ± 0.4 μM was comparable with its influx Kt of 2.03 ± 0.54 μM for wild-type PCFT (P = 0.15; Fig. 4, top). However, there was a substantial discrepancy in the relationship between the MTX influx Kt and Ki for the P425R mutant. The MTX influx Ki was 29 ± 2 μM, three times greater than the P425R influx Kt (P = 0.005) and ∼15 times greater than the wild-type MTX influx Ki (P = 0.0009). Hence, there was a marked and selective loss of affinity of the mutated carrier for MTX relative to pemetrexed (Table 1).
Fig. 5.
Dixon analysis of the kinetics of MTX inhibition of pemetrexed influx. [3H]pemetrexed influx was assessed in wild-type PCFT (top) and the P425R mutant PCFT (bottom) at 2 concentrations, 0.5 and 1.5 μM, in the absence and presence of nonlabeled MTX at the indicated concentrations. Transport was measured at pH 5.5, over 1 min at 37°C. Point of intersection is consistent with competitive inhibition; the arrow represents a perpendicular from that point to the intersection with the x-axis, which represents the MTX influx Ki. Ki values noted are based on the average of 4 experiments performed on different days, one of which is represented for the wild-type and P425R mutant PCFTs.
Impact of sulfhydryl modification of the P425C PCFT mutant by MTS reagents.
Cys-reactive MTS reagents were used to localize the P425 residue relative to the aqueous translocation pathway and the substrate binding pocket (6, 16). In this approach, inhibition of transport by a water-soluble reagent, when a residue is substituted with Cys, suggests a location in proximity to the aqueous translocation pathway. If inhibition is diminished by pretreatment with a transport substrate, the residue is likely located within or close to the binding pocket. This analysis could be applied to evaluate the location of the P425 residue since 60∼70% of wild-type PCFT activity was preserved for the Cys substituted (P425C) PCFT (Fig. 2). Two Cys-active water-soluble reagents were used, the positively charged MTSET and the negatively charged MTSES, at concentrations of 1 ∼ 10 mM (6). Experiments were performed with wild-type PCFT as a negative control. Neither reagent suppressed MTX influx mediated by P425C PCFT (data not shown), this despite the fact that the P425R mutation had a profound suppressive effect on the affinity of the carrier for this drug.
To exclude the possibility that the P425 residue is accessible and modified by these sulfhydryl reagents, but this does not result in any functional consequence, an attempt was made to immunoprecipitate a potential MTSEA-PCFT conjugate with biotin. However, P425C PCFT was not labeled by the MTSEA-biotin sulfhydryl reagent in contrast to the E292C mutant (on a Cys-less PCFT background) used as a positive control (36), further confirmation that the P425 residue is not accessible to the aqueous translocation pathway or the extracellular compartment (data not shown).
Analysis of a potential interaction between the P425R and K422 residues as a basis for the loss of function.
Further experiments were designed to evaluate whether there might be a charge interaction between the K422 residue and the adjacent P425R mutant that was the basis for the loss of function. A K422D mutant was generated and was functional as previously reported (25). Then, the K422D mutation was introduced into the P425R background to generate a K422D/P425R PCFT mutant so that the P425R was now opposed by the opposite charge. However, this did not result in a restoration of function. (data not shown).
DISCUSSION
Since the cloning of PCFT, three approaches have been utilized to characterize the structure-function properties of this carrier: 1) systematic site-directed mutagenesis, 2) analyses of mutant residues identified in subjects with HFM, and 3) unbiased PCR-based random mutagenesis (35). These studies have defined residues critical to PCFT function as described in the Introduction. The P425R mutant protein, the focus of the current study, preserved sufficient expression and function to allow a detailed characterization of its properties. While the P425R mutant PCFT was detected previously by immunohistochemistry, it was not detected by Western blot with an antibody to the protein (33). In the current study, the PCFT constructs were HA tagged in the COOH terminus and were readily detected with an anti-HA antibody. This study revealed a marked and highly selective loss in the affinities of the mutated carrier for its folate and antifolate substrates. Transport of 5-methylTHF and 5-formylTHF, both reduced folates, was most adversely affected. The former is the major dietary folate and its impaired intestinal absorption was responsible for the folate-deficiency syndrome associated with this mutation (33). Because of the severity of the defect in 5-methylTHF transport and the lack of any correction with high levels of this substrate, the achievement of sufficient intestinal absorption via the mutated PCFT would not be possible even at very high doses of this folate. Folic acid transport was better preserved and increased with an increase in concentration; however, the properties of this folate, in particular its tight binding to, and presumed inactivation of folate receptors required for transport across the choroid plexus, have raised questions regarding its suitability as a folate substrate particularly when reduced folates are available for the treatment of HFM (2a). MTX transport was even better preserved and, in this case, there was an even greater restoration of function with an increase in concentration. However, it was pemetrexed transport that was the best preserved and the degree of preservation was independent of drug concentration.
The analysis of influx kinetics for MTX and pemetrexed provided clarification of the basis for the differences in impact of the P425R mutation. For pemetrexed, there was a small (∼30%) but significant fall in the influx Kt; the influx Kt for MTX was increased by a factor of five when transport via the wild-type and P425R mutant PCFTs was compared. The possibility that this might be attributed to an interaction between these antifolates at different sites of the carrier was excluded by the documentation that MTX is a competitive inhibitor of pemetrexed influx for both the wild-type and mutated carriers. Hence, while it would appear that these folates interact with a similar binding domain, it is clear that MTX binding to the carrier has undergone a major and selective change associated with the mutation. This became even more apparent when the Ki for MTX inhibition of pemetrexed influx was found to be >3 times greater than its influx Kt for the P425R mutant PCFT, and this was ∼10–15 times greater than the MTX influx Ki or Kt mediated by the wild-type carrier. It is of interest that the R376Q mutation in a subject with HFM altered substrate binding with greater retention of binding for pemetrexed than MTX. However, unlike the current study, there was a substantial rise in the pemetrexed influx Kt, although to a lesser extent than observed for MTX, 5-formylTHF, and 5-methylTHF (11). This is further evidence for the substantial difference in pemetrexed binding relative to other folate/antifolate substrates.
For a transport system such as PCFT, the measured influx Kt is a complex term that encompasses the dissociation constant for the carrier-substrate-proton complex at the external cell membrane plus a series of additional terms that describe: 1) the rate of conformational change from the outward to inward facing conformation, 2) the dissociation of proton then folate from the carrier, 3) the restoration of the outward facing conformation, and 4) the association between proton and the carrier, all of which contribute to the rate of carrier cycling as expressed by the influx Vmax. While values for the various reactions that describe cycling of the carrier have not been defined, it is clear that the rate of proton dissociation from carrier within the cell and the rate of conformational change of the carrier from its inward to outward states can both be rate limiting. Hence, the rate of cycling (influx Vmax) is enhanced when folate substrate is present intracellularly when the carrier changes from its inward to outward facing conformation (24, 27). Likewise, when proton-coupling is abolished (as with mutation of the Glu185 residue), the influx Vmax is markedly decreased when there is a transmembrane proton gradient, a change that occurs without any alteration, at all, in influx Kt (24). What is clear, however, from the marked increase in the MTX influx Ki , a measure of binding distinct from subsequent events, is that there is a marked decrease in the affinity of the P425R mutant for this substrate relative to wild-type carrier not quantitatively reflected in the measured Kt. Hence, the contribution of the subsequent events in the carrier cycle must be sufficiently great compared with the dissociation constant to “dilute” the magnitude of the change in binding to the mutated carrier to account for the discrepancy between the influx Kt and Ki values for MTX.
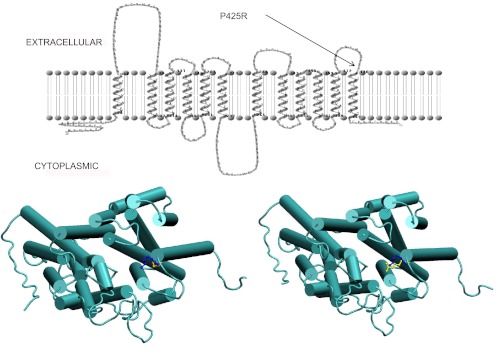
What makes the P425 mutation so unusual is its location, so remote from the folate binding site that a selective impact on folate substrate binding would not be expected. Based on the confirmed topology of this transporter (36) (Fig. 6, top), and a structural homology model (Fig. 6, bottom), P425 connects the helical transmembrane 12th segment with the highly hydrophilic, electropositive, external loop that forms an outer layer of the molecule, a typical location for a proline helix-breaker. P425 does not appear to have direct access to the aqueous translocation pathway, consistent with the lack of inhibition of transport by sulfhydryl reagents and the lack of accessibility to biotinylation. The only charged residue in close spatial proximity to P425 is K422 approximately one helix turn away at the helix cap position (Fig. 6). It is possible that there is interference between these like-charged R425 and K422 residues in the P425R mutant carrier (Fig. 6). Hence, while the substitution of a Pro with Arg should result in more flexibility at this site, the two positive charges in close proximity are likely to be repulsive and distort the tip of the helix or, at least make the structural environment more rigid, which, in turn, distorts other transmembrane domains that ultimately disturb the conformation of the folate binding pocket. Supporting this electropositive repulsion is the observation that substitution of the P425 residue with the negative charged Asp or the comparably sized, neutral, Phe, preserved function. However, when this charge repulsion was eliminated in a K422D/P425R PCFT construct, there was no restoration of function. While a positive result would confirm this hypothesis, this negative finding does not necessarily exclude the possibility of a repulsive electropositive environment in this region. Moreover, the possibility cannot be excluded that R425 is buried in the membrane lipid bilayer.
Fig. 6.
Localization of the P425 residue based on a homology model and the 2-dimensional topology of PCFT. Top: confirmed topology of PCFT (36) indicating the external location of the P425 residue at the junction of the 6th external loop and the 12th transmembrane domain (TMD). Bottom: homology model of PCFT. Bottom left: WT-PCFT, looking into the aqueous translocation pathway from the extracellular compartment. Bottom right: P425R-PCFT, looking into the aqueous translocation pathway from the extracellular compartment. The P425 residue is indicated in yellow; the K422 residue is indicated in blue.
The selectivity in binding associated with the P425R PCFT mutation might be related to the slightly smaller size of the pemetrexed molecule, due to an 11-member fused pyrrolopyrimidine ring structure in contrast to the 12-member pteridine ring-based structure of all the other substrates, allowing an easier entry into a constricted binding pocket. Likewise, the selective disruption of the interaction of MTX and the other folates, but not pemetrexed, with critical residues in a distorted folate binding site may account for the selectivity of this phenomenon. The structural changes associated with the P425R mutation, beyond the modest decrease in expression, could certainly account for the small decrease in Vmax observed for both MTX and pemetrexed reflecting a modest impaired rate of oscillation of the carrier between its conformational states.
GRANTS
This work was supported by National Cancer Institute Grant CA-82621 (to I. D. Goldman).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: D.S.S., R.Z., and I.D.G. conception and design of research; D.S.S. performed experiments; D.S.S., R.Z., E.H.Y., A.F., and I.D.G. analyzed data; D.S.S., R.Z., and I.D.G. interpreted results of experiments; D.S.S., E.H.Y., and A.F. prepared figures; D.S.S., E.H.Y., and A.F. drafted manuscript; D.S.S., R.Z., and I.D.G. edited and revised manuscript; R.Z., A.F., and I.D.G. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. John Blanchard from the Department of Biochemistry for helpful discussions and Clarissa Czekster for assistance in the analysis of the data.
REFERENCES
- 1. Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res 31: 3497–3500, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Diop-Bove NK, Wu J, Zhao R, Locker J, Goldman ID. Hypermethylation of the human proton-coupled folate transporter (SLC46A1) minimal transcriptional regulatory region in an antifolate-resistant HeLa cell line. Mol Cancer Ther 8: 2424–2431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2a. Diop-Bove N, Kronn D, Goldman ID. Hereditary folate malabsorption. In: GeneReviews (Online), edited by Pagon RA, Bird TD, Dolan CR, Stephens K. Seattle, WA: University of Washington, 2008. June 17 [updated 2011 Dec 08] [Google Scholar]
- 3. Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics 5: 113, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Fiser A, Sali A. Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol 374: 461–491, 2003 [DOI] [PubMed] [Google Scholar]
- 5. Hanna N, Shepherd FA, Fossella FV, Pereira JR, De Marinis F, Von Pawel J, Gatzemeier U, Tsao TC, Pless M, Muller T, Lim HL, Desch C, Szondy K, Gervais R, Shaharyar Manegold C, Paul S, Paoletti P, Einhorn L, Bunn PA., Jr Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol 22: 1589–1597, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Karlin A, Akabas MH. Substituted-cysteine accessibility method. Methods Enzymol 293: 123–145, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Lasry I, Berman B, Glaser F, Jansen G, Assaraf YG. Hereditary folate malabsorption: a positively charged amino acid at position 113 of the proton-coupled folate transporter (PCFT/SLC46A1) is required for folic acid binding. Biochem Biophys Res Commun 386: 426–431, 2009 [DOI] [PubMed] [Google Scholar]
- 8. Lasry I, Berman B, Straussberg R, Sofer Y, Bessler H, Sharkia M, Glaser F, Jansen G, Drori S, Assaraf YG. A novel loss of function mutation in the proton-coupled folate transporter from a patient with hereditary folate malabsorption reveals that Arg 113 is crucial for function. Blood 112: 2055–2061, 2008 [DOI] [PubMed] [Google Scholar]
- 9. Madhusudhan MS, Marti-Renom MA, Sanchez R, Sali A. Variable gap penalty for protein sequence-structure alignment. Protein Eng Des Sel 19: 129–133, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Madhusudhan MS, Webb BM, Marti-Renom MA, Eswar N, Sali A. Alignment of multiple protein structures based on sequence and structure features. Protein Eng Des Sel 22: 569–574, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mahadeo K, Diop-Bove N, Shin D, Unal E, Teo J, Zhao R, Chang MH, Fulterer A, Romero MF, Goldman ID. Properties of the Arg376 residue of the proton-coupled folate transporter (PCFT-SLC46A1) and a glutamine mutant causing hereditary folate malabsorption. Am J Physiol Cell Physiol 299: C1153–C1161, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Qiu A, Jansen M, Sakaris A, Min SH, Chattopadhyay S, Tsai E, Sandoval C, Zhao R, Akabas MH, Goldman ID. Identification of an intestinal folate transporter and the molecular basis for hereditary folate malabsorption. Cell 127: 917–928, 2006 [DOI] [PubMed] [Google Scholar]
- 14. Rai BK, Fiser A. Multiple mapping method: a novel approach to the sequence-to-structure alignment problem in comparative protein structure modeling. Proteins 63: 644–661, 2006 [DOI] [PubMed] [Google Scholar]
- 15. Rai BK, Madrid-Aliste CJ, Fajardo JE, Fiser A. MMM: a sequence-to-structure alignment protocol. Bioinformatics 22: 2691–2692, 2006 [DOI] [PubMed] [Google Scholar]
- 16. Riegelhaupt PM, Frame IJ, Akabas MH. Transmembrane segment 11 appears to line the purine permeation pathway of the Plasmodium falciparum equilibrative nucleoside transporter 1 (PfENT1). J Biol Chem 285: 17001–17010, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sali A, Blundell TL. Comparative protein modelling by satisfaction of spatial restraints. J Mol Biol 234: 779–815, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Scagliotti GV, Parikh P, Von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, De Marinis F, Simms L, Sugarman KP, Gandara D. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26: 3543–3551, 2008 [DOI] [PubMed] [Google Scholar]
- 19. Shin DS, Min SH, Russell L, Zhao R, Fiser A, Goldman ID. Functional roles of aspartate residues of the proton-coupled folate transporter (PCFT; SLC46A1); a D156Y mutation causing hereditary folate malabsorption. Blood 116: 5162–5169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sippl MJ. Recognition of errors in three-dimensional structures of proteins. Proteins 17: 355–362, 1993 [DOI] [PubMed] [Google Scholar]
- 21. Soding J. Protein homology detection by HMM-HMM comparison. Bioinformatics 21: 951–960, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Tusnady GE, Simon I. The HMMTOP transmembrane topology prediction server. Bioinformatics 17: 849–850, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Unal ES, Zhao R, Chang MH, Fiser A, Romero MF, Goldman ID. The functional roles of the His247 and His281 residues in folate and proton translocation mediated by the human proton-coupled folate transporter SLC46A1. J Biol Chem 284: 17846–17857, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Unal ES, Zhao R, Goldman ID. Role of the glutamate 185 residue in proton translocation mediated by the proton-coupled folate transporter SLC46A1. Am J Physiol Cell Physiol 297: C66–C74, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Unal ES, Zhao R, Qiu A, Goldman ID. N-linked glycosylation and its impact on the electrophoretic mobility and function of the human proton-coupled folate transporter (HsPCFT). Biochim Biophys Acta 1178: 1407–1414, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Vogelzang NJ, Rusthoven JJ, Symanowski J, Denham C, Kaukel E, Ruffie P, Gatzemeier U, Boyer M, Emri S, Manegold C, Niyikiza C, Paoletti P. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 21: 2636–2644, 2003 [DOI] [PubMed] [Google Scholar]
- 27. Wang Y, Zhao R, Goldman ID. Characterization of a folate transporter in HeLa cells with a low pH optimum and high affinity for pemetrexed distinct from the reduced folate carrier. Clin Cancer Res 10: 6256–6264, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Wollack JB, Makori B, Ahlawat S, Koneru R, Picinich SC, Smith A, Goldman ID, Qiu A, Cole PD, Glod J, Kamen B. Characterization of folate uptake by choroid plexus epithelial cells in a rat primary culture model. J Neurochem 104: 1494–1503, 2008 [DOI] [PubMed] [Google Scholar]
- 29. Zhao R, Babani S, Gao F, Liu L, Goldman ID. The mechanism of transport of the multitargeted antifolate, MTA-LY231514, and its cross resistance pattern in cell with impaired transport of methotrexate. Clin Cancer Res 6: 3687–3695, 2000 [PubMed] [Google Scholar]
- 30. Zhao R, Diop-Bove N, Visentin M, Goldman ID. Mechanisms of membrane transport of folates into cells and across epithelia. Annu Rev Nutr 31: 177–201, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhao R, Gao F, Hanscom M, Goldman ID. A prominent low-pH methotrexate transport activity in human solid tumor cells: Contribution to the preservation of methotrexate pharmacological activity in HeLa cells lacking the reduced folate carrier. Clin Cancer Res 10: 718–727, 2004 [DOI] [PubMed] [Google Scholar]
- 32. Zhao R, Matherly LH, Goldman ID. Membrane transporters and folate homeostasis: intestinal absorption and transport into systemic compartments and tissues. Expert Rev Mol Med 11: e4, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhao R, Min SH, Qiu A, Sakaris A, Goldberg GL, Sandoval C, Malatack JJ, Rosenblatt DS, Goldman ID. The spectrum of mutations in the PCFT gene, coding for an intestinal folate transporter, that are the basis for hereditary folate malabsorption. Blood 110: 1147–1152, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhao R, Min SH, Wang Y, Campanella E, Low PS, Goldman ID. A role for the proton-coupled folate transporter (PCFT-SLC46A1) in folate receptor-mediated endocytosis. J Biol Chem 284: 4267–4274, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhao R, Shin DS, Diop-Bove N, Ovits CG, Goldman ID. Random mutagenesis of the proton-coupled folate transporter (PCFT, SLC46A1), clustering of mutations and the bases for associated losses of function. J Biol Chem 286: 24150–24158, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhao R, Unal ES, Shin DS, Goldman ID. Membrane topological analysis of the proton-coupled folate transporter (PCFT-SLC46A1) by the substituted cysteine accessibility method. Biochemistry 49: 2925–2931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]