Abstract
The ion gradients generated by the Na-K-ATPase play a critical role in epithelia by driving transepithelial transport of various solutes. The efficiency of this Na-K-ATPase-driven vectorial transport depends on the integrity of epithelial junctions that maintain polar distribution of membrane transporters, including the basolateral sodium pump, and restrict paracellular diffusion of solutes. The review summarizes the data showing that, in addition to pumping ions, the Na-K-ATPase located at the sites of cell-cell junction acts as a cell adhesion molecule by interacting with the Na-K-ATPase of the adjacent cell in the intercellular space accompanied by anchoring to the cytoskeleton in the cytoplasm. The review also discusses the experimental evidence on the importance of a specific amino acid region in the extracellular domain of the Na-K-ATPase β1 subunit for the Na-K-ATPase trans-dimerization and intercellular adhesion. Furthermore, a possible role of N-glycans linked to the Na-K-ATPase β1 subunit in regulation of epithelial junctions by modulating β1-β1 interactions is discussed.
Keywords: Na-K-ATPase β1 subunit, epithelial junctions, trans-dimerization
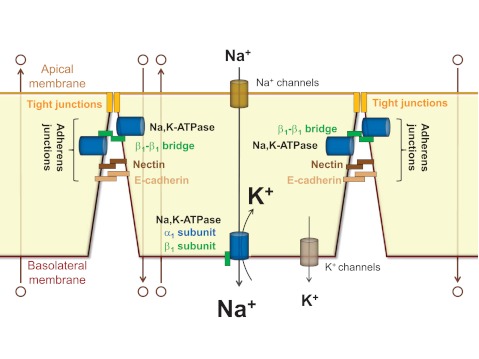
the na-k-atpase plays a critical role in epithelia by driving transepithelial Na+-dependent transport of various solutes and water. For example, in renal tubules the ion gradients generated by the Na-K-ATPase drive Na+ reabsorption and the secondary active transport (reabsorption or secretion) of numerous solutes, including other ions, glucose, and amino acids (15). In alveolar epithelium, the transepithelial Na+ gradient created by Na-K-ATPase is responsible for water reabsorption from alveolar spaces which is critical for normal gas exchange (46, 68, 79). This driving force of the Na-K-ATPase depends on the tight junctions that maintain polar distribution of basolateral and apical membrane transporters and restrict paracellular diffusion of solutes (66). The tight junctions, in turn, depend on the presence of the adherens junctions that initiate cell-cell adhesion, stabilize intercellular contacts, and trigger signaling pathways required for the formation and maintenance of the tight junctions (3, 21, 28) (Fig. 1).
Fig. 1.
The Na-K-ATPase drives transepithelial transport of various solutes by generating ion gradients across the basolateral membrane and by maintaining the integrity of the intercellular junctions. The Na-K-ATPase located in the basolateral membrane generates the ion gradients that drive vectorial transepithelial transport of Na+ and also Na+-dependent transport of water and other solutes (open circles). The efficiency of this transepithelial transport is ensured by the tight and adherens junctions. In addition to pumping ions, the Na-K-ATPase acts as a cell adhesion molecule in adherens junctions, thus further facilitating transepithelial transport by contributing to the integrity of the intercellular junctions.
Both tight and adherens junctions are formed by cell adhesion molecules (CAMs), the integral proteins embedded in the adjacent membranes, that undergo the trans (cell-to-cell)-dimerization via the interactions between their extracellular domains (23). This trans-dimerization triggers association of the cytoplasmic domain of CAM to the cytoskeleton via anchoring proteins, which stabilizes cell-cell junctions (23, 44, 66). In addition, trans-dimerization of CAMs can induce or modulate the interaction between their cytoplasmic domains and signaling molecules and hence initiate junction-associated signal transduction (66). In epithelial cells, the major CAMs of tight junctions are occludin and claudins, while CAMs of adherens junctions include a well-characterized E-cadherin and the more recently identified nectins (1, 16, 45, 62, 69). As found for both E-cadherin and nectin-1, not only trans-dimerization of CAMs, but also their cis-dimerization in the same membrane is crucial for intercellular adhesion (22, 48, 84). Particularly, cis-dimerization of nectin-1 is critical for initiation of cell-cell contact (48), while cis-dimerization of E-cadherin is important for stabilization of adherens junctions (22).
The Na-K-ATPase consists of a catalytic α subunit and an N-glycosylated β subunit required for maturation and membrane targeting of the enzyme. Additional regulatory subunits of the Na-K-ATPase belong to a family of seven single-span transmembrane proteins containing an FXYD motif in the transmembrane region (17, 67). Expressed in a tissue-specific manner, FXYD proteins associate with the Na-K-ATPase α-β heterodimer in a number of cells as subsidiary subunits and may modulate the kinetic properties of the enzyme (17, 18, 43). Of the four known isoforms of the Na-K-ATPase α subunit (α1, α2, α3, and α4) and three isoforms of the Na-K-ATPase β subunit (β1, β2, and β3) the α1 and β1 are the major isoforms in epithelial cells (6, 13). The Na-K-ATPase β1 subunit has three N-glycosylation sites, all occupied by N-glycans (42). N-glycans are added to the subunit in the endoplasmic reticulum (ER) and then modified by ER- and Golgi-resident glycosidases and glycosyltransferases. Prevention of N-glycosylation of the β1 subunit has little effect on α1-β1 assembly (4, 87), trafficking of the pump to the plasma membrane (35, 80), or Na-K-ATPase activity (4, 35, 70, 71). However, N-glycans have been found important for initiation, maintenance, and regulation of epithelial junctions (80, 81).
In the present review, we summarize the data showing that the Na-K-ATPase acts as CAM in adherens junctions (Fig. 2) and thus facilitates transepithelial transport not only by pumping ions, but also by maintaining the integrity of the intercellular junctions (Fig. 1).
Fig. 2.
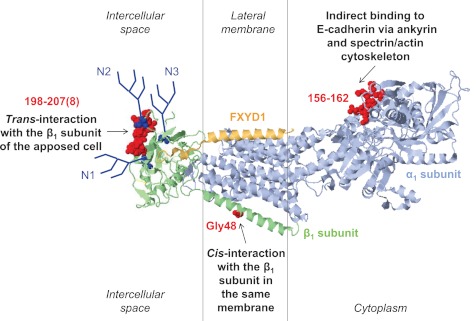
A model showing that the Na-K-ATPase acts as a cell adhesion molecule in adherens junctions. The Na-K-ATPase facilitates cell-cell adhesion by undergoing intercellular trans-dimerization via its β1 subunit (52, 65, 74) and binding to the cytoplasmic domain of E-cadherin via its α1 subunit and ankyrin/spectrin cytoskeleton (29, 50, 51), while clustering of the Na-K-ATPase molecules in the same membrane by cis-dimerization of the β1 subunits presumably stabilizes the junctional complex (2). The 198–207(8) amino acid region important for trans-dimerization of the Na-K-ATPase (75), Gly48 important for cis-dimerization of the Na-K-ATPase (2), and 156–162 residues important for ankyrin binding (88) are shown in red on a high-resolution structure of the Na-K-ATPase (2ZXE) (64). The presence of asparagine-linked glycans (dark blue) is required for normal β1 trans-dimerization, and alterations in N-glycan structure can modulate β1-β1 interactions and intercellular adhesion (74, 80, 81).
Role of Ion Transport Activity and Signaling Function of the Na-K-ATPase in Intercellular Adhesion
Transport by the Na-K-ATPase is important for cell-cell adhesion. Inhibition of Na-K-ATPase activity by ouabain in various epithelia prevents tight junction formation (56, 59), triggers disassembly of existing junctions (11, 12, 32), or increases their permeability (57, 58). During blastocyst formation in early mouse embryos, ouabain treatment, K+ depletion, or knockdown of the Na-K-ATPase β1 subunit disrupts the normal formation of tight junctions (41, 82). In Drosophila, the Na-K-ATPase is required for the formation of septate junctions (reviewed in Ref. 30). In most cases, the ouabain-dependent effects on cell adhesion are similar to the effects detected upon incubation of cells at low K+ concentration or in the presence of the Na+-ionophore gramicidin that increased intracellular concentration of Na+ (11, 59), demonstrating that the maintenance of low intracellular Na+ by the Na-K-ATPase is crucial for intercellular junctions. However, recent studies have shown that low levels of ouabain that do not inhibit ion pumping activity also modulate the degree of sealing of the tight junctions and distribution of the tight junction proteins, claudins (31). Treatment of cells with ouabain and ouabain-like compounds at nontoxic levels is known to activate signaling cascades (reviewed in Ref. 36). Several Na-K-ATPase-mediated signaling pathways, including Src, ERK1/2, MAPK, epidermal growth factor receptor, inositol 1,4,5-triphosphate receptor, phosphatidylinositol 3-kinase, RhoA and PLCγ-1, are implicated in the regulation of intercellular junctions (reviewed in Refs. 8, 10, 61). Therefore, both the ion-pumping activity and the signaling function of the Na-K-ATPase play a role in regulating intercellular junctions.
The Na-K-ATPase as an Important Component of an Epithelial Junctional Complex
Numerous studies have demonstrated colocalization of the Na-K-ATPase with junctional proteins in epithelial cell monolayers (reviewed in Refs. 8, 61). The Na-K-ATPase remains colocalized with the adherens junction proteins, E-cadherin and β-catenin, even after internalization induced by disruption of intercellular junctions between MDCK cells by Ca2+ depletion (80). Density gradient centrifugation analysis of the epithelial junctional complex shows that the Na-K-ATPase codistributes with the adherens junction proteins, E-cadherin, β-catenin, and α-catenin, as well as with the tight junction protein, occludin (83). The cytoplasmic domains of both E-cadherin and the Na-K-ATPase α1 subunit are connected to spectrin/actin cytoskeleton via an anchoring protein, ankyrin (29, 49–51). The high-affinity ankyrin binding site has been identified to be in the second cytoplasmic loop of the Na-K-ATPase α1 subunit (88) (Fig. 2).
Both E-cadherin and the Na-K-ATPase acquire resistance to nonionic detergents after formation of junctions between epithelial cells (49, 51, 81). Such junction-dependent resistance to nonionic detergents is a common property of all CAMs of both tight and adherens junctions (78). This resistance results from stable association of the cytoplasmic domains of CAMs with the cytoskeleton in response to intercellular trans-interaction of their extracellular domains at the site of cell-cell junctions. MDCK cells transformed with Moloney sarcoma virus (MSV-MDCK), which are not able to form functional tight junctions (5), express low levels of both E-cadherin and the Na-K-ATPase β1 subunit compared with normal MDCK cells (5, 55, 60). Overexpression of the Na-K-ATPase β1 subunit in E-cadherin-transfected MSV-MDCK cells increases resistance of E-cadherin to detergent extraction (60). These results indicate that the presence of the Na-K-ATPase β1 subunit is important for association of E-cadherin with the cytoskeleton. Moreover, detergent resistance of both Na-K-ATPase and E-cadherin in normal MDCK cells is decreased by the removal of N-glycans from the β1 subunit (80, 81). This implies that the intracellular association of the Na-K-ATPase α1 subunit with the cytoskeleton and E-cadherin is facilitated by N-glycan-mediated interactions of the extracellular domain of the Na-K-ATPase β1 subunit with particular CAMs present at the site of cell-cell contact. No direct interaction between the Na-K-ATPase β1 subunit and E-cadherin or occludin has been detected by coimmunoprecipitation in MDCK cells (74). On the other hand, the presence of interactions between the Na-K-ATPase β1 subunits of neighboring cells in epithelial cell monolayers was first hypothesized (65) and later demonstrated by fluorescence resonance energy transfer (52) and coimmunoprecipitation (52, 74). These results suggest that the Na-K-ATPase α-β heterodimer itself can act as CAM by undergoing both trans-dimerization via its β1 subunit and association with the cytoskeleton via its α1 subunit. To prove or disprove this hypothesis, it is essential to establish whether trans-dimerization of β1 subunits is important for either initiation of cell-cell contact, or maintenance of intercellular junctions, or both.
Trans-Dimerization of the Na-K-ATPase Is Important for Both Initiation and Maintenance of Intercellular Junctions
Overexpression of the Na-K-ATPase β1 subunit and E-cadherin, but not of E-cadherin alone, facilitates formation of tight junctions in MSV-MDCK cells (60). In normal MDCK cell monolayers, transient induction of the β1 subunit biosynthesis increases the rate of developing high transepithelial resistance (9). These results are consistent with possible contribution of the interactions between the Na-K-ATPase β1 subunits to the formation and/or stabilization of epithelial junctions. To determine whether this is the case, the effects of modulating these β1-β1 interactions on stability of adherens junctions and paracellular permeability of tight junctions have been studied in MDCK cell monolayers (74). When stably expressed in these cells, the exogenous Na-K-ATPase β1 subunits, either dog or rat, or their unglycosylated mutants, replace approximately half of the endogenous β1 subunits in the α1-β1 complexes without a change in the total amount or ion-pumping activity of the Na-K-ATPase (74, 76, 81). This occurs because of competition of the endogenous and exogenous β1 subunits for binding to the endogenous α1 subunits in the ER. The ER quality control system allows export only of assembled α-β complexes to the Golgi, thereby maintaining an equimolar ratio of α and β subunits in the plasma membrane, whereas the number of α1 subunits in the ER determines the amount of the α-β complexes (76). The presence in the plasma membrane of both endogenous β1 subunit and yellow fluorescent protein (YFP)-tagged exogenous β1 subunit enables assessment of β1-β1 interactions by detecting the amount of the endogenous β1 subunits that co-immunoprecipitate with YFP-β1 (74).
Such assessment does not discriminate between cis- and trans- β1-β1 interactions, since both endogenous and exogenous β1 subunits are expressed in the same cell. Disruption of intercellular junctions by Ca2+ depletion decreases the amount of β1-β1 complexes by 50% (74), showing that at least half of them are formed between adjacent cells. In agreement with these data, increasing the quantity of non-transfected MDCK cells in mixed monolayers of these cells with MDCK cells expressing both the exogenous and endogenous dog β1 subunits proportionally increases the amount of the endogenous β1 subunits that co-immunoprecipitates with the exogenous β1 subunits (74). The results show that a significant portion of β1-β1 complexes formed is intercellular. If the exogenous and endogenous β1 subunits interacted only within the same membrane, addition of nontransfected cells would not change the number of mixed complexes between the exogenous and endogenous β1 subunits.
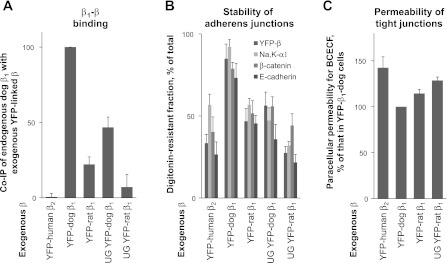
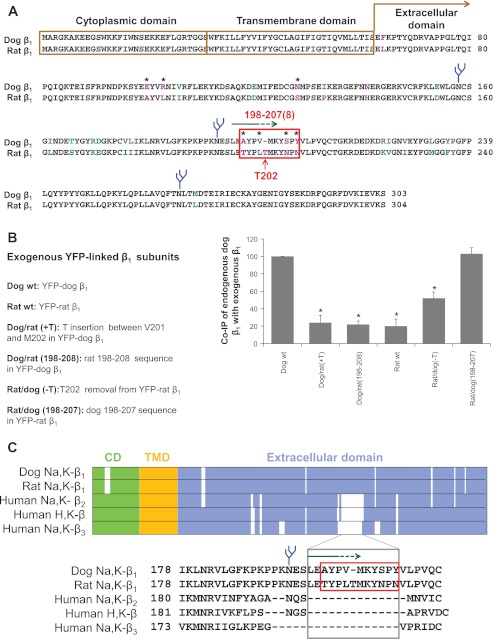
The removal of N-glycans from either dog or rat β1 subunit weakens its interaction with the endogenous dog β1 subunit (Fig. 3A) (74), confirming that β1-β1 interactions depend on the presence of N-glycans in the extracellular domain. The endogenous β1 subunit of MDCK cells that are of dog origin interacts more efficiently with the exogenous dog β1 subunit than with the exogenous rat β1 subunit (Fig. 3A) (52, 74). The cytoplasmic and transmembrane domains of rat and dog β1 subunits are identical, while the extracellular domains contain 26 amino acid mismatches, 11 of which are substantially different (Fig. 4A). Since the number and positions of N-glycans are the same in rat and dog subunits, these results show the importance of particular amino acid residues for β1-β1 binding. Consistent with this conclusion, almost no interaction is detected between the Na-K-ATPase β2 and β1 subunits that differ even more in the amino acid sequence (Fig. 3A).
Fig. 3.
The integrity of intercellular junctions depends on the Na-K-ATPase β1 subunit dimerization. A: the interactions between yellow fluorescent protein (YFP)-linked β1 or β2 subunits expressed in MDCK cells with the endogenous β1 subunits were assessed by coimmunoprecipitation (co-IP) using anti-YFP antibody followed by a Western blot analysis of precipitated YFP-linked subunits and coprecipitated endogenous subunits. Densitometric quantification for each cell line was performed by dividing the signal from the anti-β1 antibody by the corresponding signal from the anti-YFP antibody. The comparative bar graph shows these ratios as a percentage of the ratio obtained in the YFP-β1-dog cell line. The amount of coimmunoprecipitated endogenous Na-K-ATPase β1 subunits is less with rat than with dog exogenous β1 subunits. For both dog and rat exogenous subunits, the amount of coimmunoprecipitated endogenous Na-K-ATPase β1 subunits is less with the unglycosylated subunits than with fully glycosylated subunits. B: mature MDCK cell monolayers expressing various YFP-linked β subunits of the Na-K-ATPase were lysed either before or after a 30-min preincubation with 1% digitonin, which was then replaced by a cell lysis buffer. The amount of YFP-linked β subunits, Na-K-ATPase α1 subunit, β-catenin, and E-cadherin before and after preincubation with 1% digitonin was determined by a Western blot analysis of total cell lysates. Densitometric quantification for each cell line shows the amount of each protein in cells after digitonin treatment as a percentage of its amount before digitonin treatment. C: cells expressing various YFP-β constructs were maintained on porous Transwell inserts for 6 days after becoming confluent. Paracellular permeability for the membrane-impermeable fluorescent dye, BCECF, which was added to the bottom of the well, was determined as a rate of dye accumulation in the upper chamber of the insert. UG, unglycosylated by mutating all three N-glycosylation sites by Asn/Gln substitution. Error bars, ±SD (n = 3). The results presented in the figure were originally published in Ref. 74 (Journal of Biological Chemistry, ©The American Society for Biochemistry and Molecular Biology).
Fig. 4.
The 198–207 amino-acid region of the dog β1 subunit is important for β1-β1 interaction. A: computational alignment of amino acid sequences of the Na-K-ATPase dog β1 subunit (NP_001003283.1) and rat β1 subunit (NP_037245.2). The amino acid residues that are identical, similar, and different are shown in black, green, and purple font, respectively. The most variable region between dog and rat sequence (red box) overlaps with the epitope of the cell-adhesion-blocking anti-β1 subunit antibody (green arrow). The species-specific residues exposed at the surface of the extracellular domain (stars) were identified by mapping on a high-resolution structure of the Na-K-ATPase (2ZXE) (64). N-glycans are shown in blue. B: positions of the amino acid residues mutated in the 198–207(8) amino-acid regions of YFP-dog β1 and YFP-rat β1 are shown at the left. The wild-type (wt) and mutated fusion proteins were stably expressed in MDCK cells. Coimmunoprecipitation of the endogenous β1 subunits with expressed YFP-linked wild-type and mutated subunits followed by a Western blot analysis was used to assess β1-β1 interactions. The results of densitometric quantification shown at the right indicate that the rat-like mutations in the 198–207 region of YFP-dog β1 decrease its binding to the endogenous β1 subunit, while the dog-like mutations in the 198–208 region of YFP-rat β1 improve its interaction with the endogenous β1 subunit. Error bars, ±SD (n = 3). *Significant difference from the dog wt, P < 0.01, Student's t-test. C: multiple alignment of various isoforms of the β subunits of the P2-type ATPases shows that the amino acid sequence downstream of the second N-glycosylation site that has been found important for trans-dimerization of the Na-K-ATPase β1 subunits (74) is absent in other β subunit isoforms of the Na-K-ATPase and homologous H-K-ATPase. CD, cytoplasmic domain; TMD, transmembrane domain. [From Tokhtaeva et al. (75).]
The impairment of β1-β1 binding by removing N-glycans or by altering the amino acid sequence in one of the interacting subunits decreases detergent resistance of the Na-K-ATPase itself and also of the adherens junctional proteins, E-cadherin and its cytoplasmic partner β-catenin (Fig. 3B) (74, 81), indicating that stability of the adherens junctional complexes does depend on β1-β1 dimerization. It is known that stable adherens junctions are required for normal functioning of the tight junctions (3, 21, 28). Accordingly, the impairment of β1-β1 binding also increases the paracellular permeability (Fig. 3C) (74, 81). Conversely, improvement of β1-β1 binding by reduction of N-glycan branching with specific inhibitors decreases both extractability of junctional proteins and paracellular permeability (Fig. 5) (74, 81). Therefore, trans-dimerization of β1 subunits is important for integrity of mature intercellular junctions.
Fig. 5.
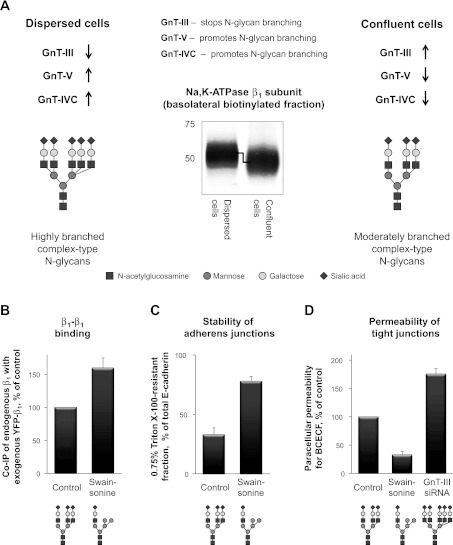
The integrity of intercellular junctions can be regulated by remodeling of N-glycans linked to the Na-K-ATPase β1 subunit. A: a scheme showing how the expression of the Golgi glycosyltransferases controls branching of N-glycans during formation of the mature cell monolayers from dispersed MDCK cells. The stop-branching enzyme, N-acetylglucosamine-glycosyltransferase (GnT)-III, is upregulated, while the branching promoting enzymes, GnT-V and GnT-IVC, are downregulated in confluent cells compared with dispersed cells. As a result, the degree of branching of N-glycans, including those linked to the Na-K-ATPase β1 subunit, is less in confluent cells than in dispersed cells. Accordingly, the β1 subunit present in the basolateral membranes of confluent cells has a higher electrophoretic mobility compared with that in dispersed cells. B–D: decreasing the degree of N-glycan branching by exposure of cells to the inhibitor of N-glycan processing, swainsonine, improves β1-β1 interactions (B), increases the resistance of E-cadherin to Triton X-100 extraction (C), and decreases the paracellular permeability of MDCK cell monolayers for BCECF (D). Conversely, increasing the degree of N-glycan branching by silencing of a stop-branching enzyme, GnT-III, increases the paracellular permeability (D). siRNA, small interfering RNA. The figure summarizes results originally published in Refs. 74 and 81 (Journal of Biological Chemistry, ©The American Society for Biochemistry and Molecular Biology).
This trans-dimerization of β1 subunits is also important for initiation of cell-cell contact. Overexpression of the β1 subunit increases adhesiveness of nonpolarized Chinese hamster ovary cells as detected by a cell aggregation assay (65). Furthermore, cell junction formation between surface-attached MDCK cells is inhibited by an antibody against the extracellular domain of the β1 subunit (80). The mutagenic removal of N-glycans from the β1 subunit reduces the rate of cell-cell contact formation between dispersed MDCK cells (80). Also, aggregation of rat lung epithelial cells is inhibited by the rat-like mutant of the extracellular domain of the dog β1 subunit added to the cell culture media (75).
The detection of β1-β1 complexes in MDCK cells separated from each other by Ca2+ depletion (74) indicates the presence of not only trans-, but also cis-β1-β1 interactions within the same membrane. Mutating Gly48 in the transmembrane domain of the Na-K-ATPase β1 subunit inhibits oligomerization of the transmembrane domains β1 subunit fused to the maltose binding protein within Escherichia coli membranes (2). The same mutation in the full-length Na-K-ATPase β1 subunit inhibits cell aggregation of β1-overexpressing MSV-MDCK cells (2), suggesting that the Na-K-ATPase β1 subunits undergo cis-oligomerization within the same plasma membrane (Fig. 2) and that this interaction facilitates cell aggregation.
Therefore, the Na-K-ATPase acts as CAM in adherens junctions, i.e., it facilitates both initiation and stabilization of cell-cell junctions via intercellular trans-dimerization followed by intracellular association with the cytoskeleton. Remarkably, the two main functions of CAM, trans-dimerization and anchorage to the cytoskeleton, are segregated between two subunits of the Na-K-ATPase, the β1 and α1, respectively (Fig. 2). It is possible that cis-dimerization of the Na-K-ATPase molecules within the same membrane via transmembrane domains of the β1 subunits also contributes to the formation and/or stabilization of the junctional complex.
The Putative β1-β1 Trans-Dimerization Domain
The interaction between two dog subunits is more effective than the interaction between rat and dog subunits (Fig. 3A), suggesting the importance of species-specific amino acid regions in β1-β1 interaction. Mapping the residues that are different in dog and rat subunits on the high-resolution structure of the Na-K-ATPase (2ZXE)(64) identified the highly variable between dog and rat region that is exposed on the surface of the extracellular domain of the β1 subunit facing a neighboring cell (Fig. 2). It contains four residues that are different in the dog and rat subunits and one residue (Thr) that is present in rat, but not in dog subunits (Fig. 4A). This region overlaps with the epitope for the antibody (Fig. 4A, green arrow) that reacts with dog, but not rat, β1 subunits and inhibits formation of cell-cell contacts between dispersed MDCK cells (74, 80).
To determine whether this region is important for β1-β1 interaction, rat-like and dog-like mutations in dog and rat β1 subunits, respectively, have been introduced into these species-specific sequences. Similar to the wild-type exogenous β1 subunits, stably expressed mutants replace about half of the endogenous β1 subunits with no change in the total amount of the pumps in the membrane (75). Insertion of a rat-specific Thr202 into the exogenous dog β1 subunit, either alone or together with four rat-like amino acid substitutions in the 198–207 sequence, impairs its interaction with the endogenous dog β1 subunit down to the level observed between rat β1 and dog β1 subunits (Fig. 4B). Conversely, the removal of a rat-unique Thr202 from the rat β1 subunits improves its binding to the dog β1 subunit. Four dog-like replacements in the 198–208 region performed in addition to the Thr removal further improved binding of the rat exogenous β1 subunit to the endogenous dog β1 subunit up to the level observed between the exogenous and endogenous dog β1 subunits (Fig. 4B), (75). These results show the importance of the 198–207 amino acid sequence for binding between dog β1 subunits (Fig. 2).
The same region is involved in the interaction between two rat β1 subunits, since the rat-like mutations in the 198–207 sequence of a secreted protein containing the extracellular domain of the dog β1 subunit increase its binding to the YFP-linked rat β1 subunit in vitro (75). In addition, the same mutations resulted in less efficient aggregation between rat epithelial cells followed by addition of the extracellular domain of the β1 subunit to the cell suspension (75). The data indicate that the extracellular domain of the dog β1 subunit with rat-like mutations in the 198–207 sequence competitively inhibits intercellular binding between rat β1 subunits and hence reduces cell-cell adhesion, while the wild-type extracellular domain of the dog β1 subunit binds poorly to the rat β1 subunit and does not prevent cell aggregation. Therefore, the interaction between two rat β1 subunits is also mediated by amino acid residues within the rat 198–208 sequence that determines species specificity (Fig. 2).
The absence of a Thr residue between residues 201 and 202 is critical for binding between two dog β1 subunits (75). On the other hand, the two rat β1 subunits effectively interact with one another (52), and their 198–208 regions, which contain the Thr residues, are important for this interaction (75). These results indicate that the amino acid residues critical for β1-β1 binding are located both upstream and downstream of the Thr insertion position. The insertion or removal of the Thr residues in one of the two interacting subunits likely misaligns these binding residues. Remarkably, the 198–207/8 region is present only in the β1 subunit, which is the major β subunit isoform in epithelial cells, but not in the other β subunit isoforms of the Na-K-ATPase and the homologous H-K-ATPase (Fig. 4C). It is possible that, during evolution, this region was conserved in the β1 subunits to enable an additional role for the Na-K-ATPase in the formation and maintenance of epithelial junctions. This comparison also suggests that the amino acid residues responsible for the adhesive role of the Na-K-ATPase β2 subunit (AMOG) (19) reside in a different region of the β2 subunit. Interestingly, Gly48 that was found important for cis-dimerization of the β1 subunits is not conserved in the Na-K-ATPase β2 and β3 subunits, but is present in the H-K-ATPase β subunit, consistent with the results suggesting an oligomeric state of the heterodimeric α1β1 Na-K-ATPase and gastric αβ H-K-ATPase (34, 63, 72).
Modulation of Intercellular Adhesion by Glycosyltransferase-Dependent Remodeling of N-Glycans of the Na-K-ATPase β1 Subunit
N-linked glycosylation is a very common modification of the transmembrane and secreted proteins. This modification is initiated cotranslationally in the ER lumen by covalent addition of a 14-residue N-glycan to the asparagine of the N-glycosylation site in the polypeptide chain and is completed posttranslationally in the Golgi. The majority of known membrane proteins contain N-glycosylation sites, suggesting that N-glycans have important biological functions. The significance of N-glycans, though, has been underestimated, limiting their roles to modulating stability, solubility, and susceptibility of proteins to aggregation. However, recent studies have shown that N-glycans have critical roles in protein folding, ER quality control, protein trafficking, intracellular signaling, cell adhesion, cell migration, and carcinogenesis (7, 24, 25, 53, 54).
For both dog and rat β1 subunits expressed in MDCK cells, the amount of coprecipitated endogenous β1 subunits is significantly greater with normally glycosylated exogenous subunits than with their unglycosylated mutants. Since N-glycans are not required for proper folding of the β1 subunits (73), these results indicate that N-glycans are involved in β1-β1 interaction. This interaction is not mediated by lectins that bind either sialic acid residue or galactose residue on N-glycan termini, because β1-β1 binding is not impaired by removal of sialic acid or galactose residues or by cell incubation with lactose that is known to inhibit galactose-lectin binding (73). Furthermore, the involvement of other lectins that bind to mannose or N-acetylglucosamine residues is doubtful, since these residues are not exposed on the N-glycan termini of the β1 subunits (77). In addition, the importance of the 198–207/8 amino acid domain for trans-dimerization is an argument against lectin involvement, since the presence of a lectin molecule between bulky hydrophilic N-glycans would not allow direct amino acid-mediated interaction between two β1 subunits of adjacent cells. Therefore, N-glycans are presumably required for stabilizing the amino acid-mediated interactions between extracellular domains of the two β1 subunits, suggesting that β1-β1 interactions can be modified by alterations in the β subunit N-glycosylation status.
Recent studies have demonstrated that both β1-β1 dimerization and intercellular adhesion indeed can be modulated by alterations in the structure of N-glycans. The highly diverse structures of N-glycans are generated during their processing in the Golgi due to the activity of various glycosyltransferases. The heterogeneity of N-glycans arises from variations in the number of branches (from 1 to 5) and the length and/or composition of individual branches (Fig. 5A). The action of N-acetylglucosamine-glycosyltransferases (GnTs) determines the number of branches in an N-glycan, while the activity of other glycosyltransferases, such as sialyltransferase and β-1,4-galactosyltransferase, is responsible for elongation of individual branches. The degree of N-glycan branching can be reduced pharmacologically, by exposing cells to the inhibitor of N-glycan processing, swainsonine. This treatment increases coprecipitation of the endogenous β1 subunit with the exogenous β1 subunit, indicating the improvement of β1-β1 interactions (Fig. 5B). In parallel, swainsonine treatment increases resistance of E-cadherin to extraction by digitonin (74) or by Triton X-100 (Fig. 5C) (81) and decreases the paracellular permeability (Fig. 5D) (81). Conversely, promoting N-glycan branching by silencing GnT-III, the enzyme that stops branching of N-glycans, increases the paracellular permeability of MDCK cell monolayers (Fig. 5D) (81). Cell exposure to swainsonine affects glycosylation of not only the Na-K-ATPase β1 subunit, but also many other cellular glycoproteins, including E-cadherin. Alterations in N-glycosylation of E-cadherin also influence cell-cell adhesion (37, 89). Importantly, the effects of swainsonine on detergent resistance of E-cadherin and paracellular permeability of MDCK cell monolayers are significantly diminished in a cell line expressing the unglycosylated Na-K-ATPase β1 subunit (81), indicating that the decreased branching of the β1 subunit N-glycans contributes to the tightening and stabilization of cell-cell junctions. Therefore, the fewer branches are in N-glycans of the β1 subunit, the tighter are intercellular junctions.
During formation of mature MDCK cell monolayers from dispersed cells, along with a gradual decrease in the paracellular permeability of cell monolayers, N-glycans linked to the β1 subunit gradually decrease in size (81). Similarly, the size of N-glycans linked to E-cadherin is decreased in confluent cells compared with dispersed cells (37, 81). Consistent with these observations, the relative molecular weight of N-glycans linked to the N-cadherin decreases as nonconfluent retinal pigmented epithelial cells become confluent (86). Also, the N-glycans linked to E-cadherin, Na-K-ATPase β1 subunit, β1-integrin, Na+/Ca2+ exchanger, and polycystin-2 are smaller in relatively tight renal epithelia compared with the leaky epithelium of small intestine (81), suggesting the inverse correlation between the size of N-glycans and tightness of epithelia.
Both the expression level and the activity of GnT-III, the enzyme that stops branching of N-glycans, are increased in epithelial GE11 cells cultured under dense conditions compared with dispersed cells (26). Similarly, expression of GnT-III gradually increases during development of mature cell monolayers from dispersed MDCK cells (Fig. 5A) (81). In parallel, two of the enzymes that promote N-glycan branching, GnT-V and GnT-IVC, are downregulated (Fig. 5A) (81). In contrast, the expression of the two key enzymes involved in elongation of individual branches, sialyltransferase and β-1,4-galactosyltransferase, does not change (81).
Upregulation of a stop-branching enzyme and downregulation of branching-promoting enzymes would decrease the number of branches in N-glycans, including those attached to the Na-K-ATPase β1 subunit. The reduced degree of N-glycan branching can explain the increase in electrophoretic mobility of the Na-K-ATPase β1 subunit observed during transformation of dispersed cells to confluent cell monolayers (Fig. 5A) (81). Decrease in branching of the β1 subunit N-glycans, in turn, increases detergent resistance of adherens junction proteins and decreases the paracellular permeability of MDCK cell monolayers (Fig. 5B) (81). At least some of the signaling pathways involved in regulation of expression of branching glycosyltransferases are implicated in the regulation of intercellular adhesion. Upregulation of GnT-III expression is observed only in epithelial cells that express basal levels of E-cadherin and GnT-III but not in E-cadherin-deficient cells, such as fibroblasts (26). In addition, upregulation of GnT-III depends on the presence of α-catenin and formation of E-cadherin-catenin-actin complex (90), while its downregulation can be mediated by the Wnt/β-catenin pathway (85).
Formation of a tight cell monolayer from dispersed MDCK cells is accompanied by decreasing cell motility. The higher degree of N-glycan branching in relatively motile dispersed epithelial cells is consistent with the data on the correlation between the high motility of malignant cells and increased branching of N-glycans (20, 33, 54, 90, 91). Thus, inhibition of cell migration by reducing N-glycan branching during development of epithelial cell monolayers may contribute to the contact inhibition of locomotion, an important property of normal epithelial cells. These data show that epithelial cells possess a mechanism that regulates cell-cell adhesion and cell migration by remodeling of N-glycans, including those of the Na-K-ATPase β1 subunit. Furthermore, dimerization of the Na-K-ATPase plays a key role in regulation of stability and tightness of epithelial junctions via N-glycan-mediated modulation of the β1-β1 bridges.
The evidence presented recently on the absence of unassembled β1 subunits in the plasma membrane of epithelial cells (9, 76) indicates that the β1 subunits can regulate cell adhesion only as components of the α-β complexes. Since the α 1 and β1 subunits are present in equimolar amounts in the α-β complexes, intercellular adhesion cannot be modulated by altering the β1 subunit abundance on the cell surface independently of ion transport activity. On the other hand, cells can regulate cell adhesion by remodeling the structure of N-glycans of the β1 subunits without affecting the quantity and, hence, activity of the pumps.
Concluding Remarks and Future Directions
The ion-pumping activity of the Na-K-ATPase and its role in signal transduction are important for formation and maintenance of intercellular junctions in epithelial cell monolayers. In addition, the Na-K-ATPase is a structural component of the epithelial junctional complex. The Na-K-ATPase acts as CAM in adherens junctions by undergoing intercellular trans-dimerization via its β1 subunit and by linking to the cytoplasmic domain of E-cadherin via its α1 subunit and ankyrin/spectrin cytoskeleton. Intercellular trans-interactions between the β1 subunits are required for integrity of epithelial junctions. The species-specific, extracellular 198–207(8) amino acid sequence of the β1 subunit is important for β1-β1 trans-dimerization, while N-glycans stabilize the amino acid-mediated interactions between β1 subunits. Intercellular adhesion can be regulated via N-glycan-mediated modulation of β1-β1 interactions. This modulation occurs because of cell-adhesion-dependent regulation of the level of the specific glycosyltransferases that control the degree of N-glycan branching of the Na-K-ATPase β1 subunit. The precise signaling pathways involved in the relationship between cell-cell adhesion, cell motility, and the activities of specific glycosyltransferases remain to be elucidated.
An additional layer of regulation of β1-β1 bridges and intercellular adhesion can arise from the interaction of the Na-K-ATPase with FXYD proteins. Overexpression of FXYD5 in cancer cell lines impairs cell-cell adhesiveness and promotes experimental cancer metastasis in animals following injection of the transfectants (27, 47). Both an E-cadherin-dependent mechanism and an E-cadherin-independent mechanism have been proposed to explain these effects of FXYD5 (47). Interestingly, overexpression of FXYD5 in renal epithelial cells decreases transepithelial electrical resistance, increases paracellular permeability to macromolecules, and modifies the glycosylation status of the Na-K-ATPase β1 subunit without any effect on expression of E-cadherin (38–40). These data suggest that FXYD5 may weaken the β1-β1 bridges by inhibiting amino acid-mediated intercellular β1-β1 interactions and/or by preventing normal glycosylation of the β1 subunit and, hence, impair intercellular adhesion. Similarly, the presence of FXYD3 delays the processing to a fully glycosylated β1 subunit in Xenopus oocytes (14), which implies a potential role of FXYD3 in modulating β1-β1 interactions. However, the nature of the involvement of FXYD proteins in regulation of β1-β1 interactions and cell adhesion in epithelia remains to be explored.
GRANTS
The work was supported by National Institutes of Health Grants DK-077149 and R37-HL-48129.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
O.V. conception and design of research; O.V. and L.A.D. analyzed the data; O.V., L.A.D., and G.S. interpreted the results of the experiments; O.V. prepared the figures; O.V. drafted the manuscript; O.V., L.A.D., E.T., and G.S. edited and revised the manuscript; O.V. approved the final version of the manuscript; E.T. performed the experiments.
ACKNOWLEDGMENTS
The authors thank Dr. Jack Kaplan and Dr. Sigrid Langhans for careful reading of the review and helpful suggestions.
REFERENCES
- 1.Anderson JM, Van Itallie CM. Physiology and function of the tight junction. Cold Spring Harb Perspect Biol 1: a002584, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barwe SP, Kim S, Rajasekaran SA, Bowie JU, Rajasekaran AK. Janus model of the Na,K-ATPase beta-subunit transmembrane domain: distinct faces mediate alpha/beta assembly and beta-beta homo-oligomerization. J Mol Biol 365: 706–714, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baum B, Georgiou M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J Cell Biol 192: 907–917, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beggah AT, Jaunin P, Geering K. Role of glycosylation and disulfide bond formation in the beta subunit in the folding and functional expression of Na,K-ATPase. J Biol Chem 272: 10318–10326, 1997 [DOI] [PubMed] [Google Scholar]
- 5.Behrens J, Mareel MM, Van Roy FM, Birchmeier W. Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol 108: 2435–2447, 1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blanco G, Mercer RW. Isozymes of the Na-K-ATPase: heterogeneity in structure, diversity in function. Am J Physiol Renal Physiol 275: F633–F650, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Caramelo JJ, Parodi AJ. Getting in and out from calnexin/calreticulin cycles. J Biol Chem 283: 10221–10225, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cereijido M, Contreras RG, Shoshani L, Larre I. The Na+-K+-ATPase as self-adhesion molecule and hormone receptor. Am J Physiol Cell Physiol 302: C473–C481, 2012 [DOI] [PubMed] [Google Scholar]
- 9.Clifford RJ, Kaplan JH. β-Subunit overexpression alters the stoicheometry of assembled Na-K-ATPase subunits in MDCK cells. Am J Physiol Renal Physiol 295: F1314–F1323, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Contreras RG, Flores-Beni Tez D, Flores-Maldonado C, Larre I, Shoshani L, Cereijido M. Na+,K+-ATPase and hormone ouabain:new roles for an old enzyme and an old inhibitor. Cell Mol Biol (Noisy-le-grand) 52: 31–40, 2006 [PubMed] [Google Scholar]
- 11.Contreras RG, Flores-Maldonado C, Lazaro A, Shoshani L, Flores-Benitez D, Larre I, Cereijido M. Ouabain binding to Na+,K+-ATPase relaxes cell attachment and sends a specific signal (NACos) to the nucleus. J Membr Biol 198: 147–158, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Contreras RG, Shoshani L, Flores-Maldonado C, Lazaro A, Cereijido M. Relationship between Na(+),K(+)-ATPase and cell attachment. J Cell Sci 112: 4223–4232, 1999 [DOI] [PubMed] [Google Scholar]
- 13.Crambert G, Hasler U, Beggah AT, Yu C, Modyanov NN, Horisberger JD, Lelievre L, Geering K. Transport and pharmacological properties of nine different human Na, K-ATPase isozymes. J Biol Chem 275: 1976–1986, 2000 [DOI] [PubMed] [Google Scholar]
- 14.Crambert G, Li C, Claeys D, Geering K. FXYD3 (Mat-8), a new regulator of Na,K-ATPase. Mol Biol Cell 16: 2363–2371, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feraille E, Doucet A. Sodium-potassium-adenosinetriphosphatase-dependent sodium transport in the kidney: hormonal control. Physiol Rev 81: 345–418, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Furuse M. Molecular basis of the core structure of tight junctions. Cold Spring Harb Perspect Biol 2: a002907, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Geering K. Functional roles of Na,K-ATPase subunits. Curr Opin Nephrol Hypertens 17: 526–532, 2008 [DOI] [PubMed] [Google Scholar]
- 18.Geering K. FXYD proteins: new regulators of Na-K-ATPase. Am J Physiol Renal Physiol 290: F241–F250, 2006 [DOI] [PubMed] [Google Scholar]
- 19.Gloor S, Antonicek H, Sweadner KJ, Pagliusi S, Frank R, Moos M, Schachner M. The adhesion molecule on glia (AMOG) is a homologue of the beta subunit of the Na,K-ATPase. J Cell Biol 110: 165–174, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu J, Taniguchi N. Potential of N-glycan in cell adhesion and migration as either a positive or negative regulator. Cell Adh Migr 2: 243–245, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gumbiner BM. Regulation of cadherin-mediated adhesion in morphogenesis. Nat Rev Mol Cell Biol 6: 622–634, 2005 [DOI] [PubMed] [Google Scholar]
- 22.Harrison OJ, Jin X, Hong S, Bahna F, Ahlsen G, Brasch J, Wu Y, Vendome J, Felsovalyi K, Hampton CM, Troyanovsky RB, Ben-Shaul A, Frank J, Troyanovsky SM, Shapiro L, Honig B. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure 19: 244–256, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hartsock A, Nelson WJ. Adherens and tight junctions: structure, function and connections to the actin cytoskeleton. Biochim Biophys Acta 1778: 660–669, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hebert DN, Bernasconi R, Molinari M. ERAD substrates: which way out? Semin Cell Dev Biol 21: 526–532, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Hebert DN, Garman SC, Molinari M. The glycan code of the endoplasmic reticulum: asparagine-linked carbohydrates as protein maturation and quality-control tags. Trends Cell Biol 15: 364–370, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Iijima J, Zhao Y, Isaji T, Kameyama A, Nakaya S, Wang X, Ihara H, Cheng X, Nakagawa T, Miyoshi E, Kondo A, Narimatsu H, Taniguchi N, Gu J. Cell-cell interaction-dependent regulation of N-acetylglucosaminyltransferase III and the bisected N-glycans in GE11 epithelial cells. Involvement of E-cadherin-mediated cell adhesion. J Biol Chem 281: 13038–13046, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K, Hirohashi S. Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci USA 99: 365–370, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Irie K, Shimizu K, Sakisaka T, Ikeda W, Takai Y. Roles and modes of action of nectins in cell-cell adhesion. Semin Cell Dev Biol 15: 643–656, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Kizhatil K, Davis JQ, Davis L, Hoffman J, Hogan BL, Bennett V. Ankyrin-G is a molecular partner of E-cadherin in epithelial cells and early embryos. J Biol Chem 282: 26552–26561, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Krupinski T, Beitel GJ. Unexpected roles of the Na-K-ATPase and other ion transporters in cell junctions and tubulogenesis. Physiology (Bethesda) 24: 192–201, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Larre I, Lazaro A, Contreras RG, Balda MS, Matter K, Flores-Maldonado C, Ponce A, Flores-Benitez D, Rincon-Heredia R, Padilla-Benavides T, Castillo A, Shoshani L, Cereijido M. Ouabain modulates epithelial cell tight junction. Proc Natl Acad Sci USA 107: 11387–11392, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larre I, Ponce A, Fiorentino R, Shoshani L, Contreras RG, Cereijido M. Contacts and cooperation between cells depend on the hormone ouabain. Proc Natl Acad Sci USA 103: 10911–10916, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau KS, Dennis JW. N-glycans in cancer progression. Glycobiology 18: 750–760, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Laughery M, Todd M, Kaplan JH. Oligomerization of the Na,K-ATPase in cell membranes. J Biol Chem 279: 36339–36348, 2004 [DOI] [PubMed] [Google Scholar]
- 35.Laughery MD, Todd ML, Kaplan JH. Mutational analysis of alpha-beta subunit interactions in the delivery of Na,K-ATPase heterodimers to the plasma membrane. J Biol Chem 278: 34794–34803, 2003 [DOI] [PubMed] [Google Scholar]
- 36.Li Z, Xie Z. The Na/K-ATPase/Src complex and cardiotonic steroid-activated protein kinase cascades. Pflügers Arch 457: 635–644, 2009 [DOI] [PubMed] [Google Scholar]
- 37.Liwosz A, Lei T, Kukuruzinska MA. N-glycosylation affects the molecular organization and stability of E-cadherin junctions. J Biol Chem 281: 23138–23149, 2006 [DOI] [PubMed] [Google Scholar]
- 38.Lubarski I, Asher C, Garty H. FXYD5 (dysadherin) regulates the paracellular permeability in cultured kidney collecting duct cells. Am J Physiol Renal Physiol 301: F1270–F1280, 2011 [DOI] [PubMed] [Google Scholar]
- 39.Lubarski I, Karlish SJ, Garty H. Structural and functional interactions between FXYD5 and the Na+-K+-ATPase. Am J Physiol Renal Physiol 293: F1818–F1826, 2007 [DOI] [PubMed] [Google Scholar]
- 40.Lubarski I, Pihakaski-Maunsbach K, Karlish SJ, Maunsbach AB, Garty H. Interaction with the Na,K-ATPase and tissue distribution of FXYD5 (related to ion channel). J Biol Chem 280: 37717–37724, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Madan P, Rose K, Watson AJ. Na/K-ATPase beta1 subunit expression is required for blastocyst formation and normal assembly of trophectoderm tight junction-associated proteins. J Biol Chem 282: 12127–12134, 2007 [DOI] [PubMed] [Google Scholar]
- 42.Miller RP, Farley RA. All three potential N-glycosylation sites of the dog kidney (Na+ + K+)-ATPase beta-subunit contain oligosaccharide. Biochim Biophys Acta 954: 50–57, 1988 [DOI] [PubMed] [Google Scholar]
- 43.Mishra NK, Peleg Y, Cirri E, Belogus T, Lifshitz Y, Voelker DR, Apell HJ, Garty H, Karlish SJ. FXYD proteins stabilize Na,K-ATPase: amplification of specific phosphatidylserine-protein interactions. J Biol Chem 286: 9699–9712, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miyoshi J, Takai Y. Molecular perspective on tight-junction assembly and epithelial polarity. Adv Drug Deliv Rev 57: 815–855, 2005 [DOI] [PubMed] [Google Scholar]
- 45.Miyoshi J, Takai Y. Nectin and nectin-like molecules: biology and pathology. Am J Nephrol 27: 590–604, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Mutlu GM, Sznajder JI. Mechanisms of pulmonary edema clearance. Am J Physiol Lung Cell Mol Physiol 289: L685–L695, 2005 [DOI] [PubMed] [Google Scholar]
- 47.Nam JS, Hirohashi S, Wakefield LM. Dysadherin: a new player in cancer progression. Cancer Lett 255: 161–169, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Narita H, Yamamoto Y, Suzuki M, Miyazaki N, Yoshida A, Kawai K, Iwasaki K, Nakagawa A, Takai Y, Sakisaka T. Crystal structure of the cis-dimer of nectin-1: implications for the architecture of cell-cell junctions. J Biol Chem 286: 12659–12669, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nelson WJ, Hammerton RW, McNeill H. Role of the membrane-cytoskeleton in the spatial organization of the Na,K-ATPase in polarized epithelial cells. Soc Gen Physiol Ser 46: 77–87, 1991 [PubMed] [Google Scholar]
- 50.Nelson WJ, Shore EM, Wang AZ, Hammerton RW. Identification of a membrane-cytoskeletal complex containing the cell adhesion molecule uvomorulin (E-cadherin), ankyrin, and fodrin in Madin-Darby canine kidney epithelial cells. J Cell Biol 110: 349–357, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nelson WJ, Veshnock PJ. Ankyrin binding to (Na+ + K+)ATPase and implications for the organization of membrane domains in polarized cells. Nature 328: 533–536, 1987 [DOI] [PubMed] [Google Scholar]
- 52.Padilla-Benavides T, Roldan ML, Larre I, Flores-Benitez D, Villegas-Sepulveda N, Contreras RG, Cereijido M, Shoshani L. The polarized distribution of Na+,K+-ATPase: role of the interaction between beta subunits. Mol Biol Cell 21: 2217–2225, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Petrescu AJ, Milac AL, Petrescu SM, Dwek RA, Wormald MR. Statistical analysis of the protein environment of N-glycosylation sites: implications for occupancy, structure, and folding. Glycobiology 14: 103–114, 2004 [DOI] [PubMed] [Google Scholar]
- 54.Pinho SS, Seruca R, Gartner F, Yamaguchi Y, Gu J, Taniguchi N, Reis CA. Modulation of E-cadherin function and dysfunction by N-glycosylation. Cell Mol Life Sci 68: 1011–1020, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rajasekaran AK, Hojo M, Huima T, Rodriguez-Boulan E. Catenins and zonula occludens-1 form a complex during early stages in the assembly of tight junctions. J Cell Biol 132: 451–463, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rajasekaran AK, Rajasekaran SA. Role of Na-K-ATPase in the assembly of tight junctions. Am J Physiol Renal Physiol 285: F388–F396, 2003 [DOI] [PubMed] [Google Scholar]
- 57.Rajasekaran SA, Gopal J, Espineda C, Ryazantsev S, Schneeberger EE, Rajasekaran AK. HPAF-II, a cell culture model to study pancreatic epithelial cell structure and function. Pancreas 29: e77–e83, 2004 [DOI] [PubMed] [Google Scholar]
- 58.Rajasekaran SA, Hu J, Gopal J, Gallemore R, Ryazantsev S, Bok D, Rajasekaran AK. Na,K-ATPase inhibition alters tight junction structure and permeability in human retinal pigment epithelial cells. Am J Physiol Cell Physiol 284: C1497–C1507, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Rajasekaran SA, Palmer LG, Moon SY, Peralta Soler A, Apodaca GL, Harper JF, Zheng Y, Rajasekaran AK. Na,K-ATPase activity is required for formation of tight junctions, desmosomes, and induction of polarity in epithelial cells. Mol Biol Cell 12: 3717–3732, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rajasekaran SA, Palmer LG, Quan K, Harper JF, Ball WJ, Jr, Bander NH, Peralta Soler A, Rajasekaran AK. Na,K-ATPase beta-subunit is required for epithelial polarization, suppression of invasion, and cell motility. Mol Biol Cell 12: 279–295, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajasekaran SA, Rajasekaran AK. Na,K-ATPase and epithelial tight junctions. Front Biosci 14: 2130–2148, 2009 [DOI] [PubMed] [Google Scholar]
- 62.Shapiro L, Weis WI. Structure and biochemistry of cadherins and catenins. Cold Spring Harb Perspect Biol 1: a003053, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shin JM, Grundler G, Senn-Bilfinger J, Simon WA, Sachs G. Functional consequences of the oligomeric form of the membrane-bound gastric H,K-ATPase. Biochemistry 44: 16321–16332, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Shinoda T, Ogawa H, Cornelius F, Toyoshima C. Crystal structure of the sodium-potassium pump at 2.4 A resolution. Nature 459: 446–450, 2009 [DOI] [PubMed] [Google Scholar]
- 65.Shoshani L, Contreras RG, Roldan ML, Moreno J, Lazaro A, Balda MS, Matter K, Cereijido M. The polarized expression of Na+,K+-ATPase in epithelia depends on the association between beta-subunits located in neighboring cells. Mol Biol Cell 16: 1071–1081, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Steed E, Balda MS, Matter K. Dynamics and functions of tight junctions. Trends Cell Biol 20: 142–149, 2010 [DOI] [PubMed] [Google Scholar]
- 67.Sweadner KJ, Rael E. The FXYD gene family of small ion transport regulators or channels: cDNA sequence, protein signature sequence, and expression. Genomics 68: 41–56, 2000 [DOI] [PubMed] [Google Scholar]
- 68.Sznajder JI. Alveolar edema must be cleared for the acute respiratory distress syndrome patient to survive. Am J Respir Crit Care Med 163: 1293–1294, 2001 [DOI] [PubMed] [Google Scholar]
- 69.Takai Y, Irie K, Shimizu K, Sakisaka T, Ikeda W. Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci 94: 655–667, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Takeda K, Noguchi S, Sugino A, Kawamura M. Functional activity of oligosaccharide-deficient (Na,K)ATPase expressed in Xenopus oocytes. FEBS Lett 238: 201–204, 1988 [DOI] [PubMed] [Google Scholar]
- 71.Tamkun MM, Fambrough DM. The (Na+ + K+)-ATPase of chick sensory neurons. Studies on biosynthesis and intracellular transport. J Biol Chem 261: 1009–1019, 1986 [PubMed] [Google Scholar]
- 72.Taniguchi K, Kaya S, Abe K, Mardh S. The oligomeric nature of Na/K-transport ATPase. J Biochem 129: 335–342, 2001 [DOI] [PubMed] [Google Scholar]
- 73.Tokhtaeva E, Munson K, Sachs G, Vagin O. N-glycan-dependent quality control of the Na,K-ATPase beta(2) subunit. Biochemistry 49: 3116–3128, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tokhtaeva E, Sachs G, Souda P, Bassilian S, Whitelegge JP, Shoshani L, Vagin O. Epithelial junctions depend on intercellular trans-interactions between the Na,K-ATPase β1 subunits. J Biol Chem 286: 25801–25812, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tokhtaeva E, Sachs G, Sun H, Dada LA, Sznajder JI, Vagin O. Identification of the amino-acid region involved in the intercellular interaction between the Na,K-ATPase β1 subunits. J Cell Sci. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tokhtaeva E, Sachs G, Vagin O. Assembly with the Na,K-ATPase alpha(1) subunit is required for export of beta(1) and beta(2) subunits from the endoplasmic reticulum. Biochemistry 48: 11421–11431, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Treuheit MJ, Costello CE, Kirley TL. Structures of the complex glycans found on the beta-subunit of (Na,K)-ATPase. J Biol Chem 268: 13914–13919, 1993 [PubMed] [Google Scholar]
- 78.Tsukamoto T, Nigam SK. Tight junction proteins form large complexes and associate with the cytoskeleton in an ATP depletion model for reversible junction assembly. J Biol Chem 272: 16133–16139, 1997 [DOI] [PubMed] [Google Scholar]
- 79.Vadasz I, Raviv S, Sznajder JI. Alveolar epithelium and Na,K-ATPase in acute lung injury. Intensive Care Med 33: 1243–1251, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vagin O, Tokhtaeva E, Sachs G. The role of the beta1 subunit of the Na,K-ATPase and its glycosylation in cell-cell adhesion. J Biol Chem 281: 39573–39587, 2006 [DOI] [PubMed] [Google Scholar]
- 81.Vagin O, Tokhtaeva E, Yakubov I, Shevchenko E, Sachs G. Inverse correlation between the extent of N-glycan branching and intercellular adhesion in epithelia. Contribution of the Na,K-ATPase beta1 subunit. J Biol Chem 283: 2192–2202, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Violette MI, Madan P, Watson AJ. Na+/K+-ATPase regulates tight junction formation and function during mouse preimplantation development. Dev Biol 289: 406–419, 2006 [DOI] [PubMed] [Google Scholar]
- 83.Vogelmann R, Nelson WJ. Fractionation of the epithelial apical junctional complex: reassessment of protein distributions in different substructures. Mol Biol Cell 16: 701–716, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wu Y, Jin X, Harrison O, Shapiro L, Honig BH, Ben-Shaul A. Cooperativity between trans and cis interactions in cadherin-mediated junction formation. Proc Natl Acad Sci USA 107: 17592–17597, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Xu Q, Akama R, Isaji T, Lu Y, Hashimoto H, Kariya Y, Fukuda T, Du Y, Gu J. Wnt/beta-catenin signaling down-regulates N-acetylglucosaminyltransferase III expression: the implications of two mutually exclusive pathways for regulation. J Biol Chem 286: 4310–4318, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Youn YH, Hong J, Burke JM. Cell phenotype in normal epithelial cell lines with high endogenous N-cadherin: comparison of RPE to an MDCK subclone. Invest Ophthalmol Vis Sci 47: 2675–2685, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zamofing D, Rossier BC, Geering K. Inhibition of N-glycosylation affects transepithelial Na+ but not Na+-K+ATPase transport. Am J Physiol Cell Physiol 256: C958–C966, 1989 [DOI] [PubMed] [Google Scholar]
- 88.Zhang Z, Devarajan P, Dorfman AL, Morrow JS. Structure of the ankyrin-binding domain of alpha-Na,K-ATPase. J Biol Chem 273: 18681–18684, 1998 [DOI] [PubMed] [Google Scholar]
- 89.Zhao H, Liang Y, Xu Z, Wang L, Zhou F, Li Z, Jin J, Yang Y, Fang Z, Hu Y, Zhang L, Su J, Zha X. N-glycosylation affects the adhesive function of E-cadherin through modifying the composition of adherens junctions (AJs) in human breast carcinoma cell line MDA-MB-435. J Cell Biochem 104: 162–175, 2008 [DOI] [PubMed] [Google Scholar]
- 90.Zhao Y, Sato Y, Isaji T, Fukuda T, Matsumoto A, Miyoshi E, Gu J, Taniguchi N. Branched N-glycans regulate the biological functions of integrins and cadherins. FEBS J 275: 1939–1948, 2008 [DOI] [PubMed] [Google Scholar]
- 91.Zhao YY, Takahashi M, Gu JG, Miyoshi E, Matsumoto A, Kitazume S, Taniguchi N. Functional roles of N-glycans in cell signaling and cell adhesion in cancer. Cancer Sci 99: 1304–1310, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]